- 1Department of Nephrology, Tianjin Medical University General Hospital, Tianjin, China
- 2Department of General Surgery, Tianjin Medical University General Hospital, Tianjin, China
- 3Department of General Surgery, Jizhou District People’s Hospital, Tianjin, China
- 4Key Laboratory of Digital Technology in Medical Diagnostics of Zhejiang Province, Dian Diagnostics Group Co., Ltd., Hangzhou, Zhejiang, China
Introduction: The prevalence of thyroid nodules and malignancies in the elderly is a growing concern. Thyroid nodules in this population have unique characteristics, requiring careful treatment strategies that balance risks and benefits. Oncocytic carcinoma of the thyroid (OCA) is a rare, aggressive subtype with diagnostic challenges.
Methods: This case features an 84-year-old patient who presented with a neck mass and symptoms of asphyxia. Clinical evaluation, imaging studies, and biopsy were conducted to assess the nature of the thyroid lesion. Molecular testing, including genetic analysis, was performed to identify specific mutations associated with OCA and guide treatment decisions.
Results: The patient was diagnosed with oncocytic carcinoma of the thyroid. The molecular testing revealed specific genetic mutations indicative of OCA, confirming the diagnosis. The presence of these mutations guided the treatment plan, emphasizing the importance of molecular diagnostics in managing thyroid malignancies, especially in the elderly.
Discussion: This case illustrates the complexities of diagnosing and treating thyroid malignancies in the elderly. Biopsy and molecular testing provided diagnostic accuracy and informed treatment. Individualized approaches are essential for better outcomes, especially in aggressive subtypes, balancing the risks and benefits of intervention.
1 Introduction
As life expectancy increases, populations are rapidly aging, leading to a substantial rise in the elderly population. The prevalence of thyroid nodules increases with age, being detectable in 25–50% of individuals over 60 years old. However, the proportion of these nodules that are malignant in elderly individuals is comparable to, or even lower than, that in younger populations (1, 2). Compared to younger adults, elderly patients (≥65 years) with thyroid malignancies tend to have larger tumor volumes, higher proportions of Stage IV cases, and more extrathyroidal extension. Notably, the distribution of histological subtypes among elderly thyroid malignancies differs, with an elevated incidence of follicular thyroid carcinoma (FTC), OCA, medullary thyroid carcinoma (MTC), and anaplastic thyroid carcinoma (ATC) but a lower proportion of papillary thyroid carcinoma (PTC) (1, 2). Therefore, tumor progression is often faster in elderly patients, resulting in a poorer prognosis (3). Clinicians assessing thyroid nodules in elderly patients must carefully balance the potential harms of thyroid cancer overdiagnosis and the risks associated with delayed diagnosis to determine appropriate treatment strategies. In diagnosing and treating thyroid nodules in the elderly, comprehensive consideration of the patient’s circumstances is crucial. This includes a higher incidence of high-risk thyroid cancer, accompanied by multiple comorbidities, declining physiological function, cognitive abilities, and an increased risk of treatment complications. When making diagnostic and therapeutic decisions, a careful balance between the risks and benefits of thyroid nodule diagnosis and treatment must be exercised (4). This case report focuses on an elderly patient with oncocytic thyroid carcinoma presenting with asphyxia.
2 Case presentation
An 84-year-old female presented to the emergency department with asphyxia. Seven days before admission to our hospital, the patient with a history of hypertension, coronary heart disease, and paroxysmal atrial fibrillation for 3 years, developed a cough and productive sputum, along with dyspnea and choking sensation after drinking water. Her symptoms worsened, leading to respiratory distress and altered consciousness. The patient was initially treated at a local hospital with interventions including endotracheal intubation, mechanical ventilation, antimicrobial therapy, and nutritional support. Subsequently, the patient was transferred to our hospital and admitted to the Intensive Care Unit (ICU). Endotracheal intubation was maintained, connected to mechanical ventilation. Physical examination revealed clear consciousness, secured endotracheal intubation, a visible anterior neck mass with a soft consistency, fixed position, tracheal compression with right deviation, and no palpable enlargement of superficial lymph nodes in the anterior neck. Laboratory investigation showed thyroid stimulating hormone of 1.754 mIU/L (0.350-4.940), Free T3 of 3.40 pmol/L (2.43-6.01), and free T4 of 8.91 pmol/L (9.01-19.05). Neck ultrasonography indicated an enlarged left thyroid lobe with predominantly hyperechoic nodules containing vascularity, measuring approximately 7.2 × 3.8 × 6.9 cm (TI-RADS 3) in size. Contrast-enhanced computed tomography of the neck revealed a solid lesion in the left thyroid lobe causing tracheal compression and deviation, with endotracheal intubation (Figure 1). Because of the rapid progression, ultrasound-guided coarse needle biopsy (US-CNB) was performed to rule out ATC, revealing eosinophilic hyperplastic nodules. Molecular testing identified TERT promoter C228T mutations and negative results for BRAF, HRAS, KRAS, NRAS, TP53, RET, NTRK1, NTRK3, PAX8, and THADA. On the fourth day of admission, the patient underwent surgery under general anesthesia following the results of US-CNB. Intraoperatively, tumor compression of the left recurrent laryngeal nerve was detected, and due to the potential risk of bilateral recurrent laryngeal nerve injury leading to severe respiratory complications in consideration of the patient’s advanced age, only left thyroidectomy and isthmusectomy were performed, avoiding total thyroidectomy. The surgery was successful, and the endotracheal tube was removed on the second postoperative day. The patient had no respiratory distress but experienced hoarseness, likely due to tumor compression and intraoperative anatomical manipulation affecting the recurrent laryngeal nerve. The patient was discharged on the ninth postoperative day. Pathological examination of the resected specimen revealed an eosinophilic cell tumor of the thyroid with focal necrosis, suspected extrathyroidal extension, vascular tumor thrombus, indicating the possibility of oncocytic cell carcinoma. Immunohistochemistry demonstrated positive staining for CK19, TG, TTF-1, and CD56, negative staining for Galectin-3 and TPO, and positive CD34 vascular staining (Figure 2).
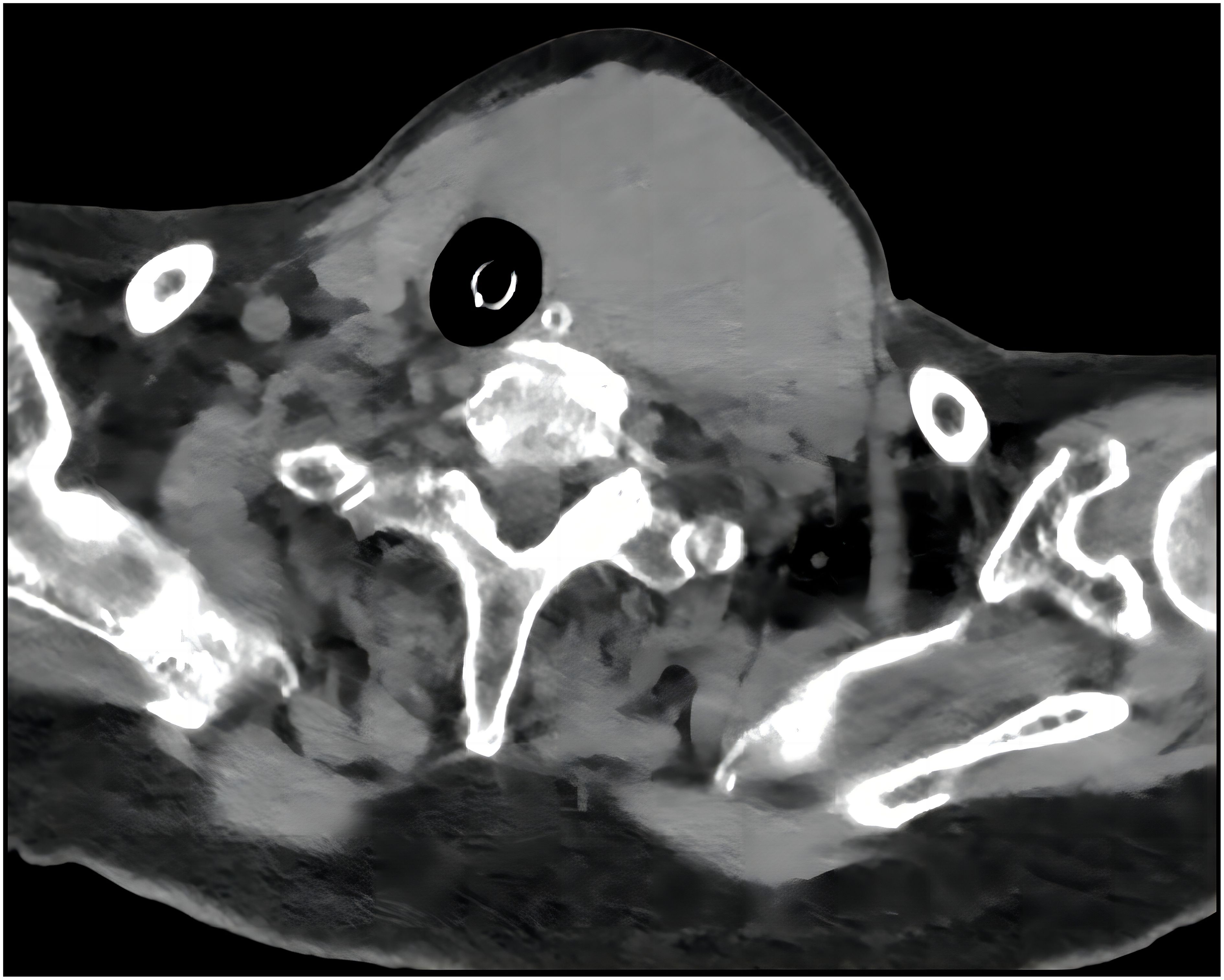
Figure 1. A CT of the neck revealed a solid lesion in the left thyroid lobe causing tracheal compression and deviation with endotracheal intubation.
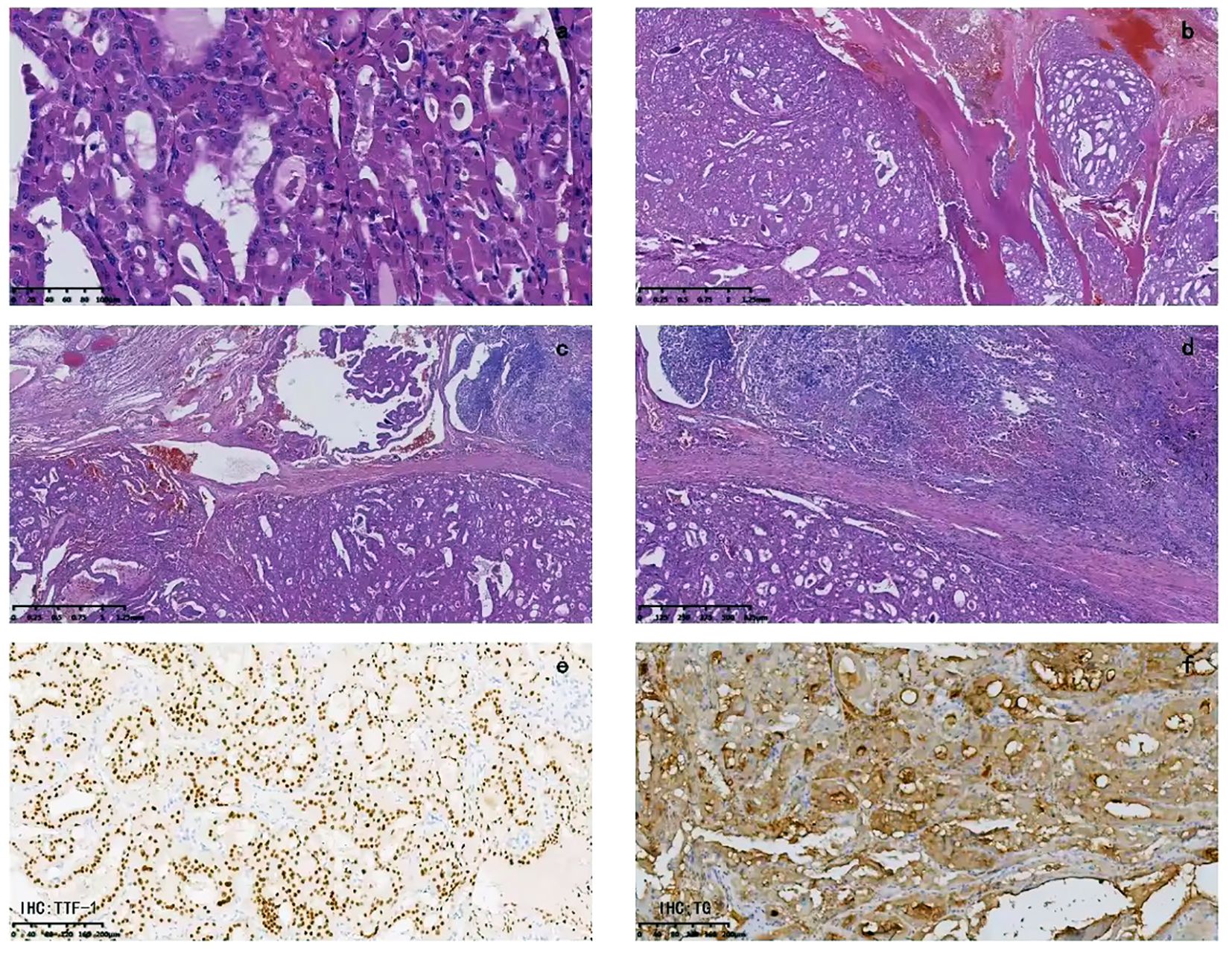
Figure 2. Pathological examination: (A) The tumor cells are cuboidal, mostly polygonal, with abundant cytoplasm filled with eosinophilic granular substances. The nuclei vary in size, being round, oval, or irregular in shape, with deep chromatin staining. Nuclear division can be seen, and nucleoli are visible in some areas. The cell boundaries are clear. The tumor cells are arranged in a follicular or trabecular pattern, with the follicular structure being predominant. There are areas with unevenly thickened colloid inside the follicular cavity. (B) Capsular invasion is visible, and outside the capsule, there are tumor cell nodules of varying sizes. (C) Tumor thrombi can be seen inside the vessels. (D) Focal necrosis is visible. (E) Immunohistochemistry for TTF-1 is positive. (F) Immunohistochemistry for TG is positive.
3 Discussion and conclusion
OCA, formerly known as Hürthle cell carcinoma (HCC), is a rare subtype of differentiated thyroid cancer and accounts for about 3-5% of all differentiated thyroid cancers (5–7). It is associated with poorer prognosis due to increased invasiveness and metastasis rates (6, 8). According to the WHO Classification for Thyroid Cancers 2022 (5th edition), the term “oncocytic carcinoma of the thyroid” is used to refer to invasive malignant follicular cell neoplasms composed of at least 75% oncocytic cells where the nuclear features of PTC and high-grade features are absent. OCAs are subclassified into minimally invasive (those with capsular invasion only), encapsulated angioinvasive, and widely invasive (those with gross invasion through the gland) tumors due to differences in clinical outcome (9). OCA typically occurs at an older age, with an average age exceeding 55 years, roughly 10 years later than the mean age of diagnosis for FTC. Although OCA is more common in women (with a 1.6 to 1 female-to-male ratio), it has a lower female-to-male ratio than is seen with FTC (6, 10). It usually presents as a slowly enlarging, painless, solitary thyroid nodule. Diagnosing OCA is challenging, as it can often be indistinguishable from benign oncocytic adenomas on thyroid ultrasonography. Differentiating between oncocytic adenomas and OCA through fine-needle aspiration biopsy (FNAB) is limited by sampling constraints (11).
Preoperative diagnosis of OCA presents significant challenges. A comprehensive diagnostic strategy can enhance accuracy, reduce missed and misdiagnosed cases, and provide better treatment options for patients. According to NCCN guidelines, preoperative evaluation should include neck ultrasound, CT, MRI, and molecular diagnostics to develop an optimal treatment plan. A retrospective study in South Korea on OCA revealed that among patients with confirmed OCA, 65.2% were classified as Bethesda Category IV, corresponding follicular neoplasm or suspicious for follicular neoplasm (FN/SFN), while atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS), suspicious for malignancy (SM), and malignant categories accounted for 8.7%, 5.4%, and 7.6%, respectively (12). Researchers have noted that managing thyroid nodules with indeterminate cytology, such as AUS/FLUS, remains a major challenge in endocrine pathology and thyroidology (13). The estimated risk of malignancy (ROM) for AUS/FLUS was 5-15% in the first edition of TBSRTC, which increased to 10-30% in the second edition, reflecting improved understanding and clinical management of these nodules (14). Recent studies suggest that the third edition of TBSRTC may further subdivide Category III into IIIA (without nuclear atypia) and IIIB (with nuclear atypia) to better reflect differing malignancy risks (14). This subdivision can help formulate more specific management recommendations based on the presence of nuclear atypia. Additionally, different management guidelines recommend fine-needle aspiration (FNA) for nodules of varying sizes. For instance, the 2015 American Thyroid Association (ATA) guidelines recommend FNA for nodules ≥10 mm with high-suspicion sonographic patterns to improve diagnostic performance and avoid unnecessary surgery (15). Larger cutoffs, such as 15 mm, may enhance the association between nodule size and malignant histopathology (15). In conclusion, subdividing cytological categories and incorporating molecular diagnostics play a crucial role in improving diagnostic accuracy (13–15).
OCAs have been shown to have recurrent DNA mutations, including RAS, EIF1AX, TERT, TP53, NF1, and CDKN1A genes (16–18). Research focused on follicular thyroid tumors indicated that TERT mutations are rare in follicular thyroid adenomas (FTA) but more common in FTC, and the presence of TERT mutations was associated with shorter patient survival, suggesting that TERT promoter mutations may serve as early genetic events occurring in follicular thyroid tumors, potentially leading to malignant transformation (19). TERT promoter mutations, specifically C228T and C250T, are mutually exclusive and more common in widely invasive OCAs than in minimally invasive cases (16, 20, 21).
Treatment decisions for elderly patients often prioritize conservative approaches due to shorter life expectancy, comorbidities, and higher surgical risks. However, differentiated thyroid cancers in the elderly tend to be more aggressive, with higher proportions of poorly differentiated or undifferentiated thyroid cancers, leading to adverse clinical outcomes. Thyroid cancer is the only one that takes into account the variable age in the staging of the disease, the AJCC Cancer Staging Manual (8th edition) designates an age of 55 or older as a high-risk prognostic factor. However, the lack of tools to predict high-risk thyroid malignancies in elderly individuals, especially those who are not suitable for biopsy, contributes to limited therapeutic strategies.
In the context of treatment, younger patients often pursue aggressive approaches to avoid future tumor progression or metastasis. Conversely, elderly patients, due to shorter life expectancy and comorbidities, face elevated surgical risks. Consequently, current guidelines advocate a conservative approach to assessing and treating thyroid nodules and thyroid cancer in elderly patients (22). Studies by Park also indicate a lower proportion of elderly patients opting for total thyroidectomy (23). However, among elderly individuals, differentiated thyroid cancer often exhibits greater invasiveness, and a higher proportion of poorly differentiated thyroid cancer is observed. Furthermore, elderly patients often demonstrate lesser concern about the development and progression of their tumors. Some patients may even present with clinical complications like airway obstruction or nerve damage due to tumor progression, leading to unfavorable clinical outcomes (24). Yet, assessing the prognostic risk of thyroid cancer in elderly patients remains limited, especially when diagnostic lobectomy is not suitable due to the patient’s condition, and methods for predicting high-risk tumors and applying such experience remain considerably constrained (25).
Our patient had discovered a neck mass two years before the presentation. Ultrasound showed an enlarged left thyroid lobe with a solid nodule measuring 58×40×31 mm (TIRADS-3). Although FNAB was indicated, the patient’s advanced age and associated risks led to the decision to forego further diagnostic procedures. Retrospective studies have shown that FNAB of thyroid nodules in patients aged ≥70 is safe and accurate for precise diagnosis (26). If FNAB had been conducted in this case, Bethesda Category III or IV, “follicular neoplasm” or “suspicious for follicular neoplasm,” might have been indicated. Even when US-CNB was performed, it was not possible to distinguish between benign and malignant based on the reason for sampling. Therefore, treatment decisions for surgery may still not be made. However, TERT promoter mutational detection through molecular testing could have influenced treatment decisions by identifying potential malignant or precancerous oncocytic tumors, thus preventing progression and asphyxiation risk. The challenge in diagnosing thyroid nodules in the elderly is to screen for high-risk thyroid cancers, which is important for improving their prognosis. Cytopathology or even coarse needle biopsy is often insufficient (as in this case), and molecular testing may provide more assistance. To our knowledge, this is the first case of OCA with TERT promoter mutation as the initial presentation of asphyxia. This case aims to share the experience and contribute insights regarding the diagnosis and treatment of thyroid nodules in the elderly population.
In conclusion, this case highlights the successful management of a rapidly progressing thyroid tumor in an elderly patient. Swift surgical intervention, tailored to the patient’s age and comorbidities, led to a positive outcome. Molecular testing played a crucial role in identifying TERT promoter mutations. Despite postoperative hoarseness, the patient’s respiratory distress was resolved, underscoring the importance of individualized treatment for challenging thyroid nodules in elderly individuals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Tianjin Medical University General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XW: Writing – original draft, Writing – review & editing, Conceptualization. YL: Writing – original draft, Writing – review & editing, Conceptualization. LC: Resources, Writing – review & editing, Investigation. JZ (4th author): Resources, Writing – review & editing, Investigation. RJ: Resources, Writing – review & editing, Investigation. LZ: Investigation, Writing – review & editing, Project administration. HY: Supervision, Writing – review & editing, Conceptualization, Project administration. JZ (8th author): Supervision, Writing – review & editing, Conceptualization, Project administration.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
Author LZ and HY was employed by the company Dian Diagnostics Group Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1349114/full#supplementary-material
Abbreviations
OCA, oncocytic thyroid carcinoma; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; ATC, anaplastic thyroid carcinoma; PTC, papillary thyroid carcinoma; ICU, Intensive Care Unit; US-CNB, ultrasound-guided coarse needle biopsy; TERT, Telomerase reverse transcriptase; BRAF, v-Raf murine sarcoma viral oncogene homolog B; HRAS, Harvey rat sarcoma viral oncogene homolog; KRAS, Kirsten rat sarcoma viral oncogene homolog; NRAS, Neuroblastoma RAS viral oncogene homolog; TP53, Tumor protein 53; RET, Rearranged during transfection proto-oncogene; NTRK1, Neurotrophic receptor tyrosine kinase 1; NTRK3, Neurotrophic receptor tyrosine kinase 3; PAX8, Paired box gene 8; THADA, Thyroid adenoma associated; CK19, Cytokeratin 19; TG, Thyroglobulin; TTF-1, Thyroid transcription factor 1; CD56, Cluster of differentiation 56; TPO, Thyroid peroxidase; CD34, Cluster of differentiation 34; HCC, Hürthle cell carcinoma; WHO, World Health Organization; FNAB, fine-needle aspiration biopsy; FN/SFN, Follicular neoplasm or suspicious for follicular neoplasm; AUS/FLUS, Atypia of undetermined significance or follicular lesion of undetermined significance; SM, Suspicious for malignancy; NCCN, National Comprehensive Cancer Network; CT, computed tomography; MRI, magnetic resonance imaging; DNA, Deoxyribonucleic acid; RAS, Rat sarcoma viral oncogene; EIF1AX, Eukaryotic translation initiation factor 1A, X-linked; TP53, Tumor protein 53; NF1, Neurofibromatosis type 1; CDKN1A, Cyclin-dependent kinase inhibitor 1A; FTA, Follicular thyroid adenomas; AJCC, American Joint Committee on Cancer.
References
1. Lin JD, Chao TC, Chen ST, Weng HF, Lin KD. Characteristics of thyroid carcinomas in aging patients. Eur J Clin Invest. (2000) 30:147–53. doi: 10.1046/j.1365-2362.2000.00598.x
2. Girardi FM. Thyroid carcinoma pattern presentation according to age. Int Arch Otorhinolaryngol. (2017) 21:38–41. doi: 10.1055/s-0036-1585095
3. Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973-1991. Cancer. (1997) 79:564–73. doi: 10.1002/(ISSN)1097-0142
4. Ospina NS, Papaleontiou M. Thyroid nodule evaluation and management in older adults: A review of practical considerations for clinical endocrinologists. Endocr Pract. (2021) 27:261–8. doi: 10.1016/j.eprac.2021.02.003
5. Stojadinovic A, Hoos A, Ghossein RA, Urist MJ, Leung DH, Spiro RH, et al. Hürthle cell carcinoma: a 60-year experience. Ann Surg Oncol. (2002) 9:197–203. doi: 10.1007/BF02557374
6. Goffredo P, Roman SA, Sosa JA. Hurthle cell carcinoma: a population-level analysis of 3311 patients. Cancer. (2013) 119:504–11. doi: 10.1002/cncr.27770
7. Nagar S, Aschebrook-Kilfoy B, Kaplan EL, Angelos P, Grogan RH. Hurthle cell carcinoma: an update on survival over the last 35 years. Surgery. (2013) 154:1263–71. doi: 10.1016/j.surg.2013.06.029
8. Bhattacharyya N. Survival and prognosis in Hürthle cell carcinoma of the thyroid gland. Arch Otolaryngol Head Neck Surg. (2003) 129:207–10. doi: 10.1001/archotol.129.2.207
9. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. (2022) 33:27–63. doi: 10.1007/s12022-022-09707-3
10. Haigh PI, Urbach DR. The treatment and prognosis of Hürthle cell follicular thyroid carcinoma compared with its non-Hürthle cell counterpart. Surgery. (2005) 138:1152–7. doi: 10.1016/j.surg.2005.08.034
11. Dahl LD, Myssiorek D, Heller KS. Hurthle cell neoplasms of the thyroid. Laryngoscope. (2002) 112:2178–80. doi: 10.1097/00005537-200212000-00009
12. Jin M, Kim ES, Kim BH, Kim HK, Kang YE, Jeon MJ, et al. Clinicopathological characteristics and disease-free survival in patients with hürthle cell carcinoma: A multicenter cohort study in South Korea. Endocrinol Metab (Seoul). (2021) 36:1078–85. doi: 10.3803/EnM.2021.1151
13. Sengul I, Sengul D. Comment on: "Evaluating treatment options in managing thyroid nodules with indeterminate cytology of TBSRTC in thyroidology: addendum aut non? Rev Assoc Med Bras. (1992) 68(2022):973–4. doi: 10.1590/1806-9282.20220383
14. Sengul I, Sengul D. Blurred lines for management of thyroid nodules in the era of atypia of undetermined significance/ follicular lesion of undetermined significance: novel subdivisions of categories IIIA and IIIB in a possible forthcoming The Bethesda System for Reporting Thyroid Cytopathology, 3rd edition; amending versus unnecessary? Rev Assoc Med Bras. (1992) 67:1385–6. doi: 10.1590/1806-9282.20210763
15. Sengul I, Sengul D. Focusing on thyroid nodules in suspense: 10-15 mm with repeat cytology, Category III, the Bethesda System for Reporting Thyroid Cytopathology, TBSRTC. Rev Assoc Med Bras. (1992) 67:166–7. doi: 10.1590/1806-9282.67.02.20200828
16. Ganly I, Makarov V, Deraje S, Dong Y, Reznik E, Seshan V, et al. Integrated genomic analysis of hürthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell. (2018) 34:256–270.e5. doi: 10.1016/j.ccell.2018.07.002
17. Gopal RK, Kübler K, Calvo SE, Polak P, Livitz D, Rosebrock D, et al. Widespread chromosomal losses and mitochondrial DNA alterations as genetic drivers in hürthle cell carcinoma. Cancer Cell. (2018) 34:242–55.e5. doi: 10.1016/j.ccell.2018.06.013
18. Ganly I, Ricarte Filho J, Eng S, Ghossein R, Morris LG, Liang Y, et al. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid Malignancy. J Clin Endocrinol Metab. (2013) 98:E962–72. doi: 10.1210/jc.2012-3539
19. Wang N, Liu T, Sofiadis A, Juhlin CC, Zedenius J, Höög A, et al. TERT promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (FTA) and atypical FTA. Cancer. (2014) 120:2965–79. doi: 10.1002/cncr.28800
20. Landa I, Ganly I, Chan TA, Mitsutake N, Matsuse M, Ibrahimpasic T, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. (2013) 98:E1562–6. doi: 10.1210/jc.2013-2383
21. Chindris AM, Casler JD, Bernet VJ, Rivera M, Thomas C, Kachergus JM, et al. Clinical and molecular features of Hürthle cell carcinoma of the thyroid. J Clin Endocrinol Metab. (2015) 100:55–62. doi: 10.1210/jc.2014-1634
22. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2015) 26:1–133. doi: 10.1089/thy.2015.0020
23. Park HS, Roman SA, Sosa JA. Treatment patterns of aging Americans with differentiated thyroid cancer. Cancer. (2010) 116:20–30. doi: 10.1002/cncr.24717
24. Syed A, Vanka SA, Escudero I, Ismail R, Krayem H. Oncocytic cell carcinoma of the thyroid: A case report and an overview of the diagnosis, treatment modalities, and prognosis. Cureus. (2022) 14:e30298. doi: 10.7759/cureus.30298
25. McLeod DS, Carruthers K, Kevat DA. Optimal differentiated thyroid cancer management in the elderly. Drugs Aging. (2015) 32:283–94. doi: 10.1007/s40266-015-0256-y
Keywords: thyroid neoplasms, carcinoma, asphyxia, mutation, aged
Citation: Wang X, Liu Y, Chen L, Zhang J, Jiang R, Zhang L, Yan H and Zhang J (2024) Oncocytic cell carcinoma of the thyroid with TERT promoter mutation presenting as asphyxia in an elderly: a case report. Front. Endocrinol. 15:1349114. doi: 10.3389/fendo.2024.1349114
Received: 04 December 2023; Accepted: 29 July 2024;
Published: 16 August 2024.
Edited by:
Vladimir Neychev, University of Central Florida, United StatesReviewed by:
Demet Sengul, Giresun University, TürkiyeBarbara Maria Jarzab, Maria Skłodowska-Curie National Research Institute of Oncology, Poland
Copyright © 2024 Wang, Liu, Chen, Zhang, Jiang, Zhang, Yan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, emhhbmdsZWkzQGRhemQuY24=; Han Yan, eWFuaGFuODkwMzI5QDEyNi5jb20=; Jie Zhang, emp1bHRyYTIwMDJAdG11LmVkdS5jbg==
†These authors share first authorship
‡These authors share last authorship
 Xiqian Wang1†
Xiqian Wang1† Lei Zhang
Lei Zhang Han Yan
Han Yan