
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 14 May 2024
Sec. Thyroid Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1372824
Background: IgA nephropathy (IgAN), the most common type of glomerulonephritis, has great individual differences in prognosis. Many studies showed the relationship between thyroid hormones and chronic kidney disease. However, the relationship between free thyroxine (FT4), as a thyroid hormone, and IgAN is still unclear. This study aimed to evaluate the impact of FT4 on IgAN prognosis.
Methods: This retrospective study involved 223 patients with biopsy-proven IgAN. The renal composite outcomes were defined as: (1) ESRD, defined as eGFR < 15 ml/(min·1.73 m2) or initiation of renal replacement therapy (hemodialysis, peritoneal dialysis, renal transplantation); (2) serum creatinine doubled from baseline; (3) eGFR decreased by more than 50% from baseline. The predictive value was determined by the area under the curve (AUC). Kaplan-Meier and Cox proportional hazards analyses assessed renal progression and prognosis.
Results: After 38 (26–54) months of follow-up, 23 patients (10.3%) experienced renal composite outcomes. Kaplan-Meier survival curve analysis showed that the renal survival rate of the IgAN patients with FT4<15.18pmol/L was lower than that with FT4≥15.18pmol/L (P < 0. 001). Multivariate Cox regression model analysis showed that FT4 was a protective factor for poor prognosis of IgAN patients, whether as a continuous variable or a categorical variable (HR 0.68, 95%CI 0.51–0.90, P =0.007; HR 0.04, 95%CI 0.01–0.20, P <0.001). ROC curve analysis showed that FT4 combined with t score had a high predictive value for poor prognosis of IgAN patients (AUC=0.881, P<0.001).
Conclusion: FT4 was a protective factor for IgAN. In addition, FT4 combined with tubular atrophy/interstitial fibrosis had a high predictive value for poor prognosis of IgAN.
IgA nephropathy (IgAN) is the most prevalent primary glomerulonephritis worldwide and carries a considerable lifetime risk of renal failure (1). 20%-50% of patients develop end-stage renal disease (ESRD) approximately 20 years after diagnosis (2–4). The prognosis of IgAN varies greatly, and the prognosis of individual patients remains difficult to predict. More indicators are urgently needed to predict the prognosis of IgAN patients to improve treatment strategies.
The thyroid gland interacts with the kidney. Thyroid hormone can directly affect renal growth and development, hemodynamics, and electrolyte balance (5). The kidney is also involved in thyroid hormone metabolism and elimination. In addition, IgAN and some thyroid diseases are affected by autoimmunity (6, 7). Free thyroxine is an important member of the thyroid hormone, but its effect on IgAN has not been studied.
In this study, we explored the effect of free thyroxine (FT4) on the prognosis of IgAN for the first time. Additionally, we demonstrated the predictive value of FT4 combined with renal tubular atrophy/interstitial fibrosis for the poor prognosis of IgAN.
We retrospectively analyzed the clinical data of patients with primary IgAN who were admitted to the First People’s Hospital of Changzhou from January 1, 2018 to December 31, 2021. Renal biopsies were performed on all these patients. Inclusion criteria were as follows: (1) Estimated glomerular filtration rate (eGFR) > 15 ml/(min·1.73 m 2) during renal biopsy; (2) Complete baseline data. Exclusion criteria were as follows: (1) lupus nephritis, Henoch-Schonlein purpura nephritis, hepatitis B virus-related nephritis and other secondary IgAN patients; (2) complicated with other primary glomerular diseases; (3) complicated with other infectious diseases, malignant tumors or serious organic diseases. (4) loss of follow-up or death due to non-renal causes. Loss of follow-up was defined as no follow-up record at our hospital after renal puncture. This study was approved by the Ethics Committee of the First People’s Hospital of Changzhou (2024 #11).
The general data and laboratory test results of the patients at the time of IgAN diagnosis were collected from the clinical medical record system as baseline data. Gender, age, proportion of hypertension, body mass index(BMI), hemoglobin, albumin, globulin,TSH, free triiodothyronine (FT3), FT4,Thyroid disease and treatment, serum creatinine (Scr), uric acid, 24h urine protein, urine red blood cell count (URBC) and treatment methods were collected. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (Supplementary Table 1). Hypertension was defined as SBP≥140 mmHg and/or DBP≥90 mmHg. Anemia was defined as hemoglobin concentration <120g/L for men and <110g/L for women. Hyperuricemia was defined as uric acid concentration >420µmol/L in males and > 360µmol/L in females. Hypoalbuminemia was defined as serum albumin <30g/L.
All renal biopsy specimens were evaluated by experienced pathologists and nephrologists who were blinded to the patients’ information. The updated Oxford classification of IgAN (8) we used for the evaluation of pathological lesions in biopsy specimens was as follows: (1) mesangial hypercellularity(M): more than 3 mesangial cells in more than 50% of the mesangial area was defined as M1, otherwise M0; (2) endocapillary hypercellularity (E): hypercellularity in the lumen of glomerular capillaries was defined as E1, otherwise E0; (3) segmental glomerulosclerosis (S): any variable degree of loop involvement, including segmental sclerosis of the glomerulus, was defined as S1,otherwise S0; (4) tubular atrophy and interstitial fibrosis (T): lesion size ≤ 25% was defined as T0, 26%-50% as T1, and > 50% as T2; (5) cellular or fibrocellular crescents (C): no crescent was defined as C0, < 25% as C1, and≥25% as C2.
Follow-up was performed through the hospital medical record system or by telephone. The follow-up period ended in August 2023. The renal composite outcomes were defined as: (1) ESRD, defined as eGFR < 15 ml/(min·1.73 m2) or initiation of renal replacement therapy (hemodialysis, peritoneal dialysis, renal transplantation); (2) serum creatinine doubled from baseline; (3) eGFR decreased by more than 50% from baseline.
We used SPSS 26.0 to analyze all data. Quantitative variables with normal distribution were expressed mean ± SD, and comparison between groups use independent sample t-test; Non-normal distribution quantitative variables were represented as median and interquartile range (IQR), and Mann-Whitney U test was used for comparison between groups. Categorical data were expressed as frequencies (percentages), and comparison between groups was analyzed using the chi-square test or Mann-Whitney U test. Kaplan-Meier survival curves were drawn to compare the renal survival rates of the two groups. Univariate and multivariate Cox regression models were used to analyze the risk factors affecting renal composite outcomes in IgAN patients. The multivariate Cox regression analysis included the variables with P-value<0.05 in univariate Cox regression analysis. ROC curve was used to analyze the predictive efficacy of different indicators for renal composite outcomes in IgAN patients. The predictive value was determined by the AUC. P < 0. 05 was considered statistically significant. In addition, sensitivity analyses were performed by excluding patients with thyroid diseases and thyroid hormone replacement therapy.
A total of 223 IgAN patients were enrolled (Figure 1). The results of ROC curve analysis showed that the best cut-off value of FT4 for predicting renal composite outcomes in IgAN patients was 15.18 pmol/L. The AUC of FT4 for predicting renal composite outcomes in IgAN patients was 0. 777, and the sensitivity and specificity were 87.0% and 65.0%, respectively (Figure 2).
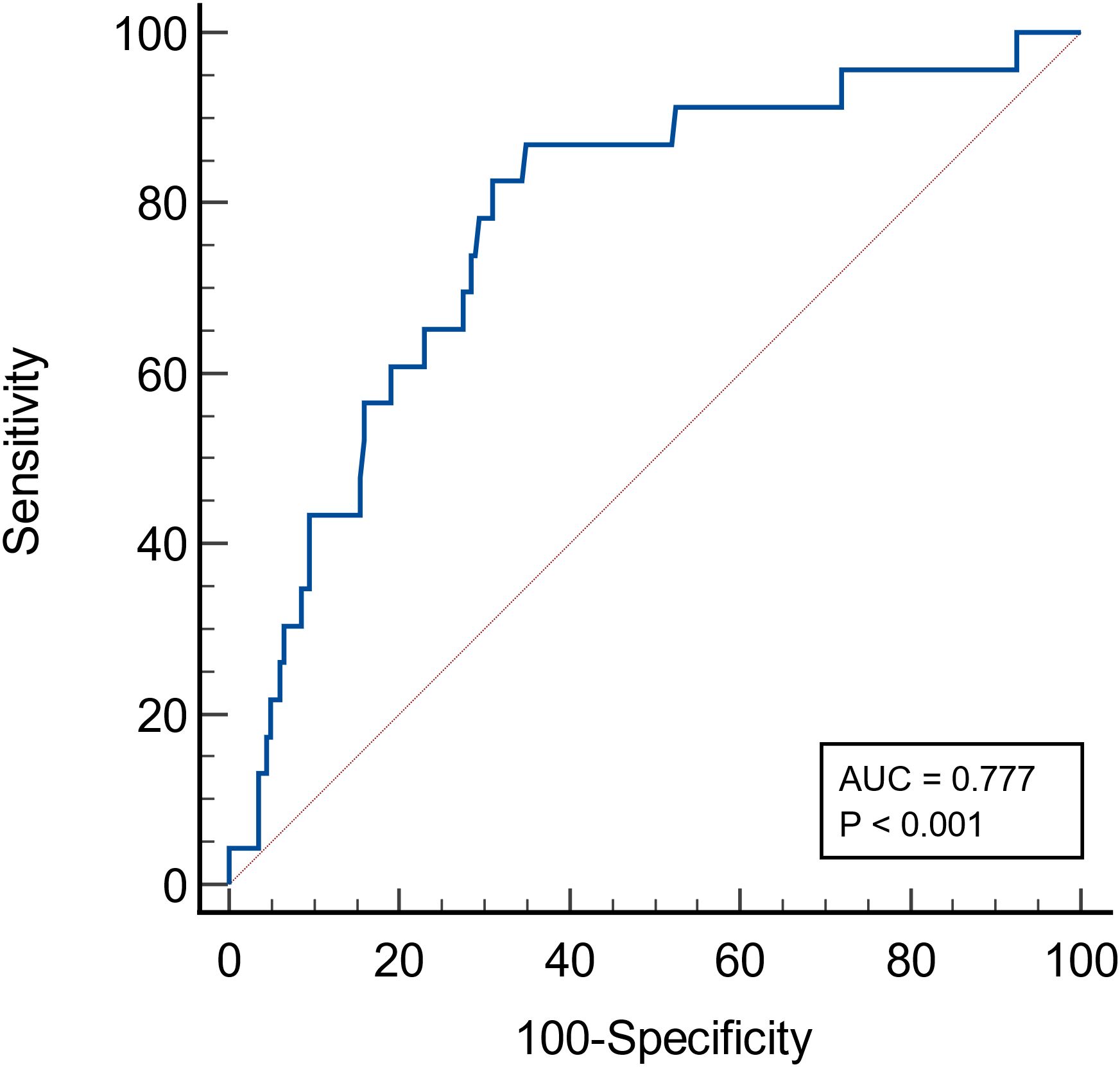
Figure 2 ROC curve of the probability of FT4 in predicting renal composite outcomes in patients with IgA nephropathy.
Table 1 described the baseline characteristics of 223 IgAN patients at the time of renal biopsy. Their median age was 39 (30–50) years, and 123 (55.2%) patients were male. 88 (39.5%) patients had hypertension. The median serum creatinine at renal biopsy was 94 (70–121) µmol/l, and the median 24h urine protein was 2 (1.13–3.43) g/day. 8 (3.6%) patients had hypothyroidism, 17 (7.6%) patients had subclinical hypothyroidism, 5 (2.2%) patients had low T3 syndrome and one (0.5%) patient had subclinical hyperthyroidism. 4 (1.8%) patients were treated with supplemental thyroid hormone, of whom three had hypothyroidism, and one had subclinical hypothyroidism. Compared with the renal survival group, the non-renal survival group had lower levels of FT3 and FT4, and higher levels of serum creatinine and 24-hour urinary protein. In addition, the non-renal survival group had significantly higher proportion of anemia, hypoalbuminemia, and hyperuricemia (56.5% vs. 12.5%, P<0.001; 39.1% vs 16.0%, P=0.015; 69.6% vs. 44.0%, P=0.020), but lower proportion of ACEI/ARB using to reduce urinary protein (26.1 vs. 55.5%, P=0.007).
There were significant differences in the proportion of tubular atrophy/interstitial fibrosis and cellular/fibrous crescents between the two groups. The proportion of T2 and C2 in the non-renal survival group was significantly higher than that in the renal survival group (52.2% vs 6.0%, P<0.001; 17.4% vs. 2.0%, P=0.011). Mesangial cell proliferation, segmental glomerulosclerosis, and endocapillary cell proliferation were not significantly different between the two groups (Table 2).
The median follow-up time was 38 (26–54) months. During the follow-up period, 23 patients (10.3%) experienced renal composite outcomes. Kaplan-Meier survival curve analysis showed that the renal survival rate of the patients with FT4<15.18pmol/L was lower than that with FT4≥15.18pmol/L(P < 0. 001) (Figure 3).
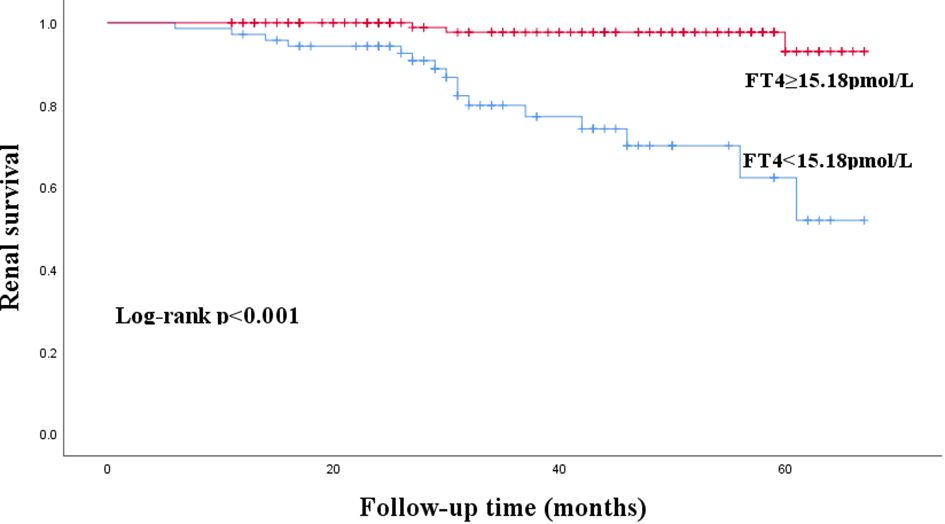
Figure 3 Kaplan-Meier curves for renal composite outcomes in patients with IgA nephropathy according to FT4.
Univariate Cox regression model analysis showed that anemia (HR 8.2, 95%CI 3.57–18.84, P<0.001), hypoalbuminemia (HR 3.76, 95%CI 1.62–8.72, P=0.002), serum creatinine (HR 1.03, 95%CI 1.02–1.03, P<0.001), 24h urine protein (HR 1.14, 95%CI 1.04–1.23, P=0.003), T1 (HR 3.5, 95%CI 1.07–11.5, P=0.039), T2 (HR 28.54, 95%CI 10.08–80.75, P<0.001), C2 (HR 8.47, 95%CI 2.43–29.61, P=0.001), TSH (HR 1.08, 95%CI 1.02–1.15, P=0.015), and hypothyroidism (HR 5.46, 95%CI 1.59–18.79, P=0.007) were risk factors for renal composite outcomes in IgAN patients (P < 0. 05) (Table 3). Besides, FT3 (HR 0.36, 95%CI 0.23–0.58, P<0.001), FT4 (HR 0.70, 95%CI 0.60–0.81, P<0.001), FT4≥15.18pmol/L (HR 0.08, 95%CI 0.02–0.27, P<0.001), and using ACEI/ARB to treat (HR 0.33, 95%CI 0.13–0.84, P=0.020) were protective factors for IgAN patients. However, thyroid hormone replacement therapy for patients with abnormal thyroid function did not affect the prognosis of IgAN (HR 3.98, 95%CI 0.53–29.78, P=0.178).
Multivariate Cox regression model analysis showed that even after adjusting these confounding factors, FT4 (HR 0.68, 95%CI 0.51–0.90, P =0.007) was still a protective factor for IgAN patients when considered as a continuous variable (Table 4). And the risk in IgAN patients with FT4≥15.18pmol/L is 0.04 times than that in patients with FT4<15.18pmol/L (HR 0.04, 95%CI 0.01–0.20, P <0.001) (Table 5). In addition, a higher serum creatinine and TSH were independently associated with renal composite outcomes in IgAN patients (HR 1.03, 95%CI 1.01–1.04, P<0.001 or HR 1.03, 95%CI 1.02–1.04, P<0.001; HR 1.08, 95%CI 1.01–1.14, P=0.021 or HR 1.12, 95%CI 1.05–1.19, P=0.001).
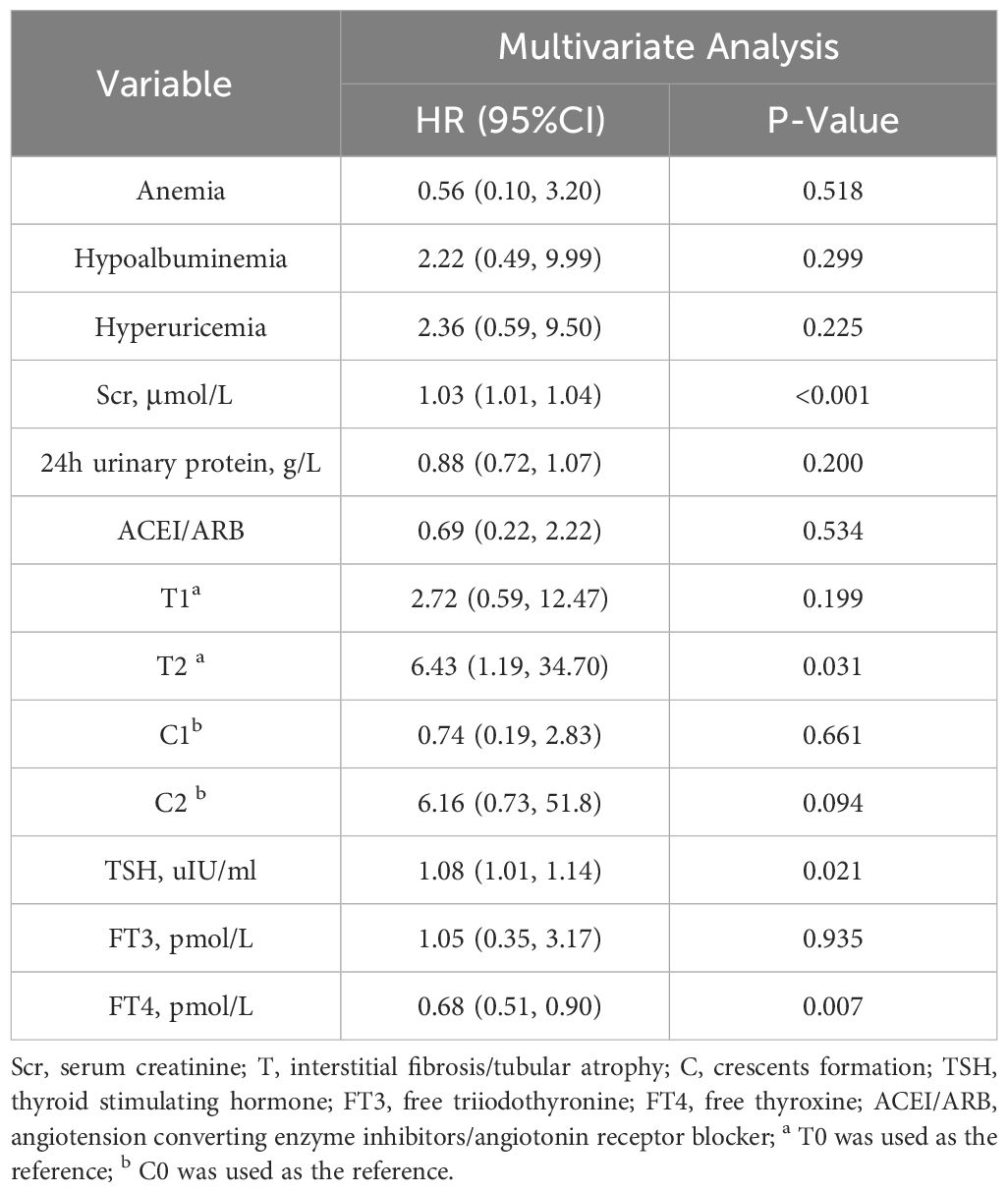
Table 4 Multivariate Cox analysis of renal composite outcomes in IgAN patients (FT4 was a continuous variable).
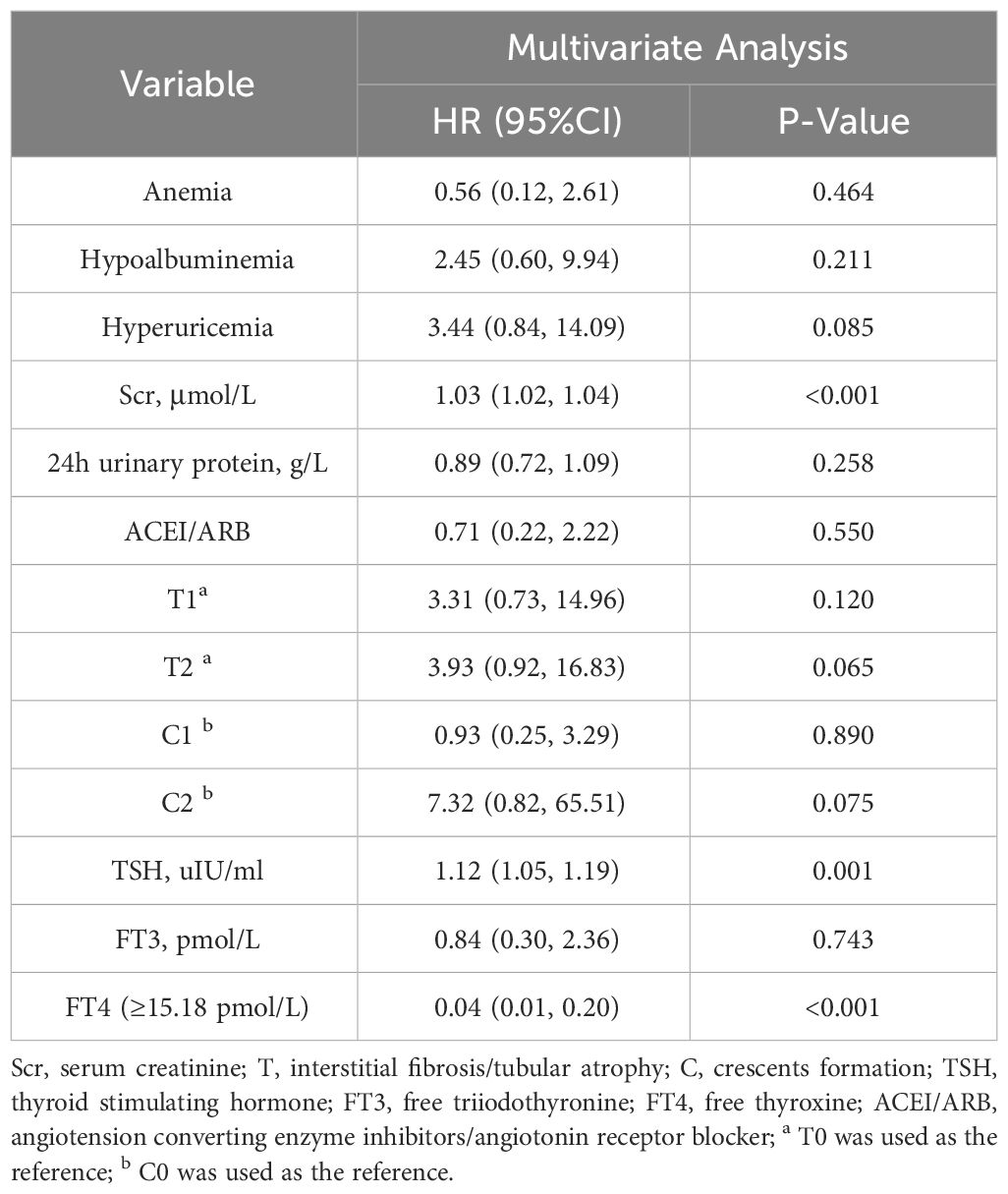
Table 5 Multivariate Cox analysis of renal composite outcomes in IgAN patients (FT4 was a categorical variable).
To predict the renal composite outcomes, the AUC of probability of FT4 was 0.777, with a sensitivity of 87.0% and a specificity of 65.0% (Table 6). The AUC of probability of T-score was 0.789, with a sensitivity of 73.9% and a specificity of 75.5%. The AUC of probability of TSH was 0.533, with a sensitivity of 43.5% and a specificity of 69.5%. The AUC of the joint probability of FT4 and T-score in predicting the renal composite outcomes was 0.881, with a sensitivity of 82.6% and a specificity of 85.5%. Moreover, it was larger than that predicted by these two indexes alone (P<0.05) (Tables 6, 7, Figure 4).
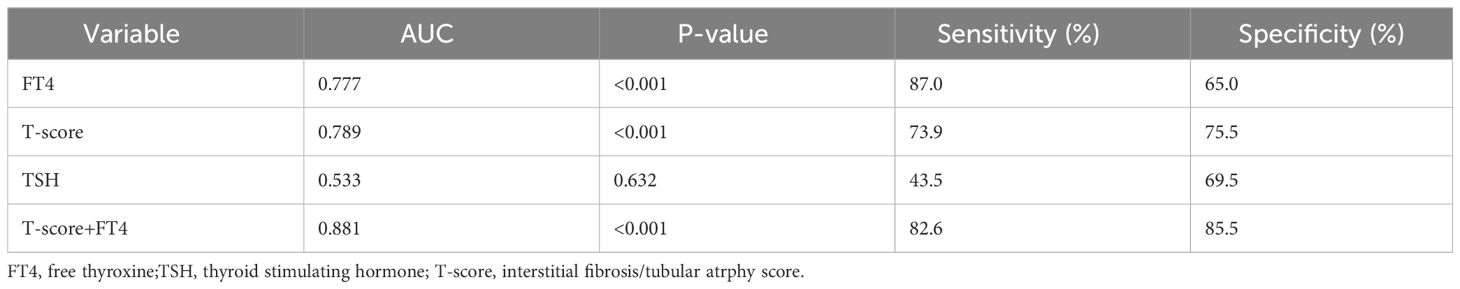
Table 6 ROC curve analysis of the probability in predicting renal composite outcomes in patients with IgA nephropathy.
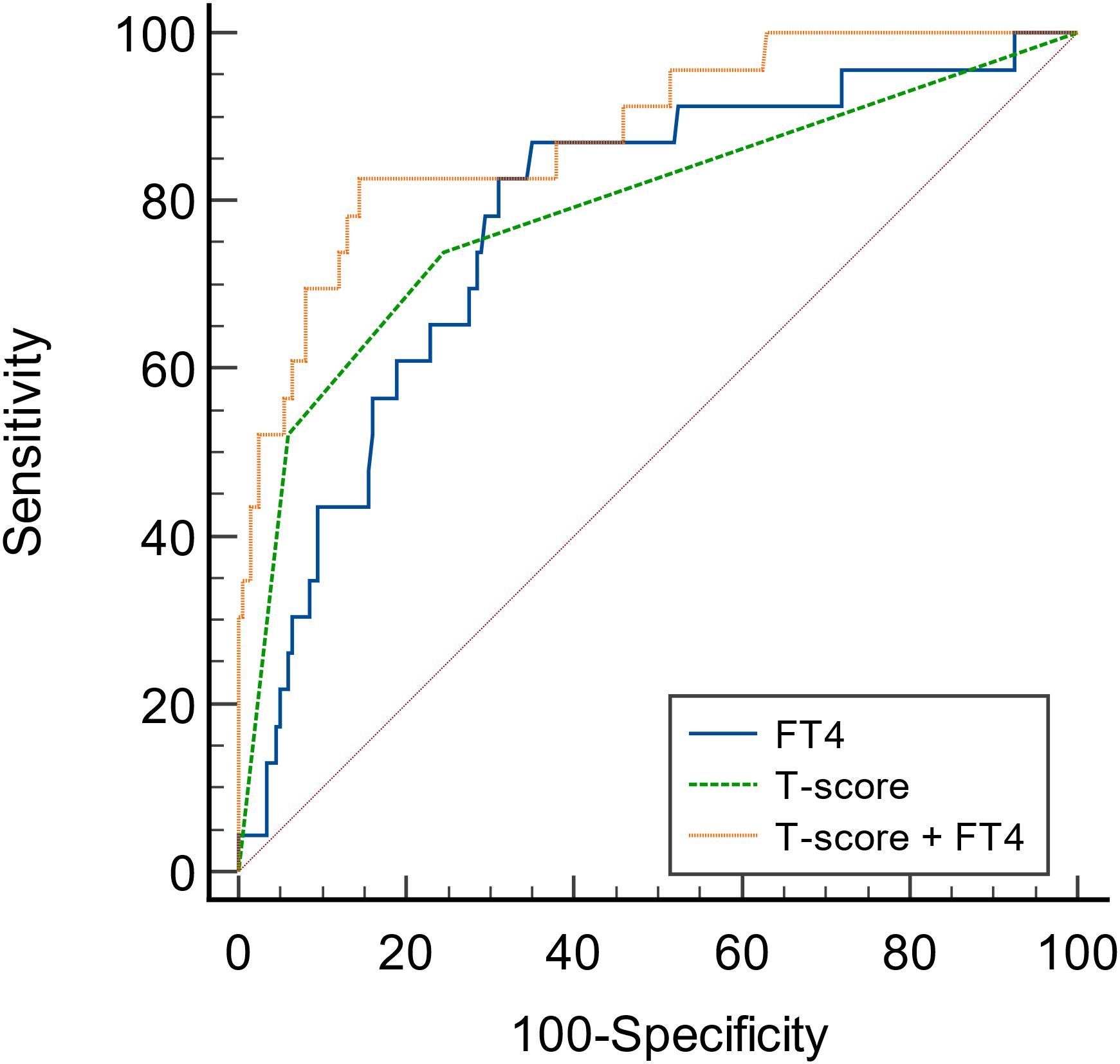
Figure 4 ROC curves of the probability of FT4 and T-score in predicting renal composite outcomes in patients with IgA nephropathy.
We conducted sensitivity analyses to determine the potential effect of confounding due to thyroid diseases and thyroid hormone replacement therapy. We excluded patients with thyroid diseases and thyroid hormone replacement therapy, and found that FT4, whether as a continuous variable or a categorical variable, was still a protective factor for the prognosis of IgAN (Supplementary Tables 2-4). Additionally, FT4 combined with T-score still had a high predictive value for poor prognosis of IgAN patients (Supplementary Table 5, Supplementary Figure 1). Therefore, thyroid diseases and thyroid hormone replacement therapy did not significantly affect the predictive performance of FT4. However, TSH was no longer an independent risk factor for the poor prognosis of IgAN patients.
This study demonstrated that FT4 was a protective factor and TSH was an independent risk factor for renal progression of IgAN patients, even after adjusting for covariates such as anemia, hypoalbuminemia, Hyperuricemia, 24h urinary protein, FT3, renal tubular atrophy/interstitial fibrosis, cellular or fibrocellular crescents, and treatment regimen. But, after excluding patients with thyroid disease and thyroid replacement therapy, TSH was no longer the risk factor for IgAN patients. Additionally, univariate Cox regression analysis showed that lower FT3 and hypothyroidism were associated with poor prognosis in IgAN patients. However, these associations disappeared after adjusting for confounding factors. Previous studies mainly focused on the relationship between thyroid hormones and chronic kidney disease (CKD). This study is the first to explore the relationship between thyroid hormones and the prognosis of IgAN.
Thyroid hormones can affect the function of the kidney and play a crucial role in renal physiological homeostasis. Hypothyroidism is common in CKD. Previous studies have mainly shown the relationship between hypothyroidism and renal function and its role in renal poor prognosis in CKD (9, 10). Similar to these previous studies, our study found that hypothyroidism was associated with poor prognosis in IgAN. However, most of the IgAN patients included in this study had thyroid hormone levels within the normal range. The best cut-off value of FT4 obtained by ROC curve was 15.18 pmol/L, which was higher than the FT4 value defined by hypothyroidism. Similar to the findings in CKD population, the IgAN patients with a lower FT4 level had worse prognosis. However, our results also contradict the findings of some studies. In a prospective study, higher levels of FT4 within the normal range were associated with an increased risk of developing CKD, a rapid decline in GFR, and an increased risk of complications (11). In euthyroid patients with diabetic nephropathy, elevated TSH and FT4 were associated with decreased eGFR in diabetic patients (12). A larger cohort with longer follow-up is needed to further verify the relationship between thyroid hormones and IgAN.
Similar to the relationship between hypothyroidism and CKD, lower FT4 may contribute to the progression of renal function in IgA nephropathy through the following aspects (1). Hemodynamic changes: Lower FT4 cause decreased cardiac contractility, decreased circulating blood volume, and renal hypoperfusion (13). Besides, lower FT4 reduce the synthesis of vascular endothelial relaxation factors, leading to arterial stiffness and increased systemic vascular resistance (14). In addition, the sensitivity to β-adrenoceptor is decreased in patients with lower FT4, and the expression and release of renin are decreased, resulting in the decreased activity of the renin-angiotensin-aldosterone system (RAAS) (2, 15). Glomerular changes: Lower FT4 lead to adaptive preglomerular vasoconstriction caused by filtrate overload due to inadequate reabsorption of sodium and water in the proximal tubule. This reduces renal blood flow, leading to prerenal renal injury (16). On the other hand, lower FT4 cause changes in glomerular structure, such as glomerular basement membrane thickening or mesangial matrix widening (17), which further reduces renal blood flow (3). Tubular changes: Lower FT4 regulate renal water and electrolyte metabolism mainly by affecting urine concentration and dilution and renal tubular reabsorption (18, 19). The above mechanisms synergistically lead to the progression of renal function. In addition, Benedetti et al.’s preclinical studies have shown that alterations in thyroid hormones are critical in podocyte pathology associated with diabetic nephropathy (20, 21). They identified thyroid hormone receptor α1 (TRα1) as a key regulator of DN pathogenesis. Although TRa1 has not been studied in IgA nephropathy, it could be an interesting mechanism to clarify in the future.
This study showed that serum creatinine is another independent risk factor for renal composite outcomes in patients with IgAN. The consistency between serum creatinine and renal function is close to poor prognosis of patients.
Renal biopsy is the gold standard for the diagnosis of IgAN, and it can also predict the prognosis of IgAN relatively. As one of the pathological indicators of IgAN, the predictive effect of T on renal prognosis has been widely validated by in previous studies (22). In the univariate Cox regression analysis of this study, T-score was also associated with the prognosis of IgAN. And in the multivariate Cox regression analysis with FT4 as a continuous variable, T2 was still an independent factor for poor prognosis of IgAN. Therefore, we combined FT4 and T-score in order to get more accurate predictive efficacy. The results showed that the combination of FT4 and T-score was the best than these two alone. As mentioned in previous studies, T-score is an independent risk factor for poor prognosis of IgAN, and this study further found that FT4 combined with kidney biopsy can greatly improve the predictive value of patients’ prognosis, which is important for more accurate and comprehensive clinical judgment of the prognosis of IgAN patients.
However, this study still has some limitations. This study is a single-center retrospective study, so the study may suffer from selection bias and a center-specific effect. Further prospective, multi-center studies with a larger sample size and longer follow-up, are needed for validation.
FT4 was a protective factor for renal progression of IgAN patients. In addition, FT4 combined with tubular atrophy/interstitial fibrosis had a high predictive value for poor prognosis of IgAN.
The original contributions presented in the study are included in the article/Supplementary material; further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
BY: Data curation, Investigation, Methodology, Software, Writing – original draft. WZ: Data curation, Investigation, Methodology, Software, Writing – original draft. LC: Data curation, Investigation, Writing – original draft. LT: Data curation, Investigation, Writing – original draft. YN: Data curation, Investigation, Writing – original draft. MY: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. YY: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Changzhou Science and Technology Youth Talent Support Program (Grant No. KY20221408), Funding from Young Talent Development Plan of Changzhou Health Commission (Grant No. CZQM2023002) and Changzhou Key Medical Discipline (Grant No. CZXK202204).
The authors thank Professor Bin Xu for statistical guidance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1372824/full#supplementary-material
1. Stamellou E, Seikrit C, Tang SCW, Boor P, Tesař V, Floege J, et al. IgA nephropathy. Nat Rev Dis Primers. (2023) 9:67. doi: 10.1038/s41572-023-00476-9
2. Radford MG Jr., Donadio JV Jr., Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. (1997) 8:199–207. doi: 10.1681/ASN.V82199
3. Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PloS One. (2014) 9:e91756. doi: 10.1371/journal.pone.0091756
4. Magistroni R, D’Agati VD, Appel GB, Kiryluk K. New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int. (2015) 88:974–89. doi: 10.1038/ki.2015.252
5. Iglesias P, Díez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. (2009) 160:503–15. doi: 10.1530/EJE-08-0837
6. Rodrigues JC, Haas M, Reich HN. IgA nephropathy. Clin J Am Soc Nephrol. (2017) 12:677–86. doi: 10.2215/CJN.07420716
7. McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. (2012) 42:252–65. doi: 10.1007/s12020-012-9703-2
8. Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. (2017) 91:1014–21. doi: 10.1016/j.kint.2017.02.003
9. Chang YC, Chang CH, Yeh YC, Chuang LM, Tu YK. Subclinical and overt hypothyroidism is associated with reduced glomerular filtration rate and proteinuria: a large cross-sectional population study. Sci Rep. (2018) 8:2031. doi: 10.1038/s41598-018-19693-4
10. Lo JC, Chertow GM, Go AS, Hsu CY. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. (2005) 67:1047–52. doi: 10.1111/j.1523-1755.2005.00169.x
11. Huang X, Ding L, Peng K, Lin L, Wang T, Zhao Z, et al. Thyroid hormones associate with risk of incident chronic kidney disease and rapid decline in renal function: a prospective investigation. J Transl Med. (2016) 14:336. doi: 10.1186/s12967-016-1081-8
12. Chen Y, Zhang W, Wang N, Wang Y, Wang C, Wan H, et al. Thyroid parameters and kidney disorder in type 2 diabetes: results from the METAL study. J Diabetes Res. (2020) 2020:4798947. doi: 10.1155/2020/4798947
13. Cini G, Carpi A, Mechanick J, Cini L, Camici M, Galetta F, et al. Thyroid hormones and the cardiovascular system: pathophysiology and interventions. BioMed Pharmacother. (2009) 63:742–53. doi: 10.1016/j.biopha.2009.08.003
14. Kotsis V, Alevizaki M, Stabouli S, Pitiriga V, Rizos Z, Sion M, et al. Hypertension and hypothyroidism: results from an ambulatory blood pressure monitoring study. J Hypertens. (2007) 25:993–9. doi: 10.1097/HJH.0b013e328082e2ff
15. Vargas F, Moreno JM, Rodríguez-Gómez I, Wangensteen R, Osuna A, Alvarez-Guerra M, et al. Vascular and renal function in experimental thyroid disorders. Eur J Endocrinol. (2006) 154:197–212. doi: 10.1530/eje.1.02093
16. Zimmerman RS, Ryan J, Edwards BS, Klee G, Zimmerman D, Scott N, et al. Cardiorenal endocrine dynamics during volume expansion in hypothyroid dogs. Am J Physiol. (1988) 255:R61–6. doi: 10.1152/ajpregu.1988.255.1.R61
17. Suher M, Koc E, Ata N, Ensari C. Relation of thyroid disfunction, thyroid autoantibodies, and renal function. Ren Fail. (2005) 27:739–42. doi: 10.1080/08860220500243338
18. Cadnapaphornchai MA, Kim YW, Gurevich AK, Summer SN, Falk S, Thurman JM, et al. Urinary concentrating defect in hypothyroid rats: role of sodium, potassium, 2-chloride co-transporter, and aquaporins. J Am Soc Nephrol. (2003) 14:566–74. doi: 10.1097/01.ASN.0000053417.33945.63
19. Chen YC, Cadnapaphornchai MA, Yang J, Summer SN, Falk S, Li C, et al. Nonosmotic release of vasopressin and renal aquaporins in impaired urinary dilution in hypothyroidism. Am J Physiol Renal Physiol. (2005) 289:F672–8. doi: 10.1152/ajprenal.00384.2004
20. Benedetti V, Lavecchia AM, Locatelli M, Brizi V, Corna D, Todeschini M, et al. Alteration of thyroid hormone signaling triggers the diabetes-induced pathological growth, remodeling, and dedifferentiation of podocytes. JCI Insight. (2019) 4(18):e130249. doi: 10.1172/jci.insight.130249
21. Mourouzis I, Lavecchia AM, Xinaris C. Thyroid hormone signalling: from the dawn of life to the bedside. J Mol Evol. (2020) 88:88–103. doi: 10.1007/s00239-019-09908-1
Keywords: IgA nephropathy, free thyroxine (FT4), tubular atrophy/interstitial fibrosis, renal prognosis, renal progression
Citation: Yang B, Zhou W, Cui L, Tian L, Ni Y, Yang M and Yang Y (2024) The predictive value of free thyroxine combined with tubular atrophy/interstitial fibrosis for poor prognosis in patients with IgA nephropathy. Front. Endocrinol. 15:1372824. doi: 10.3389/fendo.2024.1372824
Received: 18 January 2024; Accepted: 29 April 2024;
Published: 14 May 2024.
Edited by:
Joseph V. Martin, Rutgers University Camden, United StatesReviewed by:
Polyxeni Mantzouratou, National and Kapodistrian University of Athens Medical School, GreeceCopyright © 2024 Yang, Zhou, Cui, Tian, Ni, Yang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Yang, eWFuZ21pbjE1MTZAY3pmcGguY29t; Yan Yang, NjA5Mzc3Mjk1QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.