- 1Department of Urology, Xiangya Hospital, Central South University, Changsha, China
- 2Department of Urology, Tianjin Medical University General Hospital, Tianjin, China
The existing research on the association between apolipoproteins (Apos) and erectile dysfunction (ED) primarily relies on observational studies and does not distinguish between organic and psychogenic causes when diagnosing ED. It is difficult to believe that Apos play a role in psychogenic ED. To address these issues, our study explored the causal relationship between lipoproteins and ED using Mendelian randomization (MR) analysis and differentiate between organic and psychogenic ED through the use of nocturnal penile tumescence and rigidity (NPTR) monitoring. Multivariate MR analysis revealed significant causal associations between high-density lipoprotein (HDL), Apo A1, and Apo B/A1 with ED (OR and 95% CI were 0.33 (0.14-0.78), 3.58 (1.52-8.43), and 0.30 (0.13-0.66)). we conducted statistical and analytical analyses on the data of 212 patients using multivariate analyses and receiver operating characteristic (ROC) curves. Patients with organic ED had significantly lower levels of HDL, Apo A1 and Apo A1/B, whereas patients with organic ED had considerably higher levels of Apo B and low-density lipoprotein (LDL). The diagnostic value of Apos in predicting the risk of organic ED was evaluated using ROC curves. The results indicated that Apo A1 and Apo A1/B demonstrated good predictive value. HDL, Apo A1, and Apo A1/B have been identified as risk factors for ED in our study. Furthermore, our research highlights the significance of Apo A1 and Apo A1/Apo B in the development of organic ED and suggests their potential use as indicators to assess the risks associated with organic ED.
Introduction
Erectile dysfunction (ED) denotes the persistent incapacity to attain and/or sustain a sufficiently robust erection to achieve satisfactory sexual performance (1). Approximately 10-25% of men are affected by ED, a prevalent sexual condition worldwide (2). It is expected that the frequency and prevalence rates of ED will rise. It is projected that around 322 million individuals will be diagnosed with ED by 2025 (3).
Numerous studies have demonstrated a shared pathophysiological basis of ED and cardiovascular diseases (CVDs) in terms of vascular insufficiency (4). Dyslipidemia, a disorder of lipoprotein metabolism, is widely recognized as the primary risk factor for CVD. Moreover, clinicians have utilized lipoproteins such as low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), triglycerides (TG), and apolipoproteins (Apos) to predict ED due to the close pathophysiological relationship between CVD and ED (5, 6). Notably, Apos like Apo B, Apo A1, and Apo A1/B have proven to be highly effective in predicting CVD in the general population, as they indicate vascular endothelium damage (7, 8). A previous study has suggested that Apo B, Apo A1, and Apo A1/Apo B can also serve as risk factors for ED (9). However, determining the respective contribution of each factor to ED is challenging due to the complex interrelationships among these symptoms, even though many cases of ED can be attributed to a combination of dyslipidemia risk factors.
ED is a common male sexual dysfunction that can be caused by various factors, such as vascular, neurogenic, hormonal, and anatomic abnormalities, as well as psychological and mixed factors (10). The International Index of Erectile Function (IIEF-5), a widely used multidimensional tool, is valuable for evaluating male sexual function and serves as a useful resource for examining ED. It is recommended to utilize this tool as a starting point for ED testing and to assess the severity of ED (11). Additionally, nocturnal penile tumescence and rigidity (NPTR) occurs during rapid eye movement sleep independently of sexual stimulation, resulting in sleep-related erections. This concept is based on the idea that if a man with ED experiences normal nocturnal erectile activity, the cause may be ‘psychogenic’ (12). Psychogenic factors such as stress, anxiety, distress, depression, and relationship problems do not typically affect sleep-related erections. On the other hand, men with normal erectile function usually experience 4-6 episodes of involuntary nocturnal erections lasting 20-50 minutes within a 6-8 hour sleep cycle (13). NPTR monitoring was the first objective test used for diagnosing ED and has been considered a useful and reliable method for distinguishing between psychogenic and organic ED (14).
Despite the numerous assertions made, a significant portion of the research relied on observation. However, it is important to note that observational studies are limited in their ability to establish causality. Therefore, it is necessary to employ other research methods to minimize the influence of confounding factors. One such method is Mendelian randomization (MR) analysis, which offers distinct advantages over observational studies. MR analysis effectively addresses confounding and reverse causality by utilizing instrumental variables (IVs) in the form of single nucleotide polymorphisms (SNPs), rather than genetic variation. This approach is particularly valuable when examining risk factors that may be prone to measurement inaccuracies (15). Additionally, the introduction of multivariable MR (MVMR) as a new MR model allows for the direct evaluation of each risk factor’s individual impact, thereby mitigating the potential influence of other related risk factors (16, 17).
The primary objective of this study is to utilize both single variable MR (SVMR) and MVMR to assess the overall and immediate impacts of lipid characteristics on the risk of ED initially. Previous studies have used the IIEF-5 diagnostic modality for ED and the genome-wide association study (GWAS) database. However, the IIEF-5 diagnosis encompasses both organic and psychogenic causes of ED, and it is difficult to believe that Apos play a role in psychogenic ED. Therefore, we employed NPTR monitoring for ED diagnosis, which provides a more objective means to distinguish between organic and psychogenic ED. This helps us verify the correlation between Apos and organic ED, excluding psychogenic ED.
Materials and methods
MR design
MR has three primary conjectures. Hypothesis 1 suggests a strong correlation between genetic variants used as independent variables and traits associated with lipids. Hypothesis 2 states that the independent variables used should not be connected to any potential confounding variables. Hypothesis 3 proposes that the risk of the outcome should only be influenced by exposed genetic variants through risk factors, rather than through alternative pathways. In order to investigate the potential cause-and-effect relationship between lipoproteins and ED, we conducted a MR study using the logic of two-sample MR. The purpose of the SVMR analysis was to examine the correlation between individual lipoproteins and ED outcomes, while the MVMR analysis aimed to compare the independent role of lipoprotein-associated traits in ED outcomes. The study design of this experiment is illustrated in Figure 1.
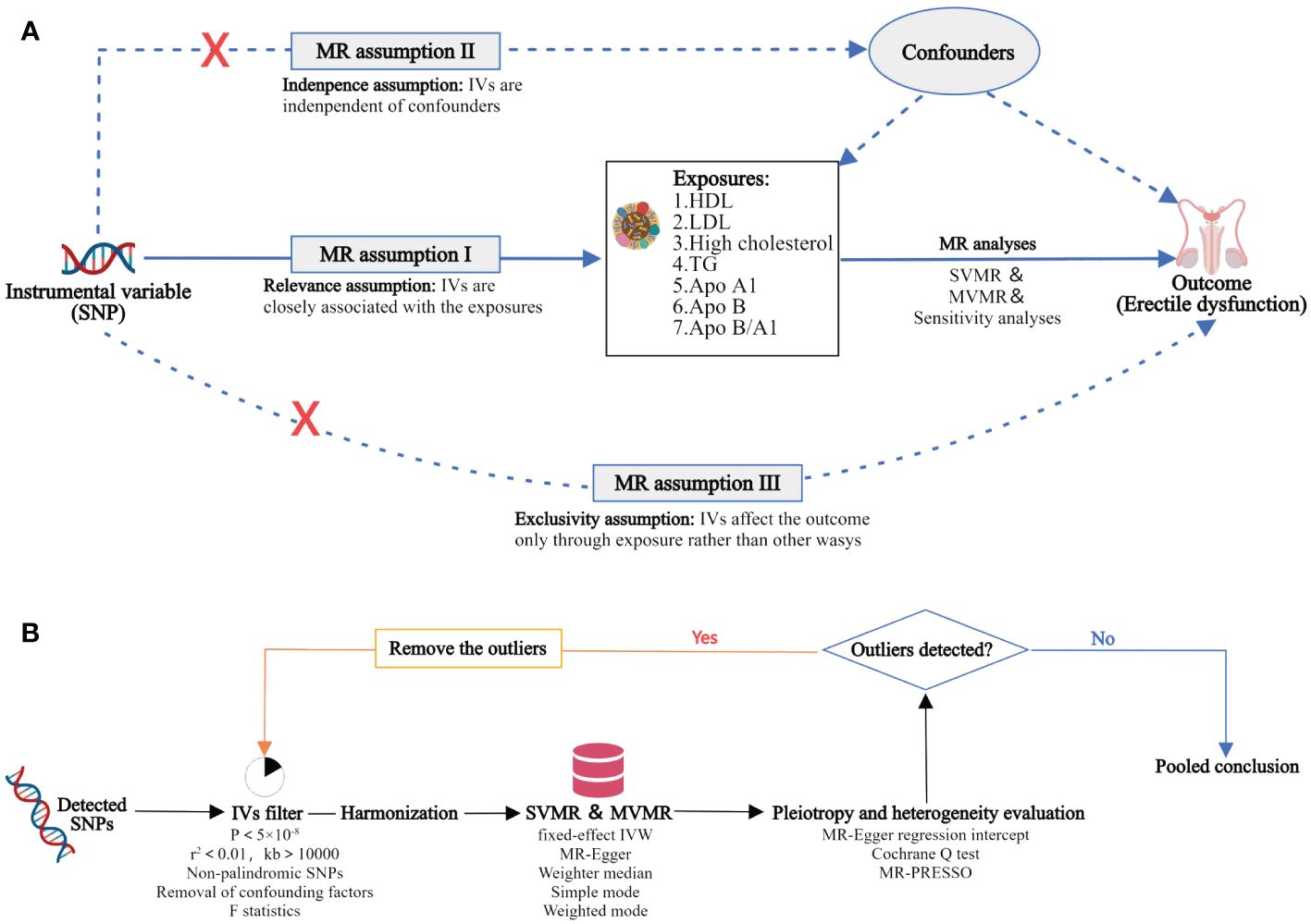
Figure 1 Overview of the MR study design and analysis strategy. (A): Study design overview: The study examines the effects of 7 lipoproteins, namely HDL, LDL, high cholesterol, TG, Apo A1, Apo B, and Apo B/A1. The MR framework is based on three fundamental MR assumptions. (B): MR analysis strategy: Initially, qualified SNPs are filtered as IVs. Subsequently, sensitivity analyses are conducted to assess the robustness of the results, along with the evaluation of heterogeneity and pleiotropy. MR, Mendelian randomization; SNP, single nucleotide polymorphism; IV, instrumental variable; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides; Apo, apolipoprotein; SVMR, single variable MR; MVMR, multivariate MR; MR‐PRESSO, MR Pleiotropy RESidualSum and Outlier.
Sample source
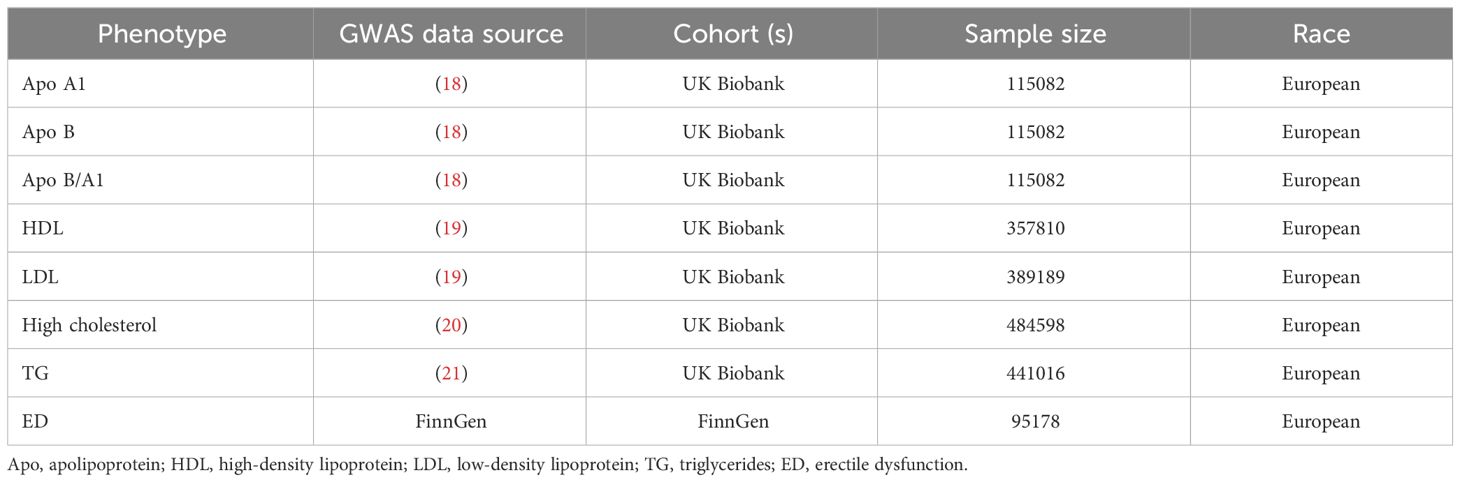
SNPs identified by large samples of GWASs were used as IVs to represent exposures of lipoproteins, including LDL, HDL, high cholesterol, TG, and Apos. Table 1 presents a comprehensive breakdown of the sample information in this study.
We made use of the Apo A1, ApoB, and Apo B/A data sourced from Tom G Richardson et al. After analyzing the information from The UK Biobank, a conclusion was reached (18). HDL, LDL, high cholesterol and TG data were obtained from The UK Biobank (http://www.ukbiobank.ac.uk/, accessed on 18 October 2023) (19–21).
In this study, the GWAS dataset of ED from FinnGen (https://r7.finngen.fi/, accessed on 20 October 2023) were used as outcome datasets. The study included a total of 1154 cases and 94024 controls, all of whom were men from European Finland. ED was identified based on the International Classification of Diseases, 10th Revision (ICD-10) codes (N48.4 and F52.2), or through medical records of ED intervention, such as surgical procedures or oral medication, or self-reported information provided by the participants.
Selection of IVs
To ensure statistical power, we selected SNPs with significant associations with lipoproteins (p < 5 × 10−8) as independent variables, as shown in Figure 1. We then excluded chain disequilibrium responses by applying thresholds of r2 < 0.001 and Kb > 10,000. Subsequently, we retrieved the effect estimates of the selected independent variables from the ‘ED outcome’ dataset. To address the second hypothesis of MR, we utilized the PhenoScannerV2 database (http://www.phenoscanner.medschl.cam.ac.uk/), eliminating SNPs associated with ED outcomes and confounding variables (22). Earlier studies have indicated that fasting glucose, uric acid, and hypertension could potentially contribute to the development of ED (23–25). To ensure the accuracy of our study, we excluded fasting glucose, uric acid, and hypertension as potential confounding factors. We also avoided using palindromic SNPs, which are characterized by having the same nucleotides paired together in a DNA molecule (26). In order to meet the Mendelian first hypothesis, we used R2 as a genetic tool to explain the proportion of trait variance. R2 was calculated using the formula R2 = 2 × (1 − MAF) × MAF × β2/(SE2 × N), where N represents the sample size, β is the effect size, SE is the standard error, and MAF is the minimum allele frequency for each SNP. Additionally, we computed an F statistic to assess the overall potency of the selected SNPs in explaining phenotypic variability. The equation used for this calculation was F = β²/SE². A value of F greater than 10 indicates that the IV SNPs are highly effective in minimizing potential bias (27), while a value of F less than or equal to 10 suggests that the SNPs are weak IVs.
MR analysis
We conducted five MR techniques to assess the impact of lipoproteins on ED outcomes. The main MR approach used was the inverse variance weighted (IVW) method, while MR Egger, weighted median, simple mode, and weighted mode techniques were also employed as alternative options (28, 29). In our study, we utilized MVMR to assess the independent causal effects of lipoprotein characteristics on the occurrence of ED. The results of the MR analyses were reported as odds ratios (ORs) with corresponding 95% confidence intervals (CIs). The MR Egger and MR Pleiotropy RESidualSum and Outlier (MR‐PRESSO) method was used to assess the multiple effects. A p-value exceeding 0.05 indicated the absence of any multiplicity of effects. The Cochran’s Q statistic was examined using mr_egger and IVW, and a p-value greater than 0.05 indicated no heterogeneity. Furthermore, a ‘leave‐one‐out’ sensitivity analysis was performed to demonstrate that the impact of lipoproteins on ED outcomes remained unchanged by individual SNPs.
Study subjects and exclusive criteria
The clinical data for this study were collected retrospectively from the medical records of 224 male patients who underwent physical examinations at the urology clinic from March 2019 to August 2022. Selected cases were required to have had persistent ED or suspected ED for more than 6 months with regular heterosexual relations (at least once per week) with a partner.
Exclusion criteria were advanced age (≥ 60 years), the presence of sleep disturbances, supportive medication prescriptions that may affect erectile function, pelvic trauma, spinal cord injuries, known history of atherosclerotic coronary heart disease, thyroid diseases, penile deformities, old or simultaneous neurologic diseases, hypogonadism, and other hormonal disorders. Patients who refused to respond to surveys and examinations were also excluded. A total of 212 patients who met the inclusion and exclusion criteria were included in the study. Prior to obtaining written permission, patients were provided with information about the purpose of the study, potential benefits, and potential hazards.
Baseline data collection
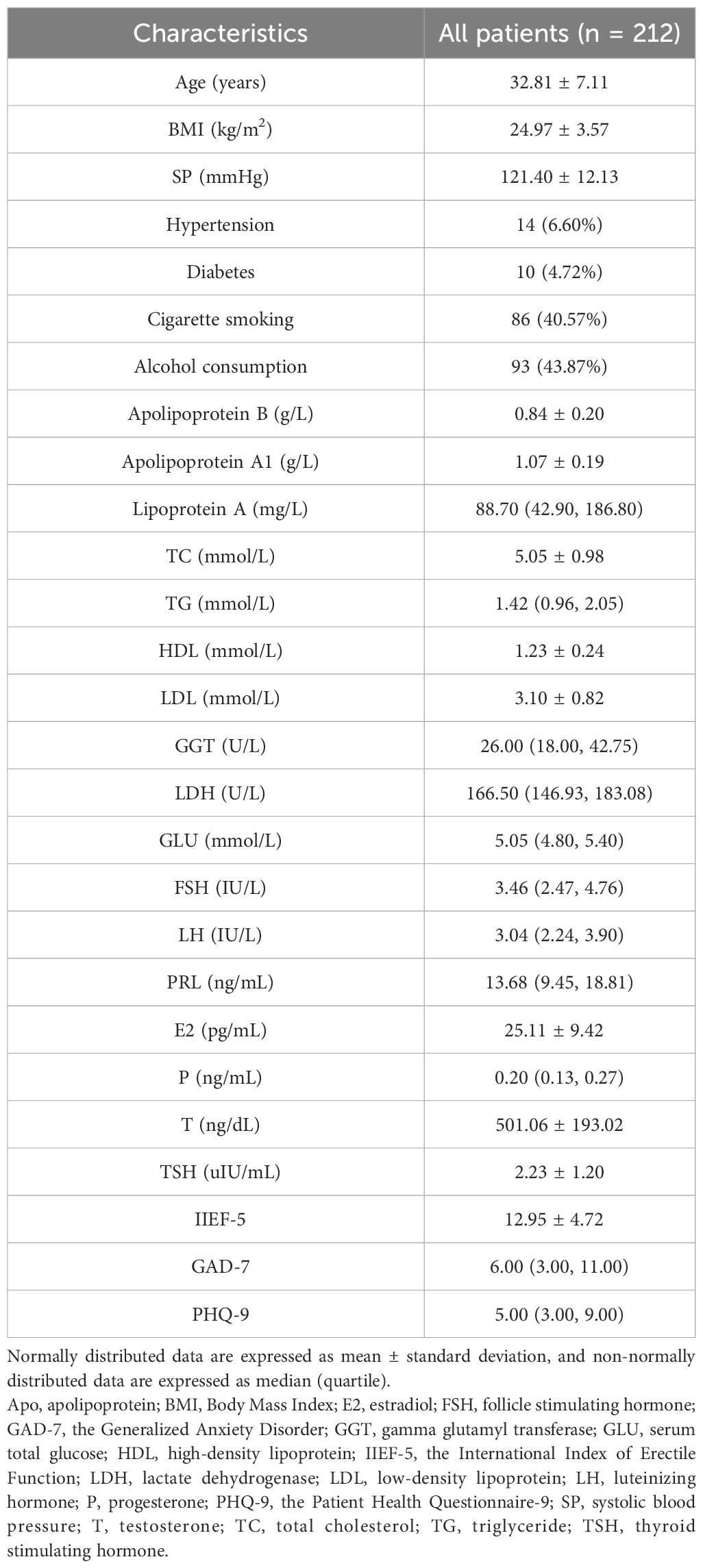
All participants were required to complete a detailed data collection form. The form included questions about their age, height, weight, marital status, fertility status, family history of diabetes, hypertension, and coronary artery disease. Additionally, participants were asked about their drinking and smoking habits. The form also included assessments such as the Generalized Anxiety Disorder (GAD-7) score, the Patient Health Questionnaire-9 (PHQ-9) score, and IIEF-5 score (30). The GAD-7 was used to evaluate symptoms of anxiety, while the PHQ-9 was used to determine symptoms of depression (31, 32). A comprehensive physical examination was conducted on all patients, encompassing measurements of weight, height, body mass index (BMI), blood pressure, diabetes, hypertension, and the status of coronary artery disease. Additionally, thyroid hormones, sex hormones, and lipid profiles were assessed, including serum total glucose (GLU), thyroid-stimulating hormone (TSH), estradiol (E), progesterone (P), luteinizing hormone (LH), prolactin (PRL), testosterone (T), follicle stimulating hormone (FSH), Apo B, Apo A1, lipoprotein A, LDL, TG, HDL, TC, gamma glutamyl transferase (GGT), and lactate dehydrogenase (LDH).
NPTR monitoring
The NPTR assessment was conducted on all participants using RigiScan monitoring. According to the study “Forensic identification of male sexual dysfunction” (GB/T37237-2018) conducted in China, effective penile erection criteria include RigiScan tests showing an average hardness of both the head and the root of the penis at maximum erection as greater than or equal to 60%, with a duration of more than or equal to 10 minutes (33). A multi-center study in Chinese men revealed that normal NPTR monitoring during 8 hours of sleep at night showed more than 2 effective erections of the penis, each lasting more than 10 minutes, with an erection hardness exceeding 60% of the normal erection (34). Based on the criteria adopted in this study, more than 2 effective erections lasting more than 10 minutes were required, with erection hardness values exceeding 60%, and satisfaction of both the tip and base of the penis at least once each. Prior to going to sleep, participants were instructed to ensure restful sleep by avoiding napping, alcohol and caffeine intake, and emptying the bladder and bowel. Based on the results of NPTR monitoring, patients were categorized into organic ED and psychogenic ED groups.
Statistical analysis
The R 4.3.4 software (R Foundation for Statistical Computing, Vienna, Austria) was utilized for conducting MR analyses in this study. At various points, packages such as ‘TwoSampleMR’, ‘MR-PRESSO’, ‘forestplot’, and ‘MendelianRandomization’ were employed. Statistical significance of causality was attributed to SVMR and MVMR analyses, with a p-value of less than 0.05.
In the case-control study, statistical analysis of all case data was performed using SPSS Statistics (version 26.0; SPSS for Windows). The one-sided Kolmogorov-Smirnov test was used to examine the presence of a normal distribution. The student’s t-test was employed for the purpose of comparing continuous variables with a normal distribution, which were presented as the average ± standard deviation (SD). The chi square test or Fisher’s exact chi-square test was used for categorical variables. For quantitative data that were not normally distributed, the median (quartile) was reported, and the non-parametric tests were used for comparison. Receiver operating characteristic (ROC) curve analysis was also performed to ensure the cut-off values for the relative variables. Additionally, binary logistic regression analyzes were carried out to determine the ORs and accompanying 95% CIs for potential effective variables, such as Apo B, Apo A1, and Apo A1/Apo B, in the prediction of organic vs psychogenic ED. A threshold of p <.05 (two-tailed) was used to determine statistical significance.
Results
Single variable MR
By performing a series of IVs selection steps, we identified the following IVs: 48 SNPs as Apo A1, 36 SNPs as Apo B, 52 SNPs as Apo B/A1, 277 SNPs as HDL, 146 SNPs as LDL, 67 SNPs as high cholesterol, and 224 SNPs as TG. These selection steps included criteria such as p<5×10-8, r2<0.001 and kb>10,000, non-palindromic, F-value > 10. Additionally, we excluded SNPs associated with fasting glucose, uric acid, and hypertension using the PhenoScannerV2 database (Figure 1). The p-value of the fixed effect IVW for all seven lipoproteins was greater than 0.05, indicating that there is no causal relationship between ED formation and the results of the MR-Egger regression. Furthermore, the Cochrane Q test, MR-PRESSO, and ‘leave-one-out’ sensitivity analyses demonstrated that the data was not significantly heterogeneous or pleiotropic (Supplementary Figures 1, 2). Given the robust interactions observed among the seven lipoproteins, additional MVMR analysis was conducted to evaluate their individual effects.
Multivariate MR
The outcomes of the MVMR analysis are presented in Figure 2. To account for the covariance of Apo A1 and Apo B with Apo B/A1, we divided them into two groups for the MVMR analysis. When considering confounders such as fasting glucose, uric acid, and hypertension, HDL, LDL, Apo A1, and Apo B/A1 were all found to have a causal association with the development of ED. The corresponding p-values were 0.012, 0.041, 0.004, and 0.003, respectively. The OR and 95% CI were 0.34 (0.15-0.79), 2.7 (1.04-6.99), 3.37 (1.47-7.72), and 0.31 (0.14-0.66). After excluding these confounders, HDL, Apo A1, and Apo B/A1 remained causally associated with the development of ED. The p-values were 0.011, 0.003, and 0.003, with corresponding OR and 95% CI of 0.33 (0.14-0.78), 3.58 (1.52-8.43), and 0.30 (0.13-0.66), respectively. Sensitivity analyses using MR-Egger regression, Cochrane Q test, and MR-PRESSO indicated no significant heterogeneity or pleiotropy in the data.
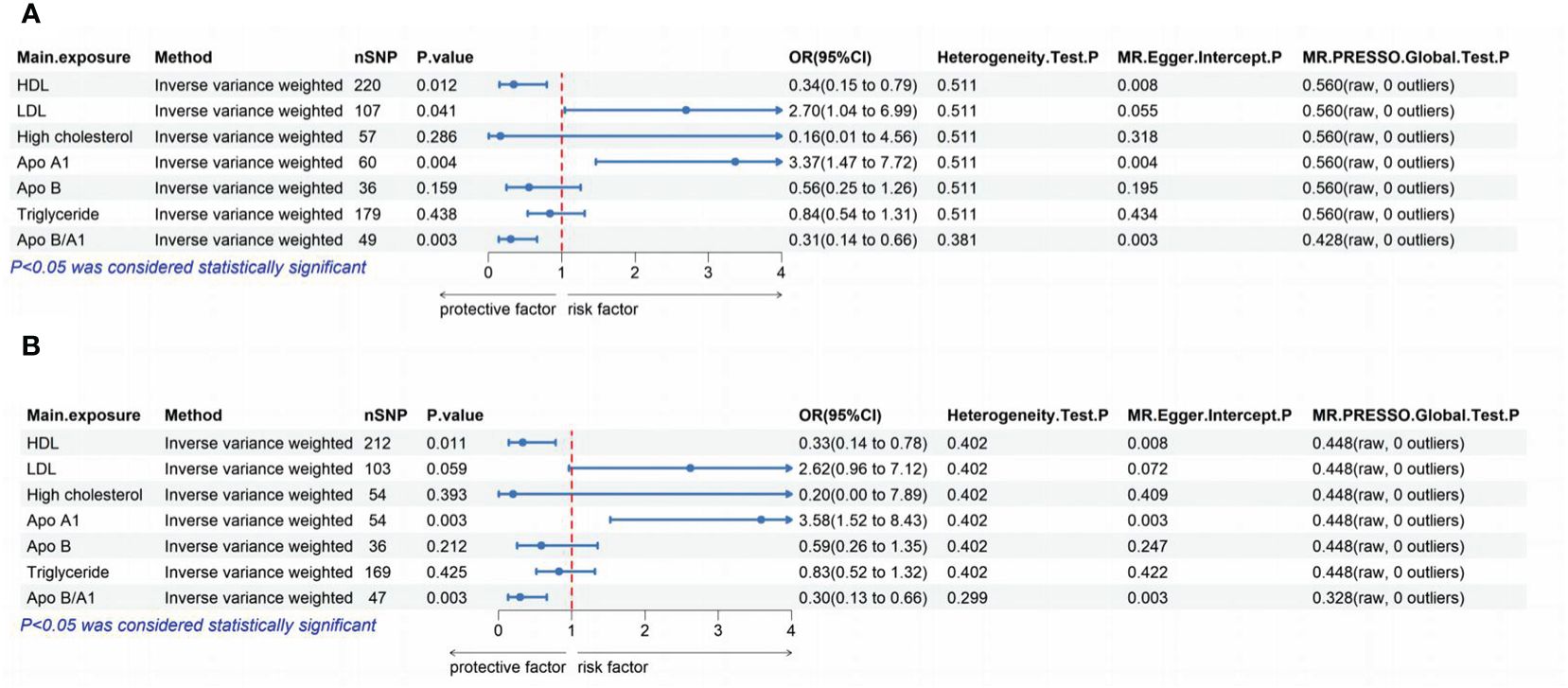
Figure 2 The lipoproteins were analyzed to determine their independent effects using MVMR. (A): The independent effects of each of the 7 lipoproteins were assessed without excluding confounders such as fasting glucose, uric acid, and hypertension. (B): The independent effects of each of the 7 lipoproteins were analyzed after excluding confounders such as fasting glucose, uric acid, and hypertension. MR, Mendelian Randomization; MVMR, multivariate MR; SNP, single nucleotide polymorphism; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Apo, apolipoprotein; OR, odds ratio; CI, confidence interval; MR‐PRESSO, MR Pleiotropy RESidualSum and Outlier.
Case-control study
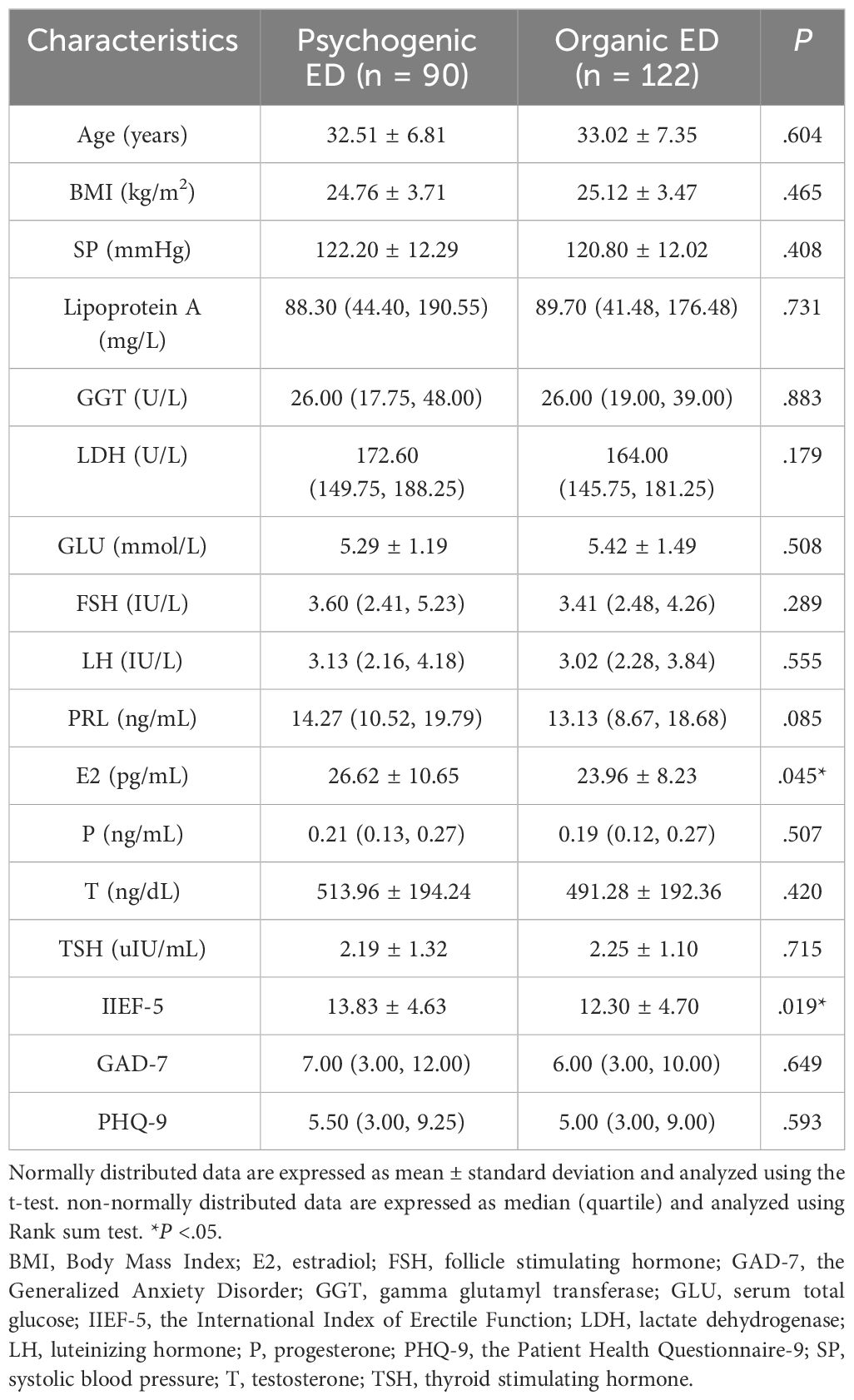
The research included a total of 212 patients. Table 2 presents the main social, biochemical, and clinical characteristics of all the patients. The indicators for all patients were analyzed for correlation, and the results are shown in Supplementary Figure 3. Table 3 provides information on the main social demographic, biochemical and clinical characteristics of patients in the normal NPTR (psychogenic ED) and abnormal NPTR (organic ED) outcome groups. Based on the NPTR results, 90 participants had psychogenic ED, while 122 had organic ED. Our investigation revealed no significant differences in age or SP between the two groups (32.51 ± 6.81 vs. 33.02 ± 7.35, 122.20 ± 12.29 vs. 120.80 ± 12.02, respectively). Compared to the organic ED group, the psychogenic ED group had significantly higher IIEF-5 scores (P = .019). Although not statistically significant, the psychogenic ED group had higher GAD-7 and PHQ-9 scores compared to the group of organic ED (P = .649 and P = .593). Furthermore, E2 levels were significantly decreased in the organic ED group (P = .045). Regarding the lipid profile, Figure 3 demonstrates that the average levels of Apo B and LDL were considerably higher in the organic ED group compared to the psychogenic ED group (P = .024 and P = .029). It is worth mentioning that there was a significant decrease in the levels of Apo A1, Apo A1/B, and HDL (P = .019, P <.001, and P = .027). However, there were no significant differences in TC or TG levels between the two groups (P = .265 and P = .548).
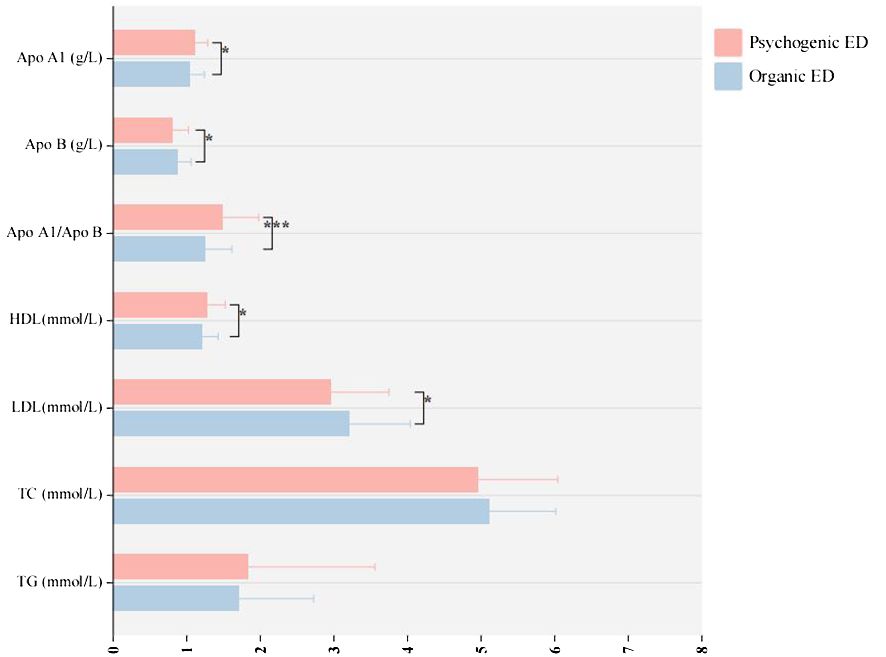
Figure 3 The study examines the variations in lipoprotein levels between organic and psychogenic ED. ED, erectile dysfunction; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; TC, total cholesterol; Apo, apolipoprotein. *p < 0.05; ***p < 0.005.
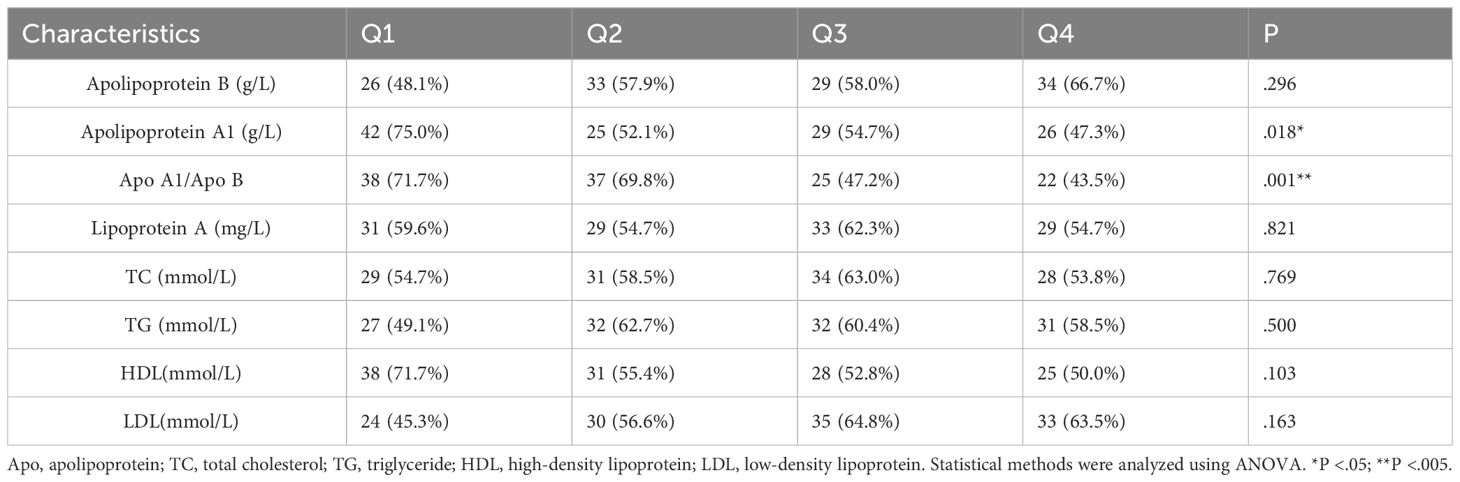
In our study, we divided the 8 lipid profile objectives into grades (Q1, Q2, Q3, and Q4) using four quartiles. We then analyzed the proportion of individuals with organic ED and psychogenic ED based on different lipid profile levels (Table 4). The results showed that patients with higher Apo A1 and Apo A1/Apo B ratios had a significantly lower proportion of organic ED compared to those with psychogenic ED (P = .018 and P = .001, respectively). Additionally, we performed binary logistic regression analysis (Table 5) to investigate the relationship between various lipid profiles and the prevalence of organic ED. Our findings revealed that the presence of organic ED was significantly influenced by Apo A1, Apo A1/B and HDL (P = .033, P = .001 and P=.007, respectively), while Apo B, lipoprotein A, TC, TG and LDL did not show significant associations (P = .091, P = .682, P = .695, P = .398 and P=.561, respectively) in our study.
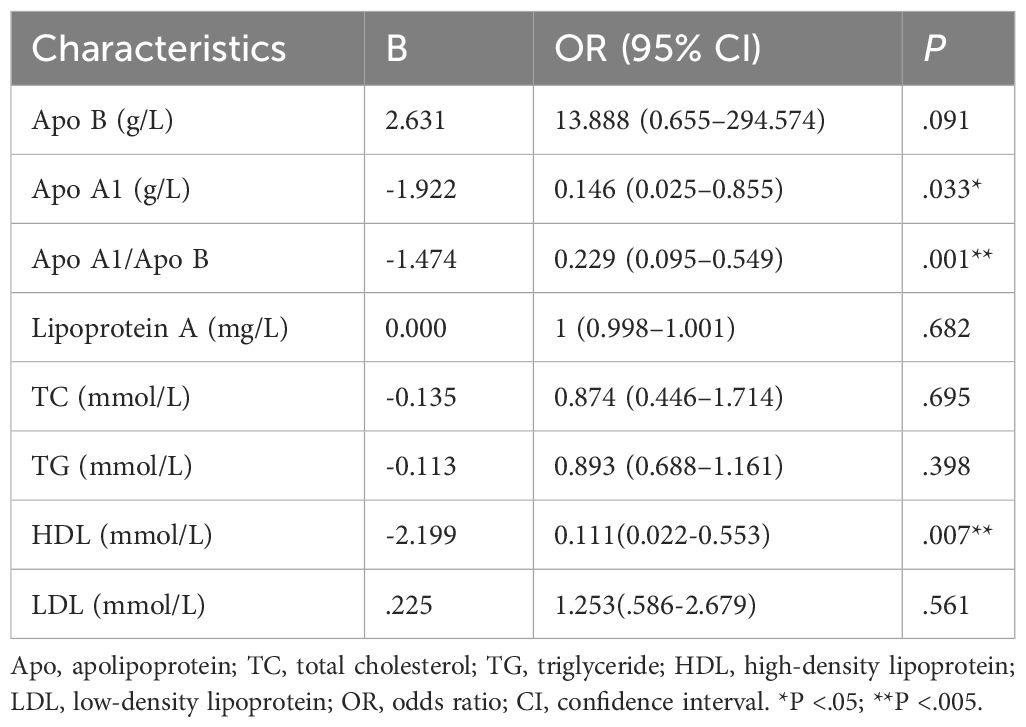
Table 5 Based on binary logistic regression analyses, correlations between lipid profiles and occurrence of abnormal NPTR results.
Apo A1 and Apo A1/B were analyzed using ROC curves to differentiate between organic and psychogenic ED (Figure 4). The diagnosis of organic ED was investigated based on the NPTR results. Out of the 8 markers, only two (Apo A1 and Apo A1/B) showed a moderate diagnostic value for organic ED, as indicated by the ROC curves (the area under the curve (AUC) = 0.61, 95% CI: 0.54–0.69, P = .005, cut-off < 0.95; AUC = 0.66, 95% CI: 0.58–0.73, P <.001, cut-off < 1.26, respectively).
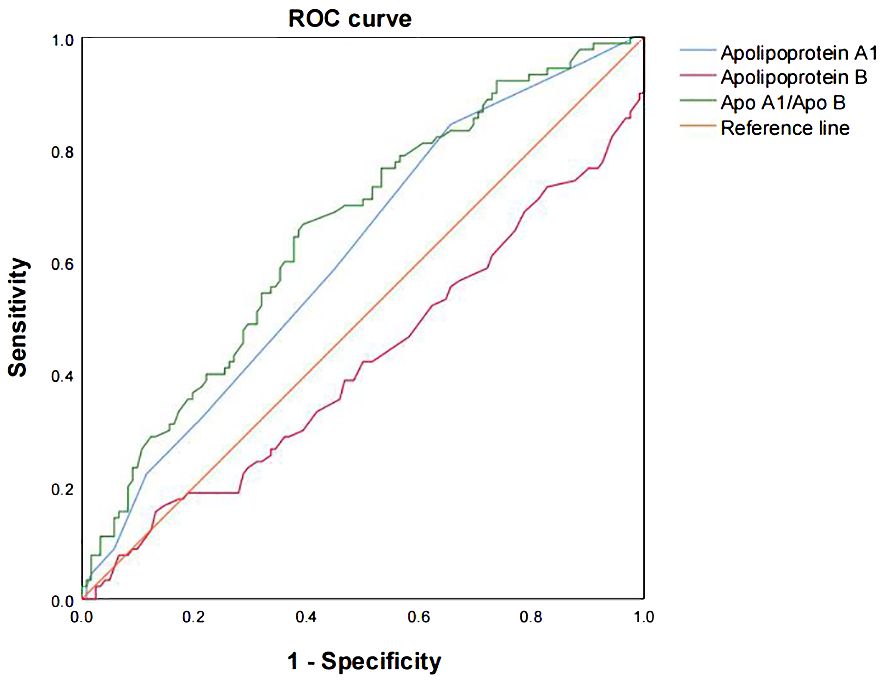
Figure 4 The ROC curves were analyzed to evaluate the prediction scores of serum Apo A1 and Apo A1/B in relation to the different NPTR scores. The ROC curve was used to diagnose organic ED based on the various NPTR results. The AUC values for Apo A1, Apo B, and Apo A1/B were 0.61 (95% CI: 0.54–0.69, P = .005, cut-off < 0.95), 0.42 (95% CI: 0.34–0.50, P = .051, cut-off < 1.09), and 0.66 (95% CI: 0.58–0.73, P <.001, cut-off < 1.26), respectively. Apo, apolipoprotein; AUC, the area under the curve; CI, confidence interval; ROC, receiver operating characteristic.
Discussion
ED is a multifaceted condition involving various factors like vascular, neurogenic, hormonal, psychogenic, vascular, and structural cave variables (35). Recent research has indicated that ED may be a potential precursor to CVD. Studies conducted in a clinical setting have demonstrated a powerful link between the emergence of ED and cardiovascular risk factors such as age, smoking, hypertension, obesity, diabetes, and dyslipidemia (36).
Our MR study did not find any significant causality in the SVMR of the seven lipoproteins, which aligns with previous MR studies (37). The probable explanation for this lack of causality is the intricate interplay among these lipoproteins (38), which cannot be disregarded solely based on SVMR. We further analyzed their respective independent effects using MVMR, and found that HDL, LDL, Apo A1, and Apo B/A1 were causally associated with the development of ED. However, after accounting for confounding factors such as fasting glucose, uric acid, and hypertension, the causal relationship between LDL and ED occurrence was no longer significant. This suggests that the influence of diabetes, hyperuricemia, and hypertension on ED occurrence may be mediated through their effects on LDL.
Our case-control study found no significant relationship between age and the prevalence of organic ED. This observation may be attributed to the fact that the patients attending Chinese andrology clinics were predominantly young. It is worth noting that the mean IIEF-5 score was lower in the organic ED group. Although the GAD-7 and PHQ-9 scores were higher in the psychogenic ED group compared to the organic ED group, this difference was not statistically significant. In contrast to a previous study, our findings indicate that NPTR monitoring is effective in distinguishing between organic and psychogenic ED (39).
Several risk factors are common to both ED and CVD (40, 41). It is important to recognize the connection between ED and CVD in order to effectively prevent and treat both conditions (42, 43). Additionally, ED significantly impacts patients’ quality of life. Therefore, it is crucial to examine the functions of Apo B, Apo A1, and Apo A1/Apo B in diagnosing organic ED, as these lipid profile markers have diagnostic significance in CVD (44). By doing so, medical professionals in various departments, even those unfamiliar with andrology, will be better equipped to treat and prevent CVD and ED in patients.
In the liver, Apo A1 is produced, an individual glycoprotein that makes up lipoprotein A, which is covalently linked to the lipoprotein Apo B-100 (45). The properties of Apo A1 can be influenced by various factors, including its size, sequence polymorphism, the type of lipoproteins it binds to, and the level of inflammation in the arterial wall. These factors suggest that Apo A1 may play a role in preventing atherosclerosis (46). On the other hand, Apo B, which is produced through cleavage and degradation of low density lipoproteins, contributes to the development of atherosclerosis (47). Apo B leads to vascular dysfunction, deposition of lipids and blood vessels in the walls of blood vessels, and the formation of foam cells (48). Considering this, it is possible that Apo A1’s ability to restore the artery wall could potentially prevent the development of organic ED in individuals.
A lower Apo A1/B ratio may indicate a higher risk of CVD (49). The ratio of HDL/LDL, represented by Apo A1/B, is inversely and favorably correlated with the probability of vascular damage (50). In our research, we found that Apo A1 and Apo A1/B were significantly lower in the organic ED compared to psychogenic ED. Apo A1 and Apo A1/B showed moderate diagnostic values for distinguishing between organic and psychogenic ED, according to the ROC curves. Among these results, Apo A1/B had the best diagnostic value for predicting organic ED. We also validated these findings using binary logistic regression analysis.
It is noteworthy that MVMR findings suggest link between HDL, Apo A1, and Apo B/A1 and the emergence of ED (OR and 95% CI 0.33 (0.14-0.78), 3.58 (1.52-8.43) and 0.30 (0.13-0.66), respectively). However, in the case-control study, binary logistic regression analyses indicate a potential association between HDL, Apo A1 and Apo A1/B and the occurrence of ED (OR and 95% CI of 0.11 (0.02-0.55), 0.15 (0.03-0.90) and 0.23 (0.10-0.55)). These findings support a causal relationship between HDL, Apo A1, and Apo A1/B and the development of ED. However, a paradox exists regarding the role of Apo A1 and Apo A1/B. This could be attributed to the diagnostic criteria used in MR, which do not clearly differentiate between organic and psychogenic ED. It is unlikely that there is a necessary connection between psychogenic ED and Apos. This highlights the need for a more definitive diagnosis of ED, considering its diagnostic aspects, to improve treatment and prognosis. Additionally, further research is required to understand the specific mechanism of action of Apo A1 in ED.
The mass spectra of other lipids, such as lipoprotein A, TC, and TG levels, did not provide any valuable information for diagnosing organic ED. Prior studies have demonstrated a link between elevated lipoprotein levels and a significantly increased risk of cardiovascular events in individuals with CVD (51, 52). However, despite higher levels of lipoprotein A in patients with organic ED, no significant correlation was observed. This could be attributed to the relatively young age of the participants and the exclusion of patients with CVD in our study.
Our research revealed that organic ED patients exhibited significantly lower levels of E2. The role of E2 in male sexual function remains unidentified, and studies investigating the impact of estrogens on erectile function have yielded inconsistent findings (53–55). Notably, our study findings differed significantly from previous research. This discrepancy may be attributed to the fact that the control group, comprising individuals with psychogenic ED, did not represent a healthy population, as evidenced by high levels of anxiety and depression indicated in the questionnaires. Consequently, these factors may have contributed to the reported increase in E2 levels (56).
It is important to note that this study has certain limitations. To begin with, MR analysis places a great emphasis on pleiotropy. The existence of pleiotropy may violate the basic MR assumptions and further distort the findings. We applied two methods MR-Egger and MR-PRESSO to detect the horizontal pleiotropy. Although sensitivity analyses did not reveal clear pleiotropy, we cannot completely exclude the possible pleiotropy of genetic variants. Additionally, the case-control study lacked a normal healthy population as a control group and had a limited sample size, with data collected from only one center. Despite having a larger sample size compared to similar studies, it is important to interpret the results with caution. Lastly, we did not conduct concurrent sleep monitoring to directly observe the sleep of the patients. However, we did provide patients with instructions on how to ensure a good night’s sleep prior to the study.
This research has certain advantages. At present, there is a scarcity of studies investigating the correlation between Apos and ED. This research, involving both European and Chinese participants, was created to supplement this area of study. Moreover, the MR design is advantageous as it permits the derivation of causal inference rather than a correlation. The MR analyses took into account a thorough IV selection and multiple sensitivity analyses, guaranteeing adherence to three fundamental assumptions in MR. Furthermore, in case-control studies, unlike previous studies that relied heavily on the subjective IIEF-5 score, our study utilized NPTR monitoring results to distinguish between organic and psychogenic ED. As a result, we confirmed that Apos are linked to organic ED, but not to psychogenic ED.
Conclusion
The MR study and case-control study have both validated the indispensable contribution of HDL, ApoA1, and ApoA1/B in the progression of ED. The differential diagnosis of ED between organic and psychogenic by NPTR testing further suggests that organic ED is characterized by reduced serum Apo A1 and Apo A1/B levels, as well as elevated Apo B levels. On the other hand, psychogenic ED does not exhibit these same associations. These findings suggest that Apo A1 and Apo A1/Apo B, which serve as serum indicators for organic ED risk, are valuable and play a critical role in the etiology of ED. Monitoring the levels of Apo A1 and Apo A1/B in the general population shows promise as a technique for identifying individuals with organic ED.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://r5.finngen.fi/, http://www.ukbiobank.ac.uk, https://gwas.mrcieu.ac.uk/. Clinical data are available from the corresponding authors upon reasonable request.
Ethics statement
The studies involving humans were approved by Tianjin Medical University General Hospital, Reg. No.ZYY-IRB-ZD-004(F)-002-02. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. All subjects in the original genome-wide association studies provided informed consent. The original studies provide access to ethical review and approval for GWAS datasets.
Author contributions
ZZ: Conceptualization, Data curation, Software, Writing – original draft, Writing – review & editing. MY: Conceptualization, Software, Writing – original draft. YL: Data curation, Software, Writing – original draft. YP: Formal analysis, Software, Writing – original draft. SW: Data curation, Methodology, Software, Writing – original draft. MX: Formal analysis, Visualization, Writing – original draft. HZ: Formal analysis, Visualization, Writing – original draft. XL: Supervision, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1359015/full#supplementary-material
Supplementary Figure 1 | Examining the causal association between lipoprotein traits and ED traits using SVMR. MR: Mendelian randomization; SNP: single nucleotide polymorphism; HDL: high-density lipoprotein; LDL: low-density lipoprotein; Apo: apolipoprotein; OR: odds ratio; CI: confidence interval; MR‐PRESSO: MR Pleiotropy RESidualSum and Outlier.
Supplementary Figure 2 | “Leave-one-out” sensitivity analysis was conducted for lipoproteins. The sensitivity analyses included: (A) Apo A1, (B) Apo B, (C) Apo B/A1, (D) HDL, (E) LDL, (F) high cholesterol, and (G) TG. MR: Mendelian randomization; HDL: high-density lipoprotein; LDL: low-density lipoprotein; Apo: apolipoprotein; TG: triglyceride.
Supplementary Figure 3 | Correlation analysis was conducted on collected indicators for all patients. Apo: apolipoprotein; BMI: Body Mass Index; E2: estradiol; FSH: follicle stimulating hormone; GAD-7: Generalized Anxiety Disorder; GGT: gamma glutamyl transferase; GLU: serum total glucose; HDL: high-density lipoprotein; IIEF-5: the International Index of Erectile Function; LDH: lactate dehydrogenase; LDL: low-density lipoprotein; LH: luteinizing hormone; P: progesterone; PHQ-9: the Patient Health Questionnaire-9; SP: systolic blood pressure; T: testosterone; TC: total cholesterol; TG: triglyceride. *P <.05; **P <.005; ***P <.001.
Abbreviations
Apo, apolipoprotein; AUC, the area under the curve; BMI, Body Mass Index; CI, confidence interval; CVD, cardiovascular diseases; E2, estradiol; ED, erectile dysfunction; FSH, follicle stimulating hormone; GAD-7, the Generalized Anxiety Disorder; GGT, gamma glutamyl transferase; GLU,serum total glucose; GWAS, the genome-wide association study; HDL, high-density lipoprotein; IIEF-5, the International Index of Erectile Function; IV, instrumental variable; IVW, the inverse variance weighted; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; LH, luteinizing hormone; MAF, the minimum allele frequency for each SNP; MR, Mendelian randomization; MR‐PRESSO, MR Pleiotropy RESidualSum and Outlier; MVMR, multivariable MR; N, the sample size; NPTR, the nocturnal penile tumescence and rigidity; OR, odds ratio; P, progesterone; PHQ-9, the Patient Health Questionnaire-9; PRL, prolactin; ROC, receiver operating characteristic; SD, standard deviation; SE, the standard error; SNP,single nucleotide polymorphism; SP, systolic blood pressure; SVMR, single variable MR; T, testosterone; TC, total cholesterol; TG, triglyceride; TSH, thyroid stimulating hormone; β, the effect size.
References
1. Najari BB, Kashanian JA. Erectile dysfunction. JAMA. (2016) 316:1838. doi: 10.1001/jama.2016.12284
2. Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. (2000) 163:460–3. doi: 10.1016/S0022-5347(05)67900-1
3. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. (1999) 84:50–6. doi: 10.1046/j.1464-410x.1999.00142.x
4. Terentes-Printzios D, Ioakeimidis N, Rokkas K, Vlachopoulos C. Interactions between erectile dysfunction, cardiovascular disease and cardiovascular drugs. Nat Rev Cardiol. (2022) 19:59–74. doi: 10.1038/s41569-021-00593-6
5. Rezanezhad B, Borgquist R, Willenheimer R, Elzanaty S. Association between serum levels of testosterone and biomarkers of subclinical atherosclerosis. Aging Male. (2018) 21:182–6. doi: 10.1080/13685538.2017.1412422
6. Yu XH, Zhao J, Zhang SC, Li JH, Zheng JB, Li Z, et al. The impact of age, BMI and sex hormone on aging males' symptoms and the international index of erectile function scores. Aging Male. (2017) 20(4):235–40. doi: 10.1080/13685538.2017.1361399
7. Chaudhary RK, Shamsi BH, Chen HM, Tan T, Tang KF, Xing JP. Risk factors for erectile dysfunction in patients with cardiovascular disease. J Int Med Res. (2016) 44:718–27. doi: 10.1177/0300060515621637
8. Lima LM, Carvalho MDG, Sousa MO. Apo B/apo A-I ratio and cardiovascular risk prediction. Arq Bras Cardiol. (2007) 88:e187–90. doi: 10.1590/s0066-782x2007000600014
9. Li X, Li D. The suggestive effect of Apo A, Apo B, and Apo A/Apo B on erectile dysfunction. J Sex Med. (2021) 18:448–56. doi: 10.1016/j.jsxm.2020.12.004
10. Katz J, Yue S, Xue W, Gao H. Increased odds ratio for erectile dysfunction in COVID-19 patients. J Endocrinol Invest. (2022) 45:859–64. doi: 10.1007/s40618-021-01717-y
11. Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. (2002) 14:226–44. doi: 10.1038/sj.ijir.3900857
12. Hackett G, Kell P, Ralph D, Dean J, Price D, Speakman M, et al. British Society for Sexual Medicine guidelines on the management of erectile dysfunction. J Sex Med. (2008) 5(8):1841–65. doi: 10.1111/j.1743-6109.2008.00773.x
13. Bradley WE, Timm GW, Gallagher JM, Johnson BK. New method for continuous measurement of nocturnal penile tumescence and rigidity. Urology. (1985) 26:4–9. doi: 10.1016/0090-4295(85)90243-2
14. Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. (2010) 57(5):804–14. doi: 10.1016/j.eururo.2010.02.020
15. Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. (2019) 48:713–27. doi: 10.1093/ije/dyy262
16. Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. (2015) 181:251–60. doi: 10.1093/aje/kwu283
17. Sanderson E. Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med. (2021) 11:a038984. doi: 10.1101/cshperspect.a038984
18. Richardson TG, Leyden GM, Wang Q, Bell JA, Elsworth B, Davey Smith G, et al. Characterising metabolomic signatures of lipid-modifying therapies through drug target mendelian randomisation. PloS Biol. (2022) 20(2):e3001547. doi: 10.1371/journal.pbio.3001547
19. Mbatchou J, Barnard L, Backman J, Marcketta A, Kosmicki JA, Ziyatdinov A, et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat Genet. (2021) 53(7):1097–103. doi: 10.1038/s41588-021-00870-7
20. Dönertaş HM, Fabian DK, Valenzuela MF, Partridge L, Thornton JM. Common genetic associations between age-related diseases. Nat Aging. (2021) 1(4):400–12. doi: 10.1038/s43587-021-00051-5
21. Richardson TG, Sanderson E, Palmer TM, Ala-Korpela M, Ference BA, Davey Smith G, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PloS Med. (2020) 17(3):e1003062. doi: 10.1371/journal.pmed.1003062
22. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35(22):4851–3. doi: 10.1093/bioinformatics/btz469
23. Yilmaz M, Karaaslan M, Tonyali S, Celik M, Toprak T, Odabas O. Triglyceride-Glucose Index (TyG) is associated with erectile dysfunction: A cross-sectional study. Andrology. (2021) 9:238–44. doi: 10.1111/andr.12904
24. Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. (2020) 16:380–90. doi: 10.1038/s41584-020-0441-1
25. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. (2013) 381:153–65. doi: 10.1016/S0140-6736(12)60520-0
26. Bull CJ, Bonilla C, Holly JM, Perks CM, Davies N, Haycock P, et al. Blood lipids and prostate cancer: a Mendelian randomization analysis. Cancer Med. (2016) 5(6):1125–36. doi: 10.1002/cam4.695
27. Burgess S, Thompson SG, CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036
28. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
29. Zhu G, Zhou S, Xu Y, Gao R, Li H, Su W, et al. Mendelian randomization study on the causal effects of COVID-19 on childhood intelligence. J Med Virol. (2022) 94(7):3233–9. doi: 10.1002/jmv.27736
30. Andrade WS, Oliveira P, Laydner H, Ferreira EJ, Barreto-Filho JA. Severity of erectile dysfunction is highly correlated with the syntax score in patients undergoing coronariography. Int Braz J Urol. (2016) 42:123–31. doi: 10.1590/S1677-5538.IBJU.2015.0002
31. Levis B, Benedetti A, Thombs BD, DEPRESsion Screening Data (DEPRESSD) Collaboration. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. (2019) 365:l1476. doi: 10.1136/bmj.l1476
32. Hinz A, Klein AM, Brähler E, Glaesmer H, Luck T, Riedel-Heller SG, et al. Psychometric evaluation of the Generalized Anxiety Disorder Screener GAD-7, based on a large German general population sample. J Affect Disord. (2017) 210:338–44. doi: 10.1016/j.jad.2016.12.012
33. State Administration of Market Administration CNSA. Forensic medical assessment for male sexual dysfunction: GB/T37237-2018. Beijing: China Standard Publishing House (2019).
34. Zhang Y. Chinese expert consensus on the clinical application of RigiScan in the diagnosis and treatment of erectile dysfunction. Chin J Hum Sexuality. (2019) 12:5–10.
35. Chew KK, Bremner A, Stuckey B, Earle C, Jamrozik K. Is the relationship between cigarette smoking and male erectile dysfunction independent of cardiovascular disease? Findings from a population-based cross-sectional study. J Sex Med. (2009) 6:222–31. doi: 10.1111/j.1743-6109.2008.00971.x
36. Barrett-Connor E. Heart disease risk factors predict erectile dysfunction 25 years later (the Rancho Bernardo Study). Am J Cardiol. (2005) 96:3M–7M. doi: 10.1016/j.amjcard.2005.07.012
37. Xiong Y, Zhang F, Zhang Y, Wang W, Ran Y, Wu C, et al. Insights into modifiable risk factors of erectile dysfunction, a wide-angled Mendelian Randomization study. J Adv Res. (2023) 58:149–61. doi: 10.1016/j.jare.2023.05.008
38. Formanowicz D, Radom M, Rybarczyk A, Tanaś K, Formanowicz P. Control of cholesterol metabolism using a systems approach. Biol (Basel). (2022) 11:430. doi: 10.3390/biology11030430
39. Marshall P, Morales A, Surridge D. Diagnostic significance of penile erections during sleep. Urology. (1982) 20:1–6. doi: 10.1016/0090-4295(82)90526-X
40. Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, Montorsi P, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. (2014) 65(5):968–78. doi: 10.1016/j.eururo.2013.08.023
41. Vlachopoulos C, Jackson G, Stefanadis C, Montorsi P. Erectile dysfunction in the cardiovascular patient. Eur Heart J. (2013) 34:2034–46. doi: 10.1093/eurheartj/eht112
42. Li DJ, Liao ZC, Zhang XB, Tang YX, Zu XB, Wang L, et al. Perception of the association between erectile dysfunction and cardiovascular disease among Chinese physicians: an online survey. J Int Med Res. (2020) 48(3):300060519894187. doi: 10.1177/0300060519894187
43. Li D, Li X, Peng E, Liao Z, Tang Z. Do urologists really recognize the association between erectile dysfunction and cardiovascular disease? Sex Med. (2020) 8:195–204. doi: 10.1016/j.esxm.2019.12.002
44. Katzke VA, Sookthai D, Johnson T, Kühn T, Kaaks R. Blood lipids and lipoproteins in relation to incidence and mortality risks for CVD and cancer in the prospective EPIC-Heidelberg cohort. BMC Med. (2017) 15:218. doi: 10.1186/s12916-017-0976-4
45. Scanu AM, Fless GM. Lipoprotein (a). Heterogeneity and biological relevance. J Clin Invest. (1990) 85:1709–15. doi: 10.1172/JCI114625
46. Milasan A, Jean G, Dallaire F, Tardif JC, Merhi Y, Sorci-Thomas M, et al. Apolipoprotein A-I modulates atherosclerosis through lymphatic vessel-dependent mechanisms in mice. J Am Heart Assoc. (2017) 6(9):e006892. doi: 10.1161/JAHA.117.006892
47. Hammer A, Kager G, Dohr G, Rabl H, Ghassempur I, Jürgens G. Generation, characterization, and histochemical application of monoclonal antibodies selectively recognizing oxidatively modified apoB-containing serum lipoproteins. Arterioscler Thromb Vasc Biol. (1995) 15:704–13. doi: 10.1161/01.atv.15.5.704
48. Lei X, Basu D, Li Z, Zhang M, Rudic RD, Jiang XC, et al. Hepatic overexpression of the prodomain of furin lessens progression of atherosclerosis and reduces vascular remodeling in response to injury. Atherosclerosis. (2014) 236(1):121–30. doi: 10.1016/j.atherosclerosis.2014.06.015
49. Lahoud R, Chongthammakun V, Wu Y, Hawwa N, Brennan DM, Cho L. Comparing SF-36® scores versus biomarkers to predict mortality in primary cardiac prevention patients. Eur J Intern Med. (2017) 46:47–55. doi: 10.1016/j.ejim.2017.05.026
50. Sabino AP, De Oliveira Sousa M, Moreira Lima L, Dias Ribeiro D, Sant'Ana Dusse LM, Das Graças Carvalho M, et al. ApoB/ApoA-I ratio in young patients with ischemic cerebral stroke or peripheral arterial disease. Transl Res. (2008) 152(3):113–8. doi: 10.1016/j.trsl.2008.06.005
51. Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. (2009) 302(4):412–23. doi: 10.1001/jama.2009.1063
52. Patel AP, Wang M, Pirruccello JP, Ellinor PT, Ng K, Kathiresan S, et al. Lp(a) (Lipoprotein[a]) concentrations and incident atherosclerotic cardiovascular disease: new insights from a large national biobank. Arterioscler Thromb Vasc Biol. (2021) 41(1):465–74. doi: 10.1161/ATVBAHA.120.315291
53. Wu X, Liu G, Zhang Y, Zhang W, Huang H, Jiang H, et al. High estradiol level is associated with erectile dysfunction: A systematic review and meta-analysis. Andrologia. (2022) 54(7):e14432. doi: 10.1111/and.14432
54. Chen HR, Tian RH, Li P, Chen HX, Xia SJ, Li Z. Estradiol is an independent risk factor for organic erectile dysfunction in eugonadal young men. Asian J Androl. (2020) 22:636–41. doi: 10.4103/aja.aja_135_19
55. Castelló-Porcar AM, Martínez-Jabaloyas JM. Testosterone/estradiol ratio, is it useful in the diagnosis of erectile dysfunction and low sexual desire? Aging Male. (2016) 19:254–8. doi: 10.1080/13685538.2016.1253672
Keywords: apolipoprotein, Mendelian randomization, diagnosis, erectile dysfunction, the nocturnal penile tumescence and rigidity test
Citation: Zhang Z, Yan M, Li Y, Pan Y, Wang S, Xu M, Zhou H and Liu X (2024) The indicative effects of apolipoproteins on organic erectile dysfunction: bridging Mendelian randomization and case-control study. Front. Endocrinol. 15:1359015. doi: 10.3389/fendo.2024.1359015
Received: 20 December 2023; Accepted: 24 April 2024;
Published: 13 June 2024.
Edited by:
Claus Yding Andersen, University of Copenhagen, DenmarkReviewed by:
Xiansheng Zhang, First Affiliated Hospital of Anhui Medical University, ChinaYuxuan Song, Peking University People’s Hospital, China
Copyright © 2024 Zhang, Yan, Li, Pan, Wang, Xu, Zhou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqiang Liu, eGlhb3FpYW5nbGl1MUAxNjMuY29t
†These authors have contributed equally to this work
‡ORCID: Xiaoqiang Liu, orcid.org/orcid.org/0000-0003-3524-6783
 Zhexin Zhang1†
Zhexin Zhang1† Mo Yan
Mo Yan Yuezheng Li
Yuezheng Li Yang Pan
Yang Pan Shangren Wang
Shangren Wang Hang Zhou
Hang Zhou Xiaoqiang Liu
Xiaoqiang Liu