
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 27 February 2024
Sec. Thyroid Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1349041
This article is part of the Research Topic Papillary Thyroid Cancer: Prognostic Factors and Risk Assessment View all 21 articles
Background: Thyroglobulin antibody (TgAb) has been found to be associated with the occurrence and development of differentiated thyroid cancer (DTC) for several years, but there is still controversy over whether thyroid peroxidase antibody (TPOAb) is related to differentiated thyroid cancer.
Methods: We scrutinized relevant studies published up to July 2023 across four major databases including PubMed, Embase, Cochrane Library, and Web of Science, to examine the association between TPOAb and DTC. Clinical outcome measures include the incidence of DTC, tumor size, extrathyroidal invasion, lymph node metastasis, multifocality, recurrence and bilaterality.
Results: 12 original studies were included, involving a total of 20,330 subjects. Our analysis of the included studies revealed that TPOAb+ individuals exhibited a higher risk of developing DTC (OR=1.57 [95% CI: 1.00–2.45], p=0.049) than TPOAb– individuals. Furthermore, TPOAb+ DTC patients were more prone to present with bilateral (OR=1.40 [95% CI: 1.21–1.62], p<0.00001) and multifocal (OR=1.40 [95% CI: 1.23-1.60], p<0.00001) tumors than TPOAb– patients. Sensitivity analysis indicated a high sensitivity for these three findings. No significant differences in the risk of extrathyroidal extension and lymph node metastasis, recurrence rate, tumor size, were observed between TPOAb+ and TPOAb– DTC patients.
Conclusion: The presence of TPOAb is correlated with an increase prevalence of DTC. However, its effectiveness as a prognostic marker for DTC patients warrants further investigation.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023448824.
Thyroid cancer is one of the most prevalent endocrine malignancies, with its annual incidence increasing in numerous countries and regions. However, the mortality rate remains consistently low (1, 2). Differentiated thyroid cancer (DTC) is the most frequently diagnosed type, accounting for over 95% of cases (3). DTC comprises two main subtypes, with papillary thyroid cancer (PTC) account for 90% of cases and follicular thyroid cancer accounting for 7–8% (4–6). In recent years, increasing studies have reported the association of thyroid cancer with various risk factors, including radiation exposure, environmental factors, iodine intake, serum TSH levels and Hashimoto’s thyroiditis (HT) (7–9). Among these factors, thyroid autoimmunity is closely linked to the development of DTC.
Thyroid peroxidase (TPO), an enzyme located in the apical membrane of thyroid follicular cells, is involved in the biosynthesis of thyroid hormone (10). Thyroid peroxidase antibody (TPOAb) is predominantly produced by lymphocytes infiltrating the thyroid gland and is one of the most common autoantibodies against the thyroid (11). It can cause thyroid cell damage through the activation of the complement system and cell cytotoxicity (12, 13). TPOAb is a hallmark thyroid autoimmune antibody in autoimmune thyroiditis (AIT) (14, 15). It is also intricately linked to several other diseases. In 1998, Smyth et al. observed significantly higher TPOAb positivity in breast cancer patients than in those with benign breast disease. Some studies have shown that TPO is expressed in breast cancer and peri-cancerous tissues, and the antigenic and biochemical properties of TPO in breast tissues are similar to those in thyroid tissues, with only minor differences in post-translational modifications (16–18). Furthermore, disease-free and overall survival were longer in TPOAb+ breast cancer patients than in their TPOAb– breast cancer counterparts (19). Numerous subsequent studies have yield similar findings (20–22). Sharma and collaborators proposed that TPOAb might cross-react with lactoperoxidase expressed in breast tissue, potentially explaining the coexistence of TPOAb and breast cancer (23). Additionally, several studies have highlighted a strong association between TPOAb and various adverse pregnancy outcomes (24–26). Furthermore, two separate fine needle aspiration cytology studies from the same center identified TPOAb as an independent risk factor for thyroid malignancy (27, 28). Nonetheless, while many studies have explored the relationship between thyroglobulin antibody (TgAb), another serum marker of AIT, and DTC, the association between TPOAb and DTC remains unclear (29–31).
Previously, a variety of diseases or molecules have been found to be associated with the prognosis of DTC, such as graves’ disease, HT, thyrotropin, thyroglobulin (Tg), BRAF mutation, estrogen receptor and VEGF pathway, etc (32–38). And we aimed to conduct a comprehensive meta-analysis and systematic review to elucidate the relationship between TPOAb and DTC. By analyzing extensive large-scale research data, we expect to provide highly in-depth theoretical support for the clinical application of TPOAb in the early diagnosis, formulation of treatment strategies, and prognosis evaluation of DTC.
This systematic review was registered (CRD42023448824) in PROSPERO. We continue to update this systematic review with the latest information.
Our systematic literature search encompassed four databases: PubMed, Embase, Web of Science, and Cochrane Library, until July 2023. The search was conducted using the following terms: “thyroid peroxidase antibody” AND “papillary thyroid cancer” OR “follicular thyroid cancer” OR “Hürthle cell thyroid cancer” OR ‘‘differentiated thyroid cancer.” Supplementary Table S1 provides a comprehensive outline of the search strategy. We selected relevant articles through the evaluation of titles, abstracts, and full texts. Two researchers independently made the selections and reviewed the abstracts and full texts. In instances where multiple original studies involving the same population were published, we selected the most recent and comprehensive study.
Studies that met the following criteria were included (1): prospective, retrospective, randomized controlled trial, or case-control study types (2), investigation of TPOAb levels in patients diagnosed with DTC (3), classification of patients based on their TPOAb levels (4), availability of relevant data, and (5) publication in English.
Studies that meet any of the following criteria were excluded (1): review articles, letters, comments, editorials, case reports, or laboratory-animal research (2), incomplete data or unavailability of raw data, or (3) duplication of studies originating from the same dataset.
Two researchers independently screened articles, with any disputes resolved through negotiation or with the assistance of a third author.
We extracted the following information from each included article (1): last name of first author (2), publication year (3), study period (4), country (5), study type (6), sample size (7), total number of TPOAb± patients (8), mean tumor sizes in TPOAb± groups (9), lymph node metastasis in TPOAb± groups (10), extrathyroidal extension in TPOAb± groups (11), tumor multifocality in TPOAb± groups (12), tumor bilaterality in TPOAb± groups, and (13) cancer recurrence in TPOAb± groups.
In this meta-analysis, two reviewers used the Newcastle Ottawa quality assessment scale (NOS) to evaluate the quality of the included studies. The NOS assesses the selection of study subjects, comparability between groups, and measurement or exposure of outcomes. Each study received a score ranging from 0 to 9 points. Studies with scores exceeding 6 were considered high quality, those with scores between 4 and 6 were considered moderate quality, and those with scores below 4 were considered low quality. Any discrepancies or inconsistencies were resolved through consensus with a third author.
We conducted statistical analyses using Review Manager 5.4 (Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration) and Stata 14 (StataCorp LP, College Station, Texas). The forest plots were generated using Review Manager 5.4. Odds ratio (OR) with a 95% confidence interval (Cl) was used to assess the strength of the association between TPOAb and DTC. Weighted mean difference and 95% Cl were calculated for continuous outcomes. In all meta-analyses, the Cochrane Q p-value and l² statistic were used to assess heterogeneity. A random-effect model was employed to merge results when the p-value was < 0.05 or I² > 50%, indicating significant heterogeneity; otherwise, a fixed-effect model was used. Statistical significance was set at p < 0.05 was considered statistically significant. Publication bias was assessed using Egger test plots in Stata 14.
We conducted a comprehensive literature search using PubMed, Embase, Cochrane Library, and Web of Science databases as well as by examining references in relevant reviews up to July 2023. Initially, we identified 878 records. After removing 218 duplicated articles, we screened titles and abstracts, resulting in the selection of 40 studies out of 660 articles. Following a full-text review and application of inclusion and exclusion criteria, we included 12 original studies in the systematic review and meta-analysis. A visual representation of this selection process, following the PRISMA guidelines, is provided in Figure 1.
The key characteristics of the 12 eligible studies are summarized in Table 1 (39–50). These 12 case-control studies encompassed 20,330 participants across the United States, China, Greece, Serbia, Korea, and Turkey. We categorized nine studies as having moderate quality and three as high quality. The sample size of the included studies ranged from 179 to 5770. We explored DTC prevalence, tumor size, extrathyroidal extension, lymph node metastasis, tumor multifocality, tumor bilaterality, and cancer recurrence between TPOAb+ and TPOAb– patients across these studies.
To explore the association between TPOAb positivity and DTC prevalence, nine studies (39, 41–45, 48–50) were included. DTC prevalence was 62% among 2141 TPOAb+ patients and 46% among 9837 TPOAb– patients. However, no significant association between different TPOAb levels and the risk of developing DTC (OR=1.57 [95% CI: 1.00–2.45], p=0.05) was shown in the forest plot (Figure 2). As the p-value of 0.05 falls exactly on the critical threshold, repeated calculations were conducted using Stata 14, and a value of p=0.049 was ascertained. Egger’s regression model did not indicate any publication bias (p=0.45), but a high level of heterogeneity was observed.
In our meta-analysis, two studies (42, 44) were selected to compare tumor size with various TPOAb levels. The results did not reveal a significant relationship between different TPOAb levels and tumor size (p=0.54; Figure 3A). However, the number of studies was insufficient for assessing publication bias, and a high level of heterogeneity was observed.
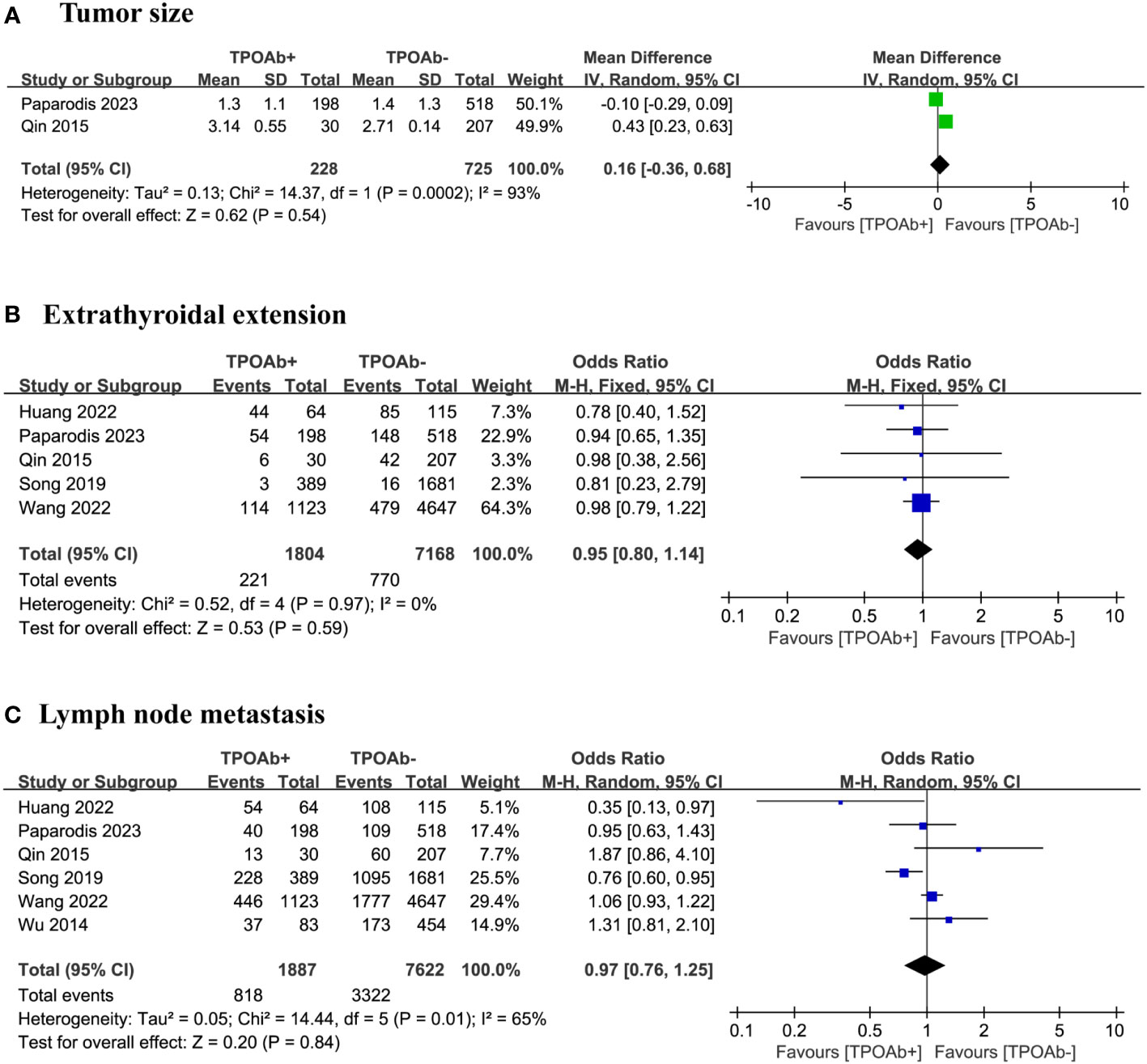
Figure 3 TPOAb positivity and risk of (A) tumor size, (B) extrathyroidal extension and (C) lymph node metastasis.
Our analysis focused on five studies (40, 42, 44, 46, 47) to determine whether the risk of extrathyroidal extension differs among DTC patients with various TPOAb levels. Among the 1804 TPOAb+ and 7168 TPOAb– patients, little difference in the risk of extrathyroidal extension was found, 12% and 11%, respectively. The forest plot did not indicate a significant association between different TPOAb levels and the risk of extrathyroidal extension (OR=0.95 [95% CI: 0.80–1.14], p=0.59; Figure 3B). Egger’s regression model showed no publication bias (p=0.19), and a low level of heterogeneity was observed.
We included six studies (40, 42, 44, 46–48) to explore the relationship between TPOAb positivity in DTC patients and the risk of lymph node metastasis. Among the 1887 TPOAb+ and 7662 TPOAb– patients, little difference in the risk of lymph node metastasis was found, 43% and 44%, respectively. The forest plot did not show a significant association between different TPOAb levels and the risk of lymph node metastasis (OR=0.97 [95%CI 0.76-1.25] p=0.84; Figure 3C). Egger’s regression model indicated no publication bias (p=0.82), and a moderate level of heterogeneity was observed.
We included three studies (40, 44, 47) to examine the association between TPOAb positivity and the risk of tumor multifocality in DTC patients. Multifocal tumors occurred in 38% of the 1217 TPOAb+ patients compared with 31% of the 4969 TPOAb– patients. The forest plot indicate the risk of tumor multifocality in TPOAb+ patients was significantly higher than that in TPOAb– patients (OR=1.40 [95% CI: 1.23–1.60], p<0.00001; Figure 4A). Egger’s regression model indicated no publication bias (p=0.05), and a low level of heterogeneity was observed.
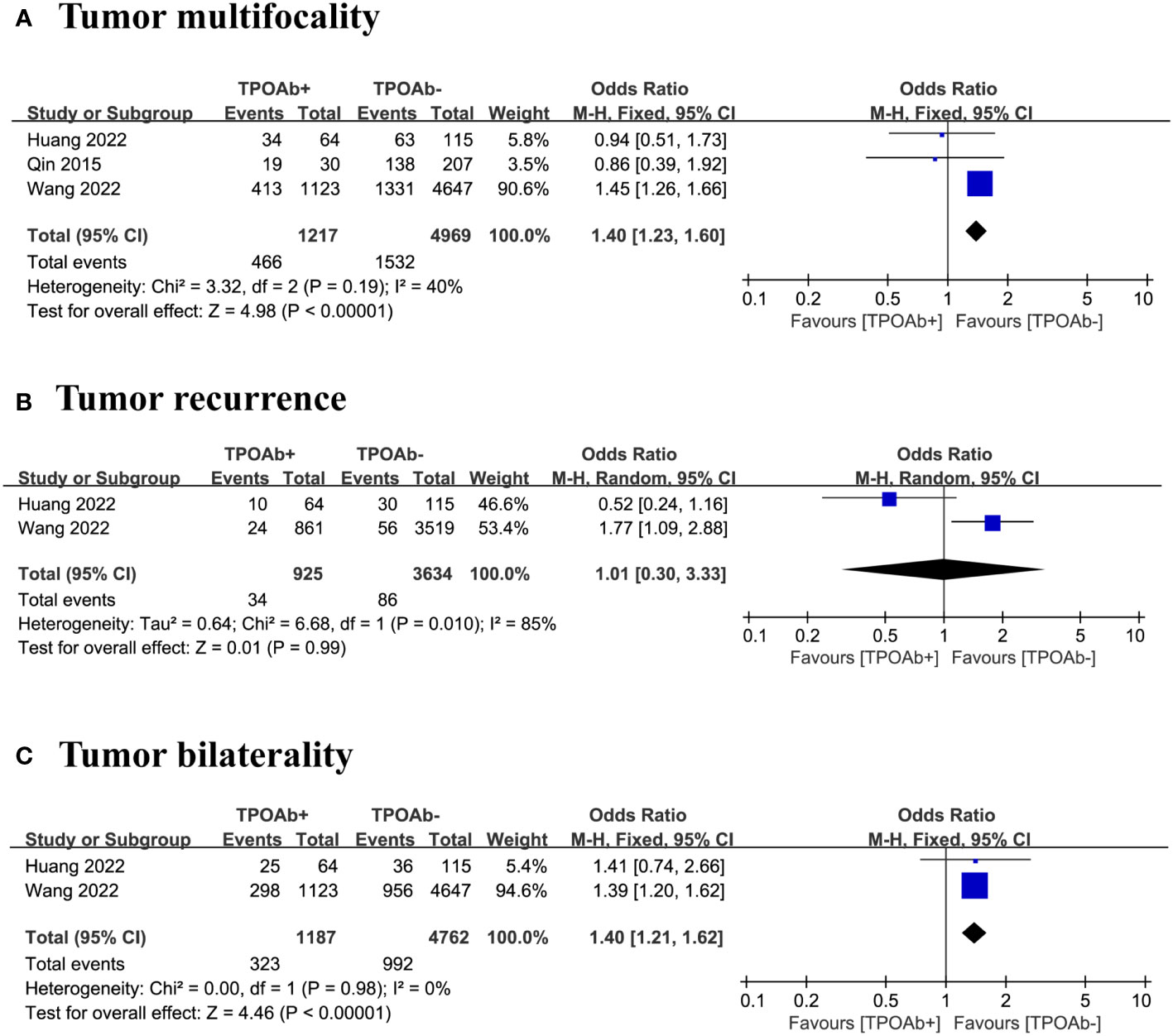
Figure 4 TPOAb positivity and risk of (A) tumor multifocality, (B) tumor recurrence and (C) tumor bilaterality.
Our analysis focused on two studies (40, 47) to evaluate the association between TPOAb positivity and the risk of tumor recurrence in DTC patients. Tumor recurrence occurred in 4% of the 925 TPOAb+ patients compared with 2% of the 3634 TPOAb– patients. The forest plot did not reveal a significant relationship between different TPOAb levels and the risk of tumor recurrence (OR=1.01 [95% CI: 0.30–3.33], p=0.99; Figure 4B). However, the number of studies was insufficient for assessing publication bias, and a high level of heterogeneity was observed.
We included three studies (40, 47) to evaluate the association between TPOAb positivity and the risk of tumor bilaterality in DTC patients. Of the 1187 TPOAb+ patients, 27% had bilateral tumors compared with 21% of the 4762 TPOAb– patients. The risk of tumor bilaterality in TPOAb+ patients was significantly higher than that in TPOAb– patients (OR=1.40 [95% CI: 1.21–1.62], p<0.00001; Figure 4C). However, the number of studies is insufficient for assessing publication bias, and a low level of heterogeneity was observed.
We conducted a sensitivity analysis to assess the stability of the results, systematically excluding each article and performing a meta-analysis on the remaining literature. Except for the high sensitivity of the results for TPOAb positivity and DTC prevalence and its association with the risk of bilateral and multifocal tumors of DTC, the remaining findings exhibited no substantial alterations, suggesting some degree of result instability.
Our meta-analysis included 12 original studies encompassing 20,330 patients from six countries for investigating associations between TPOAb and DTC prevalence, along with various prognostic factors. Our findings reveal that TPOAb+ individuals were at a higher risk of developing DTC than TPOAb– individuals (p=0.049). Furthermore, TPOAb+ DTC patients were more likely than TPOAb– DTC patients to present with multifocal and bilateral tumors, and this difference was statistically significant. However, no significant difference in tumor size, recurrence rate, risk of extrathyroidal extension, and lymph node metastasis was observed between TPOAb+ and TPOAb– DTC patients.
We observed an association between TPOAb and DTC prevalence, but the results may be subject to controversy. Upon analysis using Review Manager, we obtained a calculated p-value of 0.05, while recalculation using Stata 14 yielded a p-value of 0.049, which was statistically significant. This discrepancy might be because Review Manager rounded the p-value, and ultimately, the p-value was determined to be 0.049. Nevertheless, both the heterogeneity and sensitivity of this result were relatively high. Several factors may account for this observed heterogeneity. First, variations in the cutoff values for defining TPOAb positivity were evident, ranging from a minimum of >5.61 IU/mL to a maximum of >100 IU/mL. Second, differences existed in the methods and instruments used for TPOAb determination. Third, differences in the study populations across diverse regions may have contributed to heterogeneity. Lastly, the presence or absence of AIT in TPOAb+ patients could have influenced the results. The available data were not sufficient for a comprehensive subgroup analysis. Furthermore, the observed sensitivity issues were likely related to ethnic and regional differences. In the nine included articles, three studies contributing to the elevated sensitivity were from joint research conducted between the United States and Greece, Serbia, and Turkey, while the remaining six studies were conducted in China. Located in Asia, China differs geographically from the other four countries primarily situated in Europe and North America. As DTC prevalence varies across regions, geographic factors might play a pivotal role (51, 52). Although the p-value was <0.05, this does not inherently guarantee result stability or high reliability. Further validation through additional independent studies is essential to establish the reliability of the results.
We initially observed that TPOAb+ DTC patients are more likely to exhibit bilateral and multifocal tumors. However, Wang’s study (47) with a considerably higher weight share in both results had a significant influence. Upon exclusion of the Wang’s study, we found no significant association between TPOAb and DTC multifocality (OR=0.91 [95% CI: 0.56–1.48], p=0.70) and bilaterality (OR=1.41 [95% CI: 0.74–2.66], p=0.29). Consequently, no conclusive significant association was established in this study, emphasizing the need for further exploration of the association of TPOAb positivity with DTC multifocality and bilaterality. Moreover, we found the TPOAb+ and TPOAb– DTC patients exhibited no significant differences in tumor size, recurrence rate, risk of extrathyroidal extension, and lymph node metastasis. Nevertheless, several studies have reported that TgAb+ DTC patients face a higher risk of lymph node metastasis and recurrence (53–57).
Some studies have demonstrated that persistent or recurrent thyroid cancer can induce the production of TgAb, so the time of TPOAb detection is particularly important (58, 59). Other studies have indicated an association between positive TPOAb results 7-10 years before diagnosis and thyroid cancer. However, most of the original studies included in this analysis conducted TPOAb testing prior to surgery without specifying the exact duration of TPOAb positivity before this period, potentially impacting the credibility of the results (60). Further large prospective studies are necessary for validation. Additionally, if patients were identified with thyroid cancer due to testing positive for TPOAb and subsequently included in the selection process, it could have led to an increased rate of DTC diagnosis in TPOAb+ patients, potentially introducing selection bias. Moreover, because patients with autoimmune thyroid diseases tend to be more vigilant about their thyroid function, they may detect small subclinical tumors during ongoing medical surveillance. In contrast, patients without related diseases might not undergo continuous surveillance, potentially leading to the undiagnosed status of some subclinical tumors. This discrepancy could also introduce bias into the results.
Given that TPOAb is a vital diagnostic marker of HT, considering the influence of AIT when examining the association between TPOAb and DTC is necessary. HT is known to cause substantial immune cell infiltration in the thyroid gland (61), and similar immune cell infiltration is observed around DTC (62, 63). The potential interplay between the two and its effect on DTC progression and prognosis pose intriguing questions. HT appears to be linked to an increased risk of thyroid cancer (64). When it coexists with thyroid cancer, it seems to further elevate the likelihood of thyroid cancer development (64–66). However, it may confer a protective effect against lymph node metastasis, extrathyroidal extension, and distant metastasis (64, 67, 68). Additionally, some studies have shown that immune cell infiltration surrounding PTC resulting from HT is positively correlated with a lower recurrence rate, higher overall survival, and reduced risk of extrathyroidal extension and distant metastasis (69, 70). Moreover, some studies have found that HT is associated with smaller tumor size, lower rate of aggressive PTC variants and lower risk, and DTC patients with HT have a higher clinical remission rate and longer recurrence-free survival (71, 72). However, the accuracy of “non-HT status” as a negative prognostic marker is poor, and it cannot improve the specificity of predicting prognosis. In one meta-analysis, TgAb was distinguished from HT, and TgAb+ patients with HT exhibited larger tumor sizes and a higher risk of extrathyroidal extension, tumor multifocality, lymph node metastasis, and cancer persistence than those without HT (29). Therefore, although HT can promote the development of DTC, it seems to be a protective factor for the prognosis of DTC. However, if we consider the single effect of TgAb positivity without considering HT, it seems to become a risk factor. The studies included in our meta-analysis did not differentiate TPOAb from HT, making it challenging to determine whether our conclusions might have been confounded by HT. Further research is warranted to reach a more definitive conclusion.
Our analysis indicates that TPOAb positivity may be associated with an increased prevalence of DTC. However, TPOAb does not serve as a robust prognostic factor for TPOAb+ DTC patients. Our meta-analysis is based on a limited number of included studies, and we anticipate that future research will provide additional large-scale research data to further inform our understanding.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
HZ: Writing – original draft. LT: Writing – review & editing. XW: Writing – review & editing. XS: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by National Natural Science Foundation of China (Grant No. 82170804).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1349041/full#supplementary-material
1. Chen DW, Lang B, McLeod D, Newbold K, Haymart MR. Thyroid cancer. Lancet. (2023) 401:1531–44. doi: 10.1016/S0140-6736(23)00020-X
2. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. (2016) 12:646–53. doi: 10.1038/nrendo.2016.110
3. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. (2016) 388:2783–95. doi: 10.1016/S0140-6736(16)30172-6
4. Schlumberger M, Leboulleux S. Current practice in patients with differentiated thyroid cancer. Nat Rev Endocrinol. (2021) 17:176–88. doi: 10.1038/s41574-020-00448-z
5. Yip L, Sosa JA. Molecular-directed treatment of differentiated thyroid cancer: advances in diagnosis and treatment. JAMA Surg. (2016) 151:663–70. doi: 10.1001/jamasurg.2016.0825
6. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. (2022) 33:27–63. doi: 10.1007/s12022-022-09707-3
7. Bogović CT, Ilić TM, Girotto N, Grbac IS. Risk factors for thyroid cancer: what do we know so far? Acta Clin Croat. (2020) 59:66–72. doi: 10.20471/acc.2020.59.s1.08
8. Feldt-Rasmussen U. Hashimoto’s thyroiditis as a risk factor for thyroid cancer. Curr Opin Endocrinol Diabetes Obes. (2020) 27:364–71. doi: 10.1097/MED.0000000000000570
9. McLeod D, Zhang L, Durante C, Cooper DS. Contemporary debates in adult papillary thyroid cancer management. Endocr Rev. (2019) 40:1481–99. doi: 10.1210/er.2019-00085
10. Godlewska M, Banga PJ. Thyroid peroxidase as a dual active site enzyme: Focus on biosynthesis, hormonogenesis and thyroid disorders of autoimmunity and cancer. Biochimie. (2019) 160:34–45. doi: 10.1016/j.biochi.2019.02.003
11. Dhillon-Smith RK, Coomarasamy A. TPO antibody positivity and adverse pregnancy outcomes. Best Pract Res Clin Endocrinol Metab. (2020) 34:101433. doi: 10.1016/j.beem.2020.101433
12. Chardès T, Chapal N, Bresson D, Bès C, Giudicelli V, Lefranc MP, et al. The human anti-thyroid peroxidase autoantibody repertoire in Graves’ and Hashimoto’s autoimmune thyroid diseases. Immunogenetics. (2002) 54:141–57. doi: 10.1007/s00251-002-0453-9
13. Godlewska M, Gawel D, Buckle AM, Banga JP. Thyroid peroxidase revisited - what’s new? Horm Metab Res. (2019) 51:765–69. doi: 10.1055/a-1057-9469
14. Dwivedi SN, Kalaria T, Buch H. Thyroid autoantibodies. J Clin Pathol. (2023) 76:19–28. doi: 10.1136/jcp-2022-208290
15. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. (2017) 8:521. doi: 10.3389/fimmu.2017.00521
16. Godlewska M, Krasuska W, Czarnocka B. Biochemical properties of thyroid peroxidase (TPO) expressed in human breast and mammary-derived cell lines. PloS One. (2018) 13:e0193624. doi: 10.1371/journal.pone.0193624
17. Godlewska M, Arczewska KD, Rudzińska M, Łyczkowska A, Krasuska W, Hanusek K, et al. Thyroid peroxidase (TPO) expressed in thyroid and breast tissues shows similar antigenic properties. PloS One. (2017) 12:e0179066. doi: 10.1371/journal.pone.0179066
18. Muller I, Giani C, Zhang L, Grennan-Jones FA, Fiore E, Belardi V, et al. Does thyroid peroxidase provide an antigenic link between thyroid autoimmunity and breast cancer? Int J Cancer. (2014) 134:1706–14. doi: 10.1002/ijc.28493
19. Smyth PP, Shering SG, Kilbane MT, Murray MJ, McDermott EW, Smith DF, et al. Serum thyroid peroxidase autoantibodies, thyroid volume, and outcome in breast carcinoma. J Clin Endocrinol Metab. (1998) 83:2711–16. doi: 10.1210/jcem.83.8.5049
20. Jiskra J, Barkmanova J, Limanova Z, Lánská V, Smutek D, Potlukova E, et al. Thyroid autoimmunity occurs more frequently in women with breast cancer compared to women with colorectal cancer and controls but it has no impact on relapse-free and overall survival. Oncol Rep. (2007) 18:1603–11. doi: 10.3892/or.18.6.1603
21. Shi XZ, Jin X, Xu P, Shen HM. Relationship between breast cancer and levels of serum thyroid hormones and antibodies: a meta-analysis. Asian Pac J Cancer Prev. (2014) 15:6643–47. doi: 10.7314/apjcp.2014.15.16.6643
22. Turken O, NarIn Y, DemIrbas S, Onde ME, Sayan O, KandemIr EG, et al. Breast cancer in association with thyroid disorders. Breast Cancer Res. (2003) 5:R110–13. doi: 10.1186/bcr609
23. Sharma S, Singh AK, Kaushik S, Sinha M, Singh RP, Sharma P, et al. Lactoperoxidase: structural insights into the function,ligand binding and inhibition. Int J Biochem Mol Biol. (2013) 4:108–28.
24. Abbassi-Ghanavati M, Casey BM, Spong CY, McIntire DD, Halvorson LM, Cunningham FG. Pregnancy outcomes in women with thyroid peroxidase antibodies. Obstet Gynecol. (2010) 116:381–86. doi: 10.1097/AOG.0b013e3181e904e5
25. Liu H, Shan Z, Li C, Mao J, Xie X, Wang W, et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid. (2014) 24:1642–49. doi: 10.1089/thy.2014.0029
26. Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyreroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. Bmj. (2011) 342:d2616. doi: 10.1136/bmj.d2616
27. Boi F, Lai ML, Marziani B, Minerba L, Faa G, Mariotti S. High prevalence of suspicious cytology in thyroid nodules associated with positive thyroid autoantibodies. Eur J Endocrinol. (2005) 153:637–42. doi: 10.1530/eje.1.02020
28. Boi F, Minerba L, Lai ML, Marziani B, Figus B, Spanu F, et al. Both thyroid autoimmunity and increased serum TSH are independent risk factors for Malignancy in patients with thyroid nodules. J Endocrinol Invest. (2013) 36:313–20. doi: 10.3275/8579
29. Lee Z, Eslick GD, Edirimanne S. Investigating antithyroglobulin antibody as a prognostic marker for differentiated thyroid cancer: A meta-analysis and systematic review. Thyroid. (2020) 30:1601–12. doi: 10.1089/thy.2019.0368
30. Spencer C, Fatemi S. Thyroglobulin antibody (TgAb) methods - Strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. (2013) 27:701–12. doi: 10.1016/j.beem.2013.07.003
31. Viola N, Agate L, Caprio S, Lorusso L, Brancatella A, Ricci D, et al. Thyroid autoimmunity, thyroglobulin autoantibodies, and thyroid cancer prognosis. Endocr Relat Cancer. (2023) 30. doi: 10.1530/ERC-23-0042
32. Boucai L, Seshan V, Williams M, Knauf JA, Saqcena M, Ghossein RA, et al. Characterization of subtypes of BRAF-mutant papillary thyroid cancer defined by their thyroid differentiation score. J Clin Endocrinol Metab. (2022) 107:1030–39. doi: 10.1210/clinem/dgab851
33. Kim H, Jung J, Cho YS, Choi JY, Park H, Lee YB, et al. Pattern analysis for prognosis of differentiated thyroid cancer according to preoperative serum thyrotropin levels. Sci Rep. (2021) 11:22322. doi: 10.1038/s41598-021-01898-9
34. Song Y, Fu L, Wang P, Sun N, Qiu X, Li J, et al. Effect of Graves’ disease on the prognosis of differentiated thyroid carcinoma: a meta-analysis. Endocrine. (2020) 67:516–25. doi: 10.1007/s12020-019-02111-8
35. Barres B, Kelly A, Kwiatkowski F, Batisse-Lignier M, Fouilhoux G, Aubert B, et al. Stimulated thyroglobulin and thyroglobulin reduction index predict excellent response in differentiated thyroid cancers. J Clin Endocrinol Metab. (2019) 104:3462–72. doi: 10.1210/jc.2018-02680
36. Marotta V, Sciammarella C, Capasso M, Testori A, Pivonello C, Chiofalo MG, et al. Germline polymorphisms of the VEGF pathway predict recurrence in nonadvanced differentiated thyroid cancer. J Clin Endocrinol Metab. (2017) 102:661–71. doi: 10.1210/jc.2016-2555
37. Heikkilä A, Hagström J, Mäenpää H, Louhimo J, Siironen P, Heiskanen I, et al. Loss of estrogen receptor Beta expression in follicular thyroid carcinoma predicts poor outcome. Thyroid. (2013) 23:456–65. doi: 10.1089/thy.2012.0363
38. Dvorkin S, Robenshtok E, Hirsch D, Strenov Y, Shimon I, Benbassat CA. Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting Hashimotos thyroiditis. J Clin Endocrinol Metab. (2013) 98:2409–14. doi: 10.1210/jc.2013-1309
39. Chen Z, Lin Y, Lai S, Wang P, Li J, Wang L, et al. The utility of serum anti-thyroglobulin antibody and thyroglobulin in the preoperative differential diagnosis of thyroid follicular neoplasms. Endocrine. (2022) 76:369–76. doi: 10.1007/s12020-022-02993-1
40. Huang D, Zhi J, Zhang J, Qin X, Zhao J, Zheng X, et al. Relationship between thyroid autoantibodies and recurrence of papillary thyroid carcinoma in children and adolescents. Front Oncol. (2022) 12:883591. doi: 10.3389/fonc.2022.883591
41. Li L, Shan T, Sun X, Lv B, Chen B, Liu N, et al. Positive thyroid peroxidase antibody and thyroglobulin antibody are associated with better clinicopathologic features of papillary thyroid cancer. Endocr Pract. (2021) 27:306–11. doi: 10.1016/j.eprac.2020.10.017
42. Paparodis R, Livadas S, Karvounis E, Bantouna D, Zoupas I, Angelopoulos N, et al. Elevated preoperative TPO ab titers decrease risk for DTC in a linear fashion: A retrospective analysis of 1,635 cases. J Clin Endocrinol Metab. (2023) 109:e347–e355. doi: 10.1210/clinem/dgad408
43. Peng X, Zhu X, Cheng F, Zhou B, Zhu X, Zhu L. Correlation between thyroid autoantibodies and the risk of thyroid papillary carcinoma. Gland Surg. (2020) 9:950–55. doi: 10.21037/gs-20-445
44. Qin J, Yu Z, Guan H, Shi L, Liu Y, Zhao N, et al. High thyroglobulin antibody levels increase the risk of differentiated thyroid carcinoma. Dis Markers. (2015) 2015:648670. doi: 10.1155/2015/648670
45. Selek A, Cetinarslan B, Tarkun I, Canturk Z, Ustuner B, Akyay Z. Thyroid autoimmunity: is really associated with papillary thyroid carcinoma? Eur Arch Otorhinolaryngol. (2017) 274:1677–81. doi: 10.1007/s00405-016-4414-6
46. Song E, Oh HS, Jeon MJ, Chung KW, Hong SJ, Ryu JS, et al. The value of preoperative antithyroidperoxidase antibody as a novel predictor of recurrence in papillary thyroid carcinoma. Int J Cancer. (2019) 144:1414–20. doi: 10.1002/ijc.31944
47. Wang W, Wen L, Chen S, Su X, Mao Z, Ding Y, et al. Preoperative thyroid peroxidase antibody predicts recurrence in papillary thyroid carcinoma: A consecutive study with 5,770 cases. Front Oncol. (2022) 12:881024. doi: 10.3389/fonc.2022.881024
48. Wu X, Lun Y, Jiang H, Gang Q, Xin S, Duan Z, et al. Coexistence of thyroglobulin antibodies and thyroid peroxidase antibodies correlates with elevated thyroid-stimulating hormone level and advanced tumor stage of papillary thyroid cancer. Endocrine. (2014) 46:554–60. doi: 10.1007/s12020-013-0121-x
49. Zeng R, Shou T, Yang KX, Shen T, Zhang JP, Zuo RX, et al. Papillary thyroid carcinoma risk factors in the Yunnan plateau of southwestern China. Ther Clin Risk Manag. (2016) 12:1065–74. doi: 10.2147/TCRM.S105023
50. Zorić GV, Nikolić-Djurović MM, Paunović IR, Diklić AD, Bukumirić ZM, Slijepčević NA, et al. Analysis of Malignancy predictors for follicular thyroid tumors. Vojnosanit Pregl. (2020) 77:282–88. doi: 10.2298/VSP180314079Z
51. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. (2022) 10:264–72. doi: 10.1016/S2213-8587(22)00035-3
52. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
53. Chung JK, Park YJ, Kim TY, So Y, Kim SK, Park DJ, et al. Clinical significance of elevated level of serum antithyroglobulin antibody in patients with differentiated thyroid cancer after thyroid ablation. Clin Endocrinol (Oxf). (2002) 57:215–21. doi: 10.1046/j.1365-2265.2002.01592.x
54. Kim HS, Choi YJ, Yun JS. Features of papillary thyroid microcarcinoma in the presence and absence of lymphocytic thyroiditis. Endocr Pathol. (2010) 21:149–53. doi: 10.1007/s12022-010-9124-9
55. Soyluk O, Boztepe H, Aral F, Alagol F, Özbey NC. Papillary thyroid carcinoma patients assessed to be at low or intermediary risk after primary treatment are at greater risk of long term recurrence if they are thyroglobulin antibody positive or do not have distinctly low thyroglobulin at initial assessment. Thyroid. (2011) 21:1301–08. doi: 10.1089/thy.2011.0122
56. Vasileiadis I, Boutzios G, Charitoudis G, Koukoulioti E, Karatzas T. Thyroglobulin antibodies could be a potential predictive marker for papillary thyroid carcinoma. Ann Surg Oncol. (2014) 21:2725–32. doi: 10.1245/s10434-014-3593-x
57. Wassner AJ, Della VM, Jarolim P, Feldman HA, Huang SA. Prevalence and significance of thyroglobulin antibodies in pediatric thyroid cancer. J Clin Endocrinol Metab. (2017) 102:3146–53. doi: 10.1210/jc.2017-00286
58. Spencer CA. Clinical review: Clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab. (2011) 96:3615–27. doi: 10.1210/jc.2011-1740
59. Spencer CA, Takeuchi M, Kazarosyan M, Wang CC, Guttler RB, Singer PA, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. (1998) 83:1121–27. doi: 10.1210/jcem.83.4.4683
60. McLeod D, Bedno SA, Cooper DS, Hutfless SM, Ippolito S, Jordan SJ, et al. Pre-existing thyroid autoimmunity and risk of papillary thyroid cancer: A nested case-control study of US active-duty personnel. J Clin Oncol. (2022) 40:2578–87. doi: 10.1200/JCO.21.02618
61. Ralli M, Angeletti D, Fiore M, D’Aguanno V, Lambiase A, Artico M, et al. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential Malignant transformation. Autoimmun Rev. (2020) 19:102649. doi: 10.1016/j.autrev.2020.102649
62. Ferrari SM, Fallahi P, Galdiero MR, Ruffilli I, Elia G, Ragusa F, et al. Immune and inflammatory cells in thyroid cancer microenvironment. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20184413
63. Menicali E, Guzzetti M, Morelli S, Moretti S, Puxeddu E. Immune landscape of thyroid cancers: new insights. Front Endocrinol (Lausanne). (2020) 11:637826. doi: 10.3389/fendo.2020.637826
64. Xu J, Ding K, Mu L, Huang J, Ye F, Peng Y, et al. Hashimoto’s thyroiditis: A “Double-edged sword” in thyroid carcinoma. Front Endocrinol (Lausanne). (2022) 13:801925. doi: 10.3389/fendo.2022.801925
65. Ehlers M, Schott M. Hashimoto’s thyroiditis and papillary thyroid cancer: are they immunologically linked? Trends Endocrinol Metab. (2014) 25:656–64. doi: 10.1016/j.tem.2014.09.001
66. Paparodis R, Imam S, Todorova-Koteva K, Staii A, Jaume JC. Hashimoto’s thyroiditis pathology and risk for thyroid cancer. Thyroid. (2014) 24:1107–14. doi: 10.1089/thy.2013.0588
67. Wang Y, Zheng J, Hu X, Chang Q, Qiao Y, Yao X, et al. A retrospective study of papillary thyroid carcinoma: Hashimoto’s thyroiditis as a protective biomarker for lymph node metastasis. Eur J Surg Oncol. (2023) 49:560–67. doi: 10.1016/j.ejso.2022.11.014
68. Xu S, Huang H, Qian J, Liu Y, Huang Y, Wang X, et al. Prevalence of hashimoto thyroiditis in adults with papillary thyroid cancer and its association with cancer recurrence and outcomes. JAMA Netw Open. (2021) 4:e2118526. doi: 10.1001/jamanetworkopen.2021.18526
69. Cunha LL, Morari EC, Guihen AC, Razolli D, Gerhard R, Nonogaki S, et al. Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf). (2012) 77:918–25. doi: 10.1111/j.1365-2265.2012.04482.x
70. Pani F, Caria P, Yasuda Y, Makoto M, Mariotti S, Leenhardt L, et al. The immune landscape of papillary thyroid cancer in the context of autoimmune thyroiditis. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14174287
71. De Leo S, D’Elia S, Grani G, Dondi F, Bertagna F, Puxeddu E, et al. A prospective multicenter study examining the relationship between thyroid cancer treatment outcomes and the presence of autoimmune thyroiditis. Thyroid. (2023) 33:1318–26. doi: 10.1089/thy.2023.0052
Keywords: differentiated thyroid cancer, thyroid peroxidase antibody, prevalence, prognosis, meta analysis
Citation: Zhang H, Tian L, Wang X and Shi X (2024) The relationship between thyroid peroxidase antibody and differentiated thyroid cancer: a systematic review and meta-analysis. Front. Endocrinol. 15:1349041. doi: 10.3389/fendo.2024.1349041
Received: 04 December 2023; Accepted: 13 February 2024;
Published: 27 February 2024.
Edited by:
Vincenzo Marotta, AOU S. Giovanni di Dio e Ruggi D’Aragona, ItalyReviewed by:
Elisa Minaldi, University of Pisa, ItalyCopyright © 2024 Zhang, Tian, Wang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoguang Shi, eGlhb2d1YW5nc2hpX2NtdUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.