
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 05 March 2024
Sec. Pediatric Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1360139
Background: Increased risk of neoplastic events after recombinant human growth hormone (rhGH) treatment in childhood has been an ongoing concern but long-term safety data are limited.
Methods: A nationwide population-based cohort study in Sweden of patients treated with rhGH during childhood between 1985-2010, due to isolated growth hormone deficiency (GHD), small for gestational age (SGA) and idiopathic short stature (ISS). The comparison group consisted of 15 age-, sex-, and region-matched controls per patient, randomly selected from the general population. Data on neoplastic events and covariates, such as gestational age, birth weight, birth length, socioeconomic status, and height at study start, were collected through linkage with population-based registers. The cohort was followed for neoplastic events until the end of 2020.
Results: 53,444 individuals (3,408 patients; 50,036 controls) were followed for up to 35 years, with a median follow-up of 19.8 years and a total of 1,050,977 person-years. Patients showed a moderately increased hazard ratio (HR) for neoplastic events overall compared to controls (HR 1.28, 95% CI: 1.12-1.46), but only significant for males (HR 1.39, 95% CI: 1.17-1.66) and not females (HR 1.15, 95% CI: 0.94-1.41). Longer treatment duration was associated with an increased HR, but no association was found between neoplastic events and mean or cumulative dose. No increased risk of malignant neoplasms was observed for the patients compared to matched controls (HR 0.91 95% CI: 0.66-1.26).
Conclusion: No association was found between rhGH treatment during childhood for GHD, SGA, or ISS and malignant neoplastic events in early to mid-adulthood. A moderate increase in overall neoplastic events was observed due to an increased number of events in male patients.
For over 60 years, growth hormone (GH) has been used clinically, with an increasing number of patients around the world being treated in childhood to achieve increased height, even though they in many cases do not suffer from any secretory hormonal deficiency (1–3). Since its introduction, the use of GH treatment has been accompanied by concerns of potential cancer risks due to its potency as a mitogenic and anti-apoptotic hormone (4). Data from clinical conditions of GH excess (5) or impaired GH signaling (6) has demonstrated a link between GH activity and tumor development and experimental studies using animal models (7) have further elucidated this association. Additionally, epidemiological studies (8) in the general population have shown an association between increased circulating levels of GH’s central mediator, Insulin-like Growth Factor 1 (IGF-1), and certain types of cancer, underscoring the importance of remaining vigilant to this potential risk.
While several studies (9–13) investigating the association between childhood GH treatment and cancer risk have yielded reassuring results, there is still a paucity of studies of long-term risks in this area. The current evidence is based on studies with only a few years of follow-up and lack of proper comparison groups as well as data on potential confounders. With an expanding number of patients starting GH treatment, primarily to improve their stature rather than to replace a secretory hormonal deficiency, the need for valid evidence regarding the long-term implications of this treatment becomes even more pertinent.
The aim of this study was to investigate the risk of neoplasms later in life for patients treated with recombinant human GH (rhGH) in childhood due to isolated growth hormone deficiency (GHD), small for gestational age (SGA), or idiopathic short stature (ISS). To accomplish this objective, we collected outcome data spanning up to 35 years, along with comprehensive information on important covariates including socioeconomic factors, birth characteristics, and height at study start for both the treated patients and a matched comparison group.
The overall study design, cohort of rhGH treated patients and the matched comparison group has been described previously in detail (14) and will be briefly summarized.
We conducted a nationwide population-based cohort study investigating neoplastic events in Swedish patients treated with rhGH due to GHD, SGA or ISS during childhood between January 1, 1985, and December 31, 2010. The outcome data were prospectively collected from January 1, 1985, to December 31, 2020, and covariates of interest were retrieved through data linkage between Swedish health and population registers. This study was approved by the Regional Ethics Review Board in Stockholm, which waived the need for informed consent for the use of registry data.
Fifteen controls matched for sex, birth year, and geographical region were randomly selected for each patient and linkage of data was achieved using each individual’s unique personal identity numbers (15). As a result, complete data on birth characteristics, health history, vital status, emigration data, educational and income data, as well as socioeconomic data of parents could be linked to each patient and control. Treatment variables such as mean dose, duration of treatment, cumulative dose, and adult treatment, were collected from the GH-SAFETY-database (16) and the Swedish Prescribed Drug Register (17). We also gathered height data for each patient and control from several sources in order to obtain height at study start for each individual.
The primary outcome was the detection of the first neoplastic event after the start of the study, defined as the date of first rhGH treatment in the patients or the corresponding date in the matched controls. The secondary outcome was the occurrence of the first malignant neoplastic event. Information on neoplastic events was obtained from the Swedish National Cancer Register (18), the Swedish National Patient Register (19) and from the Cause of Death Register (20), which all have very high national coverage rates. Different categories of neoplastic events were defined according to the ICD codes in the 7th-10th revision of the International Classification of Diseases, see Supplementary Tables S1, S2 in Supplementary Appendix for the specific ICD codes for each outcome category.
Descriptive statistics were used to summarize baseline characteristics for patients and controls. The incidence rate (IR) of neoplastic events was calculated as the number of new cases observed during the study period divided by the person-time at risk and presented as events per 10,000 person-years with 95% confidence intervals (CIs). IRs were calculated overall and for each stratum of baseline characteristics. Differences in number of events between the patients and controls for each neoplastic category were tested using Fisher’s exact tests.
To assess time to first neoplastic event (overall or only malignant), a Cox proportional hazard model was employed. The assumption of proportional hazards was evaluated using graphical methods and tested using Schoenfeld’s residuals with no significant violation observed. The standard errors were estimated with the cluster sandwich estimator, considering the within-matched-group dependence. The follow-up duration for each participant was calculated from the study start until the date of first neoplastic event or censoring date defined as loss to follow-up (e.g. emigration), death or end of study (December 31, 2020).
The Cox regression analysis was performed with a non-adjusted, a restricted, and a fully adjusted model, to allow a comprehensive evaluation of the relationship between the exposure variable and the time to the outcome event, accounting for potential confounders. The restricted model adjusted for sex, age and height at study start, and the fully adjusted model also included birth length, birth weight, gestational age, parental education, and income. The analysis of time to the first malignant neoplastic event included all subjects, regardless of their prior history of non-malignant neoplasms. All HRs are presented with 95% CIs.
A mixed-effects model was utilized to estimate the height at the start of the study for the control group, taking multiple height measurements for each control subject into account. A detailed description of this model, the sensitivity analyses on a subset of the cohort more similar in height at study start (within 5 cm, Supplementary Tables S3, S4) or with adult treatment (Supplementary Table S5), as well as the other covariates has been reported previously (14). An additional sensitivity analysis with a two-year lag period after end of treatment for all subgroups of patients was also performed (Supplementary Tables S6, S7). To analyze potential differences in HRs based on follow-up time, a stratified analysis was also conducted, presenting HRs for overall and malignant neoplasms by duration of follow-up (0-9 years, 10-19 years, and ≥20 years) and separated by sex (Supplementary Table S8). Kaplan-Meier curves for overall and malignant neoplastic events, separated by sex, are also presented in the Supplementary Appendix, Supplementary Figures S1, S2. A two-sided P value of 0.05 or less was considered statistically significant.
All analyses were performed using Stata statistical software, version 17.0 (StataCorp, TX, USA).
The study population included 3,408 patients and 50,036 controls (Table 1). Mean age at the end of the study was 31.1 years (SD: ± 8.2) with a median follow-up time of 19.8 years (range: 0.0-35.7 years) and a total of 1,050,977 person-years.
A cumulative count of 9,004 neoplastic diagnoses were registered during follow-up, allowing for multiple diagnoses per individual: 596 in the patient group and 8,408 in the control group (Supplementary Table S1). Of these, 1,596 were malignant neoplasms with 84 in the patient group and 1,512 in the control group. The patients had a significantly higher number of neoplasms of uncertain or unknown behavior (104 in the patient group vs 1,229 in the control group, p=0.035, Supplementary Table S1), with the subcategories of neoplasms in the central nervous system or in unknown sites generating this overall difference (Supplementary Table S2). There was no overall difference between the groups concerning benign neoplasms (386 in the patient group vs 5,227 in the control group, p=0.106, Supplementary Table S1) but the patients had a significantly higher number of benign neoplasms of endocrine glands compared to the controls (13 in the patient group vs 51 in the control group, p<0.001, Supplementary Table S2). No difference was seen between the groups regarding malignant or benign bone or cartilage neoplasms (Supplementary Tables S1, S2).
In the analyses of time to first neoplastic event, a total of 7,201 events were recorded (469 in the patient group and 6,552 in the control group). Crude incidence rates (IRs) were similar among patients and controls overall, 69.7 vs 66.6 events/10,000 pyrs, as well as for males (55.6 vs 51.9 events/10,000 pyrs) and females (103.2 vs 101.4 events/10,000 pyrs) separately (Table 2). The adjusted hazard ratios (HRs) of overall neoplastic events were slightly increased in both the restricted (HR 1.36, 95% CI: 1.20-1.54) and full model (HR 1.28, 95% CI: 1.12-1.46), predominately in males (Table 3). In the sensitivity analysis starting follow-up with a two-year lag-period after end of treatment, no increased risks of overall neoplastic events were noted in the fully adjusted model, except among males in the subgroup of GHD with GHmax 5-9 (HR 1.34, 95% CI:1.03-1.74) (Supplementary Table S6).
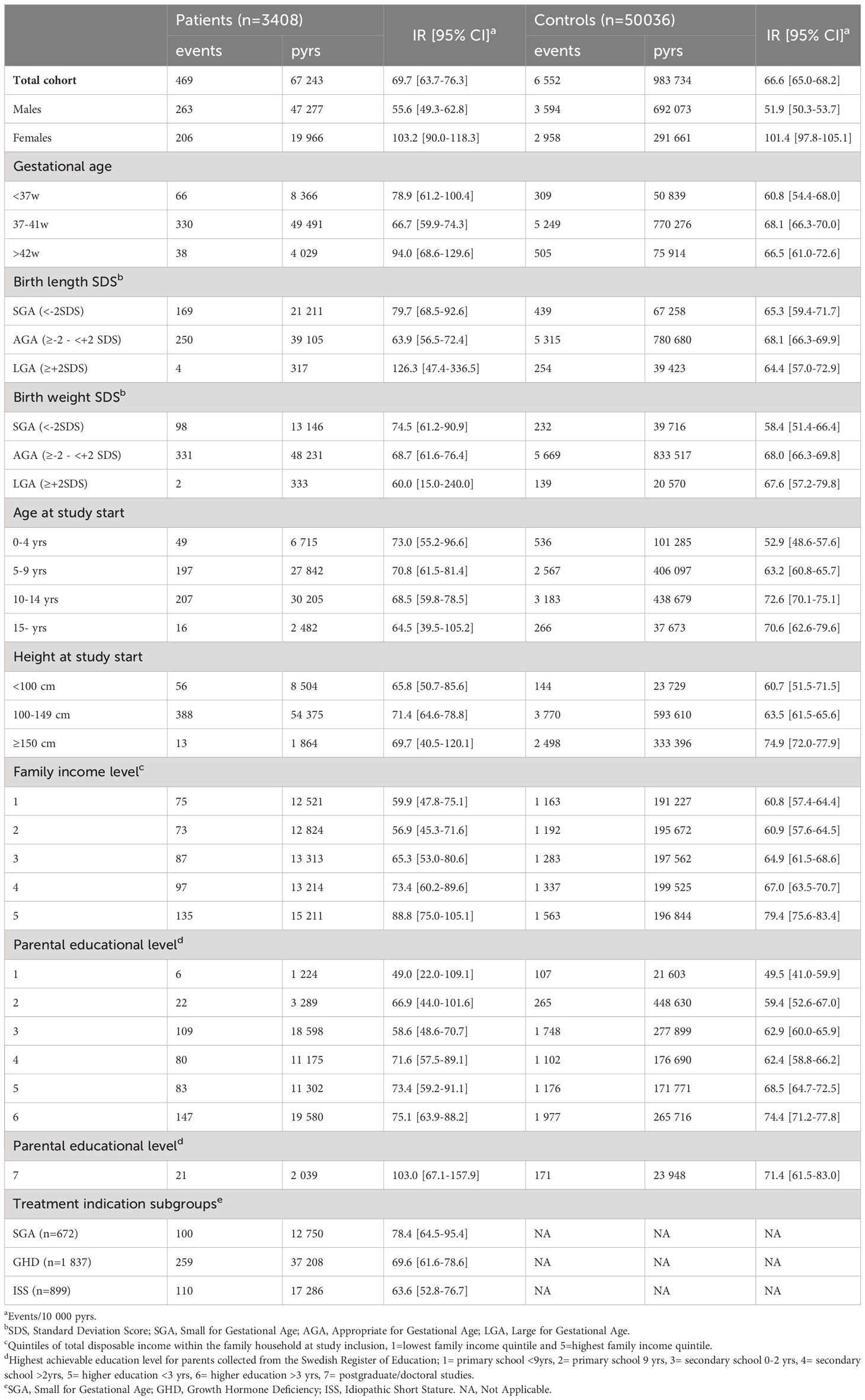
Table 2 Number of events, person-years (pyrs) and incidence rates (IRs) for neoplastic events overall (benign and malignant).
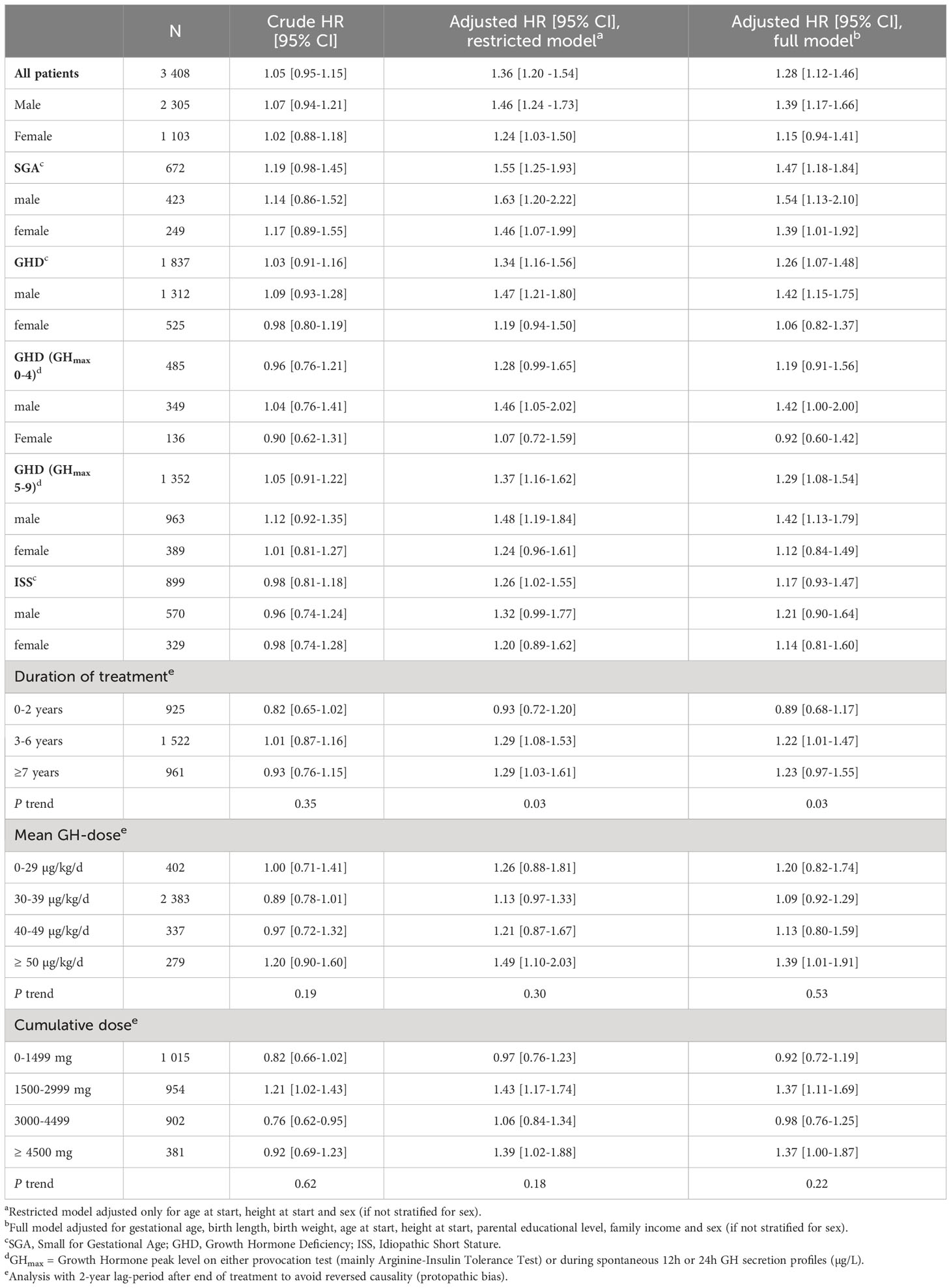
Table 3 Crude and adjusted hazard ratios for neoplastic events overall (benign and malignant) among patients compared to matched controls.
In the analyses of detailed treatment exposure, we did not observe any significant trends by different dose categories using mean or cumulative doses (Table 3). For duration of treatment, there was a significant trend over duration categories (ptrend=0.03) with the highest adjusted HRs in the group with the longest (≥7 years) treatment duration but only reaching significance in the restricted model and not in the full model (restricted model: HR 1.29, 95% CI: 1.03-1.61; full model: HR 1.23, 95% CI: 0.97-1.55, Table 3).
In the analyses of time to first malignant neoplastic event, a total of 1,381 events were observed (71 in the patients and 1,310 in the controls). Crude IR was lower for the patients compared to the controls (9.9 vs 12.6 events/10,000 pyrs) as well as the crude HR (0.78, 95% CI: 0.62-0.99) (Table 4). No significant differences were seen in the adjusted analyses with an adjusted HR of 0.93 (95% CI: 0.69-1.25) in the restricted model and 0.91 (95% CI: 0.66-1.26) in the full model. The analysis with a two-year lag-period after end of treatment, confirmed this finding showing no increased HR for malignant neoplastic events among patients compared to controls (Supplementary Table S7).
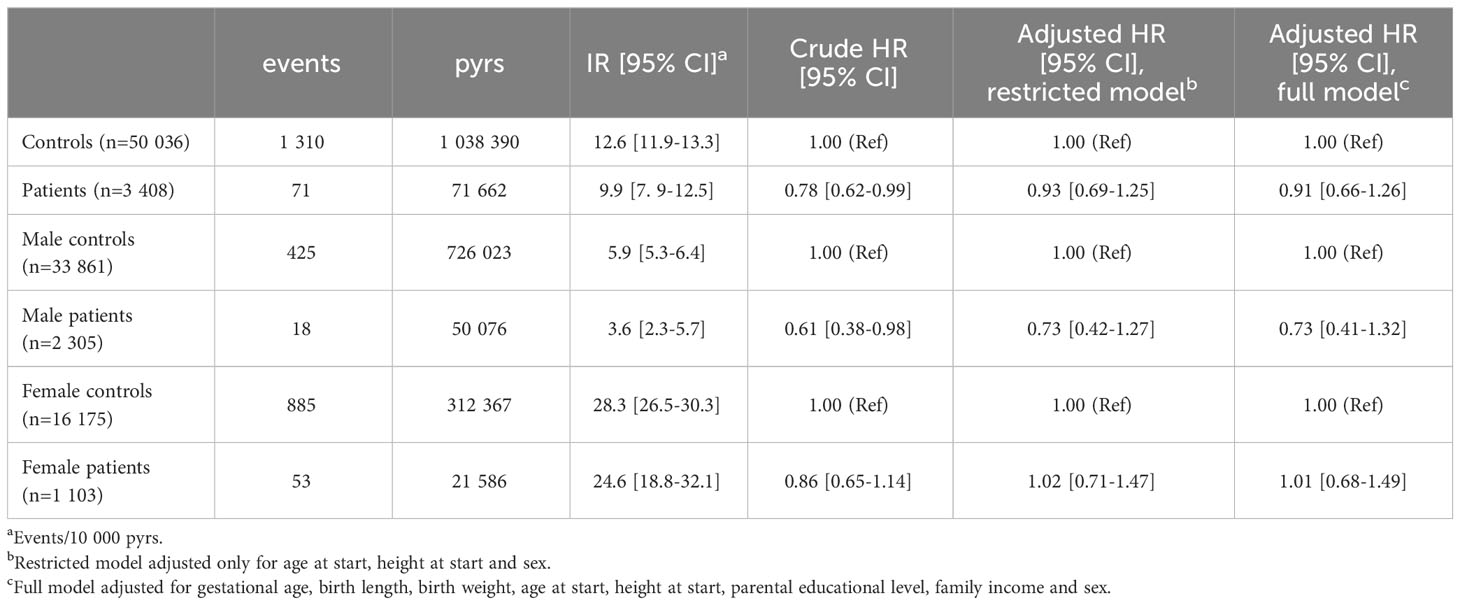
Table 4 Incidence rates (IRs) and hazard ratios (HRs) of malignant neoplasms in patients compared to matched controls.
In the sensitivity analysis, only including patients and controls similar in height at study start, a slight increased HR was detected for neoplastic events overall in the patient group in the restricted model (HR 1.32, 95% CI: 1.01-1.72) but not reaching significance in the full model (HR 1.31, 95% CI: 0.97-1.77) ( Supplementary Table S4). Similar non-significant point estimates were seen for malignant neoplastic events but with even wider confidence intervals due to few events (Supplementary Table S4). The analysis of patients with adult treatment showed increased HRs for overall neoplastic events but not for malignant neoplastic events (Supplementary Table S5). The stratified analyses of HRs for overall and malignant neoplasms by duration of follow-up did not detect any clear differences in risk based on follow-up duration (Supplementary Table 8; Supplementary Figures S1, S2).
This nationwide population-based cohort study of Swedish patients treated with rhGH in childhood due to GHD, ISS or SGA with up to 35 years of follow-up, did not detect an increased risk of malignant neoplastic events. Regarding overall neoplastic events, including benign and unspecific neoplasms, a moderately increased occurrence in the patient group was seen, caused by an increased risk in male patients, and for those with the longest duration of treatment. This increase was caused by a higher frequency of benign and unspecific neoplasms and markedly diminished in the sensitivity analysis introducing a two-year lag-period after end of treatment. The present study thus supports the overall safety of rhGH-treatment to pediatric patients with GHD, ISS or SGA regarding the long-term risk of neoplastic events, and in particular regarding the risk of malignant neoplasms.
The potential cancer risk associated with GH treatment has been an ongoing concern since its introduction due to the role of the GH-IGF-1 signaling pathways in fundamental cellular processes of mitosis, growth and cell survival, along with supporting experimental evidence of its impact on tumor formation (21, 22). In the late 1980s, increased risk of leukemia associated with GH-treatment was reported from Japan (23) but could not be confirmed in later studies showing no increased risk in patients without previous risk factors (24). In 2002, a cohort study of patients previously treated with human pituitary-derived GH reported an increased relative mortality due to colorectal cancer and Hodgkin lymphoma but based on very few cases (25).
Large post-marketing databases have been initiated to detect adverse event signals, including neoplasms, in rhGH-treated patients. Most publications from these cohorts align with our study, showing no increased risk of cancer incidence or mortality in patients without an underlying increased cancer risk (9, 12, 26–30). Although these databases have gathered significant patient-years, the average follow-up is only about four years, which restricts conclusions on long-term risks. Given the typically prolonged latency period for malignancy development, a comprehensive follow-up period is necessary to detect any potential association with prior GH treatment.
Addressing some of the limitations in previous studies, a joint collaboration of eight European countries (SAGhE) was initiated in 2009 (31). In 2012, a publication from the French sub-cohort of GHD, ISS and SGA patients, reported no overall increased cancer-related deaths but an increased site-specific mortality due to bone tumors, based on three fatal cases (32). The final analysis of the whole SAGhE-cohort, similarly reported an increased site-specific mortality for bone tumors, based on the three included French cases, but no overall increased cancer mortality or increased cancer incidence in the GHD, ISS or SGA patients (13). In a subsequent study involving the same French sub-cohort, an additionally elevated incidence of bone tumors was reported (33).
Despite the extended follow-up period of around 17 years in the SAGhE-based publications, methodological challenges persist, preventing the ability to draw firm conclusions regarding the long-term cancer risks. The cohorts are largely heterogenous, lack information on adult treatment, and only crude comparisons with the general population were performed with no information of potential confounding factors or adjusted analyses taking such factors into account. The present study attempts to address several of these issues with a randomly selected and matched comparison group as well as data on important covariates such as birth characteristics, socioeconomic factors and height at study start for both the patients and controls. By controlling for all these factors, amongst other unmeasurable height-associated confounding factors, we aimed, as far as possible, to isolate how rhGH-treatment exposure in childhood might affect future risk of neoplasia.
In the present study, we only observed a moderate rise in the risk of overall neoplastic events and no increased risk of malignant neoplasms or tumors, benign or malignant, in bone or cartilage tissue. In the dose-response analyses, an overall significant trend with longer treatment duration was observed but not for higher mean or cumulative doses. If a higher exposure to rhGH treatment was indeed associated with an increased risk for future neoplastic events, one would expect to observe a similar trend for cumulative dose as well. Our stratified analysis, examining potential increases in risks of neoplasms based on follow-up length, also failed to detect any discernible difference, further reinforcing our overall findings.
In a previous study on GHD patients treated with rhGH as adults, Child et al. reported increased relative risks of primary cancer in those with a childhood onset GHD and those in the lowest age group (<35 years) (34). In our subgroup of adult-treated patients, we could not see an increase in malignant neoplasms, only an increased risk for overall neoplastic events indicating that this finding is underpinned by benign or uncertain neoplasms.
Despite considerable efforts to address numerous methodological challenges associated with investigating this subject, our study also has limitations, the foremost being the absence of untreated controls. However, instead of relying solely on comparisons with the general population, we established a comparison group that closely resembled our patients to isolate the effect of rhGH-treatment exposure. This was achieved by randomly selected age-, sex- and region-matched controls with adjustment of multiple potential confounders such as birth characteristics, socioeconomic factors, and height at study start. Secondly, a risk of detection bias could be present in our study, possibly explaining the increased number of benign tumors in endocrine glands, and we have addressed this by adding sensitivity analyses with a lag-period not only for the dose-response analyses, but also for all subgroup analyses. We could see that this analysis diminished any differences between the groups even further, reinforcing our main finding of similar neoplastic risks between patients and controls. Thirdly, in some of our subgroup analyses we had few events which created some uncertainty regarding our reported point estimates and increases the risk of type II errors. However, most of our analyses exhibit adequate statistical power, supporting the validity of our reported findings. Lastly, even if this is to date the longest follow-up of childhood rhGH-treated patients, the relative youth of our cohort restrict us to infer about risks in later adulthood and further motivates continuous surveillance of these patients into older ages.
In this nationwide population-based cohort study conducted in Sweden, encompassing a follow-up period of up to 35 years for children treated with rhGH due to GHD, ISS or SGA, we did not detect an increased risk of malignant neoplastic events in early to mid-adulthood. Only a moderate increase in overall neoplastic events was observed for a subgroup of patients, reinforcing our overall reassuring results. While continued monitoring of previously treated patients is still necessary, the present study represents the most comprehensive evidence available to date regarding the long-term cancer safety of rhGH treatment for the above-mentioned indications.
The datasets presented in this article are not readily available because the collected data for this study, received from the National Board of Health in Sweden and Statistics Sweden, cannot be shared with third parties based on legal agreements in data delivery and in accordance with prevailing laws and regulations governing the management of personal data in Sweden.
The studies involving humans were approved by the Regional Ethics Review Board in Stockholm (dnr: 2010/578-31/1, 2011/109-32-1, 2011/305-32, 2014/1775-32 and 2017/515-32). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study only used registry data.
AT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MB: Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. KS: Methodology, Supervision, Writing – review & editing. KA-W: Data curation, Methodology, Supervision, Validation, Writing – review & editing. LS: Data curation, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was supported by grants from the Swedish Research Council (2020-02025; to LS), grants from the Karolinska Institute (to LS), and from the Swedish Governmental grants under the ALF agreement by Region Stockholm (ALF; RS2021-0855; to LS, ALF; 2021-500619 to AT), and by Sahlgrenska University Hospital (ALFGBG-719041, ALFGBG-812951 and ALFGBG-965451; to KA-W). AT was also supported by Region Stockholm (clinical postdoctoral appointment). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
We thank the rhGH-treated individuals, their families, and the team of pediatricians and nurses that gathered clinical data during the follow-up years, which made this study possible. We would also like to acknowledge the Swedish GH treatment group, also Steering Committee members of the National GH Register from 1985 until 2013: (Kerstin Albertsson-Wikland [chair] Stefan Aronson, Peter Bang, Jovanna Dahlgren, Maria Elfving, Jan Gustafson, Lars Hagenäs, Anders Häger, Berit Kriström, Claude Marcus, Christian Moell, Karl Olof Nilsson, Svante Norgren, Martin Ritzén, Torsten Tuvemo, Ulf Westgren, Otto Westphal, and Jan Åman) for their support of this project. In addition, we also acknowledge the principal investigators of the different rhGH clinical trials 1985-2010 for GHD, ISS and SGA: Claude Marcus and Klas Ekström for the MiniMax study (TRN 151:142/01), Olle Söder for the NESGAS study, Jovanna Dahlgren for the SGA trial (TRN 2009/529-31), and Kerstin Albertsson-Wikland for all the other included clinical trials 1985-2010 for GHD, ISS and SGA (TRN89-071, TRN88-080, TRN88-177, TRN89-070-01, TRN89-071-01, TRN98-0198-003, TRA6280-003).
AT reports participation in advisory board for Pfizer. LS reports lecture honoraria from Merck, Novo Nordisk, and Pfizer, travel support from Novo Nordisk and participation in adjudication committee for Aetherna-Icon and advisory boards for Pfizer and Novo Nordisk.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1360139/full#supplementary-material
1. Danowitz M, Grimberg A. Clinical indications for growth hormone therapy. Adv Pediatr. (2022) 69:203–17. doi: 10.1016/j.yapd.2022.03.005
2. Ranke MB, Wit JM. Growth hormone - past, present and future. Nat Rev Endocrinol. (2018) 14:285–300. doi: 10.1038/nrendo.2018.22
3. Albertsson-Wikland K. Growth hormone in children with idiopathic short stature. Bmj. (2011) 342:d1248. doi: 10.1136/bmj.d1248
4. Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. (2011) 7:11–24. doi: 10.1038/nrendo.2010.171
5. Fleseriu M, Langlois F, Lim DST, Varlamov EV, Melmed S. Acromegaly: pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol. (2022) 10:804–26. doi: 10.1016/S2213-8587(22)00244-3
6. Laron Z, Werner H. Laron syndrome - A historical perspective. Rev Endocr Metab Disord. (2021) 22:31–41. doi: 10.1007/s11154-020-09595-0
7. Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol. (2013) 87:201–23. doi: 10.1016/j.critrevonc.2013.01.005
8. Knuppel A, Fensom GK, Watts EL, Gunter MJ, Murphy N, Papier K, et al. Circulating insulin-like growth factor-I concentrations and risk of 30 cancers: prospective analyses in UK biobank. Cancer Res. (2020) 80:4014–21. doi: 10.1158/0008-5472.CAN-20-1281
9. Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. (2010) 95:167–77. doi: 10.1210/jc.2009-0178
10. Stochholm K, Kiess W. Long-term safety of growth hormone-A combined registry analysis. Clin Endocrinol (Oxf). (2018) 88:515–28. doi: 10.1111/cen.13502
11. Backeljauw P, Kanumakala S, Loche S, Schwab KO, Miller BS, Levy R, et al. Safety and effectiveness of Omnitrope(®) (somatropin) in PATRO Children: a multi-center, post-marketing surveillance study comparison of US and international cohort data. Eur J Pediatr. (2022) 181:2367–78. doi: 10.1007/s00431-022-04409-8
12. Wilton P, Mattsson AF, Darendeliler F. Growth hormone treatment in children is not associated with an increase in the incidence of cancer: experience from KIGS (Pfizer International Growth Database). J Pediatr. (2010) 157:265–70. doi: 10.1016/j.jpeds.2010.02.028
13. Swerdlow AJ, Cooke R, Beckers D, Borgstrom B, Butler G, Carel JC, et al. Cancer risks in patients treated with growth hormone in childhood: the SAGhE European cohort study. J Clin Endocrinol Metab. (2017) 102(5):1661–72. doi: 10.1210/jc.2016-2046
14. Tidblad A, Bottai M, Kieler H, Albertsson-Wikland K, Sävendahl L. Association of childhood growth hormone treatment with long-term cardiovascular morbidity. JAMA Pediatr. (2021) 175(2):e205199. doi: 10.1001/jamapediatrics.2020.5199
15. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. (2009) 24:659–67. doi: 10.1007/s10654-009-9350-y
16. Albertsson-Wikland K, Martensson A, Savendahl L, Niklasson A, Bang P, Dahlgren J, et al. Mortality is not increased in recombinant human growth hormone-treated patients when adjusting for birth characteristics. J Clin Endocrinol Metab. (2016) 101:2149–59. doi: 10.1210/jc.2015-3951
17. Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. (2007) 16:726–35. doi: 10.1002/pds.1294
18. Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. (2009) 48:27–33. doi: 10.1080/02841860802247664
19. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. (2011) 11:450. doi: 10.1186/1471-2458-11-450
20. Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, et al. The Swedish cause of death register. Eur J Epidemiol. (2017) 32:765–73. doi: 10.1007/s10654-017-0316-1
21. Boguszewski CL, Boguszewski M. Growth hormone's links to cancer. Endocr Rev. (2019) 40:558–74. doi: 10.1210/er.2018-00166
22. Werner H, Laron Z. Role of the GH-IGF1 system in progression of cancer. Mol Cell Endocrinol. (2020) 518:111003. doi: 10.1016/j.mce.2020.111003
23. Watanabe S, Tsunematsu Y, Fujimoto J, Komiyama A, Delemaare-van de Waal H, Odink R, et al. Leukaemia in patients treated with growth hormone. Lancet. (1988) 331:1159–60. doi: 10.1016/S0140-6736(88)91968-X
24. Allen DB, Rundle AC, Graves DA, Blethen SL. Risk of leukemia in children treated with human growth hormone: review and reanalysis. J Pediatr. (1997) 131:S32–6. doi: 10.1016/S0022-3476(97)70008-8
25. Swerdlow AJ, Higgins CD, Adlard P, Preece MA. Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959-85: a cohort study. Lancet (London England). (2002) 360:273–7. doi: 10.1016/S0140-6736(02)09519-3
26. Child CJ, Zimmermann AG, Chrousos GP, Cummings E, Deal CL, Hasegawa T, et al. Safety outcomes during pediatric GH therapy: final results from the prospective geNeSIS observational program. J Clin Endocrinol Metab. (2019) 104:379–89. doi: 10.1210/jc.2018-01189
27. Mo D, Hardin DS, Erfurth EM, Melmed S. Adult mortality or morbidity is not increased in childhood-onset growth hormone deficient patients who received pediatric GH treatment: an analysis of the Hypopituitary Control and Complications Study (HypoCCS). Pituitary. (2014) 17:477–85. doi: 10.1007/s11102-013-0529-6
28. Child CJ, Zimmermann AG, Jia N, Robison LL, Bramswig JH, Blum WF. Assessment of primary cancer incidence in growth hormone-treated children: comparison of a multinational prospective observational study with population databases. Hormone Res Paediatr. (2016) 85:198–206. doi: 10.1159/000444124
29. Sävendahl L, Polak M, Backeljauw P, Blair JC, Miller BS, Rohrer TR, et al. Long-term safety of growth hormone treatment in childhood: two large observational studies: nordiNet IOS and ANSWER. J Clin Endocrinol Metab. (2021) 106:1728–41. doi: 10.1210/clinem/dgab080
30. Maghnie M, Ranke MB, Geffner ME, Vlachopapadopoulou E, Ibáñez L, Carlsson M, et al. Safety and efficacy of pediatric growth hormone therapy: results from the full KIGS cohort. J Clin Endocrinol Metab. (2022) 107:3287–301. doi: 10.1210/clinem/dgac517
31. Swerdlow AJ, Cooke R, Albertsson-Wikland K, Borgstrom B, Butler G, Cianfarani S, et al. Description of the SAGhE cohort: A large european study of mortality and cancer incidence risks after childhood treatment with recombinant growth hormone. Hormone Res Paediatr. (2015) 84:172–83. doi: 10.1159/000435856
32. Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab. (2012) 97:416–25. doi: 10.1210/jc.2011-1995
33. Poidvin A, Carel JC, Ecosse E, Levy D, Michon J, Coste J. Increased risk of bone tumors after growth hormone treatment in childhood: A population-based cohort study in France. Cancer Med. (2018) 7:3465–73. doi: 10.1002/cam4.1602
Keywords: growth hormone, treatment, cancer, risk, long-term safety, childhood
Citation: Tidblad A, Bottai M, Smedby KE, Albertsson-Wikland K and Sävendahl L (2024) Long-term risk of neoplastic events after childhood growth hormone treatment: a population-based cohort study in Sweden. Front. Endocrinol. 15:1360139. doi: 10.3389/fendo.2024.1360139
Received: 22 December 2023; Accepted: 19 February 2024;
Published: 05 March 2024.
Edited by:
George Paltoglou, National and Kapodistrian University of Athens, GreeceReviewed by:
Laurie E. Cohen, Children’s Hospital at Montefiore, United StatesCopyright © 2024 Tidblad, Bottai, Smedby, Albertsson-Wikland and Sävendahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anders Tidblad, YW5kZXJzLnRpZGJsYWRAa2kuc2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.