- 1Reproductive Medicine Center, Department of Obstetrics and Gynecology, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2Department of Obstetrics, Department of Obstetrics and Gynecology, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China
Objective: To evaluate the association between bedtime and infertility and to identify the optimal bedtime for women of reproductive age.
Methods: We conducted a cross-sectional study using data from 3,903 female participants in the National Health and Nutrition Examination Survey (NHANES) from 2015 to 2020. The effect of bedtime on female infertility was assessed using the binary logistic regression in different models, including crude model and adjusted models. To identify the non-linear correlation between bedtime and infertility, generalized additive models (GAM) were utilized. Subgroup analyses were conducted by age, body mass index (BMI), waist circumference, physical activity total time, marital status, smoking status, drinking status and sleep duration.
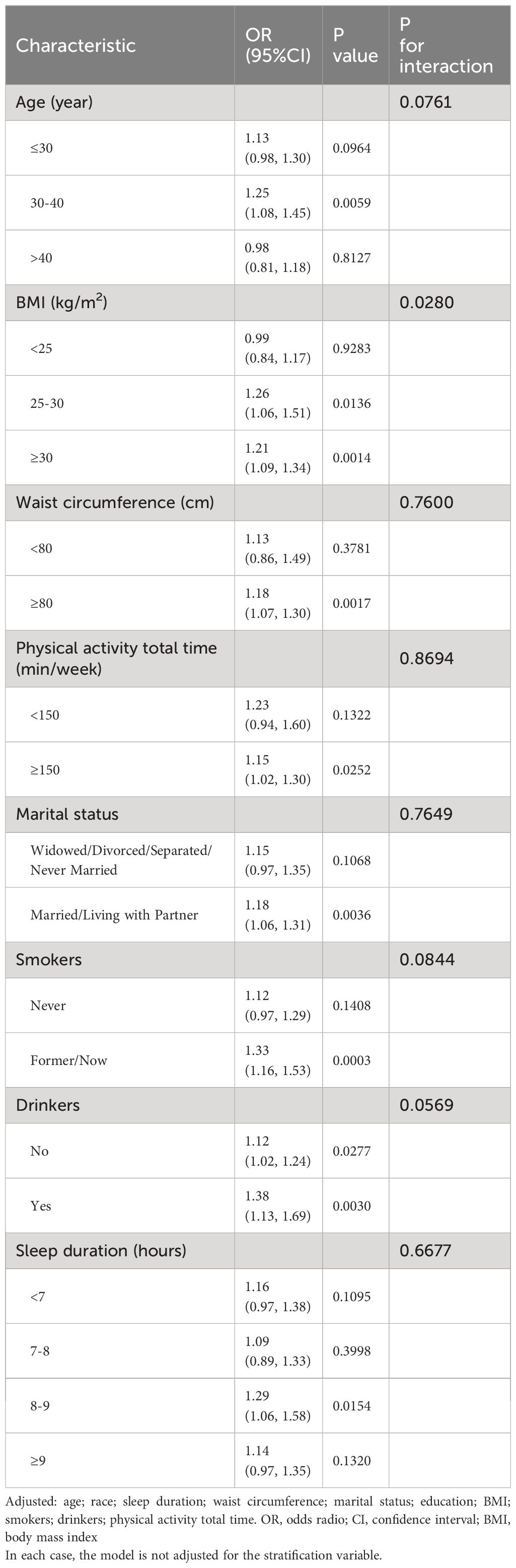
Results: After adjusting for potential confounders (age, race, sleep duration, waist circumference, marital status, education, BMI, smoking status, drinking status and physical activity total time), a non-linear relationship was observed between bedtime and infertility, with the inflection point at 22:45. To the left side of the inflection point, no significant association was detected. However, to the right of it, bedtime was positively related to the infertility (OR: 1.22; 95% CI: 1.06 to 1.39; P = 0.0049). Subgroup analyses showed that late sleepers with higher BMI were more prone to infertility than those with a lower BMI (BMI: 25–30 kg/m2: OR: 1.26; 95% CI: 1.06 to 1.51; P = 0.0136; BMI ≥ 30 kg/m²: OR: 1.21, 95% CI: 1.09 to 1.34; P = 0.0014).
Conclusion: Bedtime was non-linearly associated with infertility, which may provide guidance for sleep behavior in women of childbearing age.
Introduction
Infertility is defined as the inability to achieve a clinical pregnancy following 12 months of regular and unprotected sexual intercourse (1). Infertility is thought to affect millions of individuals and couples worldwide. The global incidence of infertility ranges from 9% to 18%, with a continuous increase in recent years (2, 3). In the United States, up to 15% of couples are affected by infertility (4). Infertility represents a widespread global health concern, highlighting the significance of identifying risk factors and prioritizing preventive measures.
Reproduction is intricately controlled by a multitude of hormonal mechanisms, and any disruptions in these hormonal regulations may potentially result in infertility. It is widely recognized that sleep patterns and alterations in circadian rhythms significantly impact the secretion of various reproductive hormones (5, 6). The circadian clock exerts its effect through the hypothalamic-pituitary-gonadal (HPG) axis and plays a key role in the regulation of reproductive function (7). Disruptions in the circadian rhythm, often originating from sleep behaviors, can lead to irregularities in the secretion of reproductive hormones, ultimately affecting reproductive function. Hence, it is crucial to further investigate the effect of sleep behaviors on infertility.
Previous studies have investigated the relationship between sleep behaviors and infertility. For example, research has indicated that unhealthy sleep behaviors due to night shift work can adversely affect reproductive function (8–10), and individuals diagnosed with infertility tend to display a nocturnal chronotype (11). These findings indicate a potential association between bedtime and infertility. However, the research on their association is limited. A previous cross-sectional study investigated the association between bedtime and infertility and found a positive linear association (12). However, it remains unclear whether a nonlinear relationship exists between bedtime and infertility, or if there might be an optimal bedtime.
The fast-paced nature of modern society has resulted in significant alterations to people’s lifestyles, particularly with regard to their sleep patterns. Due to the demands of work and study, an increasing number of individuals tend to delay their bedtime. Therefore, our study aims to explore the relationship between bedtime, a modifiable sleep behavior, and infertility, and identify an optimal bedtime. These would provide valuable health guidance for women in their childbearing age. These findings hold the potential to make substantial contributions to improving public health outcomes. This analysis was conducted using data obtained from the National Health and Nutrition Examination Survey (NHANES) database (RRID: SCR_013201), covering a nationally representative cohort of women aged 18 to 44 years. The data spanned the years 2015 to 2020.
Materials and methods
Study population
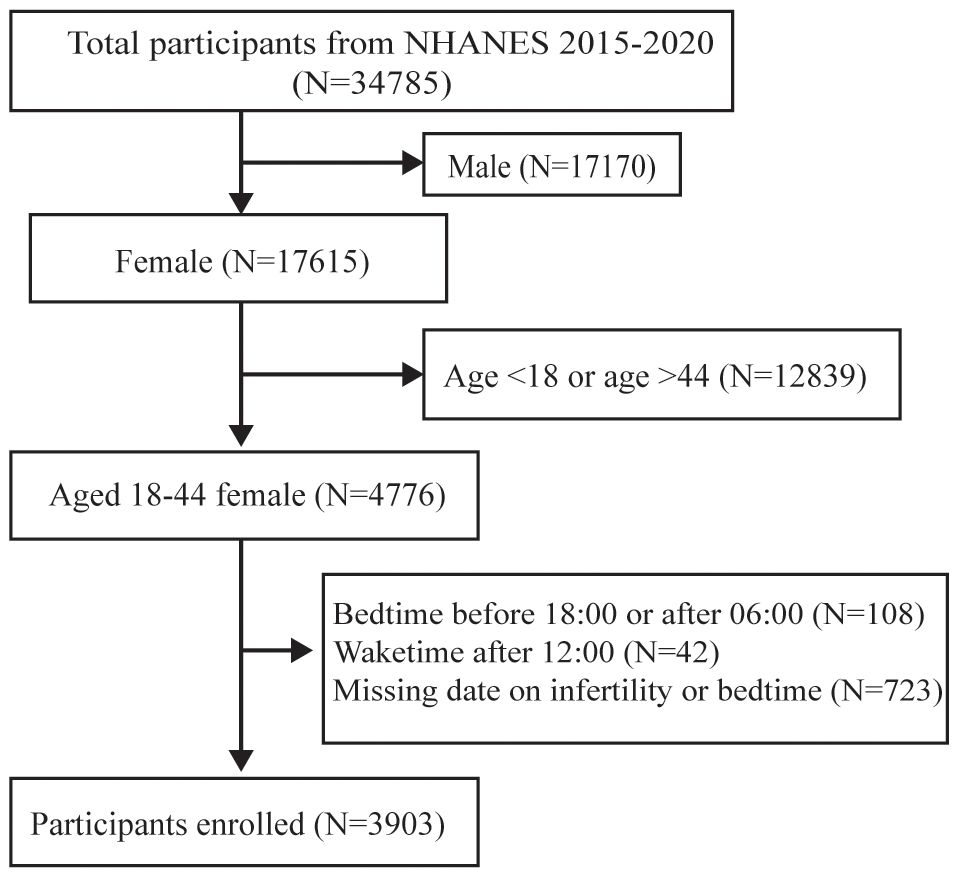
NHANES is a national cross-sectional survey designed to evaluate the health and nutritional status of the United States. The data of this study were retrieved from three consecutive cycles (2015–2016, 2017–2018, and 2019–2020) of the NHANES. A total of 3903 participants were recruited and selected according to standard exclusion criteria, as follows: 1) Male (n = 17,170); 2) age < 18 or age >44 (n = 12,839); 3) Bedtime before 18:00 or after 06:00 (n = 108); 4) Waketime after 12:00 (n=42); 5) Missing data on infertility or bedtime (n= 723). An illustration of the participant selection process is depicted in Figure 1. The NHANES project information is gathered biannually in a six-month cycle. The two periods span November 1 through April 30 and from May 1 to October 31, thus covering all the months in the year. The frequency distribution graph for ‘The two periods’ is shown in Supplementary Table S1. All research methods of the NHANES were conducted in accordance with the Declaration of Helsinki. NHANES approved by National Center for Health Statistics (NCHS) Ethics Review Board, is a major project of the NCHS within the Centers for Disease Control and Prevention (CDC). Because the NHANES database is publicly accessible, no additional ethical approvals are required. The information and resources within can be used by other researchers to replicate or reproduce the study. Access to the research design and data is available at https://www.cdc.gov/nhcs/nhanes/.
Main variables
Infertility is characterized by the inability to achieve conception following 12 months or longer of consistent and unprotected sexual intercourse, either due to diminished fertility of the individual or their partner. In our study, the evaluation of infertility status was derived from the NHANES Reproductive Health Questionnaire. Specifically, participants were asked the following inquiry, “Have you ever attempted to become pregnant over a period of at least a year without becoming pregnant?” (RHQ.074). Participants who responded with “yes” were classified as infertile.
Bedtime information was obtained from the NHANES Sleep Disorder Questionnaire, specifically through the query: “What time do you usually fall asleep on weekdays or workdays?” (SLQ.300).
Covariates
Covariates were selected according to previous researches, including age (RIDAGEYR), BMI (BMXBMI), waist circumference (BMXWAIST), race (RIDRETH3), marital status (DMDMARTZ), education (DMQ.141), physical activity (PAQ), sleep duration (SLD.012), smoking status (SMQ.040), and drinking status (ALQ). Participants reporting alcohol consumption exceeding 0 g/week were categorized as drinkers. The overall quantity of physical activity was calculated by adding time spent at work (PAD.615 and PAD.630), walking/bicycling (PAD.645), and recreational activities (PAD.660 and PAD.675).
Statistical analysis
The statistical analysis process was conducted in four steps. First, we examined the baseline characteristics of participants, who were categorized based on whether they had infertility or not, using a weighted sample. Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as percentages. Second, following the recommendation of the Strengthening the reporting ofobservational studies in epidemiology (STROBE) statement (13), logistic regression models were applied to estimate the independent correlation between bedtime and infertility before or after the adjustment of confounders. Third, the generalized additive models (GAM) were used to find the non-linear correlations between bedtime and infertility. Smooth curve fitting was performed to draw curves of the association after full adjustment. Bedtime is plotted on the X-axis, and the probability of infertility occurring is represented on the Y-axis. The probability is calculated using the formula P=1/(1+exp(-Y)). Red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. And the piecewise linear regression model was used to find the threshold impact of the bedtime on infertility. Fourth, subgroup analyses were carried out with the assistance of stratified linear regression models, and changes and interactions between subgroups were identified through likelihood ratio tests.
Data analysis was performed using R (The R Foundation; http://www.r-project.org; version 4.2.0) and EmpowerStats (www.empowerstats.net, X&Y solutions, Inc. Boston, Massachusetts). A two-sided P value of less than 0.05 was considered to indicate statistical significance.
Results
The selection of participates
Among the 34,785 participants, 30,882 were not included in the analysis. Out of these, 17,170 were male, 12,839 were with age <18 or > 44, 108 with bedtime before 18:00 or after 06:00, 42 with waketime after 12:00, and 723 showed missing information on infertility or bedtime. Consequently, the study focused on a subset of 3,903 individuals for further investigation (Figure 1).
Baseline characteristics of participants
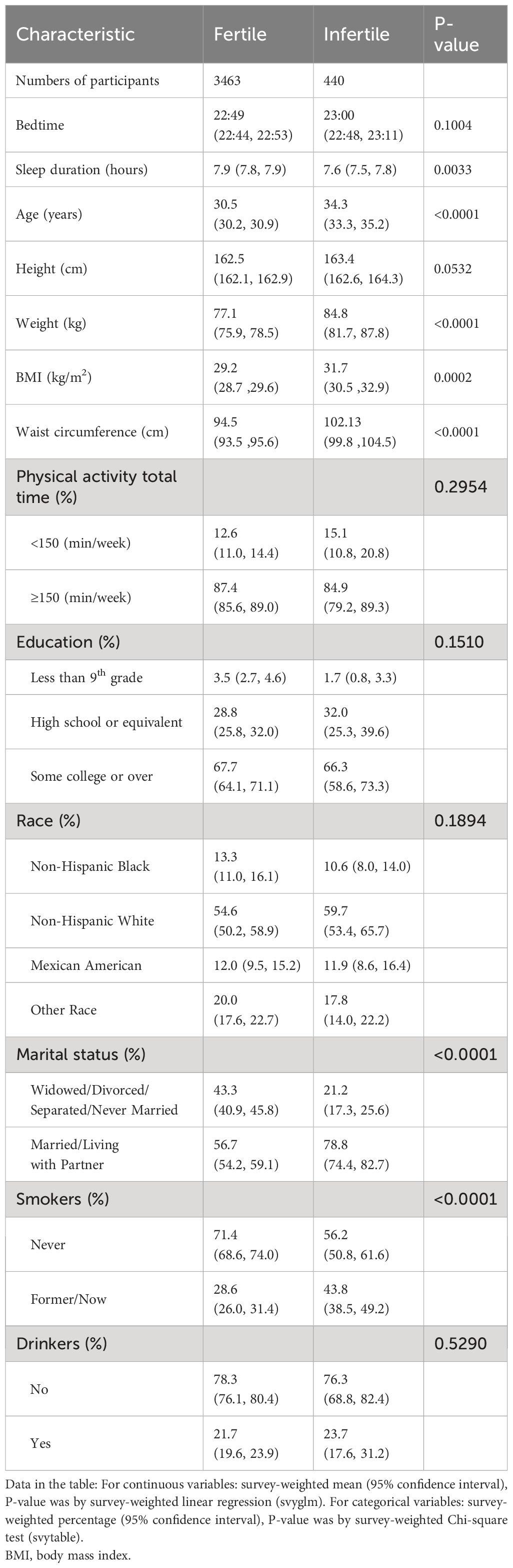
Table 1 illustrates the baseline characteristics of the participants who were selected for the study. There were 440 in the infertile group and 3463 in the fertile group. Participants with infertility had an older age (34.3 years vs. 30.5 years, P < 0.0001), higher BMI (31.7 vs 29.2, P = 0.0002), and higher waist circumference (102.1 vs. 94.5, P < 0.0001). As for the sleep-related variates, participants with infertility had significantly shorter sleep duration (7.9 hours vs. 7.6 hours, P = 0.0033). Additionally, individuals in infertility group were more likely to be smokers. However, there was no statistically significant difference in education, physical activity, race, and drinking status between fertile and infertile groups.
The relationship between bedtime and infertility
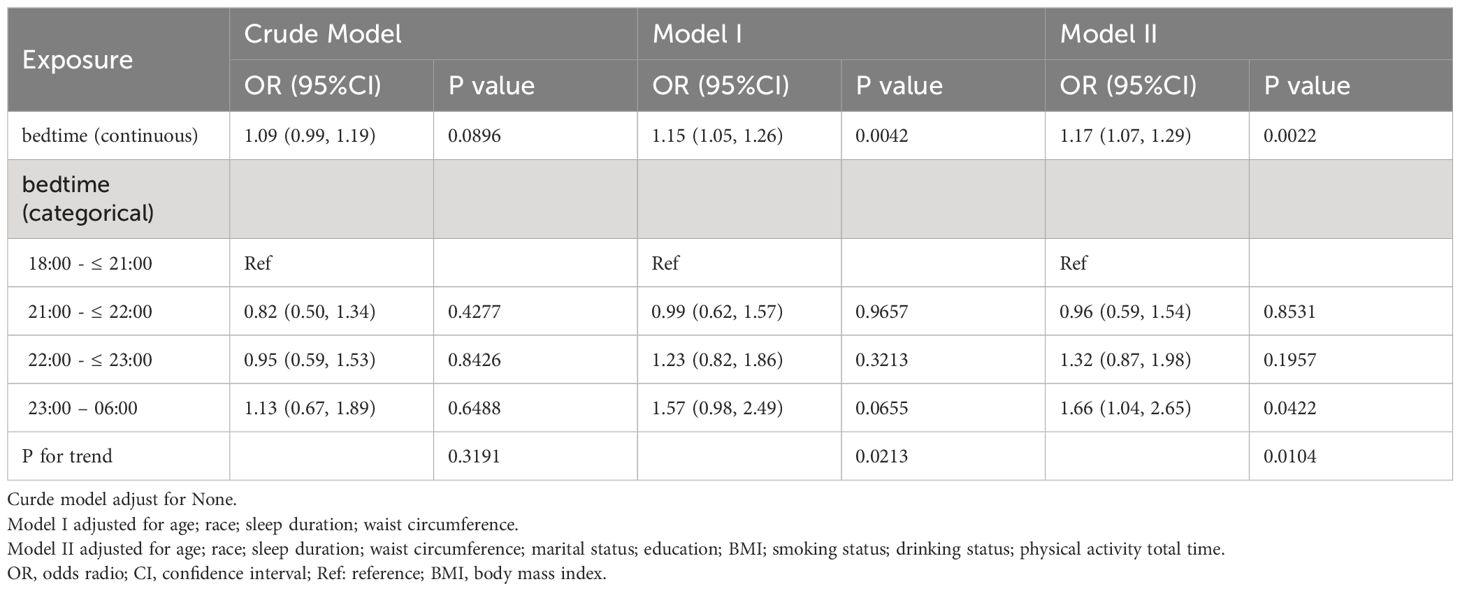
Weighted binary logistic regression models were employed to assess the correlation between bedtime and infertility (Table 2). In the crude model, bedtime didn’t show correlation with infertility (OR = 1.09, 95% confidence interval (CI): 0.99, 1.19, P = 0.0896). In the minimally adjusted model (adjusted age, race, sleep duration and waist circumference), bedtime had positive correlation with infertility (OR = 1.15, 95% CI: 1.05, 1.26, P = 0.0042). In the fully adjusted model, the association remained statistically significant (OR = 1.17, 95% CI: 1.07, 1.29, P = 0.0022). A similar trend was observed when bedtime was treated as a categorical variable in the sensitivity analysis (p for trend = 0.0104). Additionally, based on the fact that NHANES project information is gathered biannually in a six-month cycle, we included the examination period as an additional adjustment variable in Model III. As shown in Supplementary Table S2, the inclusion of this covariate did not result in significant changes to the effect estimates compared to Model II.
The analyses of non-linear relationship
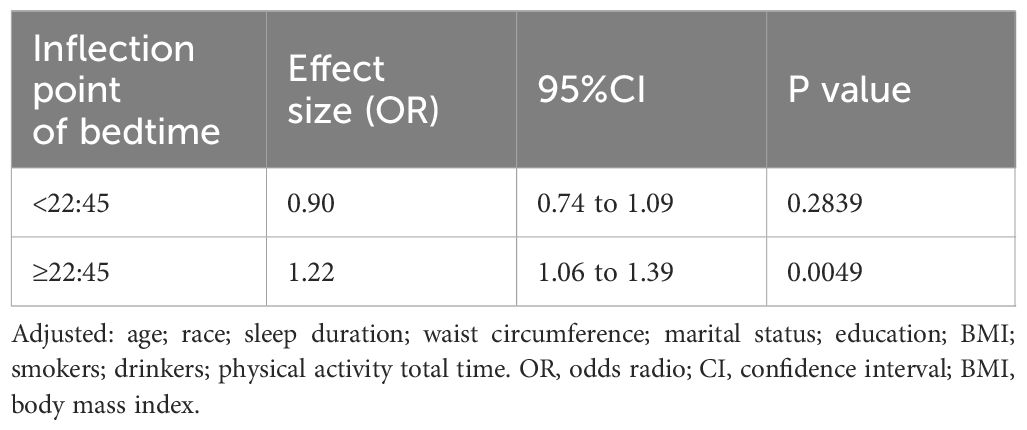
To comprehensively examine the association between bedtime and infertility, a smoothing curve fitting was performed (Figure 2). We found non-linear relationship between bedtime and infertility, after adjusting age, race, sleep duration, BMI, waist circumference, marital status, education, smoking status, drinking status and physical activity. By employing a two-piecewise linear regression model, we were able to identify that the inflection point was situated at 22:45 (Table 3). To elucidate, on the left of the inflection point, the effect size, 95% CI and P value were 0.90, 0.74 to 1.09, 0.2839. However, on the right side of the inflection point, a clear positive correlation between bedtime and infertility was observed (OR: 1.22; 95% CI: 1.06 to 1.39; P = 0.0049). In sensitivity analyses that included participants with bedtime phases from 20:00 to 4:00, the non-linear relationship and inflection points remained stable (Supplementary Figure S1, Supplementary Table S5).
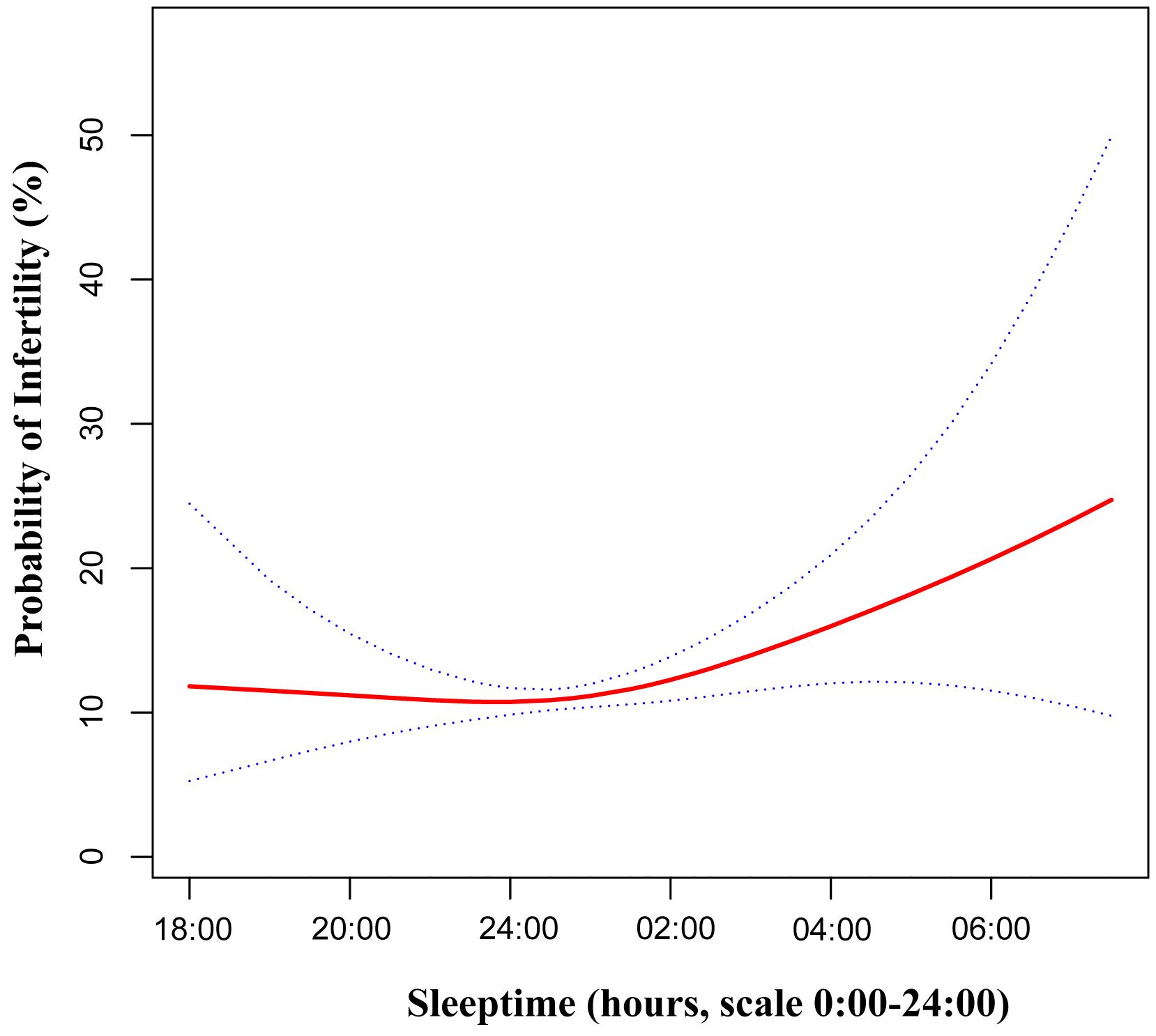
Figure 2 Adjusted associations of bedtime with female infertility. A non-linear relationship was found. Red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. Adjusted:age, race, sleep duration, BMI, waist circumference, marital status, education, smoking status, drinking status and physical activity. BMI, body mass index.
The results of subgroup analyses
The outcomes of the subgroup analyses were detailed in Table 4. The test for interaction was significant for BMI (P for interaction = 0.0280), while the test for interactions involving age, waist circumference, physical activity, marital status, smoking status, drinking status and sleep duration didn’t reach statistically significance (P values for interactions were larger than 0.05). Similarly, the time period in which participants were included did not affect the relationship between bedtime and infertility (Supplementary Table S3). Based on these findings, BMI was identified as a potential confounding variable that could modify the association between bedtime and infertility. Significant differences in effect sizes of bedtime on infertility were observed across different BMI categories. Late sleepers with a higher BMI were found to be more likely to experience infertility compared to those with a lower BMI (BMI: 25–30 kg/m2: OR: 1.26; 95% CI: 1.06 to 1.51; P = 0.0136; BMI ≥ 30 kg/m²: OR: 1.21, 95% CI: 1.09 to 1.34; P = 0.0014).
Discussion
The impact of daily lifestyle factors, such as sleep and diet, on health and biological aging, and their relationship to infertility, has been studied in the field of reproductive medicine and health sciences. Previous research has provided evidence of the significant influence of sleep and diet on biological aging and their association with infertility. For instance, Ruijie Xie et al. demonstrated that a pro-infammatory dietary pattern is associated with biological aging (14). Xu Gao and colleagues elucidated that better sleep quality can mitigate accelerated biological aging caused by air pollution (15).
In line with prior studies, a previous cross-sectional study involving 227 participants reported a higher proportion of individuals with an evening chronotype among those with infertility (11). Furthermore, another cross-sectional study comprising 2,175 participants showed a linear positive association between bedtime and infertility (12). Building upon these previous studies, our research explored in-depth by investigating the non-linear relationship between bedtime and infertility, as evidenced by Supplementary Table S4.
It’s worth noting that the previous study did not account for waist circumference and alcohol consumption as potential confounders. Waist circumference serves as an anthropometric measure used to evaluate central obesity (16). Obesity has been recognized as a risk factor contributing to infertility (17, 18). Similarly, with regards to alcohol consumption, research has indicated that it acts as a predictor for infertility in women (19). The consumption of alcohol was linked to an elevated risk of experiencing infertility (20). Hence, in the present study, we introduced waist circumference and drinking status as additional confounders to be considered in the adjustment process, and we found that the association between bedtime and infertility persisted.
Furthermore, our study has taken a step further by investigating the non-linear relationship between bedtime and infertility (Table 3). An inflection point was observed at 22:45. Prior to this inflection point, the association was not statistically significant. However, on the right side of the inflection point, a significant increase in infertility incidence was observed with a delay in bedtime. This novel finding provides valuable guidance on the ideal bedtime for women of childbearing age, potentially contributing to the improvement of their reproductive health.
Regarding the relationship between bedtime and infertility, we suggest the following mechanisms. Participants with later bedtimes were more exposed to light during the night, which may suppress melatonin production and lead to disruption of circadian rhythms (21). On the one hand, melatonin acts as a free radical scavenger, typically protecting oocytes from oxidative stress. Decreased melatonin secretion may lead to increased vulnerability of oocyte to oxidative stress injury (22). On the other hand, the disruption of circadian rhythm could affect the secretion of HPG axis-related hormones through the suprachiasmatic nucleus (SCN). Circadian rhythms regulate the rhythmic behaviors and physiological fluctuations observed in various species. In mammals, these coordinated oscillations are mediated by the SCN (23). The SCN is closely related to the secretion of gonadotropin-releasing hormone (GnRH) neurons and a variety of reproductive hormones (24). One pathway comprises direct innervation of GnRH neurons by the SCN (25, 26), while the other involves an indirect circuitry where the SCN communicates with the HPG axis via Kisspeptin neurons (27, 28). Kisspeptin neurons located in the anterior ventral periventricular area play a pivotal role in triggering the luteinizing hormone (LH) surge. Concurrently, another subset of hypothalamic kisspeptin neurons in the arcuate nucleus exerts control over the HPG axis by regulating GnRH release (29). Collectively, potential alterations in the secretion of these hormones among late sleepers could explain the elevated risk of infertility.
In the subgroup analyses performed in this study, stratification variables included age, smoking status, BMI, marital status, total physical activity time, waist circumference, drinking status, and sleep duration. We found a positive association between bedtime and infertility among participants with a BMI equal to or greater than 25 kg/m2. However, this relationship was not significant among participants with a BMI less than 25 kg/m2. The plausible explanation for this phenomenon is that the obesity could influence the secretion of HPG axis associated hormones, thereby affecting menstrual cycle and ovulation (30, 31). The disruptive effects of circadian changes could be viewed as an added challenge to the secretion of these hormones. Individuals with a high BMI are at an elevated risk of experiencing infertility due to this effect. This may provide a potential explanation for why later bedtimes are more likely to result in infertility among populations with higher BMI levels.
Our study has several significant strengths. First, we utilized data obtained from the NHANES database, well-known for its comprehensive coverage and representative nature. Second, our investigation revealed a non-linear link between bedtime and infertility. Third, the utilization of threshold effect analysis resulted in the identification of an inflection point at 22:45, providing valuable advice regarding optimal bedtime for women of reproductive age. Fourth, we conducted subgroup analyses that revealed the impact of BMI on the correlation between bedtime and infertility.
Nonetheless, our study is accompanied by certain limitations. First, despite revealing the association between bedtime and infertility, establishing causation is not feasible due to the cross-sectional nature of the study. Prospective studies are necessary to explore the causal relationship between bedtime and female infertility in the future. Second, our study relied on self-reported data, which introduces potential recall bias. Third, even though we extensively adjusted for confounding factors, cross-sectional studies may not completely capture full complexity of relationships between variables and can be susceptible to selection bias and unaccounted-for confounders which were not included or recorded in the NHANES. This could potentially influence our results. Fourth, infertility status was self-reported by women, and information related to the fertility condition of the partner was not included in the NHANES. Fifth, the dataset originates from a nationwide U.S. survey, thus requiring further validation for its applicability across diverse ethnic groups.
In conclusion, our research findings suggest a non-linear relationship between bedtime and infertility, and have identified an optimal bedtime. This provides valuable health guidance for women of childbearing age.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
All data obtained from NHANES, which was reviewed and approved by National Center for Health Statistics (NCHS) Ethics Review Board and all subjects agreed on the survey and signed written consent. The NHANES was conducted in accordance with local legislation and institutional requirements. Because the NHANES database is publicly accessible, no additional ethical approvals are required. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HZ: Data curation, Software, Visualization, Writing – original draft. JZ: Data curation, Writing – review & editing. WC: Formal analysis, Validation, Writing – original draft. HL: Data curation, Validation, Writing – original draft. JFC: Conceptualization, Investigation, Supervision, Writing – review & editing. JLC: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research and researchers were funded as follows: National Natural Science Foundation of China (82201879 to JC), Provincial Natural Science Foundation of Hunan (2022JJ40675 to JC), Hunan Provincial Health Commission general project (B202305037231 to JC), the Scientific Research Launch Project for new employees of Second Xiangya Hospital of Central South University (to JC).
Acknowledgments
We acknowledge the staff at the National Center for Health Statistics at the CDC for designing, collecting, and managing the NHANES data and releasing the data for public use.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1340131/full#supplementary-material
References
1. Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. (2018) 62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012
2. Hou C, Zhao X, Tian GG, Wu J. Stella regulates the development of female germline stem cells by modulating chromatin structure and DNA methylation. Int J Biol Sci. (2022) 18:3006–18. doi: 10.7150/ijbs.69240
3. Wang G, Liu X, Lei J. Cognitive behavioural therapy for women with infertility: A systematic review and meta-analysis. Clin Psychol Psychother. (2023) 30:38–53. doi: 10.1002/cpp.27925
4. Hutcherson NEC-, Harris JB, Karaoui LR, Lakdawala L, Lodise NM, Stone RH, et al. Infertility management and pharmacotherapy: what every pharmacist should know. J Pharm Pract. (2021) 34:635–47. doi: 10.1177/0897190020930969
5. Lateef OM, Akintubosun MO. Sleep and reproductive health. J Circadian Rhythms. (2020) 18:1. doi: 10.5334/jcr.190
6. Christensen A, Bentley GE, Cabrera R, Ortega HH, Perfito N, Wu TJ, et al. Hormonal regulation of female reproduction. Horm Metab Res. (2012) 44:587–91. doi: 10.1055/s-0032-1306301
7. Ono M, Ando H, Daikoku T, Fujiwara T, Mieda M, Mizumoto Y, et al. The circadian clock, nutritional signals and reproduction: A close relationship. Int J Mol Sci. (2023) 24:1545. doi: 10.3390/ijms24021545
8. Stocker LJ, Macklon NS, Cheong YC, Bewley SJ. Influence of shift work on early reproductive outcomes: A systematic review and meta-analysis. Obstetrics Gynecol. (2014) 124:99. doi: 10.1097/AOG.0000000000000321
9. Fernandez RC, Marino JL, Varcoe TJ, Davis S, Moran LJ, Rumbold AR, et al. Fixed or rotating night shift work undertaken by women: implications for fertility and miscarriage. Semin Reprod Med. (2016) 34:74–82. doi: 10.1055/s-0036-1571354
10. Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol. (2010) 2010:e813764. doi: 10.1155/2010/813764
11. Özçelik C, Varlı B, Gökçe A, Takmaz T, Çetin Ç, Özcan P. Evaluation of chronotype and sleep quality in infertile population and comparison with fertile population: a cross-sectional study. J Psychosomatic Obstetrics Gynecol. (2023) 44:2148523. doi: 10.1080/0167482X.2022.2148523
12. Liang Z, Liu J. Sleep behavior and self-reported infertility: A cross-sectional analysis among U.S. women. Front Endocrinol (Lausanne). (2022) 13:818567. doi: 10.3389/fendo.2022.818567
13. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
14. Xie R, Ning Z, Xiao M, Li L, Liu M, Zhang Y. Dietary inflammatory potential and biological aging among US adults: a population-based study. Aging Clin Exp Res. (2023) 35:1273–81. doi: 10.1007/s40520-023-02410-1
15. Gao X, Huang N, Guo X, Huang T. Role of sleep quality in the acceleration of biological aging and its potential for preventive interaction on air pollution insults: Findings from the UK Biobank cohort. Aging Cell. (2022) 21:e13610. doi: 10.1111/acel.13610
16. Li M-C, Mínguez-Alarcón L, Arvizu M, Chiu Y-H, Ford JB, Williams PL, et al. Waist circumference in relation to outcomes of infertility treatment with assisted reproductive technologies. Am J Obstetrics Gynecol. (2019) 220:578.e1–578.e13. doi: 10.1016/j.ajog.2019.02.013
17. Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. (2003) 9:359–72. doi: 10.1093/humupd/dmg024
18. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstetrics Gynecol. (2007) 110:1050. doi: 10.1097/01.AOG.0000287293.25465.e1
19. Tolstrup JS, Kjær SK, Holst C, Sharif H, Munk C, Osler M, et al. Alcohol use as predictor for infertility in a representative population of Danish women. Acta Obstetricia Gynecologica Scandinavica. (2003) 82:744–9. doi: 10.1080/j.1600-0412.2003.00164.x
20. Eggert J, Theobald H, Engfeldt P. Effects of alcohol consumption on female fertility during an 18-year period. Fertil Steril. (2004) 81:379–83. doi: 10.1016/j.fertnstert.2003.06.018
21. Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. (2014) 35:648–70. doi: 10.1210/er.2013-1051
22. Reiter RJ, Tamura H, Tan DX, Xu X-Y. Melatonin and the circadian system: contributions to successful female reproduction. Fertil Steril. (2014) 102:321–8. doi: 10.1016/j.fertnstert.2014.06.014
23. Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol Res Health. (2001) 25:85–93.
24. Van Loh BM, Yaw AM, Breuer JA, Jackson B, Nguyen D, Jang K, et al. The transcription factor VAX1 in VIP neurons of the suprachiasmatic nucleus impacts circadian rhythm generation, depressive-like behavior, and the reproductive axis in a sex-specific manner in mice. Front Endocrinol (Lausanne). (2023) 14:1269672. doi: 10.3389/fendo.2023.1269672
25. Van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R, Buijs RM. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J Neuroendocrinol. (1993) 5:137–44. doi: 10.1111/j.1365-2826.1993.tb00373.x
26. Van Der Beek EM, Horvath TL, Wiegant VM, Van Den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: Combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. (1997) 384:569–79. doi: 10.1002/(SICI)1096-9861(19970811)384:4<569::AID-CNE6>3.0.CO;2-0
27. Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. (2008) 57:277–87. doi: 10.1016/j.brainresrev.2007.05.006
28. Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin– GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. (2007) 30:504–11. doi: 10.1016/j.tins.2007.08.001
29. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci. (2005) 102:1761–6. doi: 10.1073/pnas.0409330102
30. Marinelli S, Napoletano G, Straccamore M, Basile G. Female obesity and infertility: outcomes and regulatory guidance. Acta Bio Medica: Atenei Parmensis. (2022) 93. doi: 10.23750/abm.v93i4.13466
Keywords: bedtime, infertility, lifestyle, non-linear relationship, cross-sectional studies
Citation: Zhang H, Zhang J, Chen W, Liu H, Chen J and Chen J (2024) Association between bedtime and female infertility: a secondary analysis from a cross-sectional study. Front. Endocrinol. 15:1340131. doi: 10.3389/fendo.2024.1340131
Received: 17 November 2023; Accepted: 06 June 2024;
Published: 20 June 2024.
Edited by:
Natalia Schlabritz-Lutsevich, LLC, United StatesReviewed by:
Giulia Brigante, University of Modena and Reggio Emilia, ItalyRuijie Xie, University of South China, China
Mingjiang Liu, University of South China, China
Copyright © 2024 Zhang, Zhang, Chen, Liu, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingfei Chen, amluZ2ZlaWNoZW5AY3N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Hanzhi Zhang1†
Hanzhi Zhang1† Jingfei Chen
Jingfei Chen Jianlin Chen
Jianlin Chen