- Department of Obstetrics and Gynecology, The First Affiliated Hospital of Zhengzhou University, Hangzhou, China
Background: In recent years, the incidence of Endometrial cancer (EC) has been on the rise due to high-fat, high-calorie diets and low-exercise lifestyles. However, the relationships between metabolic disorders and the progression of EC remain uncertain. The purpose of our study was to explore the potential association between obesity, hypertension, hyperglycemia and clinicopathologic characteristics in EC patients.
Methods: In categorical variables, Chi-square tests were used to calculate P values. Univariate logistic regression and multivariate logistic regression were used to identify the risk factors of myometrial invasion>1/2 and lymph node metastasis. Overall survival (OS) was estimated using the Kaplan-Meier method.
Results: The study included 406 individuals with EC, 62.6% had type I and 37.4% had type II. Hypertension was seen in 132 (32.5%), hyperglycemia in 75 (18.5%), and overweight or obesity in 217 (53.4%). Hypertension, hyperglycemia, and obesity are strongly associated with the clinicopathologic features of EC. Multivariate logistic regression revealed that hyperglycemia (OR=2.439,95% CI: 1.025-5.804, P = 0.044) was a risk factor for myometrial invasion depth >1/2 in patients with type I EC, and hypertension (OR=32.124,95% CI: 3.287-313.992, P = 0.003) was a risk factor for lymph node metastasis in patients with type I EC. Survival analysis found that hyperglycemia (P < 0.001) and hypertension (P = 0.002) were associated with OS in type I EC. Neither hyperglycemia, hypertension, nor obesity were associated with the prognosis in type II EC.
Conclusion: Hyperglycemia was a risk factor for myometrial invasion depth >1/2 in patients with type I EC and hypertension was a risk factor for lymph node metastasis in patients with type I EC. Hypertension and hyperglycemia were associated with poor prognosis in patients with type I EC.
1 Introduction
EC is one of the three major malignant tumors of the female reproductive system, and the incidence of EC has been on the rise due to high-fat, high-calorie diets and low-exercise lifestyles in recent years (1). It is estimated that there are about 66,200 new cases and 13,030 deaths in 2023 (2). EC can be classified into type I and type II based on clinical and molecular features (3). Type I EC is associated with excessive estrogen exposure and hormone receptor-positive status, which account for approximately 80% of all EC cases, and usually have favorable prognosis. Type II EC is primarily characterized by overexpression of estrogen and progesterone receptors (4, 5). The risks associated with EC include menarche early, menopause late, nulliparity, hormone therapy after menopause, and obesity (6).
Metabolic disorders play an important role in the development and progression of EC (7–10). Previous research has shown that women with metabolic disorders have a higher risk of EC and are also associated with a poor prognosis. Obesity is recognized as a significant risk factor for EC (11–14). In symptomatic premenopausal women, those with a BMI exceeding 25, 30, and 40 had a 3.85, 5.25, and 19.79 times greater likelihood of developing EC compared to women with a normal BMI, respectively (15). Furthermore, obese EC patients exhibit diminished responsiveness to standard treatment, an increased occurrence of distant metastases, and a poorer prognosis (16, 17). Hyperglycemia and hypertension are also essential risk factors for the development of EC and are closely related to the clinicopathological features and prognosis of EC. A meta-study revealed that diabetes was associated with an increased risk of EC (18).
However, the current understanding of the relationships between metabolic disorders and the progression of EC remains uncertain. Consequently, we performed a retrospective study to explore the potential association between obesity, hypertension, hyperglycemia and clinicopathologic characteristics in EC patients.
2 Methods
2.1 Study population
EC patients from the medical record system of the First Affiliated Hospital of Zhengzhou University (from January 1, 2012, to December 31, 2020) were included in this study. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Ethics Approval Number: 2023-KY-0305-002). Criteria for inclusion were as follows: (1) EC diagnosed by the First Affiliated Hospital of Zhengzhou University after staged surgery; (2) No neoadjuvant before surgery. Exclusion criteria:(1) Patients with other malignant tumors; (2) Patients not undergoing surgery; (3) Patients with preserved fertility; (4) Patients with incomplete case information.
2.2 Clinical data collection
Patient information was collected through the case system of the First Affiliated Hospital of Zhengzhou University as follows: age at the time of diagnosis, height, weight, blood pressure, blood glucose, date of diagnosis, surgical stage, type of pathology, pathologic grade, lymph node metastasis, parametrial invasion, stromal invasion, myometrial invasion and survival time. OS was defined as the date from the date of diagnosis to the date of death from any cause or final follow-up. BMI was classified as not overweight (BMI<25) or overweight and obesity (BMI≥25). Hyperglycemia was defined as a situation of fasting glucose>6.1 mmol/L or 2-hour glucose>7.8. Hypertension was defined by casual blood pressure >140/90 mmHg. Surgical staging according to the 2009 International Federation of Gynecology and Obstetrics (FIGO) staging criteria for EC.
2.3 Statistical analysis
The continuous variables that conform to normal distribution were expressed as mean ± SD and those that did not were expressed as median (interquartile range). In categorical variables, Chi-square tests and Fisher's exact test are used to calculate P-values. Univariate logistic regression and multivariate logistic regression were used to identify the risk factors of myometrial invasion>1/2 and lymph node metastasis. In the multivariable logistic regression analysis, only factors with a P value less than 0.1 were included. The results were expressed as odds ratio (OR). OS was estimated using the Kaplan-Meier method, and the P-values were compared using the log-rank test. All statistical analyses were performed with SPSS26. P < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of the study population
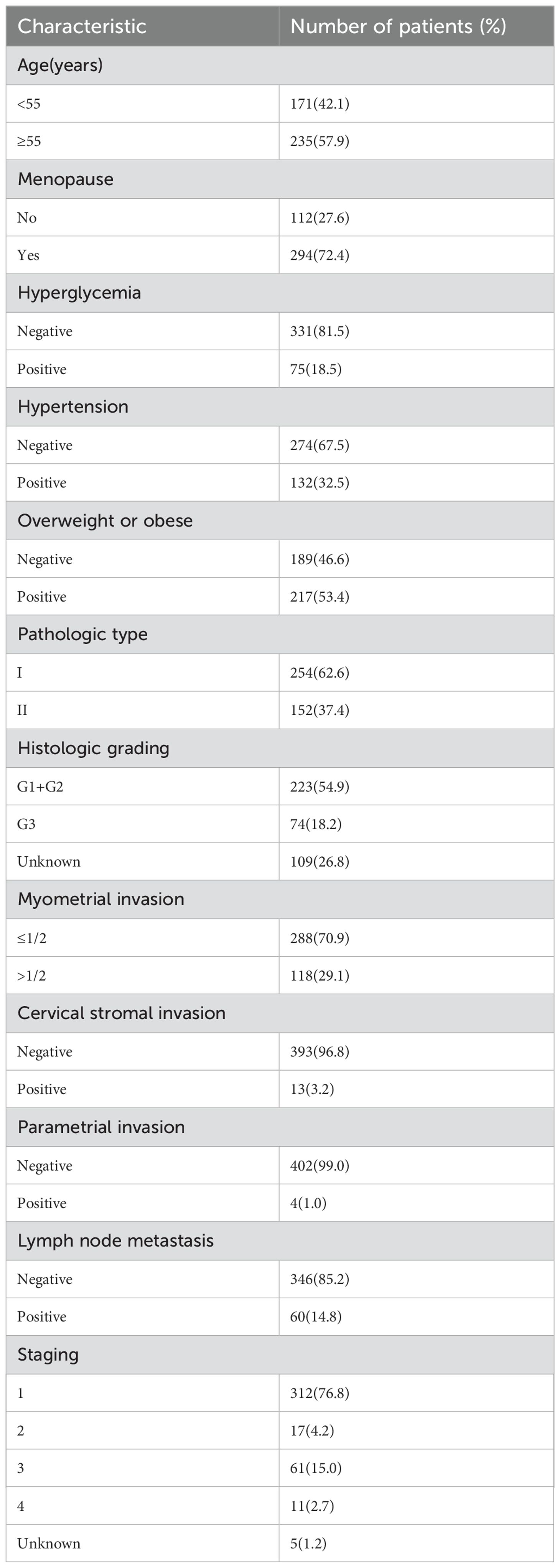
A cohort of 406 individual samples diagnosed with EC was included in our study. Of these, 254 (62.6%) had type I EC, while 152 (37.4%) had type II EC. Hypertension was present in 132 individuals (32.5%), hyperglycemia in 75 individuals (18.5%), and obesity in 217 individuals (53.4%). Out of 132 hypertensive patients, 114 took daily medication to control their blood pressure. Among 75 patients with hyperglycemia, 69 took daily medication to control their blood sugar levels.The distribution of cancer stages among the patients was as follows: 312 (76.8%) were classified as stage I, 17 (4.2%) as stage II, 61 (15.0%) as stage III, and 11 (2.7%) as stage IV. Lymph node metastasis was detected in 60 patients (14.7%), while 118 patients (29%) exhibited myometrial invasion > 1/2. Additionally, 13 patients (3.2%) displayed cervical stromal invasion (Table 1).
3.2 Correlation of hypertension, hyperglycemia, and obesity with EC clinical parameters
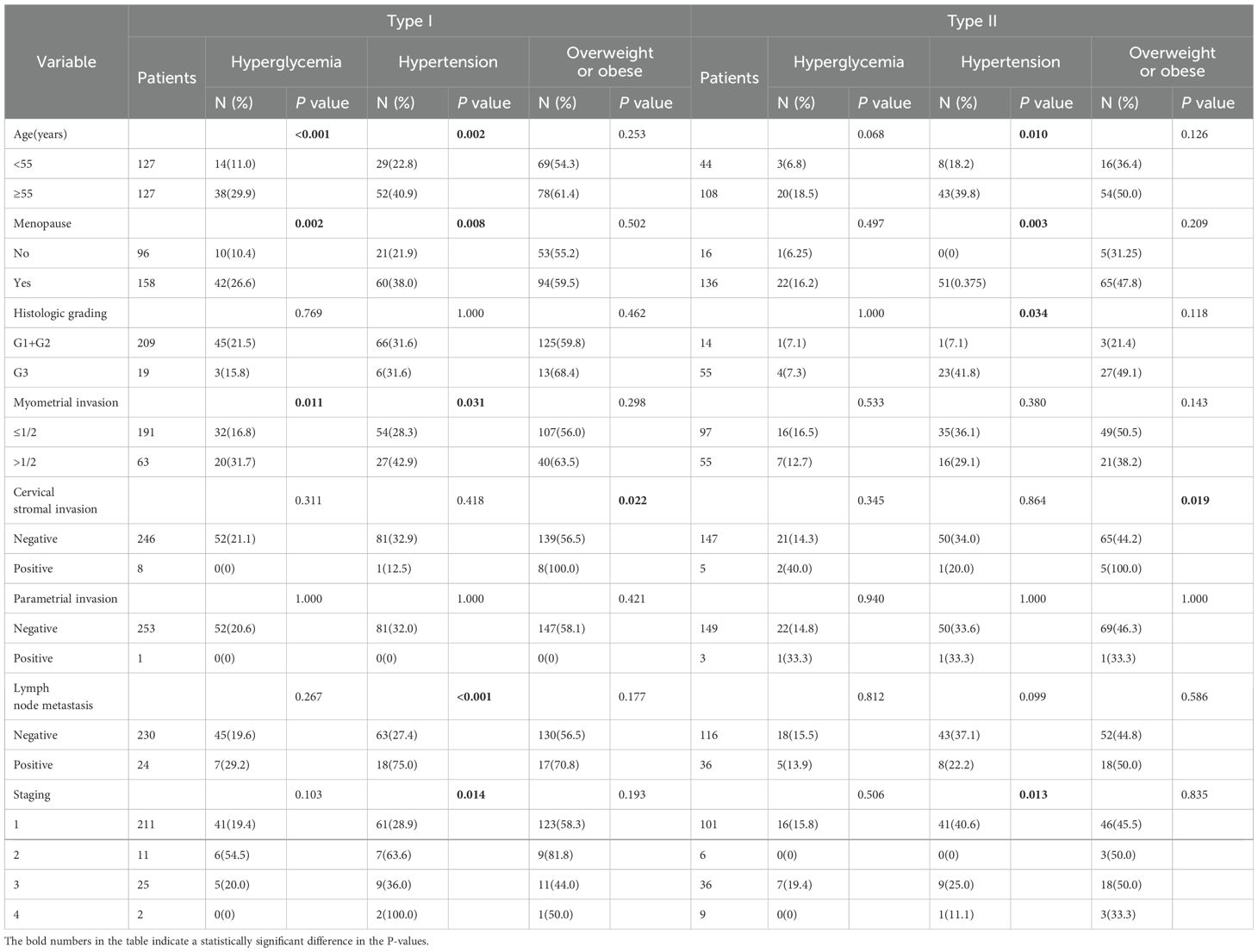
In patients with type I EC, hyperglycemia was associated with age (P < 0.001), menopause (P=0.002), and myometrial invasion depth >1/2 (P = 0.011), whereas hyperglycemia was not associated with these factors in patients with type II EC. Hypertension was correlated with age (older than 55 years, P = 0.002), menopause (P = 0.008), myometrial invasion depth >1/2 (P = 0.031), lymph node metastasis (P < 0.001), and FIGO stage (P = 0.014) in type I EC. In type II EC, hypertension is associated with advanced age (older than 55 years, P = 0.01), menopause (P = 0.001), pathologic grade(P=0.034), and FIGO stage (P = 0.013). Obesity was correlated with cervical stromal invasion in both type I (P = 0.022) and type II (P = 0.019) EC (Table 2).
3.3 Pathologic risk factors for EC: a univariate analysis
Next, we analyzed the risk factors for lymph node metastasis and myometrial invasion >1/2 for type I and type II EC, respectively, using univariate logistic analysis. These results revealed that the risk factors for lymph node metastasis in type I EC were as follows: myometrial infiltration >1/2(OR= 9.714, 95% CI: 3.804-24.809, P < 0.001), hypertension (OR = 7.952, 95% CI:3.020-20.943, P < 0.001), stage (P < 0.001), histologic grading (OR = 1.879, 95% CI: 1.187-2.973, P = 0.007) (Table 3). Risk factors for type I EC with myometrial invasion >1/2 were age (OR = 1.902, 95% CI: 1.062-3.406, P = 0.031), lymph node metastasis (OR = 9.714, 95% CI: 3.804-24.809, P < 0.001), cervical stromal invasion(OR = 9.947, 95% CI: 1.954-50.642, P = 0.006), hypertension (OR=1.903, 95%CI:1.055-3.433, P=0.033), hyperglycemia (OR=2.311, 95%CI:1.204-4.438, P=0.012 ), staging (P < 0.001), and histologic grading (OR = 2.507, 95%CI: 0.953-6.592, P=0.062) (Table 3). These results found that the risk factors for lymph node metastasis of type II EC were: menopause (OR = 3.857, 95%CI: 1.330-11.183, P=0.013), myometrial invasion > 1/2 (OR = 3.376, 95%CI: 1.557-7.321, P=0.002), cervical stromal invasion (OR = 14.375, 95%CI: 1.552-133.158, P=0.019), and histologic grading (OR = 3.375, 95%CI: 0.956-11.909, P=0.059) (Table 4). Risk factors for type II EC with myometrial infiltration >1/2 were: lymph node metastasis (OR = 3.376, 95%CI: 1.557-7.321, P=0.002), cervical stromal invasion (OR = 2.323, 95%CI: 0.253-21.317, P=0.006), stage (P=0.014), and histologic grading (OR = 8.348, 95%CI: 1.702-40.933, P=0.009) (Table 4).

Table 3. Univariate regression analysis of risk factors associated with lymph node metastasis and myometrial invasion >1/2 in type I EC.
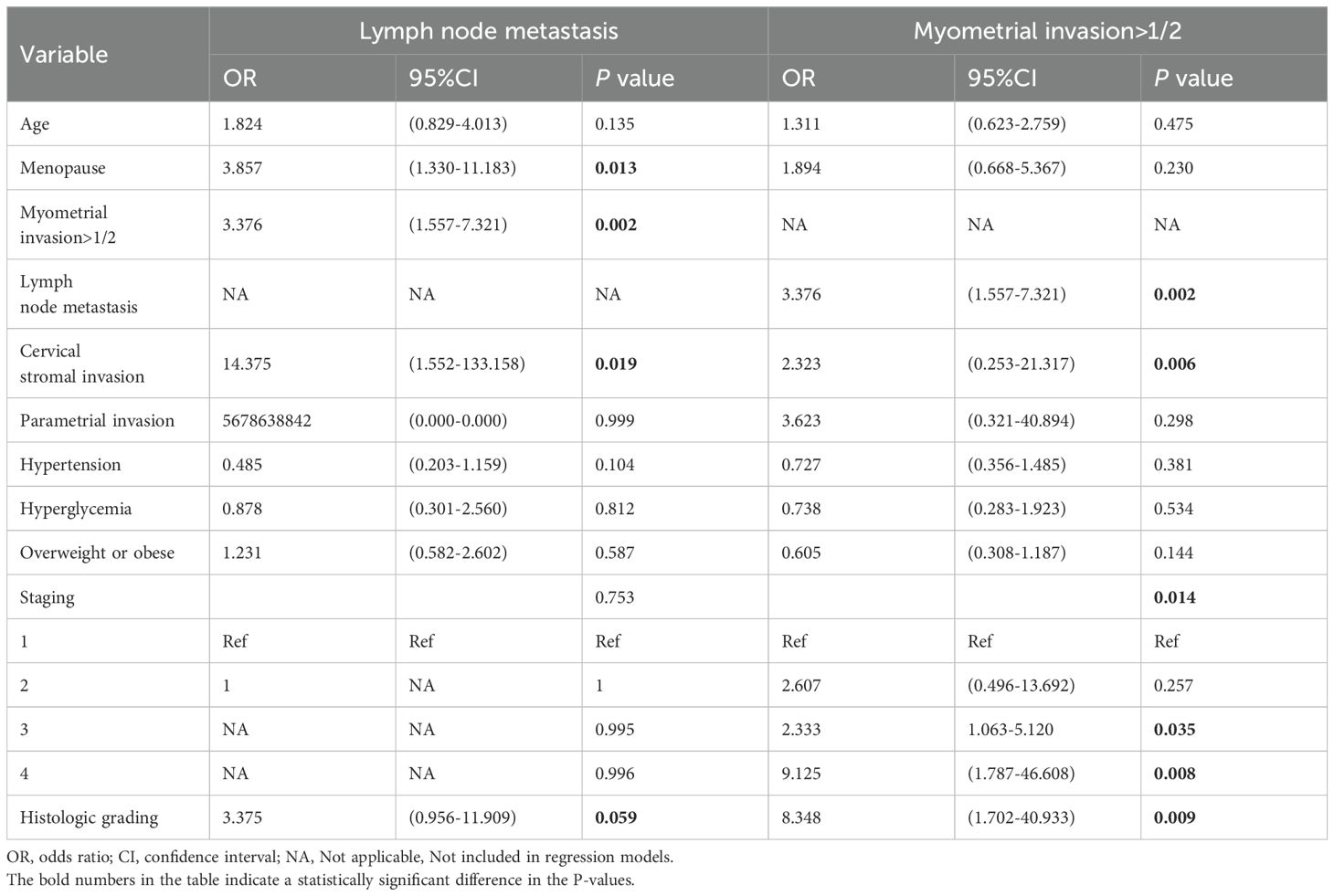
Table 4. Univariate regression analysis of risk factors associated with lymph node metastasis and myometrial invasion >1/2 in type II EC.
3.4 Pathologic risk factors for EC: a multivariate analysis
Multivariate logistic regression revealed that the factors for lymph node metastasis in type I EC were: hypertension (OR = 32.124,95% CI: 3.287-313.992, P = 0.003) and stage (P = 0.002) (Table 5). The risk factors for myometrial invasion >1/2 in type I EC were: lymph node metastasis (OR = 5.472, 95% CI: 1.014-29.515, P = 0.048), cervical stromal invasion (OR = 10.231, 95% CI: 1.353-77.373, P = 0.024), hyperglycemia (OR=2.439, 95% CI: 1.025-5.804, P = 0.044), and stage (P = 0.044) (Table 5). The risk factor for myometrial invasion >1/2 in type II EC was related to histologic grading (OR = 2.798, 95% CI: 0.836-9.364, P = 0.016) (Table 6).
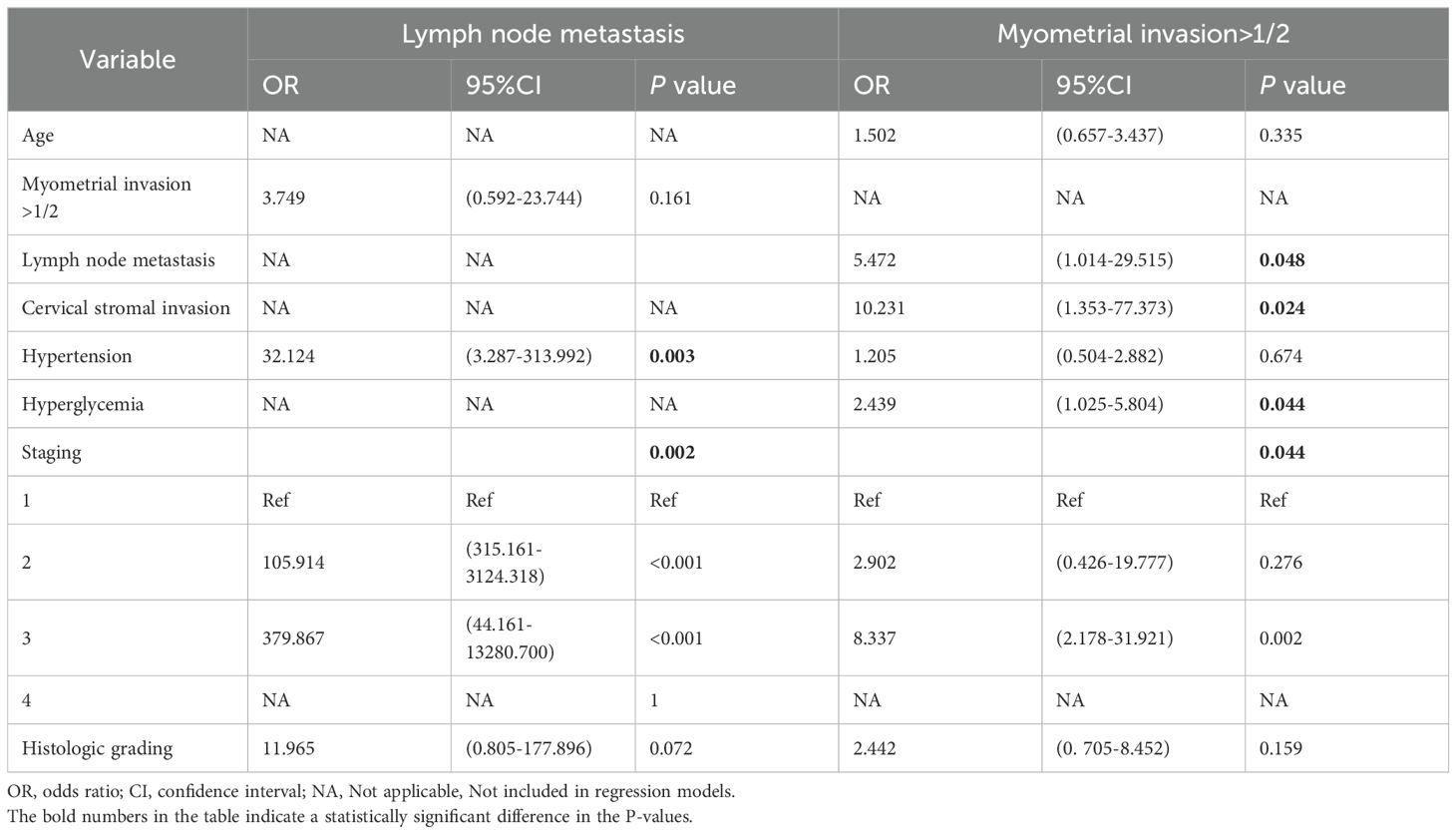
Table 5. Multivariate regression analysis of risk factors associated with lymph node metastasis and myometrial invasion >1/2 in type I EC.
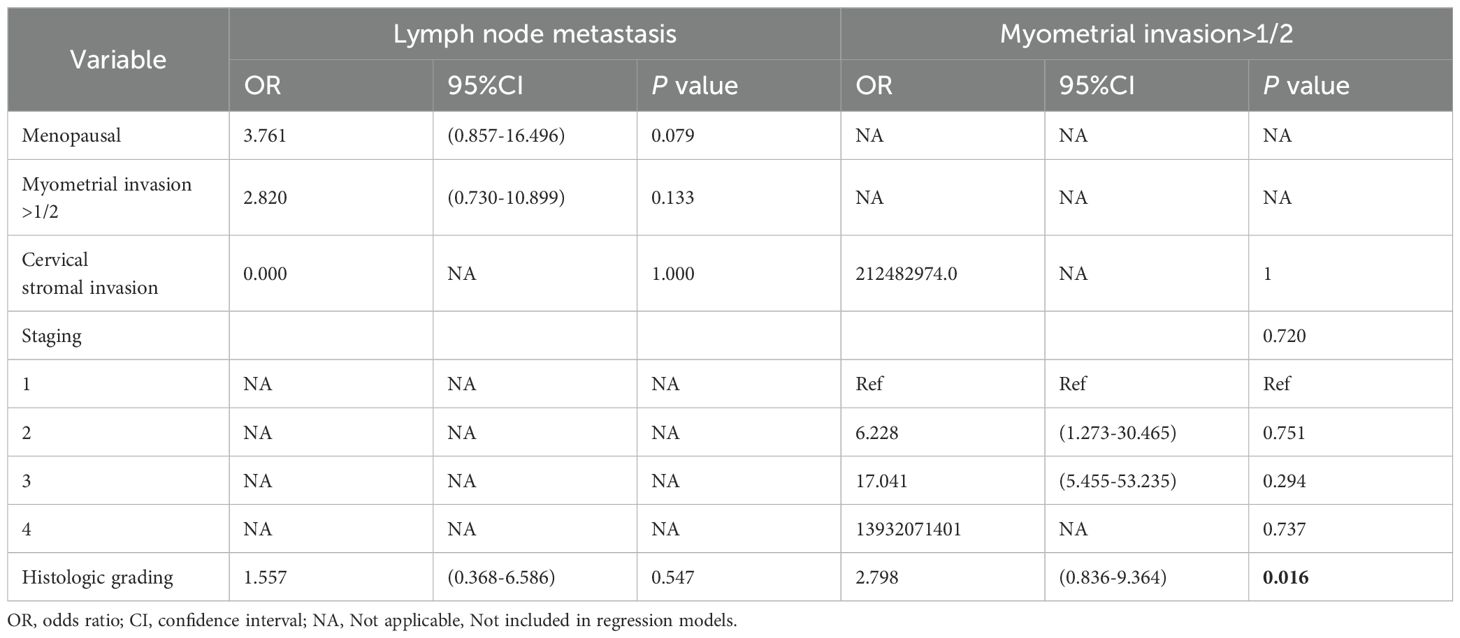
Table 6. Multivariate regression analysis of risk factors associated with lymph node metastasis and myometrial invasion >1/2 in type II EC.
3.5 Relationship between hypertension, hyperglycemia, obesity, and prognosis of patients with EC
Survival analysis found that hyperglycemia (P < 0.001) and hypertension (P = 0.002) were associated with OS in type I EC. Neither hyperglycemia, hypertension, nor obesity were associated with the prognosis of type II EC (Figure 1).
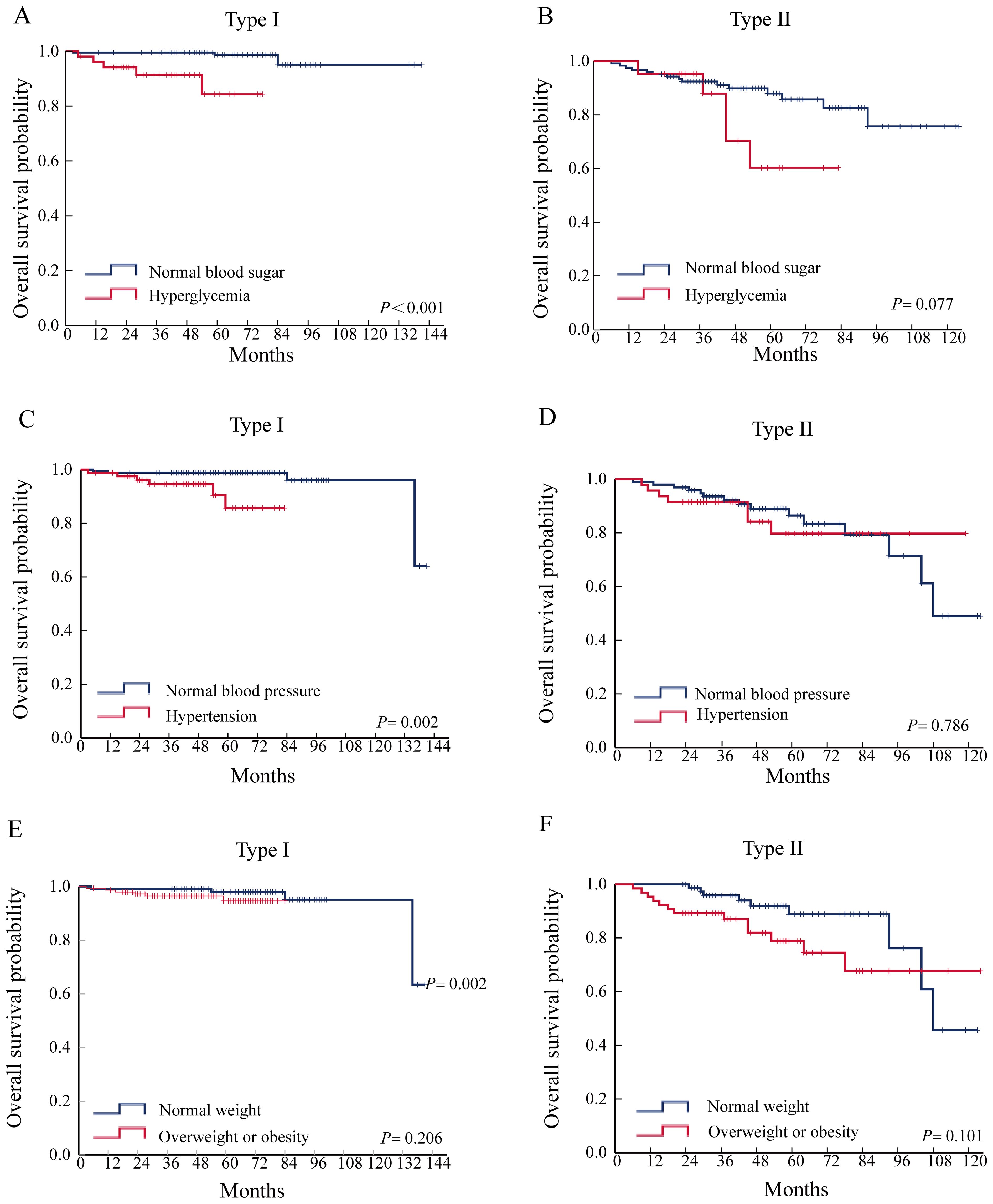
Figure 1. Relationship between hypertension, hyperglycemia, obesity, and prognosis of patients with EC. (A) Effect of hyperglycemia on OS in type I EC. (B) Effect of hyperglycemia on OS in type II EC. (C) Effect of hypertension on OS in type I EC. (D) Effect of hypertension on OS in type II EC. (E) Effect of overweight or obesity on OS in type I EC. (F) Effect of overweight or obesity on OS in type II EC.
4 Discussion
EC is one of the cancers most closely associated with metabolic disease, obesity, hypertension, and diabetes, which are known as the metabolic triad of EC (3). This study identified hypertension as a significant risk factor for lymph node metastasis in type I EC. Additionally, hypertension and hyperglycemia were found to be risk factors for deep myometrial invasion and were further associated with an unfavorable prognosis in type I EC.
Hyperglycemia is closely associated with the development and progression of many malignant tumors such as ovarian, pancreatic, and breast cancer (19, 20). A large number of epidemiologic studies on the subject have shown hyperglycemia was a risk factor for the development of EC (9, 11, 21–24). A meta-analysis that included 17 prospective studies and 12 retrospective studies found diabetes mellitus was a risk factor for EC. Another study showed a significant 15% increased risk of cancer-specific death in EC patients with preexisting diabetes compared to patients without diabetes (25). Our research has shown in type I EC patients, hyperglycemia was associated with myometrial invasion depth >1/2 and poor prognosis. A significant proportion, approximately 93%, of Type I EC, specifically the endometrioid type, exhibit an absence of the phosphatase and tensin homolog (PTEN) or harbor mutations within the PI3K/Akt/mTOR pathways, which are associated with glucose metabolism, hyperglycemia stimulates changes in the above signaling pathways to increase the proliferation, invasion, and migration of tumor cells, so hyperglycemia is associated with deep myometrial invasion in patients with type I EC (26–28). For example, one study showed that EC cells (UECC, Ishikawa, RL95-2) cultured under high glucose conditions (25 mM) were more aggressive than physiological levels (5 mM) and had increased expression of EMT pathway-related proteins (29). We speculate that hyperglycemia is associated with deep myometrial invasion of type I EC but not with type II EC, which may be caused by different molecular characteristics, and there are no reports that hyperglycemia affects the prognosis and molecular characteristics of type II EC. We look forward to future research that will shed light and address this issue.
We also found hypertension to be a risk factor for lymph node metastasis in type I EC and was associated with a poor prognosis for EC. A few studies have been conducted on the association of hypertension with the clinicopathologic characteristics of EC (30–33). One retrospective study revealed that a relative risk of EC associated with hypertension of 1.6 (34). Results of a meta-analysis suggested a strong association between hypertension and EC (35). An observational study has shown that hypertension was positively associated with mortality from a variety of cancers, and our study is consistent with this finding that hypertension is associated with poor EC outcomes (36). Hypertension is closely related to the progression of other tumors, for example, hypertension is associated with the TNM stage of gastric cancer, and with the stage, tumor size, and lymph node metastasis of thyroid cancer, in this study we found that hypertension is associated with lymph node metastasis in type I EC (37, 38). Patients with hypertension often have disorders of renin-angiotensin system (RAS), and previous studies have shown that changes in the RAS system are closely related to the occurrence and development of EC (39). For example, angiotensin II has been shown to enhance the proliferative capacity of endometrial cancer cell lines (40). A study of 30 endometrial cancer samples showed that high expression of ATP6AP2, AGTR1, and ACE1 in the RAS was associated with the spread of endometrial cancer (41). Although our study found that hypertension is not related to lymph node metastasis and prognosis of type II EC, there are still some hypertensive patients in type II EC, and some antineoplastic drugs (anti-angiogenic drugs, bevacizumab, etc.) can cause hypertension, and we should individualize the treatment of hypertensive endometrial cancer patients.
It occurrences recognized that obesity is one of the most critical risk factors for development of type I EC, and a large number of studies have shown that obesity could also affect the prognosis of patients with EC. Studies have shown that obesity was associated with a two- to five-fold increased risk of EC in pre and post-menopausal women, Every 10-year increase of the adult overweight duration (BMI ≥ 25 kg/m2) has been reported to be correlated with 17% higher risk of postmenopausal EC (42, 43). Although a large quantity of studies have demonstrated that obesity is a risk factor for EC, the effect of obesity on the prognosis of EC is still controversial Gunderson's research found that BMI is associated with all-cause mortality, but not with EC-specific mortality (44). A meta-analysis showed that higher BMI (≥30 kg/m2) was associated with increased all-cause mortality (HR = 1.34, 95%CI = 1.12-1.59) and recurrence (HR = 1.28, 95%CI = 1.06-1.56) of EC (14). Another study that included 937 cases of EC showed no association between weight and OS (45). Our study found no significant difference in the prognosis of EC patients with normal weight (BMI <25) compared to those who were overweight or obese (BMI ≥25). Future prospective studies with large samples are still needed to further determine the effect of obesity on the prognosis of EC patients, to provide postoperative health guidance for EC patients and to improve their quality of life and prolong their living time.
5 Conclusion
Hyperglycemia was a risk factor for myometrial invasion depth >1/2 in patients with type I EC and hypertension was a risk factor for lymph node metastasis in patients with type I EC. Hypertension and hyperglycemia were associated with poor prognosis in patients with type I EC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this was a retrospective study, and informed consent was waived with the approval of the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Author contributions
YW: Writing – original draft, Writing – review & editing. QL: Writing – original draft, Writing – review & editing. YS: Data curation, Writing – review & editing. WW: Data curation, Writing – original draft. XiC: Data curation, Writing – original draft. XuC: Writing – original draft, Data curation. FR: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This experiment was supported by the Excellent Youth Funding of Henan Provincial Foundation Committee (222300420091), 2021 Henan Provincial Health Young and Middle-aged Discipline Leader Project and Henan Province Medical Science and Technology Research Plan Provincial and Ministerial Co-construction Project (SBGJ202302075).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. (2022) 399:1412–28. doi: 10.1016/s0140-6736(22)00323-3
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: A Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
3. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. (2016) 387:1094–108. doi: 10.1016/s0140-6736(15)00130-0
4. Lu KH, Longo DL, Broaddus RR. Endometrial cancer. New Engl J Med. (2020) 383:2053–64. doi: 10.1056/NEJMra1514010
5. Horn LC, Meinel A, Handzel R, Einenkel J. Histopathology of endometrial hyperplasia and endometrial carcinoma: an update. Ann Diagn Pathol. (2007) 11:297–311. doi: 10.1016/j.anndiagpath.2007.05.002
6. Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Oncology. (2014) 15:e268–78. doi: 10.1016/s1470-2045(13)70591-6
7. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. (1983) 15:10–7. doi: 10.1016/0090-8258(83)90111-7
8. Wang L, Du ZH, Qiao JM, Gao S. Association between metabolic syndrome and endometrial cancer risk: a systematic review and meta-analysis of observational studies. Aging. (2020) 12:9825–39. doi: 10.18632/aging.103247
9. Trabert B, Wentzensen N, Felix AS, Yang HP, Sherman ME, Brinton LA. Metabolic syndrome and risk of endometrial cancer in the United States: a study in the SEER-medicare linked database. Cancer Epidemiol Biomarkers Prev. (2015) 24:261–7. doi: 10.1158/1055-9965.Epi-14-0923
10. Yang X, Li X, Dong Y, Fan Y, Cheng Y, Zhai L, et al. Effects of metabolic syndrome and its components on the prognosis of endometrial cancer. Front Endocrinol. (2021) 12:780769. doi: 10.3389/fendo.2021.780769
11. Arthur RS, Kabat GC, Kim MY, Wild RA, Shadyab AH, Wactawski-Wende J, et al. Metabolic syndrome and risk of endometrial cancer in postmenopausal women: a prospective study. Cancer Causes Control. (2019) 30:355–63. doi: 10.1007/s10552-019-01139-5
12. Byun D, Hong S, Ryu S, Nam Y, Jang H, Cho Y, et al. Early-life body mass index and risks of breast, endometrial, and ovarian cancers: a dose-response meta-analysis of prospective studies. Br J Cancer. (2021) 126(4):664–72. doi: 10.1038/s41416-021-01625-1
13. Dougan MM, Hankinson SE, Vivo ID, Tworoger SS, Glynn RJ, Michels KB. Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. Int J Cancer. (2015) 137:625–37. doi: 10.1002/ijc.29427
14. Kokts-Porietis RL, Elmrayed S, Brenner DR, Friedenreich CM. Obesity and mortality among endometrial cancer survivors: A systematic review and meta-analysis. Obesity. (2021) 22:e13337. doi: 10.1111/obr.13337
15. Wise MR, Jordan V, Lagas A, Showell M, Wong N, Lensen S, et al. Obesity and endometrial hyperplasia and cancer in premenopausal women: A systematic review. Am J Obstetrics Gynecology. (2016) 214:689.e1–689.e17. doi: 10.1016/j.ajog.2016.01.175
16. Secord AA, Hasselblad V, Von Gruenigen VE, Gehrig PA, Modesitt SC, Bae-Jump V, et al. Body mass index and mortality in endometrial cancer: A systematic review and meta-analysis. Gynecologic Oncol. (2016) 140:184–90. doi: 10.1016/j.ygyno.2015.10.020
17. Nagle CM, Crosbie EJ, Brand A, Obermair A, Oehler MK, Quinn M, et al. The association between diabetes, comorbidities, body mass index and all-cause and cause-specific mortality among women with endometrial cancer. Gynecologic Oncol. (2018) 150:99–105. doi: 10.1016/j.ygyno.2018.04.006
18. Saed L, Varse F, Baradaran HR, Moradi Y, Moradi Y, Khateri S, et al. The effect of diabetes on the risk of endometrial Cancer: an updated a systematic review and meta-analysis. BMC Cancer. (2019) 19:527. doi: 10.1186/s12885-019-5748-4
19. van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. (2007) 120:1986–92. doi: 10.1002/ijc.22532
20. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. (2015) 95:727–48. doi: 10.1152/physrev.00030.2014
21. Lambe M, Wigertz A, Garmo H, Walldius G, Jungner I, Hammar N. Impaired glucose metabolism and diabetes and the risk of breast, endometrial, and ovarian cancer. Cancer Causes Control. (2011) 22:1163–71. doi: 10.1007/s10552-011-9794-8
22. Park B. Associations between obesity, metabolic syndrome, and endometrial cancer risk in East Asian women. J Gynecol Oncol. (2022) 33:e35. doi: 10.3802/jgo.2022.33.e35
23. Liao C, Zhang D, Mungo C, Tompkins DA, Zeidan AM. Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? A systematic review and meta-analysis of cohort studies. Gynecol Oncol. (2014) 135:163–71. doi: 10.1016/j.ygyno.2014.07.095
24. Travier N, Jeffreys M, Brewer N, Wright CS, Cunningham CW, Hornell J, et al. Association between glycosylated hemoglobin and cancer risk: a New Zealand linkage study. Ann Oncol. (2007) 18:1414–9. doi: 10.1093/annonc/mdm135
25. McVicker L, Cardwell CR, Edge L, McCluggage WG, Quinn D, Wylie J, et al. Survival outcomes in endometrial cancer patients according to diabetes: a systematic review and meta-analysis. BMC Cancer. (2022) 22:427. doi: 10.1186/s12885-022-09510-7
26. Weigelt B, Banerjee S. Molecular targets and targeted therapeutics in endometrial cancer. Curr Opin Oncol. (2012) 24:554–63. doi: 10.1097/CCO.0b013e328354e585
27. Byrne FL, Martin AR, Kosasih M, Caruana BT, Farrell R. The role of hyperglycemia in endometrial cancer pathogenesis. Cancers. (2020) 12(5):1191. doi: 10.3390/cancers12051191
28. Han J, Zhang L, Guo H, Wysham WZ, Roque DR, Willson AK, et al. Glucose promotes cell proliferation, glucose uptake and invasion in endometrial cancer cells via AMPK/mTOR/S6 and MAPK signaling. Gynecol Oncol. (2015) 138:668–75. doi: 10.1016/j.ygyno.2015.06.036
29. Gu CJ, Xie F, Zhang B, Yang HL, Cheng J, He YY, et al. High glucose promotes epithelial-mesenchymal transition of uterus endometrial cancer cells by increasing ER/GLUT4-mediated VEGF secretion. Cell Physiol Biochem. (2018) 50:706–20. doi: 10.1159/000494237
30. Soler M, Chatenoud L, Parazzini F, Franceschi S, la Vecchia C. Hypertension and hormone-related neoplasms in women. Hypertension. (1999) 34:320–5. doi: 10.1161/01.hyp.34.2.320
31. Lindgren AM, Nissinen AM, Tuomilehto JO, Pukkala E. Cancer pattern among hypertensive patients in North Karelia, Finland. J Hum Hypertens. (2005) 19:373–9. doi: 10.1038/sj.jhh.1001834
32. Seretis A, Cividini S, Markozannes G, Tseretopoulou X, Lopez DS, Ntzani EE, et al. Association between blood pressure and risk of cancer development: a systematic review and meta-analysis of observational studies. Sci Rep. (2019) 9:8565. doi: 10.1038/s41598-019-45014-4
33. Christakoudi S, Kakourou A, Markozannes G, Tzoulaki I, Weiderpass E, Brennan P, et al. Blood pressure and risk of cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. (2020) 146:2680–93. doi: 10.1002/ijc.32576
34. Maatela J, Aromaa A, Salmi T, Pohja M, Vuento M, Grönroos M. The risk of endometrial cancer in diabetic and hypertensive patients: a nationwide record-linkage study in Finland. Ann Chir Gynaecol Suppl. (1994) 208:20–4.
35. Connaughton M, Dabagh M. Association of hypertension and organ-specific cancer: A meta-analysis. Healthcare. (2022) 10(6):1074. doi: 10.3390/healthcare10061074
36. Stocks T, Van Hemelrijck M, Manjer J, Bjørge T, Ulmer H, Hallmans G, et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension. (2012) 59:802–10. doi: 10.1161/hypertensionaha.111.189258
37. Li F, Du H, Li S, Liu J. The association between metabolic syndrome and gastric cancer in chinese. Front Oncol. (2018) 8:326. doi: 10.3389/fonc.2018.00326
38. Li LR, Song JL, Liu HQ, Chen C. Hypertension was associated with higher tumor stages in papillary thyroid cancer: A large sample single-center study. Metab Syndr Relat Disord. (2022) 20:466–72. doi: 10.1089/met.2022.0033
39. Khan NA, Elsori D, Rashid G, Tamanna S, Chakraborty A, Farooqi A, et al. Unraveling the relationship between the renin-angiotensin system and endometrial cancer: a comprehensive review. Front Oncol. (2023) 13:1235418. doi: 10.3389/fonc.2023.1235418
40. Nowakowska M, Matysiak-Burzyńska Z, Kowalska K, Płuciennik E, Domińska K, Piastowska-Ciesielska AW. Angiotensin II promotes endometrial cancer cell survival. Oncol Rep. (2016) 36:1101–10. doi: 10.3892/or.2016.4887
41. Sarah JD, Eugenie RL, Celine Corbisier de M, Yu W, Anthony P, Geoffrey O, et al. Expression of renin–angiotensin system (RAS) components in endometrial cancer. Endocrine connections. (2017) 6(1):9–19. doi: 10.1530/ec-16-0082
42. Arnold M, Jiang L, Stefanick ML, Johnson KC, Lane DS, LeBlanc ES, et al. Duration of adulthood overweight, obesity, and cancer risk in the women's health initiative: A longitudinal study from the United States. PloS Med. (2016) 13:e1002081. doi: 10.1371/journal.pmed.1002081
43. Bergström A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. (2001) 91:421–30. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t
44. Gunderson CC, Java J, Moore KN, Walker JL. The impact of obesity on surgical staging, complications, and survival with uterine cancer: a Gynecologic Oncology Group LAP2 ancillary data study. Gynecol Oncol. (2014) 133:23–7. doi: 10.1016/j.ygyno.2014.01.041
Keywords: endometrial cancer, hyperglycemia, hypertension, obesity, lymph node metastasis
Citation: Wang Y, Liu Q, Sun Y, Wu W, Cheng X, Chen X and Ren F (2024) Association between metabolic disorders and clinicopathologic features in endometrial cancer. Front. Endocrinol. 15:1351982. doi: 10.3389/fendo.2024.1351982
Received: 07 December 2023; Accepted: 09 August 2024;
Published: 26 August 2024.
Edited by:
Hermann Frieboes, University of Louisville, United StatesReviewed by:
Lingfang Wang, Zhejiang University, ChinaCanhui Cao, Huazhong University of Science and Technology, China
Copyright © 2024 Wang, Liu, Sun, Wu, Cheng, Chen and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Ren, cmVuZmFuZ0Bmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
 Yuanpei Wang
Yuanpei Wang Qianwen Liu
Qianwen Liu Yi Sun
Yi Sun Weijia Wu
Weijia Wu Fang Ren
Fang Ren