- Department of Pharmacology, School of Basic Medical Sciences, Nanjing Medical University, Nanjing, China
Corticotropin-releasing factor family peptides (CRF peptides) comprise corticotropin releasing hormone (CRH), urocortin (UCN1), UCN2 and UCN3. CRH is first isolated in the brain and later with UCNs found in many peripheral cells/tissues including the colon. CRH and UCNs function via the two types of receptors, CRF1 and CRF2, with CRH mainly acting on CRF1, UCN1 on both CRF1 &CRF2 and UCN2-3 on CRF2. Compiling evidence shows that CRH participates in inflammation and cancers via both indirect central effects related to stress response and direct peripheral influence. CRH, as a stress-response mediator, plays a significant central role in promoting the development of colitis involving colon motility, immunity and gut flora, while a few anti-colitis results of central CRH are also reported. Moreover, CRH is found to directly influence the motility and immune/inflammatory cells in the colon. Likewise, CRH is believed to be greatly related to tumorigenesis of many kinds of cancers including colon cancer via the central action during chronic stress while the peripheral effects on colitis-associated-colon cancer (CAC) are also proved. We and others observe that CRH/CRF1 plays a significant peripheral role in the development of colitis and CAC in that CRF1 deficiency dramatically suppresses the colon inflammation and CAC. However, up to date, there still exist not many relevant experimental data on this topic, and there seems to be no absolute clearcut between the central and direct peripheral effects of CRH in colitis and colon cancer. Taken together, CRH, as a critical factor in stress and immunity, may participate in colitis and CAC as a centrally active molecule; meanwhile, CRH has direct peripheral effects regulating the development of colitis and CAC, both of which will be summarized in this review.
1 Introduction
Ulcerative colitis (UC) and Crohn’s disease, the common chronic inflammation in the gastrointestinal system, are the two main forms of inflammatory bowel disease (IBD) (1, 2). The precise cause of IBD is not thoroughly known yet. It is observed that UC patients may have a dysregulated mucosal immune response to commensal gut flora, resulting in bowel inflammation characteristically restricted to the mucosal surface in the colon (3, 4). Chronic inflammation is fundamentally an immune response, which provides microenvironment for tumorigenesis and accounts for a big portion of cancer-causing factors (4), which is in concert with the case between colitis and colorectal cancer (CRC) (5, 6), although meta-analysis does not show an increased CRC risk over time of inflammation (7). CRC is one of the most common forms of malignant tumor worldwide, and patients with UC are at higher risk for developing CRC, i.e., colitis-associated colon cancer (CAC), than the general population (8). Therefore, anti-inflammation treatment is likely a useful approach for preventing the occurrence of CAC (9). However, despite constant studies and advances in conventional and/or targeted therapy, the survival rate of CRC patients is still not very high (10, 11).
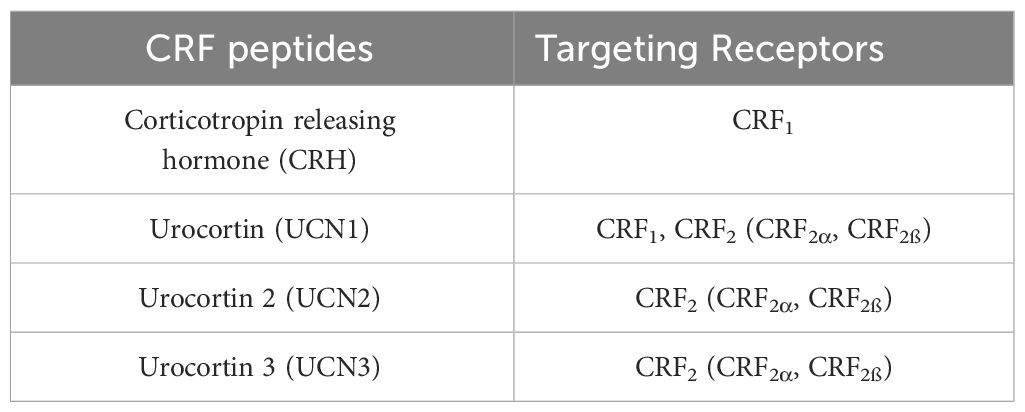
Corticotropin-releasing factor family peptides (CRF peptides) include 4 members, corticotropin releasing hormone (CRH), urocortin (UCN1), UCN2 and UCN3, mediating their effects via two distinct CRF receptor subtypes, CRF1 and CRF2, with CRH being the selective agonist of CRF1, UCN1 of both, and UCN2-3 of CRF2 (12–18) (Table 1).
Both CRF1 and CRF2 belong to the seven transmembrane domain family positively coupled to adenylate cyclase via G proteins (13–15). CRH, a 41-amino acid peptide, is observed to activate cAMP/MAPK pathway via CRF1 (19, 20). It is recognized as a primary regulator of the hypothalamic pituitary axis (HPA axis) (17, 21–23). The paraventricular nucleus (PVN) of the hypothalamus is the main source for CRH in the brain (17). CRH, UCNs and the two receptors are also reported to express widely in peripheral cells/tissues, being recognized as important cardiovascular peptides and immune/inflammatory molecules (23–28). Their presence in gastrointestinal system has been detected for a long time since decades ago (29–31). Moreover, CRH and UCN3 are detected in the human colon (32) and UCN1 mRNA is observed in the rat enteric nervous system (33). CRF1 and CRF2 are encoded by specific genes (13). CRF1 is the main type of receptor in the brain (13) while it is also abundant in some peripheral cells/tissues including skin, inflammatory cells and gastrointestinal system (13, 28, 30, 34). And CRF2α is predominantly found in neurons and CRF2β in both brain and peripheral tissues including cardiac & skeletal muscle and the gastrointestinal tract (13, 35, 36). Both CRF1 and CRF2 are distributed within the rat colon: CRF1 is found in the colonic crypts, the surface epithelium, and the lamina propria of the proximal colonic mucosa. CRF1 expression is also detected in the myenteric and submucosal nervous plexus. CRF2 expression is found to be localized mainly in the luminal surface of the crypts and in blood vessels of the submucosal layer (31). Also in the human colonic mucosa, both CRF1 and CRF2 mRNA are detected in lamina propria mononuclear cells (30). These results support a role for the two receptors’ involvement in regulating peripheral colonic effects of CRH and UCNs. Since this review focuses on the selective CRF1 agonist, CRH, more about CRH/CRF1 effects will be discussed.
The hypothalamus-pituitary-adrenal (HPA) axis, functionally a hormone stimulating cascade, mainly CRH-adrenocorticotropin (ACTH)-cortisol axis, is a critical element for stress response and immune/inflammatory processes (37). Chronic stress, characterized by activation of HPA axis and sympathetic nervous system, has been reported to be an important reason in the development of inflammation and tumorigenesis (38–40), suggesting that CRH indirectly participate in inflammation and tumorigenesis via HPA axis as a centrally active molecule. Furthermore, HPA axis communicates with the immune system at multiple levels (41, 42). Bidirectional interactions between HPA and immunity contribute to their role in inflammation and cancers: HPA activation results in secretion of CRH, ACTH and cortisol modulating the immune response while immunity-related substances, such as interleukin-1 (IL-1), IL-6 and tumor necrosis factor alpha (TNF-α) can backwards stimulate the HPA axis (42). In addition, relationship between gut microbiome and the brain, i.e. brain-gut-microbiota axis, has attracted much attention for its complicated part in stress and IBD (43–45). The imbalance of brain-gut-microbiota axis also leads to dysregulation of the HPA axis (44). Therefore, it is reasonable that CRH, as the major mediator of stress response, may take a part in IBD and CRC via brain-gut-microbiota axis. Taken together, CRH is suggested to take part in colonic inflammation and inflammation-based tumorigenesis indirectly via HPA axis and brain-gut-microbiota axis.
Moreover, peripherally direct participation of CRH in colonic inflammation has been well proved (46, 47). The expression of CRH in the large bowel of patients with UC is found considerably enhanced in mucosal inflammatory cells and slightly increased in colonic mucosal epithelial cells, suggesting CRH’s role via modulating intestinal immune/inflammatory system in UC (47). Also, it is reported that CRH may induce intestinal hyperpermeability in human colon mucosa via mast cells (48). We and others have also reported the direct peripheral role of CRH/UCNs & receptors in immunity/inflammation and cancers (42, 49–54), including colitis and colitis-associated colon cancer (CAC) (55–58).
In summary, over recent decades, CRF peptides and receptors have been found to be significantly correlated with the bowel inflammation and the development of CAC. However, controversies over the origin of CRH action sites have always been existing. Up-to-date, taken together, it is understandable that CRH, as both a centrally active endocrine hormone and peripherally active peptide, may play an important role in colitis and CAC via both indirect actions regulating chronic stress and direct peripheral effects, although there still lack experimental evidences showing direct relationship between central CRH effect and colitis/CAC and only a few investigations show the direct peripheral effects of CRH on CAC.
2 The central role of CRH in colitis and CAC
Stress, inflammation and colon cancer are highly related, forming a CRH-system driven crosstalk (38). Therefore, there may not be an absolute clearcut between the central and peripheral effects of CRH on inflammation and cancer. Up to date, little evidence has suggested a direct relationship between central CRH and the development of colitis and CAC. Instead, the central role of CRH in inflammation and cancers is mainly thought to be via mediating HPA axis as a stress mediator (38, 59, 60). Under chronic stress, the HPA axis is activated and the release of CRH from the PVN of the hypothalamus at its nerve endings in eminence, which is carried to the pituitary gland through the portal vessel, stimulating the secretion of ACTH, which in turn stimulates the secretion of cortisol from the adrenal gland. The hypothalamus-released CRH acts on CRF1 in the pituitary gland, causing ACTH release from the anterior pituitary (13, 61). About half of CRH in the brain is found to be bound with CRH binding protein (CRH-BP). In exposure to stress, the expression of CRH-BP increases in a time-dependent fashion, likely being a negative feedback mechanism for CRH’s action on CRF1 (62).
2.1 Central role of CRH/CRF1 in colitis
There exist contrary reports about CRH’s role in colitis. As summarized in the following, some researchers observe no effect or anti-inflammatory effect while many others find its pro-inflammatory actions in colitis.
2.1.1 The non-proinflammatory/anti-inflammatory role of central CRH in colitis
CRH is a 41-amino acid peptide, a primary regulator of the HPA axis and a coordinator of the gastrointestinal response to stress (22, 63, 64). The most important effect of central CRH/CRF1 is to stimulate the pituitary gland to release ACTH causing cortisol secretion of from the adrenal gland cortex, i.e., mediating the function of HPA axis (61). Since cortisol is an anti-inflammatory hormone, CRH is normally recognized to act in an anti-inflammatory fashion. However, only a few reports present consistent evidences in case of colitis. While acute colonic inflammation induced CRH secretion from PVN in the hypothalamus, CRH level is found to remain at a high level in the brain after the recovery of colitis (65), suggesting a weak link between central CRH effect and colitis. On the other hand, Gue et al. find that centrally injected CRH may have complicated influences on colitis (66). By evaluating the influence of stress and the involvement of CRH on experimental colitis in rats, it is observed that centrally injected CRH antagonist, alpha-helical CRH-(9-41) has no effect on trinitrobenzenesulfonic acid-induced colitis but enhances the effects of stress on colitis, suggesting that central CRH may only participate in controlling the process of colitis in case of stress (66). Moreover, Million et al. observe a protective role of brain CRH from stress-induced worsening of colitis (67). They assess the role of central CRH in stress-induced worsening of colitis in inbred rat strains with hypo (Lewis) and hyper (Fischer344) CRH responses to stress. It is observed that trinitrobenzenesulfonic acid induces colitis with similar severity in both strains, which is inhibited by central injection of CRH. Chronic stress aggravates colitis more in Lewis than Fischer rats, which is reversed by central injection of the CRH antagonist astressin, indicating that central CRH restrains the stress’ proinflammatory action in experimental colitis (67). Similarly, it is reported that central CRH inhibits gastric motility, which can also be abolished by the intracerebroventricular injection of astressin (68). However, its effect on the colon motility is the opposite, stimulating the movement and contributing to the process of colitis (68) (see below 1.1.2).
2.1.2 The pro-inflammatory effect of central CRH in colitis as a stress mediator
Compiling evidences show that central CRH plays indirectly a proinflammatory role in colitis. HPA axis is the critical pathway of stress response, which is elicited by physical or psychological stimuli (stressors). A stress response involves activation of sympathetic-adreno-medullar (SAM) axis, HPA axis, and immune system, and a prolonged stressor exposure constitutes a chronic stress (69).
Chronic stress is known to promote IBD (64, 69), but the underlying mechanism remains largely unresolved. IBD is a model of microbial, immune and neuropsychological integration (70, 71). It is reported that chronic stress sensitizes mice to dextran sulfate sodium (DSS)-induced colitis and enhances the infiltration of proinflammatory cells in colonic lamina propria (72). Also, a marked increase in IL-6, a stress-inducible cytokine that further activates HPA axis in a positive feedback manner, is observed (73). Moreover, IBD is presumed to be a disorder of the brain–gut-microbiome link associated with exaggerated response to stress (74, 75). Under stress, inflammation-promoting bacteria expand while transferred gut microbiota from stressed mice facilitate DSS-induced colitis, which is abrogated by broad-spectrum antibiotic treatment (72). Therefore, it is obvious that chronic stress leads to colitis via disturbing gut microbiota and hence triggering immune system response. Based on these reports, CRH, as the main stress mediator, is evidently a critical factor in the development of colitis.
Interestingly, researchers record colonic motility and reveal that restraint stress, or intracerebroventricular injections of CRH, produce significant increases in colonic motility although CRH inhibits gastric motility (68, 76), which contributes to the occurrence of abdominal pain during IBD. Central injection of astressin is observed to block exogenous CRH action and colonic response to stress, showing an antagonistic action against CRH and stress-related alterations of gastrointestinal motor function, without an intrinsic effect in rats (68). Moreover, it is observed that the colonic contractions induced by central CRH are eliminated by intracerebroventricular pretreatment with astressin (76). On the other hand, peripherally administered CRH partially mimics the stress response of the gastrointestinal motility, exaggerated in IBD patients (77), further suggesting that CRH plays an important role in modulating brain-gut functions under stress.
CRH is also found to participate in IBD during acute stress. Zhao et al. establishes a model of psychosocial stress by peripheral administration of CRH and find that CRH aggravates DSS-induced colitis via the enhancement of intestinal macrophage autophagy (78). It is observed that peripherally used CRH aggravates the severity of DSS-induced IBD, increasing overall and local inflammatory reactions and infiltration. Under the IBD-related inflammatory challenges, the autophagy levels in intestinal macrophages are significantly increased, which is further enhanced by CRH. The autophagy inhibitor, chloroquine, markedly attenuates the detrimental effects of CRH reducing the severity and inflammatory reactions (78). These results may suggest that CRH, while working centrally mimicking stress, simultaneously exacerbates DSS-induced IBD via enhancing intestinal macrophage autophagy. Therefore, it is reasonable to believe that CRH and related receptors may be a potential therapeutic target for the treatment of IBD. Another investigation also shows that peripherally administered CRH mimic the effect of acute psychological stress, leading to increased intestinal permeability characterized in IBD (79). These findings further provide new insights into the complex interplay between the central and peripheral role of CRH in IBD since CRH is administered peripherally for stress model.
2.2 Central role of CRH in colitis-associated colon cancer
Rare evidence shows a direct relationship between the central CRH and the development of cancers. However, the relationship between chronic stress and tumor development has been frequently reported and widely reviewed (38–41, 80, 81). Therefore, it is believable that the central role of CRH in tumor is likewise mainly via the indirect way through HPA axis-mediated stress.
Clinically, chronic stress is found common among cancer patients due to stressors encountered (82). Primarily, chronic stress activates the classic neuroendocrine systems, the HPA axis and the SAM, whose continuous activations have been demonstrated to take part in cancer-promoting processes by altering the tumor microenvironment (TME) (39, 81, 83). Stress hormones can promote colon cancer development through a variety of mechanisms: 1) Corresponding changes in the body’s immune function and inflammatory response (40, 83, 84); 2) Significant influence on the gut flora, i.e. the brain-gut-microbiota axis, promoting the composition of pro-inflammatory microbiome and hence resulting in colitis leading to CAC (85, 86).
Although the mechanisms might be complicated, it is believable that the central CRH, the upper element of HPA axis and stress mediator, plays a role in CAC based on that central CRH participates in colitis (see 2.1) and the cross talks between inflammation/immunity and cancer (87, 88). Recently, the microbiota has been recognized as one of the key regulators of gut-brain function. Many factors, stress in particular, can influence microbiota composition (89). Importantly, dysbiosis of the gut microbiome is found to be associated with the development of colorectal cancer (90) (see below 2.2). As precedingly described, individuals having IBD develop more easily into CAC (7, 57), and gut microbiome is involved in colon inflammation and biosynthesis of chemical carcinogens such as N-nitroso compounds that drive carcinogenesis (90–92). Meta-analysis demonstrates that in patients under stress gut microbiota perturbations are associated with loss of certain anti-inflammatory bacteria but an enrichment of pro-inflammatory bacteria (89), suggesting an interaction between the central CRH and gut flora.
It is nowadays recognized that dietary mode is related to colon microbiota (92, 93), leading to the idea that modulating the growth of beneficial microbiota in the gut by dietary and life style interventions may be a useful approach for prevention of colon cancer (94, 95). Based on the importance of the brain-gut-microbe axis during chronic stress, interfering chronic stress using CRH-related drugs might also become a useful approach for CAC prevention and treatment.
3 Peripheral roles of CRH in colitis and CAC
As precedingly described, although CRH is first isolated in CNS where it is initially recognized to be the main target site, CRH and the other CRF peptides have then been observed existing and functioning peripherally. Furthermore, the two receptors, CRF1 and CRF2 have been detected in many types of peripheral cells/tissues, such as immune cells, endothelial cells, tumor cells, etc. (13, 28, 42). CRH and the other CRF peptides are revealed to have a variety of direct peripheral functions in cardiovascular system, gastrointestinal system and immune system (13, 42). Besides the centrally indirect influence via mediating stress, CRH is also reported by our group and others to play a direct peripheral role in inflammation and tumors, including colitis and CAC (13, 42, 43, 50, 57, 58).
3.1 Peripheral participation of CRH in colitis
CRH & UCNs and the receptors are observed to be closely related to gastrointestinal system (12, 13, 96, 97) and reported to be implicated in colitis (43, 98, 99).
Firstly, CRH, the selective CRF1 ligand/agonist, is reported to play a significant role in the gastrointestinal motility by stimulating enteric nervous system (100) and evidence supporting that peripheral CRH & CRF1 directly take part in brain-gut sensitization is increasing (43). As mentioned above, IBD displays chronic abdominal pain or discomfort due to altered gut motility and visceral sensation (1, 100). Moreover, peripheral injection of CRH or UCN1 inhibits human gastric emptying and motility through interaction with CRF2, but stimulates colonic motility through activation of CRF1 (101). CRH induces motility of the descending colon in both healthy subjects and colitis patients, the latter with greater motility indexes. Paralally, abdominal symptoms evoked by CRH in colitis patients last significantly longer than in healthy controls (101). Moreover, rectal electric stimulation-induced significantly higher motility indices of the colon in colitis patients (vs healthy controls) are suppressed after administration of the selective antagonist of CRH, alphahCRH (αhCRH). Consistently, αhCRH significantly reduces the ordinate scale of abdominal pain evoked by the electric stimulation in colitis patients without changing ACTH and serum cortisol levels (102). Interestingly, peripheral administration of CRH is observed to aggravate visceral sensorimotor function as well as ACTH response in IBD patients (43, 100, 103). However, αhCRH is found to suppress higher motility among IBD patients, reducing the abdominal pain without plasma ACTH & cortisol change (43), suggesting the dominant peripheral effect. Furthermore, αhCRH is observed to block colorectal distention-induced sensitization of the visceral perception in rats (43, 102, 104). All these results demonstrate that besides its central action, CRH/CRF1 enhances colon motility, contributing to abdominal pain peripherally.
Secondly, peripheral CRH may play a role in colitis via influencing immune/inflammatory processes (42). Our group find that expressions of UCN1 and CRH are enhanced in the colon of wild type (Crhr1+/+) mice during azoxymethane (AOM) and DSS treatment (57). CRF1 has a proinflammatory and therefore a carcinogenetic (see below) effect in the mouse colon. The extent and severity of inflammation are drastically decreased in Crhr1-/- mice with much lower inflammatory cytokines’ expression, grade of dysplasia and numbers of ulceration in the colon mucosa. Moreover, accompanying the markedly lower proinflammatory cytokines, IL-1, IL-6, and TNF-α, the anti-inflammatory factor, IL-10 is increased in Crhr1-/- mice. Our results are consistent with the reports that CRF1 activation promotes inflammation (13, 42, 50, 103). However, in case of innate immunity deficiency, the opposite effect of CRH/CRF1 is observed (98). Chaniotou group investigate the role of CRH in an innate immunity–dependent mouse model of IBD (98). CRH-/- mice are observed to have more colonic inflammation than CRH+/+ mice in DDS-induced colitis model. Moreover, as precedingly described, it is observed that, CRH further enhances the promoted autophagy levels in intestinal macrophages in IBD patients, which is markedly attenuated by the autophagy inhibitor, chloroquine, reducing CRH-induced severity and inflammatory reactions (76). These results may suggest that CRH, while working centrally mimicking stress, simultaneously exacerbates DSS-induced IBD via enhancing intestinal macrophage autophagy. Therefore, it is reasonable to believe that CRH and related receptors may be a potential therapeutic target for the treatment of IBD. In addition, mast cells are found to be related to CRH effects participating in the process of colitis, increasing the intestinal mucosal permeability (101).
The role of CRF2 in colitis is also complicated. It is reported that CRF2 has a counter regulatory action against CRF1, maintaining a balance between CRF1 and CRF2 during inflammation (103). On one hand, CRF2 is observed to function as a proinflammatory element while on the other hand, it displays an anti-inflammatory feature (105). Activation of CRF2 is reported to promote inflammation during acute colitis but to inhibit inflammation during chronic colitis (106). In DSS-induced colitis, mucosal repair is delayed after administration of a CRF2 antagonist (106). Moreover, CRF2 is down-regulated in human colitis (107). Taken together, a balance between CRF1 and CRF2 may decide the process of inflammation (103). This balance-theory may well interpret that both CRF1 and CRF2 are found to participate in acute inflammation while CRF2 is the main type for repair (103). The theory may lead to better understanding the pathophysiology and provide novel therapeutic options targeting altered signaling balance of CRF1 and CRF2 in IBD.
3.2 Peripheral CRH’s role in colitis-associated colon cancer
CRH is present in the colonic mucosa of UC patients and acts as a proinflammatory factor modulating the intestinal immune system (29, 47). Furthermore, UCN1, the unselective agonist for CRF1 and CRF2, is found to be synthesized and secreted in plasma cells, related to the inflammation in colonic mucosa (108). In addition, in DSS-induced mouse colitis, CRF1 deficiency is observed to contribute to the relief of colon inflammation (57). These reports suggest that direct activation of CRF1 exerts an effect of exacerbating colitis and hence may promote CAC. Up-to-date, only a few experimental reports have been presented on the direct peripheral role of CRH & UCNs in CAC.
In 2014, we first investigate the functions of CRF1 signaling on the development of CAC by using CRF1 deficient mice in AOM and DSS-induced CAC model. And the results show that in WT (Crhr1+/+) mice, CRF1 and its endogenous ligands (UCN1 and CRH) are significantly enhanced in the colon during AOM and DSS treatment. Interestingly, in Crhr1-/- mice, tumorigenesis is dramatically reduced, accompanied by lower inflammatory responses, i.e., decreased IL-1β, IL-6, TNF-α level and macrophage infiltration. Moreover, a reduced activation of NF-κB and STAT3 phosphorylation, together with decreased proliferating & enhanced apoptotic cells in the colon are observed (57). The pro-tumorigenesis effect is further confirmed by our in vitro experiments (58). CRH enhances colon cancer cell proliferation, promoting colony formation. Furthermore, tube formation assay shows that CRH treatment significantly promotes angiogenesis of HUVECs. Further investigation shows that CRH/CRF1 significantly upregulates IL-6 and VEGF level through activating NF-κB. And the VEGF silence abolishes the tube formation induced by CRH. The CRH-induced IL-6 promotes STAT3 phosphorylation, whose inhibition by Stattic significantly inhibits the CRH-induced cell proliferation (58). Our data is consistent with a newly reported experiment, demonstrating that CRF1 deficiency inhibits CRC in AOM/DSS model (56). Taken together, CRH/CRF1 signaling promotes human colon cancer cell proliferation via NF-κB/IL-6/STAT3 and tumor angiogenesis via NF-κB/VEGF signaling pathway. Our results provide evidence to support a critical role for the CRH/CRF1 signaling in colon cancer progression and suggest its potential utility as a new therapeutic target for CAC. Based on the above, it is believable that CRH/CRF1 has a proinflammatory and therefore a pro-tumorigenic effect in terms of CAC, which might be a direction for developing new therapeutic approaches for inflammation and CAC prevention & treatment.
It is observed by Baritaki group that human colon tissues from CRC patients and CRC cell lines show decreased CRF2 expression (109). Contrary to CRH/CRF1, UCN2/CRF2 signaling inhibits cell proliferation, migration, invasion and colony formation. Furthermore, IL-1β, IL-6 and IL-6R mRNAs are diminished in CRC-CRF2+ cells. In CRC patients’ colon samples, CRF2 mRNA expression is inversely correlated with IL-6R (109). These results are in concert with the report that CRF2 deficiency worsens CRC in AOM/DSS model (56). However, opposite effect of CRF2 is also reported, i.e. CRF2 may promote the development of CRC (110). Also, a blood sample analysis suggests that CRF2 represent a risk factor for CRC development in Mexican patients (111), which raises a controversial question as well. Recently, researchers have reported the methylation status of both CRF1 and CRF2, and point out that this examination may become a promising screening approach for CAC (112, 113).
In addition, it is well established that there exists a link between gut microbiota and colitis & colon cancer (87, 114–116). As precedingly described, CRH exerts an effect on gut flora mainly as a central stress mediator via brain-gut axis. However, up-to-date, rare experimental evidence shows direct peripheral effects of CRH on gut flora.
4 Summary
Emerging evidence suggests that uncontrolled inflammation is a major risk factor for the development of cancer. A typical example for inflammation and inflammation-based tumor is colitis and CAC, strongly supported by the fact that patients with UC have a much higher risk for CAC. This review aims to mainly summarize the reports about CRH’ roles in the development of colitis and CAC, both central and peripheral, hoping to be helpful in giving a clue to future drug design of CRH relevance, as having been studied (55).
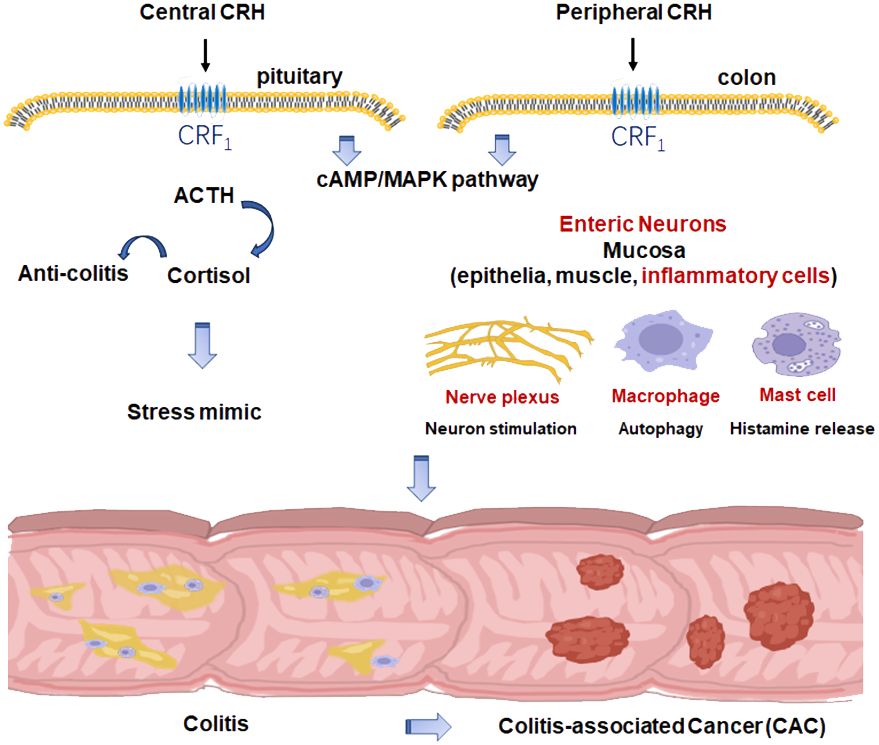
As summarized in Figure 1, CRH, as the main stress mediator, may participate in colitis and CAC via CRF1 as a central factor. Meanwhile, CRH and UCNs have been proved to play an important role in the development of colitis and CAC peripherally in which CRF1 may dominantly function as a pro-inflammatory and pro-tumorigenesis element while CRF2 may do oppositely. However, there exists no clearcut between CRH’s central and peripheral effects in colitis and CAC because of cross-talks between HPA axis and the immune system, and also between central and myenteric neurons.
However, there still lack experimental evidences for a direct relationship between central CRH and colitis/CAC, while there are also relatively few investigations on CRH’s peripheral effects on CAC. Given that CRH has a crucial role in stress and gastrointestinal system, with further evidence-reveal in the future, CRH may become a promising therapeutic target for colitis and CAC.
Author contributions
CZ: Writing – original draft, Writing – review & editing. SL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China No. 81773724 & 82273918.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. (2012) 380:1606–19. doi: 10.1016/S0140-6736(12)60150-0
2. Segal JP, LeBlanc JF, Hart AL. Ulcerative colitis: an update. Clin Med (Lond). (2021) 21:135–9. doi: 10.7861/clinmed.2021-0080
3. Meynier M, Baudu E, Rolhion N, Defaye M, Straube M, Daugey V, et al. AhR/IL-22 pathway as new target for the treatment of post-infectious irritable bowel syndrome symptoms. Gut Microbes. (2022) 14:2022997. doi: 10.1080/19490976.2021.2022997
4. Murata M. Inflammation and cancer. Environ Health Prev Med. (2018) 23:50. doi: 10.1186/s12199-018-0740-1
5. Shah SC, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology. (2022) 162:715–730.e3. doi: 10.1053/j.gastro.2021.10.035
6. Rogler G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. (2014) 345:235–41. doi: 10.1016/j.canlet.2013.07.032
7. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. (2001) 48:526–35. doi: 10.1136/gut.48.4.526
8. Olen O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. (2020) 395:123–31. doi: 10.1016/S0140-6736(19)32545-0
9. Suh SS, Hong JM, Kim EJ, Jung SW, Kim SM, Kim JE, et al. Anti-inflammation and anti-cancer activity of ethanol extract of antarctic freshwater microalga, micractinium sp. Int J Med Sci. (2018) 15:929–36. doi: 10.7150/ijms.26410
10. Ruan H, Leibowitz BJ, Zhang L, Yu J. Immunogenic cell death in colon cancer prevention and therapy. Mol Carcinog. (2020) 59:783–93. doi: 10.1002/mc.23183
11. Jahanafrooz Z, Mosafer J, Akbari M, Hashemzaei M, Mokhtarzadeh A, Baradaran B. Colon cancer therapy by focusing on colon cancer stem cells and their tumor microenvironment. J Cell Physiol. (2020) 235:4153–66. doi: 10.1002/jcp.29337
12. Rivier J, Spiess J, Vale W. Characterization of rat hypothalamic corticotropin-releasing factor. Proc Natl Acad Sci U.S.A. (1983) 80:4851–5. doi: 10.1073/pnas.80.15.4851
13. Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann N Y Acad Sci. (1999) 885:312–28. doi: 10.1111/j.1749-6632.1999.tb08687.x
14. Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U.S.A. (1993) 90:8967–71. doi: 10.1073/pnas.90.19.8967
15. Chang CP, Pearse RV 2nd, O'Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. (1993) 11:1187–95. doi: 10.1016/0896-6273(93)90230-O
16. Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U.S.A. (1995) 92:836–40. doi: 10.1073/pnas.92.3.836
17. Dedic N, Chen A, Deussing JM. The CRF family of neuropeptides and their receptors - mediators of the central stress response. Curr Mol Pharmacol. (2018) 11:4–31. doi: 10.2174/1874467210666170302104053
18. Jahn O, Tezval H, van Werven L, Eckart K, Spiess J. Three-amino acid motifs of urocortin II and III determine their CRF receptor subtype selectivity. Neuropharmacology. (2004) 47:233–42. doi: 10.1016/j.neuropharm.2004.03.018
19. Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, et al. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol. (2002) 16:1638–51. doi: 10.1210/me.16.7.1638
20. Bayatti N, Zschocke J, Behl C. Brain region-specific neuroprotective action and signaling of corticotropin-releasing hormone in primary neurons. Endocrinology. (2003) 144:4051–60. doi: 10.1210/en.2003-0168
21. de Andrade JS, Viana MB, Abrao RO, Bittencourt JC, Cespedes IC. CRF family peptides are differently altered by acute restraint stress and chronic unpredictable stress. Behav Brain Res. (2014) 271:302–8. doi: 10.1016/j.bbr.2014.06.014
22. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. (2016) 6:603–21. doi: 10.1002/cphy.c150015
23. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. (2002) 53:865–71. doi: 10.1016/S0022-3999(02)00429-4
24. Rademaker MT, Richards AM. Urocortins: Actions in health and heart failure. Clin Chim Acta. (2017) 474:76–87. doi: 10.1016/j.cca.2017.09.003
25. Kageyama K, Teui K, Tamasawa N, Suda T. Regulation and roles of urocortins in the vascular system. Int J Endocrinol. (2012) 2012:873723. doi: 10.1155/2012/873723
26. Dermitzaki E, Venihaki M, Tsatsanis C, Gravanis A, Avgoustinaki PD, Liapakis G, et al. The multi-faceted profile of corticotropin-releasing factor (CRF) family of neuropeptides and of their receptors on the paracrine/local regulation of the inflammatory response. Curr Mol Pharmacol. (2018) 11:39–50. doi: 10.2174/1874467210666170109164430
27. Gravanis A, Margioris AN. The corticotropin-releasing factor (CRF) family of neuropeptides in inflammation: potential therapeutic applications. Curr Med Chem. (2005) 12:1503–12. doi: 10.2174/0929867054039008
28. Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. (2004) 25:563–8. doi: 10.1016/j.tips.2004.09.007
29. Kawahito Y, Sano H, Kawata M, Yuri K, Mukai S, Yamamura Y, et al. Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology. (1994) 106:859–65. doi: 10.1016/0016-5085(94)90743-9
30. Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, et al. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. (2000) 21:1799–809. doi: 10.1016/S0196-9781(00)00335-1
31. Chatzaki E, Crowe PD, Wang L, Million M, Tache Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. (2004) 90:309–16. doi: 10.1111/j.1471-4159.2004.02490.x
32. Saruta M, Takahashi K, Suzuki T, Fukuda T, Torii A, Sasano H. Urocortin 3/stresscopin in human colon: possible modulators of gastrointestinal function during stressful conditions. Peptides. (2005) 26:1196–206. doi: 10.1016/j.peptides.2005.01.014
33. Harada S, Imaki T, Naruse M, Chikada N, Nakajima K, et al. Urocortin mRNA is expressed in the enteric nervous system of the rat. Neurosci Lett. (1999) 267:125–8. doi: 10.1016/S0304-3940(99)00349-3
34. Nozu T, Tsuchiya Y, Kumei S, Takakusaki K, Okumura T. Peripheral corticotropin-releasing factor (CRF) induces stimulation of gastric contractions in freely moving conscious rats: role of CRF receptor types 1 and 2. Neurogastroenterol Motil. (2013) 25:190–7. doi: 10.1111/nmo.12050
35. Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, Arias CM, et al. A soluble mouse brain splice variant of type 2alpha corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci U.S.A. (2005) 102:2620–5. doi: 10.1073/pnas.0409583102
36. Sztainberg Y, Kuperman Y, Issler O, Gil S, Vaughan J, Rivier J, et al. A novel corticotropin-releasing factor receptor splice variant exhibits dominant negative activity: a putative link to stress-induced heart disease. FASEB J. (2009) 23:2186–96. doi: 10.1096/fj.08-128066
37. Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. (1995) 332:1351–62. doi: 10.1056/NEJM199505183322008
38. Baritaki S, de Bree E, Chatzaki E, Pothoulakis C. Chronic stress, inflammation, and colon cancer: A CRH system-driven molecular crosstalk. J Clin Med. (2019) 8(10):1669. doi: 10.3390/jcm8101669
39. Tian W, Liu Y, Cao C, Zeng Y, Pan Y, Liu X, et al. Chronic stress: impacts on tumor microenvironment and implications for anti-cancer treatments. Front Cell Dev Biol. (2021) 9:777018. doi: 10.3389/fcell.2021.777018
40. Vignjevic Petrinovic S, Milosevic MS, Markovic D, Momcilovic S. Interplay between stress and cancer-A focus on inflammation. Front Physiol. (2023) 14:1119095. doi: 10.3389/fphys.2023.1119095
41. Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou M, et al. Chronic stress promotes cancer development. Front Oncol. (2020) 10:1492. doi: 10.3389/fonc.2020.01492
42. Bamberger CM, Bamberger AM. The peripheral CRH/urocortin system. Ann N Y Acad Sci. (2000) 917:290–6. doi: 10.1111/j.1749-6632.2000.tb05395.x
43. Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. (2007) 42 Suppl 17:48–51. doi: 10.1007/s00535-006-1942-7
44. Huo R, Zeng B, Zeng L, Cheng K, Li B, Luo Y, et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front Cell Infect Microbiol. (2017) 7:489. doi: 10.3389/fcimb.2017.00489
45. Bhatt S, Kanoujia J, Mohana Lakshmi S, Patil CR, Gupta G, Chellappan DK, et al. Role of brain-gut-microbiota axis in depression: emerging therapeutic avenues. CNS Neurol Disord Drug Targets. (2023) 22:276–88. doi: 10.2174/1871527321666220329140804
46. Webster EL, Torpy DJ, Elenkov IJ, Chrousos GP. Corticotropin-releasing hormone and inflammation. Ann N Y Acad Sci. (1998) 840:21–32. doi: 10.1111/j.1749-6632.1998.tb09545.x
47. Kawahito Y, Sano H, Mukai S, Asai K, Kimura S, Yamamura Y, et al. Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut. (1995) 37:544–51. doi: 10.1136/gut.37.4.544
48. Wallon C, Yang PC, Keita AV, Ericson AC, McKay DM, Sherman PM, et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. (2008) 57:50–8. doi: 10.1136/gut.2006.117549
49. Xu Y, Zhang R, Chen J, Zhang Q, Wang J, Hu J, et al. Urocortin promotes the development of vasculitis in a rat model of thromboangiitis obliterans via corticotrophin-releasing factor type 1 receptors. Br J Pharmacol. (2009) 157:1368–79. doi: 10.1111/j.1476-5381.2009.00210.x
50. Wu Y, Hu J, Zhang R, Zhou C, Xu Y, Guan X, et al. Enhanced intracellular calcium induced by urocortin is involved in degranulation of rat lung mast cells. Cell Physiol Biochem. (2008) 21:173–82. doi: 10.1159/000113759
51. Wu Y, Zhang R, Zhou C, Xu Y, Guan X, Hu J, et al. Enhanced expression of vascular cell adhesion molecule-1 by corticotrophin-releasing hormone contributes to progression of atherosclerosis in LDL receptor-deficient mice. Atherosclerosis. (2009) 203:360–70. doi: 10.1016/j.atherosclerosis.2008.05.059
52. Zhu C, Zhou J, Li T, Mu J, Jin L, Li S. Urocortin participates in LPS-induced apoptosis of THP-1 macrophages via S1P-cPLA2 signaling pathway. Eur J Pharmacol. (2020) 887:173559. doi: 10.1016/j.ejphar.2020.173559
53. Jin L, Zhu C, Wang X, Li C, Cao C, Yuan J, et al. Urocortin attenuates TGFbeta1-induced Snail1 and slug expressions: inhibitory role of Smad7 in Smad2/3 signaling in breast cancer cells. J Cell Biochem. (2015) 116:2494–503. doi: 10.1002/jcb.25194
54. Zhu C, Sun Z, Li C, Guo R, Li L, Jin L, et al. Urocortin affects migration of hepatic cancer cell lines via differential regulation of cPLA2 and iPLA2. Cell Signal. (2014) 26:1125–34. doi: 10.1016/j.cellsig.2014.02.002
55. Martinez V, Tache Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. (2006) 12:4071–88. doi: 10.2174/138161206778743637
56. Lee Y, Ma EL, Patel M, Kim G, Howe C, Pothoulakis C, et al. Corticotropin-releasing hormone receptor alters the tumor development and growth in apcmin/+ Mice and in a chemically-induced model of colon cancer. Int J Mol Sci. (2021) 22(3):1043. doi: 10.3390/ijms22031043
57. Liu Y, Fang X, Yuan J, Sun Z, Li C, Li R, et al. The role of corticotropin-releasing hormone receptor 1 in the development of colitis-associated cancer in mouse model. Endocr Relat Cancer. (2014) 21:639–51. doi: 10.1530/ERC-14-0239
58. Fang X, Hong Y, Dai L, Qian Y, Zhu C, Wu B, et al. CRH promotes human colon cancer cell proliferation via IL-6/JAK2/STAT3 signaling pathway and VEGF-induced tumor angiogenesis. Mol Carcinog. (2017) 56:2434–45. doi: 10.1002/mc.22691
59. Yamaoka K, Uotsu N, Hoshino E. Relationship between psychosocial stress-induced prefrontal cortex activity and gut microbiota in healthy Participants-A functional near-infrared spectroscopy study. Neurobiol Stress. (2022) 20:100479. doi: 10.1016/j.ynstr.2022.100479
60. Yi SQ, An JX, Liao CC, Lei S, Jin H, Tuo BG. The role of corticotropin-releasing hormone (CRH) family in tumors. Neoplasma. (2021) 68:907–16. doi: 10.4149/neo_2021_210219N226
61. Kageyama K, Suda T. Role and action in the pituitary corticotroph of corticotropin-releasing factor (CRF) in the hypothalamus. Peptides. (2009) 30:810–6. doi: 10.1016/j.peptides.2008.12.007
62. Stinnett GS, Westphal NJ, Seasholtz AF. Pituitary CRH-binding protein and stress in female mice. Physiol Behav. (2015) 150:16–23. doi: 10.1016/j.physbeh.2015.02.050
63. Lenz HJ, Burlage M, Raedler A, Greten H. Central nervous system effects of corticotropin-releasing factor on gastrointestinal transit in the rat. Gastroenterology. (1988) 94:598–602. doi: 10.1016/0016-5085(88)90229-6
64. Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. (2005) 54:1481–91. doi: 10.1136/gut.2005.064261
65. Greenwood-Van Meerveld B, Johnson AC, Schulkin J, Myers DA. Long-term expression of corticotropin-releasing factor (CRF) in the paraventricular nucleus of the hypothalamus in response to an acute colonic inflammation. Brain Res. (2006) 1071:91–6. doi: 10.1016/j.brainres.2005.11.071
66. Gue M, Bonbonne C, Fioramonti J, More J, Del Rio-Lacheze C, Comera C, et al. Stress-induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am J Physiol. (1997) 272:G84–91. doi: 10.1152/ajpgi.1997.272.1.G84
67. Million M, Tache Y, Anton P. Susceptibility of Lewis and Fischer rats to stress-induced worsening of TNB-colitis: protective role of brain CRF. Am J Physiol. (1999) 276:G1027–36o. doi: 10.1152/ajpgi.1999.276.4.G1027
68. Martinez V, Rivier J, Wang L, Tache Y. Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J Pharmacol Exp Ther. (1997) 280:754–60.
69. Chu B, Marwaha K, Sanvictores T, Ayers D. Physiology, Stress Reaction. Treasure Island (FL: StatPearls (2023). ineligible companies. Disclosure: Komal Marwaha declares no relevant financial relationships with ineligible companies. Disclosure: Terrence Sanvictores declares no relevant financial relationships with ineligible companies. Disclosure: Derek Ayers declares no relevant financial relationships with ineligible companies.
70. Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. (2000) 47:861–9. doi: 10.1136/gut.47.6.861
71. Tavakoli P, Vollmer-Conna U, Hadzi-Pavlovic D, Grimm MC. A review of inflammatory bowel disease: A model of microbial, immune and neuropsychological integration. Public Health Rev. (2021) 42:1603990. doi: 10.3389/phrs.2021.1603990
72. Gao X, Cao Q, Cheng Y, Zhao D, Wang Z, Yang H, et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci U.S.A. (2018) 115:E2960–9. doi: 10.1073/pnas.1720696115
73. Girotti M, Donegan JJ, Morilak DA. Influence of hypothalamic IL-6/gp130 receptor signaling on the HPA axis response to chronic stress. Psychoneuroendocrinology. (2013) 38:1158–69. doi: 10.1016/j.psyneuen.2012.11.004
74. Labanski A, Langhorst J, Engler H, Elsenbruch S. Stress and the brain-gut axis in functional and chronic-inflammatory gastrointestinal diseases: A transdisciplinary challenge. Psychoneuroendocrinology. (2020) 111:104501. doi: 10.1016/j.psyneuen.2019.104501
75. Fukudo S, Kanazawa M. Gene, environment, and brain-gut interactions in irritable bowel syndrome. J Gastroenterol Hepatol. (2011) 26 Suppl 3:110–5. doi: 10.1111/j.1440-1746.2011.06631.x
76. Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, et al. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. (2007) 292:G1037–44. doi: 10.1152/ajpgi.00419.2006
77. Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. (1998) 42:845–9. doi: 10.1136/gut.42.6.845
78. Zhao SB, Wu JY, He ZX, Song YH, Chang X, Xia T, et al. Corticotropin releasing hormone promotes inflammatory bowel disease via inducing intestinal macrophage autophagy. Cell Death Discov. (2021) 7:377. doi: 10.1038/s41420-021-00767-8
79. Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. (2014) 63:1293–9. doi: 10.1136/gutjnl-2013-305690
80. Cui B, Luo Y, Tian P, Peng F, Lu J, Yang Y, et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J Clin Invest. (2019) 129:1030–46. doi: 10.1172/jci121685
81. Chen X, Wang M, Yu K, Xu S, Qiu P, Lyu Z, et al. Chronic stress-induced immune dysregulation in breast cancer: Implications of psychosocial factors. J Transl Int Med. (2023) 11:226–33. doi: 10.2478/jtim-2021-0050
82. Gil F, Costa G, Hilker I, Benito L. First anxiety, afterwards depression: psychological distress in cancer patients at diagnosis and after medical treatment. Stress Health. (2012) 28:362–7. doi: 10.1002/smi.2445
83. Hou N, Zhang X, Zhao L, Zhao X, Li Z, Song T, et al. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem Biophys Res Commun. (2013) 439:471–6. doi: 10.1016/j.bbrc.2013.08.101
84. Peters S, Grunwald N, Rummele P, Endlicher E, Lechner A, Neumann ID, et al. Chronic psychosocial stress increases the risk for inflammation-related colon carcinogenesis in male mice. Stress. (2012) 15:403–15. doi: 10.3109/10253890.2011.631232
85. Tan HE. The microbiota-gut-brain axis in stress and depression. Front Neurosci. (2023) 17:1151478. doi: 10.3389/fnins.2023.1151478
86. Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
87. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. (2013) 13:759–71. doi: 10.1038/nrc3611
88. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
89. Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry. (2021) 78:1343–54. doi: 10.1001/jamapsychiatry.2021.2573
90. Zhang W, An Y, Qin X, Wu X, Wang X, Hou H, et al. Gut microbiota-derived metabolites in colorectal cancer: the bad and the challenges. Front Oncol. (2021) 11:739648. doi: 10.3389/fonc.2021.739648
91. Dalal N, Jalandra R, Bayal N, Yadav AK, Harshulika M, Sharma M, et al. Gut microbiota-derived metabolites in CRC progression and causation. J Cancer Res Clin Oncol. (2021) 147:3141–55. doi: 10.1007/s00432-021-03729-w
92. Yoo W, Zieba JK, Foegeding NJ, Torres TP, Shelton CD, Shealy NG, et al. High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science. (2021) 373:813–8. doi: 10.1126/science.aba3683
93. Loke YL, Chew MT, Ngeow YF, Lim WWD, Peh SC. Colon carcinogenesis: the interplay between diet and gut microbiota. Front Cell Infect Microbiol. (2020) 10:603086. doi: 10.3389/fcimb.2020.603086
94. Farhana L, Banerjee HN, Verma M, Majumdar APN. Role of microbiome in carcinogenesis process and epigenetic regulation of colorectal cancer. Methods Mol Biol. (2018) 1856:35–55. doi: 10.1007/978-1-4939-8751-1_3
95. Leclercq S, Starkel P, Delzenne NM, de Timary P. The gut microbiota: A new target in the management of alcohol dependence? Alcohol. (2019) 74:105–11. doi: 10.1016/j.alcohol.2018.03.005
96. Lembo T, Plourde V, Shui Z, Fullerton S, Mertz H, Tache Y, et al. Effects of the corticotropin-releasing factor (CRF) on rectal afferent nerves in humans. Neurogastroenterol Motil. (1996) 8:9–18. doi: 10.1111/j.1365-2982.1996.tb00237.x
97. Porcher C, Peinnequin A, Pellissier S, Meregnani J, Sinniger V, Canini F, et al. Endogenous expression and in vitro study of CRF-related peptides and CRF receptors in the rat gastric antrum. Peptides. (2006) 27:1464–75. doi: 10.1016/j.peptides.2005.10.023
98. Chaniotou Z, Giannogonas P, Theoharis S, Teli T, Gay J, Savidge T, et al. Corticotropin-releasing factor regulates TLR4 expression in the colon and protects mice from colitis. Gastroenterology. (2010) 139:2083–92. doi: 10.1053/j.gastro.2010.08.024
99. Chatoo M, Li Y, Ma Z, Coote J, Du J, Chen X. Involvement of corticotropin-releasing factor and receptors in immune cells in irritable bowel syndrome. Front Endocrinol (Lausanne). (2018) 9:21. doi: 10.3389/fendo.2018.00021
100. Mancinelli R, Azzena GB, Diana M, Forgione A, Fratta W. In vitro excitatory actions of corticotropin-releasing factor on rat colonic motility. J Auton Pharmacol. (1998) 18:319–24. doi: 10.1046/j.1365-2680.1998.1860319.x
101. Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. (2004) 16 Suppl 1:137–42. doi: 10.1111/j.1743-3150.2004.00490.x
102. Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, et al. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. (2004) 53:958–64. doi: 10.1136/gut.2003.018911
103. Nozu T, Okumura T. Corticotropin-releasing factor receptor type 1 and type 2 interaction in irritable bowel syndrome. J Gastroenterol. (2015) 50:819–30. doi: 10.1007/s00535-015-1086-8
104. Tanaka Y, Kanazawa M, Kano M, Morishita J, Hamaguchi T, Van Oudenhove L, et al. Differential activation in amygdala and plasma noradrenaline during colorectal distention by administration of corticotropin-releasing hormone between healthy individuals and patients with irritable bowel syndrome. PloS One. (2016) 11:e0157347. doi: 10.1371/journal.pone.0157347
105. Li B, Lee C, Filler T, Hock A, Wu RY, Li Q, et al. Inhibition of corticotropin-releasing hormone receptor 1 and activation of receptor 2 protect against colonic injury and promote epithelium repair. Sci Rep. (2017) 7:46616. doi: 10.1038/srep46616
106. Hoffman JM, Baritaki S, Ruiz JJ, Sideri A, Pothoulakis C. Corticotropin-releasing hormone receptor 2 signaling promotes mucosal repair responses after colitis. Am J Pathol. (2016) 186:134–44. doi: 10.1016/j.ajpath.2015.09.013
107. Chatzaki E, Anton PA, Million M, Lambropoulou M, Constantinidis T, Kolios G, et al. Corticotropin-releasing factor receptor subtype 2 in human colonic mucosa: down-regulation in ulcerative colitis. World J Gastroenterol. (2013) 19:1416–23. doi: 10.3748/wjg.v19.i9.1416
108. Saruta M, Takahashi K, Suzuki T, Torii A, Kawakami M, Sasano H. Urocortin 1 in colonic mucosa in patients with ulcerative colitis. J Clin Endocrinol Metab. (2004) 89:5352–61. doi: 10.1210/jc.2004-0195
109. Rodriguez JA, Huerta-Yepez S, Law IK, Baay-Guzman GJ, Tirado-Rodriguez B, Hoffman JM, et al. Diminished expression of CRHR2 in human colon cancer promotes tumor growth and EMT via persistent IL-6/Stat3 signaling. Cell Mol Gastroenterol Hepatol. (2015) 1:610–30. doi: 10.1016/j.jcmgh.2015.08.001
110. Ducarouge B, Pelissier-Rota M, Laine M, Cristina N, Vachez Y, Scoazec JY, et al. CRF2 signaling is a novel regulator of cellular adhesion and migration in colorectal cancer cells. PloS One. (2013) 8:e79335. doi: 10.1371/journal.pone.0079335
111. Ramirez-Guerrero AA, Gonzalez-Villasenor CO, Leal-Ugarte E, Gutierrez-Angulo M, Ramirez-Flores M, Delgado-Enciso I, et al. Association between genetic variant rs2267716 of CRHR2 gene with colorectal cancer. J Investig Med. (2022) 70:947–52. doi: 10.1136/jim-2021-002047
112. Panagopoulou M, Cheretaki A, Karaglani M, Balgkouranidou I, Biziota E, Amarantidis K, et al. Methylation status of corticotropin-releasing factor (CRF) receptor genes in colorectal cancer. J Clin Med. (2021) 10(12):2680. doi: 10.3390/jcm10122680
113. Kobayashi M, Matsubara N, Nakachi Y, Okazaki Y, Uchino M, Ikeuchi H, et al. Hypermethylation of corticotropin releasing hormone receptor-2 gene in ulcerative colitis associated colorectal cancer. In Vivo. (2020) 34:57–63. doi: 10.21873/invivo.11745
114. Sanchez-Alcoholado L, Ordonez R, Otero A, Plaza-Andrade I, Laborda-Illanes A, Medina JA, et al. Gut microbiota-mediated inflammation and gut permeability in patients with obesity and colorectal cancer. Int J Mol Sci. (2020) 21(18):6782. doi: 10.3390/ijms21186782
115. Chattopadhyay I, Dhar R, Pethusamy K, Seethy A, Srivastava T, Sah R, et al. Exploring the role of gut microbiome in colon cancer. Appl Biochem Biotechnol. (2021) 193:1780–99. doi: 10.1007/s12010-021-03498-9
Keywords: CRH, CRF receptors, stress, colitis, colitis-associated colon cancer
Citation: Zhu C and Li S (2024) Role of CRH in colitis and colitis-associated cancer: a combinative result of central and peripheral effects?. Front. Endocrinol. 15:1363748. doi: 10.3389/fendo.2024.1363748
Received: 31 December 2023; Accepted: 19 March 2024;
Published: 28 March 2024.
Edited by:
Yanan Ma, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Xiang Sun, St. Jude Children’s Research Hospital, United StatesQidong Li, University of Texas MD Anderson Cancer Center, United States
Weizhou Yue, Northeastern University, United States
Miklos Lengyel, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2024 Zhu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengnan Li, c25saUBuam11LmVkdS5jbg==
 Chao Zhu
Chao Zhu Shengnan Li
Shengnan Li