
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 14 January 2025
Sec. Adrenal Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1372683
This article is part of the Research TopicAdrenal Related Hypertension: From Bench to Bedside, volume IIView all 8 articles
 Takumi Kitamoto1*
Takumi Kitamoto1* Yutaro Ruike1
Yutaro Ruike1 Hisashi Koide1
Hisashi Koide1 Kosuke Inoue2
Kosuke Inoue2 Yoshiro Maezawa1
Yoshiro Maezawa1 Masao Omura3
Masao Omura3 Kazuki Nakai3
Kazuki Nakai3 Yuya Tsurutani3
Yuya Tsurutani3 Jun Saito3
Jun Saito3 Katsuhiko Kuwa4
Katsuhiko Kuwa4 Koutaro Yokote1
Koutaro Yokote1 Tetsuo Nishikawa3
Tetsuo Nishikawa3Several decades have passed since the description of the first patient with primary aldosteronism (PA). PA was initially classified in two main forms: aldosterone-producing adenoma (APA) and idiopathic hyperaldosteronism (IHA). However, the pathogenesis of PA has now been shown to be far more complex. For this reason, the traditional classification needs to be updated. Given the recent advancements in our understanding of PA pathogenesis, we should reevaluate how frequent PA cases are, beginning with the reconstruction of the screening strategy. Recent studies consistently indicated that PA has been identified in 22% of patients with resistant hypertension and 11% even in normotensives. The frequency is influenced by the screening strategy and should be based on understanding the pathogenesis of PA. Progress has been made to promote our understanding of the pathogenesis of PA by the findings of aldosterone driver mutations, which have been found in normotensives and hypertensives. In addition, much clinical evidence has been accumulated to indicate that there is a spectrum in PA pathogenesis. In this review, we will summarize the recent progress in aldosterone measurement methods based on LC-MS/MS and the current screening strategy. Then, we will discuss the progress of our understanding of PA, focusing on aldosterone driver mutations and the natural history of PA. Finally, we will discuss the optimal strategy to improve screening rate and case detection.
We searched MEDLINE for articles published from 1 January 1955 to 29 February 2024, using the search terms “primary aldosteronism,” “Conn’s syndrome,’ “hyperaldosteronism,” “screening test,” and “aldosterone measurement”. We mainly focused on English-language publications of the past 5 years (1 June 2019 to 29 February 2024) and selected relevant and highly referenced studies published before this timeframe.
Several decades have passed since the first description of primary aldosteronism (PA) due to aldosterone-producing adenomas (APA) (1). Subsequently, it was observed that some cases of PA lack the classical biological characteristics of hypokalemia or high plasma (or serum) aldosterone concentration (2). Most cases of PA are classified as APA or bilateral adrenal hyperplasia, usually diagnosed as idiopathic hyperaldosteronism (IHA). The former is a surgically curable form of PA, representing more than 5% of patients with hypertension (3), whereas the latter is treated with a mineralocorticoid receptor antagonist (MRA). Additionally, patients with PA exhibit a 1.7- to 3.5-fold increased risk of cardiovascular and cerebrovascular complications (4–7) than essential hypertensives, and early subtype diagnosis is crucial to reversing the excess risk of vascular complications and achieving a better prognosis. To simplify the initial step in diagnosing PA, from 1981, the plasma aldosterone-to-renin activity ratio (ARR) or plasma aldosterone/direct renin ratio (ADRR) has been introduced, given their superiority over the isolated measurements of plasma (or serum) aldosterone concentration and plasma renin activity (PRA) or direct renin concentration (DRC) (8). Since then, ARR and ADRR have played a primary role in screening tests for PA (9–15). In decades, numerous robust prospective studies have established that the prevalence of PA is 3%–19% in all patients with hypertension (3, 16–36), and more patients showed positive results of screening tests in referral centers than in primary care clinics (3, 22, 30, 37). Several models have demonstrated that screening all patients with resistant hypertension for PA is cost-effective (38–40). However, merely less than 2%–5% of patients expected to have PA had been screened (41–44). The factors contributing to this low screening rate may vary across countries. However, the following points seem to be shared: 1) low awareness of PA, 2) the difficulty of changing medications before screening tests, and 3) the need to consider dietary salt intake and the position for blood collection. All these factors contribute to the complexity of the screening phase.
Therefore, this review aimed to restructure the case detection strategy for PA based on numerous novel discoveries over the last few decades, particularly aldosterone driver mutations and the natural history of PA. Moreover, we aimed to discuss an alternative screening strategy that focuses on renin suppression as a biomarker of PA.
PA is a state of hypertension caused by inappropriate aldosterone secretion. Here, “inappropriate” refers to an inappropriate secretory response to salt intake. The body maintains the sodium and fluid balance through the renin–angiotensin–aldosterone system (RAAS) in response to salt intake. PA is a state of hypertension caused by inappropriate aldosterone secretion in response to salt intake, which is independent of renin secretion. Renin secretion is affected by 1) salt restriction (45), 2) fluid volume depletion with diuretics, 3) the use of RAAS inhibitors, such as MRA, angiotensin-II receptor blockers (ARB), and angiotensin-converting enzyme inhibitors, and 4) other hormones, such as glucocorticoids, estrogens, and progestogens (46, 47). Other factors that increase renin levels include renovascular hypertension and pregnancy (high levels of progesterone antagonize aldosterone action in the mineralocorticoid receptor [MR]). Factors suppressing renin secretion include renal failure, β-adrenergic blockers, a-methyldopa, clonidine, and nonsteroidal anti-inflammatory agents. Antidepressants such as selective serotonin reuptake inhibitors elevate aldosterone and renin; however, whether this results in lowering ARR is debatable (48, 49). An overview of the clinically significant factors affecting aldosterone, renin, and ARR levels is summarized in Table 1.
In PA, stimulation of renin secretion is blunted because of the feedback effects of aldosterone hypersecretion. In other words, the renin levels remained relatively low in response to these stimuli (50). Regardless of the fluctuations in aldosterone concentration, extracellular fluid volume expansion persists, resulting in continuous renin suppression. If renin-independent aldosterone excess persists, the distal nephron will reabsorb sodium into the body, and potassium will flow out, resulting in hypertension and hypokalemia as the typical PA phenotype.
Since screening tests rely on plasma aldosterone and renin levels, measurement reliability is crucial for interpreting clinical outcomes.
The reliability of routine tests depends on the measurement performance. Among the common methods for measuring aldosterone, radioimmunoassays (RIA), liquid chromatography–mass spectrometry (LC-MS/MS), and chemiluminescent enzyme immunoassays (CLEIA) are widely used because of their unique strengths and limitations. RIA, a long-established method, offers practicality and ease of use; however, it suffers from cross-reactivity and variability due to low antibody specificity. LC-MS/MS, which is considered the gold standard, provides unparalleled accuracy and sensitivity (51–53), yet its high cost, time demands, and technical requirements limit its feasibility for high-throughput testing. CLEIA improves upon RIA, with better specificity and compatibility with standardized reference materials, making it suitable for routine clinical use. Each method plays a valuable role in the clinical and research settings, catering to different accuracy and accessibility requirements.
We have undertaken standardization of aldosterone concentration measurements traceable to the International System of Units. We have assembled a Certified Reference Materials (CRM) and a Designated Comparison Method (DCM) (54). A new CLEIA-based test kit, approved for in vitro diagnostics, was established using these standards and demonstrated alignment with LC-MS/MS results, supporting CLEIA’s reliability as a routine method (55). Despite variations in RIA due to antibody specificity (55), LC-MS/MS provided a stable reference.
Notably, the median LC-MS/MS value was 48.5 pg/mL compared with 120 pg/mL of RIA (SPAC-S®), prompting a proposal to lower the ARR screening cutoff to 55 pmol/mU (PACLC-MS/MS/DRC) compared with 70 pmol/mU (PACRIA/DRC) and set a cutoff value after saline infusion test to 83 pmol/L (PACLC-MS/MS) (56, 57).
Two types of renin measurement, PRA and DRC, are currently available (9). Both PRA and DRC have methodological limitations. First of all, it is important to highlight that low renin activity could not be accurately measured in the specimens obtained from patients with PA (58).
In particular, PRA is dependent on the generation of angiotensin I, which can result in significant variability in low-renin states owing to reduced renin secretion (58). This often leads to an underestimation of renin activity (59). However, DRC provides a more stable measurement, as it directly quantifies renin concentration without relying on angiotensin I production (60). Consequently, the DRC is less affected by fluctuations in substrate levels and remains relatively stable even in low-renin states. In contrast, the PRA tends to show greater variability and is more susceptible to pre-analytical errors (61).
The poor correlation between the PRA and DRC in low-renin states is particularly relevant for PA screening (62). In such cases, PRA may underestimate renin levels due to its dependence on angiotensin I, which can affect the ARR used in screening. DRC’s stability makes it a potentially more reliable indicator of renin levels in these cases, thus potentially improving the accuracy of PA screening by reducing false-negative results when ARR is used.
Furthermore, the correlation between the PRA and DRC can be poor, particularly in low-renin states (62). This is because PRA reflects the overall activity of the renin–angiotensin system (RAS) and is more sensitive to feedback control, whereas DRC measures renin levels more directly. This distinction is crucial in PA screening as each method has different implications for accuracy and reliability depending on the renin levels.
Additionally, the variability in the renin substrate among the samples can lead to decreased PRA when the substrate concentration is insufficient for a 90-min reaction (63). If specimens are left at room temperature after collection, angiotensin levels can increase, causing inaccuracies in the PRA. PRA measurements are most accurate when specimens are chilled; however, this can lead to inaccurate DRC values. Therefore, proper handling conditions are essential to obtain reliable renin measurements and improve the overall accuracy of PA screening.
Understanding these possible errors is necessary before testing to interpret the results accurately. PRA measurements are affected by several conditions, resulting in poor reproducibility between laboratories (64–66). The DRC procedure is inexpensive, requires a short test time, and has superior specimen handling and reproducibility. However, PRA is currently the mainstream test because of low correlation of DRC with PRA ≤ 1.0 ng/mL/h. Some studies have demonstrated that DRC showed lower sensitivity than PRA when used as a screening test in a ratio to aldosterone levels compared with PRA (58, 67, 68). Nevertheless, a method that adequately correlates with PRA over a wide range has been developed (69) and may be widely used in the future because of its simplicity.
Each guideline from a different country has specific criteria for screening tests. The Japan Endocrine Society (JES) revised its guidelines for PA in 2021 (15). In Table 2, we summarize the current statements for screening strategies compared with the Endocrine Society guidelines (9), which have been adopted in many research reports. The Endocrine Society guideline recommends targeted screening for PA in specific high-risk groups, such as patients with resistant hypertension, hypokalemia, or adrenal incidentalomas, as well as those with a family history of early-onset hypertension. This selective approach aimed to efficiently identify PA in patients most likely to have the condition based on clinical indicators. In contrast, the JES guidelines advocate a broader screening approach and recommend testing for all patients with hypertension. This inclusive strategy reflects the findings that PA often presents without hypokalemia (70) and poses a higher cardiovascular risk (7, 71, 72) and that early diagnosis and treatment can be more cost-effective over time (38, 39). By screening all patients with hypertensions, the JES aims to improve PA detection rates and address the underdiagnosis of PA in the general hypertensive population.
Both guidelines converge on prioritizing high-prevalence groups but differ in the scope of initial screening due to varied emphasis on risk factors, cost-effectiveness, and the broader impact of PA on cardiovascular health.
Each guideline has recommended the use of ARR or ADRR as screening tests (9, 13–15, 73). Since ARR and ADRR are strongly influenced by renin value, the combined value of PACRIA (≥120 pg/mL in JES, ≥150 pg/mL in the United State [U.S.]) and ARR (≥200 pg/mL per ng/mL/h) or ADRR (≥24 pg/mL per mU/L) are recommended. However, we should be aware that more than 35% of patients with PA, particularly those with bilateral PA, have a PACRIA < 150 pg/mL (74). PACCLEIA cutoff is also mentioned in the JES guidelines. They recommended judging the screening test positive when PACCLEIA ≥ 60 pg/mL and ARR ≥ 200 as positive. An ARR between 100 and 200 is provisionally positive and set as a borderline range until the PACCLEIA is generalized and its optimal cutoff is established. When the active renin concentration (ARC) is measured instead of PRA, they recommended judging the screening test positive when ARR (PACCLEIA/ARC) ≥40 and PAC ≥60 pg/mL and ARR between 20 and 40 set as a borderline range.
Japanese and U.S. PA experts now specify that screening with normal blood collection is acceptable to enhance the screening rate. Many drugs and conditions do not hinder the detection of typical PA (75). In cases where the initial test is inconclusive or strongly suspicious, adjustment of interfering anti-hypertensive drugs to the ARR/ADRR thresholds (see Table 2 for the withdrawal period of each drug) and blood collection early in the morning, in the supine position after overnight fasting, are recommended (76–79). Confirmatory tests should be performed to confirm inappropriate aldosterone secretion and to exclude false-negative results. Evidence regarding the number of tests to be performed or the superiority of any confirmatory test is unavailable. When patients desire surgical treatment, performing adrenal venous sampling (AVS) is recommended for the subtype diagnosis. From these comparisons, a general agreement on the issues and methods of screening tests is observed.
Several studies have evaluated the prevalence of PA using the ARR (or ADRR) as a screening strategy, as summarized in Table 3. The prevalence of positive screening results was variable across the studies (3, 16–36, 80–82), ranging from 3% to 19% when considering the general hypertensive population and from 21% to 68% in patients with resistant hypertension, whereas the prevalence of confirmed PA cases was 3%–19% and 7%–22%, respectively. The factors causing the variable prevalence of PA are unstandardized screening strategies (i.e., different thresholds, and collection in different positions) and methodological issues (as discussed early). In addition to medications, several factors influence the ARR, potentially impacting on the accuracy of PA screening, such as pulsatility of aldosterone secretion, sodium intake, ethnicity, and posture (Table 1). Aldosterone secretion is not constant but rather pulsatile, with natural fluctuations throughout the day and night. One study demonstrated the high reproducibility of the ARR in multiple measurements taken on the same patient over time (83); however, many studies have indicated a large variability in plasma aldosterone levels in patients with or without PA (84–88). Dietary sodium restriction led to a misinterpretation of screening test, with normal results in 52% of patients with PA (89). Ethnic differences can influence the baseline aldosterone and renin levels. Certain ethnic groups, such as African Americans, tend to have lower renin levels (90). Body position affects aldosterone and renin levels. For example, standing increases renin secretion due to decreased renal perfusion, which raises renin levels and lowers the ARR. Prevalence studies have shown a wide range in the proportion of APAs in patients with PA, which impacts the aldosterone and renin values and serum potassium levels in each cohort. These factors can affect ARR measurements and screening accuracy for PA. A recent study using LC-MS/MS or CLEIA showed that more than half of the patients had an ARR ≤ 30 ng/mL/h at least once, below the screening threshold (91). PAC is highly variable, and the boundary between normal and abnormal cannot be determined independently.
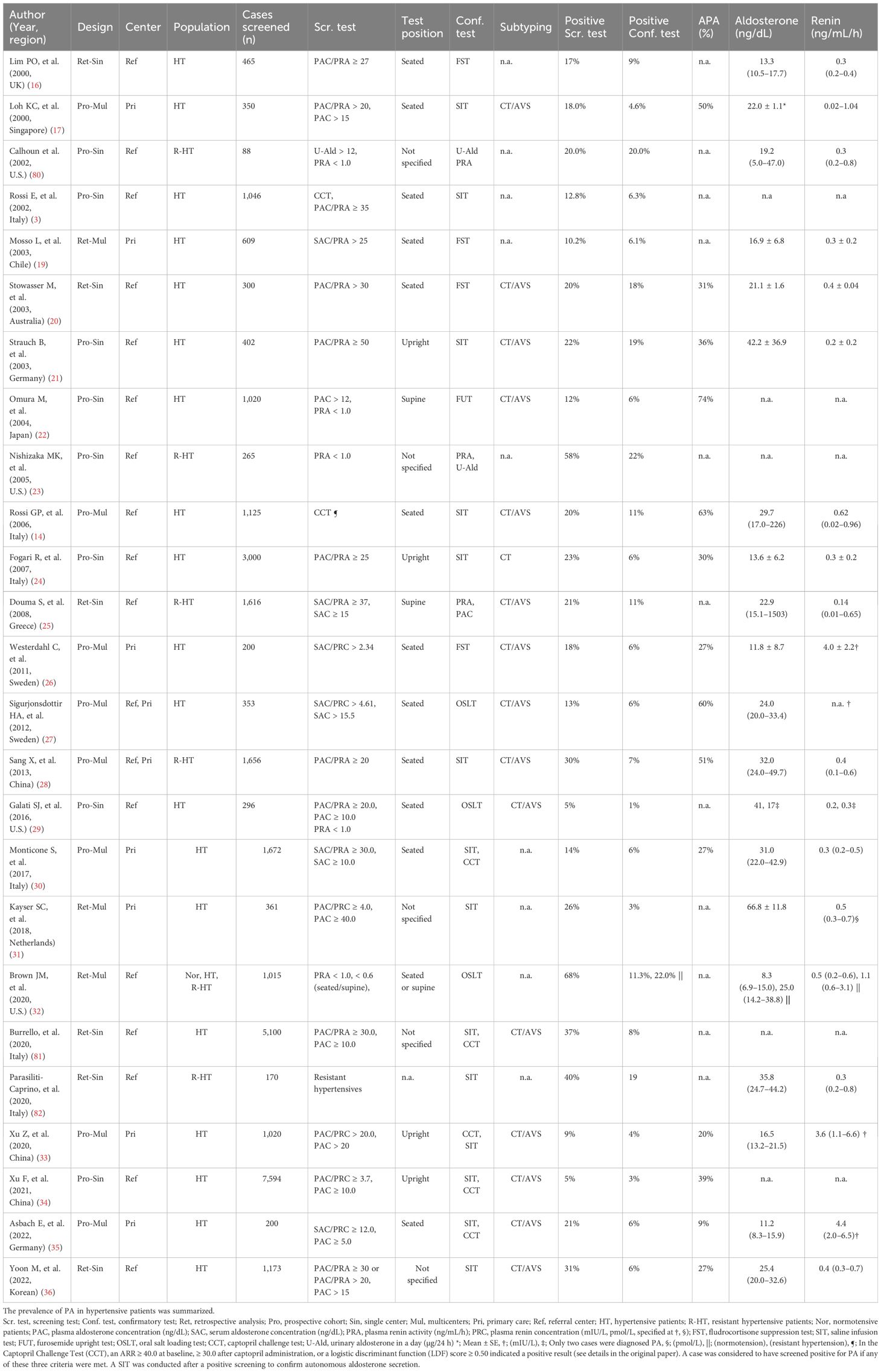
Table 3. Prevalence of confirmed PA cases and positive screening results in patients with hypertension.
A better screening strategy is required for accurate PA case detection. Numerous discussions are available regarding the need for standardization. However, international agreements on screening tests are yet to be reached. The reasons seem to be the following: 1) matching the measurement system of each institution is almost impossible, 2) setting body position and time for each measurement in patients is challenging in daily practice; and 3) serum or plasma aldosterone cutoff values vary according to measurement conditions and probably according to ethnicity. For example, the KCNJ5 somatic mutations, an aldosterone driver mutation causing severe forms of PA is known to show large ethnic differences in frequency (92–98). As we will discuss in the next section, we will delve into aldosterone driver mutations, which will advance our understanding of PA’s pathophysiology of PA to better discuss screening strategies.
Our understanding of the pathophysiology of PA has significantly advanced since the discovery of aldosterone driver mutations, which have been observed even in the adrenals of normotensive individuals and APAs and have shown sex and ethnic differences. Somatic mutations in the gene encoding KCNJ5 in APA (99) cells were first reported in 2011. Following this discovery, ATP2B3 and ATP1A1 (100), and CACNA1D (101) somatic mutations were identified. Recent work has demonstrated that more APAs carry CACNA1D mutations in KCNJ5 wild-type APAs when CYP11B2 immunohistochemistry-guided high-throughput sequencing is used instead of Sanger sequencing (102). More than 90% of APAs harbored any of these aldosterone driver mutations. Since 2011, several studies have reported the frequency of KCNJ5 mutations (92–96, 99, 103–119) (Table 4). The frequency of KCNJ5 mutation in APA is higher in eastern countries [70.5 (43.2–74.7) (%) (95, 96, 103–110)] than in western nations [41.0 (35.5–51.8%) (92–94, 99, 102, 111–119)]; however, KCNJ5 mutation is commonly the dominant mutation across the countries. We previously discussed the clinical impact of KCNJ5 mutations on APAs (120).
In summary, the typical clinical characteristics of APAs harboring KCNJ5 mutations are female dominance, higher aldosterone production capacity, and induction of hypokalemia, compared with KCNJ5-wild APAs. While the frequency of KCNJ5 mutations in APAs differs between Asians and Westerners, the plasma aldosterone levels of KCNJ5-mutated APAs are similar between the two groups [KCNJ5 mutated vs. wild APA: PACRIA 46.9 (40.1–59.8) and PRA 0.3 (0.2–0.4) in Asia, and PACRIA 48.0 (31.2–116.2) and PRA 0.3 (0.2–0.5) in Western countries] (Table 4). The distribution of somatic mutations in APAs might have caused variability in the aldosterone values of patients with PA in these studies. The underlying cause of the distinctive frequency of KCNJ5 mutations has not yet been elucidated. Whether this is due to a selection bias among patients with APA enrolled in the studies or environmental factors, such as ethnicity, remains to be investigated. In parallel with the research on somatic mutations in APA, efforts have been extended to normotensive and IHA cases. Approximately half of the adrenals from normotensive participants contained aldosterone-producing micronodules (APMs; formerly known as aldosterone-producing cell clusters), termed by CYP11B2-positive clusters (121), and more than 40% of APM harbored CACNA1D or ATP1A1 somatic mutations (122, 123). The most frequent mutation identified in these patients was that of the CACNA1D gene, which was found to almost exclusively cause IHA (124), even if this interpretation is limited by the scarcity of surgically resected IHA samples. The differential distribution of somatic mutations, such as KCNJ5 mutations that appeared uniquely in APAs and CACNA1D, which was exclusively observed in IHA, might partly explain the distinctive clinical characteristics of APA and IHA (125). Moreover, APMs in the adrenal glands of normotensive increase with aging (123, 126, 127). These findings support the concept of a continuum pathophysiology of PA from normotensive to participants with hypertension (128). A recent study using expression quantitative trait loci analysis identified the risk loci for PA (129). These discoveries have led us to conceive novel ideas for the methods for early diagnosis of PA (130–132). Aldosterone driver mutations that increase with age have been observed in normotensive patients, and some mutations display sex differences. Furthermore, different mutations demonstrate different clinical behaviors in aldosterone overproduction. In light of these points, defining a certain threshold for absolute aldosterone values for the boundary between PA and non-PA cases should be complicated.
The identification of aldosterone driver mutations in the adrenal glands of normotensive participants also raises the question of whether aldosterone secretion abnormalities occur before the onset of hypertension. The answer to this question will clarify the natural history of PA, allowing us to reconsider when and how screening tests should be performed. A study in 2017 examined 210 normotensive participants with a PRA below 1.0 ng/mL/h, of which 14% were subsequently diagnosed with PA (128). Although no significant difference was observed in the ARR between confirmed PA cases and controls, aldosterone levels were significantly higher in the PA group. Furthermore, even among suspected and unconfirmed PA cases, 20% of them received a confirmed PA diagnosis over 5 years, with one-third of cases showing a unilateral subtype. These results indicated that the pathogenesis of PA is continuous and progressive.
Another finding from these studies is that the ARR may not always accurately reflect the pathogenesis of PA. A recent meta-analysis evaluating the sensitivity and specificity of ARR to detect patients with PA demonstrated a wide variation in sensitivity from 10% to 100% and specificity from 70% to 100% (133). Of note, 3 of 10 studies reported ARR sensitivity of less than 50%, suggesting a limited ability of ARR to adequately identify patients with PA. A recent study used the amount of aldosterone excreted daily in the urine instead of the ARR to detect PA. Using 24-h urinary aldosterone excretion can address diurnal aldosterone variations in a screening test. As salt intake is a major factor in diagnosing PA (89), this study confirmed the salt intake and analyzed cases of renin suppression (32). The results showed that 22% of patients with resistant hypertension and 11% of normotensives had PA. The sensitivity of ARR in this study was less than 30%. Furthermore, a continuum of aldosterone levels and biomarkers of MR activity, such as urinary sodium–potassium ratio, was observed from normotension to hypertension resistance. This finding has been confirmed in a recent elegant study (134). This human physiological study demonstrates a continuum of dysregulated aldosterone production in the low-renin phenotype. Based on a series of studies, we speculated that in patients with PA, dysregulated aldosterone secretion in response to salt leads to renin suppression and demonstrates a continuous and progressive pathophysiology. In the natural history of PA, blood pressure is determined by individual sensitivity, and hypertension occurs when an aldosterone hypersecretion reaches a certain threshold. Suppressed renin seems to be an early biomarker for the detecting PA.
Whether high aldosterone levels per se cause cardiovascular diseases should be investigated. Extraordinarily high aldosterone levels due to chronic sodium deficiency never induce high blood pressure but rather low or normal blood pressure or any cardiovascular or renal damage (135). Thus, inappropriate aldosterone secretion—inappropriate for salt intake (136)—should be a key player in excessive vascular risk. Renin, receiving a feedback inhibition by aldosterone, may serve as a valuable biomarker for identifying dysregulated aldosterone secretion (137, 138).
A well-designed study has added new evidence regarding the association between suppressed renin, high aldosterone levels, and cardiovascular disease (139). The authors demonstrated an association between serum aldosterone concentration and coronary artery calcium (CAC) scores, a marker of subclinical atherosclerosis, in a multiethnic population without antihypertensive medication. The striking result of their study was that a marked association between aldosterone levels and CAC score and an increased risk of all-cause mortality were observed only among individuals with low renin levels. They also showed that the association between elevated aldosterone levels and subclinical atherosclerosis was only partially mediated by blood pressure, indicating the direct cardiovascular damage of aldosterone independently of hypertension. More recently, one study clarified whether renin-independent aldosteronism (i.e., subclinical PA), which fails to diagnose PA using current diagnostic criteria, is involved in cardiovascular disease. Elevated ARR, independent of brachial blood pressure, was associated with greater arterial stiffness and adverse cardiac remodeling, which was also observed in normotensive participants (140). Therefore, a low renin phenotype seems to be necessary to predict cardiovascular complications due to dysregulated aldosterone secretion.
In contrast, whether reversal of renin suppression ameliorates the excess risk of cardiovascular complications due to dysregulated aldosterone secretion should be investigated. Several studies have demonstrated that in patients with PA, adrenalectomy and MRA can ameliorate the unfavorable effects of excess aldosterone to achieve similar mortality rates in patients with essential hypertension (141, 142). Additionally, a recent large retrospective cohort study demonstrated that patients with APA that undergo surgical adrenalectomy had a significantly lower risk for cardiovascular events than patients with essential hypertension by 40% (6). In medically treated PA patients, the same investigators demonstrated different outcomes between the two subpopulations with unsuppressed or suppressed PRA. Surprisingly, the former showed an identical risk profile to that of essential hypertensives, whereas the latter showed an almost three times higher risk. A similar association was observed in the occurrence of atrial fibrillation (143). Therefore, we may need to start a renin check to estimate future cardiovascular risk due to dysregulated aldosterone secretion, which is also useful for monitoring surgical or medical treatment efficacy in patients with PA.
As observed in other endocrine disorders (e.g., hyperthyroidism and hyperparathyroidism), the dysregulated hormones are not always beyond the normal range, and the hormone-receiving feedback loop is more sensitive in reflecting the disease. This may also be true for patients with PA. PA disrupts the homeostatic feedback loop between aldosterone and salt status (136). As we have overviewed, PA is a disease with a spectrum, and inappropriate aldosterone secretion increases gradually. Furthermore, aldosterone secretion is affected by diurnal variations and salt sensitivity, which vary widely between individuals. Additionally, aldosterone driver mutations, such as KCNJ5 and CACNA1D mutations, significantly affect aldosterone secretion, with large sex and ethnic differences. Therefore, rather than setting a certain threshold for aldosterone levels to detect PA, using renin suppression as a feedback loop for inappropriate aldosterone secretion early in its natural history is reasonable. However, establishing a clear threshold for PRA suppression is challenging. Therefore, we should begin with the values used in the current guidelines (PRA <1.0 ng/mL/h) as a standard to accumulate further knowledge.
We propose that individual hormone levels of renin and aldosterone can help diagnose PA (Figure 1A). We demonstrated the prevalence of PA at a general outpatient clinic in 2004, where endocrine markers of secondary hypertension, such as renovascular hypertension, Cushing’s syndrome, and pheochromocytoma, were evaluated (22). We used PACRIA (>12 ng/dL) and PRA (<1.0 ng/mL/h) individually for screening in this study, finding a prevalence of PA of 6.0%, which is consistent with a recent report in a primary care setting (30). For individuals exhibiting a low-renin phenotype, a PACCLEIA exceeding 10 ng/dL (or equivalently, a PACRIA greater than 20 ng/dL) or the presence of hypokalemia (serum potassium less than 3.5 mEq/L) may be sufficient to diagnose PA without the need for further confirmatory testing (9). Notably, a few patients with PA show high plasma renin levels due to comorbidity (144) (such as excess cortisol secretion, chronic kidney disease, nephrotic syndrome, liver dysfunction, and chronic heart failure).
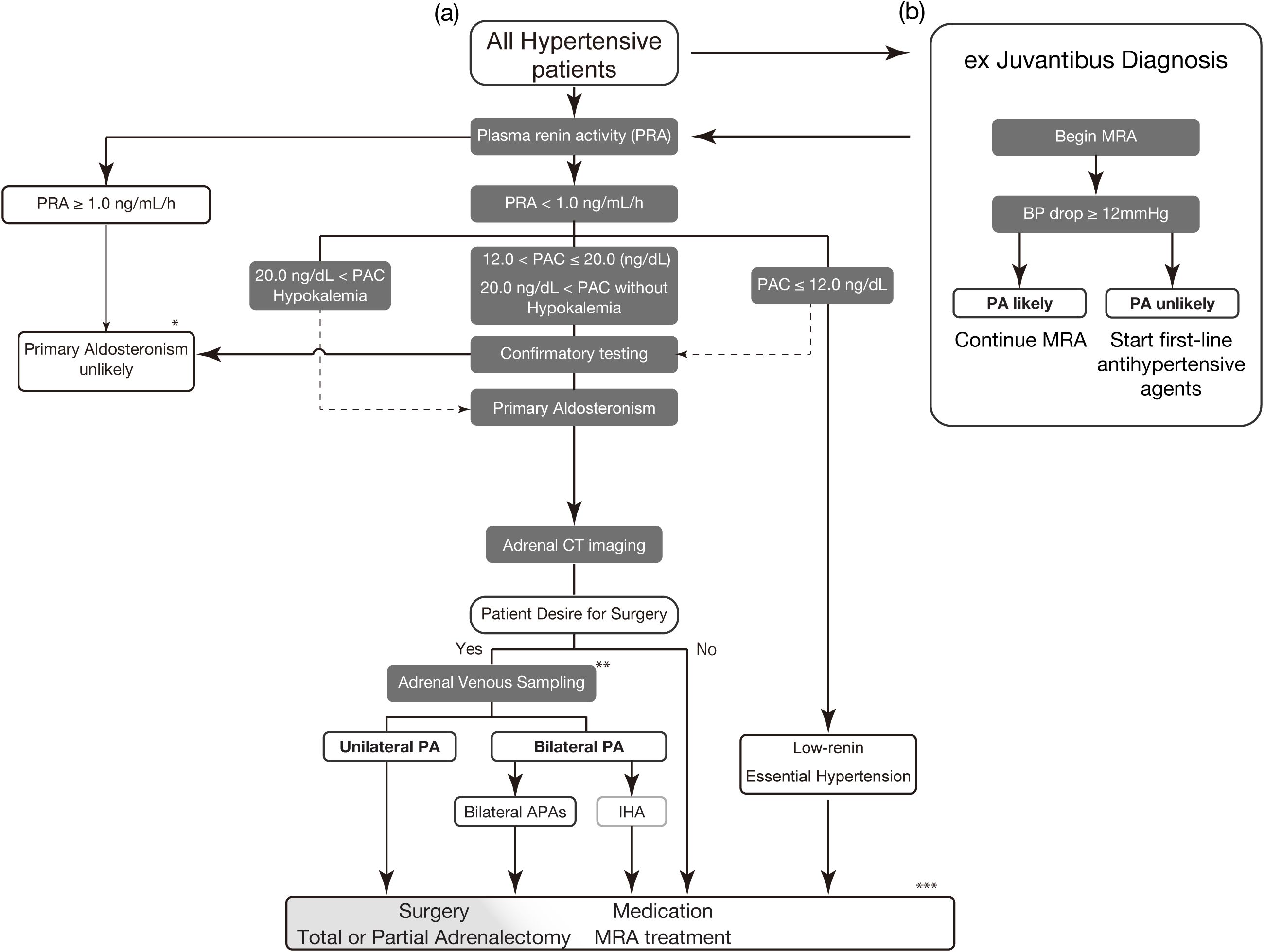
Figure 1. Proposed treatment strategy for primary aldosteronism (A) The screening for PA in all hypertensive patients should begin with PRA evaluation. Withdrawal of interfering antihypertensive drugs is preferable. However, many medications and conditions do not significantly hinder the detection of typical PA. RIA is used for PAC values in this figure. Low-renin indicates PRA <1.0 ng/mL/h. Physicians should perform confirmatory testing for cases with PAC of less than 12.0 ng/dL if PA is clinically suspected. Confirmatory testing options include the oral sodium loading test, captopril challenge test, saline infusion test, and 24-h urinary aldosterone excretion (>12 µg/24 h) after sodium intake correction (9). (B) If the proper evaluation of renin is difficult due to interfering factors, proceed with ex juvantibus diagnosis. Begin administration of MRA (e.g., spironolactone 25 mg/day) for 4 weeks to see whether there is a drop in blood pressure. A drop of 12 mmHg or more is suspected of a high likelihood of PA. * Reconsider the diagnosis if the case has the factors increasing renin (e.g., excess cortisol secretion, chronic kidney disease, nephrotic syndrome, liver dysfunction, and chronic heart failure) ** Consider segment-selective AVS if the clinical diagnosis and conventional AVS diagnosis are inconsistent. *** Partial adrenalectomy is a treatment option for cases with bilateral APAs. PAC, plasma aldosterone concentration; PRA, plasma renin activity; CT, computed tomography; PA, primary aldosteronism; APA, aldosterone-producing adenoma; IHA, idiopathic hyperaldosteronism; MRA, mineral corticoid receptor antagonist.
In settings in which proper evaluation of reninemia is not feasible, MRA is a useful strategy for ex Juvantibus diagnosis (Figure 1B). Renin is more sensitive than the ARR for detecting PA (8, 20, 145). A low-renin phenotype indicates extracellular fluid volume expansion or an MR-activated state (146–149). In patients with hypertension but without a PA diagnosis, those with suppressed renin levels experience a greater blood pressure reduction from MRA treatment, particularly if they have higher plasma aldosterone levels within the normal range (150, 151). This suggests that such patients may represent a wider spectrum of potential patients with PA (6, 141, 142, 152–154). We referred to a recent Commentary from Dr. Funder (155), who proposed to begin the administration of spironolactone 25 mg/day for 4 weeks and measure the blood pressure response. In hypertensives, a drop of less than 10 mmHg indicated a low probability of PA, whereas a drop of 12 mmHg or more suggested a high likelihood of PA. The same applies to newly developed hypertension, where spironolactone is prescribed 25 mg/day for 4 weeks. If blood pressure falls within the normal range, continue; otherwise, prescribe first-line antihypertensive agents. These steps are an effective strategy to ensure that as many patients with PA as possible receive the necessary medical treatment, regardless of the medical environment. Treatment with MRA carries certain risks, such as hyperkalemia and a decline in glomerular filtration rate. Occasionally, MRA may not effectively reverse renin suppression. In such situations, or if the patients wish to explore the possibility of curative treatment, they should be referred to an appropriate specialized center for reevaluation of the diagnosis of PA.
The perceptions of primary care physicians who see patients with PA are also critical for lowering the hurdles for PA screening. Actions are needed to increase knowledge of PA among these physicians, including its high prevalence and minor presentation of hypokalemia. The rapid immunoassay for plasma aldosterone and renin may lessen the hurdle for their measurement and encourage screening procedures (69). This will contribute to an increase in the population diagnosed with PA by more than >1% (156).
To design a better screening method, we addressed the following questions: 1) Is early intervention for normotensive renin-independent aldosteronism beneficial for the patient’s prognosis? 2) What is the cutoff for PRA and DRC to stratify the population according to excess cardiovascular risk due to hyper-aldosteronism? 3) What are the most cost-effective screening methods? 4) What is the clinically helpful definition of renin-independent aldosteronism and essential hypertension and vice versa? These answers will help us design a better screening algorithm for PA. Additionally, we need evidence that the algorithm can identify all cases that benefit from PA treatment at an early stage. Finally, we emphasize that evidence using the PAC value by CLEIA is warranted. Accumulated clinical data from larger samples will facilitate the development of a new screening strategy for PA.
TK: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. YR: Writing – review & editing. HK: Writing – review & editing. KI: Writing – review & editing. YM: Writing – review & editing. MO: Writing – review & editing. KN: Writing – review & editing. YT: Writing – review & editing. JS: Writing – review & editing. KK: Writing – review & editing. KY: Writing – review & editing. TN: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from the Ministry of Health, Labor, and Welfare, Japan (23FC1041 to TN and JS), JSPS KAKENHI Grant Numbers JP22K16422 (TK).
We thank all the medical staff in the endocrinology department of Yokohama Rosai Hospital and Dr. Kenichi Sakamoto (Rutgers Robert Wood Johnson Medical School) for the fruitful discussions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Conn JW, Cohen EL, Rovner DR, Nesbit RM. Normokalemic primary aldosteronism. A detectable cause of curable “Essential” Hypertension. JAMA. (1965) 193:200–6. doi: 10.1001/jama.1965.03090030022005
3. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. (2006) 48:2293–300. doi: 10.1016/j.jacc.2006.07.059
4. Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Internal Med. (2008) 168:80–5.
5. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. (2005) 45:1243–8. doi: 10.1016/j.jacc.2005.01.015
6. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. (2018) 6:51–9. doi: 10.1016/S2213-8587(17)30367-4
7. Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol Jan. (2018) 6:41–50. doi: 10.1016/S2213-8587(17)30319-4
8. Hiramatsu K, Yamada T, Yukimura Y, Komiya I, Ichikawa K, Ishihara M, et al. A screening test to identify aldosterone-producing adenoma by measuring plasma renin activity: results in hypertensive patients. Arch Internal Med. (1981) 141:1589–93. doi: 10.1001/archinte.1981.00340130033011
9. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2016) 101:1889–916. doi: 10.1210/jc.2015-4061
10. Wu VC, Hu YH, Er LK, Yen RF, Chang CH, Chang YL, et al. Case detection and diagnosis of primary aldosteronism - The consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. (2017) 20. doi: 10.1016/j.jfma.2017.06.004
11. Whelton Paul K, Carey Robert M, Aronow Wilbert S, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. (2017) 71:e13–e115. doi: 10.1161/HYP.0000000000000065
12. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39:3021–104. doi: 10.1093/eurheartj/ehy339
13. Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro MC, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension∗. J Hypertension. (2020) 38.
14. Rossi GP, Bisogni V, Bacca AV, Belfiore A, Cesari M, Concistre A, et al. The 2020 Italian Society of Arterial Hypertension (SIIA) practical guidelines for the management of primary aldosteronism. Int J Cardiol Hypertension. (2020) 5:100029. doi: 10.1016/j.ijchy.2020.100029
15. Naruse M, Katabami T, Shibata H, Sone M, Takahashi K, Tanabe A, et al. Japan Endocrine Society clinical practice guideline for the diagnosis and management of primary aldosteronism 2021. Endocrine J. (2022) 69:327–59. doi: 10.1507/endocrj.ej21-0508
16. Lim PO, Dow E, Brennan G, Jung RT, MacDonald TM. High prevalence of primary aldosteronism in the Tayside hypertension clinic population. J Hum Hypertens May. (2000) 14:311–5. doi: 10.1038/sj.jhh.1001013
17. Loh KC, Koay ES, Khaw MC, Emmanuel SC, Young WF. Prevalence of primary aldosteronism among Asian hypertensive patients in Singapore. Article. J Clin Endocrinol Metab. (2000) 85:2854–9. doi: 10.1210/jc.85.8.2854
18. Rossi E, Regolisti G, Negro A, Sani C, Davoli S, Perazzoli F. High prevalence of primary aldosteronism using postcaptopril plasma aldosterone to renin ratio as a screening test among Italian hypertensives. Am J Hypertens. (2002) 15:896–902. doi: 10.1016/s0895-7061(02)02969-2
19. Mosso L, Carvajal C, Gonzalez A, Barraza A, Avila F, Montero J, et al. Primary aldosteronism and hypertensive disease. Hypertension. Aug. (2003) 42:161–5. doi: 10.1161/01.HYP.0000079505.25750.11
20. Stowasser M, Gordon RD, Gunasekera TG, Cowley DC, Ward G, Archibald C, et al. High rate of detection of primary aldosteronism, including surgically treatable forms, after ‘non-selective’ screening of hypertensive patients. J Hypertens. (2003) 21:2149–57. doi: 10.1097/00004872-200311000-00025
21. Strauch B, Zelinka T, Hampf M, Bernhardt R, Widimsky J Jr. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens. (2003) 17:349–52. doi: 10.1038/sj.jhh.1001554
22. Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. (2004) 27:193–202. doi: 10.1291/hypres.27.193
23. Nishizaka MK, Pratt-Ubunama M, Zaman MA, Cofield S, Calhoun DA. Validity of plasma aldosterone-to-renin activity ratio in African American and white subjects with resistant hypertension. Am J Hypertens. (2005) 18:805–12. doi: 10.1016/j.amjhyper.2005.01.002
24. Fogari R, Preti P, Zoppi A, Rinaldi A, Fogari E, Mugellini A. Prevalence of primary aldosteronism among unselected hypertensive patients: a prospective study based on the use of an aldosterone/renin ratio above 25 as a screening test. Hypertens Res Feb. (2007) 30:111–7. doi: 10.1291/hypres.30.111
25. Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet Jun 7. (2008) 371:1921–6. doi: 10.1016/S0140-6736(08)60834-X
26. Westerdahl C, Bergenfelz A, Isaksson A, Nerbrand C, Valdemarsson S. Primary aldosteronism among newly diagnosed and untreated hypertensive patients in a Swedish primary care area. Scand J Prim Health Care. (2011) 29:57–62. doi: 10.3109/02813432.2011.554015
27. Sigurjonsdottir HA, Gronowitz M, Andersson O, Andersson O, Eggertsen R, Herlitz H, Sakinis A, et al. Unilateral adrenal hyperplasia is a usual cause of primary hyperaldosteronism. Results Swedish screening study. BMC Endocr Disord. (2012) 12:17. doi: 10.1186/1472-6823-12-17
28. Sang X, Jiang Y, Wang W, Yan L, Zhao J, Peng Y, et al. Prevalence of and risk factors for primary aldosteronism among patients with resistant hypertension in China. J Hypertens. (2013) 31:1465–71. doi: 10.1097/HJH.0b013e328360ddf6
29. Galati SJ, Cheesman KC, Springer-Miller R, Hopkins SM, Krakoff L, Bagiella E, et al. Prevelence of primary aldosteronism in an urban hypertensive population. Endocr Pract Nov. (2016) 22:1296–302. doi: 10.4158/E161332.OR
30. Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. (2017) 69:1811–20. doi: 10.1016/j.jacc.2017.01.052
31. Kayser SC, Deinum J, de Grauw WJ, Schalk BW, Bor HJ, Lenders JW, et al. Prevalence of primary aldosteronism in primary care: a cross-sectional study. Br J Gen Pract. (2018) 68:e114–22. doi: 10.3399/bjgp18X694589
32. Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, et al. The unrecognized prevalence of primary aldosteronism: A cross-sectional study. Ann Intern Med. (2020) 173:10–20. doi: 10.7326/M20-0065
33. Xu Z, Yang J, Hu J, Song Y, He W, Luo T, et al. Primary aldosteronism in patients in China with recently detected hypertension. J Am Coll Cardiol. (2020) 75:1913–22. doi: 10.1016/j.jacc.2020.02.052
34. Xu F, Gao Z, Wang G, Gao Y, Guo Y, Guo Y, et al. Prevalence, subtype classification, and outcomes of treatment of primary aldosteronism: A prospective study in China. Endocr Pract. (2021) 27:478–83. doi: 10.1016/j.eprac.2020.10.007
35. Asbach E, Kellnar A, Bekeran M, Schelling J, Bidlingmaier M, Reincke M. Prevalence of primary aldosteronism in newly diagnosed hypertensive patients in primary care. Exp Clin Endocrinol Diabetes. (2022) 130:801–5. doi: 10.1055/a-1938-4242
36. Yoon M, Hong N, Ha J, Lee CJ, Ku CR, Rhee Y, et al. Prevalence and clinical characteristics of primary aldosteronism in a tertiary-care center in Korea. Hypertens Res. (2022) 45:1418–29. doi: 10.1038/s41440-022-00948-7
37. Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies–a review of the current literature. Horm Metab Res. (2012) 44:157–62.
38. Lubitz CC, Economopoulos KP, Sy S, Johanson C, Kunzel HE, Reincke M, et al. Cost-effectiveness of screening for primary aldosteronism and subtype diagnosis in the resistant hypertensive patients. Circulation: Cardiovasc Qual Outcomes. (2015) 8:621–30. doi: 10.1161/circoutcomes.115.002002
39. Sato M, Morimoto R, Seiji K, Iwakura Y, Ono Y, Kudo M, et al. Cost-effectiveness analysis of the diagnosis and treatment of primary aldosteronism in Japan. Horm Metab Res. (2015) 47:826–32. doi: 10.1055/s-0035-1559645
40. Woode ME, Wong K, Reid CM, Stowasser M, Russell G, Gwini S, et al. Cost-effectiveness of screening for primary aldosteronism in hypertensive patients in Australia: a Markov modelling analysis. J Hypertension. (2023) 41:1615–25. doi: 10.1097/hjh.0000000000003513
41. Jaffe G, Gray Z, Krishnan G, Stedman M, Zheng Y, Han J, et al. Screening rates for primary aldosteronism in resistant hypertension. Hypertension. (2020) 75:650–9. doi: 10.1161/hypertensionaha.119.14359
42. Cohen JB, Cohen DL, Herman DS, Leppert JT, Byrd JB, Bhalla V. Testing for primary aldosteronism and mineralocorticoid receptor antagonist use among US veterans: a retrospective cohort study. Ann Internal Med. (2021) 174:289–97.
43. Liu Y-Y, King J, Kline GA, Padwal RS, Pasieka JL, Chen G, et al. Outcomes of a specialized clinic on rates of investigation and treatment of primary aldosteronism. JAMA Surgery. (2021) 156:541. doi: 10.1001/jamasurg.2021.0254
44. Hundemer GL, Imsirovic H, Vaidya A, Yozamp N, Goupil R, Madore F, et al. Screening rates for primary aldosteronism among individuals with hypertension plus hypokalemia: A population-based retrospective cohort study. Hypertension. (2022) 79:178–86. doi: 10.1161/hypertensionaha.121.18118
45. Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. (2020) 12:CD004022. doi: 10.1002/14651858.CD004022.pub5
46. Newton MA, Laragh JH. Effects of glucocorticoid administration on aldosterone excretion and plasma renin in normal subjects, in essential hypertension and in primary aldosteronism. J Clin Endocrinol Metab. (1968) 28:1014–22. doi: 10.1210/jcem-28-7-1014
47. Oelkers WK. Effects of estrogens and progestogens on the renin-aldosterone system and blood pressure. Steroids. (1996) 61:166–71. doi: 10.1016/0039-128x(96)00007-4
48. Ahmed AH, Calvird M, Gordon RD, Taylor PJ, Ward G, Pimenta E, et al. Effects of two selective serotonin reuptake inhibitor antidepressants, sertraline and escitalopram, on aldosterone/renin ratio in normotensive depressed male patients. J Clin Endocrinol Metab. (2011) 96:1039–45. doi: 10.1210/jc.2010-2603
49. Young WF, Calhoun DA, Lenders JWM, Stowasser M, Textor SC. Screening for endocrine hypertension: an endocrine society scientific statement. Endocrine Rev. (2017) 38:103–22. doi: 10.1210/er.2017-00054
50. Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. (2021) 9:876–92. doi: 10.1016/S2213-8587(21)00210-2
51. Rehan M, Raizman JE, Cavalier E, Don-Wauchope AC, Holmes DT. Laboratory challenges in primary aldosteronism screening and diagnosis. Clin Biochem. (2015) 48:377–87. doi: 10.1016/j.clinbiochem.2015.01.003
52. Thuzar M, Young K, Ahmed AH, Ward G, Wolley M, Guo Z, et al. Diagnosis of primary aldosteronism by seated saline suppression test—Variability between immunoassay and HPLC-MS/MS. J Clin Endocrinol Metab. (2019) 105:e477–83. doi: 10.1210/clinem/dgz150
53. Tamura N, Watanabe E, Shirakawa R, Nakatani E, Yamada K, Hatakeyama H, et al. Comparisons of plasma aldosterone and renin data between an automated chemiluminescent immunoanalyzer and conventional radioimmunoassays in the screening and diagnosis of primary aldosteronism. PloS One. (2021) 16:e0253807.
54. Nishikawa T, Omura M, Kawaguchi M, Takatsu A, Satoh F, Ito S, et al. Calibration and evaluation of routine methods by serum certified reference material for aldosterone measurement in blood. Endocrine J. (2016) 63:1065–80. doi: 10.1507/endocrj.ej16-0304
55. Nishikawa T, Satoh F, Takashi Y, Yanase T, Itoh H, Kurihara I, et al. Comparison and commutability study between standardized liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) and chemiluminescent enzyme immunoassay for aldosterone measurement in blood. Endocrine J. (2022) 69:45–54. doi: 10.1507/endocrj.ej21-0278
56. Fries CM, Bae YJ, Rayes N, Sandner B, Isermann B, Stumvoll M, et al. Prospective evaluation of aldosterone LC-MS/MS-specific cutoffs for the saline infusion test. Eur J Endocrinology. (2020) 183:191–201. doi: 10.1530/eje-20-0030
57. Guo Z, Poglitsch M, McWhinney BC, Ungerer JPJ, Ahmed AH, Gordon RD, et al. Aldosterone LC-MS/MS assay-specific threshold values in screening and confirmatory testing for primary aldosteronism. J Clin Endocrinol Metab. (2018) 103:3965–73. doi: 10.1210/jc.2018-01041
58. Sealey JE, Gordon RD, Mantero F. Plasma renin and aldosterone measurements in low renin hypertensive states. Trends Endocrinol Metab. (2005) 16:86–91. doi: 10.1016/j.tem.2005.02.006
59. Brossaud J, Corcuff JB. Pre-analytical and analytical considerations for the determination of plasma renin activity. Clin Chim Acta. (2009) 410:90–2. doi: 10.1016/j.cca.2009.09.018
60. Derkx FH, de Bruin RJ, van Gool JM, van den Hoek MJ, Beerendonk CC, Rosmalen F, et al. Clinical validation of renin monoclonal antibody-based sandwich assays of renin and prorenin, and use of renin inhibitor to enhance prorenin immunoreactivity. Clin Chem Jul. (1996) 42:1051–63.
61. Deinum J, Derkx FH, Schalekamp MA. Improved immunoradiometric assay for plasma renin. Clin Chem. (1999) 45:847–54. doi: 10.1093/clinchem/45.6.847
62. Leung AA, Orton DJ, Chin A, Sadrzadeh H, Kline GA. Novel approach to establishing an aldosterone: renin ratio cutoff for primary aldosteronism. Hypertension. (2017) 69:450–6. doi: 10.1161/hypertensionaha.116.08407
63. Unger N, Lopez Schmidt I, Pitt C, Walz M, Philipp T, Mann K, et al. Comparison of active renin concentration and plasma renin activity for the diagnosis of primary hyperaldosteronism in patients with an adrenal mass. Eur J Endocrinology. (2004) 150:517–23. doi: 10.1530/eje.0.1500517
64. Dorrian CA, Toole BJ, Alvarez-Madrazo S, Kelly A, Connell JMC, Wallace AM. A screening procedure for primary aldosteronism based on the Diasorin Liaison® automated chemiluminescent immunoassay for direct renin. Ann Clin Biochemistry: Int J Lab Med. (2010) 47:195–9. doi: 10.1258/acb.2010.009230
65. Morganti A, obotEsgftvoDldr a. A comparative study on inter and intralaboratory reproducibility of renin measurement with a conventional enzymatic method and a new chemiluminescent assay of immunoreactive renin. J Hypertension. (2010) 28:1307–12. doi: 10.1097/HJH.0b013e32833857ad
66. Gruson D, Maisin D, Lison P, Maiter D, Persu A. Two-site automated chemiluminescent assay for measurement of immunoreactive renin. Biomarkers. (2011) 16:605–9. doi: 10.3109/1354750X.2011.614015
67. Li X, Goswami R, Yang S, Li Q. Aldosterone/direct renin concentration ratio as a screening test for primary aldosteronism: A meta-analysis. J Renin Angiotensin Aldosterone Syst. (2016) 17:147032031665745. doi: 10.1177/1470320316657450
68. Shanik MH, Romao IJ, Velayati S. Aldosterone/direct renin concentration ratio versus aldosterone/plasma renin activity for diagnosis of primary hyperaldosteronism: case presentation. J Endocrine Society. (2021) 5:A113–4. doi: 10.1210/jendso/bvab048.228
69. Morimoto R, Ono Y, Tezuka Y, Kudo M, Yamamoto S, Arai T, et al. Rapid screening of primary aldosteronism by a novel chemiluminescent immunoassay. Hypertension. (2017) 70:334–41. doi: 10.1161/HYPERTENSIONAHA.117.09078
70. Heinrich DA, Adolf C, Rump LC, Quack I, Quinkler M, Hahner S, et al. Primary aldosteronism: key characteristics at diagnosis: a trend toward milder forms. Eur J Endocrinology. (2018) 178:605–11. doi: 10.1530/eje-17-0978
71. Savard S, Amar L, Plouin P-F, Steichen O. Cardiovascular complications associated with primary aldosteronism. Hypertension. (2013) 62:331–6.
72. Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, et al. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism. Hypertension. (2018) 71:530–7. doi: 10.1161/hypertensionaha.117.10263
73. Huang KH, Yu CC, Hu YH, Chang CC, Chan CK, Liao SC, et al. Targeted treatment of primary aldosteronism - The consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. (2018) 2. doi: 10.1016/j.jfma.2018.01.006
74. Stowasser M, Gordon RD. Primary aldosteronism—careful investigation is essential and rewarding. Mol Cell Endocrinology. (2004) 217:33–9. doi: 10.1016/j.mce.2003.10.006
75. Murase K, Nagaishi R, Takenoshita H, Nomiyama T, Akehi Y, Yanase T. Prevalence and clinical characteristics of primary aldosteronism in Japanese patients with type 2 diabetes mellitus and hypertension. Endocrine J. (2013) 60:967–76. doi: 10.1507/endocrj.ej13-0060
76. Tiu S-C, Choi C-H, Shek C-C, Ng YW, Chan FK, Ng CM, et al. The use of aldosterone-renin ratio as a diagnostic test for primary hyperaldosteronism and its test characteristics under different conditions of blood sampling. J Clin Endocrinol Metab. (2005) 90:72–8. doi: 10.1210/jc.2004-1149
77. Mulatero P, Rabbia F, Milan A, Paglieri C, Morello F, Chiandussi L, et al. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension. (2002) 40:897–902. doi: 10.1161/01.hyp.0000038478.59760.41
78. Seifarth C, Trenkel S, Schobel H, Hahn EG, Hensen J. Influence of antihypertensive medication on aldosterone and renin concentration in the differential diagnosis of essential hypertension and primary aldosteronism. Clin Endocrinology. (2002) 57:457–65. doi: 10.1046/j.1365-2265.2002.01613.x
79. Nagasawa M, Yamamoto K, Rakugi H, Takeda M, Akasaka H, Umakoshi H, et al. Influence of antihypertensive drugs in the subtype diagnosis of primary aldosteronism by adrenal venous sampling. J Hypertension. (2019) 37.
80. Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. (2002) 40:892–6. doi: 10.1161/01.hyp.0000040261.30455.b6
81. Burrello J, Monticone S, Losano I, Cavaglià G, Buffolo F, Tetti M, et al. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension. (2020) 75:1025–33. doi: 10.1161/hypertensionaha.119.14063
82. Parasiliti-Caprino M, Lopez C, Prencipe N, Lucatello B, Settanni F, Giraudo G, et al. Prevalence of primary aldosteronism and association with cardiovascular complications in patients with resistant and refractory hypertension. J Hypertension. (2020) ;38.
83. Rossi GP, Seccia TM, Palumbo G, Belfiore A, Bernini G, Caridi G, et al. Within-patient reproducibility of the aldosterone: renin ratio in primary aldosteronism. Hypertension. (2010) 55:83–9. doi: 10.1161/hypertensionaha.109.139832
84. Vieweg WV, Veldhuis JD, Carey RM. Temporal pattern of renin and aldosterone secretion in men: effects of sodium balance. Am J Physiol. (1992) 262:F871–7. doi: 10.1152/ajprenal.1992.262.5.F871
85. Siragy HM, Vieweg WV, Pincus S, Veldhuis JD. Increased disorderliness and amplified basal and pulsatile aldosterone secretion in patients with primary aldosteronism. J Clin Endocrinol Metab. (1995) 80:28–33. doi: 10.1210/jcem.80.1.7829626
86. Tanabe A, Naruse M, Takagi S, Tsuchiya K, Imaki T, Takano K. Variability in the renin/aldosterone profile under random and standardized sampling conditions in primary aldosteronism. J Clin Endocrinol Metab. (2003) 88:2489–94. doi: 10.1210/jc.2002-021476
87. Kline GA, Darras P, Leung AA, So B, Chin A, Holmes DT. Surprisingly low aldosterone levels in peripheral veins following intravenous sedation during adrenal vein sampling: implications for the concept of nonsuppressibility in primary aldosteronism. J Hypertens. (2019) 37:596–602. doi: 10.1097/HJH.0000000000001905
88. Yozamp N, Hundemer GL, Moussa M, Underhill J, Fudim T, Sacks B, et al. Variability of aldosterone measurements during adrenal venous sampling for primary aldosteronism. Am J Hypertens. (2021) 34:34–45. doi: 10.1093/ajh/hpaa151
89. Baudrand R, Guarda FJ, Torrey J, Williams G, Vaidya A. Dietary sodium restriction increases the risk of misinterpreting mild cases of primary aldosteronism. J Clin Endocrinol Metab. (2016) 101:3989–96. doi: 10.1210/jc.2016-1963
90. Spence JD, Rayner BL. Hypertension in blacks. Hypertension. (2018) 72:263–9. doi: 10.1161/hypertensionaha.118.11064
91. Yozamp N, Hundemer GL, Moussa M, Underhill J, Fudim T, Sacks B, et al. Intraindividual variability of aldosterone concentrations in primary aldosteronism. Hypertension. (2021) 77:891–9. doi: 10.1161/hypertensionaha.120.16429
92. Azizan EA, Murthy M, Stowasser M, Gordon R, Kowalski B, Xu S, et al. Somatic mutations affecting the selectivity filter of KCNJ5 are frequent in 2 large unselected collections of adrenal aldosteronomas. Hypertension. Mar. (2012) 59:587–91. doi: 10.1161/HYPERTENSIONAHA.111.186239
93. Boulkroun S, Beuschlein F, Rossi GP, Golib-Dzib JF, Fischer E, Amar L, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension. (2012) 59:592–8. doi: 10.1161/HYPERTENSIONAHA.111.186478
94. Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, et al. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension. (2014) 63:188–95. doi: 10.1161/HYPERTENSIONAHA.113.01733
95. Wu VC, Huang KH, Peng KY, Tsai YC, Wu CH, Wang SM, et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep. (2015) 5:11396. doi: 10.1038/srep11396
96. Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension. Mar. (2015) 65:622–8. doi: 10.1161/HYPERTENSIONAHA.114.03346
97. Kitamoto T, Suematsu S, Yamazaki Y, Nakamura Y, Sasano H, Matsuzawa Y, et al. Clinical and steroidogenic characteristics of aldosterone-producing adenomas with ATPase or CACNA1D gene mutations. J Clin Endocrinol Metab. (2016) 101:494–503. doi: 10.1210/jc.2015-3284
98. Nanba K, Rainey WE. GENETICS IN ENDOCRINOLOGY: Impact of race and sex on genetic causes of aldosterone-producing adenomas. Eur J Endocrinology. (2021) 185:R1–R11. doi: 10.1530/eje-21-0031
99. Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. (2011) 331:768–72. doi: 10.1126/science.1198785
100. Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. (2013) 45:440–4, 444e1-2. doi: 10.1038/ng.2550
101. Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. (2013) 45:1050–4. doi: 10.1038/ng.2695
102. De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. (2020) 75:1034–44. doi: 10.1161/hypertensionaha.119.14177
103. Cheng CJ, Sung CC, Wu ST, Lin YC, Sytwu HK, Huang CL, et al. Novel KCNJ5 mutations in sporadic aldosterone-producing adenoma reduce Kir3. 4 membrane abundance. J Clin Endocrinol Metab. (2015) 100:E155–63. doi: 10.1210/jc.2014-3009
104. Hong AR, Kim JH, Song YS, Lee KE, Seo SH, Seong MW, et al. Genetics of aldosterone-producing adenoma in korean patients. PloS One. (2016) 11:e0147590. doi: 10.1371/journal.pone.0147590
105. Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscl thrombosis. (2014) 24.
106. Mohideen SK, Mustangin M, Kamaruddin NA, Muhammad R, Jamal ARA, Sukor N, et al. Prevalence and histopathological characteristics of KCNJ5 mutant aldosterone-producing adenomas in a multi-ethnic Malaysian cohort. Original research. Front Endocrinol (Lausanne). (2019) 10:666. doi: 10.3389/fendo.2019.00666
107. Nanba K, Yamazaki Y, Bick N, Onodera K, Tezuka Y, Omata K, et al. Prevalence of somatic mutations in aldosterone-producing adenomas in Japanese patients. J Clin Endocrinol Metab. (2020) 105:e4066–73. doi: 10.1210/clinem/dgaa595
108. Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, et al. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J Clin Endocrinol Metab. (2012) 97:1311–9. doi: 10.1210/jc.2011-2885
109. Wang B, Li X, Zhang X, Ma X, Chen L, Zhang Y, et al. Prevalence and characterization of somatic mutations in chinese aldosterone-producing adenoma patients. Medicine. (2015) 94:e708. doi: 10.1097/md.0000000000000708
110. Warachit W, Atikankul T, Houngngam N, Sunthornyothin S. Prevalence of somatic KCNJ5 mutations in thai patients with aldosterone-producing adrenal adenomas. J Endocrine Society. (2018) 2:1137–46. doi: 10.1210/js.2018-00097
111. Akerstrom T, Crona J, Delgado Verdugo A, Starker LF, Cupisti K, Willenberg HS, et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PloS One. (2012) 7:e41926. doi: 10.1371/journal.pone.0041926
112. Akerstrom T, Willenberg HS, Cupisti K, Ip J, Backman S, Moser A, et al. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer. (2015) 22:735–44. doi: 10.1530/ERC-15-0321
113. Arnesen T, Glomnes N, Strømsøy S, Knappskog S, Heie A, Akslen LA, et al. Outcome after surgery for primary hyperaldosteronism may depend on KCNJ5 tumor mutation status: a population-based study from Western Norway. Langenbeck’s Arch Surgery. (2013) 398:869–74. doi: 10.1007/s00423-013-1093-2
114. Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. (2014) 64:354–61. doi: 10.1161/hypertensionaha.114.03419
115. Guo Z, Nanba K, Udager A, McWhinney BC, Ungerer JPJ, Wolley M, et al. Biochemical, histopathological, and genetic characterization of posture-responsive and unresponsive APAs. J Clin Endocrinol Metab. (2020) 105:e3224–35. doi: 10.1210/clinem/dgaa367
116. Meyer LS, Handgriff L, Lim JS, Udager AM, Kinker IS, Ladurner R, et al. Single-center prospective cohort study on the histopathology, genotype, and postsurgical outcomes of patients with primary aldosteronism. Hypertension. (2021) 78:738–46. doi: 10.1161/HYPERTENSIONAHA.121.17348
117. Nanba K, Omata K, Else T, Beck PCC, Nanba AT, Turcu AF, et al. Targeted molecular characterization of aldosterone-producing adenomas in white americans. J Clin Endocrinol Metab. (2018) 103:3869–76. doi: 10.1210/jc.2018-01004
118. Nanba K, Omata K, Gomez-Sanchez Celso E, et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension. (2019) 73:885–92. doi: 10.1161/HYPERTENSIONAHA.118.12070
119. Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinology. (2015) 83:779–89. doi: 10.1111/cen.12873
120. Kitamoto T, Nishikawa T. Clinical translationality of KCNJ5 mutation in aldosterone producing adenoma. Int J Mol Sci. (2022) 23:9042. doi: 10.3390/ijms23169042
121. Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. (2010) 95:2296–305. doi: 10.1210/jc.2009-2010
122. Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. (2015) 112:E4591–9. doi: 10.1073/pnas.1505529112
123. Omata K, Anand SK, Hovelson DH, Liu C-J, Yamazaki Y, Nakamura Y, et al. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocrine Society. (2017) 1:787–99. doi: 10.1210/js.2017-00134
124. Omata K, Satoh F, Morimoto R, Ito S, Yamazaki Y, Nakamura Y, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. Oct. (2018) 72:874–80. doi: 10.1161/HYPERTENSIONAHA.118.11086
125. Omura M, Sasano H, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Clinical characteristics of aldosterone-producing microadenoma, macroadenoma, and idiopathic hyperaldosteronism in 93 patients with primary aldosteronism. Hypertens Res. (2006) 29:883–9.
126. Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age-related autonomous aldosteronism. Circulation. (2017) 136:347–55. doi: 10.1161/circulationaha.117.028201
127. Nanba K, Vaidya A, Rainey WE. Aging and adrenal aldosterone production. Hypertension. (2018) 71:218–23. doi: 10.1161/hypertensionaha.117.10391
128. Baudrand R, Guarda FJ, Fardella C, Hundemer G, Brown J, Williams G, et al. Continuum of renin-independent aldosteronism in normotension. Hypertension. (2017) 69:950–6. doi: 10.1161/hypertensionaha.116.08952
129. Le Floch E, Cosentino T, Larsen CK, Beuschlein F, Reincke M, Amar L, et al. Identification of risk loci for primary aldosteronism in genome-wide association studies. Nat Commun. (2022) 13:5198. doi: 10.1038/s41467-022-32896-8
130. Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, et al. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension. Jan. (2016) 67:139–45. doi: 10.1161/HYPERTENSIONAHA.115.06186
131. Inoue K, Yamazaki Y, Kitamoto T, Hirose R, Saito J, Omura M, et al. Aldosterone suppression by dexamethasone in patients with KCNJ5-mutated aldosterone-producing adenoma. J Clin Endocrinol Metab. (2018) 103:3477–85. doi: 10.1210/jc.2018-00738
132. Tezuka Y, Yamazaki Y, Kitada M, Morimoto R, Kudo M, Seiji K, et al. 18-oxocortisol synthesis in aldosterone-producing adrenocortical adenoma and significance of KCNJ5 mutation status. Hypertension. (2019). doi: 10.1161/HYPERTENSIONAHA.118.12064
133. Hung A, Ahmed S, Gupta A, Davis A, Kline GA, Leung AA, et al. Performance of the aldosterone to renin ratio as a screening test for primary aldosteronism. J Clin Endocrinol Metab. (2021) 106:2423–35. doi: 10.1210/clinem/dgab348
134. Parksook WW, Brown JM, Omata K, Tezuka Y, Ono Y, Satoh F, et al. The spectrum of dysregulated aldosterone production: an international human physiology study. J Clin Endocrinol Metab. (2024). doi: 10.1210/clinem/dgae145
135. Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. (1992) 120:893–901.
136. Funder JW. Primary aldosteronism and salt. Pflugers Arch. (2015) 467:587–94. doi: 10.1007/s00424-014-1658-0
137. Ueda T, Tsurutani Y, Osada J, Inoue K, Hoshino Y, Ono M, et al. Comparison of echocardiographic changes between surgery and medication treatment in patients with primary aldosteronism. J Am Heart Assoc. (2022) 11:e023813. doi: 10.1161/JAHA.121.023813
138. Katsuragawa S, Goto A, Shinoda S, Inoue K, Nakai K, Saito J, et al. Association of reversal of renin suppression with long-term renal outcome in medically treated primary aldosteronism. Hypertension. (2023) 80:1909–20. doi: 10.1161/HYPERTENSIONAHA.123.21096
139. Inoue K, Goldwater D, Allison M, Seeman T, Kestenbaum BR, Watson KE. Serum aldosterone concentration, blood pressure, and coronary artery calcium: the multi-ethnic study of atherosclerosis. Hypertension. (2020). doi: 10.1161/HYPERTENSIONAHA.120.15006
140. Hundemer GL, Agharazii M, Madore F, Vaidya A, Brown JM, Leung AA, et al. Subclinical primary aldosteronism and cardiovascular health: A population-based cohort study. Circulation. (2024) 149:124–34. doi: 10.1161/CIRCULATIONAHA.123.066389
141. Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, et al. Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension. Sep. (2012) 60:618–24. doi: 10.1161/HYPERTENSIONAHA.112.197111
142. Marzano L, Colussi G, Sechi LA, Catena C. Adrenalectomy is comparable with medical treatment for reduction of left ventricular mass in primary aldosteronism: meta-analysis of long-term studies. Am J Hypertension. (2015) 28:312–8. doi: 10.1093/ajh/hpu154
143. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. (2018) 3:768–74. doi: 10.1001/jamacardio.2018.2003
144. Araujo-Castro M, Ruiz-Sánchez JG, Ramírez PP, Martín Rojas-Marcos P, Aguilera-Saborido A, Gómez Cerezo JF, et al. Practical consensus for the treatment and follow-up of primary aldosteronism: a multidisciplinary consensus document. Endocrine. (2024) 85:532–44. doi: 10.1007/s12020-024-03773-9
145. McKenna TJ, Sequeira SJ, Heffernan A, Chambers J, Cunningham S. Diagnosis under random conditions of all disorders of the renin-angiotensin-aldosterone axis, including primary hyperaldosteronism. J Clin Endocrinol Metab. (1991) 73:952–7. doi: 10.1210/jcem-73-5-952
146. Jose A, Crout JR, Kaplan NM. Suppressed plasma renin activity in essential hypertension. Roles plasma volume Blood pressure sympathetic nervous system. Ann Intern Med. (1970) 72:9–16. doi: 10.7326/0003-4819-72-1-9
147. Carey RM, Douglas JG, Schweikert JR, Liddle GW. The syndrome of essential hypertension and suppressed plasma renin activity. Normalization Blood Pressure spironolactone. Arch Intern Med. (1972) 130:849–54. doi: 10.1001/archinte.1972.03650060041007
148. Laragh JH, Letcher RL, Pickering TG. Renin profiling for diagnosis and treatment of hypertension. JAMA. (1979) 241:151–6. doi: 10.1001/jama.1979.03290280031022
149. Baudrand R, Vaidya A. The low-renin hypertension phenotype: genetics and the role of the mineralocorticoid receptor. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19020546
150. Williams B, Macdonald TM, Morant S, Webb DJ, Sever P, McInnes G, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. (2015) 386:2059–68. doi: 10.1016/s0140-6736(15)00257-3
151. Williams B, Macdonald TM, Morant SV, Webb DJ, Sever P, McInnes G, et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinology. (2018) 6:464–75. doi: 10.1016/s2213-8587(18)30071-8
152. Fischer E, Beuschlein F, Degenhart C, Jung P, Bidlingmaier M, Reincke M. Spontaneous remission of idiopathic aldosteronism after long-term treatment with spironolactone: results from the German Conn’s Registry. Clin endocrinology. (2012) 76:473–7.
153. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. (2018). doi: 10.1161/HYPERTENSIONAHA.118.11568
154. Satoh M, Maruhashi T, Yoshida Y, Shibata H. Systematic review of the clinical outcomes of mineralocorticoid receptor antagonist treatment versus adrenalectomy in patients with primary aldosteronism. Hypertens Res. (2019). doi: 10.1038/s41440-019-0244-4
155. Funder JW. Who and how should we screen for primary aldosteronism? Hypertension. (2023) 80:2495–500. doi: 10.1161/HYPERTENSIONAHA.123.20536
Keywords: primary aldosteronism, aldosterone measurement, screening test, low renin hypertensive, somatic mutation
Citation: Kitamoto T, Ruike Y, Koide H, Inoue K, Maezawa Y, Omura M, Nakai K, Tsurutani Y, Saito J, Kuwa K, Yokote K and Nishikawa T (2025) Shifting paradigms in primary aldosteronism: reconsideration of screening strategy via integrating pathophysiological insights. Front. Endocrinol. 15:1372683. doi: 10.3389/fendo.2024.1372683
Received: 18 January 2024; Accepted: 16 December 2024;
Published: 14 January 2025.
Edited by:
Elena Aisha Azizan, National University of Malaysia, MalaysiaReviewed by:
Piotr Glinicki, Centre of Postgraduate Medical Education, PolandCopyright © 2025 Kitamoto, Ruike, Koide, Inoue, Maezawa, Omura, Nakai, Tsurutani, Saito, Kuwa, Yokote and Nishikawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takumi Kitamoto, dC5raXRhbW90b0BjaGliYS11Lmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.