- 1Department of Endocrinology and Metabolism, Shenzhen University General Hospital, Shenzhen, Guangdong, China
- 2Department of Pathophysiology, Shenzhen University Medical School, Shenzhen, Guangdong, China
Diabetic vascular complications are prevalent and severe among diabetic patients, profoundly affecting both their quality of life and long-term prospects. These complications can be classified into macrovascular and microvascular complications. Under the impact of risk factors such as elevated blood glucose, blood pressure, and cholesterol lipids, the vascular endothelium undergoes endothelial dysfunction, characterized by increased inflammation and oxidative stress, decreased NO biosynthesis, endothelial-mesenchymal transition, senescence, and even cell death. These processes will ultimately lead to macrovascular and microvascular diseases, with macrovascular diseases mainly characterized by atherosclerosis (AS) and microvascular diseases mainly characterized by thickening of the basement membrane. It further indicates a primary contributor to the elevated morbidity and mortality observed in individuals with diabetes. In this review, we will delve into the intricate mechanisms that drive endothelial dysfunction during diabetes progression and its associated vascular complications. Furthermore, we will outline various pharmacotherapies targeting diabetic endothelial dysfunction in the hope of accelerating effective therapeutic drug discovery for early control of diabetes and its vascular complications.
1 Introduction
Diabetes is a chronic and severe metabolic disorder characterized by consistently high blood sugar levels. According to the International Diabetes Alliance statistics, the number of individuals living with diabetes worldwide in 2021 is around 537 million, with an estimated increase to 783 million in 2045 (1). The health burden of diabetes is largely due to diabetic vascular complications. Diabetic vascular complications include macrovascular complications, such as coronary artery disease (CAD), cerebrovascular disease, peripheral artery disease (PAD), and microvascular diseases, including diabetic nephropathy (DN), diabetic retinopathy (DR), diabetic neuropathy (DPN), and cardiomyopathy (Figure 1). These complications are critical factors that contribute to a huge burden on individuals with diabetes (2).
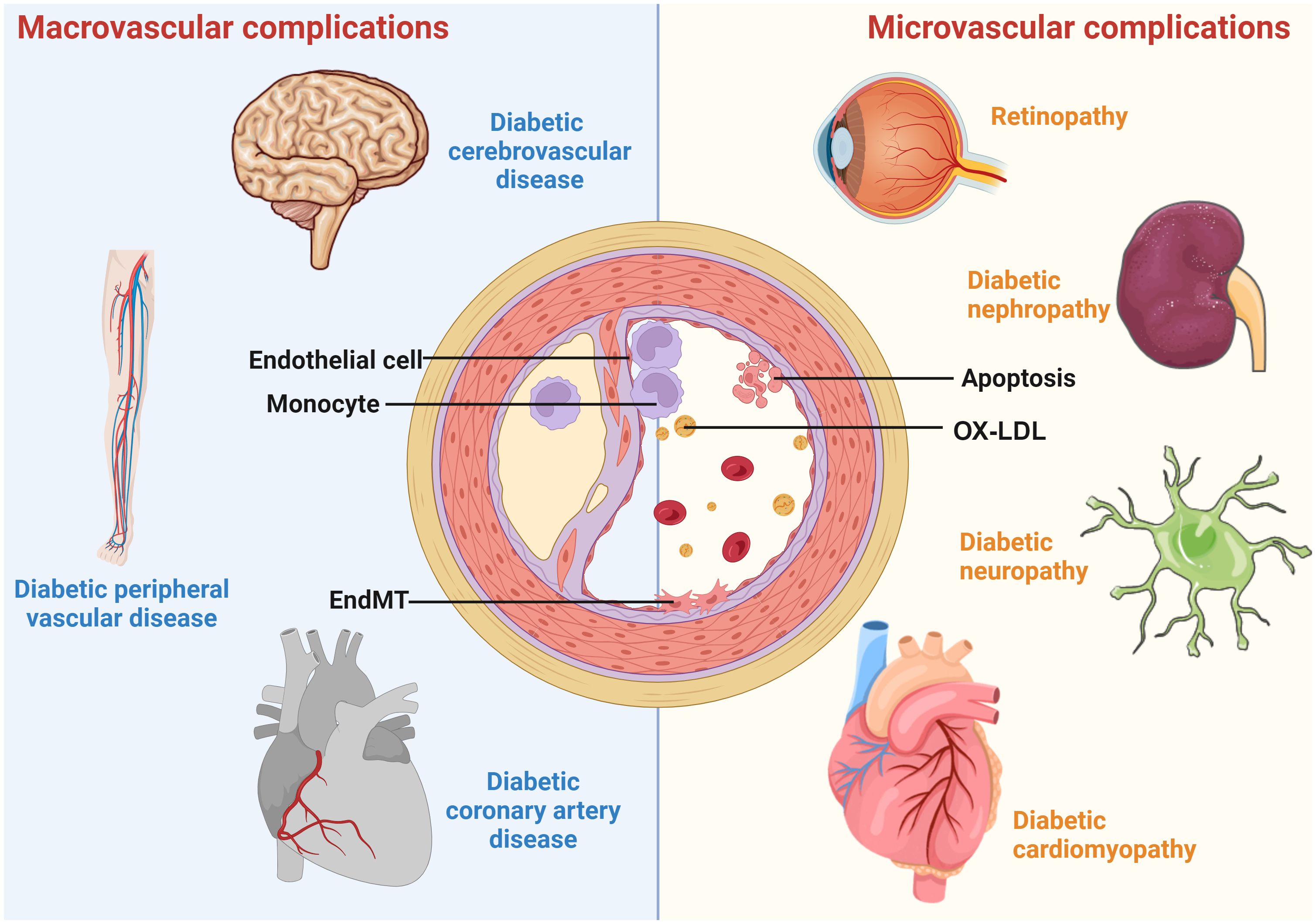
Figure 1 A schematic overview of diabetic vascular complications. Diabetic vascular complications include macrovascular complications (diabetic coronary artery disease, cerebrovascular disease, and peripheral vascular disease) and microvascular complications (diabetic retinopathy, nephropathy, cardiomyopathy, and neuropathy). Continued exposure to risk factors causes endothelial dysfunction, leading to lipid retention in the endothelium. Monocytes differentiate into macrophages, internalize modified lipoproteins, and form foam cells. Activated foam cells induce inflammation by secreting cytokines through several downstream signals. In addition, endothelial cells undergo EndMT, cell death, etc., further exacerbating the atherosclerotic process.
The vascular endothelium is a continuous lining within the vascular system that is responsible for maintaining vascular tension, angiogenesis, and hemostasis. Additionally, it provides crucial antioxidant, anti-inflammatory, and antithrombotic surfaces. Endothelial dysfunction can be narrowly defined as a decrease in vasodilation capacity or, more broadly, as any changes affecting endothelial homeostasis function. Although the interaction between endothelial dysfunction and vascular complications of diabetes is not fully understood, the existing view shows that a high glucose environment leads to the impairment of endothelium-dependent vasodilation capacity by increasing oxidative stress and inflammation, reducing vasodilation factor activity, increasing reactive oxygen species (ROS) production, promoting the uncoupling of endothelial nitric oxide synthase (eNOS), and so on (3). Endothelial dysfunction is also an important early indicator of atherosclerosis (AS) and identifies patients at a higher vascular risk, providing a “barometer” for vascular health (4). Therefore, endothelial dysfunction can be an early event in the vascular complications of diabetes. Understanding the importance of endothelial dysfunction is particularly crucial for controlling diabetic vascular complications and lowering the prevalence of diabetic vascular diseases. Additionally, researchers have established the detrimental role of endothelial dysfunction in the onset and development of serious diabetic microvascular diseases, such as heart failure, cognitive decline, and worsening metabolic dysfunction, in addition to vision loss, renal insufficiency, and neuropathy (5). We further confirmed the research keywords related to diabetic vascular complications through visual analysis and identified endothelial function as a hot topic in this field. Therefore, impaired endothelial function could become a promising target for preventing vascular complications in patients with diabetes, especially in DR and CAD (Figure 2). However, there has not been an updated review on the mechanisms and therapies of diabetic vascular complications, especially focusing on endothelial dysfunction. We aim to provide a comprehensive summary of diabetic endothelial dysfunction, which may offer a deeper insight into endothelial dysfunction to not only help prevent the deterioration of diabetes and its vascular complications but also accelerate the process of developing more beneficial therapies.
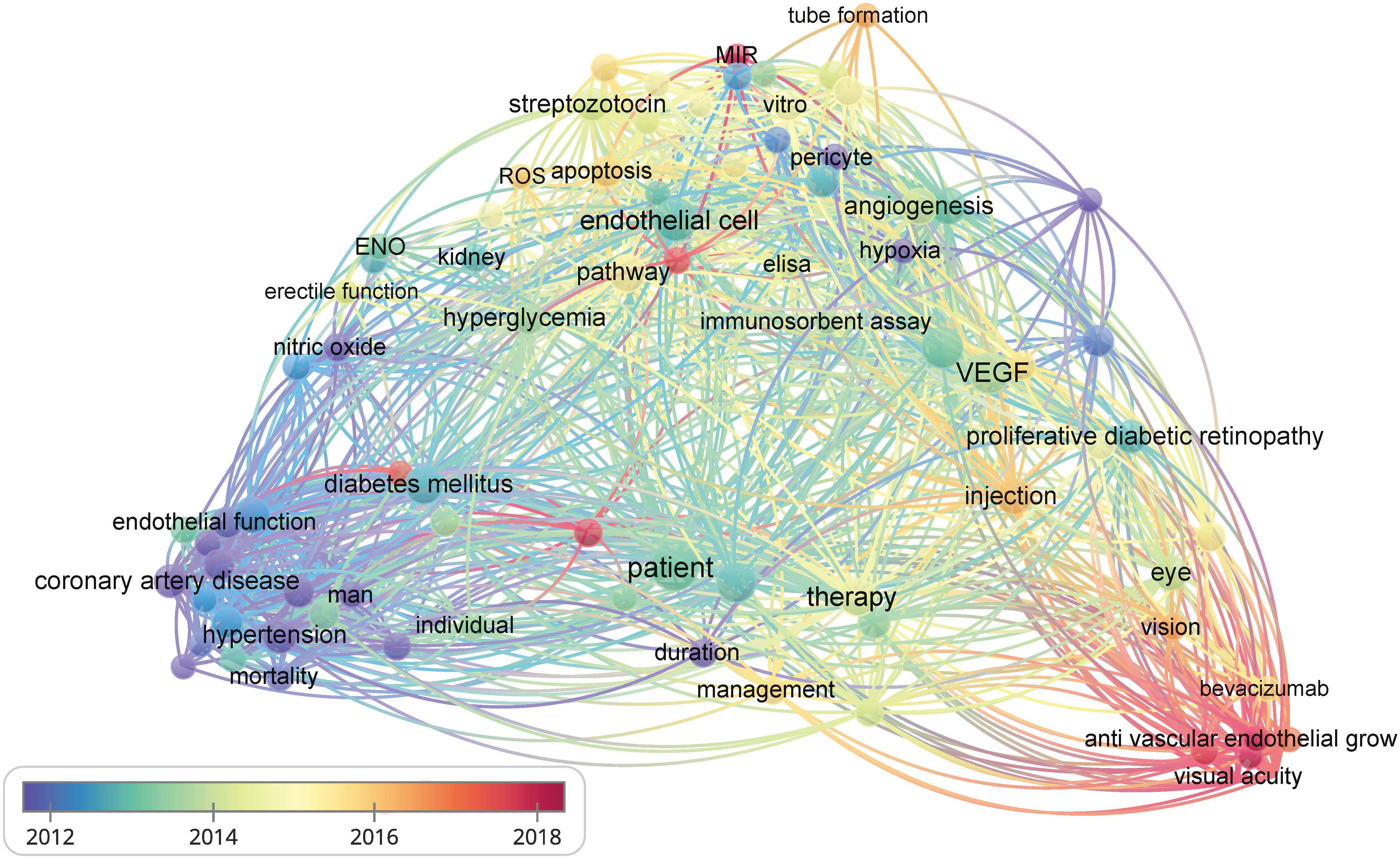
Figure 2 The bibliometric data obtained using VOSviewer. It shows the frequency of keywords in the literature related to diabetes and its vascular complications. The color of dots and lines represent the average year of publication for the corresponding term.
In this review, we present the research progress on the mechanisms of endothelial dysfunction in diabetes and its vascular complications, mainly focusing on inflammation, oxidative stress, endothelial-to-mesenchymal transition (EndMT), and cell death. We also summarized the effects and limitations of common hypoglycemic agents and novel regulators on the vascular endothelium.
2 Mechanisms of diabetic endothelial dysfunction
2.1 Inflammation
Chronic, low-grade inflammation is widely considered to be a significant factor in the development of diabetes. Hyperglycemia can activate various pathways and pro-inflammatory factors that induce endothelial dysfunction. It triggers the nonclassical nuclear factor kappa-B (NF-κB) signaling pathway, produces cytokines and chemokines, promotes inflammation, and damages β cellular function (6). Many early findings have identified the proinflammatory effect of NF-κB, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) on endothelial dysfunction in diabetes (3). TNF-α in particular can activate critical intracellular signaling molecules in various inflammatory signaling systems, such as c-Jun amino-terminal kinases (JNK) and IkappaB kinase beta (IKKβ) (7, 8), leading to impaired insulin action. Under normal conditions, insulin activates the phosphatidylinositol 3-kinase (P13K) pathway, which phosphorylates eNOS at Ser1177 in endothelial cells (ECs) to produce nitric oxide (NO) to maintain normal endothelial function (9). In an insulin-resistant state, this pathway is disrupted, leading to the imbalance between the phosphoinositide-3 kinase (PI-3K)/protein kinase B (Akt/PKB) and mitogen-activated-protein kinase (MAPK)/extracellular signal-regulated kinases (ERK) pathways. This will eventually disrupt vascular homeostasis. Activation of the MAPK pathway can release inflammatory mediators such as vascular cell adhesion protein 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), E-selectin, and plasminogen activator inhibitor-1 (PAI-1), which can compromise normal endothelial function (10). In addition, in experimental models of cardiovascular end-organ damage associated with diabetes, ANG- (1–7) has been shown to act as an anti-inflammatory agent (11). It regulates the expression of VCAM-1 induced by ANG II by reducing the nuclear translocation of NF-κB in ECs (12). WNT5A, a ligand of the Wnt signal pathway, gained attention for its pro-inflammatory effects and its role in the pathogenesis of diabetic complications (13). Researchers discovered that SETD8 (SET8) can modulate WNT5A function and control the production of proinflammatory enzymes and endothelial adhesion. Overexpression of SETD8 reduced inflammation by decreasing the activation of the NOD-like receptor pyrin domain 3 (NLRP3) inflammasome and the expression of microtubule-affinity regulating kinase 4 caused by hyperglycemia in human umbilical vein endothelial cells (HUVECs) (14, 15). Further to this, vascular endothelial growth factor (VEGF) levels change significantly in diabetic patients and play a vital role in diabetic microvascular complications (16). Activation of vascular endothelial growth factor receptor 1 (VEGFR1) in ECs attracts inflammatory cells to white adipose tissue in type 2 diabetes mellitus (T2DM). This effect can be alleviated by VEGF-B deficiency targeting the VEGFR1-EC axis (17).
Intracellular hyperglycemia can increase production of advanced glycation end products (AGEs), promoting the interaction of AGEs and the advanced glycosylation end product-specific receptor (AGER, also known as RAGE). This receptor acts as a pattern-recognition receptor that initiates specific cell signaling (18). AGE-RAGE is associated with multiple signal transduction pathways that promote atherosclerotic formation. This includes increased expression of VCAM-1, macrophage inflammatory protein-1 (MIP-1), matrix-metalloproteinase 9 (MMP9), interleukin-1β (IL-1β), and TNF-α, which mediate leukocyte adhesion and vascular inflammatory responses (19). Serum albumin glycosylation in a high glucose environment forms AGE-modified human serum albumin (AGE-HSA). In diabetic patients, it can activate the production of macrophages C-C chemokine ligand 5 (CCL-5) and IL-8, driving an increase in the inflammatory response. Inflammatory cytokines may increase the total amount of AGE-HAS in the body, leading to a vicious cycle (20).
In addition to the pathways mentioned above, hyperglycemia also genetically regulates inflammation in endothelial dysfunction. By partially suppressing signal transduction and activators of transcription 1 (STAT1), hyperglycemia upregulates the expression of proinflammatory target genes, including CCL5, CXCL10, and ICAM1. This causes tumor necrosis factor-like weak inducer of apoptosis (TWEAK) to demonstrate its pro-atherosclerotic tendency (21). Moreover, Zhu et al. found that, when exposed to high glucose levels, the activation of the mechanosensory ion channel Piezo1 upregulates genes involved in inflammatory pathways (such as IL1B). This, in turn, increases the risk of pathological mechanics-induced thrombosis by enhancing the prothrombin reaction of platelets, erythrocytes, and neutrophils, ultimately leading to disrupted blood flow (22). In this disordered blood flow state, ECs are affected by turbulent mechanical stimulation, which causes an increase in Piezo1 expression beyond the threshold, which activates the NF-κB pathway and finally leads to atherosclerosis (23).
2.2 Oxidative stress
Oxidative stress occurs when the balance between intracellular pro-oxidation and antioxidant systems is disrupted. In diabetic ECs, intracellular hyperglycemia can increase mitochondrial ROS (mtROS) generation, which accelerates endothelial dysfunction. ROS were thought to be primarily produced by the NADPH oxidase (NOX) family in phagocytic cells (24, 25). NOX1 and NOX4 were upregulated in aortas and mesenteric arteries of T2DM mice (26). Enhanced NOX4 activity and expression were also found in rats with type 1 diabetes (27). Both may lead to an increase in ROS. Angiotensin converting enzyme-2 (ACE2) promotes angiotensin (ANG)-(1-7)-dependent vasodilation and may inhibit NOX-induced ROS production in ECs. Therefore, in diabetes-induced kidney injury, mice deficient in ACE2 exhibit an increase in oxidative stress (12, 28). Ang-(1-7) treatment also attenuated the elevation of renal NOX activity in the kidneys of diabetic spontaneously hypertensive rats, reducing the degree of hyperglycemia. It significantly prevented the diabetes-induced reduction in catalase activity, as well as the reduction in PPAR-gamma mRNA and protein levels in vitro (27, 29). High levels of ROS generation or low antioxidant activity can result in endothelial mitochondria dysfunction (30). Mitochondrial calcium uptake 1 (MICU1) in the mitochondrial inner membrane regulates calcium uptake and maintains calcium homeostasis. Endothelial MICU1 expression decreased in diabetes and other vascular illnesses including diabetic cardiomyopathy. Inhibiting MICU1 resulted in an increase in mitochondrial ROS production (31). The impaired mitochondria isolated from hyperglycemia-treated cells showed increased palmitoylcarnitine oxidation, decreased pyruvate oxidation, and Uncoupling protein 2 (UCP2). These findings suggest that hyperglycemia may cause excessive mitochondrial ROS formation by increasing fatty acid oxidative damage or uncoupling the mitochondrial oxidative phosphorylation system (32). ECs motility, proliferation, angiogenesis, and death are all reliant on mitochondrial metabolism (33). Therefore, the damaged mitochondrial metabolism induced by hyperglycemia may impact normal EC function, leading to endothelial dysfunction.
Sun indicated that oxidative stress activates Sirtuin 1 (SIRT1), which deacetylates the forkhead box transcription factor O1 (FOXO1) and traps it in the nucleus, enhancing its transcriptional activity. FOXO1 enhancement may inhibit the repair of glycolysis-dependent oxidative DNA damage, which can worsen endothelial oxidative damage (34). However, another study showed that SIRT1 promotes insulin secretion and pancreatic beta cell survival by interacting with FOXO, which can alleviate diabetes (35). Additionally, restoring aortic SIRT1 levels can significantly improve endothelial dysfunction and vascular compliance by enhancing eNOS activity and inhibiting NOX-associated oxidative stress (36). SIRT1 can improve vascular function in diabetes by deacetylating the 66-kDa Src homology 2 domain-containing protein (p66Shc) and reducing ROS production (37). The differences in experimental materials, the degree of FOXO activation, and the complicated functions of SIRT1 may lead to the differences in the above research findings. Besides, healthy ECs normally undergo a metabolic shift to glycolysis to facilitate prompt DNA repair responses after H2O2 treatment. However, overactivation of FOXO1 in hyperglycemia can cause DNA damage, leading to endothelial dysfunction (34). Consequently, the function of SIRT1 and FOXO in diabetic endothelial function needs further exploration.
2.3 Endothelial-to-mesenchymal transition
EndMT is a process in which ECs transform into mesenchymal stem cells under certain pathological situations, such as high oxidative stress, disturbed metabolism, hypoxia, and shear stress force (38). Notably, EndMT has been discovered to exist in a majority of diabetic complications (39). If a significant proportion of ECs undergo EndMT, this may lead to an endothelial layer disruption resulting in plaque erosion and finally accelerating the progression of atherosclerotic disease (40).
Hyperglycemia activates many downstream signaling pathways to initiate EndMT in diabetic Ecs (39). These signaling pathways can be divided into TGF-β signaling and non-TGF-β signaling (41). TGF-β signaling contains canonical Smad2/3-mediated pathways and noncanonical Smad2/3-independent signaling pathways such as MAPK, RhoA, and c-Jun NH2-terminal kinases (JNK). Non-TGF-β signaling mainly includes Notch signaling, the canonical Wnt pathway, and other regulatory mechanisms mediated by cytokines and some inflammatory mediators (42). Of all the signaling pathways, EndMT is thought to be most significantly influenced by the TGF-β signaling pathway, specifically the canonical downstream Smad pathway (43).
Hyperglycemia induces EndMT by directly increasing TGF-β signaling and non-TGF-β signaling. TGF-β signaling is activated by hyperglycemia as a part of EC metabolic memories, which can cause EndMT in Ecs even after the culture condition is changed to normal glucose levels (44). By directly increasing the production of angiotensin II (Ang II), hyperglycemia causes EndMT in primary human aortic endothelial cells, which damages the endothelium (45). The negative effects of Ang II/AT1 pathway in Ecs can be counteracted by the Ang (1-7)/Mas pathway. The equilibrium of Ang II and Ang (1-7) might be a crucial factor in the pathophysiological events that result in endothelial dysfunction (12, 46). Additionally, the crosstalk of the TGF-β pathway and Wnt/β-catenin signaling also regulates EndMT and contributes to endothelial problems in diabetic situations, since increased β-catenin expression enhances TGF-β sensitivity in hyperglycemic situations (47).
Except for the direct role of hyperglycemia in EndMT, the higher context of inflammation and oxidative stress can regulate EndMT in many ways. NF-κB activation-mediated EndMT can be induced by IL-1β plus TGF-β2 or TNF-α and IL-6 stimulation of ECs (48, 49). EndMT can also be induced by oxidative stress. The expression of endothelial and fibrotic markers changed with increasing dosages of H2O2 (0.1–10 μM) and initiated the transformation process in HUVECs. This was accompanied by increased expression of TGF-β1 and TGF-β2 and was found to be reliant on activin receptor-like kinase 5 (ALK5) expression, Smad3 activation, and NF-κB activity (50). Moreover, SIRT1 can block EndMT in human ECs via deacetylating the Smad4 pathway (51). AMP-activated protein kinase (AMPK) activates SIRT1, which can inhibit Smad2/3 nuclear translocation, thereby decreasing TGFβ-induced EndMT. This suggests that AMPK may be involved in EndMT-induced diabetic endothelial damage (52, 53).
2.4 Cell death
Cell death can be classified into two categories: programmed and accidental. The main form of accidental cell death is called necrosis, where intracellular contents are released because of the rupture of the plasma membrane (54). Regulated cell death caused by an intracellular program is referred to as programmed cell death and includes apoptosis, autophagy, pyroptosis, ferroptosis, and necroptosis. Regulated cell death influences various pathophysiological situations, including inflammation, immunology, tissue homeostasis, and cell growth. Diabetic endothelial injury is significantly associated with cell death (55).
Apoptosis is a crucial feature of vascular injury under some circumstances, such as high levels of ox-LDL, AGE, oxidative stress in the blood, and disturbed blood flow (3). A recent study reported that TNF-related apoptosis-inducing ligand-deficient diabetes mice fed a high-fat diet displayed accelerated AS. These mice also showed more widespread necrotic cores in plaques and enhanced macrophage infiltration and apoptosis in islets (56). Endothelial WW domain-containing E3 ubiquitin protein ligase 2 (WWP2) can preserve cell survival and prevent apoptosis by targeting DEAD-box helicase 3 X-linked (DDX3X) for K63-linked polyubiquitination and proteasomal degradation, thus preventing endothelial injury (57). In addition, knocking down nuclear factor erythroid 2-related factor 2 (Nrf2) increases apoptosis and hinders tube formation in endothelial progenitor cells (EPCs) sourced from healthy donors and wild-type mice. However, overexpression of Nrf2 reduced apoptosis and restored tube formation in EPCs obtained from diabetic patients and db/db mice (58).
Autophagy is a life-sustaining process that helps maintain endothelial function (59). It can be classified into two categories: selective and non-selective. Selective autophagy such as mitophagy, lysophagy, and ER-phagy specifically targets and engulfs substrates. High glucose levels suppress the CAV1-CAVIN1-LC3B-mediated autophagic degradation of CAV1, a component of caveolae. This process promotes the transcytosis of LDL across ECs, ultimately resulting in the subendothelial retention of atherogenic lipids (60). The impairment of autophagosome-lysosome fusion and the inhibition of autophagy-related 14 (Atg14) expression, meditated by FOXO1, leads to autophagic apoptosis in ECs induced by AGEs (59). Most studies have shown that mitophagy protects hyperglycemia-treated ECs. To counteract mitochondrial ROS and prevent aging, high glucose increases mitophagy in human aortic ECs (61). Hyperglycemia dramatically inhibited mitophagy in HUVECs by downregulating PTEN-induced kinase 1 (PINK1), Parkin, LC3 II, Beclin-1, and autophagy-related gene 5 (ATG5) (62). Another study found that diabetic mice have higher levels of PINK1 and Parkin in the vascular wall. The PINK1-Parkin pathway induces mitophagy to prevent metabolic stress-induced endothelium damage (63). However, increases or decreases in PINK1 and Parkin conflict. The difference may be attributed to empirical materials and the high-glucose duration of treatment. Hence, more research is required to confirm the precise changes in Parkin and PINK1 levels.
Ferroptosis is a type of regulated necrosis that depends on iron and results from significant damage to cell membranes due to lipid peroxidation. It typically occurs alongside significant iron accumulation and lipid peroxidation during cell death (64). Vascular ECs exposed to high glucose levels in vitro exhibited higher levels of ferroptosis-associated proteins (65). The expression of heme oxygenase 1 (HMOX1) markedly increased in diabetic mice and diabetic ECs treated with high glucose and high lipid. HMOX1 upregulation is associated with increased ferroptosis in diabetes-induced endothelial injury. Suppression of HMOX1 can effectively rescue the negative effects of high glucose and high lipids by rebalancing iron and oxidative stress and blocking excessive ferroptosis (66). Moreover, glutathione peroxidase 4 (GPX4), an antioxidant enzyme, facilitates the removal of excess lipid peroxides. It’s a crucial upstream regulator of ferroptosis (67). The intracellular GPX4 reduction in hyperglycemia environments then leads to an overabundance of ROS and Fe2+ in various cells, including ECs. These elements are considered critical factors in the initiation of ferroptosis (68). Hyperglycemia and IL-1β leads to ECs ferroptosis and dysfunction by downregulating xCT (the substrate-specific subunit of system Xc-) expression through activation of p53-XcT-glutathione (GSH) axis (69).
Pyroptosis is a recently discovered form of programmed cell death that involves an early disruption of the plasma membrane, leading to the extracellular leakage of intracellular contents (70). In recent years, researchers have extensively studied the connection between pyroptosis and diabetic microvascular complications. In diabetic retinopathy, treatment of human retinal microvascular endothelial cells (HRMECs) with hyperglycemia resulted in decreased cell viability and increased caspase1 activity, indicating hyperglycemia’s induction of HRMECs pyroptosis (71). In addition, the pyroptotic pathway mediated by caspase1-GSDMD was activated by hyperglycemia in glomerular endothelial cells (GECs), which induced the development of diabetic nephropathy (72).
In summary, hyperglycemia and related risk factors, including high lipids and a disordered blood flow state, contribute to endothelial dysfunction through several pathways, such as increased inflammation, reactive oxygen species (ROS) production, EndMT, and cell death. The compromised endothelium is responsible for initiating vascular dysfunction, which may progress into more severe vascular problems without continuous glucose control and treatment over time (Figure 3).
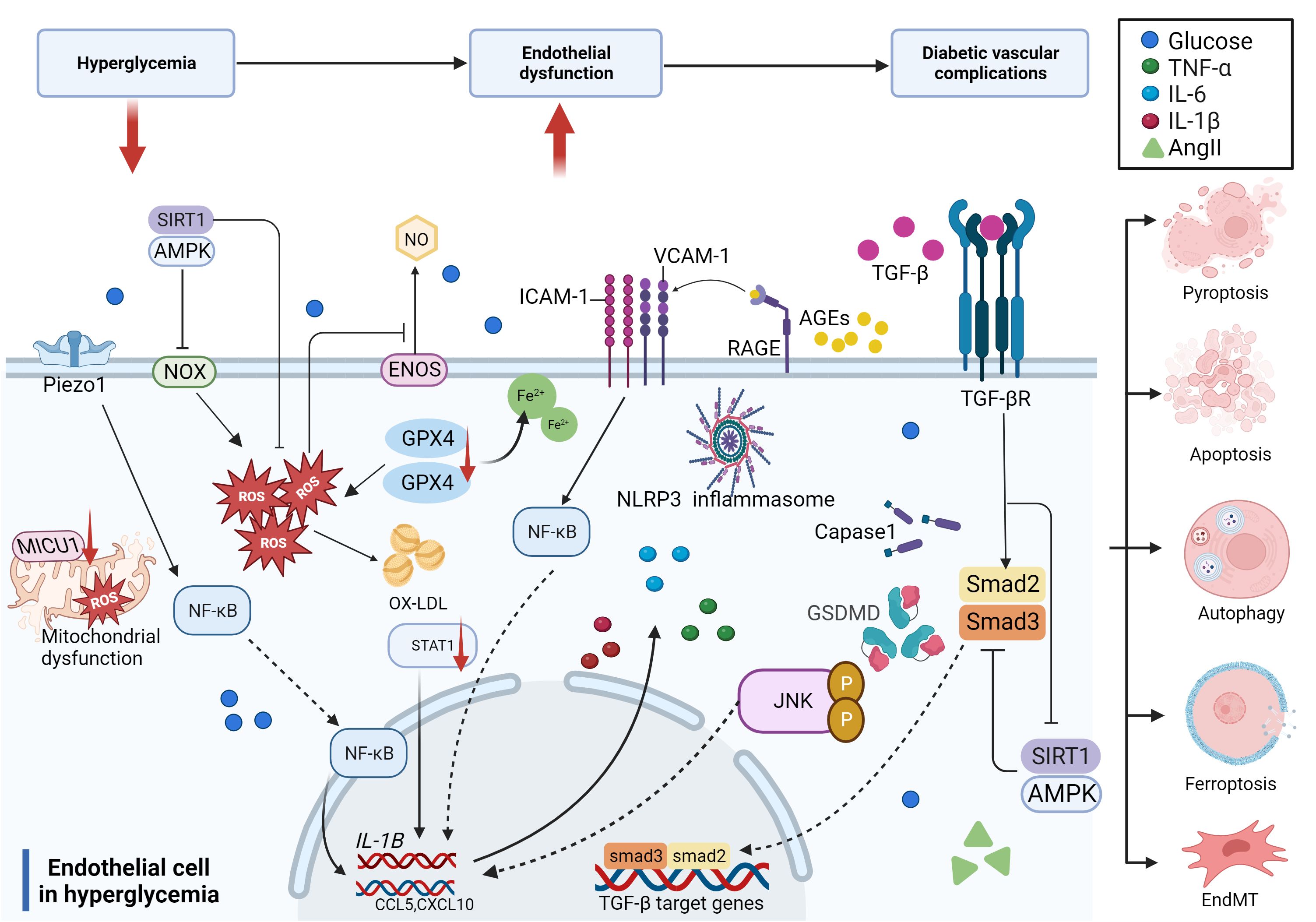
Figure 3 Molecular mechanisms of diabetic endothelial dysfunction. The mechanisms of diabetic endothelial dysfunction are complicated and include oxidative stress, inflammation, EndMT, and cell death. Most of these related factors work together to accelerate the process of diabetic endothelial dysfunction through different signaling pathways, which ultimately lead to diabetic vascular complications.
3 Diabetic macrovascular complications
Diabetic macroangiopathy mainly contains AS in large and medium arteries (aorta, coronary, basilar, and peripheral arteries), leading to vascular disorders including coronary artery disease (CAD), peripheral artery disease (PAD), and cerebrovascular disease. AS is characterized by the accumulation of fatty and/or fibrous material in the intima. Endothelial dysfunction is required for the initiation of AS, which has been identified as a major cause of diabetic macrovascular complications (73).
3.1 Diabetic atherosclerosis
Previous research has demonstrated that diabetes mellitus and AS share the same endothelial pathological response (74). As mentioned above, hyperglycemia can lead to endothelial dysfunction via various mechanisms. Endothelial dysfunction is indeed the key factor in triggering AS, so diabetes can promote the onset and development of AS.
As previously mentioned, many studies have proved that high glucose induces ROS production, which in turn causes AS. Excessive ROS can accelerate AS by directly activating proinflammatory pathways and indirectly by increasing the formation of ox-LDL, promoting insulin resistance, activating ubiquitination-related pathways, and decreasing the activation of adiponectin, AMPK, and eNOS (24). In hyperglycemic macrovasculature damage, ROS and inflammatory cytokines cause signaling dysregulation and EndMT. EndMT produces mesenchymal cells that express and deposit ECM proteins, accelerating the development of AS by acting as a support for developing plaque (75, 76). Hyperglycemia-induced TGF-β signaling activation also prompts EndMT and AS (77). Eventually, atherosclerotic plaque encroaches on the artery lumen, causing flow-limiting lesions and serious vascular consequences. Thus, without diabetes control and therapy, a damaged endothelium will worsen, causing AS and other vascular complications in diabetes (Figure 4).
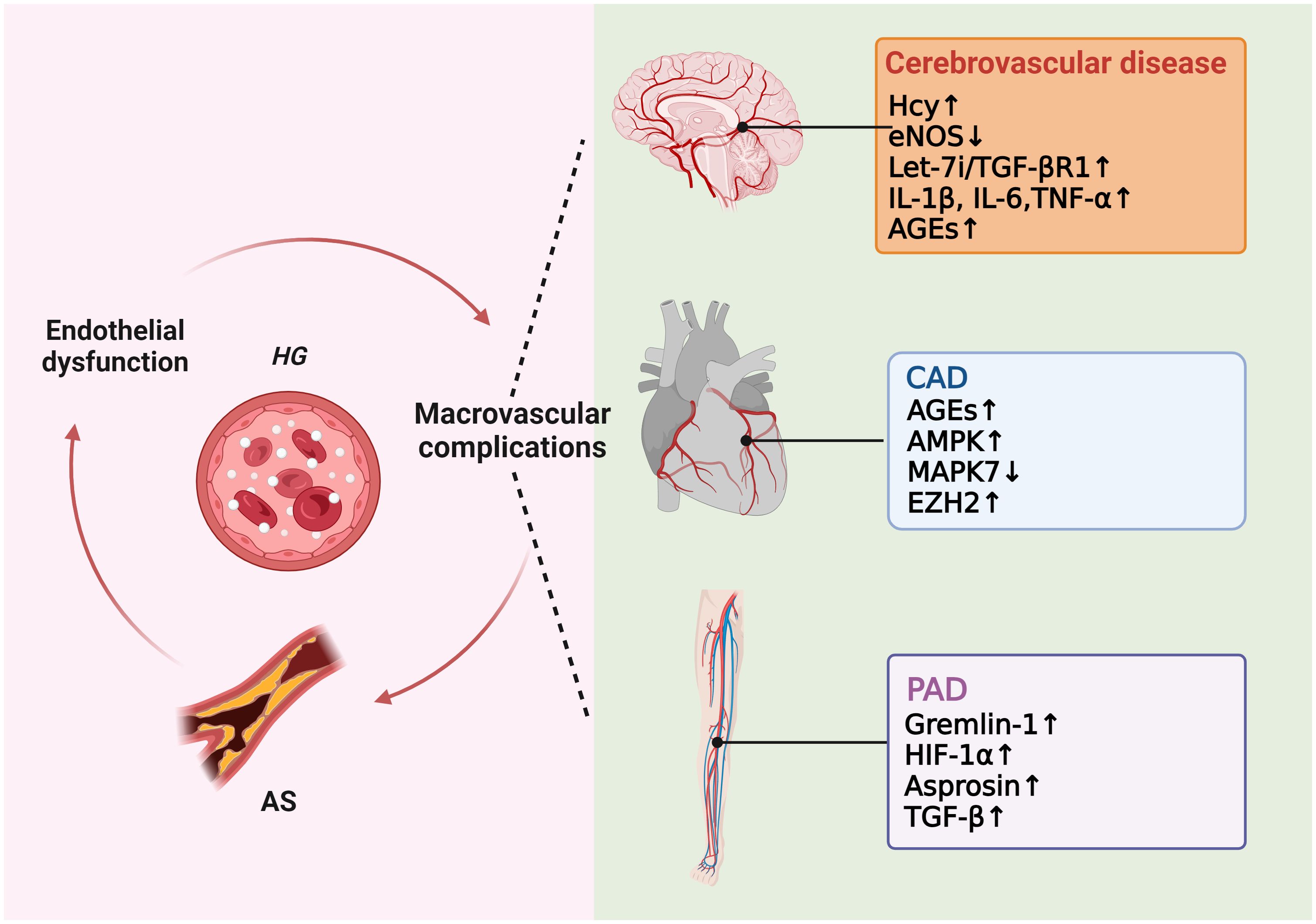
Figure 4 Molecular mechanisms of diabetic macrovascular complications. Hyperglycemia leads to endothelial dysfunction, atherosclerosis (AS), and diabetic macrovascular disorders, including coronary artery disease (CAD), cerebrovascular disease, and peripheral vascular disease. These conditions affect each other in a vicious cycle if they are not treated appropriately.
3.2 Diabetic coronary artery disease
Despite the significance of all T2DM complications, cardiovascular disease (CAD) remains the leading cause of morbidity and mortality in individuals with diabetes (78). It is worth highlighting that people with T2DM have a higher risk of developing cardiovascular diseases compared to those without diabetes, with the risk being two to four times higher (79).
CAD and its related complications are believed to be strongly associated with oxidative stress (80). As stated above, diabetics have high oxidative stress due to the inequality between the production of ROS and the protection systems of both enzymatic and non-enzymatic antioxidants. This can lead to endothelial dysfunction and AS of the coronary arteries (81). CAD has been linked to AGEs accumulation in patients diagnosed with T2DM (82). Diabetic patients with obstructive CAD have higher serum AGEs concentrations compared to T2DM patients without obstructive CAD (83). In comparison to those with CAD alone, patients with CAD and T2DM had impaired endothelium-dependent vasodilation and enhanced mitochondrial H2O2 production and AMPK activation in primary human saphenous vein endothelial cells mediated by mtROS (84). Additionally, the elevated plasma concentration of TNF-α and its receptor observed in the coronary arterioles of db/db mice enlightens the role of inflammation in DM patients with CAD (85). With the increase in intima/media thickness ratio in CAD patients, atheroprotective Mitogen-activated protein kinase 7 (MAPK7) expression decreased, whereas Zeste Homolog 2 (EZH2) expression increased. MAPK7-EZH2 reciprocity may indicate an autoregulatory feedback loop in ECs that maintains endothelial homeostasis. Disruptions in this relationship that promotes EZH2 expression can cause endothelial dysfunction and EndMT, aggravating the severity of human CAD (86).
3.3 Diabetic cerebrovascular disease and stroke
Numerous studies have shown increased infarct size, edema, and hemorrhage in hyperglycemic animals as compared to their normal counterparts (87). Ischemic stroke is the most common cerebrovascular complications of diabetes (88). Stroke related to diabetes is often caused by extracranial carotid artery disease and intracranial large and small vessel diseases. It is the second highest cause of death among patients with T2DM (79).
Endothelial dysfunction aggravated by diabetes is an essential factor in stroke development in diabetic patients and may contribute to endothelial dysfunction after cerebral ischemia (88, 89). Additionally, in the normal cerebral microcirculation and major cerebral arteries, endothelium generates NO both basally and in response to various vasoactive stimuli. However, in diabetic encephalopathy, there is a decrease in the expression of VEGF and eNOS. This leads to a diminished vascular autoregulatory response and impaired cerebral artery endothelial function, further worsening diabetic cerebrovascular disease (90). As a risk factor for cerebrovascular diseases in DM patients, serum homocysteine (Hcy) increases significantly in T2DM patients (91). Hcy harms vascular endothelium directly by oxidative stress and endoplasmic reticulum stress or indirectly by the cytokine and the immune response (92, 93). Ischemic stroke initiates a let-7i/TGF-βR1 double-negative feedback loop that causes EndMT in ECs and vascular fibrosis. This suggests that EndMT could be a target for treating cerebral vascular fibrosis (94).
Diabetic macroangiopathy and microangiopathy exacerbate each other. Macrovessel obstruction may lead to brain perfusion deficiency and microvascular diseases. Microvascular dysfunction also affects macrovessel collateral circulation, increases the risk of stroke, and worsens prognosis (95). As the main part of the cerebral microvascular system, blood-brain barrier (BBB) dysfunction may worsen cerebral macroangiopathy dysfunction by affecting cerebral microangiopathy indirectly. Higher expression of proinflammatory factors such as IL-1β, IL-6, and TNF-α and accumulation of AGEs in a diabetes-induced inflammatory environment can all harm the BBB (95, 96). Tight junctions (TJs) intercellularly among ECs effectively close gaps between adjacent cells. They are reduced by DM in the parenchymal blood vessels, which disrupts the BBB and makes it easier for albumin and inflammatory factors to enter the brain (97).
3.4 Diabetic peripheral vascular disease
Peripheral arterial disease (PAD), an occlusive atherosclerotic disease in the arteries of the lower limbs, is the most common initial manifestation of cardiovascular disease in T2DM (98). Despite this, it is frequently overlooked. Patients with diabetes may experience diabetic foot ulcers (DFUs) and PAD as two typical displays of diabetic peripheral vascular disease. DFU is a common cause of lower limb loss by amputation and are long-term consequences of diabetes. Diabetes-related mortality has DFUs as a major contributing factor (99).
Bapir et al. assessed endothelial function by flow-mediated dilation (FMD). They discovered that the femoral artery (FA)-FMD was considerably lowered in T2DM patients compared to healthy individuals, indicating a limb arterial endothelial impairment of T2DM patients (100). The levels of AGEs, MDA, and TNF-α in diabetic patients with DFU is significantly higher. These elements are necessary for the secretion of soluble vascular endothelial growth factor receptor-1 (sVEGFR-1), which ultimately decreases the expression of VEGF compared to the diabetic group without DFU and interrupts the wound healing process in diabetic patients (101). Other scientists found that VEGF, Gremlin-1, and HIF-1α increased in DFU patients. As an oxygen concentration-dependent transcription factor, HIF-1α can control a variety of target genes, most notably VEGF. Gremlin-1 is a coactivator of VEGF and can induce microangiogenesis, which is a process involved in the pathogenesis of diabetic lower limb ulcers (102). Consequently, these all lead to more severe DFU. Metabolomics studies have shown that amino acid metabolism disorders are the main metabolic hallmarks of PAD patients. In T2DM patients with lower extremity PAD, asprosin levels are very high, which promotes EndMT by activating the TGF-β pathway (103). Neutrophils are more suitable for neutrophil extracellular trap (NET)osis in diabetic wounds. NETosis delays diabetic wound healing by inducing EndMT via the Hippo-Yes-associated protein (YAP) pathway. The Hippo pathway mediates ECs dysfunction and regulates angiogenesis by inducing EndMT via the transcription factor Smad2 (104).
4 Diabetic microvascular complications
4.1 Diabetic nephropathy
Hyperglycemia is a key factor in the onset of DN and has been identified as one of the significant long-term consequences of DM that affects the microvasculature (105, 106).
Glomerular endothelial cells (GECs) are specialized vascular cells found in the kidney that help maintain renal homeostasis by forming the walls of the glomerular tufts. The inflammatory environment of diabetes can result in GECs dysfunction, leading to proteinuria and renal fibrosis. The GECs of diabetic mice have a high representation of genes involved in the oxidative stress pathway. Hyperglycemia raises leucine-rich α-2 glycoprotein 1 (LRG1) and G protein-coupled receptor 56 (GPR56) mRNA and protein levels. LRG1 promotes DN pathogenesis by enhancing TGF-β/activin receptor-like kinase 1 signaling in ECs (107). The overexpression of GPR56 reduced the phosphorylation and expression of eNOS, which may impair normal endothelial function (108). Additionally, elevated glucose levels lowered lysine methyltransferase 8 (SETD8) expression and increased myeloid zinc finger 1 (MZF1) expression. Their changes raise the expression of p-p65 and other endothelial inflammatory markers in hyperglycemic HGECs by adjusting Wingless-type family member 5 (WNT5A) transcription, which eventually causes damage to GECs (14). DN patients have higher levels of VCAM-1 and ICAM-1. Moreover, pro-inflammatory cytokines can bind to receptors on GECs, activating the NF-κB signal pathway (61, 109, 110). These results highlight the interactions between oxidative stress and inflammation in the context of hyperglycemia as major stimulators of DN endothelial dysfunction.
EndMT also plays a role in the initial phases of DN development as numerous fibroblasts in the kidneys of diabetics originate from the endothelium, which is triggered by TGF-β (111). Gremlin-1 actively upregulates the expression of TGF-β in DN, which results in a more severe development of kidney fibrosis (112, 113). Rho-associated kinase 1(ROCK1), an effector of TGF-β, is elevated in hyperglycemic environments and stimulates EndMT in DN (114). Ang-(1-7) treatment can normalize the levels of ROCK1 and ROCK2 in a diabetes-related context, providing a beneficial effect (115). In addition, ECs of the kidney are responsible for maintaining the glomerular filtration barrier (GFB). EndMT negatively affects the functional abilities of the glomerular filtration barrier (GFB) by decreasing the production of endothelial junctional proteins and increasing the deposition of extracellular matrix (ECM) proteins (116, 117). In early DN, ATP-binding cassette A1 (ABCA1) loss caused damage to glomerular endothelial cells through endoplasmic reticulum stress (ERS)-induced inflammation and apoptosis, which finally resulted in DN progression (118). Hyperglycemia significantly induced cytotoxicity and increased the expression of necroptosis markers in rat GECs. This result suggested that endothelial dysfunction induced by cell death can also partially explain the mechanisms underlying DN (119).
4.2 Diabetic retinopathy
Diabetic retinopathy (DR) is a common microvascular complication of diabetes mellitus and is the main cause of visual loss in the elderly (120). Two stages comprise the pathological process of diabetic retinopathy: non-proliferative (NPDR) and proliferative (PDR). Microvasculopathy in the DR retina is characterized by reduced capillary flexibility, heightened vascular permeability, localized inflammation, and the presence of growth factors that facilitate the development of neovascularization (95).
Hyperglycemia leads to the impairment of retinal endothelial cells (RECs) by ischemia, oxidative stress, and the release of pro-inflammatory factors. Patients with DR may experience increased vessel wall permeability and capillary occlusion as a result of increased adhesion molecule expression and decreased vasodilation found in retinal microvessels (121). Retinal binding protein 3 (RBP3) reduces inflammatory cytokines and inhibits VEGF’s activities in the retina, which may prevent the advancement of DR (122). In addition, excessive ROS (H2O2) accumulation in pathological situations such as hyperglycemia can activate the transcription factor HIF-1α, which increases the expression of inflammatory mediators and VEGF, damaging retinal microvessels (123, 124). Li et al. recently reported the anti-oxidative stress effect of F-box and WD repeat domain containing 7 (FBXW7) in ECs. FBXW7 alleviates harmful processes that lead to DR, such as DNA damage, mitochondrial dysfunction, ROS elimination, and PARP overactivation (125).
The death of RECs may accelerate the development of DR. In a hyperglycemic setting, increased homocysteine levels elevate the activity of dynamin-related protein 1 (Drp1) by causing its nitrosylation in RECs. This further fractures the mitochondria and elevates the apoptosis of RECs, finally aggravating diabetic retinopathy (126). Hyperglycemia-induced pyroptosis in human retinal microvascular endothelial cells (HRMECs) is characterized by increased caspase-1 activity, IL-β, NLRP1, NOX4, TXNIP, and NLRP3 expression (71). AGEs cause HRMECs and corneal ECs to undergo pyroptosis through cleaved caspase 1 and active gasdermin (GSDM), leading to blindness (127, 128). Hyperglycemia may promote TRIM46-mediated GPX4 ubiquitination, thereby lowering the expression of GPX4 in retinal capillary ECs, leading to more EC ferroptosis (129). RECs also help maintain the blood-retinal barrier (BRB). Loss of endothelial properties through EndMT affects REC barrier function and increased matrix protein deposition in mesenchymal cells may thicken the basement membrane, increasing vascular permeability in DR (39, 130, 131).
4.3 Diabetic neuropathy
Endothelial dysfunction often develops in the early stages of diabetes, impacting both the peripheral nervous system (PNS) and central nervous system (CNS), which can result in sciatic nerve irritation and diabetic neuropathy (132). DPN is a length-dependent injury of peripheral nerves that starts proximally in the feet and eventually moves to the hands. It is also a common consequence of diabetic microvascular disease (133). The thickening of the basement membrane and endothelial dysfunction with anomalies in endoneurium capillaries are characteristics of the diabetic nerve (133). Recent research has identified additional pro-oxidant enzymes, including NOXs, which are now known to be powerful ROS producers in DPN (134). Particularly, Nox2 and Nox4 are overexpressed in diabetic nerves and are involved in the degeneration of both structural and functional nerves (135, 136). Diabetic sciatic nerves contain less desert hedgehog. Inhibiting hedgehog signaling in ECs is sufficient to cause blood nerve barrier (BNB) breakdown and neuropathy. In addition, VEGFA increased BNB permeability induced by desert hedgehog deficiency and the severity of microangiopathy (137). Therefore, diabetic endothelial dysfunction in the neurovascular system is closely related to the state of oxidative stress.
4.4 Diabetic cardiomyopathy
Diabetic cardiomyopathy (DCM) is characterized by abnormal cardiac structure and function in the absence of cardiac risk factors in patients with diabetes, which can result in heart failure (HF) (138). According to a meta-analysis, diabetes reduces long-term survival and hospitalization in acute and chronic HF patients (139).
The development of diabetic cardiomyopathy is probably due to a combination of factors, such as endothelial dysfunction, glucose toxicity, mitochondrial dysfunction, and lipotoxicity (140). The diabetic heart has increased fatty acid oxidation but decreased glucose oxidation. It also exhibits elevated levels of pro-inflammatory cytokines and higher leukocyte infiltration (141, 142). Fibrosis is one characteristic of diabetic cardiomyopathy. EndMT can contribute to activated cardiac myofibroblasts. Therefore, in hyperglycemia, increased EndMT leads to excessive fibroblast activation and causes extracellular matrix protein overproduction, interstitial fibrosis, and a thicker basement membrane (143, 144). Moreover, both RAGE and AGEs increase in diabetes. Knocking out RAGE can decrease the degree of EndMT, accompanied by decreased expression of autophagy-related proteins (LC3BII/I and Beclin 1), and alleviate cardiac fibrosis in mice (145). The expression of mitochondrial calcium uptake 1 (MICU1) was decreased in the myocardial microvascular endothelial cells (CMECs) of diabetic mice. The reduction of MICU1 in diabetic CMECs showed a significant increase in inducible nitric oxide synthase (iNOS) enzymes related to nitrification stress and inflammation-related molecules. These changes led to exacerbated cardiac hypertrophy and fibrosis (31).
Impaired NO signaling is closely correlated with diabetic cardiomyopathy. In diabetic rat hearts, eNOS level decreased in ECs when compared to non-diabetic controls (146). However, myocardial BH4 oxidation and NOS dysfunction are not always present in the cardiomyopathic phenotype or as early indicators of diabetes mellitus, suggesting a more significant role of vascular endothelium in diabetic cardiomyopathy (147). Moreover, substantial inflammatory reactions in diabetic cardiac tissue cause elevated levels of IL-1β, IL-18, IL-6, and TNF-α. EPC dysfunction leads to adverse remodeling in the diabetic heart, which can be improved by bone morphogenetic protein-7 (BMP-7) (148). Though the role of ECs in diabetic cardiomyopathy is not directly confirmed, there has been extensive discussion on the crosstalk between ECs and cardiomyocytes, especially their metabolisms and angiogenesis functions mediated by VEGF (149). For instance, a study has suggested that cardiomyocyte-derived exosomes can modulate endothelial glucose transport and metabolism by sending glucose transporters and the associated glycolytic enzymes to the ECs (150). Thus, adverse changes in cardiomyocytes can indirectly affect myocardial ECs by their metabolism interaction. Nevertheless, a deeper understanding of ECs in diabetic cardiomyopathy still needs further investigation (Figure 5).
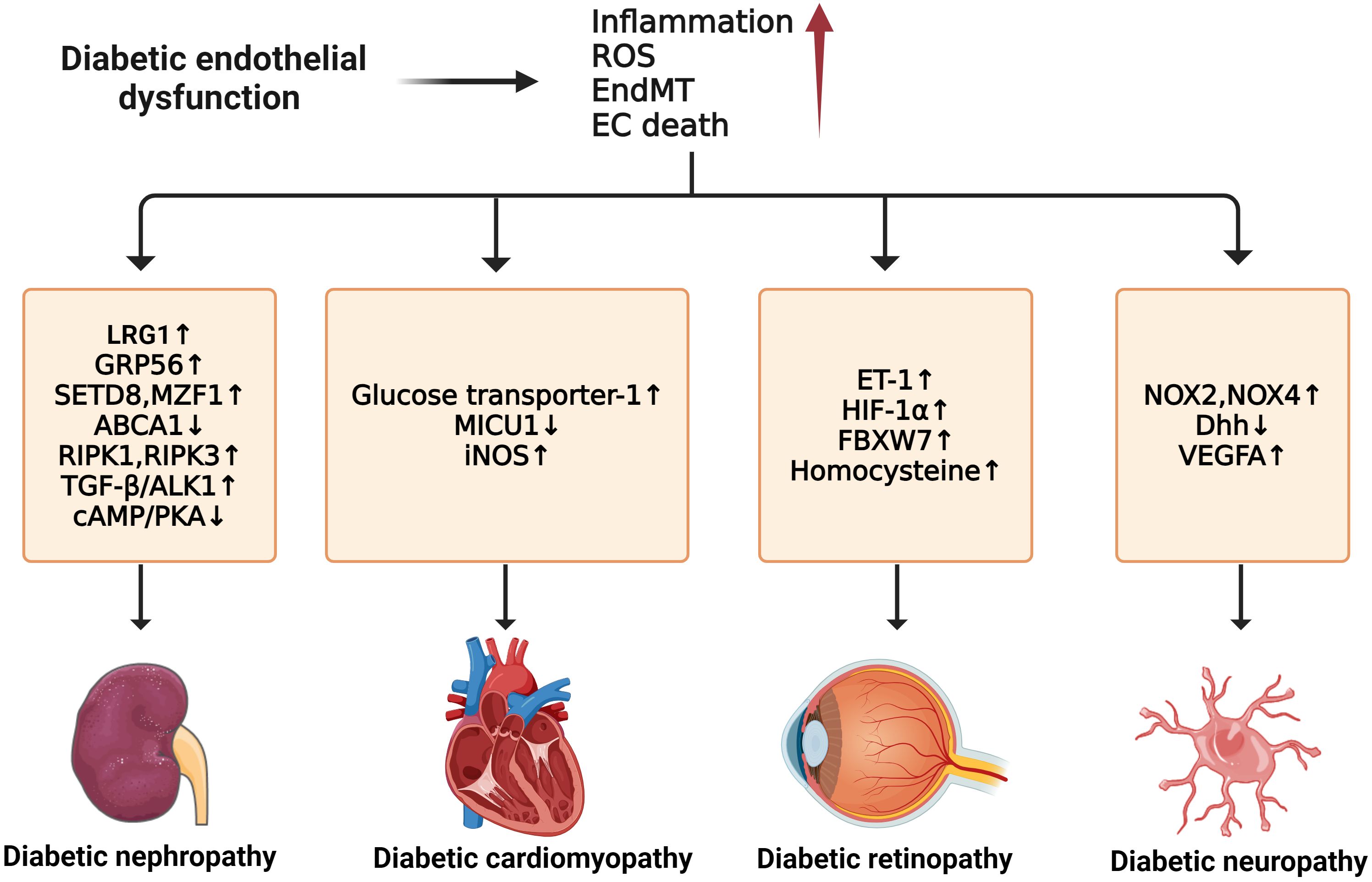
Figure 5 Molecular mechanisms of diabetic microvascular complications. In diabetic endothelial dysfunction, individuals are susceptible to microvascular complications such as diabetic retinopathy, diabetic nephropathy, diabetic neuropathy, and diabetic cardiomyopathy. The development of these complications is due to some common factors such as increased inflammation, ROS, EndMT, and endothelial cell death. For diabetic neuropathy, the upregulation of NOX2, NOX4, and VEGFA and the downregulation of Dhh can enhance its progression. Diabetic nephropathy can be accelerated by the upregulation of LRG1, GRP56, RIPK1, RIPK3, and TGF-β/ALK1 and the downregulation of ABCA1 and cAMP/PKA. In diabetic retinopathy, the exacerbations of ET-1, HIF-1α, FBXW7, and Hcy can worsen the condition. For diabetic cardiomyopathy, higher expression of GLU-1, MICU1, and iNOS contributes to its development.
5 Current therapies
5.1 Metformin
Metformin is a first-line therapy for treating type 2 diabetes due to its robust and pleiotropic effects on glucose metabolism. Metformin can protect the endothelium beyond lowering blood glucose levels. It has various effects on ECs (3, 4). Improved endothelium-dependent vasodilation from in vivo and in vitro studies showed a positive impact of metformin treatment on vascular endothelial function in diabetes (151).
Metformin can regulate the expression of inflammatory markers and slow the progression of diabetic vascular complications, including DN and PAD, by inhibiting inflammation in diabetes. It also has a favorable impact on certain cardiovascular risk markers (152, 153). Metformin inhibits NF-κB activation via AMPK activation and blockade of the PI3K-Akt pathway, which reduces the expression of proinflammatory and adhesion molecule genes (154, 155). In vivo investigations found that metformin can prevent diabetic nephropathy by reducing LRG1 and TGF-β1/ALK1-induced kidney angiogenesis and decreasing inflammatory cytokine levels (156). Moreover, metformin prevented the induction of femoral artery ultrastructural changes and the expression of vascular AGEs, ET-1, and iNOS, dyslipidemia. This resulted in an improvement in lower extremity arterial endothelial function by reducing the inflammation state in a rat model of diabetes-induced lower extremity arterial disease (LEAD) for 12 weeks. This resulted in an improvement in lower extremity arterial endothelial function (157). Metformin decreases the production of ROS from mitochondria by stimulating both AMPK-dependent and AMPK-independent pathways in human leukocytes and ECs (158, 159). It also regulates EC function, survival, proliferation, and senescence through the SIRT1 and LKB1/AMPK pathways (95). It restores Hedgehog pathway activity to suppress hyperglycemia-induced autophagosome production in diabetic retinal vasculature and cultured HUVECs (160). Metformin has been shown to protect vascular ECs by enhancing autophagy flux and diminishing lipid accumulation (161).It attenuated the development of diabetes-accelerated AS by reducing Dynamin-related protein (Drp1)-mediated mitochondrial fission in an AMPK-dependent manner (162).
However, metformin has some noticeable side effects, such as nausea, vomiting, bloating, a metallic taste in the mouth, abdominal pain, cramps, and changes in intestinal motility, which may result in loose stools and diarrhea that can be challenging to manage. Around 25% of patients experience gastrointestinal side effects and about 5% are unable to tolerate metformin (163). Additionally, metformin has been linked to increased levels of homocysteine and vitamin B12 deficiency, which can exacerbate diabetic neuropathy (164).
5.2 Dipeptidyl peptidase-4 inhibitors
Dipeptidyl peptidase-4 inhibitors (DPP-4i) are commonly used treatments for patients with T2DM and include vildagliptin, linagliptin, sitagliptin, teneligliptin, and others. It blocks the breakdown of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) to decrease blood glucose levels. In the vascular system, DPP4 is widely expressed by ECs, cardiomyocytes, and many other cell types (165), implying its role in diabetic vascular disease.
Increasing data suggests DPP-4i inhibitors have a protective role in endothelium and AS, independent of their hypoglycemic effects (3, 165). DPP-4i treatment decreased carotid intima-media thickness (IMT) in individuals with diabetes, which slowed the development of AS (166). Vildagliptin prevents hyperglycemia-induced endothelium damage in a GLP-1-independent way. It directly activates TRPV4 to promote Ca2+ absorption, AMPK activation, and SIRT1. In this way, it promotes endothelial-dependent vasorelaxation to protect against hyperglycemia-induced endothelial dysfunction (167). Sitagliptin’s activation of AMPK prevented the formation of ROS, the collapse of mitochondrial membrane potential, and the apoptosis of HUVECs induced by high glucose (168). It triggered the AMPK/unc-51-like autophagy activating kinase 1 signaling pathway to restore basal autophagy in EPCs, preventing EPC apoptosis. Thus, it preserves EPC angiogenic function, improving diabetic ischemia angiogenesis (169). In hyperglycemia, teneligliptin enhances HUVEC proliferation and inhibits HUVEC apoptosis by promoting B-cell lymphoma 2 (BCL2) expression, decreasing proapoptotic genes (BAX and CASP3), and expressing cell-cycle inhibitor hallmarks (P27, P21, and P53) (170). Adenosine AMPK phosphorylation by DPP4 promotes vascular ECs apoptosis and autophagy, while microRNA 5680 inhibits this process as a DPP4i (171).
DPP-4i have a significant impact on the development and progression of diabetic vascular disorders. It protects brain microvascular ECs against high-glucose and hypoxic conditions (172). Linagliptin effectively inhibits TNF-α-induced NF-κB nuclear protein p65 accumulation and promotes activation in RECs. It also reduces TNF-α-induced vascular cytokine production, such as IL-6, IL-8, ICAM-1, and VCAM-1 (173). In diabetic kidney ECs, linagliptin inhibited TGF-β2-induced EndMT by suppressing levels of microRNA 29s (174). It can cause a decrease in endothelial toll-like receptors 2 (TLR2) expression and a subsequent increase in NO bioavailability. Linagliptin reduced ET-1-induced basilar arteries contraction in diabetic rats, improving diabetic cerebrovascular dysfunction (175). In addition, evogliptin directly interferes with pathological retinal neovascularization (NV) by blocking VEGF-induced adenosine 5′-diphosphate ribosylation factor 6 (Arf6) activation in ECs (176).
Nevertheless, research has shown an increased risk of heart failure with DPP-4i, especially saxagliptin. Patients with moderate renal dysfunction were found to have an increased risk of heart failure. Further research is necessary to comprehend the impact of declining renal function on the cardiovascular safety of DPP-4 inhibitors (177). Furthermore, the use of sitagliptin increases the odds ratio for reported pancreatitis by a factor of six. Additionally, there was an increase in reported cases of pancreatic and thyroid cancer compared to other therapies. These findings suggest that further studies on the effects of DPP-4i on the thyroid gland and exocrine pancreas in humans are necessary (178).
5.3 Glucagon-like peptide 1 receptor agonists
Glucagon-like peptide 1 (GLP1) is a type of incretin hormone released from gut endocrine cells (179). It regulates blood glucose levels by enhancing insulin secretion in pancreatic β cells and reducing glucagon release (180). Glucagon-like peptide 1 receptor agonists (GLP-1RA) are artificial mimics or analogs of human GLP-1, including exenatide and liraglutide. The cardiovascular benefits of GLP-1RA in patients with T2DM and established cardiovascular diseases have been indicated through many clinical trials (179, 181).
Previous studies have demonstrated that GLP-1RA have the ability to enhance endothelial function in diabetic patients (3, 179, 182). Exenatide decreased high-glucose-induced ROS production and the apoptotic index in CMEC. This protective effect is reliant on the downstream suppression of Rho via a cAMP/PKA-mediated pathway (183). Additionally, it protects ECs from oxidant stress through reducing autophagy, which is dependent on the restoration of histone deacetylase 6 (HDAC6) in a GLP-1R-extracellular signal-regulated kinase (ERK)1/2-dependent way (184). In vivo and in vitro studies show that exendin-4 decreases HHcy-induced ER stress and enhances endothelial function by upregulating endoplasmic reticulum oxidoreductase (ERO1α) via AMPK in ECs and arteries. AMPK activation improves EC protein folding to reduce ER stress (185). In addition, liraglutide prevents hyperglycemia-induced Smad2 phosphorylation and EndMT via the AMPK pathway, indicating the prevention of liraglutide towards hyperglycemia-induced EndMT (186). The GLP-1 metabolite GLP-1(9-36) amide enhanced human aortic EC viability in response to hypoxic injury and hydrogen peroxide treatment via a NO- and mitochondria-dependent mechanism (187). Large-scale cardiovascular outcome trials have shown that GLP-1RAs improve cardiovascular outcomes in T2DM patients with high cardiovascular risk or established atherosclerotic cardiovascular disease (181, 188, 189), mainly through their anti-atherosclerotic properties. T2DM increases carotid intima-media thickness (CIMT), while CIMT decreases in the GLP-1RA-treated group, suggesting a slowing of the atherosclerotic process (190). In the context of ischemic cardiac injury, GLP-1RA’s cardioprotective actions mainly target GLP-1R in mouse Tie2+ endothelial cells. The cardioprotective functions of liraglutide were attenuated in Glp1rTie2-/- mice (191). The cellular localization of GLP-1R in the heart may provide insight into the mechanism of GLP-1RA in diabetic MI and other cardiovascular complications. In a concentration-dependent way, liraglutide improved the function of hyperglycemia-treated HUVECs and phosphorylated Akt, eNOS, and ERK1/2 in vitro and in vivo. Therefore, liraglutide may help treat hind-limb ischemia in type 2 diabetic mice (192).
Current findings indicate that GLP-1RA have a favorable safety profile and do not increase cardiovascular risk in patients with T2DM. Ongoing trials will further assess their cardiovascular effects (193). However, exenatide and sitagliptin have similar side effects that can increase the risk of pancreatitis, thyroid cancer, and pancreatic cancer. When taken together, there is a significantly increased association with these therapies and the development of thyroid and pancreatic cancer (178). GLP-1 RA were found to be significantly associated with gastrointestinal adverse events. Among these, semaglutide had the highest risk of nausea, diarrhea, vomiting, constipation, and pancreatitis, while liraglutide had the highest risk of upper abdominal pain (194).
5.4 Sodium-glucose cotransporter 2 inhibitor
Approximately 97% of the total renal glucose reabsorption is attributed to SGLT2 (195). SGLT2i inhibits this high-capacity glucose transporter, SGLT2, in the proximal convoluted tubule and helps the kidneys excrete glucose in the urine to lower blood glucose (196). SGLT2i improved obesity, aberrant lipid metabolism, inflammation, endothelial dysfunction, and nephropathy in diabetic mice after 4-week repeated dosing (197). The main types of SGLT2i are canagliflozin, dapagliflozin, and empagliflozin.
Based on numerous clinical trials, patients with diabetic chronic heart failure and CAD showed marked improvement in their flow-mediated dilation (FMD) and blood pressure after empagliflozin and canagliflozin treatments (198). Moreover, several types of SGLT2i prevented diabetic nephropathy in rodents (199). El-Daly et al. suggest that hyperglycemia-induced oxidative stress affects protease-activated receptor 2-mediated vasodilation in the endothelium through a NOX-triggered signaling cascade, which can be suppressed by SGLT2i (200). Furthermore, the inhibition of mitochondrial fission induced by empagliflozin protected cardiac microvascular endothelial cell barrier function by suppressing mtROS production and oxidative stress, thereby preventing senescence of cardiac microvascular endothelial cells. Furthermore, it helps to maintain eNOS phosphorylation, endothelium-dependent relaxation, and the integrity of heart microvascular barrier (201). In vivo studies showed the improvement of endothelial dysfunction in diabetic ApoE-/- mice after treatment with empagliflozin. This is due to the anti-inflammatory effect of empagliflozin, which decreases the levels of TNF-α, ICAM-1, and vasoconstrictive eicosanoids (202). In addition, empagliflozin significantly increased the levels of the Beclin1 protein, the LC3B-II/I ratio, and the p-AMPK protein, inducing autophagy in HUVECs via the AMPK signaling pathway, which delays the evolution of AS (203). Li et al. found that empagliflozin reduced EndMT in the proximal tubule and ECs in diabetic mice, decreasing kidney fibrosis and potentially protecting the kidneys (204). Oral empagliflozin improved coronary endothelial function by increasing L-arginine/asymmetric dimethylarginine ratio and NO bioavailability (205).
In addition, other types of SGLT2i also improve endothelial function through different mechanisms. The Canagliflozin and Renal Events in Diabetes with Established Nephropathy clinical evaluation trial showed that SGLT2 blockade slows the progression of established diabetic kidney disease and reduces the risk of cardiovascular death, myocardial infarction, stroke, and hospitalization due to heart failure in T2DM patients (206). In human coronary artery ECs from donors with DM (D-HCAECs) exposed to TNF-α, canagliflozin reduces inflammasome activation and ROS generation. It reduces inflammasome activation by inhibiting intracellular Ca2+ and extracellular signal regulated kinase (ERK) 1/2 phosphorylation (207). Additionally, dapagliflozin reduces endoplasmic reticulum (ER) stress in human coronary artery endothelial cells (208).
However, SGLT-2i have common side effects, such as urinary tract infections, euglycemic ketoacidosis, orthostatic hypotension, dehydration, and cardiovascular adverse events (209). Additionally, they are associated with increased risks of diabetic ketoacidosis and lower-limb amputations. The risk of lower-limb amputations is higher in patients with a history of amputation and peripheral vascular disease. Dapagliflozin is specifically associated with a high risk of toe amputation (210, 211). In general, SGLT2i are a beneficial treatment option for type 2 diabetes. However, their use should be thoroughly assessed and supervised to minimize potential adverse effects.
Taken together, conventional therapeutic approaches have distinct targets aimed at alleviating endothelial dysfunction in the context of diabetic vascular complications. Here we focus on the potential benefits of metformin, DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT-2 inhibitors in the improvement of diabetic vascular disorders, especially their ability to improve inflammation, oxidative stress, cell death, and EndMT. Additionally, we discuss some common side effects of these therapies that should be noted. The objective is to identify additional therapeutic applications for these medications in treating various types of diabetic vascular complications.
6 Future directions and novel regulators
6.1 MicroRNA therapies
MicroRNAs (MiRNAs) are short, endogenous, non-coding RNAs with a normal length of 22 nucleotides that regulate gene expression post-transcriptionally. They target more than half of the transcripts that code for proteins, making them implicated in almost all animal developmental and pathological processes (212). Research indicates that they are important for preserving optimal vascular homeostasis and preventing the sequelae of diabetes-induced end-organ destruction. MiRNAs have been shown to regulate endothelial dysfunction in many aspects and also regulate the diabetic microvasculature in many diabetes-associated complications (213).
In human aortic endothelial cells, high glucose and thrombin reduce miR-146a expression when combined. Overexpression of the miR-146a mimic can downregulate IL-6 and IL-8 in hyperglycemia/thrombin-stimulated human aortic endothelial cells (HAECs) (214). Moreover, miR-146a CAN rescue senescent HUVECs. In vivo and in vitro results demonstrate that miR-146a-5p mimics inhibit endothelial interleukin-1 receptor-associated kinase-1 (IRAK-1) and ICAM-1 expression, indicating an anti-inflammatory function in vessels (215). MiR-30 promotes oxidative stress, lipid peroxidation, and endothelial dysfunction in cultured ECs by regulating exogenous fatty acid oxidation. Thus, it might represent a potential therapeutic target for diabetic microvascular dysfunction (216). Mir-20a-5p reverses the expression of high phosphatase, tensin homolog, and autophagy-related 7 and promotes AKT and mTOR phosphorylation in high glucose circumstances, inhibiting EPC autophagy and apoptosis (217). This suggests that mir-20a-5p has a protective effect against cell death induced by hyperglycemia.
The evolving role of miRNAs in diabetic vascular complications has become a hot topic in recent years. In the glomerular vascular ECs of the kidney of DN patients, the expression of miR-155 was increased. Human renal glomerular Ecs undergo an inflammatory response and apoptosis due to negative regulation of miR-155 by E26 transformation-specific sequence 1 (ETS-1) and its downstream components VCAM-1, MCP-1, and cleaved caspase-3 (218, 219). Overexpression of miR-375 in ECs increases proliferation, wound closure, spheroid sprouting, and tube formation by targeting Kruppel-like factor 5 via altering phospho-p65 NF-κB signaling in diabetic critical limb ischemia (220). Overexpression of miR-181a-5p in HUVECs impaired the endothelial barrier and decreased gene expression of OCLN variants 1 and 2, the genes that meditate occludin expression. Occludin downregulation in various organs may cause DR or DN due to elevated miR-181a-5p levels (221). In DR, research has shown that miR-15b-5p inhibits the expression of COL12A1, a gene encoding collagen type XII α 1 chain, and inhibits the proliferation, migration, and angiogenesis of hyperglycemia-induced human retinal vascular endothelial cells (222). HG induced pyroptosis in a cell culture model of DR, whereas miR-590-3p inhibited pyroptosis by targeting the NLR family pyrin domain containing 1 (NLRP1) and inactivating the NOX4 pathway (71). In human retinal microvascular endothelial cells cultured in high glucose, miR-93 expression increases dramatically, while the overexpression of miR-93 exacerbates ferroptosis by increasing ROS generation and causing Fe2+ accumulation (223). MiR-126 overexpression can reduce EC apoptosis and promote angiogenesis by modulating VEGFR2 signaling. Consequently, it may ameliorate ischemic stroke in diabetic mice (224).
However, the use of miRNA technology at the clinical level is a potential challenge due to certain physiological barriers that affect their stability. The advanced drug delivery systems such as nanoparticles, however, have emerged as novel carriers for miRNA targeting to help solve this. There are still many problems such as the complexity of advanced drug delivery systems, especially for lipid-based nanocarriers, and the concerns of safety of the substance incorporated in these systems (225). Nanoparticles also cause possible side effects including inflammatory reactions, oxidative stress, and cell apoptosis, leading to cytotoxicity (226). MiRNAs also have the potential to target multiple genes, which can lead to unintended consequences and off-target effects. Another challenge is the complexity in the tissue and cell-specific expression and functions of specific miRNAs in the same diabetic complication. This could be related to the cell-type specific patterns, different model systems and animals studied, time of sampling, or the severity of the complications in the models studied (227).
6.2 Stem cell therapies
Stem cell-based therapy is an important field of regenerative medicine that regulates endogenous stem cells to enhance tissue homeostasis and regeneration (228). Mesenchymal stem cells (MSCs) are the main cell source used in stem cell-based therapy. MSC-based cell therapy has been recognized as an effective treatment for diabetes mellitus and its complications, serving as a means of preventing diabetic damage to the vascular endothelium (229, 230). As MSCs have long been considered a key source of cells in regenerative medicine, we will also discuss the effects of MSC-derived exosomes and extracellular vesicle (EVs) on diabetic endothelial dysfunction.
In vitro, human umbilical cord-derived MSCs restored high glucose-damaged HUVEC survival, wound healing, migration, angiogenesis, and senescence through a MAPK/ERK signaling mediated paracrine effect (230). Delivery of circ-Snhg11 from hypoxia-pretreated adipose-derived stem cell-originating exosomes (ADSC-HExo)-embedded GelMA hydrogels (GelMA-HExo) can improve EC survival and function by activating miR-144-3p/NFE2L2/HIF1α signaling, implicating the role of ADSC-Exos in diabetic wound healing (231). Similarly, exosomes from atorvastatin-pretreated MSCs may improve endothelial cell function via the AKT/eNOS pathway by upregulating miR-221-3p (232). MSC-derived small extracellular vesicles (sEV) therapy enhanced angiogenesis, migration, and proliferation in senescent HUVECs. It also ameliorated mitochondrial dysfunction and reduced ROS levels while providing protection against senescence-related problems. Its subcutaneous injection can promote skin wound healing in an animal model of aging and type-2 diabetes (233).
The increasing number of studies focusing on miRNA and stem cells holds the potential to improve the treatment of diabetic complications by providing a more fundamental approach (Table 1). However, it is important to note that translating stem cells research into therapies is complex and challenging. Uncontrolled proliferation of transplanted stem cells may lead to tumor formation in the treatment of diabetes. Improving stem cell efficacy is also a challenge, though there has been discussion about enhancing their differentiation potential through coculture techniques to address this (234). Stem cell translation may also lead to an unavoidable immune response. Research is ongoing on gene editing and nuclear transfer techniques in stem cells to help stem cells escape the immune response in the human body (235). Overcoming these obstacles through further research is critical to the development of effective novel therapies in the future.
7 Conclusions
Significant advancements have been made in the study of endothelial dysfunction in diabetes and its associated vascular complications. This review has discussed the fundamental mechanisms of diabetes and its associated vascular disorders, including inflammation, oxidative stress, cell death, and EndMT. Based on the findings of these studies, it is evident that there are many promising targets and therapies, such as metformin, DPP-4i, GLP-1RA, SGLT2i, miRNA, and stem cells, which hold potential for clinical use. In contrast to the more well-known processes of inflammation and oxidative stress, the mechanisms of diabetic endothelial dysfunction underlying cell death and EndMT remain poorly understood. Furthermore, there are still numerous limitations and side effects of the traditional therapies and novel regulators targeting diabetic vascular complications (Table 2). Therefore, given the uncertainties surrounding these variables and the intricate nature of alterations in diabetic endothelial dysfunction, further research is warranted to elucidate these mysteries.
Author contributions
D-RY: Visualization, Writing – original draft, Writing – review & editing. M-YW: Writing – review & editing, Writing – original draft. C-LZ: Funding acquisition, Writing – review & editing. YW: Conceptualization, Funding acquisition, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by PROJECT ENDOCRINE AND METABOLIC TALENT RESEARCH, grant number 2022-N-02-14, Shenzhen Overseas High-Level Talent (Peacock Plan) Project Research Initiation Programme, grant number 827000745 and Shenzhen Municipal Science and Technology Innovation Commission Stability Support Program for Higher Education Institutions (20231120180418002).
Acknowledgments
Figure 2 was created with VOSviewer software https://www.vosviewer.com/. Figures 1, 3–5 were created using https://www.biorender.com/ (accessed on 20 December 2023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Global diabetes data report 2000 — 2045. Available at: https://diabetesatlas.org/data (Accessed August 1, 2023).
2. Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. (2022) 18:525–39. doi: 10.1038/s41574-022-00690-7
3. Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. (2021) 73:924–67. doi: 10.1124/pharmrev.120.000096
4. Xu J, Zou M-H. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. (2009) 120:1266–86. doi: 10.1161/CIRCULATIONAHA.108.835223
5. Horton WB, Barrett EJ. Microvascular dysfunction in diabetes mellitus and cardiometabolic disease. Endocr Rev. (2021) 42:29–55. doi: 10.1210/endrev/bnaa025
6. Meyerovich K, Ortis F, Cardozo AK. The non-canonical NF-κB pathway and its contribution to β-cell failure in diabetes. J Mol Endocrinol. (2018) 61:F1–6. doi: 10.1530/JME-16-0183
7. Arkan MC, Hevener AL, Greten FR, Maeda S, Li Z-W, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. (2005) 11:191–8. doi: 10.1038/nm1185
8. Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. (2002) 420:333–6. doi: 10.1038/nature01137
9. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt- dependent phosphorylation. Nature. (1999) 399:601–5. doi: 10.1038/21224
10. Janus A, Szahidewicz-Krupska E, Mazur G, Doroszko A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediators Inflammation. (2016) 2016:3634948. doi: 10.1155/2016/3634948
11. El-Hashim AZ, Renno WM, Raghupathy R, Abduo HT, Akhtar S, Benter IF. Angiotensin-(1–7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-κB-dependent pathways. Br J Pharmacol. (2012) 166:1964–76. doi: 10.1111/j.1476-5381.2012.01905.x
12. Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, et al. The ACE2/angiotensin-(1–7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1–7). Physiol Rev. (2018) 98:505–53. doi: 10.1152/physrev.00023.2016
13. Lopez-Bergami P, Barbero G. The emerging role of Wnt5a in the promotion of a pro-inflammatory and immunosuppressive tumor microenvironment. Cancer Metastasis Rev. (2020) 39:933–52. doi: 10.1007/s10555-020-09878-7
14. Wang F, Hou W, Li X, Lu L, Huang T, Zhu M, et al. SETD8 cooperates with MZF1 to participate in hyperglycemia-induced endothelial inflammation via elevation of WNT5A levels in diabetic nephropathy. Cell Mol Biol Lett. (2022) 27:30. doi: 10.1186/s11658-022-00328-6
15. Wang J, Shen X, Liu J, Chen W, Wu F, Wu W, et al. High glucose mediates NLRP3 inflammasome activation via upregulation of ELF3 expression. Cell Death Dis. (2020) 11:383. doi: 10.1038/s41419-020-2598-6
16. Zhang Q, Fang W, Ma L, Wang Z-D, Yang Y-M, Lu Y-Q. VEGF levels in plasma in relation to metabolic control, inflammation, and microvascular complications in type-2 diabetes: A cohort study. Medicine. (2018) 97:e0415. doi: 10.1097/MD.0000000000010415
17. Falkevall A, Mehlem A, Folestad E, Ning FC, Osorio-Conles Ó, Radmann R, et al. Inhibition of VEGF-B signaling prevents non-alcoholic fatty liver disease development by targeting lipolysis in the white adipose tissue. J Hepatol. (2023) 78:901–13. doi: 10.1016/j.jhep.2023.01.014
18. Byun K, Yoo Y, Son M, Lee J, Jeong G-B, Park YM, et al. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol Ther. (2017) 177:44–55. doi: 10.1016/j.pharmthera.2017.02.030
19. Bongarzone S, Savickas V, Luzi F, Gee AD. Targeting the receptor for advanced glycation endproducts (RAGE): A medicinal chemistry perspective. J Med Chem. (2017) 60:7213–32. doi: 10.1021/acs.jmedchem.7b00058
20. Shen C-Y, Li K-J, Wu C-H, Lu C-H, Kuo Y-M, Hsieh S-C, et al. Unveiling the molecular basis of inflamm-aging induced by advanced glycation end products (AGEs)-modified human serum albumin (AGE-HSA) in patients with different immune-mediated diseases. Clin Immunol. (2023) 252:109655. doi: 10.1016/j.clim.2023.109655
21. Ratajczak W, Atkinson SD, Kelly C. The TWEAK/Fn14/CD163 axis—implications for metabolic disease. Rev Endocr Metab Disord. (2022) 23:449–62. doi: 10.1007/s11154-021-09688-4
22. Zhu W, Guo S, Homilius M, Nsubuga C, Wright SH, Quan D, et al. PIEZO1 mediates a mechanothrombotic pathway in diabetes. Sci Trans Med. (2022) 14:eabk1707. doi: 10.1126/scitranslmed.abk1707
23. Albarrán-Juárez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, et al. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med. (2018) 215:2655–72. doi: 10.1084/jem.20180483
24. Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, et al. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. (2018) 20:247–60. doi: 10.1016/j.redox.2018.09.025
25. Vermot A, Petit-Härtlein I, Smith SME, Fieschi F. NADPH oxidases (NOX): an overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants (Basel). (2021) 10:890. doi: 10.3390/antiox10060890
26. Liang C-F, Liu JT, Wang Y, Xu A, Vanhoutte PM. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler Thromb Vasc Biol. (2013) 33:777–84. doi: 10.1161/ATVBAHA.112.301087
27. Njeim R, Braych K, Ghadieh HE, Azar NS, Azar WS, Dia B, et al. VEGF-A: A novel mechanistic link between CYP2C-derived EETs and nox4 in diabetic kidney disease. Diabetes. (2023) 72:947–57. doi: 10.2337/db22-0636
28. Lovren F, Pan Y, Quan A, Teoh H, Wang G, Shukla PC, et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol. (2008) 295:H1377–1384. doi: 10.1152/ajpheart.00331.2008
29. Dhaunsi GS, Yousif MHM, Akhtar S, Chappell MC, Diz DI, Benter IF. Angiotensin-(1-7) prevents diabetes-induced attenuation in PPAR-gamma and catalase activities. Eur J Pharmacol. (2010) 638:108–14. doi: 10.1016/j.ejphar.2010.04.030
30. Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. (2011) 124:444–53. doi: 10.1161/CIRCULATIONAHA.110.014506
31. Shi X, Liu C, Chen J, Zhou S, Li Y, Zhao X, et al. Endothelial MICU1 alleviates diabetic cardiomyopathy by attenuating nitrative stress-mediated cardiac microvascular injury. Cardiovasc Diabetol. (2023) 22:216. doi: 10.1186/s12933-023-01941-1
32. Broniarek I, Koziel A, Jarmuszkiewicz W. The effect of chronic exposure to high palmitic acid concentrations on the aerobic metabolism of human endothelial EA.hy926 cells. Pflugers Arch - Eur J Physiol. (2016) 468:1541–54. doi: 10.1007/s00424-016-1856-z
33. Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. (2010) 11:61–74. doi: 10.1007/s11154-010-9134-4
34. Sun D, Chen S, Li S, Wang N, Zhang S, Xu L, et al. Enhancement of glycolysis-dependent DNA repair regulated by FOXO1 knockdown via PFKFB3 attenuates hyperglycemia-induced endothelial oxidative stress injury. Redox Biol. (2022) 59:102589. doi: 10.1016/j.redox.2022.102589
35. Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. (2010) 5:253–95. doi: 10.1146/annurev.pathol.4.110807.092250
36. Yang K, Velagapudi S, Akhmedov A, Kraler S, Lapikova-Bryhinska T, Schmiady MO, et al. Chronic SIRT1 supplementation in diabetic mice improves endothelial function by suppressing oxidative stress. Cardiovasc Res. (2023) 119:(12):cvad102. doi: 10.1093/cvr/cvad102
37. Kumar S, Kim Y-R, Vikram A, Naqvi A, Li Q, Kassan M, et al. Sirtuin1-regulated lysine acetylation of p66Shc governs diabetes-induced vascular oxidative stress and endothelial dysfunction. Proc Natl Acad Sci USA. (2017) 114:1714–9. doi: 10.1073/pnas.1614112114
38. Lin F, Wang N, Zhang T-C. The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life. (2012) 64:717–23. doi: 10.1002/iub.1059
39. Wang E, Wang H, Chakrabarti S. Endothelial-to-mesenchymal transition: An underappreciated mediator of diabetic complications. Front Endocrinol (Lausanne). (2023) 14:1050540. doi: 10.3389/fendo.2023.1050540
40. Kovacic JC, Dimmeler S, Harvey RP, Finkel T, Aikawa E, Krenning G, et al. Endothelial to mesenchymal transition in cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:190–209. doi: 10.1016/j.jacc.2018.09.089
41. Piera-Velazquez S, Jimenez SA. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev. (2019) 99:1281–324. doi: 10.1152/physrev.00021.2018
42. Cho JG, Lee A, Chang W, Lee M-S, Kim J. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front Immunol. (2018) 9:294. doi: 10.3389/fimmu.2018.00294
43. Xu Y, Kovacic JC. Endothelial to mesenchymal transition in health and disease. Annu Rev Physiol. (2023) 85:245–67. doi: 10.1146/annurev-physiol-032222-080806
44. Yao Y, Song Q, Hu C, Da X, Yu Y, He Z, et al. Endothelial cell metabolic memory causes cardiovascular dysfunction in diabetes. Cardiovasc Res. (2022) 118:196–211. doi: 10.1093/cvr/cvab013
45. Tang R, Li Q, Lv L, Dai H, Zheng M, Ma K, et al. Angiotensin II mediates the high-glucose-induced endothelial-to-mesenchymal transition in human aortic endothelial cells. Cardiovasc Diabetol. (2010) 9:31. doi: 10.1186/1475-2840-9-31
46. Tassone EJ, Sciacqua A, Andreozzi F, Presta I, Perticone M, Carnevale D, et al. Angiotensin (1-7) counteracts the negative effect of angiotensin II on insulin signalling in HUVECs. Cardiovasc Res. (2013) 99:129–36. doi: 10.1093/cvr/cvt065
47. Sanabria-de la Torre R, García-Fontana C, González-Salvatierra S, Andújar-Vera F, Martínez-Heredia L, García-Fontana B, et al. The contribution of wnt signaling to vascular complications in type 2 diabetes mellitus. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23136995
48. Maleszewska M, Moonen J-RAJ, Huijkman N, van de Sluis B, Krenning G, Harmsen MC. IL-1β and TGFβ2 synergistically induce endothelial to mesenchymal transition in an NFκB-dependent manner. Immunobiology. (2013) 218:443–54. doi: 10.1016/j.imbio.2012.05.026
49. Pérez L, Muñoz-Durango N, Riedel CA, Echeverría C, Kalergis AM, Cabello-Verrugio C, et al. Endothelial-to-mesenchymal transition: Cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev. (2017) 33:41–54. doi: 10.1016/j.cytogfr.2016.09.002
50. Montorfano I, Becerra A, Cerro R, Echeverría C, Sáez E, Morales MG, et al. Oxidative stress mediates the conversion of endothelial cells into myofibroblasts via a TGF-β1 and TGF-β2-dependent pathway. Lab Invest. (2014) 94:1068–82. doi: 10.1038/labinvest.2014.100
51. Li Z, Wang F, Zha S, Cao Q, Sheng J, Chen S. SIRT1 inhibits TGF-β-induced endothelial-mesenchymal transition in human endothelial cells with Smad4 deacetylation. J Cell Physiol. (2018) 233:9007–14. doi: 10.1002/jcp.26846
52. Mao W, Fan Y, Wang X, Feng G, You Y, Li H, et al. Phloretin ameliorates diabetes-induced endothelial injury through AMPK-dependent anti-EndMT pathway. Pharmacol Res. (2022) 179:106205. doi: 10.1016/j.phrs.2022.106205
53. Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. (2009) 458:1056–60. doi: 10.1038/nature07813
54. Sanz AB, Sanchez-Niño MD, Ramos AM, Ortiz A. Regulated cell death pathways in kidney disease. Nat Rev Nephrol. (2023) 19:281–99. doi: 10.1038/s41581-023-00694-0
55. Shen J, San W, Zheng Y, Zhang S, Cao D, Chen Y, et al. Different types of cell death in diabetic endothelial dysfunction. Biomed Pharmacother. (2023) 168:115802. doi: 10.1016/j.biopha.2023.115802
56. Di Bartolo BA, Chan J, Bennett MR, Cartland S, Bao S, Tuch BE, et al. TNF-related apoptosis-inducing ligand (TRAIL) protects against diabetes and atherosclerosis in Apoe–/–mice. Diabetologia. (2011) 54:3157–67. doi: 10.1007/s00125-011-2308-0
57. You S, Xu J, Yin Z, Wu B, Wang P, Hao M, et al. Down-regulation of WWP2 aggravates Type 2 diabetes mellitus-induced vascular endothelial injury through modulating ubiquitination and degradation of DDX3X. Cardiovasc Diabetol. (2023) 22:107. doi: 10.1186/s12933-023-01818-3
58. Dai X, Wang K, Fan J, Liu H, Fan X, Lin Q, et al. Nrf2 transcriptional upregulation of IDH2 to tune mitochondrial dynamics and rescue angiogenic function of diabetic EPCs. Redox Biol. (2022) 56:102449. doi: 10.1016/j.redox.2022.102449
59. Zhang H, Ge S, He K, Zhao X, Wu Y, Shao Y, et al. FoxO1 inhibits autophagosome-lysosome fusion leading to endothelial autophagic-apoptosis in diabetes. Cardiovasc Res. (2019) 115:2008–20. doi: 10.1093/cvr/cvz014
60. Bai X, Yang X, Jia X, Rong Y, Chen L, Zeng T, et al. CAV1-CAVIN1-LC3B-mediated autophagy regulates high glucose-stimulated LDL transcytosis. Autophagy. (2020) 16:1111–29. doi: 10.1080/15548627.2019.1659613
61. Sun D, Wang J, Toan S, Muid D, Li R, Chang X, et al. Molecular mechanisms of coronary microvascular endothelial dysfunction in diabetes mellitus: focus on mitochondrial quality surveillance. Angiogenesis. (2022) 25:307–29. doi: 10.1007/s10456-022-09835-8
62. Xi J, Rong Y, Zhao Z, Huang Y, Wang P, Luan H, et al. Scutellarin ameliorates high glucose-induced vascular endothelial cells injury by activating PINK1/Parkin-mediated mitophagy. J Ethnopharmacol. (2021) 271:113855. doi: 10.1016/j.jep.2021.113855
63. Wu W, Xu H, Wang Z, Mao Y, Yuan L, Luo W, et al. PINK1-parkin-mediated mitophagy protects mitochondrial integrity and prevents metabolic stress-induced endothelial injury. PloS One. (2015) 10:e0132499. doi: 10.1371/journal.pone.0132499
64. Li J, Cao F, Yin H, Huang Z, Lin Z, Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis. (2020) 11:88. doi: 10.1038/s41419-020-2298-2
65. Li S, Li Y, Wu Z, Wu Z, Fang H. Diabetic ferroptosis plays an important role in triggering on inflammation in diabetic wound. Am J Physiol Endocrinol Metab. (2021) 321:E509–20. doi: 10.1152/ajpendo.00042.2021
66. Meng Z, Liang H, Zhao J, Gao J, Liu C, Ma X, et al. HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis. Life Sci. (2021) 284:119935. doi: 10.1016/j.lfs.2021.119935
67. Jia M, Qin D, Zhao C, Chai L, Yu Z, Wang W, et al. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat Immunol. (2020) 21:727–35. doi: 10.1038/s41590-020-0699-0
68. Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. (2019) 133:144–52. doi: 10.1016/j.freeradbiomed.2018.09.014
69. Luo E-F, Li H-X, Qin Y-H, Qiao Y, Yan G-L, Yao Y-Y, et al. Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World J Diabetes. (2021) 12:124–37. doi: 10.4239/wjd.v12.i2.124
70. Zheng X, Chen W, Gong F, Chen Y, Chen E. The role and mechanism of pyroptosis and potential therapeutic targets in sepsis: A review. Front Immunol. (2021) 12:711939. doi: 10.3389/fimmu.2021.711939
71. Gu C, Draga D, Zhou C, Su T, Zou C, Gu Q, et al. miR-590-3p inhibits pyroptosis in diabetic retinopathy by targeting NLRP1 and inactivating the NOX4 signaling pathway. Invest Ophthalmol Vis Sci. (2019) 60:4215–23. doi: 10.1167/iovs.19-27825
72. Gu J, Huang W, Zhang W, Zhao T, Gao C, Gan W, et al. Sodium butyrate alleviates high-glucose-induced renal glomerular endothelial cells damage via inhibiting pyroptosis. Int Immunopharmacol. (2019) 75:105832. doi: 10.1016/j.intimp.2019.105832
73. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. (2019) 5:1–18. doi: 10.1038/s41572-019-0106-z
74. Bullon P, Newman HN, Battino M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol 2000. (2014) 64:139–53. doi: 10.1111/j.1600-0757.2012.00455.x
75. Giordo R, Ahmed YMA, Allam H, Abusnana S, Pappalardo L, Nasrallah GK, et al. EndMT regulation by small RNAs in diabetes-associated fibrotic conditions: potential link with oxidative stress. Front Cell Dev Biol. (2021) 9:683594. doi: 10.3389/fcell.2021.683594
76. Evrard SM, Lecce L, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman K-R, et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun. (2016) 7:11853. doi: 10.1038/ncomms11853
77. Medici D, Potenta S, Kalluri R. Transforming growth factor-β2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem J. (2011) 437:515–20. doi: 10.1042/BJ20101500
78. Cavender MA, Steg P, Smith SC, Eagle K, Ohman EM, Goto S, et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death. Circulation. (2015) 132:923–31. doi: 10.1161/CIRCULATIONAHA.114.014796
79. Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, et al. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. (2019) 26:25–32. doi: 10.1177/2047487319878371
80. Kibel A, Lukinac AM, Dambic V, Juric I, Selthofer-Relatic K. Oxidative stress in ischemic heart disease. Oxid Med Cell Longevity. (2020) 2020:e6627144. doi: 10.1155/2020/6627144
81. Gimbrone MA, García-Cardeña G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. (2013) 22:9–15. doi: 10.1016/j.carpath.2012.06.006
82. Kilhovd BK, Berg TJ, Birkeland KI, Thorsby P, Hanssen KF. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care. (1999) 22:1543–8. doi: 10.2337/diacare.22.9.1543
83. Kiuchi K, Nejima J, Takano T, Ohta M, Hashimoto H. Increased serum concentrations of advanced glycation end products: a marker of coronary artery disease activity in type 2 diabetic patients. Heart. (2001) 85:87–91. doi: 10.1136/heart.85.1.87
84. Mackenzie RM, Salt IP, Miller WH, Logan A, Ibrahim HA, Degasperi A, et al. Mitochondrial reactive oxygen species enhance AMP-activated protein kinase activation in the endothelium of patients with coronary artery disease and diabetes. Clin Sci (London England : 1979). (2013) 124:403. doi: 10.1042/CS20120239
85. Zhang C, Park Y, Picchi A, Potter BJ. Maturation-induces endothelial dysfunction via vascular inflammation in diabetic mice. Basic Res Cardiol. (2008) 103:407–16. doi: 10.1007/s00395-008-0725-0
86. Vanchin B, Sol M, Gjaltema RAF, Brinker M, Kiers B, Pereira AC, et al. Reciprocal regulation of endothelial–mesenchymal transition by MAPK7 and EZH2 in intimal hyperplasia and coronary artery disease. Sci Rep. (2021) 11:17764. doi: 10.1038/s41598-021-97127-4
87. Ergul A, Kelly-Cobbs A, Abdalla M, Fagan SC. Cerebrovascular complications of diabetes: focus on stroke. Endocr Metab Immune Disord Drug Targets. (2012) 12:148–58. doi: 10.2174/187153012800493477
88. Krinock MJ, Singhal NS. Diabetes, stroke, and neuroresilience: looking beyond hyperglycemia. Ann N Y Acad Sci. (2021) 1495:78–98. doi: 10.1111/nyas.14583
89. Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The vascular endothelium and human diseases. Int J Biol Sci. (2013) 9:1057–69. doi: 10.7150/ijbs.7502
90. Bogush M, Heldt NA, Persidsky Y. Blood brain barrier injury in diabetes: unrecognized effects on brain and cognition. J Neuroimmune Pharmacol. (2017) 12:593–601. doi: 10.1007/s11481-017-9752-7
91. Zhou H, Zhang X, Lu J. Progress on diabetic cerebrovascular diseases. Bosn J Basic Med Sci. (2014) 14:185–90. doi: 10.17305/bjbms.2014.4.203
92. Zhu W-G, Li S, Lin L-Q, Yan H, Fu T, Zhu J-H. Vascular oxidative stress increases dendritic cell adhesion and transmigration induced by homocysteine. Cell Immunol. (2009) 254:110–6. doi: 10.1016/j.cellimm.2008.08.001
93. Postea O, Krotz F, Henger A, Keller C, Weiss N. Stereospecific and redox-sensitive increase in monocyte adhesion to endothelial cells by homocysteine. Arterioscler Thromb Vasc Biol. (2006) 26:508–13. doi: 10.1161/01.ATV.0000201039.21705.dc
94. Chen D, Li L, Wang Y, Xu R, Peng S, Zhou L, et al. Ischemia-reperfusion injury of brain induces endothelial-mesenchymal transition and vascular fibrosis via activating let-7i/TGF-βR1 double-negative feedback loop. FASEB J. (2020) 34:7178–91. doi: 10.1096/fj.202000201R
95. Li Y, Liu Y, Liu S, Gao M, Wang W, Chen K, et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. (2023) 8:152. doi: 10.1038/s41392-023-01400-z
96. Lotan R, Ganmore I, Livny A, Shelly S, Zacharia M, Uribarri J, et al. Design and feasibility of a randomized controlled pilot trial to reduce exposure and cognitive risk associated with advanced glycation end products in older adults with type 2 diabetes. Front Nutr. (2021) 8:614149. doi: 10.3389/fnut.2021.614149
97. Venkat P, Chopp M, Chen J. Blood-brain barrier disruption, vascular impairment, and ischemia/reperfusion damage in diabetic stroke. J Am Heart Assoc. (2017) 6:e005819. doi: 10.1161/JAHA.117.005819
98. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. (2015) 3:105–13. doi: 10.1016/S2213-8587(14)70219-0
99. Saydam O, Ozgen Saydam B, Adiyaman CS, Sonmez Ince M, Eren MA, Keskin FE, et al. Diabetic foot ulcers: A neglected complication of lipodystrophy. Diabetes Care. (2020) 43:e149–51. doi: 10.2337/dc20-1655
100. Bapir M, Untracht GR, Cooke D, McVey JH, Skene SS, Campagnolo P, et al. Cocoa flavanol consumption improves lower extremity endothelial function in healthy individuals and people with type 2 diabetes. Food Funct. (2022) 13:10439–48. doi: 10.1039/D2FO02017C
101. Abd El-Khalik SR, Hafez YM, Elkholy RA. The role of circulating soluble fms-like tyrosine kinase-1 in patients with diabetic foot ulcer: A possible mechanism of pathogenesis via a novel link between oxidative stress, inflammation and angiogenesis. Microvascular Res. (2020) 130:103987. doi: 10.1016/j.mvr.2020.103987
102. Del Cuore A, Pipitone RM, Casuccio A, Mazzola MM, Puleo MG, Pacinella G, et al. Metabolic memory in diabetic foot syndrome (DFS): MICRO-RNAS, single nucleotide polymorphisms (SNPs) frequency and their relationship with indices of endothelial function and adipo-inflammatory dysfunction. Cardiovasc Diabetol. (2023) 22:148. doi: 10.1186/s12933-023-01880-x
103. You M, Liu Y, Wang B, Li L, Zhang H, He H, et al. Asprosin induces vascular endothelial-to-mesenchymal transition in diabetic lower extremity peripheral artery disease. Cardiovasc Diabetol. (2022) 21:25. doi: 10.1186/s12933-022-01457-0
104. Yang S, Wang S, Chen L, Wang Z, Chen J, Ni Q, et al. Neutrophil Extracellular Traps Delay Diabetic Wound Healing by Inducing Endothelial-to-Mesenchymal Transition via the Hippo pathway. Int J Biol Sci. (2023) 19:347–61. doi: 10.7150/ijbs.78046
105. Molitch ME, Adler AI, Flyvbjerg A, Nelson RG, So W-Y, Wanner C, et al. Diabetic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes. Kidney Int. (2015) 87:20–30. doi: 10.1038/ki.2014.128
106. Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 inhibition for the prevention and treatment of diabetic kidney disease: A review. Am J Kidney Dis. (2018) 72:267–77. doi: 10.1053/j.ajkd.2018.03.022
107. Hong Q, Zhang L, Fu J, Verghese DA, Chauhan K, Nadkarni GN, et al. LRG1 promotes diabetic kidney disease progression by enhancing TGF-β–induced angiogenesis. J Am Soc Nephrol. (2019) 30:546–62. doi: 10.1681/ASN.2018060599
108. Wu J, Wang Z, Cai M, Wang X, Lo B, Li Q, et al. GPR56 promotes diabetic kidney disease through eNOS regulation in glomerular endothelial cells. Diabetes. (2023) 72:(11):db230124. doi: 10.2337/db23-0124
109. Sun J, Zhao F, Zhang W, Lv J, Lv J, Yin A. BMSCs and miR-124a ameliorated diabetic nephropathy via inhibiting notch signalling pathway. J Cell Mol Med. (2018) 22:4840–55. doi: 10.1111/jcmm.13747
110. Rosenson RS, Chen Q, Najera SD, Lee ML, Cho DJ. Ticagrelor and the prevention of microvascular complications in diabetes patients with lower extremity arterial disease; rationale and design of the hema-kinesis trial. Cardiovasc Drugs Ther. (2018) 32:443–51. doi: 10.1007/s10557-018-6815-9
111. Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. (2008) 19:2282–7. doi: 10.1681/ASN.2008050513
112. McMahon R, Murphy M, Clarkson M, Taal M, Mackenzie HS, Godson C, et al. IHG-2, a mesangial cell gene induced by high glucose, is human gremlin. Regulation by extracellular glucose concentration, cyclic mechanical strain, and transforming growth factor-beta1. J Biol Chem. (2000) 275:9901–4. doi: 10.1074/jbc.275.14.9901
113. Murphy M, Docherty NG, Griffin B, Howlin J, McArdle E, McMahon R, et al. IHG-1 amplifies TGF-beta1 signaling and is increased in renal fibrosis. J Am Soc Nephrology : JASN. (2008) 19(9):1672–80. doi: 10.1681/ASN.2007101080
114. Peng H, Li Y, Wang C, Zhang J, Chen Y, Chen W, et al. ROCK1 induces endothelial-to-mesenchymal transition in glomeruli to aggravate albuminuria in diabetic nephropathy. Sci Rep. (2016) 6:20304. doi: 10.1038/srep20304
115. Yousif MHM, Makki B, El-Hashim AZ, Akhtar S, Benter IF. Chronic treatment with ang-(1-7) reverses abnormal reactivity in the corpus cavernosum and normalizes diabetes-induced changes in the protein levels of ACE, ACE2, ROCK1, ROCK2 and omega-hydroxylase in a rat model of type 1 diabetes. J Diabetes Res. (2014) 2014:e142154. doi: 10.1155/2014/142154
116. Sol M, Kamps JAAM, van den Born J, van den Heuvel MC, van der Vlag J, Krenning G, et al. Glomerular endothelial cells as instigators of glomerular sclerotic diseases. Front Pharmacol. (2020) 11:573557. doi: 10.3389/fphar.2020.573557
117. Liu F, Zhang S, Xu R, Gao S, Yin J. Melatonin Attenuates Endothelial-to-Mesenchymal Transition of Glomerular Endothelial Cells via Regulating miR-497/ROCK in Diabetic Nephropathy. Kidney Blood Pressure Res. (2018) 43:1425–36. doi: 10.1159/000493380
118. Zhang J, Wu Y, Zhang J, Zhang R, Wang Y, Liu F. ABCA1 deficiency-mediated glomerular cholesterol accumulation exacerbates glomerular endothelial injury and dysfunction in diabetic kidney disease. Metabolism. (2023) 139:155377. doi: 10.1016/j.metabol.2022.155377
119. Yi W, OuYang Q. Adiponectin improves diabetic nephropathy by inhibiting necrotic apoptosis. Arch Med Sci. (2019) 15:1321–8. doi: 10.5114/aoms.2018.79570
120. Lin K-Y, Hsih W-H, Lin Y-B, Wen C-Y, Chang T-J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. (2021) 12:1322–5. doi: 10.1111/jdi.13480
121. Siddiqui K, George TP, Mujammami M, Isnani A, Alfadda AA. The association of cell adhesion molecules and selectins (VCAM-1, ICAM-1, E-selectin, L-selectin, and P-selectin) with microvascular complications in patients with type 2 diabetes: A follow-up study. Front Endocrinol (Lausanne). (2023) 14:1072288. doi: 10.3389/fendo.2023.1072288
122. Fickweiler W, Park H, Park K, Mitzner MG, Chokshi T, Boumenna T, et al. Elevated retinol binding protein 3 concentrations are associated with decreased vitreous inflammatory cytokines, VEGF, and progression of diabetic retinopathy. Diabetes Care. (2022) 45:2159–62. doi: 10.2337/dc22-0165
123. D’Amico AG, Maugeri G, Rasà DM, Bucolo C, Saccone S, Federico C, et al. Modulation of IL-1β and VEGF expression in rat diabetic retinopathy after PACAP administration. Peptides. (2017) 97:64–9. doi: 10.1016/j.peptides.2017.09.014
124. D’Amico AG, Maugeri G, Bucolo C, Saccone S, Federico C, Cavallaro S, et al. Nap interferes with hypoxia-inducible factors and VEGF expression in retina of diabetic rats. J Mol Neurosci. (2017) 61:256–66. doi: 10.1007/s12031-016-0869-6
125. Li S, Deng J, Sun D, Chen S, Yao X, Wang N, et al. FBXW7 alleviates hyperglycemia-induced endothelial oxidative stress injury via ROS and PARP inhibition. Redox Biol. (2022) 58:102530. doi: 10.1016/j.redox.2022.102530
126. Kowluru RA, Mohammad G. Mitochondrial fragmentation in a high homocysteine environment in diabetic retinopathy. Antioxidants (Basel). (2022) 11:365. doi: 10.3390/antiox11020365
127. Ju J, Liu Y, Liang H, Yang B. The role of pyroptosis in endothelial dysfunction induced by diseases. Front Immunol. (2023) 13:1093985. doi: 10.3389/fimmu.2022.1093985
128. Yang K, Liu J, Zhang X, Ren Z, Gao L, Wang Y, et al. H3 relaxin alleviates migration, apoptosis and pyroptosis through P2X7R-mediated nucleotide binding oligomerization domain-like receptor protein 3 inflammasome activation in retinopathy induced by hyperglycemia. Front Pharmacol. (2020) 11:603689. doi: 10.3389/fphar.2020.603689
129. Zhang J, Qiu Q, Wang H, Chen C, Luo D. TRIM46 contributes to high glucose-induced ferroptosis and cell growth inhibition in human retinal capillary endothelial cells by facilitating GPX4 ubiquitination. Exp Cell Res. (2021) 407:112800. doi: 10.1016/j.yexcr.2021.112800
130. Cao Y, Feng B, Chen S, Chu Y, Chakrabarti S. Mechanisms of endothelial to mesenchymal transition in the retina in diabetes. Invest Ophthalmol Visual Sci. (2014) 55:7321–31. doi: 10.1167/iovs.14-15167
131. Roy S, Kim D. Retinal capillary basement membrane thickening: Role in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. (2021) 82:100903. doi: 10.1016/j.preteyeres.2020.100903
132. Maiuolo J, Gliozzi M, Musolino V, Carresi C, Nucera S, Macrì R, et al. The role of endothelial dysfunction in peripheral blood nerve barrier: molecular mechanisms and pathophysiological implications. Int J Mol Sci. (2019) 20(12):3022. doi: 10.3390/ijms20123022
133. Eid SA, Rumora AE, Beirowski B, Bennett DL, Hur J, Savelieff MG, et al. New perspectives in diabetic neuropathy. Neuron. (2023) 111:2623–41. doi: 10.1016/j.neuron.2023.05.003
134. Eid SA, Savelieff MG, Eid AA, Feldman EL. Nox, nox, are you there? The role of NADPH oxidases in the peripheral nervous system. Antioxid Redox Signal. (2022) 37:613–30. doi: 10.1089/ars.2021.0135
135. Zhao W-C, Zhang B, Liao M-J, Zhang W-X, He W-Y, Wang H-B, et al. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci Lett. (2014) 560:81–5. doi: 10.1016/j.neulet.2013.12.019
136. Eid SA, El Massry M, Hichor M, Haddad M, Grenier J, Dia B, et al. Targeting the NADPH oxidase-4 and liver X receptor pathway preserves schwann cell integrity in diabetic mice. Diabetes. (2020) 69:448–64. doi: 10.2337/db19-0517
137. Chapouly C, Yao Q, Vandierdonck S, Larrieu-Lahargue F, Mariani JN, Gadeau A-P, et al. Impaired Hedgehog signalling-induced endothelial dysfunction is sufficient to induce neuropathy: implication in diabetes. Cardiovasc Res. (2016) 109:217–27. doi: 10.1093/cvr/cvv263
138. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. (1972) 30:595–602. doi: 10.1016/0002-9149(72)90595-4
139. Dauriz M, Mantovani A, Bonapace S, Verlato G, Zoppini G, Bonora E, et al. Prognostic impact of diabetes on long-term survival outcomes in patients with heart failure: A meta-analysis. Diabetes Care. (2017) 40:1597–605. doi: 10.2337/dc17-0697
140. Knapp M, Tu X, Wu R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol Sin. (2019) 40:1–8. doi: 10.1038/s41401-018-0042-6
141. Riehle C, Bauersachs J. Of mice and men: models and mechanisms of diabetic cardiomyopathy. Basic Res Cardiol. (2018) 114:2. doi: 10.1007/s00395-018-0711-0
142. Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. (2020) 126:1501–25. doi: 10.1161/CIRCRESAHA.120.315913
143. Jordan NP, Tingle SJ, Shuttleworth VG, Cooke K, Redgrave RE, Singh E, et al. MiR-126-3p is dynamically regulated in endothelial-to-mesenchymal transition during fibrosis. Int J Mol Sci. (2021) 22:8629. doi: 10.3390/ijms22168629
144. Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. (2007) 13:952–61. doi: 10.1038/nm1613
145. Zhang L, He J, Wang J, Liu J, Chen Z, Deng B, et al. Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy. Cell Death Dis. (2021) 12:470. doi: 10.1038/s41419-021-03750-4
146. Felaco M, Grilli A, De Lutiis MA, Patruno A, Libertini N, Taccardi AA, et al. Endothelial nitric oxide synthase (eNOS) expression and localization in healthy and diabetic rat hearts. Ann Clin Lab Sci. (2001) 31:179–86.
147. Carnicer R, Duglan D, Ziberna K, Recalde A, Reilly S, Simon JN, et al. BH4 increases nNOS activity and preserves left ventricular function in diabetes. Circ Res. (2021) 128:585–601. doi: 10.1161/CIRCRESAHA.120.316656
148. Elmadbouh I, Singla DK. BMP-7 attenuates inflammation-induced pyroptosis and improves cardiac repair in diabetic cardiomyopathy. Cells. (2021) 10:2640. doi: 10.3390/cells10102640
149. Wan A, Rodrigues B. Endothelial cell-cardiomyocyte crosstalk in diabetic cardiomyopathy. Cardiovasc Res. (2016) 111:172–83. doi: 10.1093/cvr/cvw159
150. Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res. (2016) 109:397–408. doi: 10.1093/cvr/cvv260
151. Triggle CR, Ding H, Marei I, Anderson TJ, Hollenberg MD. Why the endothelium? The endothelium as a target to reduce diabetes-associated vascular disease. Can J Physiol Pharmacol. (2020) 98:415–30. doi: 10.1139/cjpp-2019-0677
152. Pernicova I, Korbonits M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. (2014) 10:143–56. doi: 10.1038/nrendo.2013.256
153. Wang Y, Jin M, Cheng CK, Li Q. Tubular injury in diabetic kidney disease: molecular mechanisms and potential therapeutic perspectives. Front Endocrinol. (2023) 14:1238927. doi: 10.3389/fendo.2023.1238927
154. Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. (2006) 47:1183–8. doi: 10.1161/01.HYP.0000221429.94591.72
155. Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, et al. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. (2006) 26:611–7. doi: 10.1161/01.ATV.0000201938.78044.75
156. Mohammad HMF, Galal Gouda S, Eladl MA, Elkazaz AY, Elbayoumi KS, Farag NE, et al. Metformin suppresses LRG1 and TGFβ1/ALK1-induced angiogenesis and protects against ultrastructural changes in rat diabetic nephropathy. Biomed Pharmacother. (2023) 158:114128. doi: 10.1016/j.biopha.2022.114128
157. Shati AA, Maarouf A, Dawood AF, Bayoumy NM, Alqahtani YA, A. Eid R, et al. Lower Extremity Arterial Disease in Type 2 Diabetes Mellitus: Metformin Inhibits Femoral Artery Ultrastructural Alterations as well as Vascular Tissue Levels of AGEs/ET-1 Axis-Mediated Inflammation and Modulation of Vascular iNOS and eNOS Expression. Biomedicines. (2023) 11:361. doi: 10.3390/biomedicines11020361
158. Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. (2006) 55:120–7. doi: 10.2337/diabetes.55.01.06.db05-0943
159. Ouslimani N, Peynet J, Bonnefont-Rousselot D, Thérond P, Legrand A, Beaudeux J-L. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism. (2005) 54:829–34. doi: 10.1016/j.metabol.2005.01.029
160. Niu C, Chen Z, Kim KT, Sun J, Xue M, Chen G, et al. Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy. (2019) 15:843–70. doi: 10.1080/15548627.2019.1569913
161. Kim H-S, Ren G, Kim T, Bhatnagar S, Yang Q, Bahk YY, et al. Metformin reduces saturated fatty acid-induced lipid accumulation and inflammatory response by restoration of autophagic flux in endothelial cells. Sci Rep. (2020) 10:13523. doi: 10.1038/s41598-020-70347-w
162. Wang Q, Zhang M, Torres G, Wu S, Ouyang C, Xie Z, et al. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of drp1-mediated mitochondrial fission. Diabetes. (2017) 66:193–205. doi: 10.2337/db16-0915
163. Mansour AA, Hamadi SS, Yaseen Alibrahim NT, Chasib MG. Predictors of metformin side effects in patients with newly diagnosed type 2 diabetes mellitus. Ibnosina J Med Biomed Sci. (2023) 15:067–73. doi: 10.1055/s-0043-1761215
164. Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J Diabetes Metab Disord. (2013) 12:17. doi: 10.1186/2251-6581-12-17
165. Liu H, Guo L, Xing J, Li P, Sang H, Hu X, et al. The protective role of DPP4 inhibitors in atherosclerosis. Eur J Pharmacol. (2020) 875:173037. doi: 10.1016/j.ejphar.2020.173037
166. Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, et al. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the study of preventive effects of alogliptin on diabetic atherosclerosis (SPEAD-A). Diabetes Care. (2016) 39:139–48. doi: 10.2337/dc15-0781
167. Gao P, Li L, Wei X, Wang M, Hong Y, Wu H, et al. Activation of transient receptor potential channel vanilloid 4 by DPP-4 (Dipeptidyl peptidase-4) inhibitor vildagliptin protects against diabetic endothelial dysfunction. Hypertension. (2020) 75:150–62. doi: 10.1161/HYPERTENSIONAHA.119.13778
168. Wu C, Hu S, Wang N, Tian J. Dipeptidyl peptidase−4 inhibitor sitagliptin prevents high glucose−induced apoptosis via activation of AMP−activated protein kinase in endothelial cells. Mol Med Rep. (2017) 15:4346–51. doi: 10.3892/mmr.2017.6501
169. Dai X, Zeng J, Yan X, Lin Q, Wang K, Chen J, et al. Sitagliptin-mediated preservation of endothelial progenitor cell function via augmenting autophagy enhances ischaemic angiogenesis in diabetes. J Cell Mol Med. (2018) 22:89–100. doi: 10.1111/jcmm.13296
170. Pujadas G, De Nigris V, Prattichizzo F, La Sala L, Testa R, Ceriello A. The dipeptidyl peptidase-4 (DPP-4) inhibitor teneligliptin functions as antioxidant on human endothelial cells exposed to chronic hyperglycemia and metabolic high-glucose memory. Endocrine. (2017) 56:509–20. doi: 10.1007/s12020-016-1052-0
171. Zhao X, Su L, He X, Zhao B, Miao J. Long noncoding RNA CA7-4 promotes autophagy and apoptosis via sponging MIR877-3P and MIR5680 in high glucose-induced vascular endothelial cells. Autophagy. (2020) 16:70–85. doi: 10.1080/15548627.2019.1598750
172. Mi D-H, Fang H-J, Zheng G-H, Liang X-H, Ding Y-R, Liu X, et al. DPP-4 inhibitors promote proliferation and migration of rat brain microvascular endothelial cells under hypoxic/high-glucose conditions, potentially through the SIRT1/HIF-1/VEGF pathway. CNS Neurosci Ther. (2019) 25:323–32. doi: 10.1111/cns.13042
173. Li H, Zhang J, Lin L, Xu L. Vascular protection of DPP-4 inhibitors in retinal endothelial cells in in vitro culture. Int Immunopharmacol. (2019) 66:162–8. doi: 10.1016/j.intimp.2018.10.040
174. Kanasaki K, Shi S, Kanasaki M, He J, Nagai T, Nakamura Y, et al. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. (2014) 63:2120–31. doi: 10.2337/db13-1029
175. Hardigan T, Abdul Y, Ergul A. Linagliptin reduces effects of ET-1 and TLR2-mediated cerebrovascular hyperreactivity in diabetes. Life Sci. (2016) 159:90–6. doi: 10.1016/j.lfs.2016.02.067
176. Seo S, Kim M-K, Kim R-I, Yeo Y, Kim KL, Suh W. Evogliptin, a dipeptidyl peptidase-4 inhibitor, attenuates pathological retinal angiogenesis by suppressing vascular endothelial growth factor-induced Arf6 activation. Exp Mol Med. (2020) 52:1744–53. doi: 10.1038/s12276-020-00512-8
177. Khalse M, Ganapathy B. Effect of dipeptidyl peptidase 4 inhibitors on cardiovascular events in type-2 diabetes patients with renal impairment: A systematic review and meta-analysis. Indian J Endocrinol Metab. (2020) 24:143. doi: 10.4103/ijem.IJEM_568_19
178. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1–based therapies. Gastroenterology. (2011) 141:150–6. doi: 10.1053/J.GASTRO.2011.02.018
179. Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. (2023) 20:463–74. doi: 10.1038/s41569-023-00849-3
180. Wang L, Cheng CK, Yi M, Lui KO, Huang Y. Targeting endothelial dysfunction and inflammation. J Mol Cell Cardiol. (2022) 168:58–67. doi: 10.1016/j.yjmcc.2022.04.011
181. Vergès B, Aboyans V, Angoulvant D, Boutouyrie P, Cariou B, Hyafil F, et al. Protection against stroke with glucagon-like peptide-1 receptor agonists: a comprehensive review of potential mechanisms. Cardiovasc Diabetol. (2022) 21:242. doi: 10.1186/s12933-022-01686-3
182. Maruhashi T, Higashi Y. Pathophysiological association between diabetes mellitus and endothelial dysfunction. Antioxidants (Basel). (2021) 10:1306. doi: 10.3390/antiox10081306
183. Wang D, Luo P, Wang Y, Li W, Wang C, Sun D, et al. Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism. Diabetes. (2013) 62:1697–708. doi: 10.2337/db12-1025
184. Cai X, She M, Xu M, Chen H, Li J, Chen X, et al. GLP-1 treatment protects endothelial cells from oxidative stress-induced autophagy and endothelial dysfunction. Int J Biol Sci. (2018) 14:1696–708. doi: 10.7150/ijbs.27774
185. Cheng CK, Luo J-Y, Lau CW, Cho WC-S, Ng CF, Ma RCW, et al. A GLP-1 analog lowers ER stress and enhances protein folding to ameliorate homocysteine-induced endothelial dysfunction. Acta Pharmacol Sin. (2021) 42:1598–609. doi: 10.1038/s41401-020-00589-x
186. Tsai T-H, Lee C-H, Cheng C-I, Fang Y-N, Chung S-Y, Chen S-M, et al. Liraglutide inhibits endothelial-to-mesenchymal transition and attenuates neointima formation after endovascular injury in streptozotocin-induced diabetic mice. Cells. (2019) 8:589. doi: 10.3390/cells8060589
187. Day SM, Yang W, Ewin S, Zhou X, Ma T. Glucagon-like peptide-1 cleavage product GLP-1 (9-36) amide enhances hippocampal long-term synaptic plasticity in correlation with suppression of Kv4.2 expression and eEF2 phosphorylation. Hippocampus. (2017) 27:1264–74. doi: 10.1002/hipo.22795
188. Goldenberg RM, Cheng AYY, Fitzpatrick T, Gilbert JD, Verma S, Hopyan JJ. Benefits of GLP-1 (Glucagon-like peptide 1) receptor agonists for stroke reduction in type 2 diabetes: A call to action for neurologists. Stroke. (2022) 53:1813–22. doi: 10.1161/STROKEAHA.121.038151
189. Bellastella G, Maiorino MI, Longo M, Scappaticcio L, Chiodini P, Esposito K, et al. Glucagon-like peptide-1 receptor agonists and prevention of stroke systematic review of cardiovascular outcome trials with meta-analysis. Stroke. (2020) 51:666–9. doi: 10.1161/STROKEAHA.119.027557
190. Luna-Marco C, de Marañon AM, Hermo-Argibay A, Rodriguez-Hernandez Y, Hermenejildo J, Fernandez-Reyes M, et al. Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biol. (2023) 66:102849. doi: 10.1016/j.redox.2023.102849
191. McLean BA, Wong CK, Kabir MG, Drucker DJ. Glucagon-like Peptide-1 receptor Tie2+ cells are essential for the cardioprotective actions of liraglutide in mice with experimental myocardial infarction. Mol Metab. (2022) 66:101641. doi: 10.1016/j.molmet.2022.101641
192. Zhu Y-X, Li Y, Ma Y, Zhang X, Du X, Gao J, et al. Liraglutide accelerates ischemia-induced angiogenesis in a murine diabetic model. J Am Heart Assoc. (2023) 12:e026586. doi: 10.1161/JAHA.122.026586
193. Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabetes Stud. (2014) 11:202–30. doi: 10.1900/RDS.2014.11.202
194. Liu L, Chen J, Wang L, Chen C, Chen L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database. Front Endocrinol. (2022) 13:1043789. doi: 10.3389/fendo.2022.1043789
195. Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol. (2021) 83:503–28. doi: 10.1146/annurev-physiol-031620-095920
196. Dhillon S. Dapagliflozin: A review in type 2 diabetes. Drugs. (2019) 79:1135–46. doi: 10.1007/s40265-019-01148-3
197. Tahara A, Takasu T, Yokono M, Imamura M, Kurosaki E. Characterization and comparison of SGLT2 inhibitors: Part 3. Effects on diabetic complications in type 2 diabetic mice. Eur J Pharmacol. (2017) 809:163–71. doi: 10.1016/j.ejphar.2017.05.019
198. Ugusman A, Kumar J, Aminuddin A. Endothelial function and dysfunction: Impact of sodium-glucose cotransporter 2 inhibitors. Pharmacol Ther. (2021) 224:107832. doi: 10.1016/j.pharmthera.2021.107832
199. Takakura S, Toyoshi T, Hayashizaki Y, Takasu T. Effect of ipragliflozin, an SGLT2 inhibitor, on progression of diabetic microvascular complications in spontaneously diabetic Torii fatty rats. Life Sci. (2016) 147:125–31. doi: 10.1016/j.lfs.2016.01.042
200. El-Daly M, Pulakazhi Venu VK, Saifeddine M, Mihara K, Kang S, Fedak P W.M. Hyperglycaemic impairment of PAR2-mediated vasodilation: Prevention by inhibition of aortic endothelial sodium-glucose-co-Transporter-2 and minimizing oxidative stress. Vasc Pharmacol. (2018) 109:56–71. doi: 10.1016/j.vph.2018.06.006
201. Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. (2018) 15:335–46. doi: 10.1016/j.redox.2017.12.019
202. Ganbaatar B, Fukuda D, Shinohara M, Yagi S, Kusunose K, Yamada H, et al. Empagliflozin ameliorates endothelial dysfunction and suppresses atherogenesis in diabetic apolipoprotein E-deficient mice. Eur J Pharmacol. (2020) 875:173040. doi: 10.1016/j.ejphar.2020.173040
203. Xu H, Fu J, Tu Q, Shuai Q, Chen Y, Wu F, et al. The SGLT2 inhibitor empagliflozin attenuates atherosclerosis progression by inducing autophagy. J Physiol Biochem. (2023) 80:27. doi: 10.1007/s13105-023-00974-0
204. Li J, Liu H, Takagi S, Nitta K, Kitada M, Srivastava SP, et al. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight. (2020) 5:e129034. doi: 10.1172/jci.insight.129034
205. Adingupu DD, Göpel SO, Grönros J, Behrendt M, Sotak M, Miliotis T, et al. SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob-/- mice. Cardiovasc Diabetol. (2019) 18:16. doi: 10.1186/s12933-019-0820-6
206. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. (2019) 380:2295–306. doi: 10.1056/NEJMoa1811744
207. Li X, Kerindongo RP, Preckel B, Kalina J-O, Hollmann MW, Zuurbier CJ, et al. Canagliflozin inhibits inflammasome activation in diabetic endothelial cells - Revealing a novel calcium-dependent anti-inflammatory effect of canagliflozin on human diabetic endothelial cells. BioMed Pharmacother. (2023) 159:114228. doi: 10.1016/j.biopha.2023.114228
208. Kapadia P, Bikkina P, Landicho MA, Parekh S, Haas MJ, Mooradian AD. Effect of anti-hyperglycemic drugs on endoplasmic reticulum (ER) stress in human coronary artery endothelial cells. Eur J Pharmacol. (2021) 907:174249. doi: 10.1016/j.ejphar.2021.174249
209. Effects of SGLT2 (Sodium-Dependent Type 2 Glucose Transporters) on Different Systems (2023). International Journal of Medical Science and Clinical Research Studies. Available at: https://ijmscr.org/index.php/ijmscrs/article/view/767 (Accessed March 1, 2024).
210. Douros A, Lix LM, Fralick M, Dell’Aniello S, Shah BR, Ronksley PE, et al. Sodium-glucose cotransporter-2 inhibitors and the risk for diabetic ketoacidosis : A multicenter cohort study. Ann Intern Med. (2020) 173:417–25. doi: 10.7326/M20-0289
211. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377:644–57. doi: 10.1056/NEJMoa1611925
212. Meng Z, Zhou D, Gao Y, Zeng M, Wang W. miRNA delivery for skin wound healing. Advanced Drug Delivery Rev. (2018) 129:308–18. doi: 10.1016/j.addr.2017.12.011
213. Zhang Y, Sun X, Icli B, Feinberg MW. Emerging roles for microRNAs in diabetic microvascular disease: novel targets for therapy. Endocr Rev. (2017) 38:145–68. doi: 10.1210/er.2016-1122
214. Wang H-J, Huang Y-L, Shih Y-Y, Wu H-Y, Peng C-T, Lo W-Y. MicroRNA-146a decreases high glucose/thrombin-induced endothelial inflammation by inhibiting NAPDH oxidase 4 expression. Mediators Inflammation. (2014) 2014:379537. doi: 10.1155/2014/379537
215. Lo W-Y, Peng C-T, Wang H-J. MicroRNA-146a-5p mediates high glucose-induced endothelial inflammation via targeting interleukin-1 receptor-associated kinase 1 expression. Front Physiol. (2017) 8:551. doi: 10.3389/fphys.2017.00551
216. Veitch S, Njock M-S, Chandy M, Siraj MA, Chi L, Mak H, et al. MiR-30 promotes fatty acid beta-oxidation and endothelial cell dysfunction and is a circulating biomarker of coronary microvascular dysfunction in pre-clinical models of diabetes. Cardiovasc Diabetol. (2022) 21:31. doi: 10.1186/s12933-022-01458-z
217. Tian D, Xiang Y, Tang Y, Ge Z, Li Q, Zhang Y. Circ-ADAM9 targeting PTEN and ATG7 promotes autophagy and apoptosis of diabetic endothelial progenitor cells by sponging mir-20a-5p. Cell Death Dis. (2020) 11:526. doi: 10.1038/s41419-020-02745-x
218. He K, Chen Z, Zhao J, He Y, Deng R, Fan X, et al. The role of microRNA-155 in glomerular endothelial cell injury induced by high glucose. Mol Biol Rep. (2022) 49:2915–24. doi: 10.1007/s11033-021-07106-1
219. Mikhail M, Vachon PH, D’Orléans-Juste P, Jacques D, Bkaily G. Role of endothelin-1 and its receptors, ETA and ETB, in the survival of human vascular endothelial cells. Can J Physiol Pharmacol. (2017) 95:1298–305. doi: 10.1139/cjpp-2017-0412
220. McCoy MG, Jamaiyar A, Sausen G, Cheng HS, Pérez-Cremades D, Zhuang R, et al. MicroRNA-375 repression of Kruppel-like factor 5 improves angiogenesis in diabetic critical limb ischemia. Angiogenesis. (2023) 26:107–27. doi: 10.1007/s10456-022-09856-3
221. Villota SD, Toledo-Rodriguez M, Leach L. Compromised barrier integrity of human feto-placental vessels from gestational diabetic pregnancies is related to downregulation of occludin expression. Diabetologia. (2021) 64. doi: 10.1007/s00125-020-05290-6
222. Wang F, Zhang M. Circ_001209 aggravates diabetic retinal vascular dysfunction through regulating miR-15b-5p/COL12A1. J Transl Med. (2021) 19:294. doi: 10.1186/s12967-021-02949-5
223. Zhan D, Zhao J, Shi Q, Lou J, Wang W. 25-hydroxyvitamin D3 inhibits oxidative stress and ferroptosis in retinal microvascular endothelial cells induced by high glucose through down-regulation of miR-93. BMC Ophthalmol. (2023) 23:22. doi: 10.1186/s12886-022-02762-8
224. Wang J, Chen S, Zhang W, Chen Y, Bihl JC. Exosomes from miRNA-126-modified endothelial progenitor cells alleviate brain injury and promote functional recovery after stroke. CNS Neurosci Ther. (2020) 26:1255–65. doi: 10.1111/cns.13455
225. Zeeshan F. “Chapter 36 - Targeting micro-ribonucleic acid (miRNA) in cancer using advanced drug delivery systems,” In: Dua K, Mehta M, de Jesus Andreoli Pinto T, Pont LG, Williams KA, Rathbone MJ, editors. Advanced Drug Delivery Systems in the Management of Cancer. Kuala Lumpur, Malaysia: Academic Press (2021). p. 461–6. doi: 10.1016/B978-0-323-85503-7.00004-3
226. Ghafouri-Fard S, Shoorei H, Noferesti L, Hussen BM, Moghadam MHB, Taheri M, et al. Nanoparticle-mediated delivery of microRNAs-based therapies for treatment of disorders. Pathol - Res Pract. (2023) 248:154667. doi: 10.1016/j.prp.2023.154667
227. Natarajan R, Putta S, Kato M. MicroRNAs and diabetic complications. J Cardiovasc Transl Res. (2012) 5:413–22. doi: 10.1007/s12265-012-9368-5
228. Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. (2022) 7:272. doi: 10.1038/s41392-022-01134-4
229. Pakpahan C, Ibrahim R, William W, Faizah Z, Juniastuti J, Lusida MI, et al. Stem cell therapy and diabetic erectile dysfunction: A critical review. World J Stem Cells. (2021) 13:1549–63. doi: 10.4252/wjsc.v13.i10.1549
230. Liu Y, Chen J, Liang H, Cai Y, Li X, Yan L, et al. Human umbilical cord-derived mesenchymal stem cells not only ameliorate blood glucose but also protect vascular endothelium from diabetic damage through a paracrine mechanism mediated by MAPK/ERK signaling. Stem Cell Res Ther. (2022) 13:258. doi: 10.1186/s13287-022-02927-8
231. Hu N, Cai Z, Jiang X, Wang C, Tang T, Xu T, et al. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta Biomater. (2023) 157:175–86. doi: 10.1016/j.actbio.2022.11.057
232. Yu M, Liu W, Li J, Lu J, Lu H, Jia W, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. (2020) 11:350. doi: 10.1186/s13287-020-01824-2
233. Xiao X, Xu M, Yu H, Wang L, Li X, Rak J, et al. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct Target Ther. (2021) 6:354. doi: 10.1038/s41392-021-00765-3
234. Peng B-Y, Dubey NK, Mishra VK, Tsai F-C, Dubey R, Deng W-P, et al. Addressing stem cell therapeutic approaches in pathobiology of diabetes and its complications. J Diabetes Res. (2018) 2018:7806435. doi: 10.1155/2018/7806435
235. Yamanaka S. Pluripotent stem cell-based cell therapy—Promise and challenges. Cell Stem Cell. (2020) 27:523–31. doi: 10.1016/j.stem.2020.09.014
Keywords: diabetes, endothelial dysfunction, vascular diseases, atherosclerotic, therapies
Citation: Yang D-R, Wang M-Y, Zhang C-L and Wang Y (2024) Endothelial dysfunction in vascular complications of diabetes: a comprehensive review of mechanisms and implications. Front. Endocrinol. 15:1359255. doi: 10.3389/fendo.2024.1359255
Received: 22 December 2023; Accepted: 08 March 2024;
Published: 05 April 2024.
Edited by:
Abhijit Chakraborty, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Ibrahim Fadil Benter, Final International University, CyprusChen Zhang, Baylor College of Medicine, United States
Copyright © 2024 Yang, Wang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Wang, d2FuZ3l1X2htdUAxNjMuY29t
 Dong-Rong Yang
Dong-Rong Yang Meng-Yan Wang2
Meng-Yan Wang2 Cheng-Lin Zhang
Cheng-Lin Zhang Yu Wang
Yu Wang