
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 22 January 2025
Sec. Renal Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1342873
 Ching-Ming Lu1,2†
Ching-Ming Lu1,2† Yuan-Hsuan Hsu1,2†
Yuan-Hsuan Hsu1,2† I-Hsin Lin1†
I-Hsin Lin1† Ko-Lin Kuo3
Ko-Lin Kuo3 Jian-Fu Liao4
Jian-Fu Liao4 Hui-Fen Huang1,2
Hui-Fen Huang1,2 Ping-Hsun Lu1,2*
Ping-Hsun Lu1,2*Renal anemia stems mainly from chronic inflammation with elevated hepcidin levels, iron deficiency, and reduced red blood cell lifespan. Inadequate erythropoietin (EPO) production, worsened kidney function, leads to symptoms such as low energy, fatigue, and impaired physical function, significantly affecting patients’ quality of life. We conducted a comprehensive search across electronic databases including PubMed, Embase, Cochrane Library, Chinese National Knowledge Infrastructure, Airiti library, and Wanfang, to compile recent clinical trials and pilot studies on conventional and complementary alternative medicine approaches for renal anemia. This discussion focuses on the hypoxia-inducible factor prolyl hydroxylase domain (HIF-PHD) axis theory, from lab research to clinical applications. It explores non-extracorporeal treatments for renal anemia, including pharmaceutical interventions, dietary strategies, and complementary and alternative medicine (CAM). The article details the effects of Roxadustat, Ferumoxytol, and Epodion.
Clinical studies show that modulating the gut microbiome can reduce inflammation and improve renal anemia. Clinical trials suggest that CAM therapy can improve renal anemia through mechanisms such as enhanced iron metabolism, anti-inflammatory effects, reduced hepcidin levels, and increased EPO and HIF expressions. By synthesizing this information, the review aims to furnish valuable insights and treatment recommendations aimed at ameliorating renal anemia in individuals grappling with chronic kidney disease.
Presently, chronic kidney disease (CKD) is delineated by markers of kidney impairment, involving imaging or proteinuria (typically assessed through the albumin-to-creatinine ratio: ACR) and diminished renal function (falling below the glomerular filtration rate: GFR thresholds, estimated from the serum creatinine concentration) (1–3). CKD constitutes a pervasive global health challenge, and its frequency has been on the rise (4, 5). As kidney function progressively declines, the prevalence and severity of anemia escalate. Sir Richard Bright initially recognized anemia as a common complication of CKD in 1836, noting a pallor in the facial complexion of patients with kidney ailments (6). Historically, anemia in CKD has been attributed to numerous symptoms accompanying the diminishing renal function, including fatigue, reduced strength, heightened dyspnea during exertion. Studies conducted earlier established a robust correlation between higher hemoglobin levels and various parameters reflecting better physical functioning and quality of life (QoL) in patients at stages 3, 4, and 5 of CKD (7–9). The incidence of anemia in individuals with CKD rises concomitantly with the decline in GFR. For instance, the prevalence of anemia climbs from 1% among patients at stage 3 CKD to 67% at stage 5 (10, 11). Furthermore, the presence of anemia in CKD is linked to an elevated risk of cardiovascular disease, left ventricular hypertrophy, increased hospitalizations, cognitive impairment, and heightened mortality (12, 13).
Multiple factors contribute to the exacerbation of anemia in CKD, with the principal factor being linked to erythropoietin (EPO) deficiency. Additional contributors to renal anemia encompass inflammation, iron deficiency, uremic inhibitors, a reduction in red blood cell survival, and vitamin B12 deficiency (14). The role of inflammation is increasingly acknowledged as a pivotal factor in renal anemia, complicating the diagnosis of either iron deficiency or a reduction in EPO renal production (15–18). Refer to Figure 1 for a proposed mechanism elucidating renal anemia. In this scenario, renal disease prompts a reduction in EPO to inhibit bone-mediated RBC production. In CKD, bones excessively secrete the fibroblast growth factor 23 (FGF-23) hormone, capable of impeding EPO and RBC production, thereby contributing to renal anemia (19). In addition, FGF-23 elicits an inflammatory response and displays immunomodulatory properties (20). Numerous investigations provide evidence supporting the role of elevated FGF-23 as a causative factor in the initiation of renal anemia, chronic inflammation, and iron deficiency in the context of CKD (19, 21–23). The inflammation response in CKD, via FGF-23, promotes livers to increase interleukin-6 (IL-6) levels and induce hepcidin production (24–26). Increased hepcidin further inhibits intestinal iron absorption and iron release, leading to functional iron deficiency. This results in elevated expression of soluble transferrin receptor (sTfR), but the iron supply remains inadequate (27) The sTfR is the extracellular fragment derived from the cleavage of the cellular transferrin receptor. Diagnosis has progressed from traditional markers like ferritin to advanced tools such as sTfR and hepcidin, enhancing the detection of both absolute and functional iron deficiency anemia. The sTfR is not dependent on inflammation (28, 29). Consequently, the surge in hepcidin, binding to its cellular receptor ferroportin, obstructs macrophage iron release and intestinal iron absorption, leading to inhibited iron release from the liver and resulting in hypoferremia (30–32). Ferritin also increased linearly with increasing hepcidin (33).The overall effect of hepcidin is to curtail the availability of iron for active EPO, a phenomenon intricately linked to pathways involved in growth retardation in CKD (34–38). In renal disease, DNA relating to EPO becomes methylated, meaning that HIF cannot bind and promotes a decrease in EPO production. In addition, transferrin, an iron-transport glycoprotein, undergoes significant changes during acute phase responses in end-stage renal disease. In maintenance hemodialysis (mHD) patients, transferrin 2, transferrin 3, and transferrin 4 serum levels decrease, influenced by renal decline, prolonged mHD, and inflammation. These changes may contribute to persistent anemia (39).
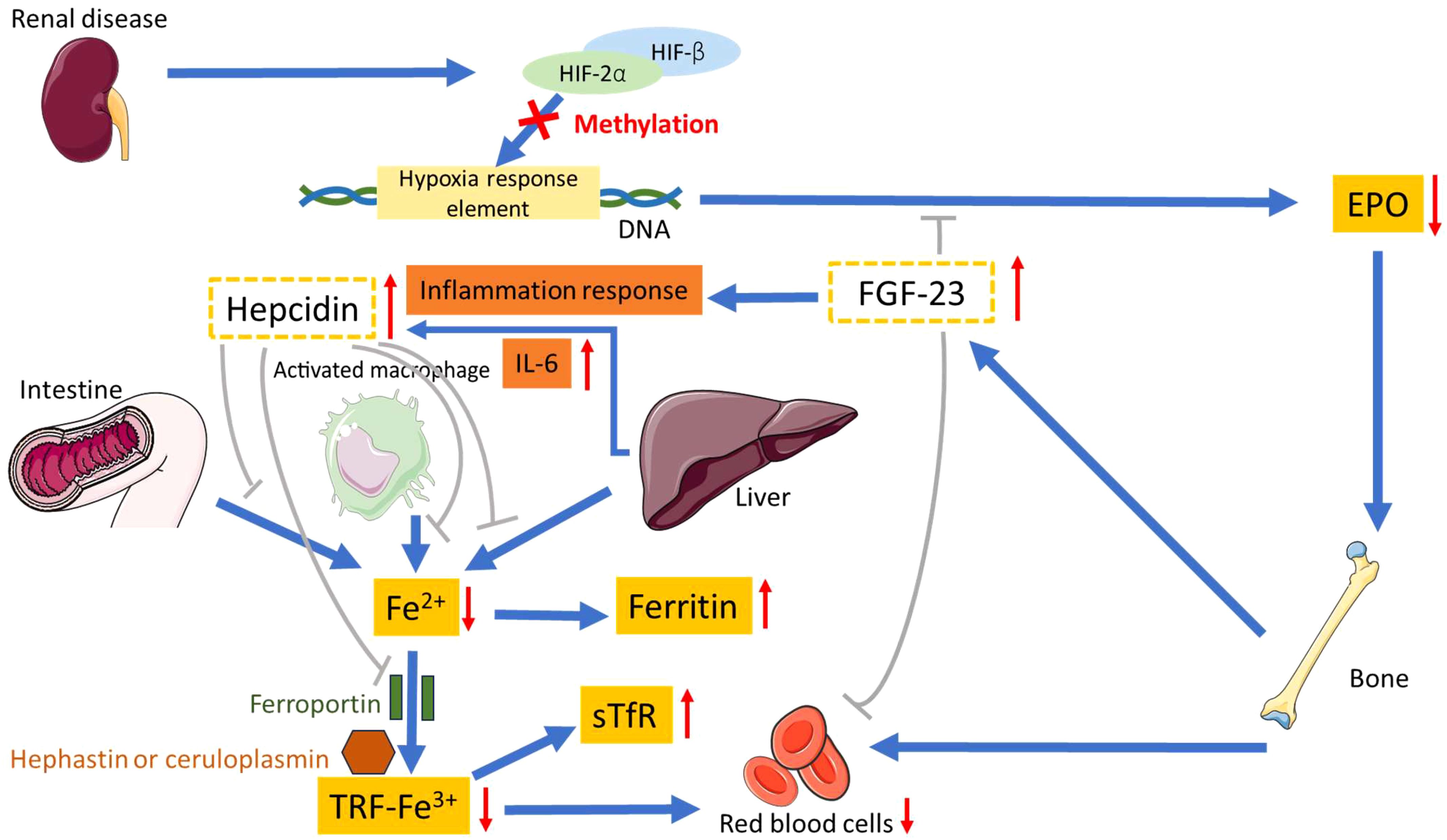
Figure 1. Proposed mechanism for renal anemia. HIF-2α, hypoxia-inducible factor-2α; HIF-β, hypoxia-inducible factor-β; EPO, erythropoietin; FGF-23, fibroblast growth factor 23; sTfR, soluble transferrin receptor; TRF, transferrin.
Figure 2 shows the therapeutic methods for renal anemia. Prolyl hydroxylase domain (PHD) oxygen sensors function as dioxygenases that modulate the activity of the hypoxia-inducible factor (HIF). This factor, in turn, governs the production of erythropoietin in both renal and hepatic contexts, orchestrating erythropoiesis in conjunction with iron metabolism (40–42). In hypoxic environments, the hydroxylation activity of PHDs experiences inhibition. This inhibition results in an elevation of the cellular concentration of HIF, leading to increased endogenous EPO production, enhanced iron absorption, and reduced levels of hepcidin. Consequently, the control of this pathway is commonly referred to as the HIF–PHD axis (42–44).
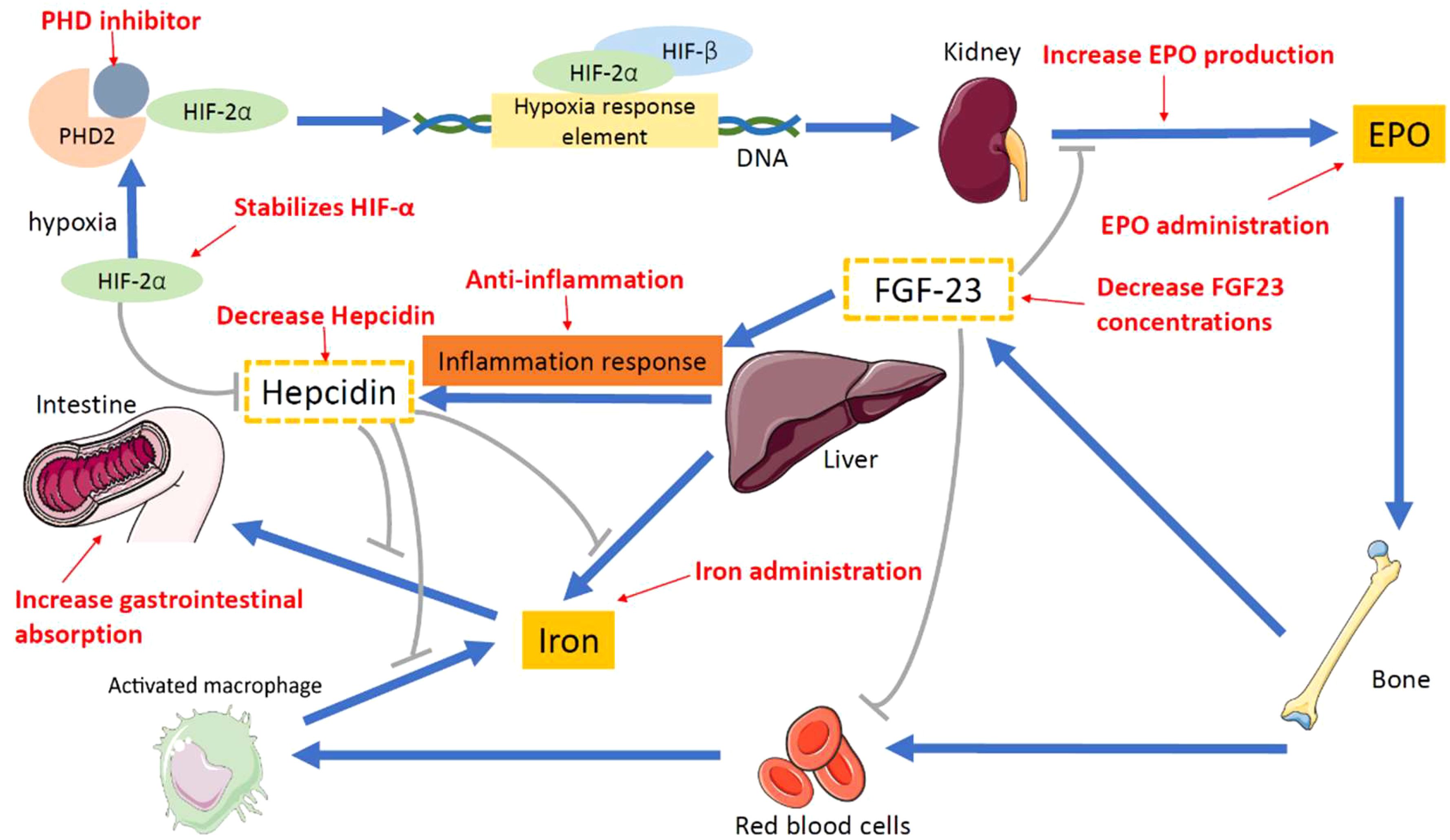
Figure 2. Proposed therapeutic methods for renal anemia. PHD inhibitor, prolyl hy-droxylase inhibitor; PHD, prolyl hydroxylase; HIF-2α, hypoxia-inducible factor-2α; HIF-β, hypoxia-inducible factor-β; EPO, erythropoietin; FGF-23, fibroblast growth factor 23.
Reviews of conventional treatments for renal anemia have been published (32, 45). However, they often entail side effects (46), necessitating exploration of alternative adjunct therapies. Current evidence suggests that dietary control, nutritional supplements, and complementary and alternative medicine (CAM) can improve renal anemia (47, 48). A comprehensive review article on medications, dietary control, nutritional supplements, and CAM for renal anemia is currently lacking. Therefore, we conducted a narrative review to assess the effectiveness of these treatments in patients with renal anemia. Given the aforementioned mechanisms, this review delineates potential non-extracorporeal approaches applicable from laboratory research to clinical application, aiming to elevate hemoglobin (Hb) levels and enhance renal function. The search encompassed databases from their inception to 1 August 2023 including Embase, Cochrane Library, PubMed, Airiti Library, Wanfang, and Chinese National Knowledge Infrastructure, using the term “renal anemia.” To augment the search scope, we conducted further scrutiny of included articles and citations, utilizing the “related articles” feature on PubMed. The structure of this paper is as follows: The initial section concentrates on pharmaceutical interventions, encompassing HIF-PHIs, iron supplements, and EPO products. The subsequent section delves into dietary control and supplementary therapies, encompassing nutraceuticals prebiotics, and probiotics. The concluding section expounds on the utilization of CAMs and supplementary therapeutic modalities.
In standard medical interventions that are implemented to control any current underlying diseases, the Hb serum concentration is mainly raised by administering drugs to slow down kidney deterioration. This therapeutic strategy involves augmenting the inherent synthesis of erythrocytes, ensuring ample iron levels for hemoglobin formation, reducing cytokine production and release, implementing antioxidative and anti-inflammatory processes, suppressing hepcidin, and addressing anemia (49), overseeing and safeguarding renal EPO-producing cells (REPs) during stressful conditions for the treatment of renal anemia (50, 51), enhancing the production of endogenous EPO, optimizing iron utilization under hypoxia (52), inhibiting prolyl hydroxylases, and modulating hepcidin activity (53). Moreover, new studies have shown that the simultaneous correction of iron deficiency and hyperphosphatemia in CKD reduces the magnitude of FGF-23 increase. Thus, using iron-based phosphate binders in CKD might mitigate cardiac and renal injury and improve survival (54). In addition, dialysis improves hematocrit levels by reducing the plasma volume and increases RBC mass by removing middle molecule uremic toxins that affect RBC survival and EPO efficiency (55). Here is a concise overview of the existing treatment approaches that can be employed to elevate Hb levels (Table 1).
By orally inhibiting hypoxia inducible factor prolyl hydroxylase (HIF-PH), Roxadustat induces erythropoiesis and modulates iron metabolism. This is achieved through the reduction of serum hepcidin levels and an increase in the absorption of iron in the intestine. Moreover, the increased levels of endogenous erythropoietin further increase hemoglobin levels and improve iron homeostasis (70–72). Additionally, Roxadustat exhibits the potential to enhance renal osteodystrophy (ROD) by concurrently handling bone remodeling. Moreover, the use of Roxadustat represents a potentially promising approach in the treatment of osteoporosis (73). During an 8week clinical trial, Roxadustat treatment was administered to 101 non-dialysis patients with stages 3–5 CKD. Subsequently, a notable reduction in both hepcidin and cholesterol levels was observed, leading to a significant elevation in hemoglobin levels (56).
Vadadustat, an innovative, adjustable, orally administered inhibitor of hypoxia inducible factor prolyl hydroxylase, can activate HIF signaling and induce endogenous erythropoietin synthesis, which stimulates iron mobilization and inhibits FGF-23 (74, 75). Vadadustat treatment in animal studies has ameliorated anemia and also decreased levels of serum urea nitrogen and creatinine concentrations alongside the appearance of kidney fibrosis markers (76). In a randomized clinical trial spanning 20 weeks, 138 non-dialysis patients with stages 3–5 CKD were subjected to Vadadustat treatment. Consistently, their hemoglobin levels showed an increase and were sustained, accompanied by heightened iron mobilization as evidenced by a significant rise in both reticulocytes and total iron binding. Moreover, Vadadustat treatment led to a notable reduction in both serum hepcidin and ferritin levels (57).
Daprodustat is an oral HIF-PH enzyme that inhibits PHD1, PHD2, and PHD3, which stimulates erythropoiesis. Meanwhile, it has the capacity to boost endogenous EPO production by stabilizing the HIF-α subunit, facilitating its dimerization with the HIF-β subunit, and activating target genes crucial for protecting against hypoxia, such as the erythropoietin gene (77). In a randomized clinical trial, 177 patients with HD switched from being treated with rhEPO to Daprodustat for 24 weeks, which worked to maintain and stabilize their Hb levels (58).
Molidustat, a orally bioavailable inhibitor of HIF-PH, emulates hypoxia by stabilizing the HIF-α subunits. It orchestrates a physiological response by triggering the transcription of erythropoietin and hypoxia inducible genes, encompassing those linked to erythropoiesis, angiogenesis, and mitochondrial metabolism (78). Following a 16week randomized clinical trial involving 121 dialysis patients, treatment with Molidustat resulted in a significant elevation of their hemoglobin levels (59, 79). Hence, Molidustat emerges as an effective and generally well-tolerated substitute for darbepoetin in the management of renal anemia.
Ferric citrate (FC) serves as an oral, calcium-free, iron-based phosphate binder, effectively lowering serum phosphorus levels by inhibiting phosphate absorption in the gastrointestinal tract and concurrently addressing anemia through iron supplementation (80). In addition, FC and HIF-PHIs have been shown to significantly decrease FGF-23 levels and renal anemia (81, 82). Moreover, numerous studies have indicated that ferric citrate may reduce circulating FGF-23 levels, potentially achieved through the modulation of dietary phosphate absorption (60, 83, 84). In a randomized clinical trial, 30 patients were treated with ferric citrate for 12 weeks, and their TSAT, ferritin, iron, and Hb were significantly increased (60).
Ferric maltol, a compound comprising ferric iron and maltol, a naturally occurring sugar derivative, demonstrates stability at a physiological environment. This complex remains securely chelated in the intestinal lumen until absorption, where the iron transport receptor on luminal enterocytes facilitates dissociation from maltol. Consequently, the absence of free iron in the gut diminishes the generation of hydroxyl radicals, minimizing the risk of gastrointestinal toxicity. In a 16week randomized clinical trial involving 111 patients, ferric maltol treatment resulted in significant increases in hemoglobin, ferritin, transferrin saturation, and serum iron levels. Notably, this treatment was well-tolerated for up to 1 year (61, 85).
Liposomal iron, a formulation of ferric pyrophosphate encapsulated within phospholipids and sucrose esters derived from the fatty acid membrane, represents an advanced generation of oral iron characterized by its superior gastrointestinal absorption, high bioavailability, and minimal incidence of side effects (62, 86). Utilizing advanced liposome-based technology as a carrier, this innovative approach ensures that iron bypasses direct contact with the gastrointestinal mucosa. Instead, absorption occurs directly in the intestine. Within the intestinal lumen, M cells in the small intestine, originating from the lymphatic system, directly absorb the liposome. Following this, macrophages incorporate the liposome intact through endocytosis into the lymphatic system, enabling it to reach hepatocytes (87). Then, lysosomal enzymes facilitate the opening of the liposome, leading to the release of iron. In a randomized clinical trial, 66 patients received oral liposomal iron for 3 months, and their Hb levels increased significantly, while the number of adverse events also decreased significantly (62).
Ferumoxytol is a formulation of iron that is delivered through an IV. There are only a limited number of gastrointestinal side effects from ferumoxytol treatment. However, its efficacy is notably enhanced in individuals with malabsorption syndromes or those who have undergone gastric surgery. Yet, it is important to note the potential for adverse events, which may encompass hypersensitivity reactions and anaphylactic shock in extremely rare instances (46, 63). In a clinical trial, 140 patients received IV ferumoxytol infusions and their Hb levels increased significantly and safety (63).
The utilization of intravenous iron treatments facilitates the prompt correction of iron-deficiency anemia. However, in comparison to iron isomaltoside, the use of ferric carboxymaltose resulted in a higher incidence of hypophosphatemia, which was mediated by fibroblast growth factor 23 (88, 89). In a randomized clinical trial involving 155 patients in the high ferritin group, a rapid and sustained achievement of consistent hemoglobin levels was observed. This outcome led to the delayed or reduced necessity for other anemia management interventions, such as erythropoiesis stimulating agents (ESAs), with no observed renal toxicity and no discernible difference in cardiovascular or infectious events (64).
The recommendation for high-dose, low-frequency intravenous iron treatments aims to potentially enhance symptoms, functional capacity, and overall quality of life for patients. In a randomized clinical trial involving 1027 individuals diagnosed with non-dialysis-dependent CKD, the administration of a single dose of iron isomaltoside resulted in a faster and transiently greater hemoglobin response compared to the treatment with multiple doses of iron sucrose. Additionally, lower rates of hypersensitivity reactions were observed, accompanied by a significant decrease in the incidence of composite cardiovascular diseases (65).
Ferric pyrophosphate citrate (FPC) stands out as a carbohydrate-free, water-soluble, complex iron salt capable of delivering iron through dialysate, effectively sustaining hemoglobin concentrations and iron balance. This innovative approach has demonstrated the potential to reduce the requirement for intravenous (IV) iron by approximately 80% (90). FPC exhibits the capability to traverse the dialyzer membrane, entering the bloodstream to directly contribute its iron to transferrin before undergoing rapid clearance. Moreover, FPC serves as a source of iron for erythropoiesis, circumventing iron sequestration within reticuloendothelial macrophages and hepcidin induced iron entrapment (90, 91). In a randomized clinical trial, which included 299 chronic hemodialysis patients, dialysate containing 2 μM FPC-iron was administered for 48 weeks. The Hb levels in the patients receiving the treatment increased and were maintained. Concurrently, FPC demonstrated a lack of increase in iron stores among the patients and exhibited a safe profile, suggesting its potential for future use (66).
Epodion, an alpha rhEPO, is a biosimilar exogenous EPO product. Epodion is a yellowish, transparent solution that can be injected intravenously (IV) or subcutaneously (SC). Moreover, in a clinical trial with 200 patients, epodion was administered for 52 weeks and resulted in an increase in Hb levels without demonstrating any adverse immunogenetic reaction (67, 92).
Methoxy polyethylene glycol epoetin beta, an attachment to an extensive methoxy polyethylene glycol polymer chain, represents a modified form of recombinant human erythropoietin epoetin. The introduction of these modifications has elevated the treatment’s efficacy, leading to less frequent administration and subsequently reducing the treatment burden on both patients and healthcare providers. However, the potential increase in cardiovascular risk associated with erythropoiesis stimulating agents prompted a randomized clinical trial involving 640 patients treated with methoxy polyethylene glycol epoetin beta compared to 644 patients receiving shorter acting epoetin alfa/beta agents, with both treatments administered to target hemoglobin levels of 10–12 g/dL. The trial results indicated that the once monthly methoxy polyethylene glycol epoetin beta treatment was non inferior to conventional, shorter acting erythropoiesis stimulating agents when assessing adverse cardiovascular events and all cause mortality (68).
Developed specifically for the management of anemia associated with chronic kidney disease, darbepoetin alfa represents a novel erythropoiesis stimulating protein (93). However, the utilization of erythropoietin stimulating agents (ESAs) may be associated with elevated blood pressure (BP), inflammation, hyperparathyroidism, and malnutrition (94). In a clinical trial, 18 patients were treated with darbepoetin alfa (DA) for 24 weeks and their Hb levels were maintained at 10–12 g/dL, while the office/ambulatory BP profiles also did not decline (69).
Renal anemia is a consequence of end-stage renal disease (ESRD). Contemporary investigations indicate a significant association between gut microbiota and the onset and progression of ESRD. Furthermore, there is a robust correlation between the gut microbiome and EPO hyporesponsiveness (EH) (95). Therefore, the modulation of gut microbiota is anticipated to represent an innovative therapeutic approach for CKD patients experiencing clinical refractory anemia. Furthermore, this intervention has the potential to decrease reliance on conventional ESA and iron agent medications, consequently mitigating the adverse effects associated with these drugs and enhancing the prognosis for individuals with kidney failure. The ensuing treatment strategies are succinctly examined below (Table 2).
Dietary fiber (DF), primarily obtained from plant-based foods, is a polysaccharide that undergoes limited digestion in the gastrointestinal tract and is instead utilized by the gut microbiota (97, 98). Nevertheless, certain bacteria present in the human gut have the capability to utilize dietary fiber (DF) as a catabolic substrate, leading to the production of substances like bile acids and short chain fatty acids (SCFAs). These compounds play a crucial role in modulating inflammation and oxidative stress. Consequently, SCFAs contribute to the overall well-being of the microbiome and mucosa, offering a range of health benefits, including antidiabetic, anticancer, antibacterial, anti-inflammatory, and antioxidative effects (99). Research findings indicate that elevated levels of short chain fatty acids (SCFAs) in feces and/or serum have been associated with alleviation of renal anemia (100, 101). Furthermore, additional studies have demonstrated that the presence of Bifidobacterium adolescentis, Lactobacillus, and Lactobacillaceae increased in the dietary fiber (DF) group. Interestingly, Lactobacillus and Lactobacillaceae were found to be positively correlated with hemoglobin (Hb) and Fe2+ levels, and inversely correlated with recombinant human erythropoietin (rhEPO) dosage (95). Therefore, the regulation of gut microbiota and modulation of short chain fatty acids (SCFAs) by dietary fiber (DF) present a potential avenue for enhancing renal anemia in individuals with end stage renal disease (ESRD).
Probiotics play a role in establishing a balance between pro- and anti-inflammatory cytokines, which could potentially be linked to renal anemia. Studies have shown that probiotic supplementation can decrease Hb fluctuations in hemodialysis patients; however, no significant increase was observed in the Hb level (96). Further, in a randomized clinical trial, 32 patients were provided with oral probiotic supplements for 3 months. Thereafter, a notable reduction was observed in both syndecan-1 and blood glucose levels, suggesting potential enhancements in metabolism and a decrease in systemic inflammation (47).
CAM therapy finds extensive application in addressing CKD with renal anemia. Mounting evidence indicates that CAM therapy holds the potential to ameliorate renal anemia by mechanisms including enhanced iron metabolism, anti-inflammatory effects, diminished hepcidin levels, and heightened expressions of EPO and HIF (102, 103). Hence, this section provides a concise overview of the positive impact of CAM therapies on CKD with renal anemia. The CAM therapeutic modalities employed for CKD encompass traditional Chinese medicine (TCM) decoction, TCM monomers, herbal monomers, acupoint application, acupoint injection, and ginger moxibustion (Table 3).
Shengxuening tablets (SXN) are an extract from silkworm excrement. The main component is chlorophyll and its derivatives, and they have a similar structure to heme, making them an effective biological iron supplement. Moreover, SXN can enhance the uptake of free iron and stimulate bone marrow cell proliferation. In a recent clinical trial, 94 patients with renal anemia undergoing mHD were randomly assigned to either the SXN group (receiving oral SXN tablets) or the FS group (oral treatment with ferrous succinate tablets). In both groups, Hb and transferrin saturation (TSAT) levels demonstrated a significant rise compared to the screening period. However, no notable distinction was observed between the two treatment groups. Nevertheless, the amount of EPO administered in the SXN group was less than that in the FS group, suggesting that the administration of SXN tablets can lead to a decrease in the utilization of EPO and effectively ameliorate renal anemia (104, 123). Furthermore, another recent clinical trial reported that SXN tablets combined with rHuEPO could increase Hb, TSAT, SF, and Hct levels and decrease the consumption of rHuEPO compared to the control group (105). Additionally, Lin et al. demonstrated that SXN tablets could improve iron metabolism and were a safe and effective treatment option, with a reduced dosage of EPO, for renal anemia in patients with stages 3–4 CKD (106).
Qingshen granules (QG) are a preparation used in TCM that has been clinically demonstrated to delay the progression of renal fibrosis in patients with CKD. Earlier investigations have also substantiated that QG can ameliorate the inflammatory condition in patients with CKD by decreasing the serum levels of IL-6, TNF-α, and hs-CRP, as well as mitigating renal fibrosis in patients concurrently experiencing chronic renal failure (123–126). In a preceding clinical study, disclosed in 2019, investigators examined the physiological data of 60 patients with CKD in stages 3–5. These participants were arbitrarily allocated into a QG group and a control group. After treatment for 12 weeks, the levels of HGB, HCT, RBC, SF, and TSAT had increased in both groups, and the levels of hs-CRP, IL-6, and hepcidin decreased; however, the improvements were more apparent within the QG group. The results indicated that QG can effectively improve renal anemia in CKD patients, potentially by enhancing iron metabolism through mitigating inflammation and reducing hepcidin levels (107).
Danggui Buxue decoction (DBD) is another TCM preparation. DBD is composed of Angelica and Astragalus and is often used to treat coronary heart disease and anemia. A study showed that DBD has Quercetin and can modulate multiple inflammatory proteins and pathways in response to renal anemia (127, 128). A recent clinical trial, which included 110 patients with renal anemia and dialysis-dependent CKD, showed a notable rise in RBCs, Hb, and HCT levels following treatment with DBD combined with L-carnitine for 3 months, compared to the control group. These results confirmed that DBD in conjunction with L-carnitine can improve anemia-related symptoms and renal function by reducing the damage of inflammatory mediators to renal tissue (108). A meta-analysis of seven studies revealed that DBD combined with conventional Western medicine (CWM) was more effective and safe than CWM alone (48).
In China, a Zishen Shengxue recipe (ZSR) consisting of eight herbs is often used to treat renal anemia with CKD. In a recent clinical study, 80 participants were enrolled and randomly allocated into two groups: EPO subcutaneous injection and EPO subcutaneous injection plus ZSR. Following 8 weeks of intervention, the ZSR group demonstrated a substantial elevation in the levels of Hb, HCT, and TSAT, in contrast to the control group, with a simultaneous significant reduction in Hep levels. Furthermore, a study by Wu et al. showed that ZSR combined with EPO had a definite clinically curative effect on patients with renal anemia, which was more advantageous than using EPO alone. A possible mechanism to explain this intervention is that Hep corrects the disorder of iron metabolism in the body and promotes the production of RBC (109).
Jianpi Shengxue tablets (JSTs) are a compound preparation of traditional Chinese and Western medicine, commonly used to treat anemia. Western medicine includes ferrous sulfate and vitamin C, while the TCM prescription includes common blood-enriching herbs, such as Codonopsis, Poria, Atractylodes macrocephala, licorice, astragalus, and gallinacean (129). Recently, a clinical trial compared the curative effects of JST and polysaccharide-iron complex capsules in the management of renal anemia without dialysis and found that the levels of Hb, RBCs, and HCT in the JST group showed a significant increase compared to the control group (110).
Jiawei Shiquandabu decoction (JSD) is a TCM preparation consisting of rhubarb, chuanxiong, ginseng, paeoniae alba, Atractylodes macrocephala, Angelica, Astragalus, cinnamon, licorice, Rehmannia and Poria. Previously, a clinical trial using JSD showed that administering JSD combined with EPO therapy for 3 months could increase the Hb and Hct levels in HD patients with renal anemia (111, 130).
Yishen Jiangzhuo decoction (YJD) is a TCM preparation that is composed of Pseudostellaria, Atractylodes macrocephala, Astragalus, Poria cocos, rhubarb, tangerine peel, angelica, mulberry, salvia, achyranthes bidentata, plantain seed, Serissa japonica, mulberry, and motherwort. YJD was shown to reduce Scr, BUN, and CRP levels, and increase RBC, Hb, and HCT levels. Moreover, the herbs in YJD, such as astragalus, angelica, and ginseng have been shown to stimulate bone marrow hematopoiesis and participate in erythropoiesis, possibly by upregulating HIF expression, activating downstream signaling, and promoting erythropoiesis (112).
Acupoint application is an external treatment method in TCM, which can be used to determine the effect of medicine and acupuncture point stimulation at the same time. A recent clinical trial with 100 patients with CKD used EPO subcutaneous injections plus acupoint application (the medicinal ingredients: Astragalus, Eucommia, Dipsacus, raw rhubarb, Angelica, motherwort, Chuanqiong, raw oysters, Radix Aconiti) at Zusanli (ST36), Geshu (BI17), Pishu (BI20), and Shenshu (BI23) for 2 months; the patients demonstrated an increase in Hb and Hct levels in the acupoint application group, surpassing those in the control group (113). In addition, acupoint application is also effective in reducing BUN and serum creatinine levels (131). In a different clinical study, 100 participants were enrolled and randomly assigned to two groups: EPO subcutaneous injection and EPO subcutaneous injection plus Wenshen Jiedu ointment (the medicinal ingredients: Aconite, Rhizoma Chuanxiong, cinnamon, Asarum, Paeoniae alba, fairy pill, rhubarb, Pinellia, agarwood) acupoint application at Zusanli (ST36) and Shenshu (BI23). Zhu et al. indicated that Hb and Hct were significantly improved after treatment with Wenshen Jiedu ointment before and after treatment and between groups (114).
Acupoint injection therapy injects drugs into acupoints, stimulates acupoints and meridians through acupuncture and medicinal liquid, and integrates meridians, acupoints, and drug effects. It represents a successful example of the clinical utilization of combined traditional Chinese and Western medicine (117, 132). A clinical trial by Cheng et al. found that the EPO Zusanli acupoint injection improved EPO resistance and enhanced the efficacy of EPO by alleviating the microinflammatory state of the body (115).
Houttuynia cordata is a TCM herb that is often used to prevent and treat colds and has been clinically proven to have anti-inflammatory effects. In a preliminary investigation released in 2006, scientists examined physiological data from 43 non-dialysis CKD patients. These individuals were arbitrarily allocated into two groups: EPO subcutaneous injection plus Houttuynia cordata Shenshu (BI23) and Zusanli (ST36) acupoint injection, and EPO subcutaneous injection. The results indicated a more significant increase in Hb after acupoint injection with Houttuynia cordata (116).
Modern pharmacological studies have shown that Astragalus has a similar effect to erythropoietin, and can promote the production, development, and maturation of various blood cells. Furthermore, a clinical trial, which included patients with stage 4 CKD, revealed that treatment with EPO subcutaneous injection plus astragalus Shenshu(BI23), Zusanli(ST36), Sanyinjiao(SP6), Pishu(BI20) acupoint injection facilitated a notable rise in Hb, Hc, and Ret levels, in contrast to the EPO subcutaneous injection group. Tan et al. indicated that Astragalus acupoint injections could yield a therapeutic effect on stage 4 renal anemia in CKD and diminish the usage of EPO, although the specific mechanism of action needs further study (117).
Moxibustion is a CAM therapy involving the combustion of dried moxa at specific acupoints on the body. The principle of the moxibustion effect is to induce a warming effect alongside radiation and pharmacology effects (130). Ginger moxibustion is a kind of indirect moxibustion, which achieves a therapeutic effect through the dual warming and medicinal effects of moxa and ginger. A clinical trial involving 80 patients with CKD found that ginger moxibustion therapy led to a significant decrease in BUN, serum CRP, and creatinine levels, while inducing Hb levels. Moreover, Liu et al. indicated that ginger moxibustion has the potential to markedly enhance the inflammatory condition in CKD patients, increase Hb levels, improve renal function, and reduce disease progression (118).
Siwu granules, encompassing Angelica, Ligusticum, and Rehmannia glutinosa, are employed in TCM and constitute a classical formula for promoting blood circulation. Wu et al. found that in rats with adenine-induced renal injury, the administration of Siwu granules in conjunction with EPO treatment elevated the expression of EPO and EPOR in renal tissues by enhancing the expression of endogenous EPO or mitigating EPO resistance. Furthermore, it was observed that in the Siwu plus EPO group, oxidative stress and inflammatory factors were inhibited, resulting in improved renal function and anemia (119).
Jian-Pi-Yi-Shen (JPYS) is a TCM formulation comprising eight herbs, often utilized in the management of CKD and associated complications, including anemia. JPYS enhanced red blood cells (RBCs), hemoglobin (HGB), and hematocrit (HCT) levels by triggering the expressions of EPO and hypoxia-inducible factor-2 alpha (HIF-2α) proteins in rats with anemia induced by 5/6 nephrectomy (103).
Panax notoginseng (PN) is a TCM herb, which is available in two forms. Moreover, it is frequently employed to address hematological issues, with unprocessed PN utilized for inflammation and pain treatment, and processed steamed PN (SPN) serving as a “blood-enriching” remedy to alleviate anemia and boost overall immunity. A study on rats with adenine-induced renal anemia suggested that treatment with SPN could alleviate renal anemia by restoring the expression of EPO mRNA in the kidneys and EPO receptor mRNA in bone marrow nucleated cells (120).
Luteolin (Lut) is a natural flavonoid found ubiquitously in the diet and possessing numerous biological activities (133). A group of HgCl2 mice displaying anemia were treated with Lut, which could alleviate the anemia. Further studies have shown that Lut inhibits PHD2 in the kidney, a finding supported by a molecular docking study, and reinstates the expression of downstream proteins of PHD2, namely HIF-2α and erythropoietin. Additionally, Lut alleviates renal oxidative stress by enhancing the expression of antioxidant enzymes downstream of HIF-2α. To sum up, Lut alleviates renal anemia in mice by blocking the PHD2/HIF-2α axis and mitigating oxidative stress (121).
Jujube polysaccharides (JPs) represent a category of active dietary glycans present in the fruit of Ziziphus jujuba. The application of jujube extract yielded positive outcomes, including the modulation of EPO through the activation of hypoxia-inducible factor (HIF) induced erythropoietin, the potential capability to recycle heme iron during erythrophagocytosis, and the bidirectional regulation of the immune response (134).Therefore, the suggested function of jujube in nourishing the blood is being proposed (135). A previous study has shown in a CKD rat model that treatment with JP substantially improved renal function and mitigated kidney pathological damage, while elevating RBC, Hb, hematocrit, and platelet counts (122). Additionally, JP stimulated the release of short-chain fatty acids (SCFAs) in rats with CKD, along with modulating the levels of kidney EPO mRNA and kidney EPO protein through HIF-α signaling.
Elevated ferritin levels and reduced iron saturation were associated with lower hemoglobin levels, with ferritin increasing further in advanced CKD stages. Anemia management in CKD varies by disease stage and dialysis status, requiring a tailored approach (136). Evaluating iron metabolism in CKD is intricate and should be individualized to ensure optimal care.
Various interventions, encompassing medications, dietary control, nutritional supplements, and CAM, can elicit distinct mechanisms to ameliorate renal anemia and enhance kidney function. These mechanisms include heightened EPO production, increased gastrointestinal absorption of iron, elevated iron concentrations, reduction in FGF-23 and hepcidin levels, inflammation inhibition, and stabilization of HIF-2α. The existing evidence underscores the potential applications of these therapeutic approaches. Furthermore, a growing body of research suggests a correlation between the intestine and renal anemia, emphasizing the role of dietary regulation and modulation of intestinal microbiota in mitigating the severity of renal anemia. Recent studies indicate that improving renal anemia levels in CKD can positively impact patient survival by mitigating mortality and pathological consequences associated with CKD. While certain Chinese medicines exhibit nephroprotective and renal anemia-improving properties, caution is warranted as some Chinese medicines may have detrimental effects on the kidneys. Notably, an increasing number of studies propose that the synergistic effects of combining Western medicine with Chinese medicine may more effectively address renal anemia and reduce reliance on Western medications like EPO. Additionally, dietary control, nutritional supplements, and CAM have fewer side effects than traditional treatments, making it a safer option for prolonged use. However, additional large-scale trials are imperative to validate their efficacy in improving renal anemia and elucidating the associated mechanisms.
C-ML: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Visualization. Y-HH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. I-HL: Writing – review & editing. K-LK: Supervision, Writing – review & editing. J-FL: Writing – review & editing. H-FH: Writing – review & editing. P-HL: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received financial support from the Buddhist Tzu Chi Medical Foundation, Taiwan (Grant Number: TCMF-CM1-111-03) and Taipei Tzu Chi Hospital (TCRD-TPE-111-07, TCRD-TPE-113-07, and TCRD-TPE-NSTC-113-06).
We extend our gratitude to all our colleagues at Taipei Tzu Chi Hospital for their invaluable assistance in conducting this study. Our sincere appreciation goes to the Core Laboratory of the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, for providing essential technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that Generative AI was used in the creation of this manuscript. Chatgpt was used to correct English grammar. OpenAI. (version 2.0, 2024). ChatGPT [Large language model]. https://chatgpt.com.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease - A systematic review and meta-analysis. PloS One. (2016) 11(7):e0158765. doi: 10.1371/journal.pone.0158765
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
2. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: A review. Jama. (2019) 322(13):1294–304. doi: 10.1001/jama.2019.14745
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
3. Webster AC, Nagler EV, Morton RL, Masson P, Kelly J, Johnson DW, et al. Chronic kidney disease. Lancet. (2017) 389(10075):1238–52. doi: 10.1016/S0140-6736(16)32064-5
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
4. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS, Gillen DL, et al. Prevalence of chronic kidney disease in the United States. Jama. (2007) 298(17):2038–47. doi: 10.1001/jama.298.17.2038
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
5. Saran R, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. (2018) 71(3 Suppl 1):A7. doi: 10.1053/j.ajkd.2018.01.002
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
6. Cases and Observations, Illustrative of Renal Disease. Accompanied with the secretion of albuminous urine. Br Foreign Med Rev. (1840) 10(20):301–29. doi: 10.1016/S0140-6736(02)80114-3
7. Finkelstein FO, Wuerth D, Finkelstein SH, Juergensen P, Kliger A, Raj DS, et al. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol. (2009) 4(1):33–8. doi: 10.2215/CJN.00630208
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
8. Finkelstein FO, Finkelstein SH. The impact of anemia treatment on health-related quality of life in patients with chronic kidney disease in the contemporary era. Adv Chronic Kidney Dis. (2019) 26(4):250–2. doi: 10.1053/j.ackd.2019.04.003
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
9. Gregg LP, Jain N, Carmody T, Lentine KL, Abbott KC, Ho PM, et al. Fatigue in CKD: epidemiology, pathophysiology, and treatment. Clin J Am Soc Nephrol. (2021) 16(9):1445–55. doi: 10.2215/CJN.19891220
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
10. Kovesdy CP, Trivedi BK, Anderson JE, Kalantar-Zadeh K, Norris KC, Kopple JD, et al. Prevalence of anaemia in adults with chronic kidney disease in a representative sample of the United States population: analysis of the 1999-2018 National Health and Nutrition Examination Survey. Clin Kidney J. (2023) 16(2):303–11. doi: 10.1093/ckj/sfac240
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
11. Astor BC, Coresh J, Heiss G, Pettitt D, Sarnak MJ, Schreiber MJ, et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988-1994). Arch Intern Med. (2002) 162(12):1401–8. doi: 10.1001/archinte.162.12.1401
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
12. KDOQI; National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. (2006) 47(5 Suppl 3):S11–145. doi: 10.1053/j.ajkd.2006.03.010
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
13. McCullough PA, Lepor NE. The deadly triangle of anemia, renal insufficiency, and cardiovascular disease: implications for prognosis and treatment. Rev Cardiovasc Med. (2005) 6(1):1–10. doi: 10.1016/j.carrev.2005.06.002
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
14. Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. (2012) 23(10):1631–4. doi: 10.1681/ASN.2011111078
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
15. Malyszko J, Mysliwiec M. Hepcidin in anemia and inflammation in chronic kidney disease. Kidney Blood Press Res. (2007) 30(1):15–30. doi: 10.1159/000098522
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
16. Ueda N, Takasawa K, Yoshikawa M, Nishiyama K, Matsui T, Yamaguchi T, et al. Impact of inflammation on ferritin, hepcidin and the management of iron deficiency anemia in chronic kidney disease. Nutrients. (2018) 10(9):1–5. doi: 10.3390/nu10091173
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
17. Lanser L, Fuchs D, Kurz K, Weiss G, Kainz A, Mittermayr M, et al. Physiology and inflammation driven pathophysiology of iron homeostasis-mechanistic insights into anemia of inflammation and its treatment. Nutrients. (2021) 13(11):1–7. doi: 10.3390/nu13113732
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
18. Costa E, Rocha-Pereira P, Rocha S, Castro E, Miranda V, Loureiro A, et al. Aging is associated with impaired renal function, INF-gamma induced inflammation and with alterations in iron regulatory proteins gene expression. Aging Dis. (2014) 5(6):356–65. doi: 10.14336/ad.2014.0500356
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
19. Czaya B, Faul C. The role of fibroblast growth factor 23 in inflammation and anemia. Int J Mol Sci. (2019) 20(17):1–9. doi: 10.3390/ijms20174195
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
20. David V, Francis C, Babitt JL. Ironing out the cross talk between FGF23 and inflammation. Am J Physiol Renal Physiol. (2017) 312(1):F1–f8. doi: 10.1152/ajprenal.00359.2016
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
21. Agoro R, White KE, Burnett-Bowie SA, Wolf M, Moe SM, Chonchol M, et al. Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia. FASEB J. (2018) 32(7):3752–64. doi: 10.1096/fj.201700667R
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
22. Baloglu I, Guney A, Tuncel M, Bayrak S, Arici B, Bakir B, et al. The relationship between FGF23 and anemia in HD and renal transplant patients. Int Urol Nephrol. (2022) 54(5):1117–22. doi: 10.1007/s11255-021-02982-9
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
23. David V, Martin A, Isakova T, Wang X, Epstein M, Goodman WG, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. (2016) 89(1):135–46. doi: 10.1038/ki.2015.290
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
24. Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. (2003) 102(3):783–8. doi: 10.1182/blood-2003-03-0672
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
25. Troutt JS, Butterfield AM, Konrad RJ. Hepcidin-25 concentrations are markedly increased in patients with chronic kidney disease and are inversely correlated with estimated glomerular filtration rates. J Clin Lab Anal. (2013) 27(6):504–10. doi: 10.1002/jcla.2013.27.issue-6
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
26. D’Angelo G. Role of hepcidin in the pathophysiology and diagnosis of anemia. Blood Res. (2013) 48(1):10–5. doi: 10.5045/br.2013.48.1.10
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
27. Vogt AS, Avner BS, Zhang H, Jackson DS, Wilson GJ, Martinez LF, et al. On iron metabolism and its regulation. Int J Mol Sci. (2021) 22(9):1–10. doi: 10.3390/ijms22094591
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
28. Iriarte-Gahete M, Ruiz-Torres M, Martin-Borreguero P, Lopez-Garcia S, Fernandez-Cuenca F, Martinez-Jimenez JL, et al. Absolute and functional iron deficiency: Biomarkers, impact on immune system, and therapy. Blood Rev. (2024) 68:101227. doi: 10.1016/j.blre.2024.101227
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
29. Tarancon-Diez L, Gomez-Rivero P, Jimenez-Aguilera L, Rodriguez-Lopez R, Sanchez-Gonzalez D, Carrillo-Cruz E, et al. Threshold ferritin concentrations reflecting early iron deficiency based on hepcidin and soluble transferrin receptor serum levels in patients with absolute iron deficiency. Nutrients. (2022) 14(22):1–9. doi: 10.3390/nu14224739
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
30. Cucuianu A, Patiu M, Rusu A. Hepcidin and multiple myeloma related anemia. Med Hypotheses. (2006) 66(2):352–4. doi: 10.1016/j.mehy.2005.08.041
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
31. Wojtaszek E, Glogowski T, Malyszko J. Iron and chronic kidney disease: still a challenge. Front Med (Lausanne). (2020) 7:565135. doi: 10.3389/fmed.2020.565135
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
32. Borawski B, Malyszko J, Bissonnette J, Van Wyck D, Coyne D, Kalantar-Zadeh K, et al. Current status of renal anemia pharmacotherapy-what can we offer today. J Clin Med. (2021) 10(18):1–23. doi: 10.3390/jcm10184149
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
33. Galetti V, Zimmermann MB, Moretti D, Wegmuller R, Zeder C, Hurrell RF, et al. Threshold ferritin and hepcidin concentrations indicating early iron deficiency in young women based on upregulation of iron absorption. EClinicalMedicine. (2021) 39:101052. doi: 10.1016/j.eclinm.2021.101052
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
34. Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr. (2017) 106(Suppl 6):1559s–66s. doi: 10.3945/ajcn.117.155804
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
35. Cui Y, Wu Q, Zhou Y. Iron-refractory iron deficiency anemia: new molecular mechanisms. Kidney Int. (2009) 76(11):1137–41. doi: 10.1038/ki.2009.357
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
36. Akchurin O, Xu Y, Puri S, Hashemi ES, Karasik E, Yu A, et al. Lack of hepcidin ameliorates anemia and improves growth in an adenine-induced mouse model of chronic kidney disease. Am J Physiol Renal Physiol. (2016) 311(5):F877–f889. doi: 10.1152/ajprenal.00089.2016
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
37. Hanudel MR, Eisenga MF, Rappaport M, Gabayan V, Esparza A, Friedmann Angeli JP, et al. Increased serum hepcidin contributes to the anemia of chronic kidney disease in a murine model. Haematologica. (2017) 102(3):e85–8. doi: 10.3324/haematol.2016.150433
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
38. Kulaksiz H, Gehrke SG, Janetzko A, Rost D, Bents K, Stremmel W, et al. Pro-hepcidin: expression and cell specific localisation in the liver and its regulation in hereditary haemochromatosis, chronic renal insufficiency, and renal anaemia. Gut. (2004) 53(5):735–43. doi: 10.1136/gut.2003.022863
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
39. Formanowicz D, Formanowicz P. Transferrin changes in haemodialysed patients. Int Urol Nephrol. (2012) 44(3):907–19. doi: 10.1007/s11255-011-9947-4
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
40. Sanghani NS, Haase VH. Hypoxia-inducible factor activators in renal anemia: current clinical experience. Adv Chronic Kidney Dis. (2019) 26(4):253–66. doi: 10.1053/j.ackd.2019.04.004
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
41. Voit RA, Sankaran VG. Stabilizing HIF to ameliorate anemia. Cell. (2020) 180(1):6. doi: 10.1016/j.cell.2019.12.010
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
42. Cao JY, Liu BC. Current insights into the role of HIF-PHD axis in renal anemia. Sheng Li Xue Bao. (2018) 70(6):623–9.
43. Schödel J, Ratcliffe PJ. Mechanisms of hypoxia signalling: new implications for nephrology. Nat Rev Nephrol. (2019) 15(10):641–59. doi: 10.1038/s41581-019-0182-z
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
44. Jelkmann W. Regulation of erythropoietin production. J Physiol. (2011) 589(Pt 6):1251–8. doi: 10.1113/jphysiol.2010.195057
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
45. Heras-Benito M. Renal anemia: current treatments and emerging molecules. Rev Clin Esp (Barc). (2023) 223(7):433–9. doi: 10.1016/j.rce.2023.04.005
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
46. Avni T, Biener A, Fishman N, Levi A, Gafter-Gvili A, Shpilberg O, et al. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clin Proc. (2015) 90(1):12–23. doi: 10.1016/j.mayocp.2014.10.007
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
47. de Araú ÉMR, da Silva AS, Coutinho ND, de Melo LGN, Camargo MRA, Alves TP, et al. Use of probiotics in patients with chronic kidney disease on hemodialysis: a randomized clinical trial. J Bras Nefrol. (2022). doi: 10.1590/2175-8239-JBN-2022-0021en
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
48. Zhao MM, Yuan Y, Mou X, Wang YL, Li F, Tian JW, et al. Efficacy and safety of Danggui Buxue Decoction in combination with western medicine treatment of anemia for renal anemia: a systematic review and meta-analysis. Ann Transl Med. (2017) 5(6):136. doi: 10.21037/atm.2017.01.17
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
49. Gluba-Brzózka A, Franczyk B, Rysz J, Banach M, Koziarska-Rościszewska M, Piotr C. The influence of inflammation on anemia in CKD patients. Int J Mol Sci. (2020) 21(3):1–23. doi: 10.3390/ijms21030725
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
50. Geng G, Qiu J, Zhang H, Li X, Liu W, Wang S, et al. Receptor-mediated mitophagy regulates EPO production and protects against renal anemia. Elife. (2021) 10:1–24. doi: 10.7554/eLife.64480
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
51. Sasaki Y, Nemoto Y, Kaneko S, Nakamura T, Matsumura T, Masuda S, et al. Erythropoietin stimulation decreases hepcidin expression through hematopoietic activity on bone marrow cells in mice. Int J Hematol. (2012) 96(6):692–700. doi: 10.1007/s12185-012-1217-4
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
52. Yan MT, Chao CT, Lin SH. Chronic kidney disease: strategies to retard progression. Int J Mol Sci. (2021) 22(18):1–23. doi: 10.3390/ijms221810084
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
53. Malyszko J. New renal anemia drugs: is there really anything new on the horizon? Expert Opin Emerg Drugs. (2014) 19(1):1–4. doi: 10.1517/14728214.2014.872239
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
54. Courbon G, Martinez-Calle M, David V. Simultaneous management of disordered phosphate and iron homeostasis to correct fibroblast growth factor 23 and associated outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. (2020) 29(4):359–66. doi: 10.1097/MNH.0000000000000614
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
55. Korbet SM. Comparison of hemodialysis and peritoneal dialysis in the management of anemia related to chronic renal disease. Semin Nephrol. (1989) 9(1 Suppl 1):9–15.
56. Chen N, Hao C, Liu BC, Lin H, Yin A, Hao L, et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. (2019) 381(11):1001–10. doi: 10.1056/NEJMoa1813599
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
57. Pergola PE, Spinowitz BS, Coyne DW, Dahl N, Friedman A, Lin T, et al. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. (2016) 90(5):1115–22. doi: 10.1016/j.kint.2016.07.019
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
58. Meadowcroft AM, Liu W, Bian A, Liu S, Desai M, Liu Y, et al. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis. Clin Kidney J. (2019) 12(1):139–48. doi: 10.1093/ckj/sfy014
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
59. Macdougall IC, Akizawa T, Berns JS, Bernhardt T, Drescher D, Reusch M, et al. Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. (2019) 14(1):28–39. doi: 10.2215/CJN.02510218
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
60. Womack R, Mudge DW, Wang E, Liu Y, Ooi E, Block GA, et al. Effect of ferric citrate versus ferrous sulfate on iron and phosphate parameters in patients with iron deficiency and CKD: A randomized trial. Clin J Am Soc Nephrol. (2020) 15(9):1251–8. doi: 10.2215/CJN.15291219
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
61. Pergola PE, Kopyt NP. Oral ferric maltol for the treatment of iron-deficiency anemia in patients with CKD: A randomized trial and open-label extension. Am J Kidney Dis. (2021) 78(6):846–856.e1. doi: 10.1053/j.ajkd.2021.03.020
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
62. Pisani A, Riccio E, Sabbatini M, Andreucci M, Del Vecchio L, Esposito P, et al. Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: a randomized trial. Nephrol Dial Transplant. (2015) 30(4):645–52. doi: 10.1093/ndt/gfu357
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
63. Khan H, Stone J, Chi T, Coulton C, Robb C, Anderson M, et al. Safety and efficacy of a single total dose infusion (1020 mg) of ferumoxytol. Ther Adv Hematol. (2021) 12:20406207211006022. doi: 10.1177/20406207211006022
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
64. Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, et al. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant. (2014) 29(11):2075–84. doi: 10.1093/ndt/gfu201
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
65. Bhandari S, Kalra PA, Kothari J, Ambühl PM, Coyne DW, Twerenbold R, et al. Safety and efficacy of iron isomaltoside 1000/ferric derisomaltose versus iron sucrose in patients with chronic kidney disease: the FERWON-NEPHRO randomized, open-label, comparative trial. Nephrol Dial Transplant. (2021) 36(1):111–20. doi: 10.1093/ndt/gfaa011
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
66. Fishbane SN, Singh AK, Cournoyer SH, Jindal KK, Fanti P, Guss CD, et al. Ferric pyrophosphate citrate (Triferic) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Transplant. (2015) 30(12):2019–26. doi: 10.1093/ndt/gfv277
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
67. Angginy N, Wibisono J, Wibisono L. Cohort prospective study to evaluate immunogenicity of epodion® (Biosimilar epoetin-a) in anemia associated with chronic kidney disease (CKD) patients. Acta Med Indones. (2022) 54(3):397–405.
68. Locatelli F, Ponikowski P, Filippatos G, Zoccali C, Anker SD, Cleland JGF, et al. Cardiovascular safety and all-cause mortality of methoxy polyethylene glycol-epoetin beta and other erythropoiesis-stimulating agents in anemia of CKD: A randomized noninferiority trial. Clin J Am Soc Nephrol. (2019) 14(12):1701–10. doi: 10.2215/CJN.01380219
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
69. Ohki K, Hasegawa M, Sano Y, Uchida M, Muto M, Yamamoto Y, et al. Effects of erythropoietin-stimulating agents on blood pressure in patients with non-dialysis CKD and renal anemia. Kidney Dis (Basel). (2020) 6(4):299–308. doi: 10.1159/000507396
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
70. Li ZL, Tu Y, Liu BC. Treatment of renal anemia with roxadustat: advantages and achievement. Kidney Dis (Basel). (2020) 6(2):65–73. doi: 10.1159/000504850
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
71. Dhillon S. Roxadustat: first global approval. Drugs. (2019) 79(5):563–72. doi: 10.1007/s40265-019-01077-1
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
72. Fishbane S, Pollock C, El-Shahawy M, Mendelssohn DC, McCullough PA, McGuire D, et al. Roxadustat versus epoetin alfa for treating anemia in patients with chronic kidney disease on dialysis: results from the randomized phase 3 ROCKIES study. J Am Soc Nephrol. (2022) 33(4):850–66. doi: 10.1681/ASN.2020111638
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
73. Li L, Zhang X, Wang W, Luo Y, Chen L, Xu M, et al. Roxadustat improves renal osteodystrophy by dual regulation of bone remodeling. Endocrine. (2023) 79(1):180–9. doi: 10.1007/s12020-022-03199-1
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
74. Ikeda Y. Novel roles of HIF-PHIs in chronic kidney disease: the link between iron metabolism, kidney function, and FGF23. Kidney Int. (2021) 100(1):14–6. doi: 10.1016/j.kint.2021.04.030
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
75. Chertow GM, Pergola PE, Agarwal R, Krempin J, Toto RD, O'Connor A, et al. Vadadustat in patients with anemia and non-dialysis-dependent CKD. N Engl J Med. (2021) 384(17):1589–600. doi: 10.1056/NEJMoa2035938
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
76. Hanudel MR, Chua K, Haase VH, Gales B, Jung G, Cohen A, et al. Amelioration of chronic kidney disease-associated anemia by vadadustat in mice is not dependent on erythroferrone. Kidney Int. (2021) 100(1):79–89. doi: 10.1016/j.kint.2021.03.019
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
77. Singh AK, Carroll K, Perkovic V, Koulouridis I, Leong RW, Berns JS, et al. Daprodustat for the treatment of anemia in patients not undergoing dialysis. N Engl J Med. (2021) 385(25):2313–24. doi: 10.1056/NEJMoa2113380
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
78. Akizawa T, Iwasaki M, Yamaguchi Y, Majikawa Y, Yamaguchi T, Onouchi Y, et al. Molidustat for Japanese patients with renal anemia receiving dialysis. Kidney Int Rep. (2021) 6(10):2604–16. doi: 10.1016/j.ekir.2021.07.015
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
79. Yamamoto H, Uchida S, Yamaguchi T, Majikawa Y, Onouchi Y, Kimura K. Efficacy and safety of molidustat for anemia in ESA-naive nondialysis patients: A randomized, phase 3 trial. Am J Nephrol. (2021) 52(10-11):871–83. doi: 10.1159/000518071
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
80. Li L, Ma X, Wang H, Chen Y, Zhang R, Liu P, et al. Ferric citrate for the treatment of hyperphosphatemia and anemia in patients with chronic kidney disease: a meta-analysis of randomized clinical trials. Ren Fail. (2022) 44(1):1112–22. doi: 10.1080/0886022X.2022.2094273
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
81. Agoro R, White KE. Anemia and fibroblast growth factor 23 elevation in chronic kidney disease: homeostatic interactions and emerging therapeutics. Curr Opin Nephrol Hypertens. (2022) 31(4):320–5. doi: 10.1097/MNH.0000000000000797
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
82. Abu-Zaid A, Hameed DA, Al-Hasani HA, Hassan RA, Omar YA, Ahmed RH, et al. The effect of iron supplementation on FGF23 in chronic kidney disease patients: a systematic review and time-response meta-analysis. Biol Trace Elem Res. (2021) 199(12):4516–24. doi: 10.1007/s12011-021-02598-1
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
83. Hanudel MR, Gales B, Jung G, Lunstroot K, Castellino S, Warady BA, et al. A review of ferric citrate clinical studies, and the rationale and design of the Ferric Citrate and Chronic Kidney Disease in Children (FIT4KiD) trial. Pediatr Nephrol. (2022) 37(11):2547–57. doi: 10.1007/s00467-022-05492-7
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
84. Ganz T, Bino A, Salusky IB. Mechanism of action and clinical attributes of auryxia(®) (Ferric citrate). Drugs. (2019) 79(9):957–68. doi: 10.1007/s40265-019-01125-w
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
85. Bazeley JW, Wish JB. Recent and emerging therapies for iron deficiency in anemia of CKD: A review. Am J Kidney Dis. (2022) 79(6):868–76. doi: 10.1053/j.ajkd.2021.09.017
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
86. de Alvarenga Antunes CV, Marinho A, Garcia V, Ferraz JG, Luque A, Dos Santos JV, et al. Treatment of iron deficiency anemia with liposomal iron in inflammatory bowel disease: efficacy and impact on quality of life. Int J Clin Pharm. (2020) 42(3):895–902. doi: 10.1007/s11096-020-01044-x
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
87. Kozubek A, Siniarska E, Piatek M, Jemiola-Rzeminska M, Michalak K. Liposomal drug delivery, a novel approach: PLARosomes. Acta Biochim Pol. (2000) 47(3):639–49. doi: 10.18388/abp.2000_3985
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
88. Wolf M, Koch TA, Bregman DB, Martin ER, Mathioudakis N, Shannon M, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. Jama. (2020) 323(5):432–43. doi: 10.1001/jama.2019.22450
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
89. Coppolino G, Bolignano D, Mazzaferro S, Gallieni M, Malindretos P, Polimeni A, et al. Iron infusion and induced hypophosphatemia: the role of fibroblast growth factor-23. Ther Apher Dial. (2020) 24(3):258–64. doi: 10.1111/1744-9987.13435
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
90. Gupta A, Crumbliss AL. Treatment of iron deficiency anemia: are monomeric iron compounds suitable for parenteral administration? J Lab Clin Med. (2000) 136(5):371–8. doi: 10.1067/mlc.2000.110368
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
91. Gupta A, Gupta R, Stenvinkel P, Kalantar-Zadeh K, Coyne DW, Kovesdy CP, et al. Dialysate iron therapy: infusion of soluble ferric pyrophosphate via the dialysate during hemodialysis. Kidney Int. (1999) 55(5):1891–8. doi: 10.1046/j.1523-1755.1999.00436.x
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
92. Angginy N, Andayani R, Wiratama BS, Prananta RA, Rochmah W, Wijayanti A, et al. Cohort prospective study to evaluate immunogenicity.pdf. Acta Med Indones - Indones J Intern Med. (2022) 54:397–405.
93. Ibbotson T, Goa KL. Darbepoetin alfa. Drugs. (2001) 61(14):2097–104; discussion 2105-6. doi: 10.2165/00003495-200161140-00007
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
94. Weir MR. Managing anemia across the stages of kidney disease in those hyporesponsive to erythropoiesis-stimulating agents. Am J Nephrol. (2021) 52(6):450–66. doi: 10.1159/000516901
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
95. Li Y, Liu X, Zhang L, Huang W, Wu L, Wang Z, et al. The prebiotic effects of soluble dietary fiber mixture on renal anemia and the gut microbiota in end-stage renal disease patients on maintenance hemodialysis: a prospective, randomized, placebo-controlled study. J Transl Med. (2022) 20(1):599. doi: 10.1186/s12967-022-03812-x
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
96. Shariaty Z, Akbari H, Ghasemi Z, Mohammadi J, Hedayati S. The effects of probiotic supplement on hemoglobin in chronic renal failure patients under hemodialysis: A randomized clinical trial. J Res Med Sci. (2017) 22:74. doi: 10.4103/jrms.JRMS_614_16
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
97. Lancaster SM, Coeuret A, Lacroix C, Zeller G, Bork P, Elinav E, et al. Global, distinctive, and personal changes in molecular and microbial profiles by specific fibers in humans. Cell Host Microbe. (2022) 30(6):848–862 e7. doi: 10.1016/j.chom.2022.03.036
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
98. So D, Seo Y, Kim HJ, Jung D, Lim H, Kim JY, et al. Dietary fibres and IBS: translating functional characteristics to clinical value in the era of personalised medicine. Gut. (2021) 70(12):2383–94. doi: 10.1136/gutjnl-2021-324891
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
99. Magliocca G, Damiani C, Porreca S, Coppolino G, Polimeni A, Malindretos P, et al. Short-chain fatty acids in chronic kidney disease: focus on inflammation and oxidative stress regulation. Int J Mol Sci. (2022) 23(10):1–24. doi: 10.3390/ijms23105354
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
100. Soriano-Lerma A, García-Fuentes E, Egües E, Pérez-Martínez E, Rodríguez-De Alba S, Martín-López M, et al. Gut microbiome-short-chain fatty acids interplay in the context of iron deficiency anaemia. Eur J Nutr. (2022) 61(1):399–412. doi: 10.1007/s00394-021-02645-6
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
101. Wang R, Wu J, Li Y, Zheng L, Feng L, Zhang X, et al. Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat Commun. (2022) 13(1):2522. doi: 10.1038/s41467-022-30240-8
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
102. Chen WD, Lin Q, Huang W, Zhang Y, Wang Y, Yang J, et al. The characteristics and prescription patterns of Chinese herbal medicine in clinical practice for the treatment of anemia. Taiwan J Obstet Gynecol. (2018) 57(4):570–7. doi: 10.1016/j.tjog.2018.06.030
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
103. Wang F, Luo M, Wang X, Zhang L, Zhang H, Huang X, et al. Jian-pi-yi-shen regulates EPO and iron recycling protein expressions in anemic rats with chronic kidney disease: accumulation of hypoxia inducible factor-2α via ERK signaling. Evid Based Complement Alternat Med. (2020) 2020:8894257. doi: 10.1155/2020/8894257
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
104. Tang XJ, Li H, Wang Y, Liu W, Zhang J, Chen X. Effect of sheng xue ning tablets on renal anemia in patients subject to maintenance hemodialysis and safety evaluation: A multi-setting prospective randomized study. Curr Med Sci. (2020) 40(2):327–31. doi: 10.1007/s11596-020-2179-z
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
105. Cheng X, Wei W, Liu Q, Zhu J. Clinical study of Shengxuening tablet combined with rHuEPO for the treatment of renal anemia of maintenance hemodialysis patients. Exp Ther Med. (2016) 12(1):157–60. doi: 10.3892/etm.2016.3307
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
106. Ju L, Zhang X, Huang F, Wei H, Zhou Q. Effect of Shengxuening in the treatment of renal anemia of CKD patients. Chin J Kidney Dis Invest. (2016) 5(5):223–6. doi: 10.3877/cma.j.issn.2095-3216.2016.05.008
107. Zhang L, Chen W, Li Z, Sun Q, Zhang J. Effect of Qingshen Granules on inflammation/hepcidin axis and iron metabolism in patients with renal anemia: a single-center, randomized controlled trial. Nan Fang Yi Ke Da Xue Xue Bao. (2019) 39:1155–9.
108. Hao L, Xu J, Wang Y, Zhang H, Chen X, Li F, et al. Clinical observation of danggui buxue decoction combined with L-carnitine in the treatment of renal anemia. World Chin Med. (2022) 17(01):108–11.
109. Xiaomei W, Zhang J, Li H, Chen X, Wang Q. Effect of Zishen Shengxue Decoction on Renal Anemia in Maintenance Hemodialysis and effects on iron metabolism and blood lipids. J Of Hebei TCM And Pharmacol. (2022) 37:44–7.
110. Xun J, Wei L, Zhang Z, Liu Y, Wang H, Chen X, et al. Comparison of therapeutic efficacy and safety of jianpi shengxue tablets and iron polysaccharide complex capsules in the treatment of nondialysis renal anemia. China Pharm. (2018) 29:1384–7. doi: CNKI:SUN:ZGYA.0.2018-10-022
111. Zhenhai Y. The effect of jiawei shiquandabu decoction combined with erythropoietin in the treatment of renal anemia. Mod Diagn Treat. (2016) 7:1202–4.
112. Haiyan R, Liang Z, Shulin H. Application effect of Yishen Jiangzhuo decoction in the treatment of renal anemia in stages 3 and 4 of chronic kidney disease. J Ningxia Med Univ. (2021) 43(02):181–5. doi: 10.16050/j.cnki.issn1674-6309.2021.02.017
113. Shi F, Tang S, Zhou J. Clinical research on acupoint application in auxiliary treatment of maintenance hemodialysis renal anemia. Chin J Integr Tradit West Nephrol. (2022) 23(08):724–5.
114. Zhu Y-j. Discussion on the therapeutic effect of wenshen jiedu ointment combined with meridian theory on renal anemia. Chin Foreign Med Treat. (2020) 39(22):178–80,183. doi: 10.16662/j.cnki.1674-0742.2020.22.178
115. Cheng Y. Observation on curative effect of EPO point injection on renal anemia. Nei Mongol J Tradit Chin Med. (2017) 23:70–71.
116. Xiong F, Guo S, Cao Y. Auxiliary therapeutic effect of acupoint injection on anemia in patients with chronic renal failure. Chin Acupunct Moxibust. (2006) 26.9:679–80.
117. Tan Y. Effect of acupoint injection on anemia index in patients with CKD stage 4 renal anemia. Shandong J Tradit Chin Med. (2015) 2015(4):271–2.
118. Liu Y, Ren Y, Hua Q. 40 cases of chronic renal failure treated by ginger partition moxibustion combined with conventional therapy. Tradit Chin Med Res. (2021) 34(08):38–42.
119. Wu Y, Chen J, Wang L, Zhang T, Sun H, Yang W, et al. Siwu granules and erythropoietin synergistically ameliorated anemia in adenine-induced chronic renal failure rats. Evid Based Complement Alternat Med. (2019) 2019:5832105. doi: 10.1155/2019/5832105
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
120. Gao M, Li F, Zhao J, Wang T, Liu S, Xu L, et al. Steamed Panax notoginseng attenuates renal anemia in an adenine-induced mouse model of chronic kidney disease. J Ethnopharmacol. (2022) 288:114941. doi: 10.1016/j.jep.2021.114941
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
121. Siyu L, Zhang Y, Xu J, Liu J, Li M, Wang L, et al. Dietary luteolin protects against renal anemia in mice. J Funct Foods. (2020) 65:103740. doi: 10.1016/j.jff.2019.103740
122. Huang S, Zhang W, Liu Q, Xu Y, Wang L, Zhao Z, et al. Jujube polysaccharides mitigated anemia in rats with chronic kidney disease: Regulation of short chain fatty acids release and erythropoietin production. J Funct Foods. (2021) 86:1–12. doi: 10.1016/j.jff.2021.104673
123. Ding L, Chen Y, Wang Z, Zhang H, Liu X, Li J, et al. Efficacy of SXN in the treatment of iron deficiency anemia: A phase IV clinical trial. Evid Based Complement Alternat Med. (2019) 2019:8796234. doi: 10.1155/2019/8796234
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
124. Liu JH, Wang Y-p. Curative clinical emcacy of qing-shen granule to changes of blood high sensitivity C-reactiVe protein and hemocysteine in damp_heat syndrome of chroIlic renal failure patients. J Chengdu Univ TCM. (2011) 34(2):30–34.
125. Wang Y-p, M Y-p. Effect of Qingshen grannule on serum tumor necrosis factors-a and soluble tumor necrosis factor receptor I in patients with sharp deterioration damp-heat symdrome of chronic renal failure. Chin J TCM WM Crit Care. (2006) 39(10):1155–9.
126. Wang Y-p, L W-j. Effect of Qinshen decotion on serum leptin and interleukin-6 in patinets with chronic renal failure and sharp deterioration of damp-heat syndrome. Chin J TCM WM Crit Care. (2005) 12:71–5.
127. Tu C, Zhang L, Wang F, Zhao R, Sun X, Li Y, et al. Exploring mechanisms by which danggui buxue decoction regulates inflammation and improves renal anemia based on network pharmacology. Natural Product Commun. (2022) 17(5):1–9. doi: 10.1177/1934578X221093905
128. Huang GC, Liu W, Chen X, Zhao J, Zhang Y, Xu L, et al. Effects of Dang-Gui-Bu-Xue-Tang, an herbal decoction, on iron uptake in iron-deficient anemia. Drug Des Devel Ther. (2016) 10:949–57. doi: 10.2147/DDDT.S94309
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
129. Chunfu L, XZ Z, Qiu T, Dongge Y. Dongge you, study on tonifying qi and producing blood mechanism of jianpi shengxue tablets. China Pharmacist. (2016) 19:209–12.
130. Peiyui Y, Chongya H, Tianshen Z. Current status of clinical research on the principle and function of moxibustion. Chin Med Herald. (2019) 16(12):31–34.
131. Zhao Y. 40 cases of chronic renal failure treated by acupoint application. World Latest Med Inform. (2013) 2013(26):115–6. doi: 10.3969/j.issn.1671-3141.2013.26.086
132. Sun TC. The application of external treatment of traditional Chinese medicine in the treatment of chronic renal failure. World Latest Med Inf. (2020) 20(95):175–6. doi: 10.3969/j.issn.1671-3141.2020.95.099
133. Cao YL, Wang X, Liu J, Zhang Y, Sun H, Zhao L, et al. Flavonoids in treatment of chronic kidney disease. Molecules. (2022) 27:2365. doi: 10.3390/molecules27072365
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
134. Chen J, Wu Y, Liu Z, Zhao X, Zhang H, Sun L, et al. The extract of Ziziphus jujuba fruit (jujube) induces expression of erythropoietin via hypoxia-inducible factor-1alpha in cultured Hep3B cells. Planta Med. (2014) 80:1622–7. doi: 10.1055/s-0034-1383049
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
135. Chen J, Tsim KWK. A review of edible jujube, the ziziphus jujuba fruit: A heath food supplement for anemia prevalence. Front Pharmacol. (2020) 11:593655. doi: 10.3389/fphar.2020.593655
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
136. Wong MMY, Zhang X, Chen L, Lee Y, Zhou Y, Sun K, et al. Anemia and iron deficiency among chronic kidney disease Stages 3–5ND patients in the Chronic Kidney Disease Outcomes and Practice Patterns Study: often unmeasured, variably treated. Clin Kidney J. (2019) 13:613–24. doi: 10.1093/ckj/sfz091
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Keywords: chronic kidney disease, diet control, dietary supplement, complementary and alternative medicine, renal anemia, conventional medical therapy
Citation: Lu C-M, Hsu Y-H, Lin I-H, Kuo K-L, Liao J-F, Huang H-F and Lu P-H (2025) Conventional and complementary alternative medicine therapies for renal anemia: a literature review. Front. Endocrinol. 15:1342873. doi: 10.3389/fendo.2024.1342873
Received: 12 December 2023; Accepted: 17 December 2024;
Published: 22 January 2025.
Edited by:
Francesco Locatelli, Alessandro Manzoni Hospital, ItalyReviewed by:
Elham Ahmadian, Tabriz University of Medical Sciences, IranCopyright © 2025 Lu, Hsu, Lin, Kuo, Liao, Huang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping-Hsun Lu, cGluZ2hzdW5sdUBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.