- 1Department of Endocrinology and Metabolism, Shunde Hospital, Southern Medical University (The First People’s Hospital of Shunde), Foshan, Guangdong, China
- 2Department of Cardiology, The First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, China
Background: Metabolic syndrome (MetS) and sarcopenia (SP) have emerged as significant public health concerns in contemporary societies, characterized by shared pathophysiological mechanisms and interrelatedness, leading to profound health implications. In this prospective cohort study conducted within a US population, we aimed to examine the influence of MetS and SP on all-cause and cardiovascular mortality.
Methods: This study analyzed data from the National Health and Nutrition Examination Survey (NHANES) III for the years 1999-2006 and 2011-2018, and death outcomes were ascertained by linkage to National Death Index (NDI) records through December 31, 2019. Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for all-cause and cardiovascular mortality. In addition, subgroup and sensitivity analyses were conducted to test the robustness of the results.
Results: Over a median follow-up period of 13.3 years (95% CI: 12.8-13.8), 1714 deaths were observed. The groups characterized by MetS−/SP+, MetS+/SP−, and MetS+/SP+ exhibited higher all-cause mortality rates in comparison to the MetS-/SP- group, with the MetS+/SP+ group (HR 1.76, 95% CI: 1.37-2.25) displaying the highest all-cause mortality. Increased cardiovascular mortality was observed in the MetS+/SP− (HR 1.84, 95% CI: 1.24-2.72), and MetS+/SP+ groups (HR 2.39, 95% CI: 1.32-4.35) compared to the MetS−/SP− group, whereas it was not statistically significant in the MetS-/SP+ group. However, among males and individuals aged < 60, the presence of both MetS and SP (MetS+/SP+ group) was found to be significantly associated with a higher risk of all-cause and cardiovascular mortality.
Conclusion: The coexistence of MetS and SP increased the risk of all-cause and cardiovascular mortality, particularly in males and in nonelderly populations. Individuals with either MetS or SP may require more careful management to prevent the development of other diseases and thereby reduce mortality.
1 Introduction
Metabolic syndrome (MetS) is a group of clinical syndromes characterized by the aggregation of multiple disease states such as abdominal obesity, hypertension, dyslipidemia, abnormal glucose metabolism, and hyperuricemia in an individual (1). According to the National Health and Nutrition Examination Survey (NHANES), the prevalence of MetS has increased dramatically among U.S. adults, from 25.3% in 1988-1994 to 36.9% in 2015-2016 (2, 3). Most studies have shown that individuals with MetS have higher cardiovascular disease morbidity and mortality (4–6). Another study showed that MetS and its components were associated with all-cause, cardiovascular disease (CVD), and diabetes mortality (7).
Age-related reductions in skeletal muscle mass and strength, and diminished physical function are known as sarcopenia (SP) (8). In an aging society, the prevalence of SP is increasing globally, with an overall prevalence of 10-27% (9). Most studies have shown that individuals in SP are associated with a high risk of all-cause mortality (10–12).
Insulin resistance and chronic inflammation, as pathophysiological mechanisms common to both MetS and SP, interact to produce deleterious metabolic effects (13–17). MetS increases the risk of physical capacity and dysfunction (18–20) and is associated with lower muscle mass and strength (21). A meta-analysis demonstrated a positive association between SP and MetS (odds ratio, OR 2.01, 95% CI, 1.63-2.47) (22). MetS and SP are thought to be bi-directionally linked, increasing the risk of mutual morbidity (22, 23). Current studies on the comorbidity of MetS and SP have focused on the risk of cardiovascular disease, diabetes, and hyperlipidemia (24), and no studies comprehensively analyze the association of MetS and SP with mortality.
MetS and SP are highly prevalent worldwide and pose a significant public health burden. It may be valuable to assess the impact of their interaction on mortality in the general population. Therefore, this study investigates the association of MetS and SP with all-cause and cardiovascular mortality among U.S. adults using a sample that is nationally representative of the U.S. population.
2 Materials and methods
2.1 Study design and participants
Data for the study were obtained from the NHANES III, a research program conducted by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention designed to assess the health and nutritional status of adults and children in the U.S. NHANES utilizes a complex, multistage, probability sampling approach to obtain data through questionnaires, interviews, mobile medical examinations, and laboratory tests (25). The NHANES data are free and available on the Web (26).
This study was conducted in accordance with the Declaration of Helsinki (27). Written informed consent was obtained from all study participants, and the program was approved by the Ethics Review Board of the National Center for Health Statistics (28).
This study analyzed data from 1999-2006 and 2011-2018 and included 80,630 participants. Participants who were aged < 18 years, pregnant females, had a history of cancer at the time of enrollment, missing data on metabolic syndrome-related components, including blood pressure (BP), fasting blood glucose (FBG), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and waist circumference (WC), missing data on the skeletal muscle mass of the extremities, and missing data on mortality were excluded, and 10,778 subjects were ultimately included in the study analysis. The flow chart of the study is shown in Figure 1.
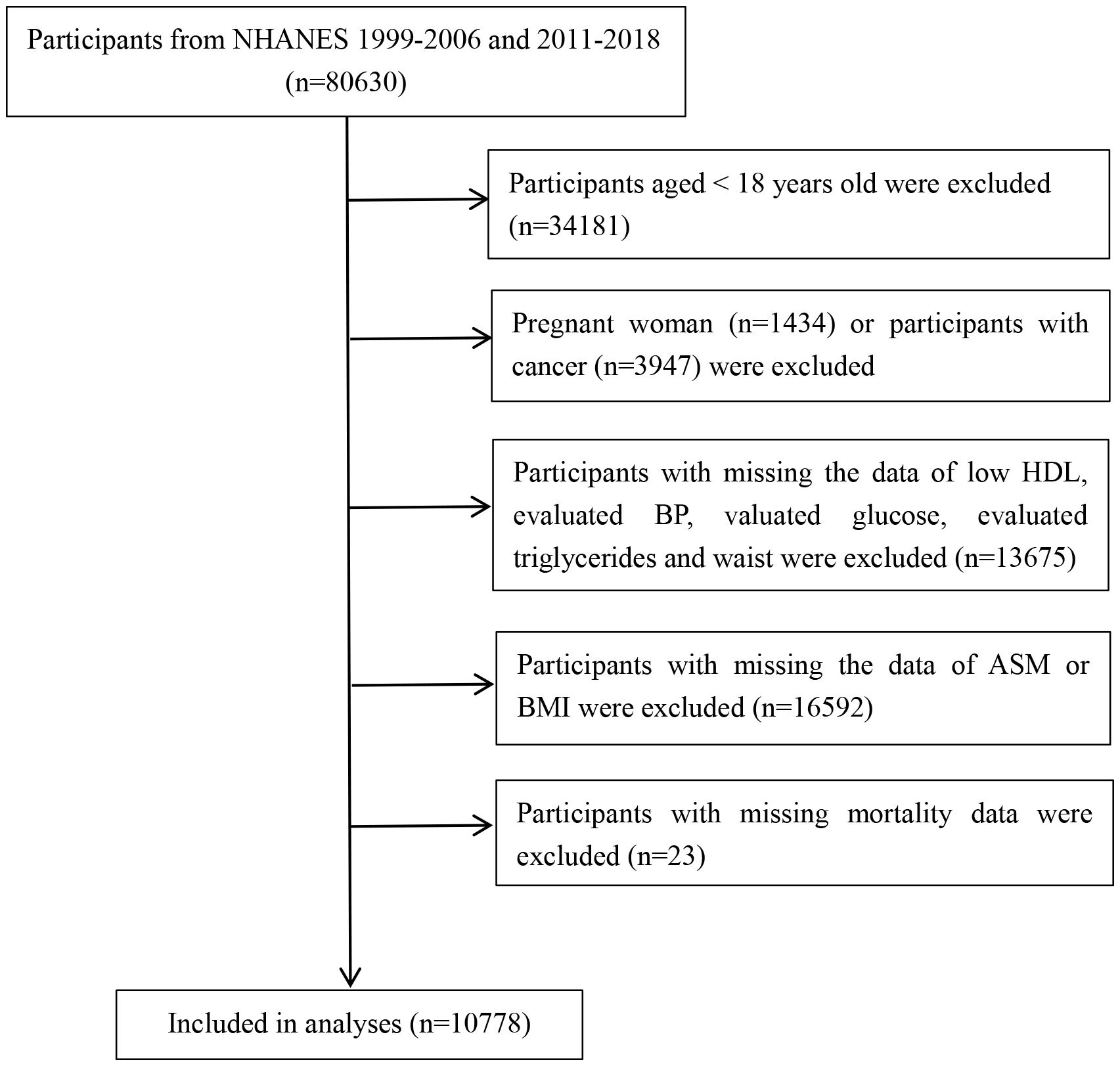
Figure 1 Study flowchart displaying the selection of patients according to exclusion criteria. BP, blood pressure; HDL, high-density lipoprotein cholesterol; ASM, appendicular skeletal muscle mass.
2.2 Ascertainment of MetS and SP
MetS was defined according to the NCEP ATP III-2005 criteria (29). People with three or more of the following criteria were diagnosed with MetS: (1) elevated WC (EWC): WC ≥ 102 cm in men and ≥ 88 cm in women; (2) elevated BP (EBP): BP ≥ 130/85 mm Hg or drug treatment of previously diagnosed hypertension; (3) reduced HDL-C (RHDL-C): < 40 mg/dL in men and < 50 mg/dL in women or specific treatment for reduced HDL-C; (4) elevated TG (ETG): TG level ≥ 150 mg/dL or drug treatment for elevated TG; and (5) elevated fasting glucose (EGLU): fasting glucose level of ≥100 mg/L or drug treatment for elevated glucose and previously diagnosed type 2 diabetes. The unit of HDL-C converted to mmol/L is equal to mg/dL*0.0259. The unit of TG converted to mmol/L is equal to mg/dL* 0.0113. The unit of FBG converted to mmol/L is equal to mg/dL*18.
Appendicular skeletal muscle mass (ASM), the sum of the lean mass of extremities, was assessed using dual-energy X-ray (DXA) (QDR Discovery; Hologic, Inc., Bedford, MA, USA). In this study, SP used ASM divided by body mass index (BMI) (ASM/BMI) with cutoff points of ≤ 0.789 in men and ≤ 0.512 in women according to the Foundation for the National Institutes of Health (FNIH) criteria, which widely used in recent research (30).
Based on these definitions, the participants were categorized into the following four groups according to the presence of MetS and SP: 1) without MetS or SP (MetS−/SP−), 2) with MetS but no SP (MetS+/SP−), 3) without MetS but with SP (MetS−/SP+), and 4) with both MetS and SP (MetS+/SP+).
2.3 Ascertainment of covariates
Study data also included sex, age, race and ethnicity, smoking status, drinking status, physical activity, marital status, education level, family poverty-to-income ratio (PIR), height, and weight. Participants’ race and ethnicity were categorized into four groups: Mexican American, non-Hispanic White, non-Hispanic Black, or others (31). Never smokers were defined as those who smoked fewer than 100 cigarettes in their lifetime, those who smoked at least 100 cigarettes in their lifetime were categorized as current smokers, and those who smoked at least 100 cigarettes in their lifetime and quit were labeled ex-smokers (32). Alcohol consumption was determined by a cutoff of ≥ 12 drinks per year, with no alcohol consumption defined as drinking < 12 drinks per year (33). Ideal physical activity was defined as at least 150 minutes of moderate or 75 minutes of vigorous physical activity per week, according to US PA guidelines (34). Educational level was categorized as less than high school, high school or equivalent, college or above, and marital status was categorized as married, separated, including widowed and divorced groups, or never married (7). Family PIR levels were grouped into three categories: 0-1.0, 1.1-3.0, and > 3.0 (35). BMI was calculated as weight (kg) divided by height squared (m2). Multiple interpolation was used for missing values of covariates.
2.4 Ascertainment of death
Mortality status was ascertained by probabilistic matching to the NDI through December 31, 2019, using a unique study identifier. Details of the matching method are available from the NCHS (36). Causes of death were classified according to the codes of ICD-10. Primary outcomes in this study were mortality from all causes, heart diseases (codes I00-I09, I11, I13, and I20-I51).
2.5 Statistical analysis
In accordance with the NHANES analysis guidelines, all analyses considered a complex survey design, including sample weights, clustering, and stratification. Missing values for covariates were supplemented using multiple interpolations. Continuous variables were expressed as weighted mean ± standard deviation for weighted characterization, while categorical variables were expressed as frequencies with weighted percentages. Differences in covariates across the groups were performed using one-way ANOVA for continuous variables and the Rao-Scott chi-square test for categorical variables with adjusted weights. Survival curves associated with all-cause and cardiovascular mortality were plotted according to the presence of MetS and SP using the Kaplan-Meier method. Cox proportional hazards models were used to calculate hazard ratios (HRs) and their 95% confidence intervals (95% CIs) for all-cause mortality and cardiovascular mortality, and P values for trends were calculated. Model 1 was unadjusted, Model 2 was adjusted for age, sex, and race/ethnicity, and Model 3 was adjusted for age, sex, race/ethnicity, education level, marital status, family PIR, smoking status, drinking status, and physical activity. We also performed stratified analyses by sex and age, obtaining P values for interactions. Cox regression analysis was performed in the SP population according to the number of abnormal metabolic components and the type of metabolic abnormality. To test the robustness of the findings, we performed three sensitivity analyses: first, we excluded participants with prior myocardial infarction or angina; second, we excluded participants with a prior history of stroke; and finally, we excluded participants who died within 2 years of the follow-up period.
All statistical analyses were performed using R (version 4.2.3; R Foundation for Statistical Computing) to account for the NHANES complex sample design, with 2-sided P < 0.05 considered statistically significant.
3 Results
3.1 Baseline characteristics
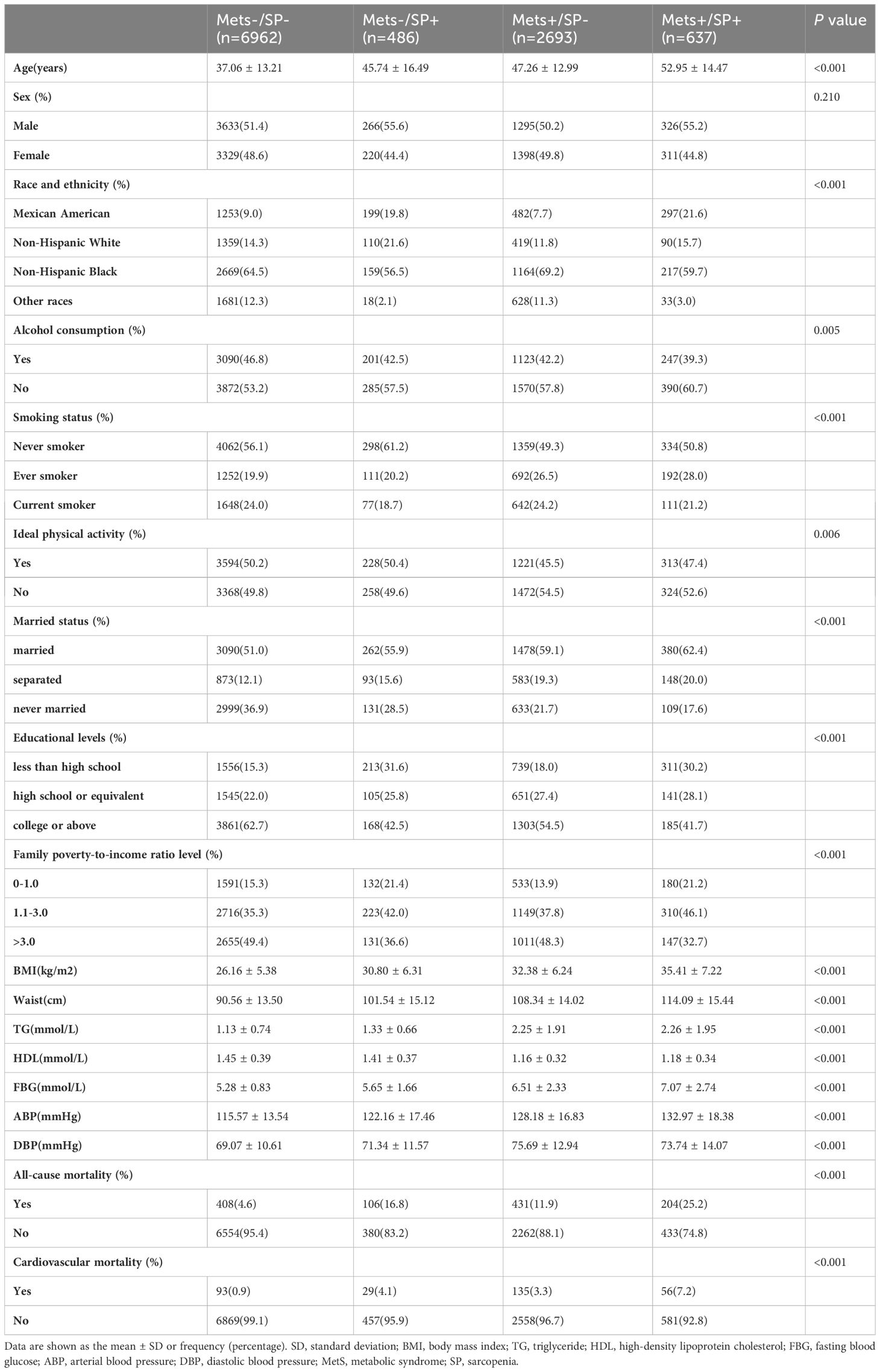
Table 1 summarizes the baseline characteristics of the four groups of subjects. Among the 10,778 subjects, 6,962 (64.6%) had neither MetS nor SP (MetS−/SP−), 486 (4.5%) had SP only (MetS−/SP+), 2693 (25.0%) had MetS only (MetS+/SP−), and 637 (5.9%) had both MetS and SP (MetS+/SP+). Subjects in the MetS+/SP+ group were significantly older than those in the other groups (P < 0.001). Subjects in the MetS+/SP+ group had higher all-cause mortality (25.2%) and cardiovascular mortality (7.2%). BMI, WC, TG, and FBG in the MetS+/SP+ group were also significantly higher than those in the other groups (P < 0.001). Differences between groups were significant except for the sex group.
3.2 Association of MetS and SP status with mortality
There were 1714 deaths during the follow-up period: 1149 (10.66%) from all-cause mortality and 313 (2.90%) from CVD. Figure 2 depicts the survival curves of the four groups of subjects and shows significant differences in overall survival and cardiovascular survival among the groups during a median follow-up duration of 13.3 years (95% CI: 12.8-13.8) (three-group log-rank P < 0.001). The MetS−/SP− group had the best survival, whereas the MetS+/SP+ group had the worst survival among all the groups.
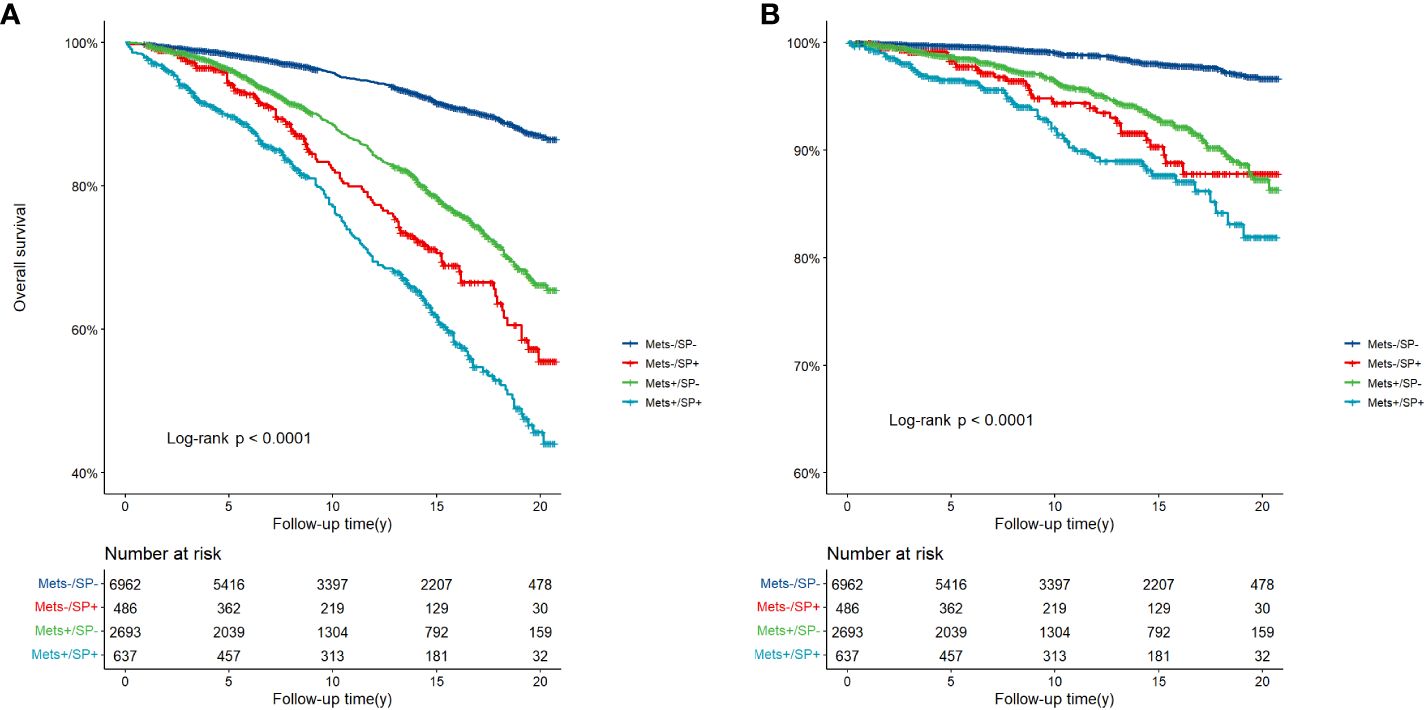
Figure 2 (A) Overall survival, and (B) CVD-related survival according to MetS and SP status. CVD, cardiovascular disease; MetS, metabolic syndrome; SP, sarcopenia.
Table 2 shows the association of MetS and SP status with all-cause and cardiovascular mortality. After adjusting for sex, age, race and ethnicity, smoking status, drinking status, physical activity, marital status, education level, and PIR, compared to the MetS−/SP− group, the risk of all-cause mortality was increased in the MetS−/SP+ group (HR 1.52, 95% CI: 1.15-2.01, P = 0.003), the MetS+/SP− group (HR 1.32, 95% CI: 1.06-1.64, P = 0.012), and the MetS+/SP+ group (HR 1.76, 95% CI: 1.37-2.25, P < 0.001). There was an increased risk of cardiovascular death in the MetS+/SP− (HR 1.84, 95% CI: 1.24-2.72, P = 0.002) and MetS+/SP+ (HR 2.39, 95% CI: 1.32-4.35, P = 0.004) groups compared with the MetS−/SP− group, whereas there was no significant difference in the MetS−/SP+ group. The HRs for all-cause mortality gradually increased in the MetS−/SP−, MetS+/SP−, MetS−/SP+, and MetS+/SP+ groups (P for trend < 0.001). A similar trend was observed for cardiovascular mortality (P for trend < 0.001).
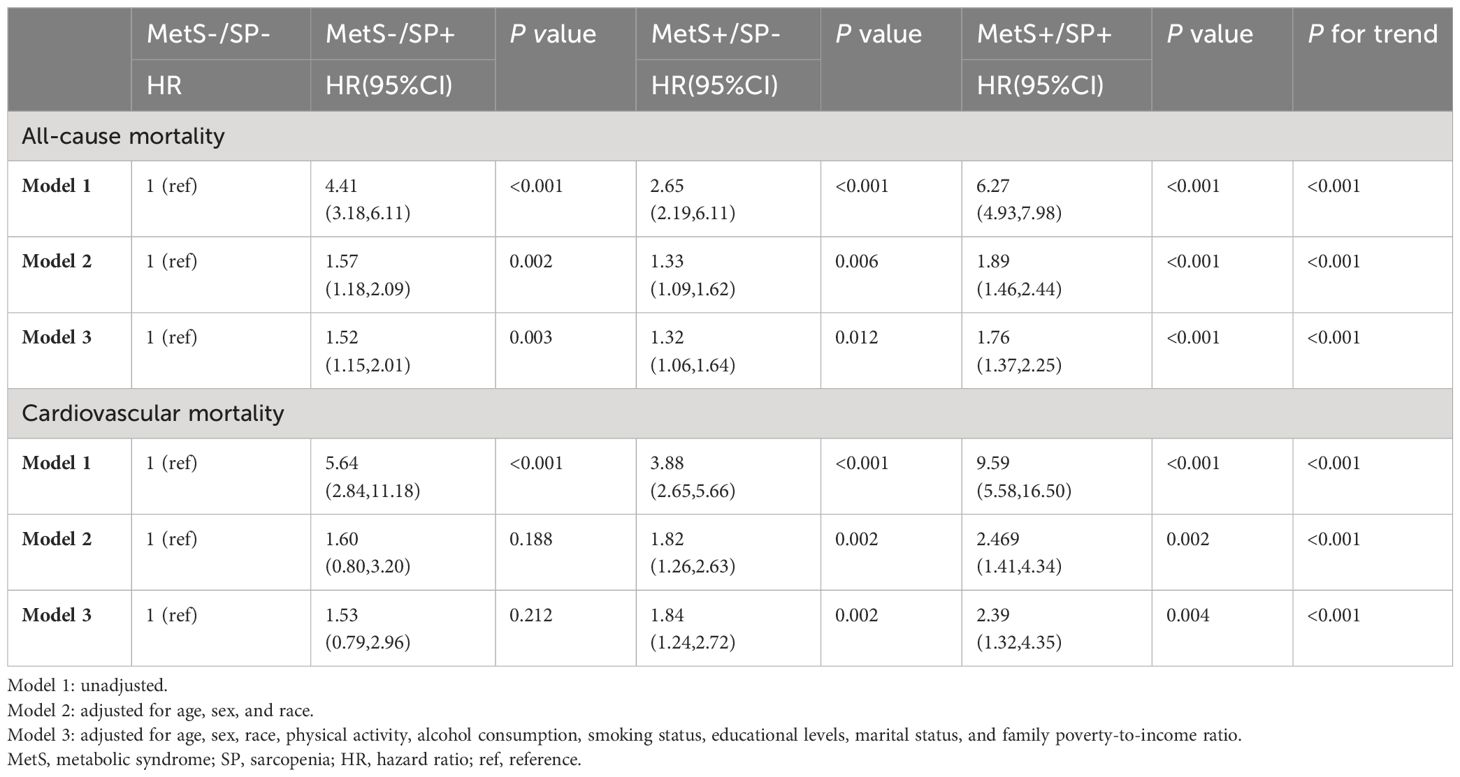
Table 2 Risks of all-cause and cardiovascular mortality according to the presence of MetS or SP status.
Table 3 presents the association of MetS and its components with all-cause and cardiovascular mortality in general and SP populations. After adjusting for covariates, when the number of metabolic abnormalities was ≥ 4, there was a significant positive association between the number of MetS components and all-cause and cardiovascular mortality. As the number of MetS components increased, the risk of all-cause and cardiovascular mortality increased. After adjusting for covariates, all five components of MetS were associated with an increased risk of all-cause and cardiovascular mortality. Only when the number of metabolic abnormalities was equal to 4 (HR 2.38, 95% CI: 1.51-4.92, P = 0.019) was associated with cardiovascular mortality in the SP population, and no other number of metabolic abnormalities was observed to be associated with all-cause and cardiovascular mortality (P > 0.05). Only ETG (HR 1.42, 95% CI: 1.00-2.00, P = 0.046) was associated with all-cause mortality, whereas the other metabolic abnormality components were not associated with all-cause and cardiovascular mortality.
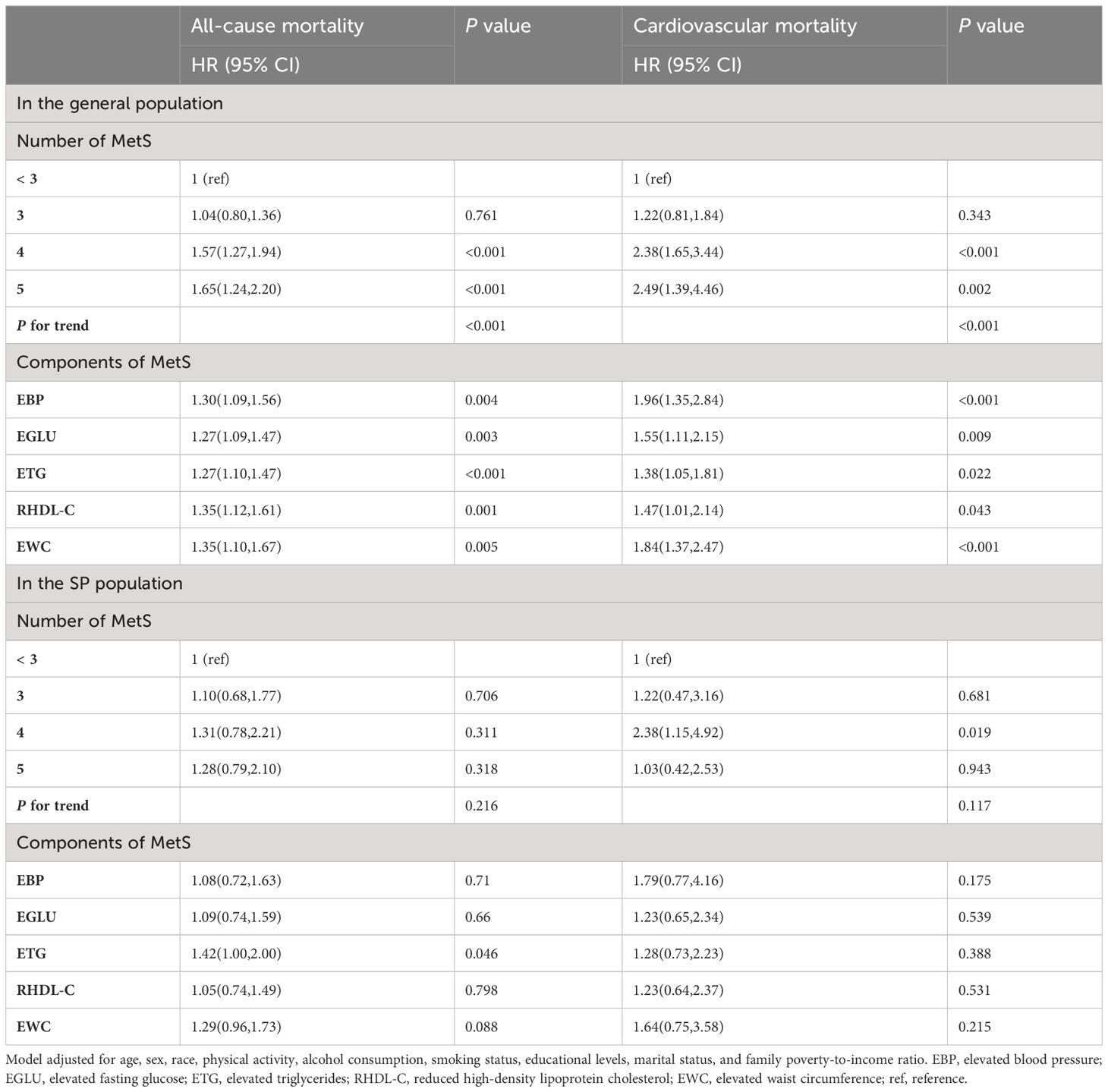
Table 3 Associations of MetS and its components with all-cause and cardiovascular mortality in the general population and in the SP population.
3.3 Subgroup analysis
The results of subgroup analyses of MetS and SP status with all-cause and cardiovascular mortality by sex and age are shown in Table 4. Compared to the MetS−/SP− group, the MetS+/SP+ group had a higher risk of all-cause mortality (HR 2.23, 95% CI: 1.53-3.24, P < 0.001) and cardiovascular mortality (HR 3.29, 95% CI: 1.37-7.88, P = 0.008) among the males, while no similar situation was observed in the females. Individuals aged < 60 years with MetS and SP had a higher risk of all-cause mortality (HR 4.48, 95% CI: 2.64-7.63, P < 0.001) and cardiovascular mortality (HR 8.88, 95% CI: 2.84-27.80, P < 0.001), whereas individuals aged ≥ 60 years with MetS and SP had higher hazard of all-cause mortality (HR 1.67, 95% CI: 1.32-2.10, P < 0.001), and cardiovascular mortality (HR 1.95, 95% CI: 1.07-3.53, P = 0.028).
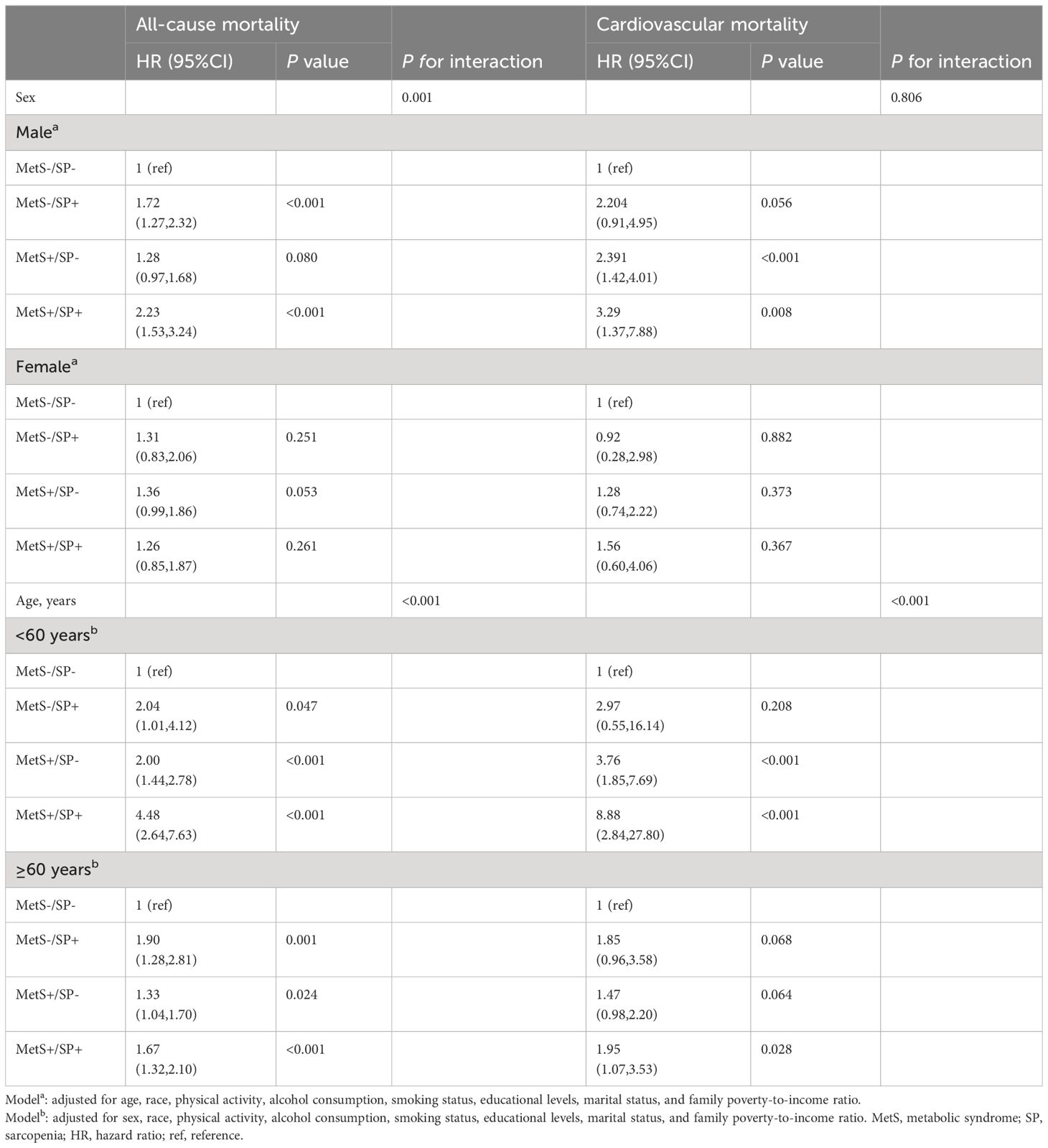
Table 4 Risks of all-cause and cardiovascular mortality according to the presence of MetS or sarcopenia status, stratified by sex and age.
3.4 Sensitivity analysis
Sensitivity analysis was performed by excluding the subjects with a previous myocardial infarction or angina (Supplementary Table 1). Compared to the MetS−/SP− group, the MetS+/SP+ group had an increased risk of all-cause mortality (HR 1.60, 95% CI: 1.21-2.10, P < 0.001) and cardiovascular mortality (HR 2.37, 95% CI: 1.17-4.80, P = 0.016). Subjects with SP only (MetS−/SP+ group) had only an increased risk of all-cause mortality (HR 1.57, 95% CI: 1.14-2.15, P = 0.005). Subjects with MetS only (MetS+/SP− group) had an increased risk of cardiovascular mortality (HR 1.72, 95% CI: 1.13-2.62, P = 0.012).
Sensitivity analysis was performed by excluding the subjects with a previous episode of stroke (Supplementary Table 2). All-cause mortality was significantly increased in the MetS−/SP+ (HR 1.55, 95% CI: 1.16-2.06, P = 0.003), MetS+/SP− (HR 1.31, 95% CI: 1.06-1.62, P = 0.013), and MetS+/SP+ (HR 1.70, 95% CI: 1.31-2.20, P < 0.001) groups compared with the MetS−/SP− group (P for trend < 0.001). Cardiovascular mortality was increased in the MetS+/SP− group (HR 1.79, 95% CI: 1.20-2.66, P = 0.004) and the MetS+/SP+ group (HR 2.38, 95% CI: 1.25-4.53, P = 0.008), but statistical significance was not reached in the MetS−/SP+ group.
Sensitivity analysis was performed by excluding the subjects who had died within two years of follow-up (Supplementary Table 3). Similarly, all-cause mortality was significantly increased in the MetS−/SP+ (HR 1.52, 95% CI: 1.09-2.11, P = 0.013), MetS+/SP− (HR 1.33, 95% CI: 1.07-1.67, P = 0.011), and MetS+/SP+ (HR 1.63, 95% CI: 1.26-2.11, P < 0.001) groups compared with the MetS−/SP− group (P for trend < 0.001). Cardiovascular mortality was significantly increased in the MetS+/SP− group (HR 1.87, 95% CI: 1.28-2.73, P = 0.001) and MetS+/SP+ group (HR 2.36, 95% CI: 1.30-4.31, P = 0.005).
4 Discussion
Our study retrospectively assessed the association of MetS and SP with all-cause and cardiovascular mortality mortality. In our study, the coexistence of MetS and SP was independently and positively associated with an elevated risk of all-cause and cardiovascular mortality after adjusting for potential confounders such as sociodemographic factors, lifestyle factors, and other factors. Mortality risks almost doubled in the MetS and SP coexistence group (HR 1.76 for all-cause mortality, HR 2.39 for cardiovascular mortality), and such a trend was observed in males (HR 2.23 for all-cause mortality, HR 3.29 for cardiovascular mortality) and nonelderly individuals (HR 4.48 for all-cause mortality, HR 8.88 for cardiovascular mortality) more significantly. Our findings suggest that the coexistence of MetS and SP increases the risk of all-cause and cardiovascular mortality, especially in male and nonelderly populations.
In our study, 56.7% (637/1123) of patients with SP showed coexistence with MetS, and 19.1% (637/3330) of patients with MetS showed coexistence with SP. The reasons for this phenomenon may be related to some common pathogenesis between the two (13–17). Several features of MetS may damage muscle health, including insulin resistance and chronic systemic inflammation, which negatively affects muscle homeostasis, leading to reduced muscle mass and strength (14, 37–39), and oxidative stress leading to mitochondrial dysfunction and impaired muscle repair of damage (39, 40), which in turn leads to decreased muscle function (4). Skeletal muscle, as the largest organ in the body, plays a crucial role in maintaining glucose homeostasis and regulating carbohydrate metabolism (41). Low muscle mass may impair blood glucose uptake by altering myokine secretion, leading to a state of insulin resistance and increasing the degree of localized inflammation and metabolic disturbances, which may facilitate the development of MetS (42–44). MetS and SP may contribute to each other’s development based on the mechanisms described above. As the incidence and prevalence of both MetS and SP are rapidly increasing in current society, the bidirectional relationship between these diseases may lead to amplified health risks in the population, and it is meaningful to study the mortality risk of MetS and SP comorbidity for an aging society.
Our study shows that all-cause and cardiovascular mortality was higher in the MetS and SP coexistence group than in the group with one disease alone, whereas there was no significant difference in the SP-only group compared with the standard control group, which is similar to the findings of Eyun Song et al. (4). The impact of MetS on cardiovascular mortality is higher than that of SP, which may be due to the common pathogenesis between MetS and CVD (4–6). MetS and SP may contribute to each other’s disease progression through some mechanism, thus increasing the risk of death. However, the possible mechanisms leading to this situation need to be explored and verified by more basic and clinical studies.
Our study shows that the effect of the state of presence of MetS and SP on all-cause and cardiovascular mortality is not identical in different populations. In the male population, all-cause and cardiovascular mortality was significantly higher in the group in which MetS and SP coexisted and was higher than in the group in which MetS or SP alone was present. In contrast, we did not observe this trend in the female population. For the nonelderly population (< 60 years), this trend was also evident, however, in the elderly population (≥ 60 years), the group with the coexistence of MetS and SP had significantly higher cardiovascular mortality than the group with only one disease. Previous studies have shown that the adverse effects of MetS on muscle mass and strength are mainly seen in young males. However, females are mostly less susceptible to the adverse effects of MetS on muscle (42). This is similar to the results of the present study, where this trend of having a higher risk of death when MetS and SP coexisted was more pronounced in male and nonelderly populations. The mechanism responsible for this phenomenon may be related to the effects of adipokines on skeletal muscle (45), which are produced and released by adipose tissue, such as lipocalin, leptin, and proinflammatory cytokines (46). Skeletal muscle is a crucial target tissue affected by these molecules, and their circulation levels are influenced by age and sex (47). In young women, serum lipocalin and leptin do not appear to be significantly associated with skeletal muscle morphology and function (48). However, in males, skeletal muscle seems more vulnerable to the impact of adipokines. Another possible explanation could be sex hormones, with MetS being associated with reduced testosterone levels (49), and testosterone being positively correlated with muscle strength (50). Since testosterone levels decrease with age (51) and women have lower testosterone levels than men, young men with relatively high testosterone levels may be particularly vulnerable.
Our research has several advantages. First, we adopted a prospective cohort study of a large, nationally representative sample. For the study population, we had a relatively adequate follow-up period and a reliable assessment of the causes of death of the study population. Second, a nationally representative community sample, standardized data collection procedures, and complete follow-up of survival times conducted by the U.S. government more than validate our study. In addition, we performed a detailed analysis of the association of MetS and SP presence status with all-cause and cardiovascular mortality, adjusting for a large number of potential confounders. The analyses were stratified to explore the effects of sex and age on the experimental results, and three sensitivity analyses were conducted to investigate the stability of the results. However, this study has several limitations. First, the results of this study may be representative of U.S. residents only, and the definition and cutoff value of SP varied by race. Therefore, the results cannot be generalized to the general population of different races and need to be further validated in other races. Second, alcohol consumption, smoking, and ideal physical activity were self-reported, which may not be accurate. Third, residual bias could not be eliminated despite adjusting for confounding mortality-related variables.
5 Conclusions
In summary, for US adults, MetS or SP is associated with a high risk of all-cause and cardiovascular mortality, and this relationship is more pronounced in males or nonelderly adults. MetS and SP as comorbidities increased the risk of all-cause and cardiovascular death compared with the presence of each condition alone. Future research is needed to reveal the mechanisms underlying the association between MetS, SP, and mortality and finding simple and practical criteria for screening patients with MetS and SP for early intervention is important for improving the healthy life expectancy of the population, which should be of concern to health care professionals.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WH: Writing – review & editing, Writing – original draft, Software, Methodology, Data curation, Conceptualization. SD: Writing – review & editing, Writing – original draft, Software, Data curation. SL: Writing – review & editing, Methodology. QM: Writing – review & editing, Data curation. LC: Writing – review & editing, Data curation. LL: Writing – review & editing, Methodology. HW: Writing – review & editing, Supervision, Methodology. JS: Writing – review & editing, Supervision, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Medical Scientific Research Foundation of Guangdong Province of China (B2021264) and the Guangdong Basic and Applied Basic Research Foundation (2021A1515110682).
Acknowledgments
The authors express their gratitude to the participants and staff of the NHANES for their invaluable contributions to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1346669/full#supplementary-material
Abbreviations
MetS, Metabolic Syndrome; SP, Sarcopenia; NHANES, National Health and Nutrition Examination Survey; CVD, Cardiovascular disease; NDI, National Death Index; HRs, Hazard Ratios; CIs, Confidence Intervals; NCHS, National Center for Health Statistics; BP, blood pressure; FBG , fasting blood glucose; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; WC, waist circumference; EWC, Elevated waist circumference; EBP, Elevated blood pressure; RHDL-C, Reduced HDL-C; ETG, Elevated TGs; EGLU, Elevated fasting glucose; ASM, Appendicular skeletal muscle mass; DXA, Dual-energy X-ray; BMI, Body mass index; FNIH, Foundation for the National Institutes of Health; PIR, poverty-to-income ratio.
References
1. Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
2. Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, national health and nutrition examination survey, 1988-2012. Preventing Chronic Disease. (2017) 14:E24. doi: 10.5888/pcd14.160287
3. Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. (2020) 323:2526–8. doi: 10.1001/jama.2020.4501
4. Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. J Am Coll Cardiol. (2005) 46:1978–85. doi: 10.1016/j.jacc.2005.06.082
5. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. (1991) 14:173–94. doi: 10.2337/diacare.14.3.173
6. Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. (2007) 49:403–14. doi: 10.1016/j.jacc.2006.09.032
7. Li W, Chen D, Peng Y, Lu Z, Kwan M-P, Tse LA. Association between metabolic syndrome and mortality: prospective cohort study. JMIR Public Health Surveillance. (2023) 9:e44073. doi: 10.2196/44073
8. Chen L-K, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Directors Assoc. (2020) 21(3):300–7.e2. doi: 10.1016/j.jamda.2019.12.012
9. Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:86–99. doi: 10.1002/jcsm.12783
10. Yuki A, Ando F, Otsuka R, Shimokata H. Sarcopenia based on the Asian Working Group for Sarcopenia criteria and all-cause mortality risk in older Japanese adults. Geriatrics Gerontology Int. (2017) 17:1642–7. doi: 10.1111/ggi.12946
11. Xu J, Wan CS, Ktoris K, Reijnierse EM, Maier AB. Sarcopenia is associated with mortality in adults: A systematic review and meta-analysis. Gerontology. (2022) 68:361–76. doi: 10.1159/000517099
12. Song E, Hwang SY, Park MJ, Jang A, Kim KJ, Yu JH, et al. Additive impact of diabetes and sarcopenia on all-cause and cardiovascular mortality: A longitudinal nationwide population-based study. Metabolism: Clin Experimental. (2023) 148:155678. doi: 10.1016/j.metabol.2023.155678
13. Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation–mechanisms and therapeutic targets. Arteriosclerosis Thrombosis Vasc Biol. (2012) 32:1771–6. doi: 10.1161/ATVBAHA.111.241869
14. Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE, Cawthon PM, Hoffman AR, et al. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatrics Society. (2011) 59:1217–24. doi: 10.1111/j.1532-5415.2011.03472.x
15. Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. (2009) 32:1993–7. doi: 10.2337/dc09-0264
16. Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. (2006) 119:526.e529–526.517. doi: 10.1016/j.amjmed.2005.10.049
17. Beenakker KGM, Ling CH, Meskers CGM, de Craen AJ, Stijnen T, Westendorp RG, et al. Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Aging Res Rev. (2010) 9:431–6. doi: 10.1016/j.arr.2010.05.005
18. Stenholm S, Koster A, Alley DE, Kanaya A, Lee JS, Newman AB, et al. Joint association of obesity and metabolic syndrome with incident mobility limitation in older men and women–results from the Health, Aging, and Body Composition Study. Journals Gerontology Ser A Biol Sci Med Sci. (2010) 65:84–92. doi: 10.1093/gerona/glp150
19. Penninx BWJH, Nicklas BJ, Newman AB, Harris TB, Goodpaster BH, Satterfield S, et al. Metabolic syndrome and physical decline in older persons: results from the Health, Aging And Body Composition Study. Journals Gerontology Ser A Biol Sci Med Sci. (2009) 64(1):96–102. doi: 10.1093/gerona/gln005
20. Carriere I, Pérès K, Ancelin ML, Gourlet V, Berr C, Barberger-Gateau P, et al. Metabolic syndrome and disability: findings from the prospective three-city study. Journals Gerontology Ser A Biol Sci Med Sci. (2014) 69:79–86. doi: 10.1093/gerona/glt101
21. Ishii S, Tanaka T, Akishita M, Ouchi Y, Tuji T, Iijima K. Metabolic syndrome, sarcopenia and role of sex and age: cross-sectional analysis of Kashiwa cohort study. PloS One. (2014) 9:e112718. doi: 10.1371/journal.pone.0112718
22. Zhang H, Lin S, Gao T, Zhong F, Cai J, Sun Y, et al. Association between sarcopenia and metabolic syndrome in middle-aged and older non-obese adults: A systematic review and meta-analysis. Nutrients. (2018) 10(3):364. doi: 10.3390/nu10030364
23. Kim SH, Jeong JB, Kang J, Ahn DW, Kim JW, Kim BG, et al. Association between sarcopenia level and metabolic syndrome. PloS One. (2021) 16:e0248856. doi: 10.1371/journal.pone.0248856
24. Sanada K, Iemitsu M, Murakami H, Gando Y, Kawano H, Kawakami R, et al. Adverse effects of coexistence of sarcopenia and metabolic syndrome in Japanese women. Eur J Clin Nutr. (2012) 66:1093–8. doi: 10.1038/ejcn.2012.43
25. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Statistics Ser 1 Programs Collection Procedures. (2013) 56:1–37.
26. NHANES survey methods and analytic guidelines. Centers for Disease Control and Prevention. Available at: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx.
27. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
29. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
30. Cheng L, Wang S. Correlation between bone mineral density and sarcopenia in US adults: a population-based study. J Orthopaedic Surg Res. (2023) 18:588. doi: 10.1186/s13018-023-04034-7
31. Wang K, Zhao Y, Nie J, Xu H, Yu C, Wang S. Higher HEI-2015 score is associated with reduced risk of depression: result from NHANES 2005-2016. Nutrients. (2021) 13(2):348. doi: 10.3390/nu13020348
32. ALHarthi SSY, Natto ZS, Midle JB, Gyurko R, O'Neill R, Steffensen B. Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J Periodontol. (2019) 90:16–25. doi: 10.1002/JPER.18-0183
33. Shao Y, Li L, Zhong H, Wang X, Hua Y, Zhou X. Anticipated correlation between lean body mass to visceral fat mass ratio and insulin resistance: NHANES 2011-2018. Front Endocrinol (Lausanne). (2023) 14:1232896. doi: 10.3389/fendo.2023.1232896
34. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for americans. Jama. (2018) 320:2020–8. doi: 10.1001/jama.2018.14854
35. Brown AF, Liang LJ, Vassar SD, Escarce JJ, Merkin SS, Cheng E, et al. Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Ann Intern Med. (2018) 168:541–9. doi: 10.7326/M17-0996
36. Wang X, Lu J, Song Z, Zhou Y, Liu T, Zhang D. From past to future: Bibliometric analysis of global research productivity on nomogram (2000-2021). Front Public Health. (2022) 10:997713. doi: 10.3389/fpubh.2022.997713
37. Alemán H, Esparza J, Ramirez FA, Astiazaran H, Payette H. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Aging. (2011) 40:469–75. doi: 10.1093/ageing/afr040
38. Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. (2007) 30:1507–12. doi: 10.2337/dc06-2537
39. Abbatecola AM, Paolisso G, Fattoretti P, Evans WJ, Fiore V, Dicioccio L, et al. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging. (2011) 15:890–5. doi: 10.1007/s12603-011-0366-0
40. Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. (2005) 102:5618–23. doi: 10.1073/pnas.0501559102
41. Sinacore DR, Gulve EA. The role of skeletal muscle in glucose transport, glucose homeostasis, and insulin resistance: implications for physical therapy. Phys Ther. (1993) 73:878–91. doi: 10.1093/ptj/73.12.878
42. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. (2009) 32 Suppl 2:S157–163. doi: 10.2337/dc09-S302
43. Eckardt K, Görgens SW, Raschke S, Eckel J. Myokines in insulin resistance and type 2 diabetes. Diabetologia. (2014) 57:1087–99. doi: 10.1007/s00125-014-3224-x
44. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. (2012) 8:457–65. doi: 10.1038/nrendo.2012.49
45. Lu W, Feng W, Lai J, Yuan D, Xiao W, Li Y. Role of adipokines in sarcopenia. Chin Med J (Engl). (2023) 136:1794–804. doi: 10.1097/CM9.0000000000002255
46. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. (2005) 115:911–919; quiz 920. doi: 10.1016/j.jaci.2005.02.023
47. Bucci L, Yani SL, Fabbri C, Bijlsma AY, Maier AB, Meskers CG, et al. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology. (2013) 14:261–72. doi: 10.1007/s10522-013-9428-5
48. Yoshiko A, Ohta M, Kuramochi R, Mitsuyama H. Serum adiponectin and leptin is not related to skeletal muscle morphology and function in young women. J Endocr Soc. (2023) 7:bvad032. doi: 10.1210/jendso/bvad032
49. Kupelian V, Hayes FJ, Link CL, Rosen R, McKinlay JB. Inverse association of testosterone and the metabolic syndrome in men is consistent across race and ethnic groups. J Clin Endocrinol Metab. (2008) 93:3403–10. doi: 10.1210/jc.2008-0054
50. Auyeung TW, Lee JS, Kwok T, Leung J, Ohlsson C, Vandenput L, et al. Testosterone but not estradiol level is positively related to muscle strength and physical performance independent of muscle mass: a cross-sectional study in 1489 older men. Eur J Endocrinol. (2011) 164:811–7. doi: 10.1530/EJE-10-0952
51. Liu PY, Beilin J, Meier C, Nguyen TV, Center JR, Leedman PJ, et al. Age-related changes in serum testosterone and sex hormone binding globulin in Australian men: longitudinal analyses of two geographically separate regional cohorts. J Clin Endocrinol Metab. (2007) 92:3599–603. doi: 10.1210/jc.2007-0862
Keywords: metabolic syndrome, sarcopenia, all-cause mortality, cardiovascular mortality, NHANES
Citation: Huang W, Deng S, Liu S, Ma Q, Cao L, Liu L, Wan H and Shen J (2024) Association of metabolic syndrome and sarcopenia with all-cause and cardiovascular mortality: a prospective cohort study based on the NHANES. Front. Endocrinol. 15:1346669. doi: 10.3389/fendo.2024.1346669
Received: 29 November 2023; Accepted: 12 March 2024;
Published: 26 March 2024.
Edited by:
Carmine Izzo, University of Salerno, ItalyReviewed by:
Xiankun Chen, Karolinska Institutet (KI), SwedenRamesh Bhandari, KLE College of Pharmacy, India
Copyright © 2024 Huang, Deng, Liu, Ma, Cao, Liu, Wan and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Shen, c2ppZXN5QHNtdS5lZHUuY24=; Heng Wan, d2FuaGRyQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Weihong Huang1†
Weihong Huang1† Qintao Ma
Qintao Ma Heng Wan
Heng Wan Jie Shen
Jie Shen