
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 24 June 2024
Sec. Reproduction
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1369684
A commentary has been posted on this article:
Commentary: Association between life’s essential 8 and male biochemical androgen deficiency: evidence from NHANES 2013-2016
Purpose: To evaluate the association of Life’s Essential 8 (LE8) and its subscales with male biochemical androgen deficiency (MBAD) and total testosterone based on the data from the national health and nutrition examination survey (NHANES) database.
Methods: Data of males aged 20 years or older from NHANES of 2013–2016 were extracted. LE8 score was calculated based on American Heart Association definitions. Total testosterone (TT) values were measured in NHANES using precise isotope dilution liquid chromatography. MBAD was defined as serum TT of <300 ng/dL. Univariate and multivariable analyses were conducted. Propensity score matching (PSM) and weighted regression after matching were added as sensitivity analyses. The generalized additive model, smooth curve fitting, and the recursive algorithm were used to determine the potential inflection points. Piecewise regression models with log-likelihood ratio test were used to quantify nonlinear effects.
Results: A total of 3094 participants who were males and aged 20 years or above were included. Out of them, 805 males were diagnosed with MBAD. After adjusting the confounders in the multivariable model, LE8 was independently associated with MBAD (OR 0.96, P < 0.001) and TT (β 2.7, P < 0.001). The association remained robust even after PSM. The non-linear relationship of LE8 behaviors score with MBAD and TT was revealed.
Conclusion: LE8 was an independent protective factor of MBAD and a feasible approach to promote male endocrine sexual function.
Testosterone is the primary androgen in males. In males, 95% of testosterone is produced in Leyden cells of the testis under the regulation of the hypothalamic-pituitary-testis axis (1). Testosterone is necessary for spermatogenesis, stimulation of sexual desire, and normal sexual function in adults. Additionally, testosterone is involved in body composition, bone and muscle formation, erythropoiesis, and iron metabolism. By 2025, it is projected that the United States will witness a prevalence of symptomatic testosterone deficiency (TD) in approximately 6.5 million males (2). The American Urological Association guideline defined TD as testosterone levels lower than 300 ng/dL with corresponding symptoms or signs (3). It manifests as impaired development of muscle and body hair, gynecomastia, decreased stature, erectile dysfunction, and sexual difficulties (4).
In 2010, the American Heart Association (AHA) introduced the concept of Life’s Simple 7 (LS7) as a set of seven key indicators of cardiovascular health (CVH) that can be targeted for the improvement of cardiovascular well-being (5). Recently, the AHA has updated LS7, incorporating sleep health as an essential component, resulting in the formulation of Life’s Essential 8 (LE8) (6). LE8 comprises four behaviors: diet, physical activity, nicotine exposure, and sleep health, along with four factors: body mass index (BMI), blood lipids, blood glucose, and blood pressure.
The AHA’s LE8 construct is a useful tool for assessing and improving CVH. By following the health behaviors and health factors outlined in LE8, individuals can lower their risk for heart disease, stroke, and other major health problems. Higher LE8 scores have been associated with a lower risk of cardiovascular disease (CVD) and all-cause mortality among aging men (7). An analysis found that people whose lifestyles better reflect CVH can reap gains in life expectancy free from major chronic disease (8). Other researchers have reported that a high LE8 score is significantly associated with a lower risk of chronic kidney disease and nonalcoholic fatty liver disease (9, 10).
LE8 is a comprehensive index and numerous studies have demonstrated its association with various health conditions. Testosterone has been linked to CVH and its risk factors in multiple research (1, 11). Given the established connection, this study aims to investigate the association of LE8 with testosterone, an area that remains unexplored.
NHANES is an ongoing series of surveys conducted by the National Center for Health Statistics at the U.S. Centers for Disease Control and Prevention. Its purpose is to assess the health and nutritional status of the U.S. population through interviews and physical examinations (http://www.cdc.gov/nchs/nhanes.htm). NHANES follows a complex, multistage probability sampling design in a 2-year cycle to ensure nationally representative data for the civilian noninstitutionalized U.S. population. More detailed information about NHANES methods and protocols can be found on their website. The NCHS Research Ethics Review Board approved the NHANES procedures and protocols, with written informed consent obtained from all participants. The reporting guidelines of Strengthening the Reporting of Observational Studies in Epidemiology were followed during this study.
Data were limited to the continuous data cycles of 2013–2014 and 2015–2016. The cohort consisted of men aged 20 years who completed a comprehensive 24-hour dietary history and underwent sex hormone testing. Participants with incomplete data regarding sex hormones and dietary recall assessments were excluded from the analysis. After further exclusion of men taking medication related to sex hormones, such as testosterone, progesterone, estrogen, or other similar substances mentioned in the NHANES questionnaire, a total of 3,094 participants were included in the study (Figure 1).
The LE8 score comprises 4 health behaviors (diet, physical activity, nicotine exposure, and sleep duration) and 4 health factors (BMI, non-high-density lipoprotein (non-HDL) cholesterol, blood glucose, and blood pressure). Dietary indicators were assessed employing the Healthy Eating Index (HEI) 2015 measured by the subjects’ 24-hour dietary review (12). Relevant information such as physical activity, nicotine exposure, sleep patterns, diabetes history, and medication usage were obtained via a self-report survey. Measurements of height, weight, and blood pressure were conducted in the physical examination. The BMI was calculated by dividing the weight (kilograms) by the height (meters) squared. Non–HDL cholesterol, plasma glucose, and hemoglobin A1c were measured from collected blood samples. The algorithm for determining the LE8 score in the NHANES dataset has been previously documented (6, 13). Each of the 8 CVH indicators was assigned a score between 0 and 100, and the overall LE8 score was calculated as an average of these indicators. Meanwhile, individuals with high CVH were categorized as having a LE8 score ranging from 80 to 100. Moderate CVH was defined as a LE8 score between 50 and 79, while low CVH referred to a LE8 score of 0 to 49 (6). The same cut-off points were applied in our research for classifying the subscales.
The primary outcomes were total testosterone (TT), free testosterone (FT), and male biochemical androgen deficiency (MBAD). TT values were measured in NHANES using precise isotope dilution liquid chromatography and tandem mass spectrometry at a single time point in the morning, afternoon, or evening. FT was calculated based on the Vermeulen Equation using the online tool Free & Bioavailable Testosterone calculator (www.issam.ch/freetesto.html) (14). MBAD was defined as serum TT of <300 ng/dL in this study according to the American Urological Association guidelines (3). Given that NHANES does not comprehensively include the symptoms and physical signs necessary for evaluating TD, and questionnaires are not recommended for reporting clinical symptoms (3, 15), this study establishes the testosterone threshold as the sole inclusion criterion for MBAD.
Potential variables confounding the association between LE8 and MBAD were included in multivariable models. The covariates included age (16–18), ethnicity (19), education level, marital status, poverty ratio, time of sample collection (venipuncture time), and self-report cardiovascular disease (CVD). Education level was classified as high school or less, some college and college graduate or above. marital status was classified as married/living with a partner, never married, and widowed/divorced/separated. The poverty ratio was categorized as ≤1.3, 1.4–3.5, and >3.5. Considering that testosterone may have circadian variation (20), the venipuncture time was categorized as morning, afternoon, or evening. CVD was defined as a self-reported history of one of the following conditions: coronary heart disease, myocardial infarction, congestive heart failure, and stroke.
Weighted mean, weighted proportions, and corresponding 95% confidence interval (CI) were used to describe the characteristics of the participants. Continuous data were compared by survey t-test, and categorical data were compared by Rao-Scott chi-square test. Odds ratio (OR), β, and corresponding 95% CI were calculated. Weighted histograms were employed in this study to assess whether the data conform to a normal distribution (Supplementary Figure S1). Ultimately, only FT was observed to exhibit pronounced non-normality, prompting us to perform a logarithmic transformation (log2) and include it in our sensitivity analysis (Supplementary Tables S7, S8). Given the lack of knowledge regarding the overall population distribution of LE8 scores, we refrained from transforming them. During regression analysis, we utilized both the original LE8 scores and a segmented version suitable for left-skewed distributions. Weighted logistic regression model and weighted linear regression were employed to calculate the independent associations of LE8 as well as its subscales with TT, FT, and MBAD. Three models were constructed with model 1 being a crude model without adjustment for covariate, model 2 adjusted for venipuncture time, age, and ethnicity, and model 3 further adjusted for marital income level, education, and self-reported CVD history of one of the following conditions: coronary heart disease, heart attack, congestive heart failure, or stroke. LE8 score was categorized according to the scoring intervals mentioned previously. The generalized additive model, smooth curve fitting, and the recursive algorithm were used to determine the potential inflection points. Piecewise regression models with log-likelihood ratio test were used to quantify nonlinear effects. A 1:1 propensity score matching (PSM) and weighted regression after matching were added as sensitivity analyses. Furthermore, given the potential impact of mental distress on hormone levels (21, 22), we excluded individuals taking psychiatric drugs and further controlled for Patient Health Questionnaire (PHQ-9) score which measures the severity of depression in subsequent sensitivity analyses. The software R 4.3.1 and Empower® (www.empowerstats.com) were utilized for conducting all statistical analyses. Two-tailed p values <0.05 were considered statistically significant.
In the NHANES Continuous Surveys from 2013–2016, 3094 individuals who were males and aged 20 years or above were considered for inclusion. Baseline characteristics of them were presented in Table 1, categorized according to their MBAD status. Before PSM, 805 males were diagnosed with MBAD. Compared to those without MBAD, participants with MBAD tended to be older and coupled and have a CVD history. The LE8 score (70.50, 95%CI 69.45–71.55) and LE8 health factors score (72.42, 95%CI 71.09–73.75) were higher in participants without MBAD, while there was no significant difference in the LE8 health behaviors score between the two groups. A total of 1610 participants were enrolled after PSM, including 805 participants with and without MBAD. Except for the study variables, other demographic characteristics were controlled for almost the same level. The homogeneity was almost achieved in these two groups (Table 1; Supplementary Figure S2).
Univariate and multivariable analysis before PSM showed that, both as a continuous variable and stratification variables, higher LE8 score as well as LE8 health factors score was associated with decreased risk of MBAD (Table 2, all OR < 1, all P < 0.001), elevated level of TT (Supplementary Table S2, all β > 0, all P < 0.001) and FT (Supplementary Table S3, all β > 0, all P < 0.05). After PSM and concerning the influence of psychiatric disorders, LE8 and LE8 health factors maintained robust results as a protective role for MBAD (Table 2; Supplementary Table S4).
Logistic regression and linear regression showed no association of LE8 health behaviors with MBAD, TT, and FT (Table 2; Supplementary Tables S2, S3). Smooth curve fitting and threshold effect analysis revealed the dose-response association between LE8 health behaviors score with MBAD and TT (Table 3, Figure 2). When the health behaviors score was below 78.75, it did not indicate a correlation with MBAD and was inversely associated with TT (β -0.55, P = 0.0171). When it exceeded 78.75, it showed a positive correlation with TT (β 1.96, P = 0.0057). However, if it surpassed the threshold of 80, it exhibited a protective effect against both TT and MBAD (OR 0.96, P = 0.0009).
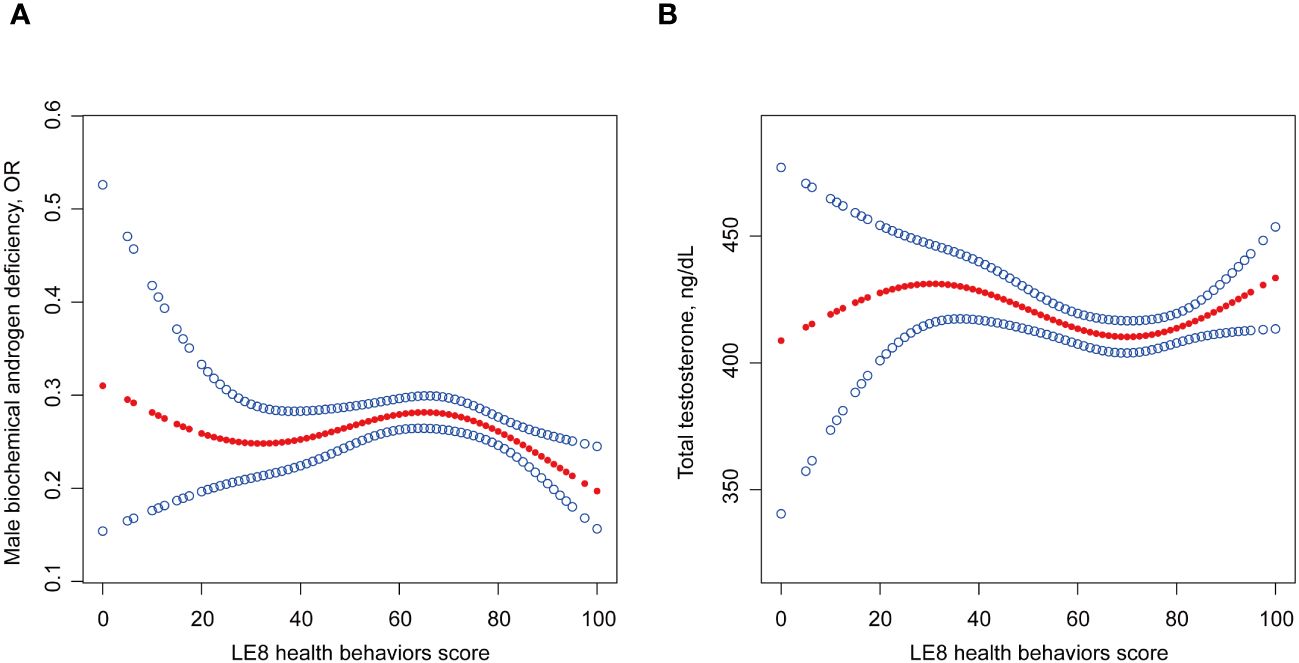
Figure 2 Non-linear relationship of LE8 with (A) MBAD and (B) TT. Age, ethnicity, time of venipuncture, marital status, poverty ratio, education levels and self-reported cardiovascular disease history were adjusted in this model. LE8, life’s essential 8; MBAD, male biochemical androgen deficiency; TT, total testosterone.
In this nationally representative cross-sectional study, we found that compared to those without MBAD, participants with MBAD tended to be older and coupled and have a CVD history. Participants without MBAD had higher LE8 score and LE8 health factors score. LE8 score and LE8 health factors score were negatively associated with the prevalence of MBAD. The association remained robust in multiple regression both before and after PSM. Moreover, our results revealed the non-linear association of LE8 health behaviors with MBAD and TT. This is the first study assessing an association between the LE8 and MBAD.
Multiple researches have evaluated the association between testosterone and CVH (23–26). Our discovery aligns with the existing understanding that testosterone is associated with CVH levels. In the present study, the association of LE8 with MBAD and testosterone remained stable after controlling for covariates and sensitivity analyses. This suggested that LE8 is a highly robust independent factor of MBAD and testosterone. The promotion of LE8 contributes to the maintenance of male reproductive function. Besides, many researchers have espoused the view that testosterone at physiological levels confers beneficial effects on the male CVH (1, 11, 27), implying that testosterone may play the role of mediator in LE8 promoting CVH. Another study has demonstrated that maintaining a high level of LE8 would effectively postpone phenotypic aging, with oxidative stress playing a crucial role as a mediator in this biological process (28). This broadened our perception of LE8, suggesting that adherence to the LE8 lifestyle may improve oxidative stress management, thereby reducing inflammation levels and ultimately promoting holistic well-being (29). The decrease in the risk of MBAD is one of the comprehensive optimal outcomes.
The underlying mechanisms of LE8 health factors in endocrine sexual function have been explored. Matteo et al.’s review extensively explored the pathogenesis of Functional Hypogonadotropic Hypogonadism (30). It elucidated that in obese patients, elevated levels of proinflammatory cytokines lead to decreased concentrations of SHBG, ultimately impacting testosterone levels. Moreover, adipose tissue can induce testosterone conversion and affect steroid survival of interstitial cells, resulting in reduced testosterone production. The article also highlights the structural changes in gonads and sexual dysfunction caused by diabetes, with insulin resistance in type 2 diabetes mellitus potentially impairing the secretion of gonadotropin-releasing hormone and decreasing testosterone levels. Another article reported a correlation between sex hormones and arterial hypertension (31). Sex hormones can regulate cardiovascular homeostasis through both slow genomic mechanisms and rapid nongenomic mechanisms, potentially providing cardiovascular protection in premenopausal women (and possibly men).
The present study revealed no significant difference in diet score, smoking score, and sleep score between individuals with MBAD and those without. Besides, there was no linear association between MBAD and LE8 health behaviors score grouped as categorized. To date, several studies on the correlation between metrics of LE8 health behaviors score and testosterone based on linear models have not reached consistent conclusions (32–40). In fact, both excessive and insufficient physical activity are associated with lower testosterone levels (34, 35). There may exist an optimal window of exercise levels where going above or below is associated with adverse outcomes. The evaluation of dietary patterns encompasses various facets, wherein diet exhibits correlations with testosterone levels in certain domains while lacking such associations in others (36–39). A meta-analysis based on 28 studies indicated that testosterone levels are higher in male smokers compared to non-smokers, while no association was found in females. This discovery challenges our preconceived notion of tobacco. The potential mechanism is that tobacco metabolites inhibit testosterone degradation (40). The research findings on the impact of sleep on testosterone levels are also contradictory (32, 33), possibly due to different cutoff values used in sleep assessments. The presence of certain covariates may also contribute to the variability in research results. These studies were unable to draw consistent conclusions about the relationship of physical activity, diet, and smoking with testosterone levels based on the linear model.
However, our study revealed a non-linear association of LE8 health behaviors with MBAD and TT. Only after the health behaviors score reached the threshold of 78.75 and 80 was it protective against TT and MBAD. This may emphasize the lifestyle as a whole, any unhealthy behavior could lead to a bucket effect. Grounded in LE8, physicians can offer the population comprehensive lifestyle guidance in primary healthcare. Testosterone therapy is currently the primary treatment of male MBAD. However, there is currently a lack of comprehensive lifestyle recommendations available for patients with MBAD. LE8 is an assessment tool that can be easily implemented in clinical settings to encourage the adoption of healthy behaviors and optimal health lifestyles. Our study expands upon the existing evidence by demonstrating that ideal CVH metrics not only have a positive impact on CVD but also play a beneficial role in reducing the burden of MBAD. Therefore, adhering to ideal CVH metrics may serve as an effective strategy for preventing and managing MBAD as well as CVD.
This study possesses several strengths. First, the more updated LE8 was utilized to reflect CVH in this study, and the components of the LE8 were analyzed with MBAD, making the findings more comprehensive and targeted. Second, we used a large nationally representative sample of US adults which allows the findings to be generalized to a broader population.
Nonetheless, certain limitations warrant consideration. Firstly, LE8 health behaviors did not demonstrate a correlation with FT in this study. However, AUA guideline recommends that all male patients with TD receive counseling on lifestyle adjustments and consider it as a treatment strategy (3). Therefore, we assume that the lack of correlation between LE8 health behaviors and FT does not hinder clinical practitioners from providing lifestyle recommendations to individuals with MBAD. Secondly, the cross-sectional design of the study precludes drawing causal inferences about the relationship between LE8 and MBAD. Therefore, we hope that readers, when interpreting the study, take into account the potential bidirectional relationship between LE8 and MBAD, especially concerning the metabolic parameters within the Health factors. Then, the potential for recall bias cannot be disregarded due to the utilization of self-report questionnaires to evaluate health behaviors. Another problem arises from the fact that serum testosterone is measured only once, while AUA guidelines recommend 2 levels due to intra-individual and diurnal variations of serum TT. Finally, although we controlled for confounding risk factors of MBAD in regression analyses, unknown or unmeasured confounding variables could still exist.
In this nationally representative sample of US adults, higher LE8 and its subscales score were independently associated with the lower prevalence of MBAD and higher levels of serum testosterone. A non-linear relationship between LE8 behaviors and MBAD was revealed in the current study. Our findings suggested that LE8 may have the potential to serve as a viable and effective method for promoting male endocrine sexual function. Further research is required to explore the longitudinal and causal relationship between LE8 and the risk of MBAD.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by National Center for Health Statistics Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
WH: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. MC: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. HZ: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. ZZ: Formal analysis, Writing – original draft, Writing – review & editing. CY: Visualization, Writing – original draft, Writing – review & editing. MH: Data curation, Writing – original draft, Writing – review & editing. BS: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the “Sanming Project of Medicine in Shenzhen” (No.SZSM202111007), the “Shenzhen Key Medical Discipline Construction Fund” (No.SZXK020), and the the “Shenzhen High-level Hospital Construction Fund”.
We acknowledge NHANES databases for their generous provision of platforms and researchers who contributed their datasets.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1369684/full#supplementary-material
1. Elagizi A, Köhler TS, Lavie CJ. Testosterone and cardiovascular health. Mayo Clinic Proc. (2018) 93:83–100. doi: 10.1016/j.mayocp.2017.11.006
2. Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. (2007) 92:4241–7. doi: 10.1210/jc.2007-1245
3. Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urology. (2018) 200:423–32. doi: 10.1016/j.juro.2018.03.115
4. Antonio A, Abraham M. The practical management of testosterone deficiency in men. Nat Rev Urol. (2015) 12:641–50. doi: 10.1038/nrurol.2015.238
5. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. (2010) 121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703
6. Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life’s essential 8: updating and enhancing the american heart association’s construct of cardiovascular health: A presidential advisory from the american heart association. Circulation. (2022) 146:e18–e43. doi: 10.1161/CIR.0000000000001078
7. Isiozor NM, Kunutsor SK, Voutilainen A, Laukkanen JA. Life’s Essential 8 and the risk of cardiovascular disease death and all-cause mortality in Finnish men. Eur J Prev Cardiol. (2023) 30(8):658–67. doi: 10.1093/eurjpc/zwad040
8. Wang X, Ma H, Li X, Heianza Y, Manson JE, Franco OH, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in UK adults. JAMA Internal Med. (2023) 183:340–9. doi: 10.1001/jamainternmed.2023.0015
9. Tang R, Wang X, Li X, Ma H, Liang Z, Heianza Y, et al. Adherence to Life’s Essential 8 and incident chronic kidney disease: a prospective study of 147,988 UK Biobank participants. Am J Clin Nutr. (2023) S0002–9165:66077–0. doi: 10.1016/j.ajcnut.2023.08.007
10. Wang L, Yi J, Guo X, Ren X. Associations between life’s essential 8 and non-alcoholic fatty liver disease among US adults. J Transl Med. (2022) 20:616. doi: 10.1186/s12967-022-03839-0
11. Stallone JN, Oloyo AK. Cardiovascular and metabolic actions of the androgens: Is testosterone a Janus-faced molecule? Biochem Pharmacol. (2023) 208:115347. doi: 10.1016/j.bcp.2022.115347
12. Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. (2018) 118:1591–602. doi: 10.1016/j.jand.2018.05.021
13. Lloyd-Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, et al. Status of cardiovascular health in US adults and children using the american heart association’s new “Life’s essential 8” Metrics: prevalence estimates from the national health and nutrition examination survey (NHANES), 2013 through 2018. Circulation. (2022) 146:822–35. doi: 10.1161/CIRCULATIONAHA.122.060911
14. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. (1999) 84:3666–72. doi: 10.1210/jcem.84.10.6079
15. Morales A, Bebb RA, Manjoo P, Assimakopoulos P, Axler J, Collier C, et al. Diagnosis and management of testosterone deficiency syndrome in men: clinical practice guideline. Can Med Assoc J. (2015) 187:1369–77. doi: 10.1503/cmaj.150033
16. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. (2001) 86:724–31. doi: 10.1210/jcem.86.2.7219
17. Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. (2002) 87:589–98. doi: 10.1210/jcem.87.2.8201
18. Marriott RJ, Murray K, Adams RJ, Antonio L, Ballantyne CM, Bauer DC, et al. Factors associated with circulating sex hormones in men: individual participant data meta-analyses. Ann Intern Med. (2023) 176:1221–34. doi: 10.7326/M23-0342
19. Lopez DS, Peskoe SB, Joshu CE, Dobs A, Feinleib M, Kanarek N, et al. Racial/ethnic differences in serum sex steroid hormone concentrations in US adolescent males. Cancer causes control : CCC. (2013) 24:10. doi: 10.1007/s10552-013-0154-8
20. Gupta SK, Lindemulder EA, Sathyan G. Modeling of circadian testosterone in healthy men and hypogonadal men. J Clin Pharmacol. (2000) 40:731–8. doi: 10.1177/00912700022009486
21. Pavlidi P, Kokras N, Dalla C. Antidepressants’ effects on testosterone and estrogens: What do we know? Eur J Pharmacol. (2021) 899:173998. doi: 10.1016/j.ejphar.2021.173998
22. Kurita N, Horie S, Yamazaki S, Otoshi K, Otani K, Sekiguchi M, et al. Low testosterone levels, depressive symptoms, and falls in older men: a cross-sectional study. J Am Med Directors Assoc. (2014) 15:30–5. doi: 10.1016/j.jamda.2013.11.003
23. Srinath R, Hill Golden S, Carson KA, Dobs A. Endogenous testosterone and its relationship to preclinical and clinical measures of cardiovascular disease in the atherosclerosis risk in communities study. J Clin Endocrinol Metab. (2015) 100:1602–8. doi: 10.1210/jc.2014-3934
24. Kwon H, Lee DG, Kang HC, Lee JH. The relationship between testosterone, metabolic syndrome, and mean carotid intima-media thickness in aging men. Aging Male. (2014) 17:211–5. doi: 10.3109/13685538.2014.958458
25. de Sousa G, Brodoswki C, Kleber M, Wunsch R, Reinehr T. Association between androgens, intima-media thickness and the metabolic syndrome in obese adolescent girls. Clin Endocrinol (Oxf). (2010) 72:770–4. doi: 10.1111/j.1365-2265.2009.03710.x
26. Soisson V, Brailly-Tabard S, Empana JP, Féart C, Ryan J, Bertrand M, et al. Low plasma testosterone and elevated carotid intima-media thickness: Importance of low-grade inflammation in elderly men. Atherosclerosis. (2012) 223:244–9. doi: 10.1016/j.atherosclerosis.2012.05.009
27. Green DJ, Chasland LC, Naylor LH, Yeap BB. New horizons: testosterone or exercise for cardiometabolic health in older men. J Clin Endocrinol Metab. (2023) 108:2141–53. doi: 10.1210/clinem/dgad175
28. Liu W, Wang J, Wang M, Hou H, Ding X, Ma L, et al. Oxidative stress factors mediate the association between life’s essential 8 and accelerated phenotypic aging: NHANES 2005–2018. Journals Gerontology Ser A Biol Sci Med Sci. (2023) 79(1): glad240. doi: 10.1093/gerona/glad240
29. Salzano S, Checconi P, Hanschmann EM, Lillig CH, Bowler LD, Chan P, et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci U S A. (2014) 111:12157–62. doi: 10.1073/pnas.1401712111
30. Spaziani M, Carlomagno F, Tarantino C, Angelini F, Vincenzi L, Gianfrilli D. New perspectives in functional hypogonadotropic hypogonadism: beyond late onset hypogonadism. Front Endocrinol. (2023) 14:1184530. doi: 10.3389/fendo.2023.1184530
31. Barton M, Prossnitz ER, Meyer MR. Testosterone and secondary hypertension: new pieces to the puzzle. Hypertension. (2012) 59:1101–3. doi: 10.1161/HYPERTENSIONAHA.112.195149
32. Hernández-Pérez JG, Taha S, Torres-Sánchez LE, Villasante-Tezanos A, Milani SA, Baillargeon J, et al. Association of sleep duration and quality with serum testosterone concentrations among men and women: NHANES 2011–2016. Andrology. (2023) 12(3):518–26. doi: 10.1111/andr.13496
33. Zhou X, Qiu S, Deng L, Zhang Z, Jin K, Su X, et al. Sleep duration and testosterone levels in community older men: results from the West China Health and Aging Trend study. Chin Med J (Engl). (2023) 136:1123–5. doi: 10.1097/CM9.0000000000002466
34. Hackney AC. Hypogonadism in exercising males: dysfunction or adaptive-regulatory adjustment? Front Endocrinol. (2020) 11:11. doi: 10.3389/fendo.2020.00011
35. Marriott RJ, Murray K, Adams RJ, Antonio L, Ballantyne CM, Bauer DC, et al. Factors associated with circulating sex hormones in men : individual participant data meta-analyses. Ann Intern Med. (2023) 176:1221–34. doi: 10.7326/M23-0342
36. Gomes GK, de Branco FMS, Santos HO, Pereira JL, Orsatti FL, de Oliveira EP. Cholesterol intake and serum total cholesterol levels are not associated with total testosterone levels in men: a cross-sectional study from NHANES 2013–2014. Lipids Health Dis. (2023) 22:168. doi: 10.1186/s12944-023-01928-7
37. Storz MA, Ronco AL. Dietary acid load is not associated with serum testosterone in men: insights from the NHANES. Nutrients. (2023) 15:3075. doi: 10.3390/nu15133075
38. Kuchakulla M, Nackeeran S, Blachman-Braun R, Ramasamy R. The association between plant-based content in diet and testosterone levels in US adults. World J Urol. (2021) 39:1307–11. doi: 10.1007/s00345-020-03276-y
39. Zhang C, Bian H, Chen Z, Tian B, Wang H, Tu X, et al. The association between dietary inflammatory index and sex hormones among men in the United States. J Urol. (2021) 206:97–103. doi: 10.1097/JU.0000000000001703
Keywords: life’s essential 8, biochemical androgen deficiency, endocrine function, reproduction, NHANES
Citation: Huang W, Chen M, Zhang H, Zhang Z, Yin C, Huang M and Shi B (2024) Association between life’s essential 8 and male biochemical androgen deficiency: evidence from NHANES 2013–2016. Front. Endocrinol. 15:1369684. doi: 10.3389/fendo.2024.1369684
Received: 12 January 2024; Accepted: 07 June 2024;
Published: 24 June 2024.
Edited by:
Arcangelo Barbonetti, University of L’Aquila, ItalyReviewed by:
Chunlin Wang, University of Rome Tor Vergata, ItalyCopyright © 2024 Huang, Chen, Zhang, Zhang, Yin, Huang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bentao Shi, c2hpYmVudGFvcGt1QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.