- 1Department of Urology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
- 2Department of Urology, The Fourth Affiliated Hospital of Soochow University, Suzhou, China
- 3Department of Urology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, China
- 4Center for Systems Biology, Department of Bioinformatics, School of Biology and Basic Medical Sciences, Soochow University, Suzhou, China
Prostate cancer (PCa) is commonly occurred with high incidence in men worldwide, and many patients will be eventually suffered from the dilemma of castration-resistance with the time of disease progression. Castration-resistant PCa (CRPC) is an advanced subtype of PCa with heterogeneous carcinogenesis, resulting in poor prognosis and difficulties in therapy. Currently, disorders in androgen receptor (AR)-related signaling are widely acknowledged as the leading cause of CRPC development, and some non-AR-based strategies are also proposed for CRPC clinical analyses. The initiation of CRPC is a consequence of abnormal interaction and regulation among molecules and pathways at multi-biological levels. In this study, CRPC-associated genes, RNAs, proteins, and metabolites were manually collected and integrated by a comprehensive literature review, and they were functionally classified and compared based on the role during CRPC evolution, i.e., drivers, suppressors, and biomarkers, etc. Finally, translational perspectives for data-driven and artificial intelligence-powered CRPC systems biology analysis were discussed to highlight the significance of novel molecule-based approaches for CRPC precision medicine and holistic healthcare.
Introduction
Prostate cancer (PCa) is the most frequently diagnosed cancer in men, representing 29% of all male cancer cases and ranking second only to lung cancer in terms of fatalities (1). The incidence and mortality of PCa in Asia are much lower than those in Europe and in the United States, but the increasing trend is much higher. The incidence of PCa is influenced by multiple factors such as age, race, and genetics, etc., and biological characteristics of the tumor, as well as the prognosis, can vary significantly among different individuals and populations. In 1941, Huggins and Hodges discovered that PCa could be treated by castration. In the early stage of the tumor, almost all PCa patients are responsive to androgen deprivation therapy (ADT). However, after a median of 18 to 24 months of treatment, nearly all patients progressed to castration-resistant prostate cancer (CRPC) (2). CRPC is a heterogeneous status with complex molecular characteristics, and its poor prognosis and high mortality rate remain to be a significant clinical challenge.
The occurrence and development of CRPC result from interactions among various carcinogenic mechanisms, which are not fully deciphered. Currently, chemotherapy, novel endocrine therapy, and immunotherapy have been used for CRPC clinical treatment, and these methods may be effective during the initial stages. However, drug resistance typically develops soon. CRPC is generally a fatal condition, with a median time to death of 1–2 years after entering this stage (3). To fight against this dilemma, biomarkers across different biological levels, e.g., genes, RNAs, proteins, and metabolites, were identified by both computational and experimental techniques for early prediction, precision prognosis and personalized therapy of CRPC, and this has increased the flourishing of molecule-based approaches for CRPC application (4).
Due to the high heterogeneity in CRPC evolution, the reliability and efficacy of current therapeutic strategies for CRPC clinical practice are still unsatisfactory. Two important issues are widely concerned across CRPC studies, i.e., what are the key signatures that could be used for indicating the development of CRPC, and what therapeutic schedules should be applied when a patient has been diagnosed with CRPC. With the accumulation of multi-omics biomedical data and technologies, a great number of biological molecules have been identified for CRPC risk prediction and personalized therapeutics.
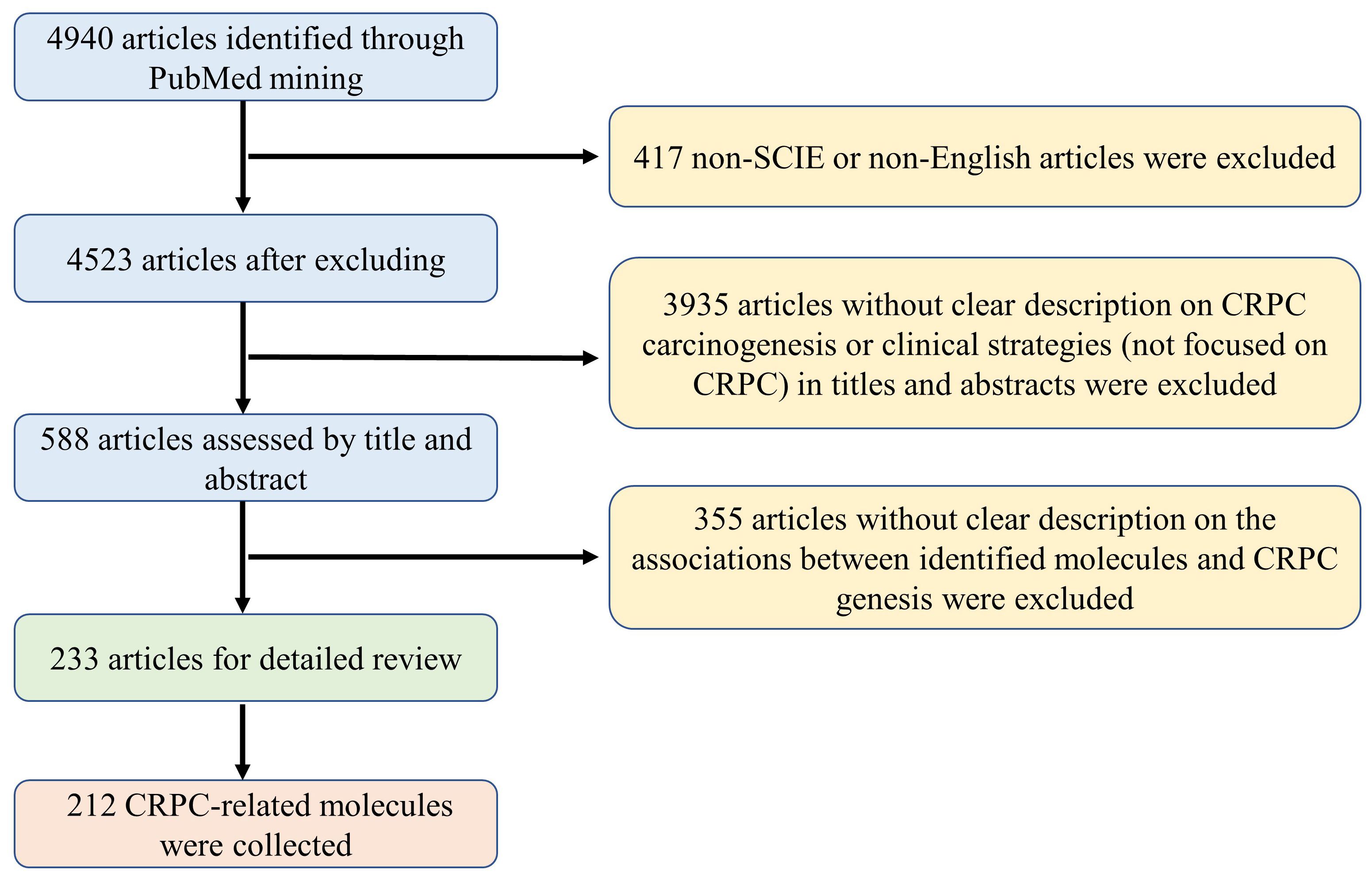
In this study, a systematic literature search was conducted to collect reported CRPC-associated molecules, e.g., genes, RNAs, proteins and metabolites etc., using the NCBI PubMed up to September 2023. The search formula was defined as “prostate cancer[tiab] AND [CRPC(tiab) OR castration-resistant (tiab)] AND [gene*(tiab) OR pathway*(tiab) OR signaling*(tiab)].” As shown in Figure 1, a total of 4940 articles were obtained from NCBI PubMed using the above search criteria. Among them, 417 articles that were not indexed in Science Citation Index Expanded or not written in English were excluded. After reviewing the titles and abstracts, 3935 articles that were not focused on CRPC studies, i.e., unrelated to the pathogenesis or clinical prevention and therapeutic strategies of CRPC, were excluded. Based on a detailed review of the remaining 588 articles, a total of 233 articles with clear description on the associations between identified molecules and CRPC genesis were included and analyzed from three perspectives: First, introducing the carcinogenesis and clinical strategies for CRPC prevention and treatment based both on androgen receptor (AR)-related and non-AR-based mechanisms. Then, conducting a comprehensive functional characterization from single molecules to integrated pathways at three aspects, i.e, drivers promoting CRPC occurrence and progression, suppressors inhibiting CRPC development, and biomarkers indicating the state transition into CRPC. Finally, discussing future directions for CRPC precision medicine and personalized therapy to indicate novel approaches and opportunities for data-driven translational CRPC studies.
CRPC carcinogenesis and clinical intervention strategies
AR-related mechanisms and therapeutic schemes
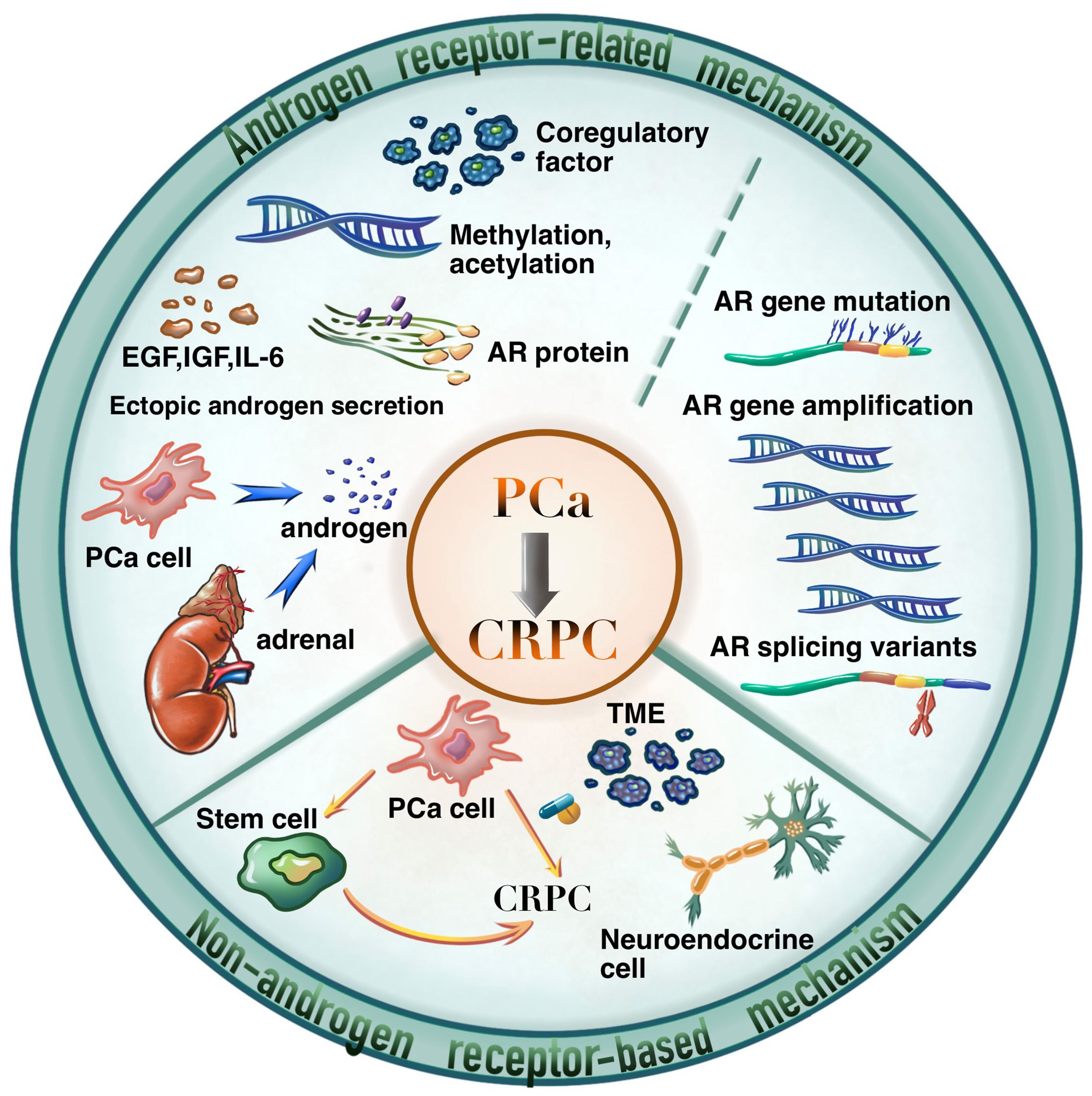
As shown in Figure 2, the carcinogenesis of CRPC could be divided into two primary aspects, i.e., the AR-related mechanisms, and the non-AR-based mechanisms. Among them, AR-related mechanisms have been widely concerned by researchers and clinical practitioners, including AR overexpression, mutations, and splice variants, abnormal AR transcription and modifications, AR-related alternative pathway activation, and abnormal androgen synthesis (5).
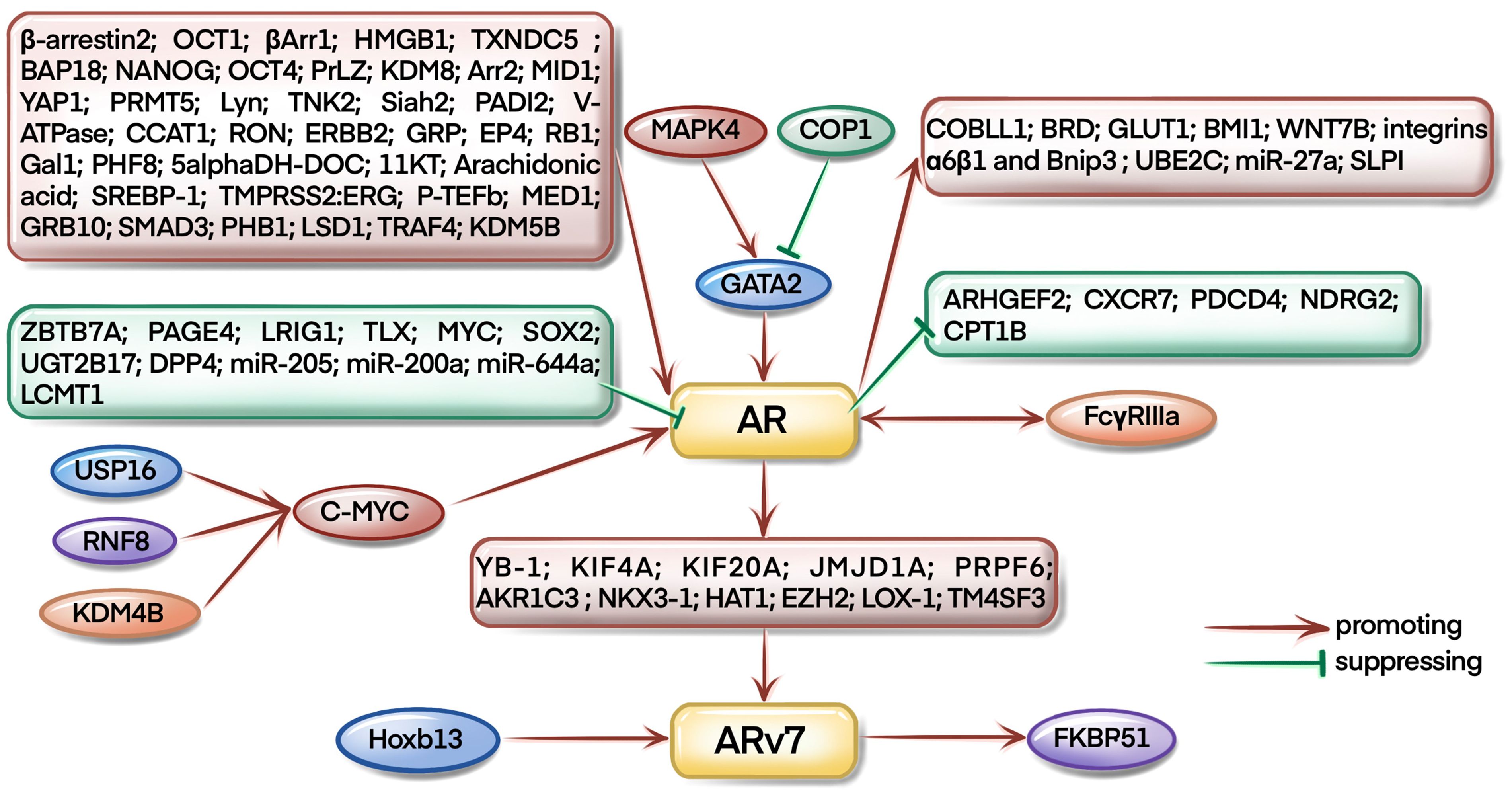
As a famous star in CRPC development, targeting AR signaling axis has already been the first-line approach for CRPC therapy. As illustrated in Figure 3, most of the studies focus on the upstream regulation of AR pathway during CRPC evolution. For example, USP16, KDM4B, and RNF8 could regulate AR signaling by mediating the expression of c-myc (6–8). Interestingly, Larsson et al. found that FcγRIIIa receptors could interact with AR receptors and affect the progression of CRPC in xenograft mouse models (9). COP1 promoted GATA2 degradation to inhibit AR expression and activation (10), however, Shen et al. found that MAPK4 activated GATA2 to regulate AR transcription in mice (11).
CRPC exhibits substantial heterogeneity in terms of its sensitivity to ADT, tissue histopathological types, and genetic profiles. In patients with metastatic CRPC (mCRPC), the occurrence rate of SPOP mutations is relatively low, however, patients carrying SPOP mutations have a relatively better prognosis and are more sensitive to treatment with novel anti-androgen drugs (12, 13). In addition, Taplin et al. found that the detection rate of ARv7 in mCRPC patients was less than 10% in a randomized trial (14), but ARv7 positive patients had poor response to treatment with novel anti-androgen drugs (15). Such variances directly impacted the responsiveness (or resistance) of patients with the same histopathological type to medications. In clinical practice, corresponding theoretical support for a uniform treatment approach to CRPC is still limited, making it challenging to achieve the desired therapeutic outcomes. Deeper exploration of the heterogeneity of CRPC among patients, identifying relevant molecular targets, understanding how these targets vary among different patient subgroups or racial populations, and how this affects treatment outcomes are significant for the personalized management of CRPC patients. With the rapid advance of sequencing techniques, the next-generation sequencing (NGS) is increasingly being widely utilized in clinical diagnosis and treatment. Therefore, it has become essential to analyze the mechanisms and pathological characteristics of CRPC, to categorize CRPC patients accordingly, and to develop personalized drug dosing plans to achieve optimal treatment outcomes.
Non-AR-based mechanisms for CRPC management
The understanding of non-AR-based mechanisms of CRPC opens new avenues for the development of novel therapies against resistance. As shown in Figure 2, the non-AR-based mechanisms are scattered but could be summarized as the following aspects according to recent literature reports.
Neuroendocrine cell-related mechanisms: Neuroendocrine CRPC could be induced by treatments such as ADT, radiotherapy, and chemotherapy, where neuroendocrine differentiation of PCa cells is the main driving force of disease development. Neuroendocrine CRPC exhibits resistance to hormone therapy with rapid progresses but does not reveal an elevation in PSA levels (16). Previous studies indicated that neuroendocrine cells were negative for PSA, and were more abundant in CRPC tumors (17). Moreover, neuroendocrine cells expressed IL-8, and CXCR2, and IL-8/CXCR2 had a significant role in benign and malignant neuroendocrine cells by interacting with p53 signaling (18). Li et al. demonstrated that CXCR2 expression could alter the phenotype of PCa cells, and the inhibition of CXCR2 expression in neuroendocrine PCa cells had the significance to re-sensitized enzalutamide-resistant PCa to enzalutamide (19).
Prostate stem cell-related mechanisms: It mainly includes the transformation of normal stem cells into malignant cells and the activation of tumor stem cells from differentiated tumor cells in response to external stimuli. Here a small subset of cells expressing CD44+/α2β1/CD133+ and lacking of AR expression are identified as prostate cancer stem cells (PCSC), and they hold the ability of proliferating even in androgen-depleted environments or under ADT (20). The research progress in targeted therapy for PCSC includes approaches targeting the prostate CSC microenvironment, targeted nanoparticles, and CAR-T cells targeting the CSC marker epithelial cell adhesion molecule (EpCAM), and some of them have already been entered into clinical trials (21, 22).
The molecular heterogeneity and variability of cellular populations within tumor microenvironment (TME): Chen et al. performed single-cell sequencing and discovered the activated endothelial cells, KLK3-high T-cell clusters, and KLK3-positive T cells in TME for CRPC progression to elucidate the significant variability presented in PCa and offered insights for pinpointing therapeutic targets and developing robust tumor biomarkers (23). In recent years, cancer immunotherapy has garnered increasing attention in cancer therapeutics. Some small-molecule tyrosine kinase inhibitors, whether used as single agents or in combination with other immunotherapies, may potentially improve clinical outcomes (24).
Deregulations in pathways including PI3K-Akt-mTOR, Wnt, Hippo, Hedgehog, and Notch etc: The PI3K-Akt-mTOR signaling pathway played a crucial role in regulating cell survival, proliferation, differentiation, and angiogenesis. It is recognized as one of the important pathway implicated in driving the progression of CRPC (25). In a randomized study conducted on mCRPC patients who had undergone prior docetaxel chemotherapy, the combination of the Akt inhibitor Ipatasertib with abiraterone was compared to abiraterone alone. It was observed that patients with PTEN loss derived a radiographic progression-free survival (rPFS) benefit from varying doses of Ipatasertib in conjunction with abiraterone (26). Robinson et al. identified abnormalities within the Wnt pathway in 18% of mCRPC patients. These abnormalities encompassed periodic alterations in adenomatous polyposis coli, β-catenin, and R-spondins within the pathway, implying a potential pivotal role of this pathway in CRPC progression (27). Currently, small molecule drugs and biological agents directed at the Wnt pathway remain in early stages of research, thus further exploration of the potential anti-tumor mechanisms induced by Wnt pathway inhibition needs to be conducted.
Mutations in the genetic architecture: In addition to epigenetic changes, molecular mutations in specific genes were found to be associated with the prognosis of patients with CRPC, which could guide clinical treatment for patients. In CRPC, inherited or systemic mutations, particularly alterations in the BRCA1 and BRCA2 genes, were linked to an unfavorable prognosis (28). In patients with metastatic hormone-sensitive PCa and mCRPC, TP53 mutations (32%) and PTEN mutations or copy number variations (20%), along with RB1 copy number variations (6%), were commonly observed (29). These genetic alterations were significantly correlated with increased tumor burden and a less favorable clinical prognosis (30, 31).
Functional classification of CRPC-related signatures: from single molecules to integrated pathways
Drivers promoting CRPC occurrence and progression
Genes positively associated with CRPC progression
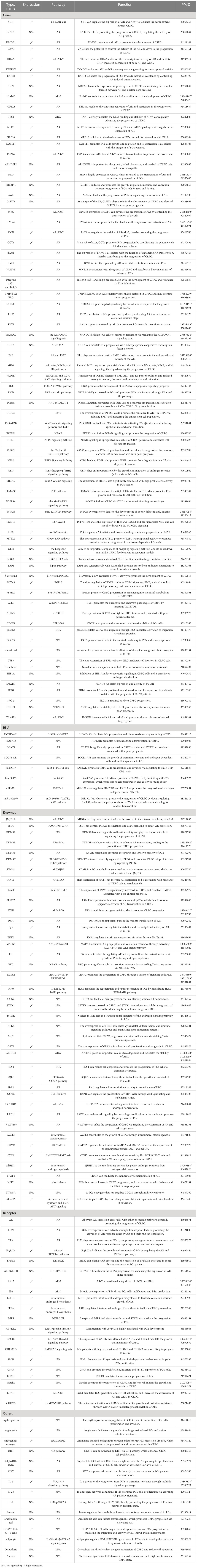
Mutation and abnormal expression of genes enriched in AR regulation play a pivotal role in CRPC progression. As shown in Table 1, more than half of the retrieved genes were involved in the regulation of AR signaling. For example, YB-1, KIF4A, KIF20A and PRPF6 have been found to regulate AR and ARv7 transcription and splicing (32–35). The progression of CRPC is a process of cross-talk among multiple signaling pathways, and some genes have been shown to regulate multiple signaling pathways. For example, Choi et al. found that the knockdown of ISL1 inhibited AR signaling and AKT/NF-κB signaling and promoted enzalutamide resistance in CRPC through epithelial to mesenchymal transition (36). PROS, PKIB and PCDH7 regulated the progression of CRPC by mediating the PI3K/AKT signaling pathway (37). In addition to mRNA transcript changes, the alternations in protein abundance, e.g., TXNDC5, SREBP-1, OCT1, β-arrestin2, and p66Shc, would also contribute to the development of CRPC (38–41). It should be noticed that AR mutations are seldom occurred in the early stages of PCa, whereas aberrant AR signal transduction and alterations in AR-related pathways are prevalently observed in advanced PCa (42). Thus, early detection of AR-related molecular alterations could offer insightful opportunities for CRPC precision diagnosis and prevention.
RNAs involved in promoting the development of CRPC
As shown in Table 1, numerous studies demonstrated the role of RNAs in CRPC development. For instance, certain specific RNA molecules could modulate the proliferation and invasive capabilities of CRPC cells, consequently influencing tumor progression. In addition, RNA could serve as a molecular marker to predict the occurrence and prognosis of CRPC. In particular, several studies showed that the elevated expression of long non-coding RNAs (lncRNAs) in CRPC was related to the degree of malignancy and drug resistance of tumors. For example, HOXD-AS1 facilitated PCa progression and chemo-resistance by recruiting WDR5 (43). HOTAIR promoted neuroendocrine differentiation in CRPC (44). CCAT1 was an oncogenic factor for CRPC progression and was highly up-regulated in CRPC, and elevated CCAT1 was associated with poor prognosis (45). SOCS2-AS1 promoted the growth of castration-resistant and androgen-dependent cells and inhibited apoptosis in PCa (46). In addition, microRNAs (miRNAs) also play an important roles in CRPC, such as miR-221 and miR-302/367, and they promoted the development of CRPC by inhibiting the expression of targeted anti-tumor proteins (47, 48).
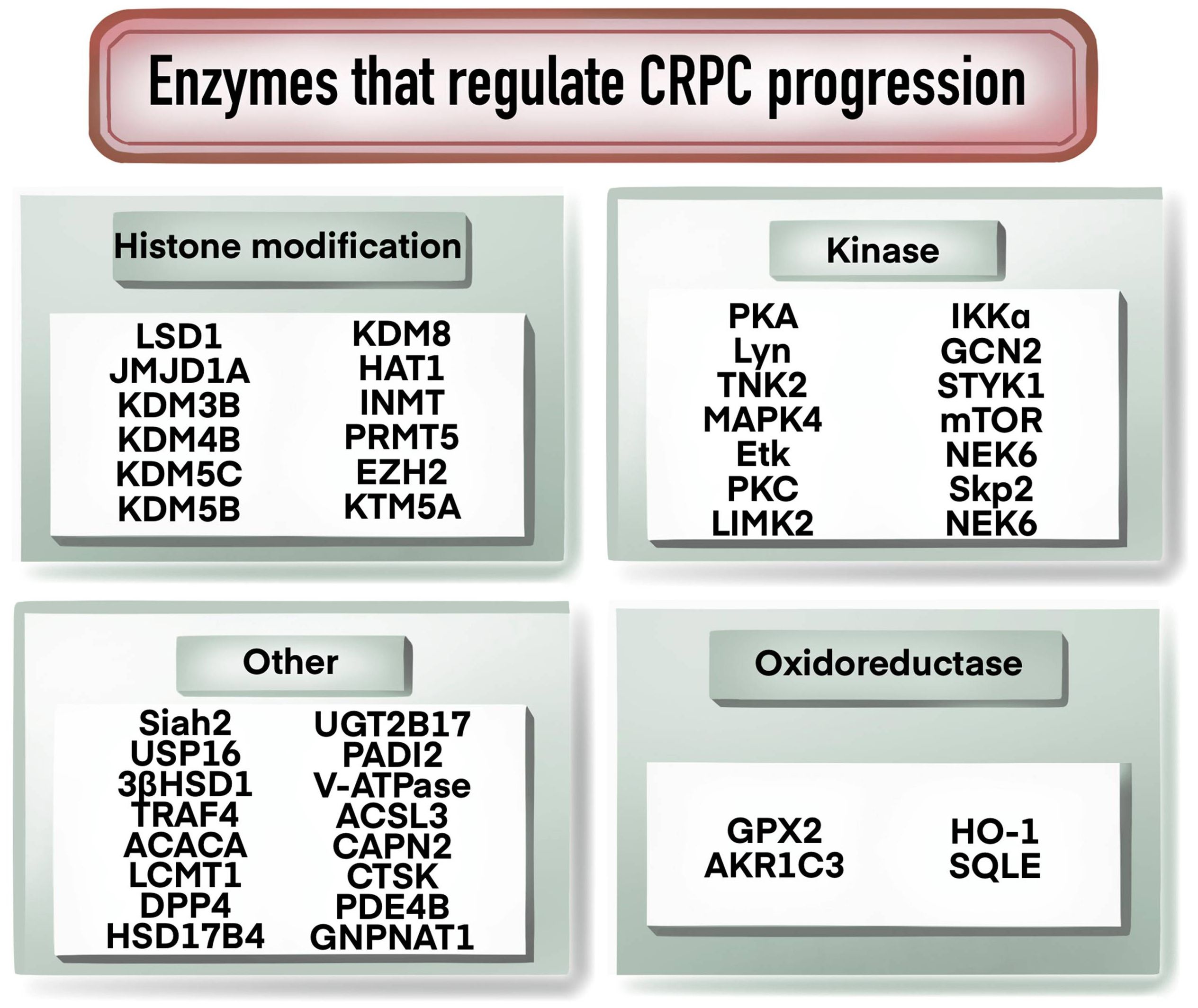
Enzymes that regulate CRPC progression
As shown in Figure 4, enzymes play an important role in the progression of CRPC, and many studies have been conducted on enzymes related to histone modification. For example, histone demethylases LSD1, JMJD1A, KDM3B, KDM4B, KDM5B and KDM5C were found to be involved in the regulation of AR, c-Myc or PTEN signaling pathways to affect the development of CRPC (49–54). KDM8 could double activate AR and JMJD5, participating in the regulation of androgen response and the regulation of PCa metabolism genes (55). Over-expression of HAT1 increased AR expression and was associated with the resistance of CRPC cells to enzalutamide (56). Methylation-modifying enzymes, kinases and oxidorereductases also played significant functions in CRPC development and progression. For example, INMT promoted the production or release of methylation of anticancer metabolites, and PRMT5 and EZH2 regulated the transcription of AR through methylation (57–59). Kinases such as PKA, Lyn, TNK2, MAPK4 and Etk are involved in CRPC progression by regulating AR signaling pathway (11, 60–62). PKC, LIMK2, IKKα, and GCN2 have been reported to be involved in CRPC regulation through various mechanisms, as listed in Table 1. Oxidoreductase such as GPX2, AKR1C3, HO-1 and SQLE have been reported to be elevated in CRPC and to contribute to CRPC progression and prognosis (63–65). Ubiquitination is a regulator in CRPC progression. For example, Siah2 regulated the transcriptional activity of AR, and USP16 promoted CRPC proliferation through deubiquitination and stabilization of c-Myc (6, 66). Other enzymes such as UGT2B17, PADI2 and V-ATPase could facilitate CRPC progression by regulating AR signaling (67–69). ACSL3 contributed to the growth of CRPC through intratumoral steroidogenesis (70). CTSK promoted the tumor growth and metastasis by IL-17/CTSK/EMT axis and mediates M2 macrophage polarization in CRPC (71).
Receptor molecules involved in CRPC progression
AR is reported to be functional in CRPC progression by mediating the effects of androgens. As shown in Table 1, it is widely acknowledged that resistance to ADT is often a result from aberrations within the AR signaling, such as mutations in AR gene or heightened expression of the AR protein. Accumulating evidence confirmed that the aberrant cross-talk between AR expression and other oncogenic pathways could promote CRPC progression (72). Some receptor molecules regulate the progression of CRPC by regulating AR directly or indirectly. For example, the overexpression of RON could activate multiple transcription factors, and it promoted AR activation of AR response genes and nuclear localization (73). FcγRIIIa facilitated the growth and metastasis of PCa by regulating the AR and PIP5K1α pathways (9). TLX plays an oncogenic role in prostate carcinogenesis by suppressing oncogene-induced senescence, and it could confer resistance to androgen deprivation and anti-androgen (74). ErbB2 stabilized AR protein, and the expression of ERBB2 was increased in some abiraterone-resistant PCa patients (75). ARv7 is considered as a key driver of ENZR in CRPC (76). Ectopic overexpression of EP4 drived PCa cells proliferation and PSA production via regulating ARv7 signaling pathway (77). Ubiquitination is an intracellular protein regulatory mechanism, which is closely related to the occurrence and progression of CRPC. It has been reported that the high expression of the ubiquitination modifying enzyme Siah2 could promote the transcriptional activity of AR and deubiquitinate the enzyme USP16 could regulate the proliferation of CRPC cells through deubiquitinating and stabilizing c-Myc. Other receptors that have been implicated in CRPC include cell surface molecular receptor and tumor immunotherapy receptor. LRH-1 and ERRα facilitate CRPC progression via promoting intratumoral androgen biosynthesis (78, 79). Interplay among EGFR and signal transducer and STAT3 could mediate the progression of PCa (80). Co-expression of AVPR1A with AVPR2 was highly correlated with the development of PCa (81). The expression of CXCR7 was elevated after ADT, and it could facilitate the growth and metastasis of CRPC via MIF/CXCR7/AKT signaling pathway (82). PCa patients with high expression of CHRM1 and CHRM3 were more likely to progress to CRPC (83). The expression of FGFR1 and Notch1 were all elevated in CRPC and they regulated the proliferation and progression of CRPC through different mechanisms (84, 85).
Other molecules
The transition from HSPC to the castration-resistant stage is also encompassed by hormones, cytokines, and cellular components. As described in Table 1, androgens are signaling molecules that are necessary for the growth and maintenance of PCa cell survival. 5alphaDH-DOC within CRPC tissues might activate the AR pathway for proliferation and survival of CRPC cells under an extremely low level of DHT (86). 11KT is a potent AR agonist and is the major active androgen in PCa patients after castration (87). Other hormones are also functional in PCa progression, tumor growth, and invasion (88–90). Cytokines are a class of secreted proteins or molecules that can regulate and influence cell-to-cell interactions and communication. In the context of CRPC, cytokines and factors mediating the interaction between tumor cells and immune cells to promote the proliferation, invasion, and metastasis of PCa cells. As stated in Table 1, IL-6 promoted the progression from PCa to castration resistance through multiple signaling pathways (91). In androgen-deprived conditions, IL-23 promoted PCa cell proliferation by activating the AR pathway signaling (92). Recent studies suggested a close relationship between abnormal fatty acid metabolism and CRPC progression. Lactate regulated the metabolic-epigenetic axis to foster metastatic potential in PCa (93). Some cells have also been reported to be functional in the advancement of CRPC. CD4lowHLA-G+ T cells may drive androgen-independent PCa progression by mediating the migration and activity of CD11blowF4/80hi macrophages (94). Platelets could synthesize testosterone in a novel mechanism, and might sustain CRPC state (95).
Suppressors inhibiting CRPC evolution
Genes that inhibit the growth of CRPC
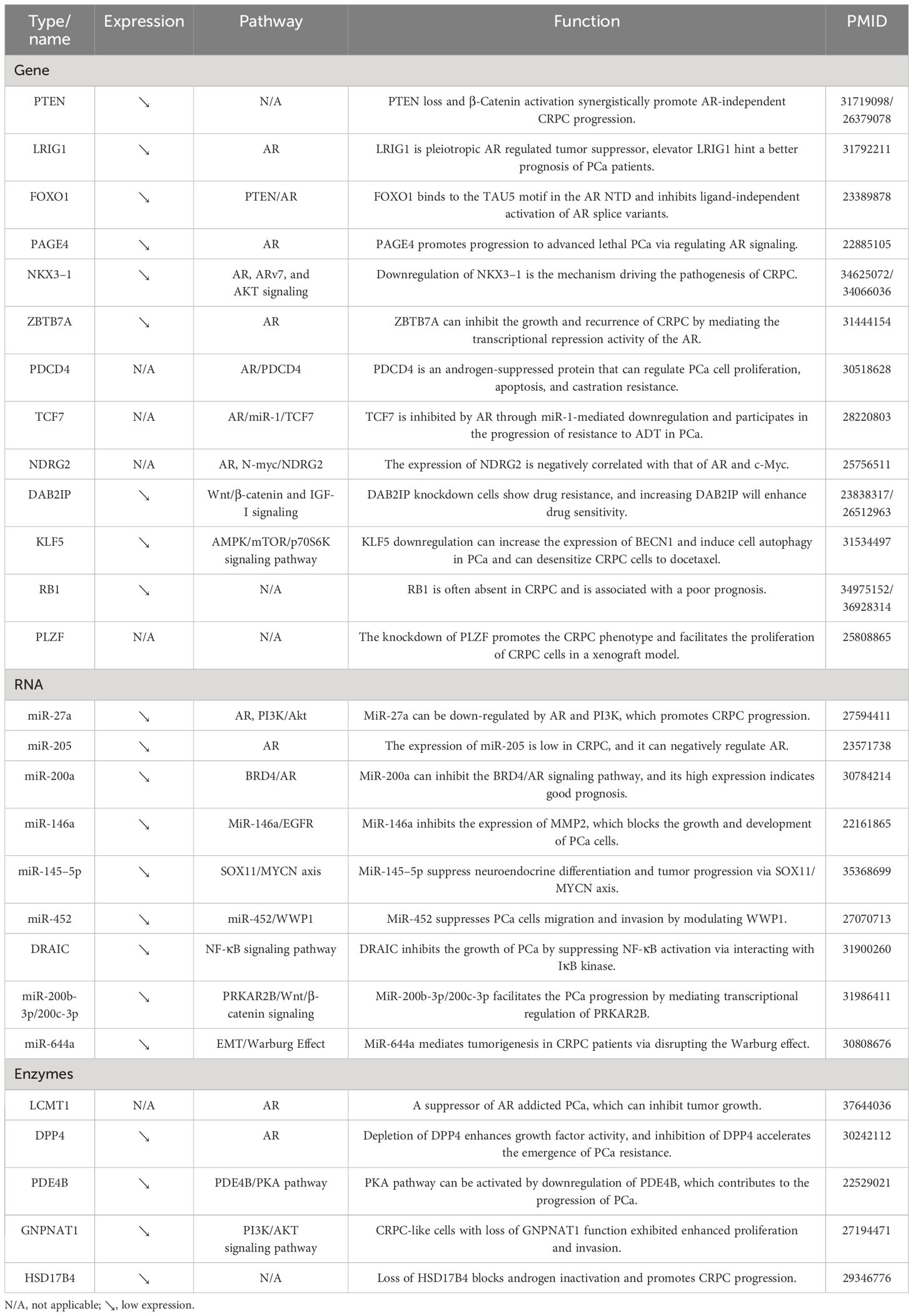
As illustrated in Table 2, several genes were found to be negatively associated with CRPC progression. For example, the expression of RB1 was negatively correlated with the prognosis of CRPC patients (96). In addition, the knockdown of PLZF promoted the CRPC phenotype and facilitated the proliferation of CRPC cells in a xenograft model (97). On the other hand, several genes, i.e., PTEN, LRIG1, PAGE4, NKX3–1, ZBTB7A, and PDCD4, could regulate the AR signaling pathway through various ways to inhibit the progression of CRPC (98–103). Furthermore, DAB2IP knockdown cells showed drug resistance, and increasing DAB2IP enhanced drug sensitivity. Besides, a study also found that it could regulate the Wnt/β-catenin and IGF-I signaling pathways (104). KLF5 downregulation increased the expression of BECN1 and induced cell autophagy in PCa. It could also desensitize CRPC cells to docetaxel through the AMPK/mTOR/p70S6K signaling pathway (105).
RNAs involved in inhibiting CRPC progression
MicroRNAs (miRNAs) are small non-coding RNA molecules that can regulate gene expression post-transcriptionally by binding to target mRNAs and inhibiting their translation or promoting their degradation. As shown in Table 2, many studies have focused on the correlation between miRNA and PCa progression. The AR signaling pathway represents the classical route of progression in CRPC. In this study, miR-205, and miR-200a were found to regulate AR signaling through different pathways to inhibit the progression of CRPC. Among them, the high expression of miR-200a indicated good prognosis (106, 107). In addition, miR-452 suppressed PCa cells migration and invasion by modulating WWP1 (108). MiR-200b-3p/200c-3p inhibited the PCa progression by mediating transcriptional regulation of PRKAR2B (109). MiR-644a mediated tumorigenesis in CRPC patients via disrupting the Warburg effect (110). In addition to miRNAs, lncRNAs are also a class of non-coding RNA molecules in CRPC development. For example, DRAIC could inhibit the growth of PCa by suppressing NF-κB activation via interacting with IκB kinase (111).
Enzymes that inhibit the development and progression of CRPC
As shown in Table 2, there is a paucity of studies investigating the inhibitory effects of enzymes on CRPC occurrence and progression. Rasool et al. discovered and demonstrated in murine models that the loss of heterologous LCMT1, along with biased protein phosphatase 2A activity, drived the progression of PCa and confers resistance to treatment (112). Depletion of DPP4 enhanced growth factor activity, and inhibition of DPP4 accelerated the emergence of PCa resistance. Kashiwagi et al. discovered that depletion of DPP4 augments growth factor activity, while inhibition of DPP4 expedited the emergence of PCa resistance (113). The study conducted by Ko et al. revealed that the emergence of CRPC was facilitated by the loss of a specific splice form of HSD17B4, which was responsible for inactivating androgen hormones (114). In addition, CRPC-like cells with loss of GNPNAT1 function exhibited augmented proliferation and invasion (115).
Biomarkers indicating the state transition into CRPC
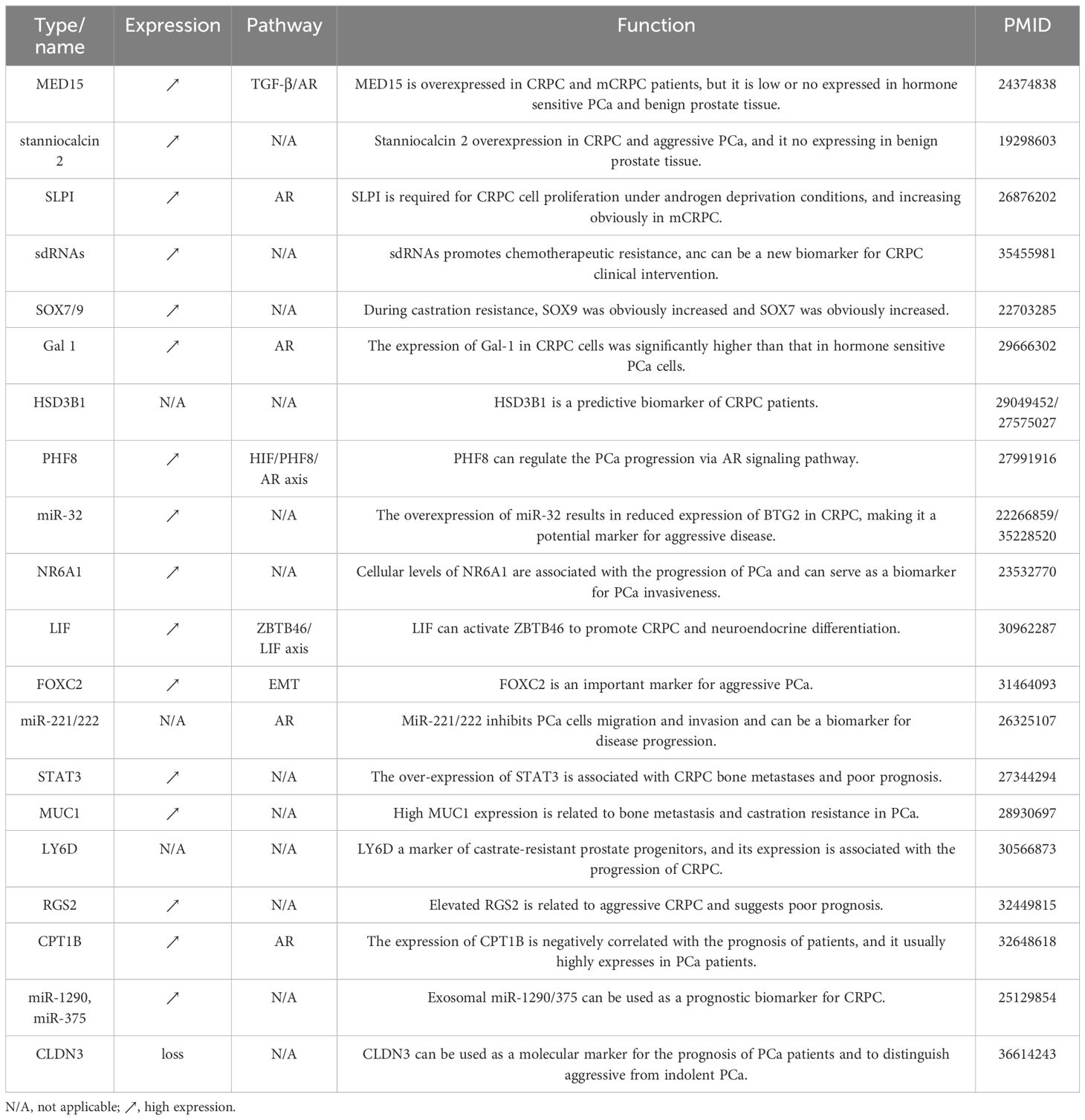
Biomarkers are important predictors indicating the state change for CRPC management. Previous studies identified plenty of potential biomarkers that may help the early detection, prognosis, and treatment response prediction of CRPC patients. As listed in Table 3, HSD3B1 is a biomarker that enhanced dihydrotestosterone synthesis from extra-gonadal precursors and has been shown to predict castration resistance in PCa in two retrospective studies (116, 117). PHF8 could promote CRPC progression through the HIF/PHF8/AR axis (118). The expression of Gal-1 in CRPC cells was significantly higher than that in hormone-sensitive PCa cells (119). MiR-32, FOXC2, and miRNA-221/222 have been found to be potential biomarkers for the progression and malignant invasion of CRPC (120–123). LIF was associated with CRPC neuroendocrine and could be used as a serum biomarker for the diagnosis of advanced PCa (124).
Bone metastasis has a negative effect on patient quality of life and contributes to fatal outcomes. Hence, timely intervention holds immense significance. As shown in Table 3, studies have shown that STAT3 and MUC1 were closely related to bone metastasis in CRPC patients (125, 126). The expressions of LY6D, RGS2, CPT1B, miR-1290, and miR-375 were related to the prognosis of CRPC patients and could be used as potential indicators for predicting prognosis (127–129). In addition, MED15 and stanniocalcin 2 were found to be overexpressed in CRPC and aggressive PCa, while they were expressed at lower levels in benign prostate tissue (130, 131). Furthermore, SLPI was a potential biomarker in the cell proliferation of CRPC under androgen deprivation conditions and its levels were observed to be increased significantly in mCRPC (132). SOX7 and SOX9 belong to the same SOX gene family, however, during castration resistance, SOX9 was found to be significantly increased, while SOX7 was observed to decrease significantly (133).
The identification of biomarkers holds both theoretical and clinical significance for CRPC risk prediction and personalized therapy. For example, the integration of biomarkers including HSD3B1, PHF8, Gal-1, and the SOX gene family facilitated the construction of computational models for CRPC early diagnosis (116, 119). The utilization of factors including LIF, NR6A1, miR-32, FOXC2, and miRNA-221/222 could improve the stratification of patients for applying personalized clinical therapeutics (122–124). Moreover, STAT3, and MUC1 indicated the possibility of bone metastasis, which would be helpful of monitoring the prognosis of CRPC patients into metastatic status (125, 126).
Translational perspectives toward CRPC holistic healthcare
Perspective 1: improving both AR and non-AR-targeted precision molecular therapy
AR plays a pivotal role in PCa, particularly in cases of clinical CRPC. In PCa cell models, AR overexpression has been frequently observed and established as a primary driving factor for PCa progression (134). Over the past few decades, numerous anti-AR drugs have been developed and approved for use across different stages of PCa. In the 1980s and 1990s, the FDA approved the first-generation AR antagonists, including flutamide, nilutamide, and bicalutamide, which had efficacy in the early stages of the disease but ultimately led to the development of resistance and progression to CRPC. With the in-depth research into the AR, second-generation AR antagonists that target the ligand-binding domain (LBD), such as Enzalutamide, Apalutamide, and Darolutamide, have been developed and applied. These agents possess higher AR binding affinity, allowing for more effective suppression of AR expression, and have led to significant improvements in patient survival rates (135–137). However, with the rapid development of resistance, these drugs only provide short-term effects and may potentially give rise to central nervous system toxicity and cardiovascular toxicity (138, 139). It is acknowledged that PCa demonstrates significant inter- and intra-tumor heterogeneity. Targeting a single molecule (e.g., AR) does not benefit all patients, and does not affect all tumor cells equally. Recent studies indicated that many non-AR-based mechanisms were involved in CRPC development, including neuroendocrine cell-related mechanisms, prostate stem cell-related mechanisms, alternations in TME, and deregulations in non-AR genes and pathways. Understanding the role of non-AR-based mechanisms in the development of castration resistance in PCa is also important for identifying new therapeutic targets or strategies against castration resistance. Moreover, as shown in Figure 5 the integration of both AR and non-AR strategies, e.g., inhibiting of AR and neuroendocrine cell-expressed CXCR2 simultaneously, may achieve a better therapeutic effect on CRPC (19).
Perspective 2: identifying molecular mechanisms based on novel programmed cell death types for CRPC personalized medicine
Tumor cells demonstrate the ability to evade apoptosis, which is an important cause of drug resistance and recurrence in cancer therapy. In recent years, novel regulated cell death pathways such as ferroptosis and pyroptosis have gained increasing attention as representatives in cancer drug discovery and application (140). In PCa studies, Wang et al. triggered ferroptosis in mice using a stable GPX4 inhibitor in a genetically engineered model, and it inhibited the growth and spread of RB-deficient PCa tumors. This finding offered promising prospects for the treatment of RB1-deficient malignant PCa (141). Wu et al. confirmed that inhibiting CDC20 could promote pyroptosis in PCa cells and boost tumor immunity in a mouse model of PCa (142). Wang et al. synthesized a series of aggregation-induced emission materials to mediate the process of ferroptosis and pyroptosis for enhancing PCa immunotherapy (143, 144). As shown in Figure 5, the identification and application of novel programmed cell death-based approaches would be an emerging direction for CRPC treatment, especially for patients with failure under traditional CRPC therapeutics.
Perspective 3: integrating multi-omics data and artificial intelligence for CRPC systems modeling and clinical application
Identifying molecular targets and understanding how these targets vary among different patient subgroups or racial groups and how this affects treatment outcomes are of clinical interest for CRPC personalized management. It is reported that there was a higher similarity at pathway level than that at single gene level in the expression of genes across different PCa datasets, which could partly explain why the single-gene based approaches cannot benefit all patient cohorts and indicate the significance for the development of network medicine-based strategies to fight against therapeutic heterogeneity in cancers (145). In the era of big data and artificial intelligence (AI), computer-aided modeling has now become an emerging approach for translational cancer researches. Compared with traditional experimental methods, computational algorithms simulate the diversity and dynamicity of disease occurrence and progression under a systems biology framework, which would promote the identification and characterization of key signatures for disease early diagnosis and personalized therapy (146, 147). The development of CRPC is a heterogenous process in which genetic, epigenetic, and environmental factors generate large-scale biological networks and contribute to the complexity in PCa phenotype from androgen dependence to castration resistance, thus it is of great significance to integrate multi-omics molecular data with image and clinical information as prior knowledge for multi-step AI model training (148). Here the AI models could be simply divided as two sub-categories based on the methods of feature selection, i.e., traditional models that manually characterize features for training, and deep learning-based techniques automatically extracting features for optimization. The typical applications of AI models for PCa studies are pathological evaluation and classification of multiple PCa status such as benign and malignant lesion identification, PCa grading and molecular subtyping, prognosis and risk stratification, prediction of time to CRPC (149–151), etc. Although there is a promising perspective of AI modeling in PCa and CRPC, the limitations and challenges are still worthy to be concerned. First, the clinical data have the characteristics of small sample size but high dimension and heterogeneity, hence how to reduce the overfitting results and address difficulties in model generalization are the leading issues to be considered. Second, the quality of datasets will directly affect the accuracy of model output. Currently, there is still a lack of in-depth research on clinical data standardization and privacy protection. Construction of PCa-related ontologies would be a possible and feasible way to provide a systematical framework for decoding the large amounts of PCa data and knowledge, and this will contribute to the development of data sharing and integration for model analyses (152). Finally, the interpretability of AI needs to be improved continually, and clinical urologists and pathologists should strengthen their professional behaviors to avoid the biases of missed diagnosis caused by AI models (153).
Conclusions
Although there has been a notable advancement in the field of CRPC research, the current clinical management of CRPC remains a challenge. The emergence of CRPC tumors is predominantly propelled by genetic and molecular events. For instance, accumulating evidence confirmed the role of AR signaling in the progression of PCa to castration resistance. However, the evolution of CRPC is a complex and dynamic process, and AR signaling is not the only clue for CRPC understanding. Hence, it is urgently needed for further elucidating the pathogenesis of CRPC by integrating molecular signatures at muti-omics levels. This review provides an updated landscape of literature-reported molecules for CRPC, which may offer novel insights and targets for translational CRPC research to facilitate the early diagnosis and personalized therapeutics of CRPC.
Author contributions
JJ: Writing – review & editing, Writing – original draft, Validation, Formal analysis, Data curation. XW: Writing – review & editing, Writing – original draft, Validation, Formal analysis, Data curation. JZ: Writing – original draft, Formal analysis, Data curation. CZ: Writing – original draft, Formal analysis. XH: Writing – original draft, Formal analysis. YH: Writing – review & editing, Validation, Funding acquisition. JH: Writing – review & editing, Validation, Supervision, Conceptualization. YL: Writing – review & editing, Writing – original draft, Validation, Supervision, Funding acquisition, Conceptualization. XW: Writing – review & editing, Writing – original draft, Validation, Supervision, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (grant number 32200533), the General Program of Jiangsu Health Commission (grant number H2019040), and the Suzhou Science and Technology Plan Project (grant number SLJ2022008).
Acknowledgments
The authors gratefully thank the academic editor and reviewers for their constructive suggestions to help improve this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA A Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. (2017) 71:630–42. doi: 10.1016/j.eururo.2016.08.002
3. Armstrong AJ, Garrett-Mayer E, De Wit R, Tannock I, Eisenberger M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. (2010) 16:203–11. doi: 10.1158/1078–0432.CCR-09–2514
4. Lozano R, Castro E, Aragón IM, Cendón Y, Cattrini C, López-Casas PP, et al. Genetic aberrations in DNA repair pathways: a cornerstone of precision oncology in prostate cancer. Br J Cancer. (2021) 124:552–63. doi: 10.1038/s41416-020-01114-x
5. Jamroze A, Chatta G, Tang DG. Androgen receptor (AR) heterogeneity in prostate cancer and therapy resistance. Cancer Lett. (2021) 518:1–9. doi: 10.1016/j.canlet.2021.06.006
6. Ge J, Yu W, Li J, Ma H, Wang P, Zhou Y, et al. USP16 regulates castration-resistant prostate cancer cell proliferation by deubiquitinating and stabilizing c-Myc. J Exp Clin Cancer Res. (2021) 40:59. doi: 10.1186/s13046–021-01843–8
7. Wu M-J, Chen C-J, Lin T-Y, Liu Y-Y, Tseng L-L, Cheng M-L, et al. Targeting KDM4B that coactivates c-Myc-regulated metabolism to suppress tumor growth in castration-resistant prostate cancer. Theranostics. (2021) 11:7779–96. doi: 10.7150/thno.58729
8. Zhou T, Wang S, Song X, Liu W, Dong F, Huo Y, et al. RNF8 up-regulates AR/ARV7 action to contribute to advanced prostate cancer progression. Cell Death Dis. (2022) 13:352. doi: 10.1038/s41419–022-04787–9
9. Larsson PF, Karlsson R, Sarwar M, Miftakhova R, Wang T, Syed Khaja AS, et al. FcγRIIIa receptor interacts with androgen receptor and PIP5K1α to promote growth and metastasis of prostate cancer. Mol Oncol. (2022) 16:2496–517. doi: 10.1002/1878–0261.13166
10. Shen T, Dong B, Meng Y, Moore DD, Yang F. A COP1-GATA2 axis suppresses AR signaling and prostate cancer. Proc Natl Acad Sci U.S.A. (2022) 119:e2205350119. doi: 10.1073/pnas.2205350119
11. Shen T, Wang W, Zhou W, Coleman I, Cai Q, Dong B, et al. MAPK4 promotes prostate cancer by concerted activation of androgen receptor and AKT. J Clin Invest. (2021) 131:e135465. doi: 10.1172/JCI135465
12. Swami U, Isaacsson Velho P, Nussenzveig R, Chipman J, Sacristan Santos V, Erickson S, et al. Association of SPOP mutations with outcomes in men with de novo metastatic castration-sensitive prostate cancer. Eur Urol. (2020) 78:652–6. doi: 10.1016/j.eururo.2020.06.033
13. Boysen G, Rodrigues DN, Rescigno P, Seed G, Dolling D, Riisnaes R, et al. SPOP-mutated/CHD1-deleted lethal prostate cancer and abiraterone sensitivity. Clin Cancer Res. (2018) 24:5585–93. doi: 10.1158/1078–0432.CCR-18–0937
14. Taplin M-E, Antonarakis ES, Ferrante KJ, Horgan K, Blumenstein B, Saad F, et al. Androgen receptor modulation optimized for response—Splice variant: A phase 3, randomized trial of galeterone versus enzalutamide in androgen receptor splice variant-7–expressing metastatic castration-resistant prostate cancer. Eur Urol. (2019) 76:843–51. doi: 10.1016/j.eururo.2019.08.034
15. Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. (2014) 371:1028–38. doi: 10.1056/NEJMoa1315815
16. Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. (2016) 22:298–305. doi: 10.1038/nm.4045
17. Huang J, Yao JL, di Sant’Agnese PA, Yang Q, Bourne PA, Na Y. Immunohistochemical characterization of neuroendocrine cells in prostate cancer. Prostate. (2006) 66:1399–406. doi: 10.1002/pros.20434
18. Chen H, Sun Y, Wu C, Magyar CE, Li X, Cheng L, et al. Pathogenesis of prostatic small cell carcinoma involves the inactivation of the P53 pathway. Endocrine-Related Cancer. (2012) 19:321–31. doi: 10.1530/ERC-11–0368
19. Li Y, He Y, Butler W, Xu L, Chang Y, Lei K, et al. Targeting cellular heterogeneity with CXCR2 blockade for the treatment of therapy-resistant prostate cancer. Sci Trans Med. (2019) 11:eaax0428. doi: 10.1126/scitranslmed.aax0428
20. Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. (2005) 65:10946–51. doi: 10.1158/0008–5472.CAN-05–2018
21. Qin D, Li D, Zhang B, Chen Y, Liao X, Li X, et al. Potential lung attack and lethality generated by EpCAM-specific CAR-T cells in immunocompetent mouse models. OncoImmunology. (2020) 9:1806009. doi: 10.1080/2162402X.2020.1806009
22. Huang W-Y, Lin J-N, Hsieh J-T, Chou S-C, Lai C-H, Yun E-J, et al. Nanoparticle targeting CD44-positive cancer cells for site-specific drug delivery in prostate cancer therapy. ACS Appl Mater Interfaces. (2016) 8:30722–34. doi: 10.1021/acsami.6b10029
23. Chen S, Zhu G, Yang Y, Wang F, Xiao Y-T, Zhang N, et al. Single-cell analysis reveals transcriptomic remodelings in distinct cell types that contribute to human prostate cancer progression. Nat Cell Biol. (2021) 23:87–98. doi: 10.1038/s41556–020-00613–6
24. Sridaran D, Bradshaw E, DeSelm C, Pachynski R, Mahajan K, Mahajan NP. Prostate cancer immunotherapy: Improving clinical outcomes with a multi-pronged approach. CR Med. (2023) 4:101199. doi: 10.1016/j.xcrm.2023.101199
25. Tortorella E, Giantulli S, Sciarra A, Silvestri I. AR and PI3K/AKT in prostate cancer: A tale of two interconnected pathways. IJMS. (2023) 24:2046. doi: 10.3390/ijms24032046
26. De Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, Font A, et al. Randomized phase II study evaluating akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin Cancer Res. (2019) 25:928–36. doi: 10.1158/1078–0432.CCR-18–0981
27. Robinson D, Van Allen EM, Wu Y-M, Schultz N, Lonigro RJ, Mosquera J-M, et al. Integrative clinical genomics of advanced prostate cancer. Cell. (2015) 161:1215–28. doi: 10.1016/j.cell.2015.05.001
28. Castro E, Romero-Laorden N, Del Pozo A, Lozano R, Medina A, Puente J, et al. PROREPAIR-B: A prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. JCO. (2019) 37:490–503. doi: 10.1200/JCO.18.00358
29. Hamid AA, Gray KP, Shaw G, MacConaill LE, Evan C, Bernard B, et al. Compound genomic alterations of TP53, PTEN, and RB1 tumor suppressors in localized and metastatic prostate cancer. Eur Urol. (2019) 76:89–97. doi: 10.1016/j.eururo.2018.11.045
30. Liu Z, Guo H, Zhu Y, Xia Y, Cui J, Shi K, et al. TP53 alterations of hormone-naïve prostate cancer in the Chinese population. Prostate Cancer Prostatic Dis. (2021) 24:482–91. doi: 10.1038/s41391–020-00302–3
31. Van der Eecken K, Vanwelkenhuyzen J, Deek MP, Tran PT, Warner E, Wyatt AW, et al. Tissue- and blood-derived genomic biomarkers for metastatic hormone-sensitive prostate cancer: A systematic review. Eur Urol Oncol. (2021) 4:914–23. doi: 10.1016/j.euo.2021.10.005
32. Shiota M, Sekino Y, Tsukahara S, Abe T, Kinoshita F, Imada K, et al. Gene amplification of YB-1 in castration-resistant prostate cancer in association with aberrant androgen receptor expression. Cancer Sci. (2021) 112:323–30. doi: 10.1111/cas.14695
33. Cao Q, Song Z, Ruan H, Wang C, Yang X, Bao L, et al. Targeting the KIF4A/AR axis to reverse endocrine therapy resistance in castration-resistant prostate cancer. Clin Cancer Res. (2020) 26:1516–28. doi: 10.1158/1078–0432.CCR-19–0396
34. Copello VA, Burnstein KL. The kinesin KIF20A promotes progression to castration-resistant prostate cancer through autocrine activation of the androgen receptor. Oncogene. (2022) 41:2824–32. doi: 10.1038/s41388–022-02307–9
35. Liu W, Wang C, Wang S, Zeng K, Wei S, Sun N, et al. PRPF6 promotes androgen receptor/androgen receptor-variant 7 actions in castration-resistant prostate cancer cells. Int J Biol Sci. (2021) 17:188–203. doi: 10.7150/ijbs.50810
36. Choi JD, Kim TJ, Jeong BC, Jeon HG, Jeon SS, Kang MY, et al. ISL1 promotes enzalutamide resistance in castration-resistant prostate cancer (CRPC) through epithelial to mesenchymal transition (EMT). Sci Rep. (2021) 11:21984. doi: 10.1038/s41598–021-01003–0
37. Ning P, Zhong J-G, Jiang F, Zhang Y, Zhao J, Tian F, et al. Role of protein S in castration-resistant prostate cancer-like cells. Endocr Relat Cancer. (2016) 23:595–607. doi: 10.1530/ERC-16–0126
38. Wang L, Song G, Chang X, Tan W, Pan J, Zhu X, et al. The role of TXNDC5 in castration-resistant prostate cancer-involvement of androgen receptor signaling pathway. Oncogene. (2015) 34:4735–45. doi: 10.1038/onc.2014.401
39. Obinata D, Takayama K, Fujiwara K, Suzuki T, Tsutsumi S, Fukuda N, et al. Targeting Oct1 genomic function inhibits androgen receptor signaling and castration-resistant prostate cancer growth. Oncogene. (2016) 35:6350–8. doi: 10.1038/onc.2016.171
40. Duan X, Kong Z, Liu Y, Zeng Z, Li S, Wu W, et al. β-arrestin2 contributes to cell viability and proliferation via the down-regulation of FOXO1 in castration-resistant prostate cancer. J Cell Physiol. (2015) 230:2371–81. doi: 10.1002/jcp.24963
41. Miller DR, Ingersoll MA, Chatterjee A, Baker B, Shrishrimal S, Kosmacek EA, et al. p66Shc protein through a redox mechanism enhances the progression of prostate cancer cells towards castration-resistance. Free Radic Biol Med. (2019) 139:24–34. doi: 10.1016/j.freeradbiomed.2019.05.015
42. Li J, Xu C, Lee HJ, Ren S, Zi X, Zhang Z, et al. A genomic and epigenomic atlas of prostate cancer in Asian populations. Nature. (2020) 580:93–9. doi: 10.1038/s41586–020-2135-x
43. Gu P, Chen X, Xie R, Han J, Xie W, Wang B, et al. lncRNA HOXD-AS1 regulates proliferation and chemo-resistance of castration-resistant prostate cancer via recruiting WDR5. Mol Ther. (2017) 25:1959–73. doi: 10.1016/j.ymthe.2017.04.016
44. Chang Y-T, Lin T-P, Tang J-T, Campbell M, Luo Y-L, Lu S-Y, et al. HOTAIR is a REST-regulated lncRNA that promotes neuroendocrine differentiation in castration resistant prostate cancer. Cancer Lett. (2018) 433:43–52. doi: 10.1016/j.canlet.2018.06.029
45. You Z, Liu C, Wang C, Ling Z, Wang Y, Wang Y, et al. LncRNA CCAT1 promotes prostate cancer cell proliferation by interacting with DDX5 and MIR-28–5P. Mol Cancer Ther. (2019) 18:2469–79. doi: 10.1158/1535–7163.MCT-19–0095
46. Misawa A, Takayama K-I, Urano T, Inoue S. Androgen-induced long noncoding RNA (lncRNA) SOCS2-AS1 promotes cell growth and inhibits apoptosis in prostate cancer cells. J Biol Chem. (2016) 291:17861–80. doi: 10.1074/jbc.M116.718536
47. Sun T, Wang X, He HH, Sweeney CJ, Liu SX, Brown M, et al. MiR-221 promotes the development of androgen independence in prostate cancer cells via downregulation of HECTD2 and RAB1A. Oncogene. (2014) 33:2790–800. doi: 10.1038/onc.2013.230
48. Guo Y, Cui J, Ji Z, Cheng C, Zhang K, Zhang C, et al. miR-302/367/LATS2/YAP pathway is essential for prostate tumor-propagating cells and promotes the development of castration resistance. Oncogene. (2017) 36:6336–47. doi: 10.1038/onc.2017.240
49. Tang D-E, Dai Y, He J-X, Lin L-W, Leng Q-X, Geng X-Y, et al. Targeting the KDM4B-AR-c-Myc axis promotes sensitivity to androgen receptor-targeted therapy in advanced prostate cancer. J Pathol. (2020) 252:101–13. doi: 10.1002/path.5495
50. Fan L, Zhang F, Xu S, Cui X, Hussain A, Fazli L, et al. Histone demethylase JMJD1A promotes alternative splicing of AR variant 7 (AR-V7) in prostate cancer cells. Proc Natl Acad Sci U.S.A. (2018) 115:E4584–93. doi: 10.1073/pnas.1802415115
51. Saraç H, Morova T, Pires E, McCullagh J, Kaplan A, Cingöz A, et al. Systematic characterization of chromatin modifying enzymes identifies KDM3B as a critical regulator in castration resistant prostate cancer. Oncogene. (2020) 39:2187–201. doi: 10.1038/s41388–019-1116–8
52. Hong Z, Wu G, Xiang Z-D, Xu C-D, Huang S-S, Li C, et al. KDM5C is transcriptionally regulated by BRD4 and promotes castration-resistance prostate cancer cell proliferation by repressing PTEN. BioMed Pharmacother. (2019) 114:108793. doi: 10.1016/j.biopha.2019.108793
53. Metzler VM, de Brot S, Haigh DB, Woodcock CL, Lothion-Roy J, Harris AE, et al. The KDM5B and KDM1A lysine demethylases cooperate in regulating androgen receptor expression and signaling in prostate cancer. Front Cell Dev Biol. (2023) 11:1116424. doi: 10.3389/fcell.2023.1116424
54. Li M, Liu M, Han W, Wang Z, Han D, Patalano S, et al. LSD1 inhibition disrupts super-enhancer-driven oncogenic transcriptional programs in castration-resistant prostate cancer. Cancer Res. (2023) 83:1684–98. doi: 10.1158/0008–5472.CAN-22–2433
55. Wang H-J, Pochampalli M, Wang L-Y, Zou JX, Li P-S, Hsu S-C, et al. KDM8/JMJD5 as a dual coactivator of AR and PKM2 integrates AR/EZH2 network and tumor metabolism in CRPC. Oncogene. (2019) 38:17–32. doi: 10.1038/s41388-018-0414-x
56. Hong Z, Xiang Z, Zhang P, Wu Q, Xu C, Wang X, et al. Histone acetyltransferase 1 upregulates androgen receptor expression to modulate CRPC cell resistance to enzalutamide. Clin Transl Med. (2021) 11:e495. doi: 10.1002/ctm2.495
57. Zhong S, Jeong J-H, Huang C, Chen X, Dickinson SI, Dhillon J, et al. Targeting INMT and interrupting its methylation pathway for the treatment of castration resistant prostate cancer. J Exp Clin Cancer Res. (2021) 40:307. doi: 10.1186/s13046-021-02109-z
58. Beketova E, Fang S, Owens JL, Liu S, Chen X, Zhang Q, et al. Protein arginine methyltransferase 5 promotes pICln-dependent androgen receptor transcription in castration-resistant prostate cancer. Cancer Res. (2020) 80:4904–17. doi: 10.1158/0008–5472.CAN-20–1228
59. Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. (2012) 338:1465–9. doi: 10.1126/science.1227604
60. Zardan A, Nip KM, Thaper D, Toren P, Vahid S, Beraldi E, et al. Lyn tyrosine kinase regulates androgen receptor expression and activity in castrate-resistant prostate cancer. Oncogenesis. (2014) 3:e115. doi: 10.1038/oncsis.2014.30
61. Mahajan K, Malla P, Lawrence HR, Chen Z, Kumar-Sinha C, Malik R, et al. ACK1/TNK2 regulates histone H4 tyr88-phosphorylation and AR gene expression in castration-resistant prostate cancer. Cancer Cell. (2017) 31:790–803.e8. doi: 10.1016/j.ccell.2017.05.003
62. Dai B, Chen H, Guo S, Yang X, Linn DE, Sun F, et al. Compensatory upregulation of tyrosine kinase Etk/BMX in response to androgen deprivation promotes castration-resistant growth of prostate cancer cells. Cancer Res. (2010) 70:5587–96. doi: 10.1158/0008–5472.CAN-09–4610
63. Liu C, Yang JC, Armstrong CM, Lou W, Liu L, Qiu X, et al. AKR1C3 promotes AR-V7 protein stabilization and confers resistance to AR-targeted therapies in advanced prostate cancer. Mol Cancer Ther. (2019) 18:1875–86. doi: 10.1158/1535–7163.MCT-18–1322
64. Miller DR, Ingersoll MA, Chou Y-W, Kosmacek EA, Oberley-Deegan RE, Lin M-F. Dynamics of antioxidant heme oxygenase-1 and pro-oxidant p66Shc in promoting advanced prostate cancer progression. Free Radic Biol Med. (2022) 193:274–91. doi: 10.1016/j.freeradbiomed.2022.10.269
65. Shangguan X, Ma Z, Yu M, Ding J, Xue W, Qi J. Squalene epoxidase metabolic dependency is a targetable vulnerability in castration-resistant prostate cancer. Cancer Res. (2022) 82:3032–44. doi: 10.1158/0008–5472.CAN-21–3822
66. Qi J, Tripathi M, Mishra R, Sahgal N, Fazil L, Ettinger S, et al. The E3 ubiquitin ligase siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell. (2013) 23:332–46. doi: 10.1016/j.ccr.2013.02.016
67. Li H, Xie N, Chen R, Verreault M, Fazli L, Gleave ME, et al. UGT2B17 expedites progression of castration-resistant prostate cancers by promoting ligand-independent AR signaling. Cancer Res. (2016) 76:6701–11. doi: 10.1158/0008–5472.CAN-16–1518
68. Wang L, Song G, Zhang X, Feng T, Pan J, Chen W, et al. PADI2-mediated citrullination promotes prostate cancer progression. Cancer Res. (2017) 77:5755–68. doi: 10.1158/0008–5472.CAN-17–0150
69. Whitton B, Okamoto H, Rose-Zerilli M, Packham G, Crabb SJ. V-ATPase inhibition decreases mutant androgen receptor activity in castrate-resistant prostate cancer. Mol Cancer Ther. (2021) 20:739–48. doi: 10.1158/1535–7163.MCT-20–0662
70. Migita T, Takayama K-I, Urano T, Obinata D, Ikeda K, Soga T, et al. ACSL3 promotes intratumoral steroidogenesis in prostate cancer cells. Cancer Sci. (2017) 108:2011–21. doi: 10.1111/cas.13339
71. Wu N, Wang Y, Wang K, Zhong B, Liao Y, Liang J, et al. Cathepsin K regulates the tumor growth and metastasis by IL-17/CTSK/EMT axis and mediates M2 macrophage polarization in castration-resistant prostate cancer. Cell Death Dis. (2022) 13:813. doi: 10.1038/s41419–022-05215–8
72. Kahn B, Collazo J, Kyprianou N. Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int J Biol Sci. (2014) 10:588–95. doi: 10.7150/ijbs.8671
73. Brown NE, Paluch AM, Nashu MA, Komurov K, Waltz SE. Tumor cell autonomous RON receptor expression promotes prostate cancer growth under conditions of androgen deprivation. Neoplasia. (2018) 20:917–29. doi: 10.1016/j.neo.2018.07.003
74. Jia L, Wu D, Wang Y, You W, Wang Z, Xiao L, et al. Orphan nuclear receptor TLX contributes to androgen insensitivity in castration-resistant prostate cancer via its repression of androgen receptor transcription. Oncogene. (2018) 37:3340–55. doi: 10.1038/s41388-018-0198-z
75. Gao S, Ye H, Gerrin S, Wang H, Sharma A, Chen S, et al. ErbB2 signaling increases androgen receptor expression in abiraterone-resistant prostate cancer. Clin Cancer Res. (2016) 22:3672–82. doi: 10.1158/1078–0432.CCR-15–2309
76. Chen Z, Wu D, Thomas-Ahner JM, Lu C, Zhao P, Zhang Q, et al. Diverse AR-V7 cistromes in castration-resistant prostate cancer are governed by HoxB13. Proc Natl Acad Sci USA. (2018) 115:6810–5. doi: 10.1073/pnas.1718811115
77. Terada N, Shimizu Y, Kamba T, Inoue T, Maeno A, Kobayashi T, et al. Identification of EP4 as a potential target for the treatment of castration-resistant prostate cancer using a novel xenograft model. Cancer Res. (2010) 70:1606–15. doi: 10.1158/0008–5472.CAN-09–2984
78. Xiao L, Wang Y, Xu K, Hu H, Xu Z, Wu D, et al. Nuclear receptor LRH-1 functions to promote castration-resistant growth of prostate cancer via its promotion of intratumoral androgen biosynthesis. Cancer Res. (2018) 78:2205–18. doi: 10.1158/0008–5472.CAN-17–2341
79. Xu Z, Ma T, Zhou J, Gao W, Li Y, Yu S, et al. Nuclear receptor ERRα contributes to castration-resistant growth of prostate cancer via its regulation of intratumoral androgen biosynthesis. Theranostics. (2020) 10:4201–16. doi: 10.7150/thno.35589
80. Lin S-R, Wen Y-C, Yeh H-L, Jiang K-C, Chen W-H, Mokgautsi N, et al. EGFR-upregulated LIFR promotes SUCLG2-dependent castration resistance and neuroendocrine differentiation of prostate cancer. Oncogene. (2020) 39:6757–75. doi: 10.1038/s41388–020-01468–9
81. Heidman LM, Peinetti N, Copello VA, Burnstein KL. Exploiting dependence of castration-resistant prostate cancer on the arginine vasopressin signaling axis by repurposing vaptans. Mol Cancer Res. (2022) 20:1295–304. doi: 10.1158/1541–7786.MCR-21–0927
82. Rafiei S, Gui B, Wu J, Liu XS, Kibel AS, Jia L. Targeting the MIF/CXCR7/AKT signaling pathway in castration-resistant prostate cancer. Mol Cancer Res. (2019) 17:263–76. doi: 10.1158/1541–7786.MCR-18–0412
83. Wang N, Yao M, Xu J, Quan Y, Zhang K, Yang R, et al. Autocrine activation of CHRM3 promotes prostate cancer growth and castration resistance via caM/caMKK-mediated phosphorylation of akt. Clin Cancer Res. (2015) 21:4676–85. doi: 10.1158/1078–0432.CCR-14–3163
84. Armstrong K, Ahmad I, Kalna G, Tan SS, Edwards J, Robson CN, et al. Upregulated FGFR1 expression is associated with the transition of hormone-naive to castrate-resistant prostate cancer. Br J Cancer. (2011) 105:1362–9. doi: 10.1038/bjc.2011.367
85. Stoyanova T, Riedinger M, Lin S, Faltermeier CM, Smith BA, Zhang KX, et al. Activation of Notch1 synergizes with multiple pathways in promoting castration-resistant prostate cancer. Proc Natl Acad Sci U.S.A. (2016) 113:E6457–66. doi: 10.1073/pnas.1614529113
86. Uemura M, Honma S, Chung S, Takata R, Furihata M, Nishimura K, et al. 5alphaDH-DOC (5alpha-dihydro-deoxycorticosterone) activates androgen receptor in castration-resistant prostate cancer. Cancer Sci. (2010) 101:1897–904. doi: 10.1111/j.1349-7006.2010.01620.x
87. Snaterse G, van Dessel LF, van Riet J, Taylor AE, van der Vlugt-Daane M, Hamberg P, et al. 11-Ketotestosterone is the predominant active androgen in prostate cancer patients after castration. JCI Insight. (2021) 6:e148507. doi: 10.1172/jci.insight.148507
88. Ye C, Chen G-H, Chen X, Qin S-F, Shi M-F, Zhou T. Upregulation of erythropoietin and erythropoietin receptor in castration-resistant progression of prostate cancer. Asian J Androl. (2020) 22:422–6. doi: 10.4103/aja.aja_80_19
89. Li S, Hu MG, Sun Y, Yoshioka N, Ibaragi S, Sheng J, et al. Angiogenin mediates androgen-stimulated prostate cancer growth and enables castration resistance. Mol Cancer Res. (2013) 11:1203–14. doi: 10.1158/1541–7786.MCR-13–0072
90. Liang Z, Cao J, Tian L, Shen Y, Yang X, Lin Q, et al. Aromatase-induced endogenous estrogen promotes tumor metastasis through estrogen receptor-α/matrix metalloproteinase 12 axis activation in castration-resistant prostate cancer. Cancer Lett. (2019) 467:72–84. doi: 10.1016/j.canlet.2019.09.001
91. Wang X, Lee SO, Xia S, Jiang Q, Luo J, Li L, et al. Endothelial cells enhance prostate cancer metastasis via IL-6→androgen receptor→TGF-β→MMP-9 signals. Mol Cancer Ther. (2013) 12:1026–37. doi: 10.1158/1535–7163.MCT-12–0895
92. Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. (2018) 559:363–9. doi: 10.1038/s41586–018-0266–0
93. Ippolito L, Comito G, Parri M, Iozzo M, Duatti A, Virgilio F, et al. Lactate rewires lipid metabolism and sustains a metabolic-epigenetic axis in prostate cancer. Cancer Res. (2022) 82:1267–82. doi: 10.1158/0008–5472.CAN-21–0914
94. Wang C, Chen J, Zhang Q, Li W, Zhang S, Xu Y, et al. Elimination of CD4lowHLA-G+ T cells overcomes castration-resistance in prostate cancer therapy. Cell Res. (2018) 28:1103–17. doi: 10.1038/s41422–018-0089–4
95. Zaslavsky AB, Gloeckner-Kalousek A, Adams M, Putluri N, Venghatakrishnan H, Li H, et al. Platelet-synthesized testosterone in men with prostate cancer induces androgen receptor signaling. Neoplasia. (2015) 17:490–6. doi: 10.1016/j.neo.2015.05.003
96. Han W, Liu M, Han D, Li M, Toure AA, Wang Z, et al. RB1 loss in castration-resistant prostate cancer confers vulnerability to LSD1 inhibition. Oncogene. (2022) 41:852–64. doi: 10.1038/s41388–021-02135–3
97. Hsieh C-L, Botta G, Gao S, Li T, Van Allen EM, Treacy DJ, et al. PLZF, a tumor suppressor genetically lost in metastatic castration-resistant prostate cancer, is a mediator of resistance to androgen deprivation therapy. Cancer Res. (2015) 75:1944–8. doi: 10.1158/0008–5472.CAN-14–3602
98. Punnoose EA, Ferraldeschi R, Szafer-Glusman E, Tucker EK, Mohan S, Flohr P, et al. PTEN loss in circulating tumor cells correlates with PTEN loss in fresh tumor tissue from castration-resistant prostate cancer patients. Br J Cancer. (2015) 113:1225–33. doi: 10.1038/bjc.2015.332
99. Patel R, Brzezinska EA, Repiscak P, Ahmad I, Mui E, Gao M, et al. Activation of β-catenin cooperates with loss of pten to drive AR-independent castration-resistant prostate cancer. Cancer Res. (2020) 80:576–90. doi: 10.1158/0008–5472.CAN-19–1684
100. Li Q, Liu B, Chao H-P, Ji Y, Lu Y, Mehmood R, et al. LRIG1 is a pleiotropic androgen receptor-regulated feedback tumor suppressor in prostate cancer. Nat Commun. (2019) 10:5494. doi: 10.1038/s41467–019-13532–4
101. Sooreshjani MA, Nikhil K, Kamra M, Nguyen DN, Kumar D, Shah K. LIMK2-NKX3.1 engagement promotes castration-resistant prostate cancer. Cancers. (2021) 13:2324. doi: 10.3390/cancers13102324
102. Sooreshjani MA, Kamra M, Zoubeidi A, Shah K. Reciprocal deregulation of NKX3.1 and AURKA axis in castration-resistant prostate cancer and NEPC models. J BioMed Sci. (2021) 28:68. doi: 10.1186/s12929-021-00765-z
103. Han D, Chen S, Han W, Gao S, Owiredu JN, Li M, et al. ZBTB7A mediates the transcriptional repression activity of the androgen receptor in prostate cancer. Cancer Res. (2019) 79:5260–71. doi: 10.1158/0008–5472.CAN-19–0815
104. Zhou J, Ning Z, Wang B, Yun E-J, Zhang T, Pong R-C, et al. DAB2IP loss confers the resistance of prostate cancer to androgen deprivation therapy through activating STAT3 and inhibiting apoptosis. Cell Death Dis. (2015) 6:e1955. doi: 10.1038/cddis.2015.289
105. Jia J, Zhang H-B, Shi Q, Yang C, Ma J-B, Jin B, et al. KLF5 downregulation desensitizes castration-resistant prostate cancer cells to docetaxel by increasing BECN1 expression and inducing cell autophagy. Theranostics. (2019) 9:5464–77. doi: 10.7150/thno.33282
106. Hagman Z, Haflidadóttir BS, Ceder JA, Larne O, Bjartell A, Lilja H, et al. miR-205 negatively regulates the androgen receptor and is associated with adverse outcome of prostate cancer patients. Br J Cancer. (2013) 108:1668–76. doi: 10.1038/bjc.2013.131
107. Guan H, You Z, Wang C, Fang F, Peng R, Mao L, et al. MicroRNA-200a suppresses prostate cancer progression through BRD4/AR signaling pathway. Cancer Med. (2019) 8:1474–85. doi: 10.1002/cam4.2029
108. Goto Y, Kojima S, Kurozumi A, Kato M, Okato A, Matsushita R, et al. Regulation of E3 ubiquitin ligase-1 (WWP1) by microRNA-452 inhibits cancer cell migration and invasion in prostate cancer. Br J Cancer. (2016) 114:1135–44. doi: 10.1038/bjc.2016.95
109. Xia L, Han Q, Chi C, Zhu Y, Pan J, Dong B, et al. Transcriptional regulation of PRKAR2B by miR-200b-3p/200c-3p and XBP1 in human prostate cancer. BioMed Pharmacother. (2020) 124:109863. doi: 10.1016/j.biopha.2020.109863
110. Ebron JS, Shankar E, Singh J, Sikand K, Weyman CM, Gupta S, et al. MiR-644a disrupts oncogenic transformation and warburg effect by direct modulation of multiple genes of tumor-promoting pathways. Cancer Res. (2019) 79:1844–56. doi: 10.1158/0008–5472.CAN-18–2993
111. Saha S, Kiran M, Kuscu C, Chatrath A, Wotton D, Mayo MW, et al. Long noncoding RNA DRAIC inhibits prostate cancer progression by interacting with IKK to inhibit NF-κB activation. Cancer Res. (2020) 80:950–63. doi: 10.1158/0008–5472.CAN-19–3460
112. Rasool R, O’Connor CM, Das CK, Alhusayan M, Verma BK, Islam S, et al. Loss of LCMT1 and biased protein phosphatase 2A heterotrimerization drive prostate cancer progression and therapy resistance. Nat Commun. (2023) 14:5253. doi: 10.1038/s41467–023-40760–6
113. Russo JW, Gao C, Bhasin SS, Voznesensky OS, Calagua C, Arai S, et al. Downregulation of dipeptidyl peptidase 4 accelerates progression to castration-resistant prostate cancer. Cancer Res. (2018) 78:6354–62. doi: 10.1158/0008–5472.CAN-18–0687
114. Ko H-K, Berk M, Chung Y-M, Willard B, Bareja R, Rubin M, et al. Loss of an androgen-inactivating and isoform-specific HSD17B4 splice form enables emergence of castration-resistant prostate cancer. Cell Rep. (2018) 22:809–19. doi: 10.1016/j.celrep.2017.12.081
115. Kaushik AK, Shojaie A, Panzitt K, Sonavane R, Venghatakrishnan H, Manikkam M, et al. Inhibition of the hexosamine biosynthetic pathway promotes castration-resistant prostate cancer. Nat Commun. (2016) 7:11612. doi: 10.1038/ncomms11612
116. Hearn JWD, AbuAli G, Reichard CA, Reddy CA, Magi-Galluzzi C, Chang K-H, et al. HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol. (2016) 17:1435–44. doi: 10.1016/S1470–2045(16)30227–3
117. Almassi N, Reichard C, Li J, Russell C, Perry J, Ryan CJ, et al. HSD3B1 and response to a nonsteroidal CYP17A1 inhibitor in castration-resistant prostate cancer. JAMA Oncol. (2018) 4:554–7. doi: 10.1001/jamaoncol.2017.3159
118. Tong D, Liu Q, Liu G, Yuan W, Wang L, Guo Y, et al. The HIF/PHF8/AR axis promotes prostate cancer progression. Oncogenesis. (2016) 5:e283. doi: 10.1038/oncsis.2016.74
119. Shih T-C, Liu R, Wu C-T, Li X, Xiao W, Deng X, et al. Targeting galectin-1 impairs castration-resistant prostate cancer progression and invasion. Clin Cancer Res. (2018) 24:4319–31. doi: 10.1158/1078–0432.CCR-18–0157
120. Scaravilli M, Koivukoski S, Gillen A, Bouazza A, Ruusuvuori P, Visakorpi T, et al. miR-32 promotes MYC-driven prostate cancer. Oncogenesis. (2022) 11:11. doi: 10.1038/s41389–022-00385–8
121. Jalava SE, Urbanucci A, Latonen L, Waltering KK, Sahu B, Jänne OA, et al. Androgen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancer. Oncogene. (2012) 31:4460–71. doi: 10.1038/onc.2011.624
122. Børretzen A, Gravdal K, Haukaas SA, Beisland C, Akslen LA, Halvorsen OJ. FOXC2 expression and epithelial-mesenchymal phenotypes are associated with castration resistance, metastasis and survival in prostate cancer. J Pathol Clin Res. (2019) 5:272–86. doi: 10.1002/cjp2.142
123. Goto Y, Kojima S, Nishikawa R, Kurozumi A, Kato M, Enokida H, et al. MicroRNA expression signature of castration-resistant prostate cancer: the microRNA-221/222 cluster functions as a tumor suppressor and disease progression marker. Br J Cancer. (2015) 113:1055–65. doi: 10.1038/bjc.2015.300
124. Liu Y-N, Niu S, Chen W-Y, Zhang Q, Tao Y, Chen W-H, et al. Leukemia inhibitory factor promotes castration-resistant prostate cancer and neuroendocrine differentiation by activated ZBTB46. Clin Cancer Res. (2019) 25:4128–40. doi: 10.1158/1078–0432.CCR-18–3239
125. Don-Doncow N, Marginean F, Coleman I, Nelson PS, Ehrnström R, Krzyzanowska A, et al. Expression of STAT3 in prostate cancer metastases. Eur Urol. (2017) 71:313–6. doi: 10.1016/j.eururo.2016.06.018
126. Lin X, Gu Y, Kapoor A, Wei F, Aziz T, Ojo D, et al. Overexpression of MUC1 and genomic alterations in its network associate with prostate cancer progression. Neoplasia. (2017) 19:857–67. doi: 10.1016/j.neo.2017.06.006
127. Barros-Silva JD, Linn DE, Steiner I, Guo G, Ali A, Pakula H, et al. Single-cell analysis identifies LY6D as a marker linking castration-resistant prostate luminal cells to prostate progenitors and cancer. Cell Rep. (2018) 25:3504–3518.e6. doi: 10.1016/j.celrep.2018.11.069
128. Linder A, Larsson K, Welén K, Damber J-E. RGS2 is prognostic for development of castration resistance and cancer-specific survival in castration-resistant prostate cancer. Prostate. (2020) 80:799–810. doi: 10.1002/pros.23994
129. Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. (2015) 67:33–41. doi: 10.1016/j.eururo.2014.07.035
130. Adler D, Menon R, Braun M, Offermann A, Queisser A, Boehm D, et al. MED15, encoding a subunit of the mediator complex, is overexpressed at high frequency in castration-resistant prostate cancer. Int J Cancer. (2014) 135:19–26. doi: 10.1002/ijc.28647
131. Tamura K, Furihata M, Chung S-Y, Uemura M, Yoshioka H, Iiyama T, et al. Stanniocalcin 2 overexpression in castration-resistant prostate cancer and aggressive prostate cancer. Cancer Sci. (2009) 100:914–9. doi: 10.1111/j.1349-7006.2009.01117.x
132. Zheng D, Gui B, Gray KP, Tinay I, Rafiei S, Huang Q, et al. Secretory leukocyte protease inhibitor is a survival and proliferation factor for castration-resistant prostate cancer. Oncogene. (2016) 35:4807–15. doi: 10.1038/onc.2016.13
133. Zhong W, Qin G, Dai Q, Han Z, Chen S, Ling X, et al. SOXs in human prostate cancer: implication as progression and prognosis factors. BMC Cancer. (2012) 12:248. doi: 10.1186/1471–2407-12–248
134. Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. (2004) 10:33–9. doi: 10.1038/nm972
135. Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. (2019) 381:121–31. doi: 10.1056/NEJMoa1903835
136. Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. (2018) 378:1408–18. doi: 10.1056/NEJMoa1715546
137. Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. (2019) 380:1235–46. doi: 10.1056/NEJMoa1815671
138. Crawford ED, Schellhammer PF, McLeod DG, Moul JW, Higano CS, Shore N, et al. Androgen receptor targeted treatments of prostate cancer: 35 years of progress with antiandrogens. J Urol. (2018) 200:956–66. doi: 10.1016/j.juro.2018.04.083
139. Rizzo A, Merler S, Sorgentoni G, Oderda M, Mollica V, Gadaleta-Caldarola G, et al. Risk of cardiovascular toxicities and hypertension in nonmetastatic castration-resistant prostate cancer patients treated with novel hormonal agents: a systematic review and meta-analysis. Expert Opin Drug Metab Toxicol. (2021) 17:1237–43. doi: 10.1080/17425255.2021.1970745
140. Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. (2022) 15:174. doi: 10.1186/s13045–022-01392–3
141. Wang M-E, Chen J, Lu Y, Bawcom AR, Wu J, Ou J, et al. RB1-deficient prostate tumor growth and metastasis are vulnerable to ferroptosis induction via the E2F/ACSL4 axis. J Clin Invest. (2023) 133:e166647. doi: 10.1172/JCI166647
142. Wu F, Wang M, Zhong T, Xiao C, Chen X, Huang Y, et al. Inhibition of CDC20 potentiates anti-tumor immunity through facilitating GSDME-mediated pyroptosis in prostate cancer. Exp Hematol Oncol. (2023) 12:67. doi: 10.1186/s40164–023-00428–9
143. Wang H, He Z, Gao Y, Feng D, Wei X, Huang Y, et al. Dual-pronged attack: pH-driven membrane-anchored NIR dual-type nano-photosensitizer excites immunogenic pyroptosis and sequester immune checkpoint for enhanced prostate cancer photo-immunotherapy. Adv Sci (Weinh). (2023) 10:2302422. doi: 10.1002/advs.202302422
144. Wang H, Jiao D, Feng D, Liu Q, Huang Y, Hou J, et al. Transformable supramolecular self-assembled peptides for cascade self-enhanced ferroptosis primed cancer immunotherapy. Advanced Mater. (2024) 36:2311733. doi: 10.1002/adma.202311733
145. Wang Y, Chen J, Li Q, Wang H, Liu G, Jing Q, et al. Identifying novel prostate cancer associated pathways based on integrative microarray data analysis. Comput Biol Chem. (2011) 35:151–8. doi: 10.1016/j.compbiolchem.2011.04.003
146. Lin Y, Qian F, Shen L, Chen F, Chen J, Shen B. Computer-aided biomarker discovery for precision medicine: data resources, models and applications. Briefings Bioinf. (2019) 20:952–75. doi: 10.1093/bib/bbx158
147. Lin Y, Zhao X, Miao Z, Ling Z, Wei X, Pu J, et al. Data-driven translational prostate cancer research: from biomarker discovery to clinical decision. J Trans Med. (2020) 18:119. doi: 10.1186/s12967–020-02281–4
148. Marrón-Esquivel JM, Duran-Lopez L, Linares-Barranco A, Dominguez-Morales JP. A comparative study of the inter-observer variability on Gleason grading against Deep Learning-based approaches for prostate cancer. Comput Biol Med. (2023) 159:106856. doi: 10.1016/j.compbiomed.2023.106856
149. Ström P, Kartasalo K, Olsson H, Solorzano L, Delahunt B, Berney DM, et al. Artificial intelligence for diagnosis and grading of prostate cancer in biopsies: a population-based, diagnostic study. Lancet Oncol. (2020) 21:222–32. doi: 10.1016/S1470–2045(19)30738–7
150. Tolkach Y, Dohmgörgen T, Toma M, Kristiansen G. High-accuracy prostate cancer pathology using deep learning. Nat Mach Intell. (2020) 2:411–8. doi: 10.1038/s42256–020-0200–7
151. Nakata W, Mori H, Tsujimura G, Tsujimoto Y, Gotoh T, Tsujihata M. Pilot study of an artificial intelligence-based deep learning algorithm to predict time to castration-resistant prostate cancer for metastatic hormone-naïve prostate cancer. Japanese J Clin Oncol. (2022) 52:1062–6. doi: 10.1093/jjco/hyac089
152. Yu C, Zong H, Chen Y, Zhou Y, Liu X, Lin Y, et al. PCAO2: an ontology for integration of prostate cancer associated genotypic, phenotypic and lifestyle data. Briefings Bioinf. (2024) 25:bbae136. doi: 10.1093/bib/bbae136
Keywords: castration-resistant prostate cancer, molecular signatures, carcinogenic mechanisms, personalized medicine, medical systems biology
Citation: Jian J, Wang X, Zhang J, Zhou C, Hou X, Huang Y, Hou J, Lin Y and Wei X (2024) Molecular landscape for risk prediction and personalized therapeutics of castration-resistant prostate cancer: at a glance. Front. Endocrinol. 15:1360430. doi: 10.3389/fendo.2024.1360430
Received: 23 December 2023; Accepted: 20 May 2024;
Published: 03 June 2024.
Edited by:
Fred Sinowatz, Ludwig Maximilian University of Munich, GermanyCopyright © 2024 Jian, Wang, Zhang, Zhou, Hou, Huang, Hou, Lin and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuedong Wei, d3hkMDQyMkAxNjMuY29t; Yuxin Lin, bGlueXV4aW5Ac3VkYS5lZHUuY24=; Jianquan Hou, eGYxOTJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jingang Jian
Jingang Jian Xin’an Wang
Xin’an Wang Jun Zhang1
Jun Zhang1 Yuhua Huang
Yuhua Huang Jianquan Hou
Jianquan Hou Yuxin Lin
Yuxin Lin Xuedong Wei
Xuedong Wei