- 1Department of General Surgery, Peking University Third Hospital, Beijing, China
- 2Department of Ultrasound, Peking University Third Hospital, Beijing, China
- 3Department of Maternal and Child Health, School of Public Health, Peking University, Beijing, China
- 4Department of Pathology, Peking University Third Hospital, School of Basic Medical Sciences, Peking University Health Science Center, Beijing, China
- 5National Key Laboratory for Multimedia Information Processing, School of Computer Science, Peking University, Beijing, China
- 6Information Management and Big Data Center, Peking University Third Hospital, Beijing, China
Purpose: Pregnant women with a diagnosis of differentiated thyroid cancer (DTC) were potentially high-risk but largely ignored study population. We aimed to explore whether gestational thyrotropin levels were associated with progression of DTC.
Methods: We conducted a retrospective cohort study at Peking University Third Hospital in Beijing, China from January 2012 to December 2022. We included pregnant women with a pre-pregnancy DTC managed by active surveillance (under-surveillance DTC) or surgical treatment (after-surgery DTC). Dynamic changes of gestational thyrotropin levels across multiple time points were characterized by both statistical (average level, change instability, longitudinal trajectory) and clinical (thyroid dysfunction, thyrotropin suppression, and achievement of thyrotropin suppression target) indicators. Outcomes were clinician-validated progression of DTC, measured separately for patients under surveillance (tumor enlargement or lymph node metastasis) and those after surgery (≥ 3 mm growth in the size of existing metastatic foci, development of new lymph node metastases, ≥ 2 mm growth in the size of existing cancer foci in the contralateral thyroid, or biochemical progression).
Results: Among 43 and 118 patients with under-surveillance and after-surgery DTC, we observed no evidence of associations between any of the quantitative or clinical indicators of gestational thyrotropin levels and progression-free survival, after a median of 2.63 (IQR: 0.90-4.73) and 4.22 (2.53-6.02) year follow-up, respectively (all P values > 0.05).
Conclusions: Gestational thyrotropin levels appeared to play a minor role in the progression of under-surveillance or after-surgery DTC. Clinicians might focus on the risk of adverse pregnancy outcomes when optimizing thyrotropin levels for pregnant women with a diagnosis of DTC.
Introduction
Thyroid cancer has been increasingly prevalent worldwide. Females are much more likely to experience thyroid cancer than males (1). The age-standardized incidence of thyroid cancer has elevated from 6.68/105 to 20.20/105 between 2005 and 2015 among females in China (1). Differentiated thyroid cancer (DTC) makes up more than ninety percent of all types of thyroid cancers (2). DTC is commonly diagnosed in women of reproductive age whose prevalence only follows breast cancer (3).
The clinical decisions for pregnant patients with DTC are generally complex. The complexity lies in the double burden of health risks for pregnant patients with DTC: adverse pregnancy outcomes and progression of DTC. Gestational thyrotropin is one important focus of prenatal care due to its critical function in the maintenance of normal pregnancy. To optimize gestational thyrotropin levels for pregnant women with DTC, it is equally important to elucidate their associations with adverse pregnancy outcomes as well as with the progression of DTC. However, the existing studies have focused more on the former than the latter associations (4, 5).
Another challenge is to assess the repeated measures of thyrotropin across the whole gestation in a fine-grained manner. Studies have mostly used the static, single-point measure, but this might refrain us from determining whether the influence of thyrotropin levels on outcomes is transient on only one occasion or accumulated along the temporal dimension. For example, the risk of the increase in thyrotropin levels for disease progression possibly differs between that occurs only in early pregnancy with subsequent disappearance and that sustains across the whole period of gestation. It is thus paramount to adopt accurate and sensitive indicators to reflect the dynamic changes in thyrotropin levels during early, middle, and late pregnancy. Several studies have used novel indicators or statistical methods to capture the average level (6), instability characteristics (7), or longitudinal trajectory of the repeated measures over time (8, 9), but these approaches have not been applied to investigate the present topic until now, to our knowledge.
“Less is more” has been recognized as the primary treatment strategy for the low-risk DTC (10, 11). Correspondingly, active surveillance has been considered as an alternative to immediate surgery for appropriately selected patients with low-risk DTC (12). Active surveillance refers to the close monitoring of cancer progression during a period without receiving immediate surgery. Despite the overall preferable prognosis of DTC, the degree of disease progression under active surveillance (briefly called “under-surveillance” below) varies greatly across individuals. Specifically, whether the level of thyrotropin relates to the progression of under-surveillance DTC has remained unresolved (13). Moreover, the relevant evidence until now has largely accumulated for the non-pregnant population (6, 14, 15). With the increasing number of pregnant women comorbid with under-surveillance DTC, it is urgent to clarify the associations of gestational thyrotropin levels with the progression of under-surveillance DTC. This has also been identified as an important research gap in the 2017 “Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum” (16).
To fill the research gaps, our study was focused on pregnant patients with under-surveillance or after-surgery DTC, comprehensively measured the characteristics of changes in gestational thyrotropin levels by multiple indicators, and examined their associations with the risk of biomedical or structural progression of DTC. Dynamic monitoring of gestational thyrotropin levels has been highlighted in the 2022 “Guidelines for prevention and management of thyroid diseases during pregnancy and perinatal period” in China. Findings of our study would thus respond in a timely matter to this call through providing more detailed evidence (17).
Methods
Study population
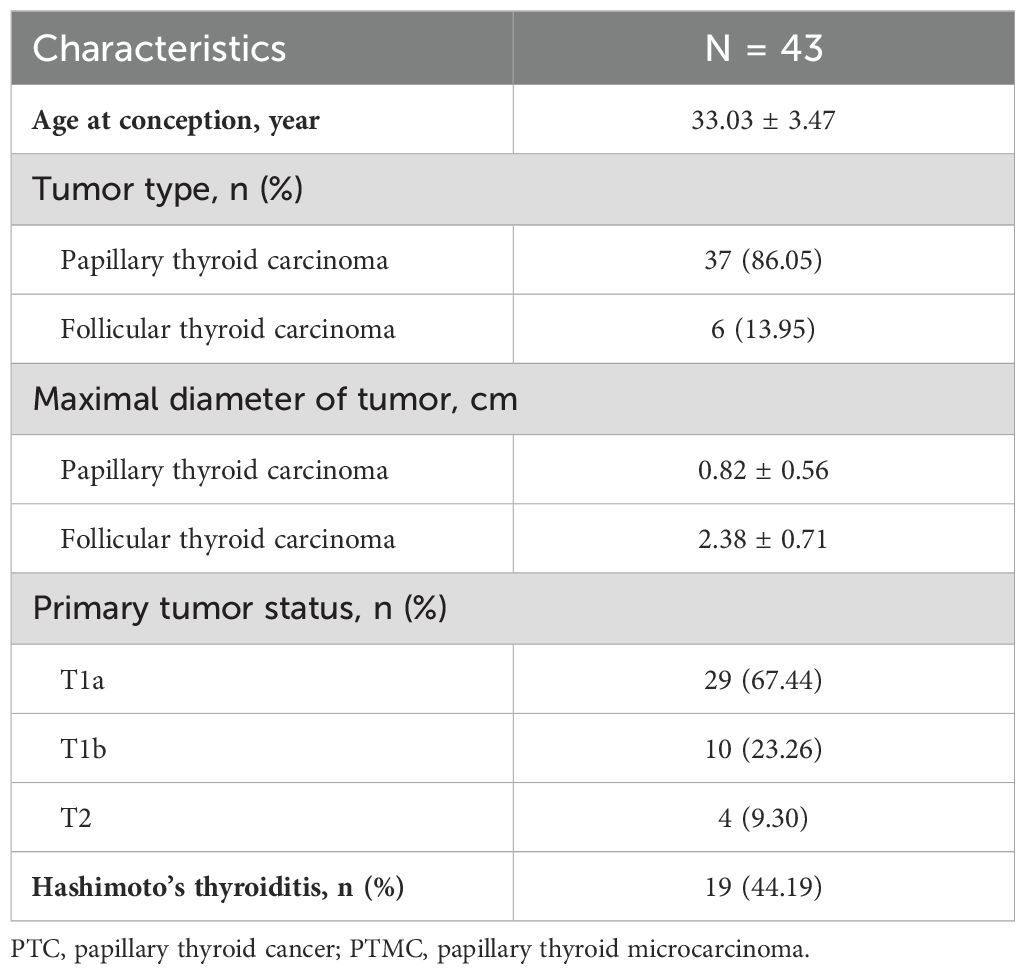
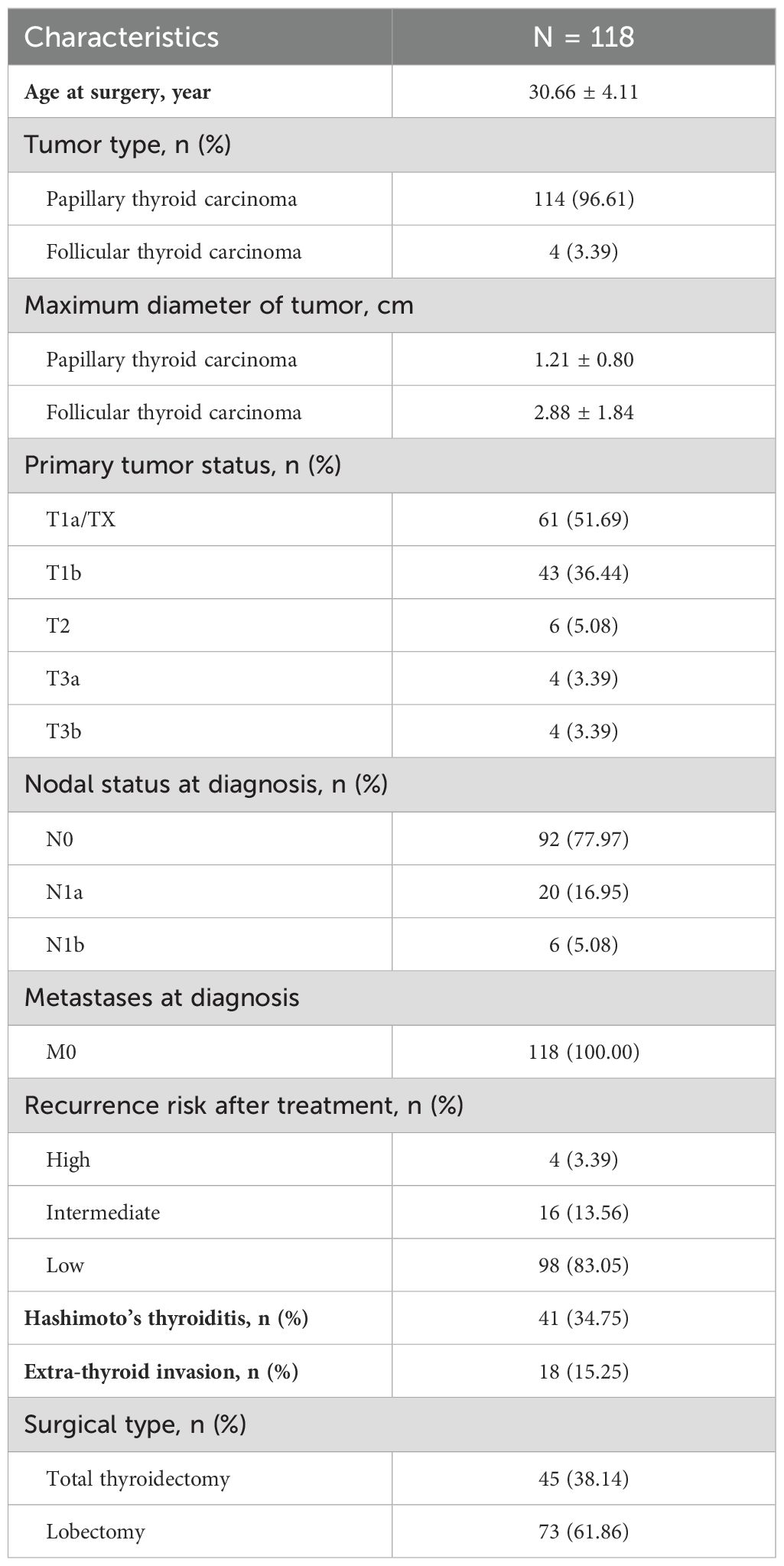
This was a retrospective cohort study conducted at Peking University Third Hospital in Beijing, China from January 2012 to December 2022. First, we selected patients if they had at least two thyrotropin measurements during gestation and were pathologically diagnosed with DTC. Then we selected those who were very similar to those in the prospective active surveillance studies as our study population 1: pregnant women with under-surveillance DTC. Inclusion criteria: 1) women receiving surgical treatment after the end of pregnancy; 2) with a duration of surveillance of no less than 6 months prior to surgery; and 3) undergoing at least two examinations of neck ultrasonography prior to surgery; Exclusion criteria: patients with lymph node metastasis or extra-thyroid invasion at baseline. For our study population 2 (pregnant women with after-surgery DTC), the inclusion criteria were: 1) women receiving surgical treatment before pregnancy; and 2) with postoperational and postpartum examinations of neck ultrasonography, or measurements of serum thyroglobulin (Tg) levels and Tg antibodies. Finally, a total of 43 and 118 patients were included as those with under-surveillance DTC and those with after-surgery DTC, respectively. This study was approved by the Medical Research Ethics Committee of Peking University Third Hospital (No. IRB00006761-M2022721).
Gestational thyrotropin levels
Thyrotropin levels were measured with commercial kits (Siemens Healthcare Diagnostics) using a fully automatic chemiluminescence immunoassay analyzer (ADVIA Centaur XP; Siemens Healthcare Diagnostics). The lowest and highest detection levels of thyrotropin were 0.008 mIU/L and 150.000 mIU/L, respectively.
Based on literature review (6–9), guidelines (17, 18) and our previous work (19, 20), we used both statistical and clinical indicators to comprehensively reflect the characteristics of the dynamic changes in gestational thyrotropin levels. Following the current Guidelines for the diagnosis and management of thyroid nodules and DTC in China (21), no levothyroxine medication was administered to under-surveillance individuals to maintain low thyrotropin levels while this medication was administered to after-surgery individuals to achieve thyrotropin suppression in this study setting. As such, specific indicators of gestational thyrotropin levels differed between our two study populations. For our study population 1 (pregnant women with under-surveillance DTC), the indicators of gestational thyrotropin levels included (1) time-weighted average of multiple measurements of thyrotropin, (2) instability of change in thyrotropin levels across multiple measurements, (3) longitudinal trajectory of thyrotropin levels across multiple measurements, and (4) thyroid dysfunction during gestation; for our study population 2 (pregnant women with after-surgery DTC), we additionally measured the gestational thyrotropin levels using (5) level of thyrotropin suppression and (6) achievement of thyrotropin suppression target based on response to therapy. Detailed definitions, calculation formula, or classification criteria for these indicators are shown in Supplemental Table 1.
Disease progression
The progression of under-surveillance DTC was assessed by tumor size enlargement (one greatest increment in one dimension of tumor size of no less than 3 mm); incident lymph node metastasis (LNM) at follow-up observation; progression (either tumor size enlargement or incident LNM).
The progression of after-surgery DTC was assessed in both structural and biochemical types. Structural progression referred to incident LNM, ≥ 3 mm growth in the size of existing metastatic foci (22), or ≥ 2 mm growth in the size of existing cancer foci in the contralateral thyroid by using neck ultrasonography. Biochemical progression referred to a ≥ 20% increase in postoperational serum Tg or Tg antibodies relative to the preoperational level (23). To ensure the accuracy of Tg measurement, we only included the measurement values of serum Tg if the patient’s Tg antibody was negative (24).
Status of disease progression in clinical records were carefully reviewed and checked by two researchers with rich experiences in clinical practice (X. L.) and data preprocessing (W.C.X.).
Statistical analyses
We first used Schoenfeld residuals to test the proportional-hazards assumption, and then we adopted Cox proportional risk models to analyze the relationship between indicators of gestational thyrotropin levels and progression-free survival. To estimate the adjusted Hazard Ratio (HR) and its 95% confidence interval (CI), the models among patients with under-surveillance DTC were adjusted for tumor type (papillary thyroid carcinoma; follicular thyroid carcinoma), tumor size at baseline, the status of Hashimoto’s thyroiditis (yes; no), and age at conception; the models among patients with after-surgery DTC were adjusted for tumor size at surgery, the status of Hashimoto’s thyroiditis (yes; no), age at surgery, and surgical type (thyroid lobectomy; total thyroidectomy). Statistical analyses were performed using R software version 4.2 and Stata software version 16.0. P values < 0.05 were considered statistically significant.
Results
Baseline characteristics of patients with under-surveillance or after-surgery DTC
Tables 1, 2 shows the baseline characteristics of patients with under-surveillance DTC (n = 43) and those with after-surgery DTC (n = 118), respectively. More than 85% of the two groups of the study population were diagnosed with low-risk (T1N0M0) papillary thyroid carcinoma. Approximately 44% patients with under-surveillance DTC and 35% of those with after-surgery DTC were comorbid with Hashimoto’s thyroiditis, respectively.
Associations of gestational thyrotropin levels with progression of under-surveillance DTC
For patients with under-surveillance DTC, the median duration of surveillance before pregnancy was 0.53 year (IQR: 0.17-2.19). As shown in Table 3, after a median of 2.63 (IQR: 0.90-4.73) year follow-up among 43 patients with under-surveillance DTC, 17 (53.13%) patients in the higher time-weighted average thyrotropin group (n = 32) and 4 (36.36%) of those in the lower time-weighted average thyrotropin group (n = 11) occurred progression of DTC. Results from the Cox proportional risk model indicated that there was no evidence of difference between the two groups in DTC progression-free survival, with adjustment for tumor type, tumor size, the status of Hashimoto’s thyroiditis, and age at conception [hazard ratio (HR): 1.51; 95% confidence interval (CI): 0.45, 5.03; P = 0.504]. There was also no evidence of associations between any of the other three indicators of gestational thyrotropin levels (instability of change, longitudinal trajectory, thyroid dysfunction) and progression of under-surveillance DTC.
Associations of gestational thyrotropin levels with progression of after-surgery DTC
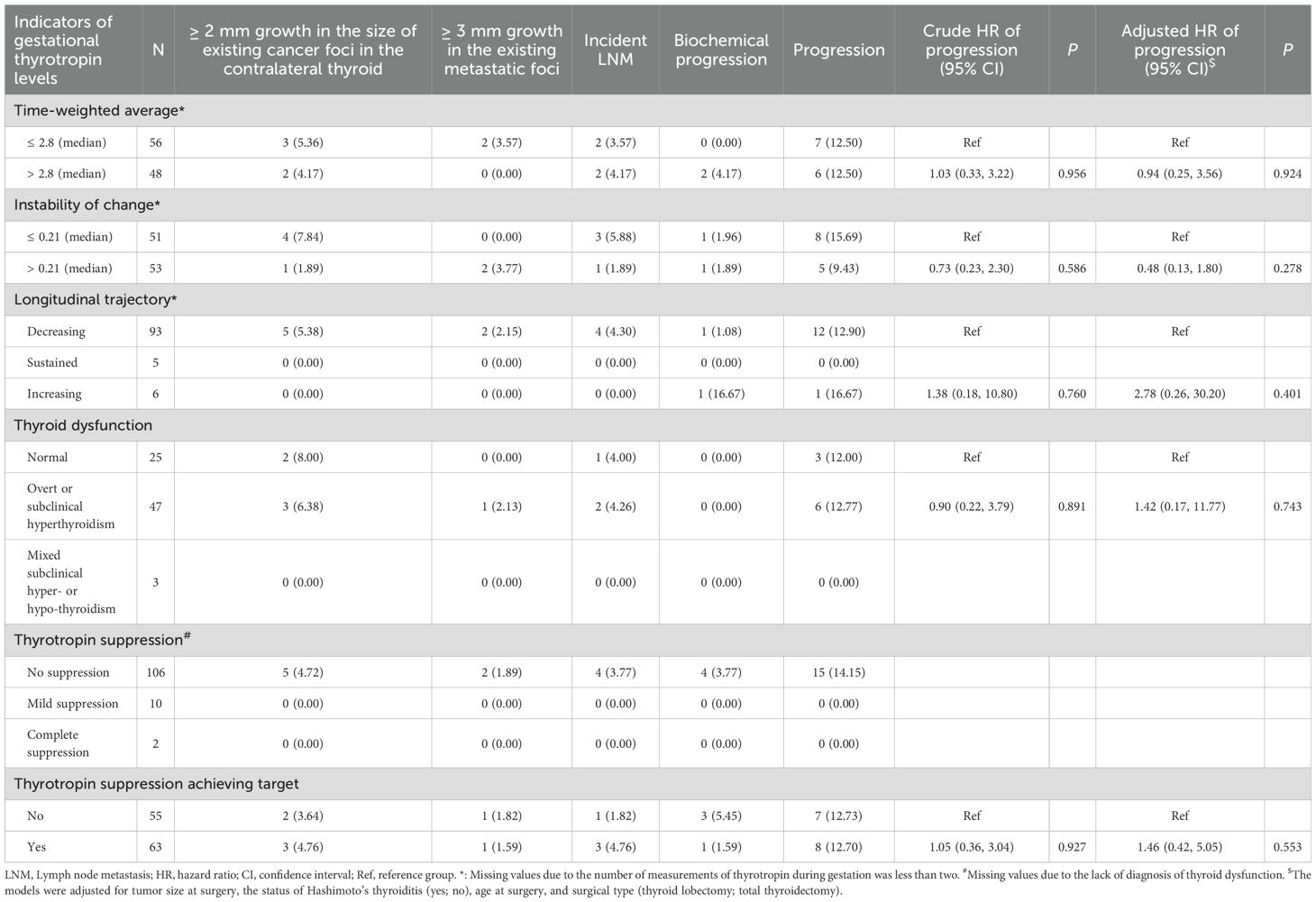
For those with after-surgery DTC, the median time interval between surgery and pregnancy was 1.36 year (IQR: 0.62-2.82). As shown in Table 4, after a median of 4.22 (IQR: 2.53-6.02) year follow-up among 118 patients with after-surgery DTC, 8 (12.70%) of patients who achieved the target of thyrotropin suppression (n = 63) and 7 (12.73%) of those not achieving the suppression target (n = 55) occurred structural or biochemical progression of DTC, respectively. The Cox proportional risk model results indicated that there was no evidence of difference between the two groups in DTC progression-free survival, with adjustment for tumor size, the status of Hashimoto’s thyroiditis, age at surgery, and surgical type (HR: 1.46; 95% CI: 0.42, 5.05; P = 0.553; Table 4; Figure 1). There was also no evidence of associations between any of the other 4 indicators of gestational thyrotropin levels (time-weighted average, instability of change, longitudinal trajectory, thyroid dysfunction) and the progression of after-surgery DTC (all P values > 0.05). Concerning the level of thyrotropin suppression during the gestation, 15 (14.15%) patients occurred after-surgery progression in the no-suppression group (n = 106) while none of the patients showed progression in the mild or complete suppression group (n = 12).
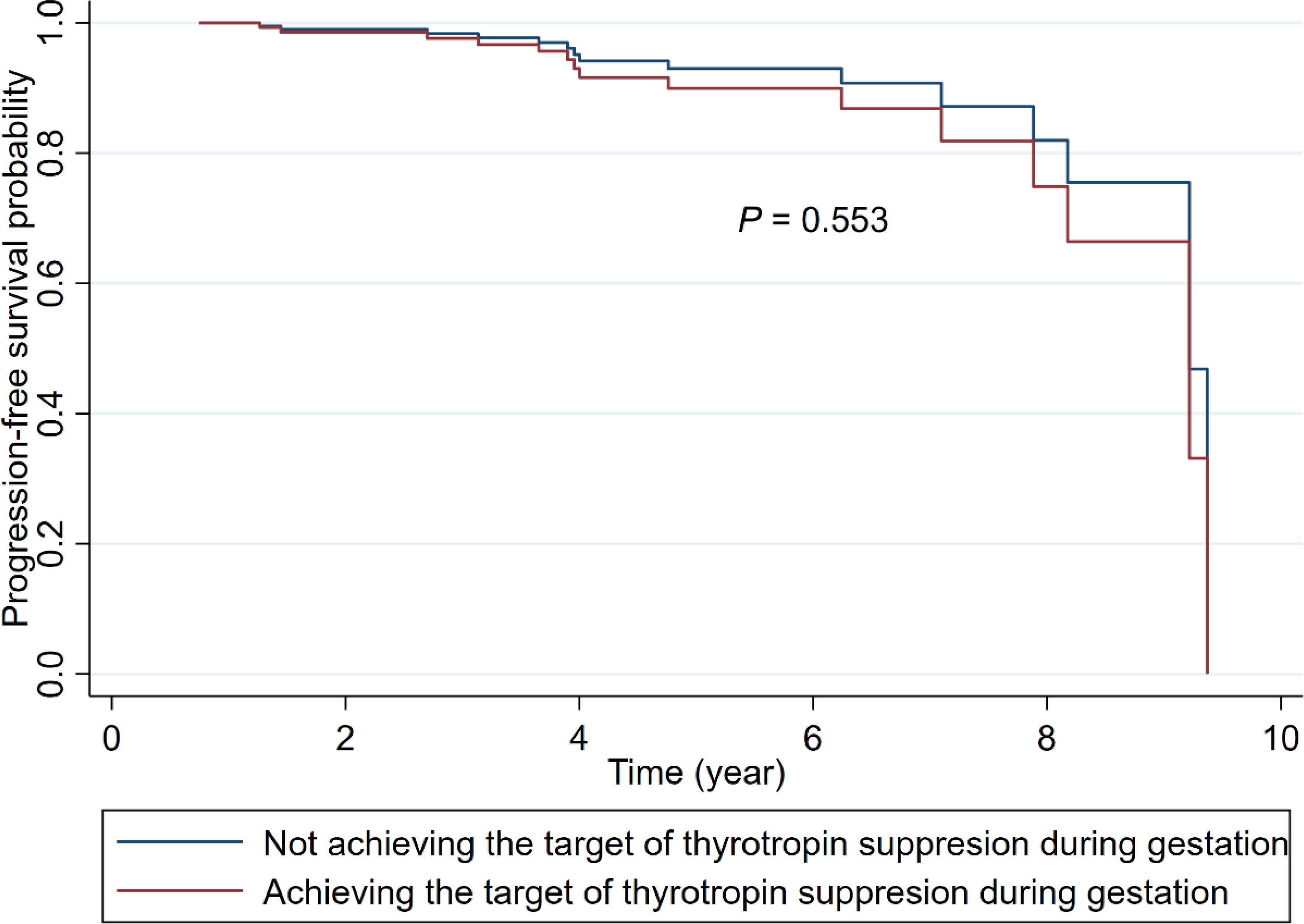
Figure 1. Adjusted Kaplan-Meier survival curves for after-surgery progression of DTC among patients achieving or not achieving the target of thyrotropin suppression during gestation. (The models were adjusted for tumor size at surgery, the status of Hashimoto’s thyroiditis (yes; no), age at surgery, and surgical type (thyroid lobectomy; total thyroidectomy).
Discussion
In this retrospective cohort study, we adopted six distinct indicators to comprehensively characterize gestational thyrotropin levels in terms of the average of thyrotropin level, instability of change in thyrotropin, longitudinal trajectory of thyrotropin, type of gestational thyroid dysfunction, level of thyrotropin suppression, and achievement of thyrotropin target during the pregnancy. We observed no evidence of associations between these indicators of gestational thyrotropin levels and disease progression in both pregnant women with under-surveillance DTC and those with after-surgery DTC.
First, our study highlighted the important clinical question regarding the monitoring and treatment of gestational thyrotropin levels among pregnant women with a DTC history, and answered this question by exploring the associations of gestational thyrotropin levels with the disease progression of DTC. In previous studies, this topic has mainly been explored among the non-pregnant population, with contradictory findings. Among patients under active surveillance, Sugitani et al. (14) did not observe the associations of baseline thyrotropin or mean thyrotropin during follow-up with the progression of T1aN0M0 papillary thyroid microcarcinoma; by contrast, Ito et al. (6) and Kim et al. (15) used time-weighted indicator of thyrotropin levels to account for the unequal time intervals across multiple measurements and found that lower thyrotropin may be related to suppression of enlargement of low-risk papillary thyroid microcarcinoma, especially for patients aged < 40 years. Among patients after surgical treatment, previous studies of associations between thyrotropin levels and progression of DTC have also been focused on the non-pregnant population. Briefly, the effect of thyrotropin suppression in decreasing the risk of recurrence has been generally acknowledged for patients with moderate-to-high-risk DTC but remained inconclusive for patients with low-risk DTC (25–27).
Notably, these findings from the non-pregnant population cannot be directly translated to the pregnant population due to the difference in thyrotropin levels between the pregnancy and non-pregnancy periods. The normal range and change pattern of thyrotropin levels during pregnancy is greatly influenced by the changes in pregnancy-specific hormones such as estrogen and human chorionic gonadotropin from the early, middle, to late pregnancy (28).
Second, our study was advantageous in not only focusing on patients with after-surgery DTC but also on those with under-surveillance DTC. Active surveillance has been acknowledged as an option for appropriately selected low-risk patients with DTC, but follow-up studies among pregnant women have been scarce, especially in China. Our study addressed this gap and the preliminary results did not observe evidence of statistical associations between gestational thyrotropin levels and disease progression in pregnant women with under-surveillance DTC. However, the results were derived from only 43 eligible patients and thus should be validated in future large-sample studies before clinical translation.
Third, our study was novel in capturing the dynamic change of gestational thyrotropin levels by using multiple statistical-method-driven and clinically meaningful indicators. Based on our literature review, previous studies have assessed thyrotropin levels using static, single-point measurement (5, 29, 30), a simple mean of multiple measurements (14), or the time-weighted average of multiple measurements (6, 15). However, these coarsely-grained indicators could only provide an average estimate of thyrotropin levels with little information about the longitudinal trajectory or instability of change over time. To capture the characteristics of multiple measurements of gestational thyrotropin levels in a fine-grained manner, our study took advantage of the latent class trajectory model (8, 9), a statistical method to simultaneously consider repeated measurements as a whole and classify the population into heterogeneous clusters with intra-cluster individuals following similar trajectory of repeated measurements. Additionally, we adopted a formula to calculate the instability of change (i.e., variability of measurements across time points) previously used in other topics (7).
In addition to the statistical-method assessment, our indicators of gestational thyrotropin levels have also considered clinical implications. We carefully evaluated pregnant women’s trimester-specific thyroid dysfunctions, including hyperthyroidism, subclinical hyperthyroidism, hypothyroidism, and subclinical hypothyroidism. We also examined the level of thyrotropin suppression during pregnancy and whether it achieved the target of thyrotropin suppression corresponding to the risk of recurrence. Our findings suggested that approximately half of pregnant patients with after-surgery DTC did not rigorously achieve the target of thyrotropin suppression during pregnancy, but this appeared to have a minor influence on the risk of disease progression.
Findings of our study should be interpreted cautiously. Our results only indicated that thyrotropin levels within the specific gestation period seemed to play a minor role in the risk of disease progression of DTC. This is not a denying to the long-lasting accumulated effect of thyrotropin levels across pre-pregnancy and postnatal periods on the disease progression of DTC. Additionally, the moderate sample size and relatively low incidence of progression limited the statistical power of multiple regression analyses and precluded us from further conducting subgroup analyses. We cannot accurately assess whether maternal advanced age, parity, assisted reproduction, and other factors moderated the exposure-outcome associations. In addition, restricted by the data available, other variables such as information on L-T4 therapy, hCG, and other hormones were not evaluated. These limitations were relatively common in the single institution-based studies. Nevertheless, this type of study had the advantage of accuracy and homogeneity in diagnosis and treatment and high-standard quality control of data integrity. All of the exposures, covariates, and outcomes used in this study had been carefully validated by experienced clinicians, ensuring the reliability of our study findings. Moreover, our study was unique in the focus on pregnant women with DTC, which was a potentially high-risk but largely ignored study population by previous studies.
The findings of our study had important implications for clinical practice. During regular check-ups for maternal thyrotropin levels during pregnancy, clinicians might pay more attention to the clinically meaningful influence on adverse pregnancy outcomes and the concern on disease progression of DTC seemed not much necessary, in most cases, according to findings of our study. Furthermore, clinicians and patients might persist in a long-term observation of thyrotropin levels during the life course, rather than the temporary fluctuations during the gestation period. Considering the severe supply-and-demand imbalance of medical resources, especially for the management of high-risk pregnant women, our findings have significant implications for resource allocation and prioritization in real-world settings.
Conclusions
Overall, the risk of progression of under-surveillance or after-surgery DTC was not associated with gestational thyrotropin levels. However, the life-long observation of thyrotropin levels might not be omitted for DTC patients. Our findings also reminded clinicians to prioritize the consideration of adverse pregnancy outcomes when optimizing thyrotropin levels for pregnant women previously diagnosed with DTC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Research Ethics Committee of Peking University Third Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this study is a retrospective study based on the historical diagnosis and treatment data of the hospital. Written informed consent cannot be obtained and has been approved by the Ethics Committee.
Author contributions
XL: Investigation, Writing – original draft. PF: Investigation, Writing – original draft. W-CX: Data curation, Formal analysis, Visualization, Writing – review & editing. FM: Investigation, Resources, Writing – review & editing. FZ: Investigation, Writing – review & editing. SZ: Investigation, Writing – review & editing. JC: Investigation, Writing – review & editing. RS: Investigation, Writing – review & editing. B-KS: Data curation, Writing – review & editing. S-BS: Investigation, Project administration, Writing – review & editing. CY: Project administration, Writing – review & editing. ZL: Conceptualization, Funding acquisition, Project administration, Methodology, Supervision, Writing – original draft, Writing – review & editing
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was funded by the National Natural Science Foundation of China (82373694), Young Elite Scientists Sponsorship Program by CAST (2023QNRC001), Beijing Education Sciences Planning Program during the 14th Five-Year Plan (BECA23111), and the Fundamental Research Funds for the Central Universities (BMU2021YJ030).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1369344/full#supplementary-material
References
1. Wang J, Yu F, Shang Y, Ping Z, Liu L. Thyroid cancer: incidence and mortality trends in China, 2005-2015. Endocrine. (2020) 68:163–73. doi: 10.1007/s12020-020-02207-6
3. Cottreau CM, Dashevsky I, Andrade SE, Li DK, Nekhlyudov L, Raebel MA, et al. Pregnancy-associated cancer: A U.S. Population-based study. J Womens Health (Larchmt). (2019) 28:250–7. doi: 10.1089/jwh.2018.6962
4. Marx H, Amin P, Lazarus JH. Hyperthyroidism and pregnancy. Bmj. (2008) 336:663–7. doi: 10.1136/bmj.39462.709005.AE
5. Zhang Y, Li Y, Shan Z, Xu Y, Li C, Xie X, et al. Association of overt and subclinical hyperthyroidism during weeks 4-8 with adverse pregnancy outcomes. J Womens Health (Larchmt). (2019) 28:842–8. doi: 10.1089/jwh.2018.7180
6. Ito Y, Miyauchi A, Fujishima M, Noda T, Sano T, Sasaki T, et al. Thyroid-stimulating hormone, age, and tumor size are risk factors for progression during active surveillance of low-risk papillary thyroid microcarcinoma in adults. World J Surg. (2023) 47:392–401. doi: 10.1007/s00268-022-06770-z
7. Taquet M, Griffiths K, Palmer EOC, Ker S, Liman C, Wee SN, et al. Early trajectory of clinical global impression as a transdiagnostic predictor of psychiatric hospitalisation: a retrospective cohort study. Lancet Psychiatry. (2023) 10:334–41. doi: 10.1016/S2215-0366(23)00066-4
8. Lennon H, Kelly S, Sperrin M, Buchan I, Cross AJ, Leitzmann M, et al. Framework to construct and interpret latent class trajectory modelling. BMJ Open. (2018) 8:e020683. doi: 10.1136/bmjopen-2017-020683
9. Herle M, Micali N, Abdulkadir M, Loos R, Bryant-Waugh R, Hübel C, et al. Identifying typical trajectories in longitudinal data: modelling strategies and interpretations. Eur J Epidemiol. (2020) 35:205–22. doi: 10.1007/s10654-020-00615-6
10. Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer - recent advances and future directions. Nat Rev Endocrinol. (2018) 14:670–83. doi: 10.1038/s41574-018-0080-7
11. Kim BW, Yousman W, Wong WX, Cheng C, McAninch EA. Less is more: comparing the 2015 and 2009 american thyroid association guidelines for thyroid nodules and cancer. Thyroid. (2016) 26:759–64. doi: 10.1089/thy.2016.0068
12. Chou RG, Dana T, Haymart M, Leung AM, Tufano RP, Sosa JA, et al. Active surveillance versus thyroid surgery for differentiated thyroid cancer: A systematic review. Thyroid. (2022) 32:351–67. doi: 10.1089/thy.2021.0539
13. Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan association of endocrine surgery task force on management for papillary thyroid microcarcinoma. Thyroid. (2021) 31:183–92. doi: 10.1089/thy.2020.0330
14. Sugitani I, Fujimoto Y, Yamada K. Association between serum thyrotropin concentration and growth of asymptomatic papillary thyroid microcarcinoma. World J Surg. (2014) 38:673–8. doi: 10.1007/s00268-013-2335-8
15. Kim HI, Jang HW, Ahn HS, Ahn S, Park SY, Oh YL, et al. High serum TSH level is associated with progression of papillary thyroid microcarcinoma during active surveillance. J Clin Endocrinol Metab. (2018) 103:446–51. doi: 10.1210/jc.2017-01775
16. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 guidelines of the american thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. (2017) 27:315–89. doi: 10.1089/thy.2016.0457
17. Writing Committee for Guidelines for Prevention and Management of Thyroid Diseases During Pregnancy and Perinatal Period, Chinese Society of Endocrinology, Chinese Medical Association, Women’s Health Care Branch of Chinese Preventive Medicine Association. Guidelines for prevention and management of thyroid diseases during pregnancy and perinatal period. Chin J Endocrinol Metab. (2022) 38:539–51. doi: 10.3760/cma.j.cn311282-20220416-00234
18. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 american thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
19. Li X, Xiao WC, Mei F, Shan R, Song SB, Sun BK, et al. The association of pregnancy with disease progression in patients previously treated for differentiated thyroid cancer: A propensity score-matched retrospective cohort study. J Womens Health (Larchmt). (2023) 32:1174–81. doi: 10.1089/jwh.2023.0172
20. Xiao WC, Li X, Shan R, Mei F, Song SB, Chen J, et al. Pregnancy and progression of differentiated thyroid cancer: A propensity score-matched retrospective cohort study. J Clin Endocrinol Metab. (2023) 109:837–43. doi: 10.1210/clinem/dgad557
21. Chinese Society of Endocrinology, Thyroid and Metabolism Surgery Group of the Chinese Society of Surgery, China Anti-Cancer Association, Chinese Association of Head and Neck Oncology, Chinese Society of Nuclear Medicine, China Anti-Cancer Association, et al. Guidelines for the diagnosis and management of thyroid nodules and differentiated thyroid cancer (Second edition). Chin J Endocrinol Metab. (2023) 39:181–226. doi: 10.3760/cma.j.cn311282-20221023-00589-1
22. Rakhlin L, Fish S, Tuttle RM. Response to therapy status is an excellent predictor of pregnancy-associated structural disease progression in patients previously treated for differentiated thyroid cancer. Thyroid. (2017) 27:396–401. doi: 10.1089/thy.2016.0501
23. Hirsch D, Levy S, Tsvetov G, Weinstein R, Lifshitz A, Singer J, et al. Impact of pregnancy on outcome and prognosis of survivors of papillary thyroid cancer. Thyroid. (2010) 20:1179–85. doi: 10.1089/thy.2010.0081
24. Spencer C, Petrovic I, Fatemi S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J Clin Endocrinol Metab. (2011) 96:1283–91. doi: 10.1210/jc.2010-2762
25. Won HR, Jeon E, Chang JW, Kang YE, Song K, Kim SW, et al. Is maintaining thyroid-stimulating hormone effective in patients undergoing thyroid lobectomy for low-risk differentiated thyroid cancer? A systematic review and meta-analysis. Cancers (Basel). (2022) 14:1470. doi: 10.3390/cancers14061470
26. Park JH, Lee YM, Lee YH, Hong SJ, Yoon JH. The prognostic value of serum thyroid-stimulating hormone level post-lobectomy in low- and intermediate-risk papillary thyroid carcinoma. J Surg Oncol. (2018) 118:390–6. doi: 10.1002/jso.v118.3
27. Lee EK, Kang YE, Park YJ, Koo BS, Chung KW, Ku EJ, et al. A multicenter, randomized, controlled trial for assessing the usefulness of suppressing thyroid stimulating hormone target levels after thyroid lobectomy in low to intermediate risk thyroid cancer patients (MASTER): A study protocol. Endocrinol Metab (Seoul). (2021) 36:574–81. doi: 10.3803/EnM.2020.943
28. Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. (1997) 18:404–33. doi: 10.1210/edrv.18.3.0300
29. Yang Y, Guo T, Fu J, Zhao J, Wang Y, He Y, et al. Association of preconception thyrotropin levels with fecundability and risk of spontaneous abortion in China. JAMA Netw Open. (2022) 5:e2228892. doi: 10.1001/jamanetworkopen.2022.28892
Keywords: differentiated thyroid cancer, thyrotropin, disease progression, pregnancy, DTC
Citation: Li X, Fu P, Xiao W-C, Mei F, Zhang F, Zhang S, Chen J, Shan R, Sun B-K, Song S-B, Yuan C-H and Liu Z (2024) Associations of gestational thyrotropin levels with disease progression among pregnant women with differentiated thyroid cancer: a retrospective cohort study. Front. Endocrinol. 15:1369344. doi: 10.3389/fendo.2024.1369344
Received: 12 January 2024; Accepted: 30 September 2024;
Published: 18 October 2024.
Edited by:
Fausto Bogazzi, University of Pisa, ItalyReviewed by:
Rhitajit Sarkar, National Institute of Diabetes and Digestive and Kidney Diseases (NIH), United StatesLida Luo, Yale University, United States
Copyright © 2024 Li, Fu, Xiao, Mei, Zhang, Zhang, Chen, Shan, Sun, Song, Yuan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Liu, bGl1emhlbmdAYmptdS5lZHUuY24=
†These authors share first authorship
‡ORCID: Shanghang Zhang, orcid.org/0000-0003-4047-3526
Zheng Liu, orcid.org/0000-0002-0405-2348
 Xin Li1†
Xin Li1† Fang Mei
Fang Mei Fan Zhang
Fan Zhang Chun-Hui Yuan
Chun-Hui Yuan Zheng Liu
Zheng Liu