- 1Department of Obstetrics and Gynecology, Hebei General Hospital, Shijiazhuang, China
- 2Department of Information Management, Hebei Center for Women and Children’s Health, Shijiazhuang, China
- 3Department of Obstetrics and Gynecology, Hebei Provincial Hospital of Chinese Medicine, Shijiazhuang, China
Objective: To explore the relationship between the exposure level of particulate matter 2.5 (PM2.5) and particulate matter 10 (PM10) in the air of pregnant women during preconception and first trimester of pregnancy and the risk of gestational diabetes mellitus (GDM).
Methods: The data of pregnant women delivered in 22 monitoring hospitals in Hebei Province from 2019 to 2021 were collected, and the daily air quality data of their cities were used to calculate the exposure levels of PM2.5 and PM10 in different pregnancy stages, and logistic regression model was used to analyze the impact of exposure levels of PM2.5 and PM10 on GDM during preconception and first trimester of pregnancy.
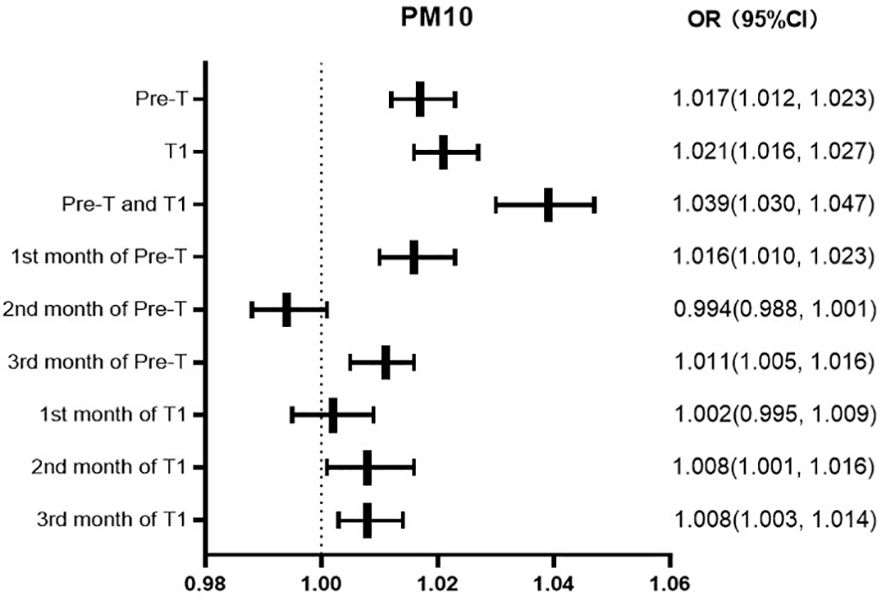
Results: 108,429 singleton live deliveries were included in the study, of which 12,967 (12.0%) women had a GDM diagnosis. The prevalence of GDM increased over the course of the study from 10.2% (2019) to 14.9% (2021). From 2019 to 2021, the average exposure of PM2.5 and PM10 was relatively 56.67 and 103.08μg/m3 during the period of preconception and first trimester of pregnancy in Hebei Province. Handan, Shijiazhuang, and Xingtai regions had the most severe exposure to PM2.5 and PM10, while Zhangjiakou, Chengde, and Qinhuangdao had significantly lower exposure levels than other regions. The GDM group had statistically higher exposure concentrations of PM2.5 and PM10 during the period of preconception, first trimester, preconception and first trimester (P<0.05). Multivariate logistic regression analysis showed that the risk of GDM increases by 4.5%, 6.0%, and 10.6% for every 10ug/m3 increase in the average exposure value of PM2.5 in preconception, first trimester, preconception and first trimester, and 1.7%, 2.1%, and 3.9% for PM10. Moreover, High exposure to PM2.5 in the first, second, and third months of preconception and first trimester is associated with the risk of GDM. And high exposure to PM10 in the first, second, and third months of first trimester and the first, and third months of preconception is associated with the risk of GDM.
Conclusion: Exposure to high concentrations of PM2.5 and PM10 during preconception and first trimester of pregnancy can significantly increase the risk of GDM. It is important to take precautions to prevent exposure to pollutants, reduce the risk of GDM, and improve maternal and fetal outcomes.
Introduction
Gestational diabetes mellitus (GDM) is one of the most common pregnancy complications and usually diagnosed in the second and third trimester. It was defined as carbohydrate intolerance of any degree with onset or first recognition during pregnancy (1). GDM increases not only the rate of adverse maternal and infant outcomes, but also the long-term risk of childhood obesity, type 2 diabetes and cardiovascular disease (2). which has a serious impact on both mothers and fetuses. With the adjustment of fertility policy, the number of pregnant women with advanced maternal age and pregnancy complications is increasing, and the incidence rate of GDM is increasing. The GDM incidence in China was reported to be 11.91% (3). How to prevent GDM is a serious public health challenge.
Air pollution is one of the most serious environmental problems in the world. Research shows that atmospheric particulate matters are one of the main risk factors for diabetes (4, 5). Although the pathophysiological mechanisms between ambient air pollution and maternal disease remain unclear, several mechanisms including systemic inflammation, oxidative stress, and endothelial dysfunction have been proposed (6, 7). Systemic inflammation and oxidative stress induced by air pollution can lead to insulin resistance, which is the underlying mechanism of GDM (8).
The susceptibility of pregnant women to pollutants increases due to physiological characteristics such as increased blood volume and accelerated respiratory rate during pregnancy. This study analyzes the data of pregnant women in 108, 429 singleton live deliveries in Hebei Province, and expounds the relationship between air pollution exposure of women during the period of pre-pregnancy and first trimester and the occurrence of GDM. The aim is to provide scientific basis for preventing exposure to pollutants, reducing the risk of GDM, improving maternal and fetal outcomes, and improving the quality of the birth population.
Methods
Study area
Hebei Province (36°05′–42°40′N, 113°27′–119°50′E), which encompasses the areas of Beijing and Tianjin, has the Bohai Sea to the east, Yanshan Mountains to the north, and Tai hang Mountains to the west.
Data collection
This is a retrospective study. Data in this study were retrieved from the monitoring information management system for pregnant women in 22 hospitals of Hebei Province China from 2019 to 2021. And the 22 monitoring hospitals are distributed in 11 cities in Hebei Province.
Inclusion criteria are single live birth and over 28 weeks of gestation. Exclusion criteria included age <20y, stillbirth, multiple births, and incomplete data. 108,429 singleton live deliveries were included in the study.
The air pollutant concentration data (PM2.5, PM10) were obtained from China Environmental Monitoring Network (http://www.cnemc.cn/). The concentration was recorded hourly for each of the following air pollutants: particulate matters (PM) with a diameter of 10 µm or less (PM10, µg/m3), PM with a diameter of 2.5 µm or less (PM2.5, µg/m3). The study was approved by the ethics committee of Hebei Women and Children’s Health Center.
Diagnostic approaches and criteria
Pregnant women at 24–28 weeks of gestation were tested for fasting 75-g oral glucose tolerance. GDM was diagnosed if one or more thresholds are met or exceeded: fasting blood glucose: 5.1 mmol/L, blood glucose of 1 hour: 10.0 mmol/L) and blood glucose of 2 hour: 8.5 mmol/L (9).
We calculated individual average concentrations of air pollutants in the following windows based on the gestational age of each pregnant woman::(1) preconception (12weeks before pregnancy, Pre-T); (2) first trimester (1–13 gestational weeks, T1).
Statistical analyses
SPSS 21.0 software was used for statistical analyses. The data description was presented as median [interquartile ranges (IQR)] for continuous variables and rank sum test is used for the comparison between groups. χ2-test is used for the comparison between groups of counting data. The multivariate logistic regression model was used to analyze the risk factors of GDM. p was set at <0.05 for statistical significance.
Results
Maternal characteristics
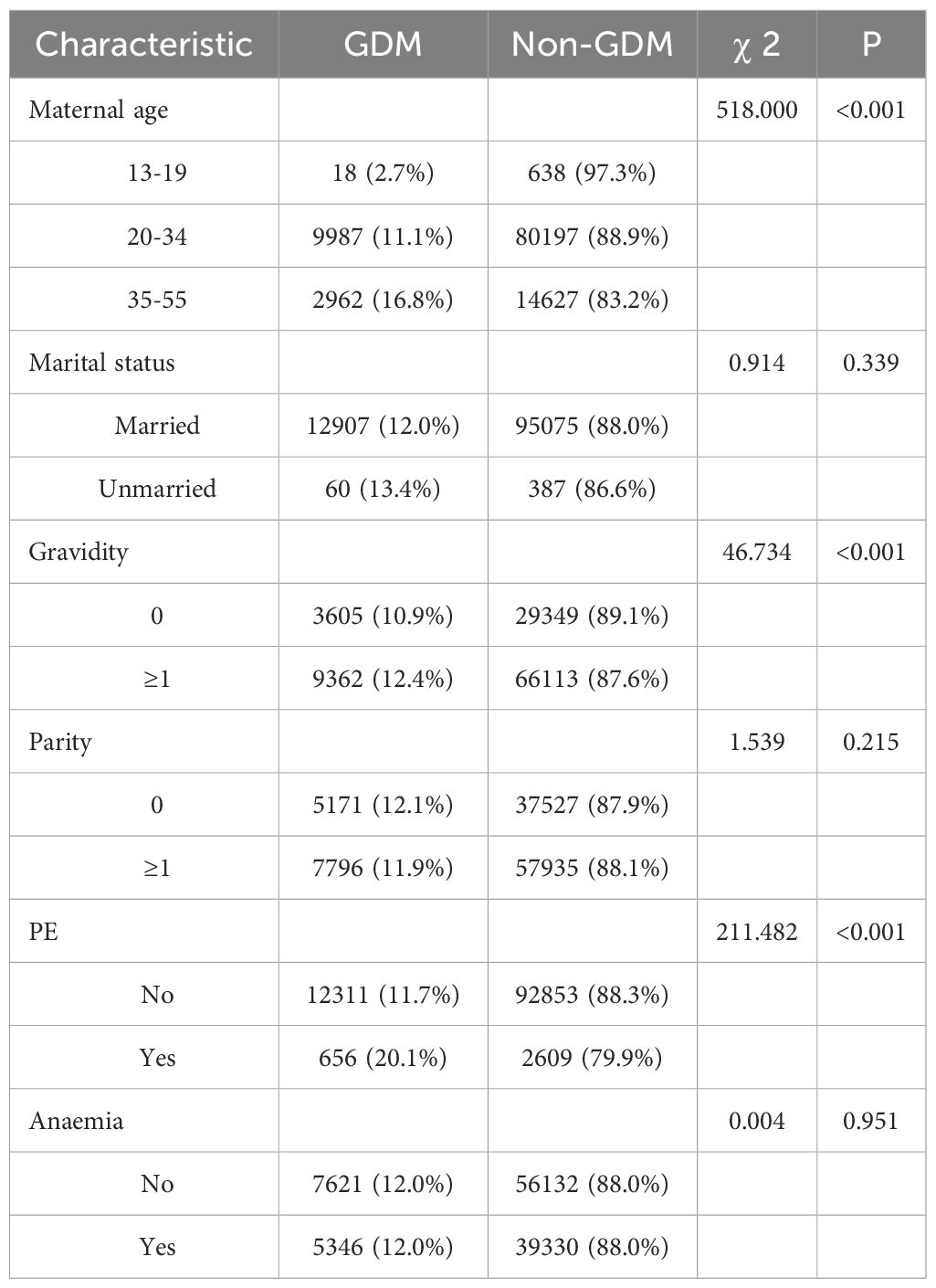
In this study, 108,429 singleton live deliveries were included in the study, of which 12,967 (12.0%) women had a GDM diagnosis. The prevalence of GDM increased over the course of the study from 10.2% (4,751/46,453) in 2019 to 14.9% (4,129/27,661) in 2021. Women with GDM were more likely to be with advanced age, multigravidity, and PE (Table 1).
Exposure of PM2.5&PM10 during the period of stage 1 and 2 among various cities in Hebei Province
From 2019 to 2021, the average exposure of PM2.5 was 56.67μg/m3 during the period of stage 1 and 2 in Hebei Province, the median was 58.83μg/m3, max 95.00μg/m3, with a minimum value of 18.83μg/m3. The average exposure of PM10 was 103.08μg/m3 during the period of pre-pregnancy and first trimester in Hebei Province, the median was 105.00μg/m3, max 165.67μg/m3, with a minimum value of 43.83μg/m3. The values of PM2.5 and PM10 are much higher than the WHO health standard (annual average concentration of 10μg/m3). From the perspective of regional distribution, Handan, Shijiazhuang, and Xingtai regions had the most severe exposure to PM2.5 and PM10, while Zhangjiakou, Chengde, and Qinhuangdao had significantly lower exposure levels than other regions (Table 2).
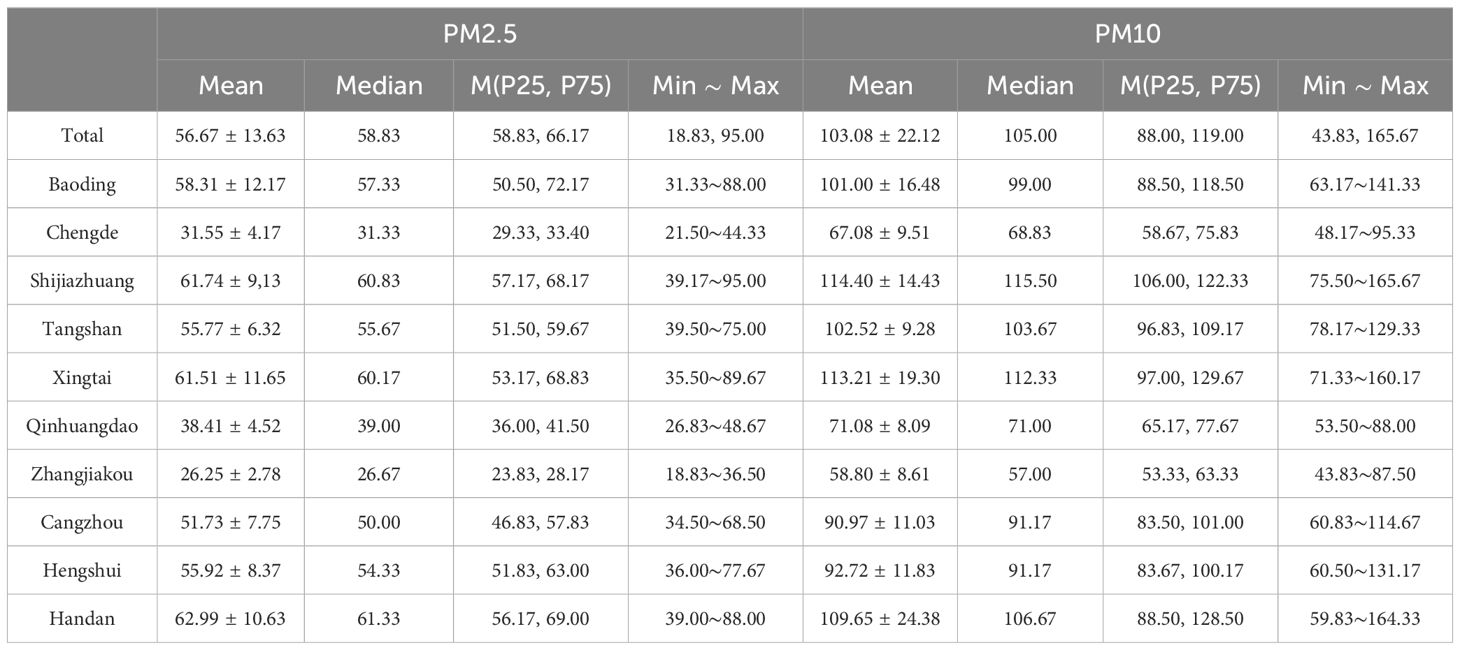
Table 2. Total exposure of PM2.5 and PM10 during the period of stage 1 and 2 in different cities in Hebei Province.
Exposure of PM2.5 and PM10 in different groups
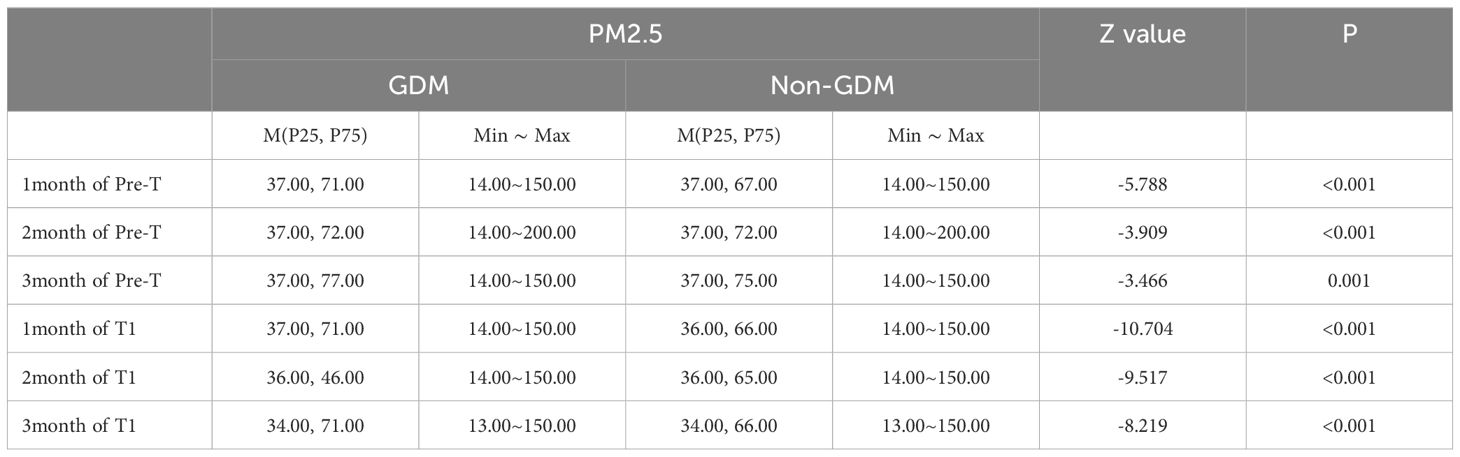
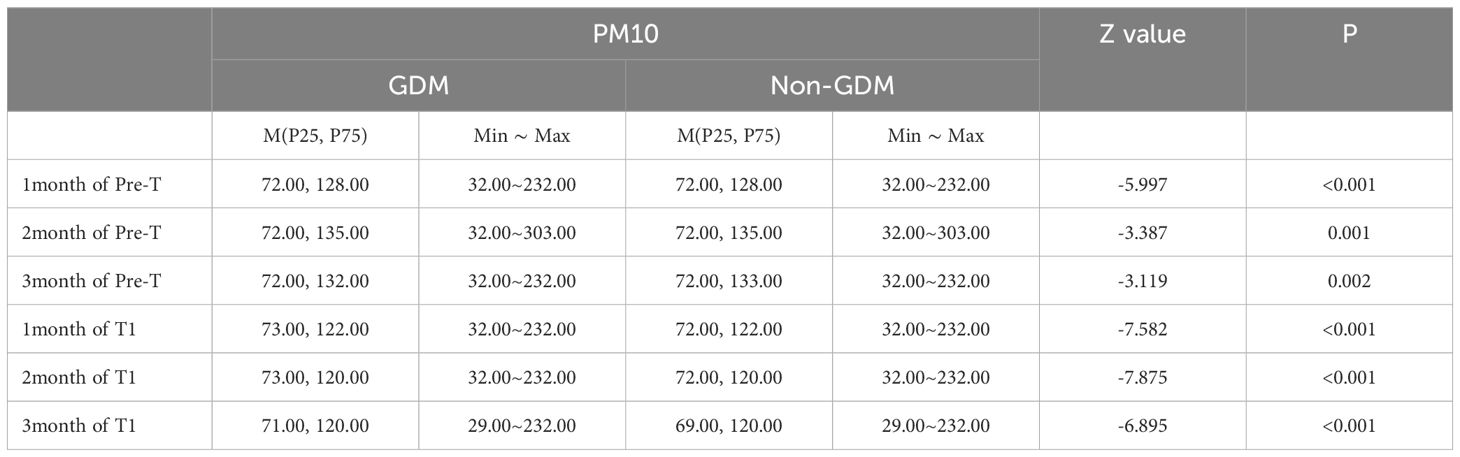
Compared to the non-GDM group, the GDM group had statistically higher exposure concentrations of PM2.5 and PM10 during the period of stage 1, stage 2, stage 1 & 2 (Tables 3, 4), and per month (Tables 5, 6) (P<0.05).
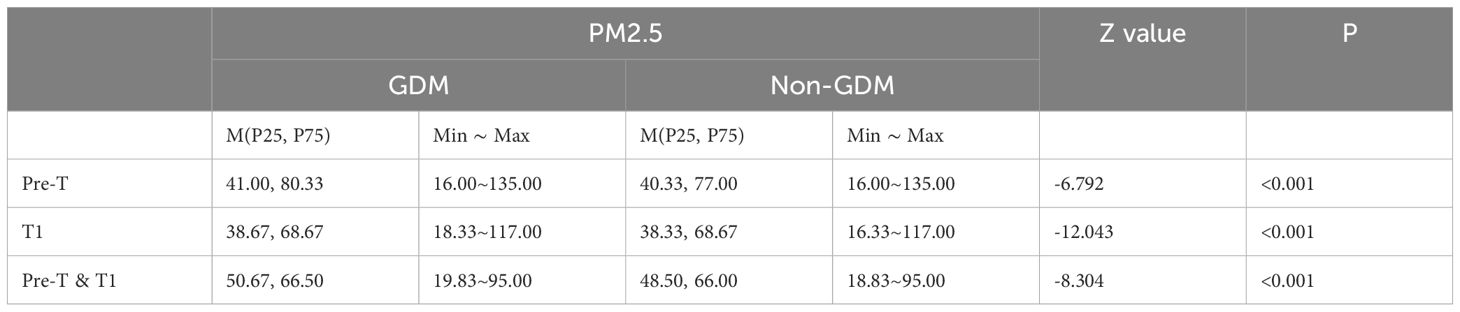
Table 3. Exposure of PM2.5 in different groups of pre-trimester, first-trimester, and pre-trimester&first-trimester.
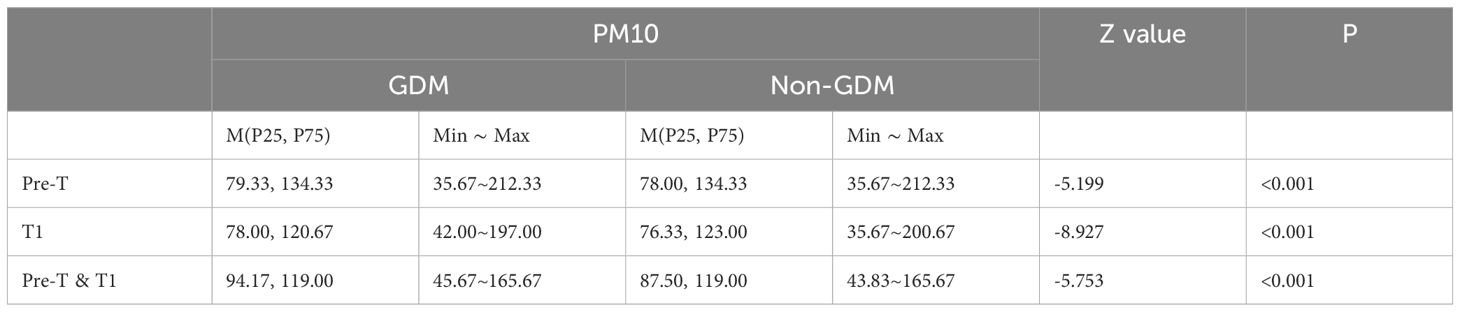
Table 4. Exposure of PM10 in different groups of pre-trimester, first-trimester, and pre-trimester&first-trimester.
The correlation between PM2.5 exposure and GDM in different periods
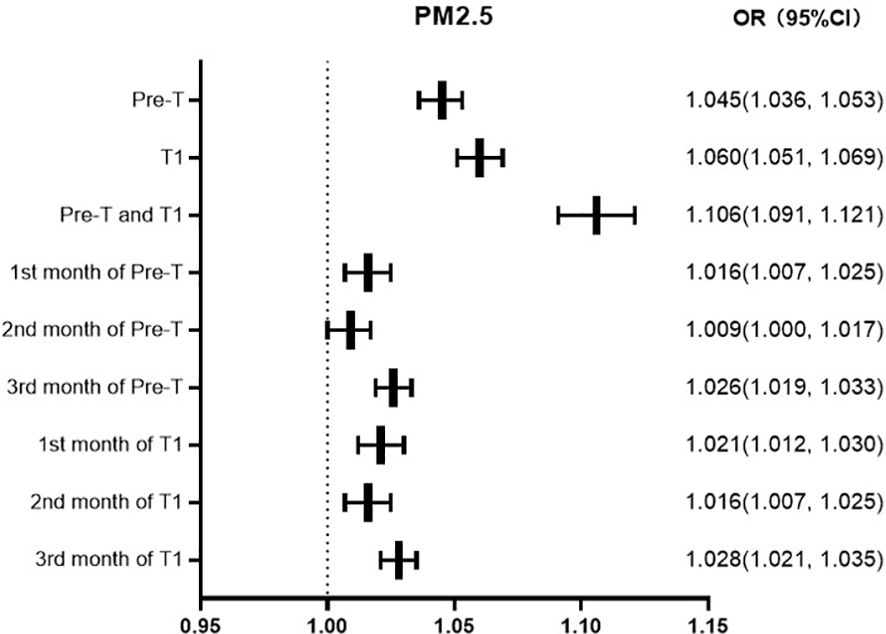
GDM was defined as the dependent variable (0=no, 1=yes), and the factors of age, marital status, times of gravidity and parity, gestational hypertension, and anemia were defined as independent variables. Binary logistic regression analysis was used for analysis. The regression results show that, with the same other factors, the risk of GDM increases by 4.5%, 6.0%, and 10.6% for every 10ug/m3 increase in the average exposure value of PM2.5 in Pre-T, T1, Pre-T and T1, respectively, and 1.6%, 0.9%, and 2.6% respectively in the first, second, and third months of Pre-T and T1.1%, 1.6%, 2.8% respectively in the first, second, and third months of T1 (Figure 1).
The correlation between PM10 exposure and GDM in different periods
The risk of GDM increases by 1.7%, 2.1%, and 3.9% for every 10ug/m3 increase in the average exposure value of PM10 in Pre-T, T1, Pre-T and T1, respectively. The risk of GDM increases by 1.6% and 1.1% for every 10ug/m3 increase in the average PM2.5 exposure value in the first and third months of Pre-T, respectively. While there was not statistically significant in the second month of Pre-T. The risk increased by 2.0%, 0.8%, and 0.8% in the first, second, and third months of T1, respectively (Figure 2).
Discussion
From 2013 to 2018, the prevalence rate of diabetes in China increased from 10.9% to 12.4%, and the overall prevalence rate of adult diabetes and pre-diabetes has reached 50.5% (10). At the same time, the prevalence of GDM is also rising. The prevalence of GDM in China shows that the prevalence of GDM is 11.91% (3) The average incidence of GDM in Hebei from 2014 to 2021 was 7.64% (11). In this study, the prevalence of GDM increased over the course of the study from 10.2% in 2019 to 14.9% in 2021. How to prevent the occurrence of GDM and reduce the disease burden of diabetes is a serious public health challenge at present.
GDM refers to the abnormal glucose metabolism of pregnant women firstly found in 24-28 weeks of pregnancy, which is one of the most common complications of pregnancy (12). GDM will not only increase the incidence rate of adverse perinatal maternal and infant outcomes, but also increase the long-term risk of obesity, type 2 diabetes and cardiovascular disease in the offspring (2, 11).
The etiology of GDM was complicated and this hyperglycemia is the result of impaired glucose tolerance due to pancreatic β-cell dysfunction on a background of chronic insulin resistance (13). In recent years, the potential risks of air pollution to human health have become a research hotspot. Air pollution is one of the most serious environmental problems in the world. Air pollution is one of the most serious environmental problems in the world, and toxicological studies have shown that air pollutants can cause widespread damage to the respiratory system, cardiovascular system, immune system, and endocrine system (14–19). Moreover, systemic inflammation, oxidative stress, and endothelial dysfunction have been proposed (6, 7). Systemic inflammation and oxidative stress induced by air pollution can lead to insulin resistance, which is the underlying mechanism of gestational diabetes mellitus (GDM) in pregnant women (8, 20, 21).
Due to differences in geographical environment, industrial structure, and other aspects, the air pollution situation in various cities in Hebei Province is polarized. Data shows that Handan City, Shijiazhuang City, and Xingtai City have consistently ranked among the top three cities in terms of PM2.5 and PM10 concentrations, while Zhangjiakou, Chengde, and Qinhuangdao have concentrations of PM2.5 and PM10 that meet or approach the national secondary standard for ambient air quality. Therefore, pregnant women in various regions of Hebei Province face an external environment with significant differences in PM2.5 and PM10 concentrations, which is helpful for stratified analysis of the impact of PM2.5 and PM10 exposure on GDM during pregnancy or while trying to get pregnant.
Recent studies (22–24) have shown a close correlation between air pollution exposure and the occurrence of GDM; However, the research conclusions of the association between air pollutant exposure and the incidence of diabetes in pregnancy are inconsistent, and the window period of pollutant exposure is also unclear. Research (25–30) shows that air pollution can induce oxidative stress, inflammatory response, and lipid metabolism dysregulation of adipokines and imbalance of gut microbiota, ultimately leading to abnormal glucose metabolism and insulin resistance which might induce GDM. Our study found that exposure to PM2.5 and PM10 during the period of preparation and first-trimester of pregnancy was associated with GDM. This is consistent with previous research results (31–34). Systemic inflammation, oxidative stress dysregulation of adipokines, and imbalance of gut microbiota induced by air pollution can lead to insulin resistance, which is the underlying mechanism of gestational diabetes mellitus (GDM) in pregnant women (8).
Previous studies (34, 35) on air pollution and GDM have mostly focused on the first and second trimester of pregnancy. And research shows that pre pregnancy may also be an important exposure window period for air pollution exposure to affect GDM (36). Multivariate logistic regression analysis showed that the risk of GDM increases by 4.5%, 6.0%, and 10.6% for every 10ug/m3 increase in the average exposure value of PM2.5 in preconception, first trimester, preconception and first trimester. And this is consistent with previous research results. Moreover, we have conducted a detailed analysis of the impact of each month on GDM occurrence, refining the exposure period to more accurately determine the critical exposure window. Particulate pollution varies in size, shape, and composition. Excepting PM2.5 and PM10, particulate pollution can be made up of a variety of components including acids, inorganic compounds, organic chemicals, soot, metals, soil or dust particles, and biological materials. These components may also be crucial to accurately assess their health effects, especially in vulnerable populations such as pregnant women. In future work, we will also further investigate the impact of other pollutants on the pregnancy outcomes of pregnant women.
Conclusions
GDM remains to be the most common metabolic disturbance during pregnancy, which has significant short and long-term complications for both the mother and the offspring. Exposure to high concentrations of PM2.5 and PM10 can increase the risk of GDM. Women in high-risk areas should pay attention to prenatal protection, especially during the preparation and early stages of pregnancy. We can develop the habit of checking the air quality index report every day to keep abreast of the level of particulate matter pollution in real time. If the air quality is poor, we can try not to go out or take protective measures when going out. In addition, we can also use air purifiers and other methods to reduce the inhalation of polluted air.
Our study had some limitations. This study is epidemiological and mechanism about the association between maternal exposure to air pollution and GDM should be deeply explored. And moreover, due to limitations of clinical data, the lack of clinical data on pregnant women’s height, weight, and weight gain during pregnancy can lead to analyzed biases. But this study has a large amount of data and high reliability of the experimental results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All participants consented in writing to participation, and the above protocols were approved by the ethics committee of Hebei Women and Children’s Health Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
M-LT: Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Funding acquisition. YJ: Data curation, Writing – original draft. L-YD: Data curation, Writing – review & editing. G-YZ: Formal analysis, Writing – review & editing. CZ: Formal analysis, Writing – original draft. G-JM: Data curation, Writing – review & editing. YS: Data curation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Youth Science and technology project of Hebei Health Commission (grant number: 20200001, 20230305).
Acknowledgments
We thank the patients, their families, and the investigators who participated in this trial.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. (2014) 103:341–63. doi: 10.1016/j.diabres.2013.10.012
2. Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. Bmj. (2022) 377:e067946. doi: 10.1136/bmj-2021-067946
3. Juan J, Yang H. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int J Environ Res Public Health. (2020) 17(24):9517. doi: 10.3390/ijerph17249517
4. Wong SF, Yap PS, Mak JW, Chan WLE, Khor GL, Ambu S, et al. Association between long-term exposure to ambient air pollution and prevalence of diabetes mellitus among Malaysian adults. Environ Health: Global Access Sci Source. (2020) 19:37. doi: 10.1186/s12940-020-00579-w
5. Aarthi GR, Mehreen Begum TS, Moosawi SA, Kusuma D, Ranjani H, Paradeepa R, et al. Associations of the built environment with type 2 diabetes in Asia: a systematic review. BMJ Open. (2023) 13:e065431. doi: 10.1136/bmjopen-2022-065431
6. Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. (2010) 121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1
7. Kelly FJ, Fussell JC. Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution. Free Radic Biol Med. (2017) 110:345–67. doi: 10.1016/j.freeradbiomed.2017.06.019
8. Park SK. Ambient air pollution and type 2 diabetes: do the metabolic effects of air pollution start early in life? Diabetes. (2017) 66:1755–7. doi: 10.2337/dbi17-0012
9. Garrison A. Screening, diagnosis, and management of gestational diabetes mellitus. Am Family Phys. (2015) 91:460–7.
10. Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and treatment of diabetes in China, 2013-2018. Jama. (2021) 326:2498–506. doi: 10.1001/jama.2021.22208
11. Tian ML, Du LY, Ma GJ, Zhang T, Ma XY, Zhang YK, et al. Secular increase in the prevalence of gestational diabetes and its associated adverse pregnancy outcomes from 2014 to 2021 in Hebei province, China. Front Endocrinol (Lausanne). (2022) 13:1039051. doi: 10.3389/fendo.2022.1039051
12. Sweeting A, Wong J, Murphy HR, Ross GP. A clinical update on gestational diabetes mellitus. Endocrine Rev. (2022) 43:763–93. doi: 10.1210/endrev/bnac003
13. Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. (2018) 19(11):3342. doi: 10.3390/ijms19113342
14. Schraufnagel DE, Balmes JR, De Matteis S, Hoffman B, Kim WJ, Perez-Padilla R, et al. Health benefits of air pollution reduction. Ann Am Thorac Soc. (2019) 16:1478–87. doi: 10.1513/AnnalsATS.201907-538CME
15. Hayes RB, Lim C, Zhang Y, Cromar K, Shao Y, Reynolds HR, et al. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int J Epidemiol. (2020) 49:25–35. doi: 10.1093/ije/dyz114
16. Orellano P, Reynoso J, Quaranta N, Bardach A, Ciapponi A. Short-term exposure to particulate matter (PM (10) and PM (2.5)), nitrogen dioxide (NO (2)), and ozone (O (3)) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ Int. (2020) 142:105876. doi: 10.1016/j.envint.2020.105876
17. Chandia-Poblete D, Cole-Hunter T, Haswell M, Heesch KC. The influence of air pollution exposure on the short- and long-term health benefits associated with active mobility: A systematic review. Sci Total Environ. (2022) 850:157978. doi: 10.1016/j.scitotenv.2022.157978
18. Pun VC, Kazemiparkouhi F, Manjourides J, Suh HH. Long-term PM2.5 exposure and respiratory, cancer, and cardiovascular mortality in older US adults. Am J Epidemiol. (2017) 186:961–9. doi: 10.1093/aje/kwx166
19. Wang B, Eum KD, Kazemiparkouhi F, Li C, Manjourides J, Pavlu V, et al. The impact of long-term PM (2.5) exposure on specific causes of death: exposure-response curves and effect modification among 53 million U.S. Medicare beneficiaries. Environ Health: Global Access Sci Source. (2020) 19:20. doi: 10.1186/s12940-020-00575-0
20. Li CY, Wu CD, Pan WC, Chen YC, Su HJ. Association between long-term exposure to PM2.5 and incidence of type 2 diabetes in Taiwan: A national retrospective cohort study. Epidemiol (Cambridge Mass). (2019) 30 Suppl1:S67–s75. doi: 10.1097/EDE.0000000000001035
21. Wu Y, Zhang S, Qian SE, Cai M, Li H, Wang C. et al: Ambient air pollution associated with incidence and dynamic progression of type 2 diabetes: a trajectory analysis of a population-based cohort. BMC Med. (2022) 20:375. doi: 10.1186/s12916-022-02573-0
22. Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Künzli N, et al. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect. (2015) 123:381–9. doi: 10.1289/ehp.1307823
23. Yang BY, Fan S, Thiering E, Seissler J, Nowak D, Dong GH, et al. Ambient air pollution and diabetes: A systematic review and meta-analysis. Environ Res. (2020) 180:108817. doi: 10.1016/j.envres.2019.108817
24. Lim CC, Thurston GD. Air pollution, oxidative stress, and diabetes: a life course epidemiologic perspective. Curr Diabetes Rep. (2019) 19:58. doi: 10.1007/s11892-019-1181-y
25. Hahad O, Lelieveld J, Birklein F, Lieb K, Daiber A, Münzel T. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. (2020) 21:4306. doi: 10.3390/ijms21124306
26. Li Y, Xu L, Shan Z, Teng W, Han C. Association between air pollution and type 2 diabetes: an updated review of the literature. Ther Adv Endocrinol Metab. (2019) 10:2042018819897046. doi: 10.1177/2042018819897046
27. Yi L, Wei C, Fan W. Fine-particulate matter (PM2.5), a risk factor for rat gestational diabetes with altered blood glucose and pancreatic GLUT2 expression. Gynecol Endocrinol. (2017) 33:611–6. doi: 10.1080/09513590.2017.1301923
28. Siddiqui K, George TP. Resistin role in development of gestational diabetes mellitus. biomark Med. (2017) 11:579–86. doi: 10.2217/bmm-2017-0013
29. Fitch MN, Phillippi D, Zhang Y, Lucero J, Pandey RS, Liu J, et al. Effects of inhaled air pollution on markers of integrity, inflammation, and microbiota profiles of the intestines in Apolipoprotein E knockout mice. Environ Res. (2020) 181:108913. doi: 10.1016/j.envres.2019.108913
30. Ghosh SS, Wang J, Yannie PJ, Ghosh S. Intestinal barrier dysfunction, LPS translocation, and disease development. J Endocr Soc. (2020) 4:bvz039. doi: 10.1210/jendso/bvz039
31. Cao L, Diao R, Shi X, Cao L, Gong Z, Zhang X, et al. Effects of air pollution exposure during preconception and pregnancy on gestational diabetes mellitus. Toxics. (2023) 11(9):728. doi: 10.3390/toxics11090728
32. Hu H, Ha S, Henderson BH, Warner TD, Roth J, Kan H, et al. Association of atmospheric particulate matter and ozone with gestational diabetes mellitus. Environ Health Perspect. (2015) 123:853–9. doi: 10.1289/ehp.1408456
33. Melody SM, Wills K, Knibbs LD, Ford J, Venn A, Johnston F. Maternal exposure to ambient air pollution and pregnancy complications in victoria, Australia. Int J Environ Res Public Health. (2020) 17(7):2572. doi: 10.3390/ijerph17072572
34. Choe SA, Eliot MN, Savitz DA, Wellenius GA. Ambient air pollution during pregnancy and risk of gestational diabetes in New York City. Environ Res. (2019) 175:414–20. doi: 10.1016/j.envres.2019.04.030
35. Yu G, Ao J, Cai J, Luo Z, Martin R, Donkelaar AV, et al. Fine particular matter and its constituents in air pollution and gestational diabetes mellitus. Environ Int. (2020) 142:105880. doi: 10.1016/j.envint.2020.105880
Keywords: air pollution, gestational diabetes mellitus, PM2.5, PM10, Hebei
Citation: Tian M-L, Jin Y, Du L-Y, Zhou G-Y, Zhang C, Ma G-J and Shi Y (2024) Air pollution exposure during preconception and first trimester of pregnancy and gestational diabetes mellitus in a large pregnancy cohort, Hebei Province, China. Front. Endocrinol. 15:1343172. doi: 10.3389/fendo.2024.1343172
Received: 23 November 2023; Accepted: 26 August 2024;
Published: 11 September 2024.
Edited by:
A. Seval Ozgu-Erdinc, Ankara City Hospital, TürkiyeReviewed by:
Farhad Saravani, Tehran University of Medical Sciences, IranReinaldo Marín, Instituto Venezolano de Investigaciones Científicas (IVIC), Venezuela
Copyright © 2024 Tian, Jin, Du, Zhou, Zhang, Ma and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei-Ling Tian, ODcwNDIyMzkxQHFxLmNvbQ==
 Mei-Ling Tian
Mei-Ling Tian Ying Jin1
Ying Jin1