- 1Unità di Medicina della Riproduzione, Centro HERA, Catania, Italy
- 2Urology Section, Department of Surgery, University of Catania, Catania, Italy
- 3Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
Background: In 2020, 38% of adults were affected by obesity, while infertility globally affected 1 in 6 people at some stage of their lives.
Body mass index (BMI) provides an easy but occasionally inaccurate estimation of body composition. To achieve a more precise assessment, bioelectric impedance analysis serves as a validated tool that administers electrical energy through surface electrodes. Phase angle as a function of the relationship between tissues resistance and reactance, is a trustworthy predictor of body composition and cell membrane integrity.
Objectives: We aim to assess whether there is an association between phase angle and seminal parameters, as well as sperm DNA fragmentation percentage.
Design: Semen samples of 520 idiopathic infertile patients were analyzed according to 2021 World Health Organization guidelines and evaluated for sperm DNA fragmentation rate. Each participants underwent bioelectric impedance analysis.
Results: Median age was 40 years old, median BMI was 26.3 kg/m2, median phase angle was 6.2°. In the logistic regression analysis adjusted for age and total intracorporeal water, phase angle (continuous) was significantly associated with oligozoospermia (odds ratio [OR]:0.4; p<0.01) and sperm morphology (OR: 0.65; p=0.05) and slightly with sperm DNA fragmentation (OR: 0.98; p=0.07). In subgroup analysis, the logistic regression analysis adjusted for the mentioned parameters showed that a phase angle between 6.2 and 7 (°) (OR: 0.63; p=0.02) and >7 (°) (OR: 0.12; p<0.01) were associated with a reduced risk of oligozoospermia compared to values <6.2 (°). Similarly, a phase angle between 6.2 and 7 (°) (OR: 0.57; p< 0.01 and OR: 0.58; p= 0.01) and PA > 7 (°) (OR: 0.12; p= 0.03 and OR: 0.21; p< 0.01) were associated with a reduced risk of lower sperm concentration and lower total sperm count, respectively, compared to a phase angle < 6.2 (°).
Conclusion: Our study suggests a negative association between phase angle and detrimental sperm parameters in male idiopathic infertility.
1 Introduction
In 2020, obesity was a global condition affecting 38% of adults aged 18 years and older (1). The expected global prevalence is projected to increase from 2.6 billion in 2020 to 4 billion by 2035 (1). Furthermore, predictions indicate that the prevalence of overweight in male and female adolescents will rise from 9% in 2020 to 19% in 2035, underscoring the importance of the issue of overweight (1). Similarly, infertility, defined as the inability to achieve pregnancy after 12 months or more of regular unprotected sexual intercourse, has been acknowledged as a significantly widespread global condition, affecting 1 in 6 people at some point in their lives (2). According to the data, up to 50% of infertility in couples can be attributed to male factors (3). Obesity has been demonstrated to be a relevant risk factor for male infertility through various mechanisms including hypogonadotropic-hyperestrogenic hypogonadism, increased testicular inflammation events, resulting from augmented adipokines, raised testicular temperature, sexual dysfunctions and epigenetic induced alteration (4). Altered environment and the subsequent increase in reactive oxygen species (ROS) lead to elevated DNA damage through direct or indirect interaction with the DNA strand (5, 6). For this reason, several studies extensively tested sperm DNA fragmentation (SDF) as a marker of male infertility such as Chavarro and Kort found a statistically significant correlation between augmented and SDF percentage (7) (8),. Their findings were subsequently confirmed by Fariello et al., who observed a higher percentage of damaged DNA in obese men compared to normal-weight and overweight patients (9). Conversely, a recent meta-analysis published in 2020, involving a total cohort of 8255 patients, concluded that the data were insufficient to demonstrate a positive association between overweight and SDF values (10).
A study involving a large group of participants found that male partner obesity increases the risk of infertility for the couple (11, 12). Moreover, when both partners are obese, the risk of infertility is further elevated (13). Despite documented evidence, the relationship between obesity and seminal parameters remains a subject of ongoing debate.
Several studies reported a negative association between overweight and quality of conventional seminal parameters. Belloc et al. found that overweight negatively affects sperm count, sperm concentration, total sperm volume and sperm motility (14). Conversely, according to Aggerholm et al., obesity only affects semen volume but has no effect on other seminal parameters (15–17). To address this endless debate, Guo conducted a meta-analysis involving 26,814 participants and observed that obese patients had no alteration in sperm motility but experienced statistically significant decreases in total sperm count, sperm concentration, and semen volume (18). Since sedentary lifestyle and consequent augmented BMI seems to contribute to male infertility, it is easy to suggest that physical activity has a potentially significant impact on seminal parameters. Indeed, case-control studies have indicated that individuals in the physically active group exhibit enhanced semen parameters, including semen volume, viability, progressive motility, total motility, and morphology, when compared to the sedentary group (19). However, it has been widely reported that, while physical activity is generally associated with improved seminal parameters, excessively intense and prolonged training may have a negative impact on male fertility. Overtraining and intense physical stress may result in hormonal imbalances, elevated testicular temperature, and subsequent oxidative stress, potentially diminishing the quality of semen parameters (20).
Hakonsen reported that obesity-related oligozoospermia can be improved with weight loss, along with enhancements in reproductive hormonal profile and SDF percentage (21, 22) while Andersen observed that increased sperm concentration and total sperm count persist if weight loss is maintained (23).
While BMI is a convenient and easily calculable metric, its indirect estimation of body composition may be prone to inaccuracies. For instance, it can overestimate fat percentage in persons with higher lean muscle mass, such as athletes, and underestimate adiposity in individuals with lower muscular mass (24). To achieve a more accurate evaluation of body composition, various alternative technologies have been developed, with one of the most promising being bioelectric impedance analysis (BIA).
BIA is a validated alternative tool that administers electrical energy through surface electrodes and records tissue responses by measuring parameters such as resistance and reactance (25).The working principle underlying BIA is Ohm’s law, which establishes that the voltage across a conductor is directly related to the resistance to current flow (26). When an electrical current is introduced to biological tissue, its components facilitate the passage of the charge. The predominant charge carriers include mobile ions in water, while the dipolar components, categorized into positive and negative charges, consist of proteins and the lipidic cell membrane. Substantially, the measurement of those electric features gives information about tissues property and composition This permits the prediction of total body water (TBW), fat mass (FM), lean body mass (LBM), and the percentage of body fat (%BF), providing an accurate estimation of adiposity (27, 28). Phase angle (PA) is a function of the relationship between resistance and reactance representing a measure of extra- and intracellular water content; since electricity flows more easily through hydrated tissue, such as muscle, PA seems the most trustworthy predictor of body composition (25, 29). Phase angle in the context of bioimpedance refers to the phase shift between the voltage and current in an electrical circuit that passes through biological tissues. BIA is a method used to measure the impedance of biological tissues to alternating electrical currents. This impedance includes both resistive (real) and capacitive/reactive (imaginary) components. Moreover, PA represents a reliable marker of membrane integrity and cell mass (30). Essentially, BIA relies on models that predict TBW as a linear function of the resistance index, considering factors such as weight, age, and gender (31). However, literature data are missing regarding the potential association between PA and sperm parameters in patients with infertility. For these reasons, our study aims to assess the existence of an association between PA and seminal parameters, including total sperm count, sperm concentration, total motility, morphology, and SDF percentage in patients with idiopathic male infertility.
2 Materials and methods
A total of 520 consecutive male patients, seeking assistance at the Unit of Reproductive Medicine in the clinic ‘Centro HERA’ for primary couple infertility, participated in this prospective study (from January 2023 to June 2023. Our study included patients aged 18 years or older who were affected by idiopathic infertility. We collected information on the patients’ age and conducted a physical examination, documenting measurements of height, weight, and BMI. Each patient performed sperm analysis, evaluation of SDF and BIA before starting any other treatment. Patients with varicocele, male accessory gland infection, genetic alterations, and hormonal diseases were excluded from the study. The current study protocol obtained approval from the Institutional Review Board at Centro HERA - UMR (Approval No. 1/2023). All subjects provided informed consent upon enrollment in the study.
2.1 Sperm analysis
Semen samples were collected through masturbation into a sterile container following 2–7 days of sexual abstinence. Analysis was conducted immediately after liquefaction. Each sample was assessed for seminal volume, sperm count, progressive motility, and morphology, in accordance with the 2021 WHO guidelines (3).
2.2 Sperm DNA fragmentation
Sperm samples underwent terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin nick end-labelling (TUNEL) staining using a commercially available kit (Dead End Fluorimetric TUNEL System; Promega, USA) following the manufacturer’s instructions. Briefly, sperm were fixed in 4% paraformaldehyde at 4°C and permeabilized with 0.2% Triton X-100 (Promega) in PBS (Nutricell). After permeabilization, the samples were incubated in 100 ml drops with a reagent mix containing terminal deoxynucleotide transferase enzyme solution and 90% staining solution (dUTP fluorescein conjugate) for 1 h at 37°C in a dark humid chamber. Subsequently, the sperm were stained with Vectashield (Vector Laboratories Inc., Burlingame, CA, USA), plus 4’,6-diamidino-2-phenylindole (DAPI) and mounted on slides for evaluation using fluorescence microscopy (Olympus BX51). The TUNEL assay results were reported as the percentage of sperm DNA fragmentation, indicating the proportion of cells with DNA damage (32).
2.3 Bioelectric impedance analysis
Participant body composition was assessed during a single-day visit (<1 hour). Individuals were assessed on a direct segmental octopolar multi-frequency device (InBody, Model 770, Cerritos, California, USA), standing with feet apart and elbows extended to avoid body contact for approximately 1 min. The bare feet made positive contact with the base electrodes at the heels and forefeet and subjects grasped two handle electrodes for direct contact with two more electrodes for each hand at thumbs and forefingers. The segmental analysis was computed with proprietary algorithms. Data obtained from the InBody 720 device were processed using the Lookin Body 3.0 program. By data analysis, biometric information for each patient were collected, including:
- Fat mass (FM).
- Lean mass (LM).
- Muscular mass (MM).
- Percentage of body mass (%BF).
- Waist to hip ratio (WHR).
- Abdominal circumference (AR).
- PA (33).
Subjects reported to the laboratory for a single testing session after a minimum of 08 hours of fasting from food, caloric beverages, caffeine, alcohol, and tobacco. Additionally, subjects refrained from strenuous exercise for a minimum of twelve hours before testing. Height (cm) and weight (Kg) were measured upon arrival at the laboratory using a calibrated scale. For all measurements, subjects were instructed to be free from metal (e.g., zippers, jewelry, hard plastic) to avoid interference with data collection accuracy. Multi-frequency bioelectrical impedance analysis (MF-BIA) using the InBody 770 device (Biospace Co.) estimated total body composition, including fat percentage, FM and LM. Subjects stood barefoot on the device’s scale for 5 minutes, with the soles of their feet positioned on four corresponding electrodes and holding the handles in both hands to contact corresponding electrodes on the thumbs and palms. Height, sex, and age were entered into the MF-BIA software, and the device collected weight. Subjects remained still for the duration of the assessment.
2.4 Statistical analysis
All statistical analyses were conducted using Stata (Stata Statistical Software: College Station, TX: Stata Corp LP). For all statistical comparisons, results were considered significant when p < 0.05. Normally distributed continuous variables were presented as median (interquartile range, IQR), and differences between groups were tested by Student’s independent t-test or Mann–Whitney U-test, depending on their normal or non-normal distribution (normality of variables’ distribution was tested by Kolmogorov–Smirnov test).
Age-adjusted linear regression models were performed to verify factors correlated with abnormal sperm parameters, expressed as beta-coefficient. The beta-coefficient represents the magnitude of the variation in the independent variable for each increase in the dependent value.
Multivariable logistic regression models were constructed to identify predictive factors of:
- Oligozoospermia, defined as < 39 million or < 15 million/ml of spermatozoa.
- Asthenospermia, defined as motility lower than 32%.
- Teratozoospermia, defined as normal morphology lower than 4%.
- Oligoasthenoteratozoospermia (OAT), defined as the coexistence of these abnormalities.
PA (reference value from 1 to 10) has been categorized into three sub-groups according to the resulting tertiles:
1) PA <6.2;
2) PA between 6.2 and 7;
3) PA >7.
A cut off of 20% has been considered for SDF according to Agarwal (34). Area under the curve (AUC) has been performed to verify accuracy of phase angle in diagnosing OAT.
3 Results
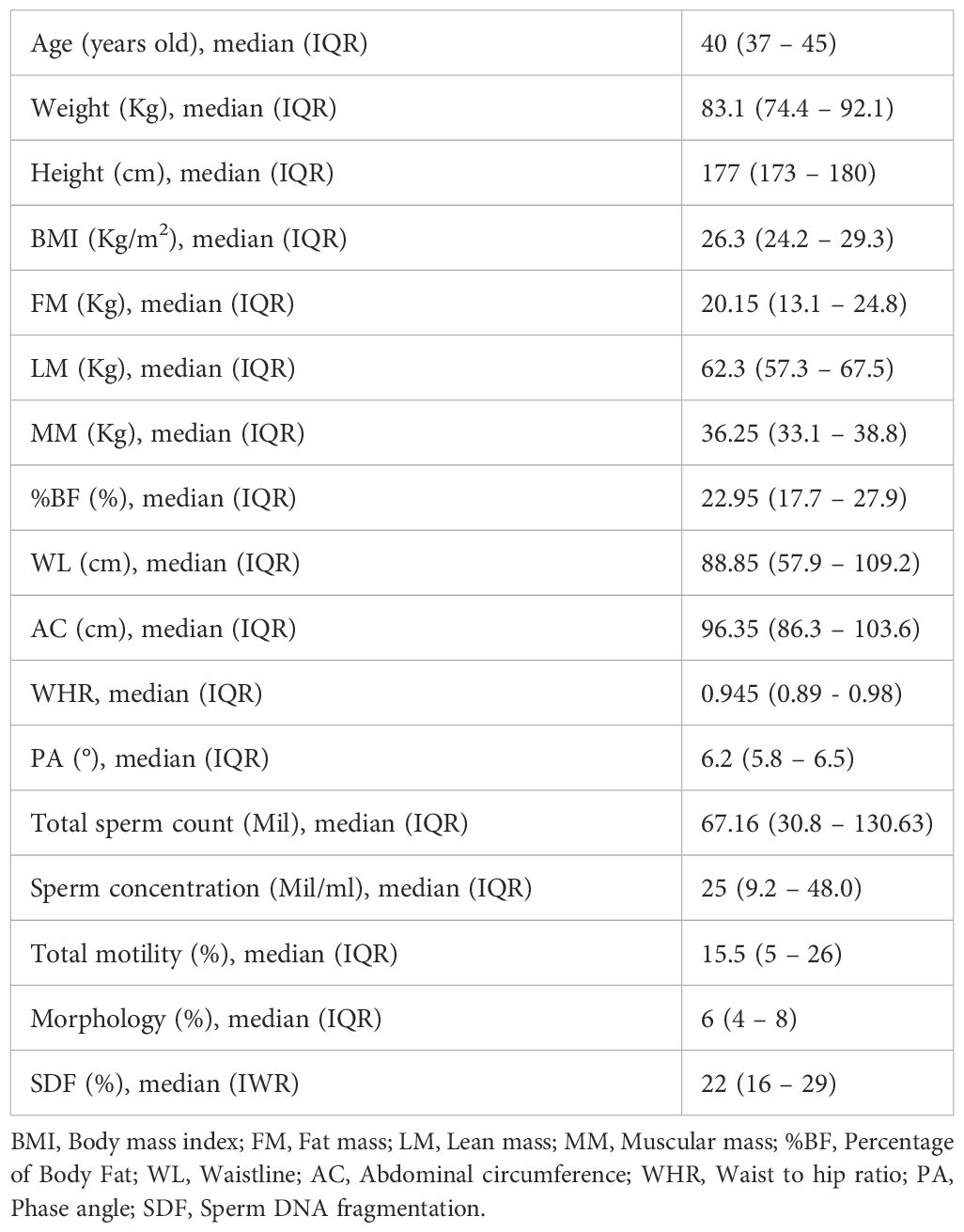
The median age was 40 years old (interquartile range [IQR]: 37.0-45.0), and the median BMI was 26.3 kg/m2 (IQR: 24.2 – 29.3). Additionally, patients’ biometric parameters were collected: median FM was 20.15 kg (IQR: 13.1 – 24.8), median percentage of fat mass was 22.95% (IQR: 17.7-27.9), median LM was 62.3 kg (IQR: 57.3 – 67.5), median MM was 36.25 kg (IQR: 33.1 – 38.8), median AC was 96.35 cm (IQR: 86.3 – 103.6), median WHR was 0.945 (IQR: 0.89- 0.98), and median PA was 6.2°(IQR: 5.8 – 6.5). Baseline characteristics of the entire cohort are listed in Table 1.
Classification of the analyzed cohort according to BMI in presented in Supplementary Table 1.
All semen analyses were conducted on sperm samples obtained after a median day of ejaculatory abstinence of 4 days (IQR: 3- 4). The semen analysis reported a median SDF of 22.0% (IQR: 16.0-29.0), median sperm concentration of 25.0 million/ml (IQR: 9.2-48.0), median total sperm count of 67.16 million (IQR: 30.8-130.63), median progressive motility of 15.5% (IQR: 5.0-26.0), and median morphology of 6.0% (IQR: 4.0-8.0). A total of 116 patients (22.3%) suffered from OAT. The prevalence of the remaining semen alterations among the cohort is listed in Supplementary Table 2.
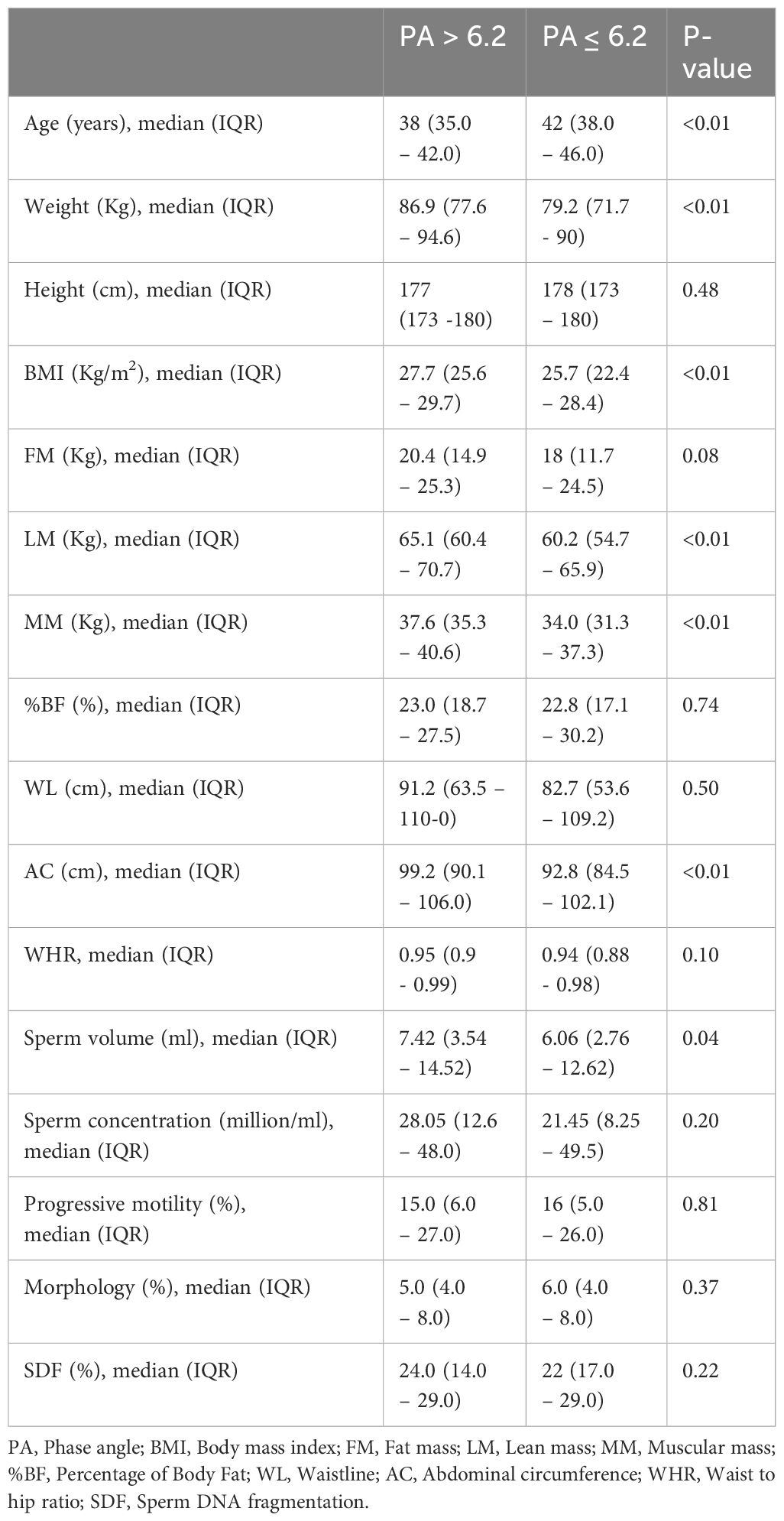
Patients with PA ≤ 6.2 had higher median age (42 vs 40; p<0.01), lower median BMI (25.7 vs 26.3; p< 0.01), lower median lean mass (60.2 vs 62.3; p<0.01), lower median muscular mass (34.0 vs 36.2; p<0.01), lower median abdominal circumference (92.8 vs 96.35; p<0.01). Other biometric data did not show significant differences between the two groups. Moreover, patients with PA ≤ 6.2 had a significantly lower total sperm count (60.6 vs 67.16; p<0.05). The other sperm parameters were not significantly influenced by PA variation (Table 2).
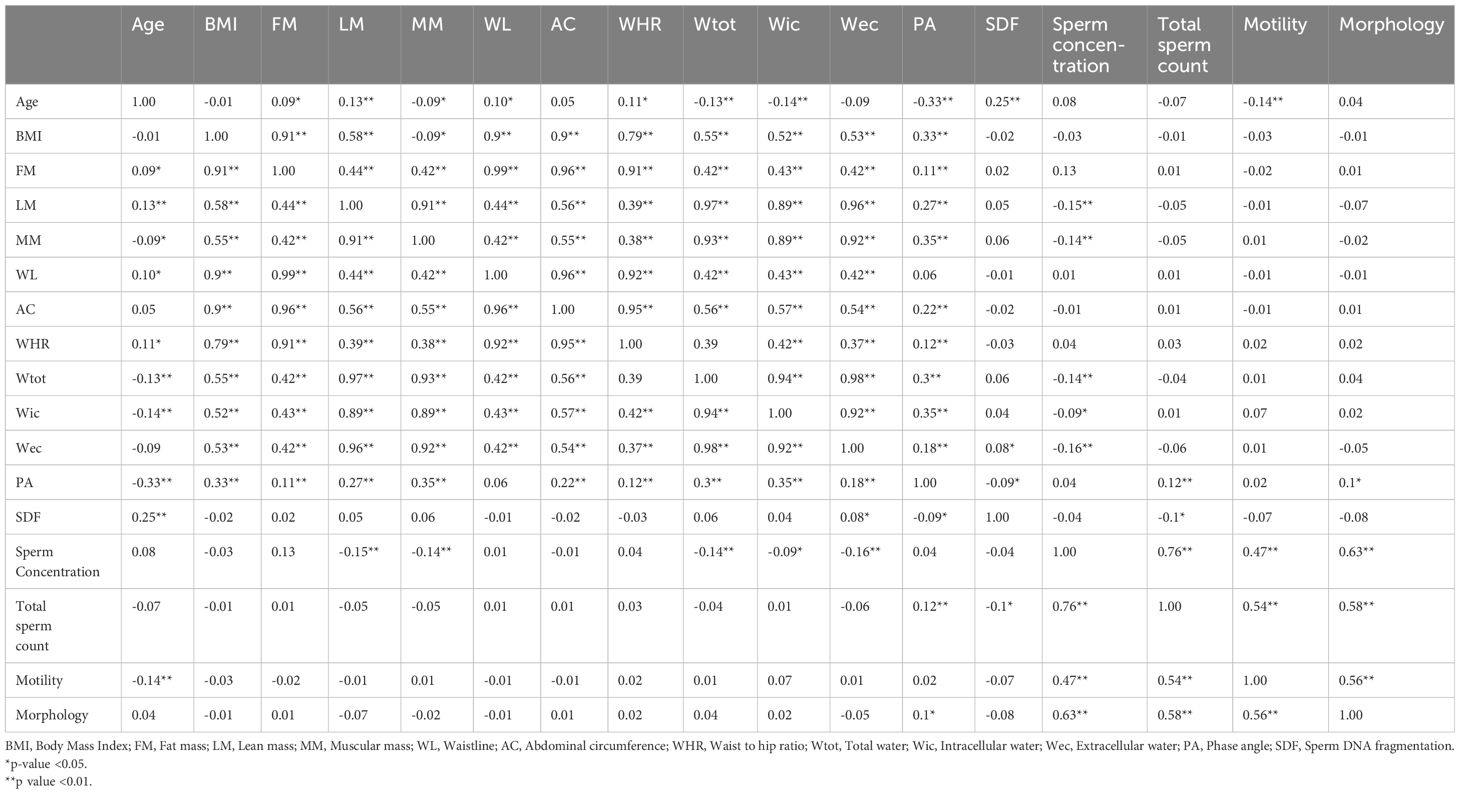
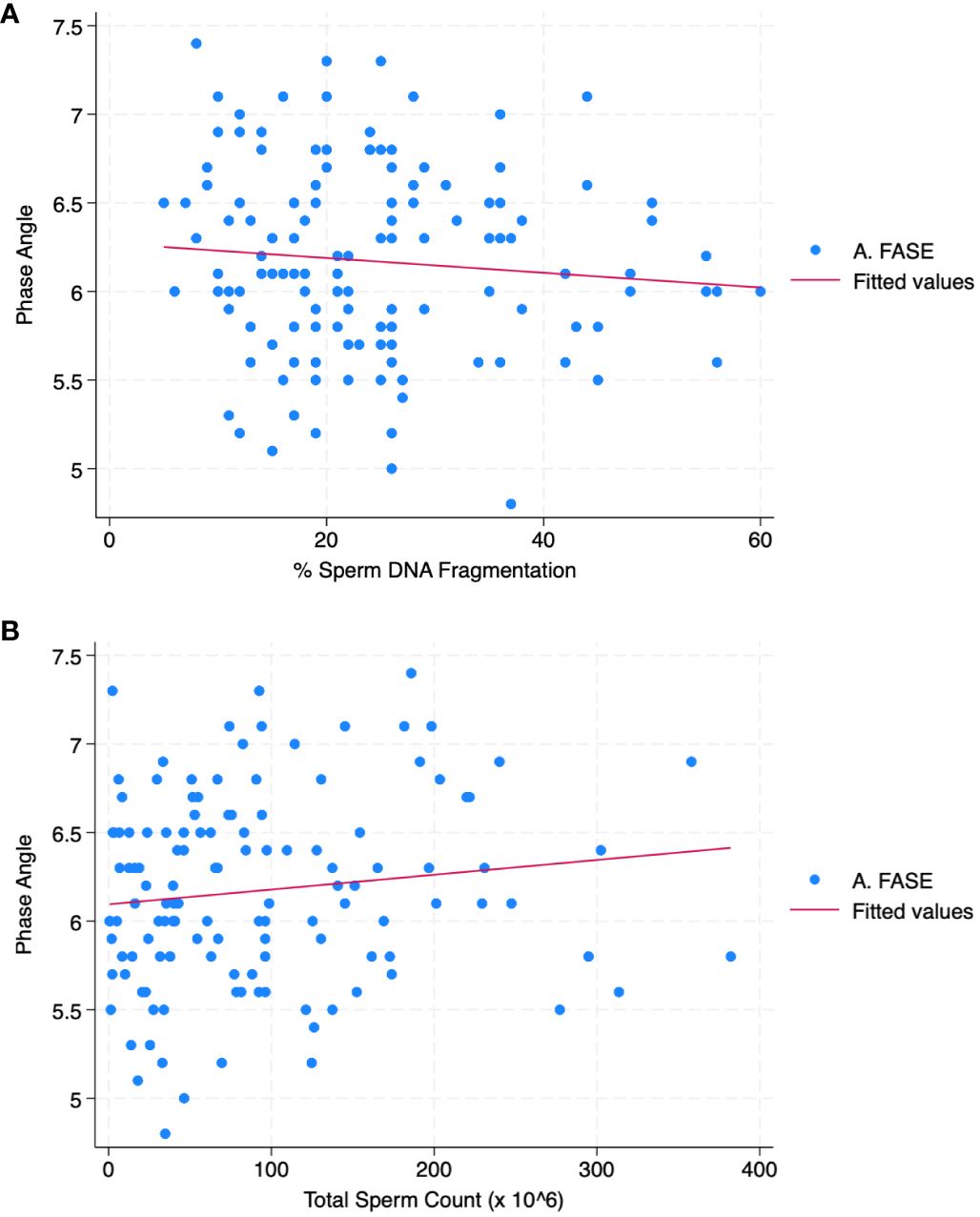
Table 3 reports the correlation analysis between all parameters. PA was correlated with SDF (r = - 0.09; p< 0.05) and total sperm count (r = 0.12; p< 0.01). Figures 1A, B shows the scatter plot of the association between phase angle and SDF (Figure 1A) and TSC (Figure 1B).
The age-adjusted linear regression analysis demonstrated that PA was positively associated with sperm count (r=0.01; p=0.01) and morphology (r=0.02; p<0.01) but not with SDF (p=0.81), sperm concentration (p=0.06) and total motility (p=0.58).
In the logistic regression analysis adjusted for age and total intracorporeal water, PA (continuous) (OR [odds ratio]: 0.4; 95%CI 0.27-0.59; p<0.01) was significantly associated with oligozoospermia but not with the SDF (OR: 0.98; p=0.07) and with sperm morphology (OR: 0.65; p=0.05).
Logistic regression analysis of PA sub-groups, adjusted for age, total intracorporeal water, and SDF showed that a PA between 6.2 and 7 (°)(OR: 0.63; 95%CI 0.42-0.94; p=0.02) and PA >7 (°)(OR: 0.12; 95%CI 0.04-0.37; p<0.01) were associated with reduced risk of oligozoospermia compared to PA <6.2 (°). Similarly, PA between 6.2 and 7 (°)(OR: 0.57; 95% CI 0.37- 0.86; p< 0.01 and OR:0.58 95% CI 0.38- 0.88; p= 0.01) and PA > 7 (°)(OR: 0.12; 95% CI 0.0.4- 0.36; p= 0.03 and OR: 0.21; 95% CI 0.07- 0.63; p< 0.01) were associated with reduced risk of lower sperm concentration and of lower total sperm count respectively, compared to PA < 6.2 (°).
PA sub-groups logistic regression analysis adjusted for age and total intracorporeal water did not show any relation with sperm morphology (p=0.98 and p=0.09).
The AUC for phase in angle in diagnosing OAT was 0.61.
4 Discussion
Herein our data suggests that patients with lower PA (°) (≤ 6.2) had detrimental sperm parameters in particular lower sperm concentration and total sperm count. Our study is the first investigating the potential association between PA and low sperm quality in patients with idiopathic male infertility.
Although the exact mechanism by which PA influences sperm parameters remains unclear, several hypotheses have been proposed. First, PA is employed to assess nutritional status and body composition based on the electrical properties of different tissues (35). Indeed, since electricity flows more easily through hydrated tissue, such as muscle, it is foreseeable that suitable PA values are predictors of better body composition (29). On the other hand, some studies claim that BIA has a limited accuracy in predicting body composition (36). BIA appears to be significantly influenced by environmental factors, ethnicity, and medical conditions. Therefore, the development of an appropriate calibration equation is necessary for different groups of participants (37).
The relationship between phase angle from BIA and sperm DNA fragmentation has not been extensively studied or validated. While BIA has been investigated in various clinical contexts, it’s utility in predicting sperm DNA fragmentation remains largely unexplored. Sperm quality and DNA integrity are influenced by multiple factors beyond cellular health, including oxidative stress, exposure to toxins, lifestyle factors, and genetic factors. While BIA may provide some insights into overall health status, it may not capture all the determinants of sperm quality and DNA fragmentation.
However, since PA is a measure of extra and intracellular water content, it serves as a direct indicator of cell membrane integrity; in fact, the smaller the PA, the weaker the cell structure, and the higher the probability of cell death (38). Various studies have reported an association between PA and biochemical markers involved in monitoring chronic diseases as well as cancer prognosis (39, 40). Given that chronic diseases promote inflammation and oxidative stress, which may be responsible for cell damage, PA can serve as an early predictor of inflammation (41). Among chronic disorders, obesity is reported to be a noteworthy promoter of inflammatory status, characterized by an increase in tumor necrosis factor-α (TNF-α), interleukin 6 (IL6), and interleukin 10 (IL10) production (42). Building upon this previous statement, patients with a higher BMI are more likely to exhibit cell membrane damage, contributing to cell fluid imbalance and lower PA values (43).
Based on these premises and considering that chronic oxidative stress is known to affect sperm quality by damaging sperm DNA, PA appears to be a promising predictor of semen quality (44).
To establish phase angle as a strong predictor of male fertility, a multifaceted approach is necessary. Firstly, extensive research is paramount. Investigating the relationship between phase angle and male fertility demands thorough exploration. This entails collecting data from diverse populations to ensure the reliability and universality of findings. Collaboration with experts across various disciplines such as nutrition, physiology, endocrinology, and reproductive medicine is essential. Their insights can illuminate the underlying mechanisms linking phase angle with male fertility, enriching our understanding. Developing diagnostic tools or algorithms that integrate phase angle measurements with other pertinent biomarkers is pivotal. These tools should accurately assess male fertility status, enhancing diagnostic precision. Validation and standardization efforts are indispensable. Validating phase angle’s predictive capacity across diverse populations and settings ensures its reliability. Standardizing measurement protocols and interpretation criteria promotes consistency and reproducibility of results. By pursuing these comprehensive steps, phase angle can emerge as a robust predictor of male fertility, facilitating more accurate diagnosis and management of male infertility issues.
Limitations of the study are important to be defined. Infertility often results from a combination of factors, including hormonal imbalances affecting ovulation or sperm production, structural issues impacting the reproductive organs, and systemic health conditions affecting fertility. Phase angle may not capture the multifaceted aspects contributing to infertility. The relationship between phase angle and infertility lacks extensive study and validation. While phase angle has been explored in various clinical contexts such as nutritional status, muscle health, and disease prognosis, its utility in predicting infertility remains largely unexplored. BIA measurements, including phase angle, can be influenced by external factors like hydration status, body temperature, skin integrity, and electrode placement. Fluctuations in these factors can affect the accuracy and reliability of BIA measurements, potentially complicating the interpretation of phase angle in predicting infertility. Individuals exhibit significant variability in phase angle values based on factors such as age, sex, body composition, and overall health status. Additionally, reference ranges for phase angle may vary across different populations and measurement techniques, making it challenging to establish universal thresholds for predicting infertility based on phase angle alone.
In summary, our data suggest an association between PA and semen parameters; however, further research is needed to fully understand the underlying mechanisms. Furthermore, a limitation of our study was the lack of data regarding the lifestyle habits and comorbidities of our patients, which may be of great relevance when evaluating fertility potential and alterations in semen parameters.
5 Conclusion
In conclusion, further research is needed to fully understand the association between semen parameters and PA. Nevertheless, this preliminary study suggests that the phase angle assessed at BIA may be associated with poor sperm quality in males affected by idiopathic infertility. Our preliminary data may support further studies that can reveal the impact of phase angle with other aspects of couple infertility in the context of assisted reproductive technology. Furthermore, these results highlight the detrimental relationship between abnormal body composition and sperm quality. Clinicians may consider these results when developing strategies to increase the phase angle and improve semen quality.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional review board of Centro HERA - UMR “Unità di Medicina della Riproduzione”. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. AL: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing – review & editing. FG: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing – review & editing. DL: Investigation, Validation, Visualization, Writing – review & editing. MA: Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. AA: Writing – review & editing, Formal analysis. SCi: Investigation, Validation, Visualization, Writing – review & editing. AG: Investigation, Validation, Visualization, Writing – review & editing. SCh: Investigation, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Authors extend their sincere appreciation to Researchers Supporting Project number (RSPD2024R750), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1354733/full#supplementary-material
References
1. Lobstein T, Jackson-Leach R, Powis J, Brinsden H, Gray M, World Obesity Federation. World Obesity Atlas 2023. (2023).
2. World Health Organization. Infertility prevalence estimates, 1990–2021 Vol. 2023. Geneva: World Health Organization (2023).
3. Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European association of urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. (2021) 80:603–20. doi: 10.1016/j.eururo.2021.08.014
4. Barbagallo F, Condorelli RA, Mongioì LM, Cannarella R, Cimino L, Magagnini MC, et al. Molecular mechanisms underlying the relationship between obesity and male infertility. Metabolites. (2021) 11:840. doi: 10.3390/metabo11120840
5. Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril. (1997) 68:519–24. doi: 10.1016/S0015-0282(97)00236-7
6. Lo Giudice A, Asmundo MG, Cimino S, Cocci A, Falcone A, Capece M, et al. Effects of long and short ejaculatory abstinence on sperm parameters: a meta-analysis of randomized-controlled-trials. Front Endocrinol (Lausanne). (2024) 15. doi: 10.5534/wjmh.230106
7. Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. (2010) 93:2222–31. doi: 10.1016/j.fertnstert.2009.01.100
8. Kort HI. Impact of body mass index values on sperm quantity and quality. J Androl. (2006) 27:450–2. doi: 10.2164/jandrol.05124
9. Fariello RM, Pariz JR, Spaine DM, Cedenho AP, Bertolla RP, Fraietta R. Association between obesity and alteration of sperm DNA integrity and mitochondrial activity. BJU Int. (2012) 110:863–7. doi: 10.1111/j.1464-410X.2011.10813.x
10. Sepidarkish M, Maleki-Hajiagha A, Maroufizadeh S, Rezaeinejad M, Almasi-Hashiani A, Razavi M. The effect of body mass index on sperm DNA fragmentation: a systematic review and meta-analysis. Int J Obes. (2020) 44:549–58. doi: 10.1038/s41366-020-0524-8
11. Craig JR, Jenkins TG, Carrell DT, Hotaling JM. Obesity, male infertility, and the sperm epigenome. Fertil Steril. (2017) 107:848–59. doi: 10.1016/j.fertnstert.2017.02.115
12. Kahn BE, Brannigan RE. Obesity and male infertility. Curr Opin Urol. (2017) 27:441–5. doi: 10.1097/MOU.0000000000000417
13. Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sørensen TIA, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. (2007) 22:1634–7. doi: 10.1093/humrep/dem035
14. Belloc S, Cohen-Bacrie M, Amar E, Izard V, Benkhalifa M, Dalléac A, et al. High body mass index has a deleterious effect on semen parameters except morphology: results from a large cohort study. Fertil Steril. (2014) 102:1268–73. doi: 10.1016/j.fertnstert.2014.07.1212
15. Aggerholm AS, Thulstrup AM, Toft G, Ramlau-Hansen CH, Bonde JP. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril. (2008) 90:619–26. doi: 10.1016/j.fertnstert.2007.07.1292
16. Duits FH, van Wely M, van der Veen F, Gianotten J. Healthy overweight male partners of subfertile couples should not worry about their semen quality. Fertil Steril. (2010) 94:1356–9. doi: 10.1016/j.fertnstert.2009.05.075
17. Shayeb AG, Harrild K, Mathers E, Bhattacharya S. An exploration of the association between male body mass index and semen quality. Reprod BioMed Online. (2011) 23:717–23. doi: 10.1016/j.rbmo.2011.07.018
18. Guo D, Wu W, Tang Q, Qiao S, Chen Y, Chen M, et al. The impact of BMI on sperm parameters and the metabolite changes of seminal plasma concomitantly. Oncotarget. (2017) 8:48619–34. doi: 10.18632/oncotarget.14950
19. Lalinde-Acevedo PC, Mayorga-Torres BJM, Agarwal A, du Plessis SS, Ahmad G, Cadavid ÁP, et al. Physically active men show better semen parameters than their sedentary counterparts. Int J Fertil Steril. (2017) 11:156–65. doi: 10.22074/ijfs.2017.4881
20. Ibañez-Perez J, Santos-Zorrozua B, Lopez-Lopez E, Matorras R, Garcia-Orad A. An update on the implication of physical activity on semen quality: a systematic review and meta-analysis. Arch Gynecol Obstet. (2019) 299:901–21. doi: 10.1007/s00404-019-05045-8
21. Håkonsen LB, Thulstrup AM, Aggerholm AS, Olsen J, Bonde JP, Andersen CY, et al. Does weight loss improve semen quality and reproductive hormones? results from a cohort of severely obese men. Reprod Health. (2011) 8:24. doi: 10.1186/1742-4755-8-24
22. Wood GJA, Tiseo BC, Paluello DV, de Martin H, Santo MA, Nahas W, et al. Bariatric surgery impact on reproductive hormones, semen analysis, and sperm DNA fragmentation in men with severe obesity: prospective study. Obes Surg. (2020) 30:4840–51. doi: 10.1007/s11695-020-04851-3
23. Andersen E, Juhl CR, Kjøller ET, Lundgren JR, Janus C, Dehestani Y, et al. Sperm count is increased by diet-induced weight loss and maintained by exercise or GLP-1 analogue treatment: a randomized controlled trial. Hum Reprod. (2022) 37:1414–22. doi: 10.1093/humrep/deac096
24. Tafeit E, Cvirn G, Lamprecht M, Hohensinn M, Moeller R, Hamlin M, et al. Using body mass index ignores the intensive training of elite special force personnel. Exp Biol Med. (2019) 244:873–9. doi: 10.1177/1535370219848986
25. Tinsley GM, Harty PS, Moore ML, Grgic J, Silva AM, Sardinha LB. Changes in total and segmental bioelectrical resistance are correlated with whole-body and segmental changes in lean soft tissue following a resistance training intervention. J Int Soc Sports Nutr. (2019) 16:25. doi: 10.1186/s12970-019-0325-4
26. Sánchez-Iglesias A, Fernández-Lucas M, Teruel JL. The electrical basis of bioimpedance. Nefrologia. (2012) 32:133–5. doi: 10.3265/Nefrologia.pre2012.Jan.11310
27. Lukaski H, Johnson P, Bolonchuk W, Lykken G. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. (1985) 41:810–7. doi: 10.1093/ajcn/41.4.810
28. Zhu S, Wang Z, Shen W, Heymsfield SB, Heshka S. Percentage body fat ranges associated with metabolic syndrome risk: results based on the third National Health and Nutrition Examination Survey (1988–1994). Am J Clin Nutr. (2003) 78:228–35. doi: 10.1093/ajcn/78.2.228
29. Campa F, Toselli S, Mazzilli M, Gobbo LA, Coratella G. Assessment of body composition in athletes: A narrative review of available methods with special reference to quantitative and qualitative bioimpedance analysis. Nutrients. (2021) 13:1620. doi: 10.3390/nu13051620
30. Ward LC, Brantlov S. Bioimpedance basics and phase angle fundamentals. Rev Endocr Metab Disord. (2023) 24:381–91. doi: 10.1007/s11154-022-09780-3
31. Jaffrin MY, Morel H. Body fluid volumes measurements by impedance: A review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys. (2008) 30:1257–69. doi: 10.1016/j.medengphy.2008.06.009
32. Cohen-Bacrie P, Belloc S, Ménézo YJR, Clement P, Hamidi J, Benkhalifa M. Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril. (2009) 91:1801–5. doi: 10.1016/j.fertnstert.2008.01.086
33. Potter AW, Nindl LJ, Soto LD, Pazmino A, Looney DP, Tharion WJ, et al. High precision but systematic offset in a standing bioelectrical impedance analysis (BIA) compared with dual-energy X-ray absorptiometry (DXA). BMJ Nutr Prev Health. (2022) 5:254–62. doi: 10.1136/bmjnph-2022-000512
34. Agarwal A, Farkouh A, Parekh N, Zini A, Arafa M, Kandil H, et al. Sperm DNA fragmentation: A critical assessment of clinical practice guidelines. World J Mens Health. (2022) 40:30–7. doi: 10.5534/wjmh.210056
35. Zhou S, Yu Z, Shi X, Zhao H, Dai M, Chen W. The relationship between phase angle, nutrition status, and complications in patients with pancreatic head cancer. Int J Environ Res Public Health. (2022) 19:6426. doi: 10.3390/ijerph19116426
36. Achamrah N, Colange G, Delay J, Rimbert A, Folope V, Petit A, et al. Comparison of body composition assessment by DXA and BIA according to the body mass index: A retrospective study on 3655 measures. PloS One. (2018) 13:e0200465. doi: 10.1371/journal.pone.0200465
37. Dehghan M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J. (2008) 7:26. doi: 10.1186/1475-2891-7-26
38. Allison RD, Ray Lewis A, Liedtke R, Dean Buchmeyer N, Frank H. Early identification of hypovolemia using total body resistance measurements in long-term care facility residents. Gend Med. (2005) 2:19–34. doi: 10.1016/S1550-8579(05)80006-3
39. Jun M-H, Kim S, Ku B, Cho J, Kim K, Yoo H-R, et al. Glucose-independent segmental phase angles from multi-frequency bioimpedance analysis to discriminate diabetes mellitus. Sci Rep. (2018) 8:648. doi: 10.1038/s41598-017-18913-7
40. Souza NC, Avesani CM, Prado CM, Martucci RB, Rodrigues VD, de Pinho NB, et al. Phase angle as a marker for muscle abnormalities and function in patients with colorectal cancer. Clin Nutr. (2021) 40:4799–806. doi: 10.1016/j.clnu.2021.06.013
41. da Silva BR, Gonzalez MC, Cereda E, Prado CM. Exploring the potential role of phase angle as a marker of oxidative stress: A narrative review. Nutrition. (2022) 93:111493. doi: 10.1016/j.nut.2021.111493
42. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. (2017) 4:851–63. doi: 10.5114/aoms.2016.58928
43. da Silva BR, Orsso CE, Gonzalez MC, Sicchieri JMF, Mialich MS, Jordao AA, et al. Phase angle and cellular health: inflammation and oxidative damage. Rev Endocr Metab Disord. (2023) 24:543–62. doi: 10.1007/s11154-022-09775-0
Keywords: bioelectric impedance analysis, male infertility, phase angle, semen analysis, sperm DNA fragmentation
Citation: Liprino A, Giacone F, Lombardo D, Asmundo MG, Russo GI, Abdelhameed AS, Cimino S, Guglielmino A and Chamayou S (2024) Phase angle at bioelectric impedance analysis is associated with detrimental sperm quality in idiopathic male infertility: a preliminary clinical study. Front. Endocrinol. 15:1354733. doi: 10.3389/fendo.2024.1354733
Received: 12 December 2023; Accepted: 05 April 2024;
Published: 24 April 2024.
Edited by:
Rossella Cannarella, Cleveland Clinic, United StatesReviewed by:
Luís Pedro Rato, Instituto Politécnico da Guarda, PortugalAfonso Morgado, Centro Hospitalar Universitário de São João (CHUSJ), Portugal
Copyright © 2024 Liprino, Giacone, Lombardo, Asmundo, Russo, Abdelhameed, Cimino, Guglielmino and Chamayou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giorgio Ivan Russo, Z2lvcmdpb2l2YW4xOTg3QGdtYWlsLmNvbQ==
 Annalisa Liprino1
Annalisa Liprino1 Filippo Giacone
Filippo Giacone Maria Giovanna Asmundo
Maria Giovanna Asmundo Giorgio Ivan Russo
Giorgio Ivan Russo Ali Saber Abdelhameed
Ali Saber Abdelhameed Sebastiano Cimino
Sebastiano Cimino