
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 16 July 2024
Sec. Translational and Clinical Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1362396
This article is part of the Research TopicNew insights: Developments in laboratory testing for the diagnosis and monitoring of endocrine related disorders and metabolic diseaseView all 9 articles
 Jianjun Wang1,2†
Jianjun Wang1,2† Han Li3†
Han Li3† Xiaoyi Wang4†
Xiaoyi Wang4† Ruizi Shi1
Ruizi Shi1 Junchao Hu1
Junchao Hu1 Xintao Zeng1
Xintao Zeng1 Hua Luo1
Hua Luo1 Pei Yang1
Pei Yang1 Huiwen Luo2
Huiwen Luo2 Yuan Cao5
Yuan Cao5 Xianfu Cai5
Xianfu Cai5 Sirui Chen1*
Sirui Chen1* Decai Wang2,5*
Decai Wang2,5*Objective: This study investigated the link between triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio and nonalcoholic fatty liver disease (NAFLD) and liver fibrosis in American adults.
Methods: Information for 6495 participants from the National Health and Nutrition Examination Survey (NHANES) 2017–2020.03 was used for this cross-sectional study. The link between TG/HDL-C ratios and NAFLD and liver fibrosis was assessed by multiple linear regression before evaluating nonlinear correlations based on smoothed curve fitting models. Stratification analysis was then applied to confirm whether the dependent and independent variables displayed a stable association across populations.
Results: TG/HDL-C ratios were positively correlated with NAFLD, with higher ratios being linked to increased prevalence of NAFLD. After adjusting for potential confounders, the odds ratios (OR) for NAFLD patients in the fourth TG/HDL-C quartile were 3.61 (95% confidence interval [CI], 2.94–4.38) (P for trend < 0.001) in comparison with those in the first quartile after adjusting for clinical variables. However, no statistical significance was noted for the ratio for liver fibrosis after adjusting for potential confounders (P for trend = 0.07). A nonlinear correlation between TG/HDL-C ratios and NAFLD was observed based on smoothed curve fitting models. However, a nonlinear relationship between the ratios and liver fibrosis was not established. In subgroup analyses, there was an interaction between smoking status and TG/HDL-C ratio in relation to the prevalence of liver fibrosis (P for interaction < 0.001).
Conclusions: Among American adults, the TG/HDL-C ratio was noted to be nonlinearly positively associated with the prevalence of NAFLD; however, this relationship was not present in liver fibrosis.
Nonalcoholic fatty liver disease (NAFLD), as a globally prevalent metabolic-related disease of the liver, affects approximately 38% of the world’s population, and as such, it significantly threatens public health (1). Most patients with NAFLD do not experience discomfort, whereas a small percentage may present with fatigue, epigastric discomfort, or vague pain (2). While its typical characteristic include excessive lipid accumulation in the liver without alcohol abuse, some NAFLD patients can further progress to nonalcoholic steatohepatitis, liver fibrosis, cirrhosis, and eventually hepatocellular carcinoma. The number of individuals at risk for this serious consequence is steadily increasing (3, 4). Although NAFLD involves complex risk factors, its prevalence is usually associated with obesity (5–7). NAFLD is associated with poor hepatic prognosis and an increased risk of extrahepatic metabolic abnormalities, including hyperlipidemia, hyperglycemia, insulin resistance (IR), and hyperuricemia (8, 9). Furthermore, cardiovascular events and extrahepatic tumorigenesis, the leading causes of extrahepatic death, are also more likely with this condition (8, 10). Despite ongoing efforts to determine drugs to treat NAFLD and liver fibrosis, there are no specific licensed drugs that can completely reverse NAFLD or liver fibrosis. The main goals of current treatment are to ameliorate metabolic sequelae and to control the risk of cardiovascular events to reduce associated mortality (11, 12). Therefore, it is of utmost importance to investigate novel treatment agents, targets, and interventional approaches for NAFLD and liver fibrosis.
The severity of hepatic steatosis and liver fibrosis is currently assessed by liver biopsy, but despite being an established standard, this practice is invasive and expensive, with limited acceptability, and hence, it is not only impractical for widespread screening of the general population but also requires caution in clinical practice (13). Therefore, there is an urgent need to identify new noninvasive tests to diagnose NAFLD. Serum markers and their combinations have several advantages. They are easy to access, low-cost, and offer high diagnostic accuracy, making them valuable for disease screening. A combination of lipids and lipoproteins proves to be more beneficial for predicting risks of NAFLD compared with individual lipid values as it captures interactions between lipid components (14, 15). Patients with NAFLD usually have lipid metabolism disorders and dyslipidemia, and serum markers may show reduced levels of high-density lipoprotein cholesterol (HDL-C) along with increased levels of low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and triglycerides (TG) (16). TG level is crucial for NAFLD development and progression. When TG level is elevated in the blood, there is an increase in the amount of TG celiac “debris” and free fatty acids transported to the liver through the bloodstream, which exceeds the liver’s ability to utilize these fatty acids, resulting in fatty infiltration of hepatocytes (17). In hepatocytes from patients with NAFLD, intracellular TG levels exceed 5% (18). As free fatty acids are oxidized in the mitochondria, hepatocytes experience oxidative stress. The excess reactive oxygen species generated in these reactions harm the hepatocytes, impairing their ability to metabolize free fatty acids and aggravating oxidative stress and lipid peroxidation, ultimately hastening the pathological process of NAFLD (19). The development of NAFLD impairs the ability of hepatocytes to oxidize, transport, and resynthesize lipids in the blood, exacerbating the increase in TG levels (20). HDL-C is essential for preventing atherosclerosis through the reverse cholesterol transport system that carries dietary cholesterol from peripheral tissues to the liver, where it is transformed into bile salts and expelled in the feces (21). HDL-C even possesses antioxidant and anti-inflammatory properties based on which it is called “good cholesterol.” Additionally, HDL-C levels showed a negatively correlated with NAFLD diagnosis (22).
The TG/HDL-C ratio exhibits a strong association with IR (23, 24). Indeed, in a study involving a multiethnic primary prevention cohort, a stronger relationship was noted between TG/HDL-C and a homeostasis model assessment of IR in comparison with other lipid parameters (25). Moreover, it can predict cardiovascular diseases, hypertension, and type 2 diabetes (26–28). Limited evidence has recently suggested that this ratio could also be linked to NAFLD, with an observational study noting an increased likelihood of developing NAFLD in children and adolescents having higher TG/HDL-C ratios (29). Similarly, in Japan, a population-based cohort study demonstrated that TG/HDL-C ratios could predict NAFLD incidence (30). Nonetheless, the relationship between the rate and NAFLD and liver fibrosis in American adults remains unclear.
To the best of the current authors’ knowledge, no epidemiological studies have explored the above association in American adults. Therefore, based on information of 6495 participants from the National Health and Nutrition Examination Survey (NHANES) 2017–2020.03, a cross-sectional study was performed for investigating how TG/HDL-C ratio was linked to NAFLD and liver fibrosis. It is expected that this study will provide new understanding of NAFLD that will guide treatment and management of the condition.
Data from the 2017–2020.03 NHANES database, a comprehensive population-based survey, was used for the current observational study. Information on the health, diet as well as socioeconomic, and demographic characteristics of the participants, were obtained through questionnaires, physical examinations, laboratory tests, and medical examinations, including anthropometric measurements and laboratory evaluations. The National Center for Health Statistics Ethics Review Committee gave approval for the NHANES survey protocol, with written informed consent also obtained from all participants. Since the NHANES database is publicly available, this study was exempted from ethical review.
Out of 15,560 American adults included from the NHANES database, participants below 18 years old (n = 5867) who did not undergo the liver ultrasound transient elastography (LUTE) test (n = 512) and with missing TG/HDL-C ratio information (n = 1442) were then excluded alongside those who had a history of autoimmune hepatitis (n = 4) or viral hepatitis (n = 214), with the latter including individuals who were positive for hepatitis C virus RNA (n = 59), hepatitis C antibody (n = 67), and hepatitis B surface antigen (n = 88). In addition, males and females with an alcohol intake of more than 30 g/d (n = 487) and 20 g/d (n = 353), respectively, indicative of substantial alcohol consumption, were not considered (31). Finally, according to the NHANES guidelines, individuals with liver stiffness measurements (LSMs) displaying an interquartile range/median > 30% were deemed unreliable and were, therefore, excluded (n = 186). As a result, this study involved 6495 participants (Figure 1).
Demographic characteristics, such as age, sex, ethnicity, educational attainment, smoking and drinking status, body mass index (BMI), household income, family income to poverty threshold ratio as well as past medical history of blood transfusion, hypertension, and diabetes, were retrieved from the NHANES database.
Laboratory variables included TG, TC, albumin (Alb), glycated hemoglobin (HbA1c), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), C-reactive protein (CRP), alanine aminotransferase (ALT), LDL-C, HDL-C, and ferritin. The measurement protocols for these variables were obtained from the literature (32, 33). The ratio of TG to HDL-C was used to obtain the TG/HDL-C ratio.
LUTE primarily aims to provide an objective measure of liver steatosis (liver fat) and liver fibrosis (liver scarring) which are two critical manifestations of liver disease. A FibroScan® machine was used for all measurements, where the assessment of one physical parameter, referred to as the controlled attenuation parameter (CAP), primarily reflected the extent of hepatic steatosis. Steatosis was considered in cases where the median CAP value was ≥ 274 dB/m (34). Liver fibrosis was determined based on LSMs and most recent guidelines of the European Association for the Study of the Liver (35). Based on thresholds of 8.2, 9.7, and 13.6 kPa, liver fibrosis was eventually categorized as F2, F3, and F4, respectively (32, 36).
In March 2020, field operations for the NHANES project were halted because of the 2019 coronavirus pandemic. Therefore, to construct a nationally representative sample, information obtained from 2019 to March 2020 and that from the NHANES 2017–2018 cycle was combined. Additionally, for the pre-epidemic data files from March 2017 to 2020, a special weighting procedure was employed by the NHANES workgroup. LUTE data were analyzed using NHANES check sample weights in accordance with the NHANES guidelines, with special examination sample weights (variable name: WTMECPRP) employed for the 2017–2020.03 cycle. R software (version 4.2.2) was used to extract, merge, clean, and analyze the NHANES dataset, with P-values < 0.05 indicating statistical significance. Categorizing the TG/HDL-C ratios into quartiles yielded four participant subgroups. For normally distributed continuous variables, data are presented as mean ± standard deviation (SD); for non-normally distributed continuous variables, data are presented as median (interquartile range, IQR); and for categorical variables, data are presented as numbers (%). Furthermore, differences between categorical data of the four subgroups were compared with chi-squared tests, while one-way analysis of variance and Kruskal–Wallis were used in the case of normally and non-normally distributed data, respectively. Correlations between TG/HDL-C ratio and clinical variables were then determined with Spearman’s correlation analyses before investigating the link between the independent and dependent variables based on multiple linear regression analysis which generated three models with varying covariates adjustments: For Model 1, no adjustments were made for covariates; Adjustments for age, sex, and ethnicity were made in the case of Model 2; and Model 3, additionally adjusted for smoking status, poverty income ratio (PIR), educational attainment, diabetes, hypertension, BMI, HbA1c and TC levels. To evaluate the stability of the associations across cohorts and to identify sensitive populations, we conducted subgroup analyses with subgroups that included age, gender, ethnicity, BMI, educational level, household income, smoking status, and diabetes. Finally, the existence of a nonlinear association between the independent and dependent variables was assessed by smoothed curve fitting analysis.
In total, 6495 participants were included in this study (Table 1). Eventually, this study included 3108 males and 3387 females. Hepatic fibrosis and NAFLD were observed in 9.3% and 42.5% of the participants, respectively. Higher TG/HDL-C ratios were linked to a higher prevalence of NAFLD, severe hepatic steatosis, and hepatic fibrosis (P < 0.05). Age, sex, ethnicity, smoking status, household income, educational attainment, BMI as well as diabetes, hypertension, TC, LDL-C, ALT, AST, GGT, Alb, HbA1c, CRP, and ferritin levels were significant factors in all TG/HDL-C quartiles (P < 0.05). However, the TG/DHL-C quartiles were not significantly different for blood transfusion (P = 0.09).
A positive correlation was noted between TG/DHL-C ratio and age, BMI, LSM, CAP, HbA1c, GGT,TC, LDL-C, ALT, AST, ferritin levels; and (r = 0.041, 0.156, 0.079, 0.309, 0.211, 0.151, 0.187, 0.113, 0.199, 0.091, 0.144, and respectively; P < 0.05) (Table 2). However, a significantly positive association was not observed with Alb and CRP levels (r = 0.023 and 0.022, and P = 0.06 and 0.08, respectively).
All models indicated that TG/HDL-C ratios and NAFLD were positively correlated (Table 3). Grouping the TG/HDL-C ratios into quartiles revealed a consistent correlation, with the association between the two strengthening as the ratio increased (P for trend < 0.001). Participants in Q2, Q3, and Q4 exhibited NAFLD odds ratios (ORs) of 2.15 (95% confidence interval [CI], 1.82–2.53), 5.01 (95% CI, 4.27–5.87), and 8.99 (95% CI, 7.64–10.58), respectively, compared with those in Q1 (all P < 0.05). Moreover, following adjustments for additional clinical variables, the corresponding NAFLD ORs for Q2, Q3, and Q4 were 1.32 (95% CI, 1.09–1.59), 2.41 (95% CI, 2.00–2.90), and 3.61 (95% CI, 2.94–4.38), respectively, in comparison with the TG/HDL-C ratio of the Q1 participants (all P < 0.05).
Additionally, TG/HDL-C ratio was positively correlated with liver fibrosis in Models 1 and 2 and was statistically significant, but this positive correlation was not statistically significant in Model 3 (Table 3). Q2, Q3, and Q4 participants exhibited ORs of 1.42 (95% CI, 1.05–1.91), 2.64 (95% CI, 2.01–3.46), and 3.06 (95% CI, 2.35–4.00) for liver fibrosis, respectively compared with the TG/HDL-C ratio of Q1 participants (P for trend < 0.001). However, following adjustments for other clinical variables in Model 3, the OR of liver fibrosis did not reach statistical significance despite enhanced association between TG/HDL-C and liver fibrosis at higher ratios (P for trend = 0.07).
By smooth curve-fitting analysis, we further investigated the potential nonlinear relationship between TG/HDL-C ratio and NAFLD and liver fibrosis. According to Figure 2, our findings indicated a nonlinear positive correlation between the TG/HDL-C ratio and NAFLD, while such a relationship does not exist in liver fibrosis.
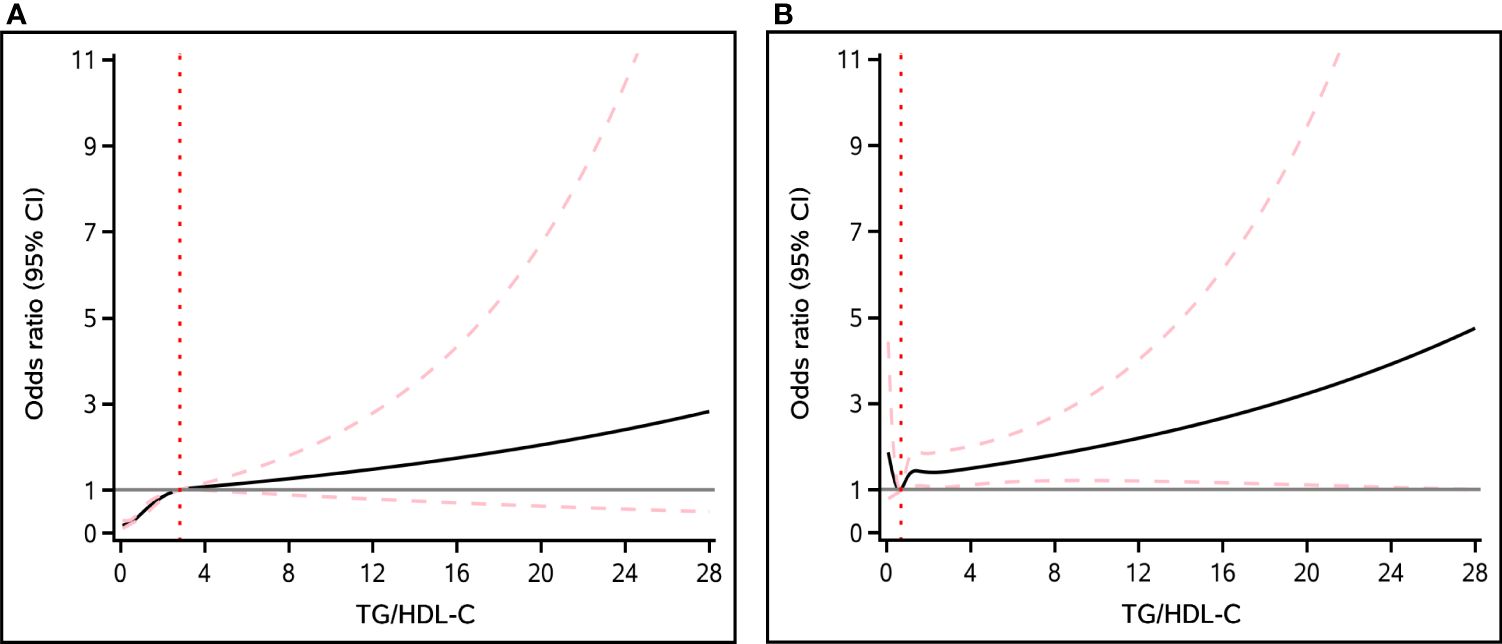
Figure 2 The nonlinear association between TG/HDL-C ratio and the risk of NAFLD (A) and liver fibrosis (B). Solid black line represents the smooth curve fit between variables. The pink dashed lines represent the 95% of confidence interval from the fit.
Stratified analyses were performed based on age, sex, BMI, ethnicity, smoking status, educational attainment, household income, hypertension, and diabetes. The results of stratified analysis of NAFLD and liver fibrosis are shown in Figures 3 and 4.The positive correlation between TG/HDL-C ratio and NAFLD exhibited a broad consistency across populations. In addition, we found an interaction between smoking status and TG/HDL-C ratio in relation to the prevalence of liver fibrosis (P for interaction < 0.001).
NAFLD is recently prevalent worldwide, and timely and effective assessment of NAFLD risk is critical for patients, with efforts to stop disease progression to advanced fibrosis or hepatocellular carcinoma (37). Currently, liver biopsy remains the established standard to assess the extent of liver steatosis and fibrosis is; however, being a harmful and invasive procedure, liver puncture should therefore be cautiously applied in clinical practice (38). LUTE is widely used for evaluating the extent of liver fibrosis and steatosis; its main advantage is that it is noninvasive, and the results are comparable to those of a liver biopsy (39, 40). Despite limited evidence on the relationship between TG/HDL-C ratio and NAFLD (29, 30, 41), it remains unclear how this ratio is linked to NAFLD and liver fibrosis in American adults. The current study examined this association in American adults, with the results demonstrating a positive correlation between TG/HDL-C ratios and NAFLD in all models. Grouping the ratios into quartiles revealed a consistent correlation that strengthened with increasing TG/HDL-C. However, although the TG/HDL-C ratios were positively correlated with liver fibrosis in all models, this positive correlation was not statistically significant in Model 3. Our findings could assist healthcare professionals in identifying high-risk patients for NAFLD and liver fibrosis, thereby enabling more informed decisions regarding patient management.
Recently, increasing evidence has suggested that TG/HDL-C ratios were linked to more unfavorable metabolic diseases, such as atherosclerosis, obesity, diabetes, and dyslipidemia (41). Our results were consistent with previous results and also showed that for participants with higher TG/HDL-C ratios, LDL-C, AST, ALT, TC, GGT, Alb, TG, CRP, blood pressure, BMI, and ferritin levels were significantly higher compared with those who presented lower TG/HDL-C ratios.
However, the mechanism underlying the link between TG/HDL-C ratio and NAFLD and liver fibrosis have not been fully elucidated. IR is an important feature of NAFLD and may, therefore, be a potential mediator. IR and lipid disorders are the starting links and the core of NAFLD. When the effector organs of insulin action (e.g., liver, skeletal muscle, adipose tissue) become less sensitive to insulin, there is a compensatory increase in insulin, eventually leading to hyperinsulinemia. Compensatory elevation of insulin can promote hepatic synthesis of TC, which also elevates LDL-C levels and leads to inactivation of lipoprotein lipase, which reduces clearance of TG. Consequently, large amounts of TG accumulate in the liver (42). Additionally, when the concentration of TG is elevated in the bloodstream, the “debris” and free fatty acids of TG celiacs transported to the liver through the bloodstream will increase, which exceeds the liver’s ability to utilize these fatty acids, ultimately triggering the development of NAFLD (43–46). Moreover, IR leads to decreased fat metabolism and increased catabolism, and the liver takes up large amounts of free fatty acids. However, the β-oxidation of fatty acids is inhibited by hyperinsulinemia, and a large amount of free fatty acids accumulates in the liver, exacerbating hepatocellular steatosis (47). As NAFLD progresses, oxidative stress and excessive lipid deposition aggravate the impairment of hepatocyte mitochondrial function, which further attenuates the metabolism of lipid pairs, thereby leading to further increases in the levels of TG, TC, and other markers (47). IR induces the secretion of larger and TG-overconcentrated very low-density lipoprotein particles while decreasing HDL-C concentration (48, 49). Therefore, IR contributes to an increase in TG/HDL-C ratio.
Recently, a strong association between IR and leptin secretion has been reported (50). Leptin is a protein-like hormone secreted by adipose tissue, and the degree of hepatic steatosis is positively correlated to its expression level (50). Persistent hyperleptinemia has therefore been linked to steatosis, liver fibrosis, and hepatocellular cancer in several observational clinical investigations, indicating that hyperleptinemia is a reliable indicator of the onset or development of NAFLD (51–53). It is currently believed that leptin promotes the development of NAFLD by centrally suppressing dietary intake and increasing sympathetic nerve activity, metabolic rates, and gluconeogenesis (54, 55).
This study has significant clinical value, as it reliably assesses hepatic steatosis and fibrosis in American adults with an adequate and representative sample size. However, certain limitations were also noted. Firstly, being a cross-sectional study, the causal association between TG/HDL-C ratio and NAFLD and liver fibrosis could not be elucidated. A longitudinal study in the future is necessary to better investigate the potential causal relationships. Secondly, the findings may not be applicable to individuals aged < 18 years since all included participants were over 18 years old. Therefore, further studies are required for investigating the association between TG/HDL-C ratios and NAFLD and liver fibrosis in this population. Thirdly, despite the fact that 6,495 participants have been included in this research, there is still a risk of insufficient sample size for a specific group, and therefore it is necessary for future studies to include more participants to demonstrate our results. Fourthly, the inherent limitations of the NHANES database resulted in several confounders that may have had an effect on the results that were not adjusted for. Finally, there are several potential influences on the occurrence and development of NAFLD and liver fibrosis. Despite including as many covariates as possible in this study and making adjustments to the models, the possibility that unaccounted confounders could have led to biased results cannot be excluded. Therefore, additional prospective studies would be required to validate the findings of this investigation and address the above limitations.
In the American adult population, the TG/HDL-C ratio was nonlinearly and positively associated with the prevalence of NAFLD; however, this relationship was not present in liver fibrosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
National Center for Health Statistics Ethics Review Committee gave approval for the NHANES survey protocol, with written informed consent also obtained from all participants. Since the NHANES database is publicly available, this study was exempted from ethical review. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JW: Writing – review & editing, Writing – original draft, Validation, Funding acquisition, Formal analysis, Data curation. HLi: Writing – original draft, Investigation, Data curation, Conceptualization. XW: Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Conceptualization. RS: Writing – original draft, Supervision, Software, Methodology, Investigation, Data curation. JH: Writing – original draft, Validation, Software, Project administration, Investigation, Formal analysis, Conceptualization. XZ: Writing – review & editing, Visualization, Validation, Supervision, Resources, Methodology, Data curation. HAL: Writing – review & editing, Validation, Project administration, Methodology, Formal analysis, Data curation. PY: Writing – review & editing, Software, Methodology, Investigation, Data curation, Conceptualization. HWL: Writing – original draft, Project administration, Methodology, Data curation. YC: Writing – original draft, Software, Investigation, Conceptualization. XC: Writing – original draft, Data curation. SC: Writing – original draft, Supervision, Investigation, Funding acquisition, Formal analysis. DW: Writing – review & editing, Writing – original draft, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by NHC Key Laboratory of Nuclear Technology Medical Transformation (Mianyang Central Hospital) (Grant No.2023HYX032), Incubation Project of Mianyang Central Hospital (2022FH010, 2023FH002, 2023FH005) and Talent Introduction Project of Mianyang Central Hospital (2023RCYJ-001).
We are grateful to the NHANES participants and staff. We appreciate all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; CI, confidence interval; CRP, C-reactive protein; GGT γ-glutamyltransferase; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; IR, insulin resistance; LDL-C, low-density lipoprotein cholesterol; LSMs, liver stiffness measurements; LUTE, liver ultrasound transient elastography; NAFLD, nonalcoholic fatty liver disease; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; PIR, poverty income ratio; TC, total cholesterol; TG, triglyceride; TG/HDL-C, triglyceride to high-density lipoprotein cholesterol ratio.
1. Wong VW, Ekstedt M, Wong GL, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. (2023) 79:842–52. doi: 10.1016/j.jhep.2023.04.036
2. Dalbeni A, Lombardi R, Henrique M, Zoncapè M, Pennisi G, Petta S, et al. Diagnostic accuracy of AGILE3+ score for advanced fibrosis in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Hepatology. (2024) 79(5):1107-1116. doi: 10.1097/HEP.0000000000000694
3. Domingues I, Leclercq IA, Beloqui A. Nonalcoholic fatty liver disease: Current therapies and future perspectives in drug delivery. J Control Release. (2023) 363:415–34. doi: 10.1016/j.jconrel.2023.09.040
4. Sharma S, Le Guillou D, Chen JY. Cellular stress in the pathogenesis of nonalcoholic steatohepatitis and liver fibrosis. Nat Rev Gastroenterol Hepatol. (2023) 20:662–78. doi: 10.1038/s41575-023-00832-w
5. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
6. Friedman SL, Pinzani M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology. (2022) 75:473–88. doi: 10.1002/hep.32285
7. Zhang X, Ha S, Lau HC, Yu J. Excess body weight: Novel insights into its roles in obesity comorbidities. Semin Cancer Biol. (2023) 92:16–27. doi: 10.1016/j.semcancer.2023.03.008
8. Foerster F, Gairing SJ, Müller L, Galle PR. NAFLD-driven HCC: Safety and efficacy of current and emerging treatment options. J Hepatol. (2022) 76:446–57. doi: 10.1016/j.jhep.2021.09.007
9. LoMonaco R, Sunny NE, Bril F, Cusi K. Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs. (2013) 73:1–14. doi: 10.1007/s40265-012-0004-0
10. Jia Y, Li D, You Y, Yu J, Jiang W, Liu Y, et al. Multi-system diseases and death trajectory of metabolic dysfunction-associated fatty liver disease: findings from the UK Biobank. BMC Med. (2023) 21:398. doi: 10.1186/s12916-023-03080-6
11. Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. (2020) 14:889–919. doi: 10.1007/s12072-020-10094-2
12. Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol. (2021) 56:951–63. doi: 10.1007/s00535-021-01796-x
13. Grander C, Grabherr F, Tilg H. Non-alcoholic fatty liver disease: pathophysiological concepts and treatment options. Cardiovasc Res. (2023) 119:1787–98. doi: 10.1093/cvr/cvad095
14. Ren XY, Shi D, Ding J, Cheng ZY, Li HY, Li JS, et al. Total cholesterol to high-density lipoprotein cholesterol ratio is a significant predictor of nonalcoholic fatty liver: Jinchang cohort study. Lipids Health Dis. (2019) 18:47. doi: 10.1186/s12944-019-0984-9
15. Zou Y, Zhong L, Hu C, Zhong M, Peng N, Sheng G. LDL/HDL cholesterol ratio is associated with new-onset NAFLD in Chinese non-obese people with normal lipids: a 5-year longitudinal cohort study. Lipids Health Dis. (2021) 20:28. doi: 10.1186/s12944-021-01457-1
16. Ahmed HS, Wang N, Carr JJ, Ding J, Terry JG, VanWagner LB, et al. The association between hepatic steatosis and incident cardiovascular disease, cancer, and all-cause mortality in a US multicohort study. Hepatology. (2023) 77:2063–72. doi: 10.1097/HEP.0000000000000286
17. Wang J, Yan S, Cui Y, Chen F, Piao M, Cui W. The diagnostic and prognostic value of the triglyceride-glucose index in metabolic dysfunction-associated fatty liver disease (MAFLD): A systematic review and meta-analysis. Nutrients. (2022) 14:4969. doi: 10.3390/nu14234969
18. Chen HK, Luo J, Li XJ, Liao WZ, Hu YQ, Guo XG. Serum folate associated with nonalcoholic fatty liver disease and advanced hepatic fibrosis. Sci Rep. (2023) 13:12933. doi: 10.1038/s41598-023-39641-1
19. Badmus OO, Hillhouse SA, Anderson CD, Hinds TD, Stec DE. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways. Clin Sci (Lond). (2022) 136:1347–66. doi: 10.1042/CS20220572
20. Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metab. (2021) 50:101238. doi: 10.1016/j.molmet.2021.101238
21. Crudele L, De Matteis C, Piccinin E, Gadaleta RM, Cariello M, Di Buduo E, et al. Low HDL-cholesterol levels predict hepatocellular carcinoma development in individuals with liver fibrosis. JHEP Rep. (2022) 5:100627. doi: 10.1016/j.jhepr.2022.100627
22. Saponaro C, Sabatini S, Gaggini M, Carli F, Rosso C, Positano V, et al. Adipose tissue dysfunction and visceral fat are associated with hepatic insulin resistance and severity of NASH even in lean individuals. Liver Int. (2022) 42:2418–27. doi: 10.1111/liv.15377
23. Ting YW, Jalaludin MY, Zaini AA, Mohamed R. Triglyceride to high-density lipoprotein cholesterol ratio is an independent predictor of liver fibrosis among pediatrics non-alcoholic fatty liver disease. Front Endocrinol (Lausanne). (2022) 13:1071350. doi: 10.3389/fendo.2022.1071350
24. Zhang Y, Wang R, Fu X, Song H. Non-insulin-based insulin resistance indexes in predicting severity for coronary artery disease. Diabetol Metab Syndr. (2022) 14:191. doi: 10.1186/s13098-022-00967-x
25. Gasevic D, Frohlich J, Mancini GB, Lear SA. The association between triglyceride to high-density-lipoprotein cholesterol ratio and insulin resistance in a multiethnic primary prevention cohort. Metabolism. (2012) 61:583–9. doi: 10.1016/j.metabol.2011.09.009
26. Mackey RH, Mora S, Bertoni AG, Wassel CL, Carnethon MR, Sibley CT, et al. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care. (2015) 38:628–36. doi: 10.2337/dc14-0645
27. Tohidi M, Hatami M, Hadaegh F, Azizi F. Triglycerides and triglycerides to high-density lipoprotein cholesterol ratio are strong predictors of incident hypertension in Middle Eastern women. J Hum Hypertens. (2012) 26:525–32. doi: 10.1038/jhh.2011.70
28. Sung KC, Reaven G, Kim S. Ability of the plasma concentration ratio of triglyceride/high-density lipoprotein cholesterol to identify increased cardio-metabolic risk in an east Asian population. Diabetes Res Clin Pract. (2014) 105:96–101. doi: 10.1016/j.diabres.2014.04.021
29. Pacifico L, Bonci E, Andreoli G, Romaggioli S, Di Miscio R, Lombardo CV, et al. Association of serum triglyceride-to-HDL cholesterol ratio with carotid artery intima-media thickness, insulin resistance and nonalcoholic fatty liver disease in children and adolescents. Nutr Metab Cardiovasc Dis. (2014) 24:737–43. doi: 10.1016/j.numecd.2014.01.010
30. Fukuda Y, Hashimoto Y, Hamaguchi M, Fukuda T, Nakamura N, Ohbora A, et al. Triglycerides to high-density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; a population-based cohort study. Liver Int. (2016) 36:713–20. doi: 10.1111/liv.12977
31. Wang X, Seo YA, Park SK. Serum selenium and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2011-2016. Environ Res. (2021) 197:111190. doi: 10.1016/j.envres.2021.111190
32. Ciardullo S, Perseghin G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metabolism. (2021) 121:154752. doi: 10.1016/j.metabol.2021.154752
33. Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999-2016. J Intern Med. (2020) 288:139–51. doi: 10.1111/joim.13069
34. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of fibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1717–30. doi: 10.1053/j.gastro.2019.01.042
35. European Association for the Study of the Liver. Electronic address:ZWFzbG9mZmljZUBlYXNsb2ZmaWNlLmV1LA== Clinical Practice Guideline Panel, Chair, EASL Governing Board representative, Panel members. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. (2022) 76:251–2. doi: 10.1016/j.jhep.2021.10.008
36. Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. (2008) 48:606–13. doi: 10.1016/j.jhep.2007.11.020
37. Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. (2018) 15:274–82. doi: 10.1038/nrgastro.2018.10
38. Arai T, Atsukawa M, Tsubota A, Mikami S, Haruki U, Yoshikata K, et al. Antifibrotic effect and long-term outcome of SGLT2 inhibitors in patients with NAFLD complicated by diabetes mellitus. Hepatol Commun. (2022) 6:3073–82. doi: 10.1002/hep4.2069
39. Arai T, Takahashi H, Seko Y, Toyoda H, Hayashi H, Yamaguchi K, et al. Accuracy of the enhanced liver fibrosis test in patients with type 2 diabetes mellitus and its clinical implications. Clin Gastroenterol Hepatol. (2024) 22(4):789-797.e8. doi: 10.1016/j.cgh.2023.11.022
40. Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. (2010) 36:1825–35. doi: 10.1016/j.ultrasmedbio.2010.07.005
41. Fan N, Peng L, Xia Z, Zhang L, Song Z, Wang Y, et al. Triglycerides to high-density lipoprotein cholesterol ratio as a surrogate for nonalcoholic fatty liver disease: a cross-sectional study. Lipids Health Dis. (2019) 18:39. doi: 10.1186/s12944-019-0986-7
42. Li H, Yu XH, Ou X, Ouyang XP, Tang CK. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog Lipid Res. (2021) 83:101109. doi: 10.1016/j.plipres.2021.101109
43. Semova I, Biddinger SB. Triglycerides in nonalcoholic fatty liver disease: guilty until proven innocent. Trends Pharmacol Sci. (2021) 42:183–90. doi: 10.1016/j.tips.2020.12.001
44. Marchesini G, Petta S, Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology. (2016) 63:2032–43. doi: 10.1002/hep.28392
45. Sahebkar A, Chew GT, Watts GF. Recent advances in pharmacotherapy for hypertriglyceridemia. Prog Lipid Res. (2014) 56:47–66. doi: 10.1016/j.plipres.2014.07.002
46. Sahini N, Borlak J. Recent insights into the molecular pathophysiology of lipid droplet formation in hepatocytes. Prog Lipid Res. (2014) 54:86–112. doi: 10.1016/j.plipres.2014.02.002
47. Cook JR, Hawkins MA, Pajvani UB. Liver insulinization as a driver of triglyceride dysmetabolism. Nat Metab. (2023) 5:1101–10. doi: 10.1038/s42255-023-00843-6
48. Lucero D, Miksztowicz V, Macri V, López GH, Friedman S, Berg G, et al. Overproduction of altered VLDL in an insulin-resistance rat model: Influence of SREBP-1c and PPAR-α. Clin Investig Arterioscler. (2015) 27:167–74. doi: 10.1016/j.arteri.2014.11.002
49. Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. (2012) 32:2104–12. doi: 10.1161/ATVBAHA.111.241463
50. Tan T, Song Z, Li W, Wang R, Zhu M, Liang Z, et al. Modelling porcine NAFLD by deletion of leptin and defining the role of AMPK in hepatic fibrosis. Cell Biosci. (2023) 13:169. doi: 10.1186/s13578-023-01124-1
51. Adolph TE, Grander C, Grabherr F, Tilg H. Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int J Mol Sci. (2017) 18:1649. doi: 10.3390/ijms18081649
52. Ikejima K, Honda H, Yoshikawa M, Hirose M, Kitamura T, Takei Y, et al. Leptin augments inflammatory and profibrogenic responses in the murine liver induced by hepatotoxic chemicals. Hepatology. (2001) 34:288–97. doi: 10.1053/jhep.2001.26518
53. Jiménez-Cortegana C, García-Galey A, Tami M, Del Pino P, Carmona I, López S, et al. Role of leptin in non-alcoholic fatty liver disease. Biomedicines. (2021) 9:762. doi: 10.3390/biomedicines9070762
54. Lonardo A, Nascimbeni F, Maurantonio M, Marrazzo A, Rinaldi L, Adinolfi LE. Nonalcoholic fatty liver disease: Evolving paradigms. World J Gastroenterol. (2017) 23:6571–92. doi: 10.3748/wjg.v23.i36.6571
Keywords: National health and nutrition examination survey, nonalcoholic fatty liver disease, cross-sectional study, triglyceride to high-density lipoprotein cholesterol ratio, liver fibrosis
Citation: Wang J, Li H, Wang X, Shi R, Hu J, Zeng X, Luo H, Yang P, Luo H, Cao Y, Cai X, Chen S and Wang D (2024) Association between triglyceride to high-density lipoprotein cholesterol ratio and nonalcoholic fatty liver disease and liver fibrosis in American adults: an observational study from the National Health and Nutrition Examination Survey 2017–2020. Front. Endocrinol. 15:1362396. doi: 10.3389/fendo.2024.1362396
Received: 28 December 2023; Accepted: 03 July 2024;
Published: 16 July 2024.
Edited by:
Pranoot Tanpaiboon, Quest Diagnostics (United States), United StatesReviewed by:
Zheng Liu, Peking University, ChinaCopyright © 2024 Wang, Li, Wang, Shi, Hu, Zeng, Luo, Yang, Luo, Cao, Cai, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sirui Chen, NjQ1Mzc2NUBxcS5jb20=; Decai Wang, ZGVjYWl3YW5nXzIwMjBAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.