- 1Department of General Surgery, The Second Hospital of Tianjin Medical University, Tianjin, China
- 2Department of General Surgery, Tianjin Medical University General Hospital, Tianjin, China
Background and purpose: Thyroid papillary carcinoma (PTC) had a high possibility of recurrence after surgery, and thyroid stimulating hormone (TSH) suppression and radioactive iodine (131I) were used for postoperative therapy. This study explored the potential mechanism of lymph node metastasis (LNM) and aimed to develop differentiated treatments for PTC.
Method: This study explored the risk factors of lymph node metastasis in PTC by analyzing the clinical information of 2073 cases. The Cancer Genome Atlas Thyroid Cancer (TCGA-THCA) and the Gene Expression Omnibus (GEO) databases of gene expression were analyzed to identify the interrelationships between gene expression to phenotype.
Results: Analyzing clinical data, we found that male gender, younger age, larger tumor size, and extra-thyroidal extension (ETE) were risk significant risk factors for lymph node metastasis(P<0.05). Conversely, thyroid function parameters such as TSH, FT3, FT4, TSH/FT3, and TSH/FT4 didn’t correlate with LNM(P>0.05), and TSH levels were observed to be higher in females(P<0.05). Gene expression analysis revealed that SLC5A5 was down-regulated in males, younger individuals, and those with lymph node metastasis, and a lower level of SLC5A5 was associated with a worse disease-free survival(P<0.05). Additionally, our examination of single-cell RNA sequencing (scRNA-seq) data indicated that SLC5A5 expression was reduced in tumors and lymph node metastasis samples, correlating positively with the expression of TSHR.
Conclusion: The impact of TSH on PTC behavior remained unclear, while the capacity for absorbing 131I in dependence on SLC5A5 showed variations across different genders and ages. We conclude that postoperative treatment of PTC should take into account the differences caused by gender and age.
1 Background
Papillary thyroid carcinoma (PTC) is one of the most frequently diagnosed malignancies of the endocrine system worldwide (1). Surgery is the preferred treatment for PTC, which has a satisfactory prognosis in an early stage (2). However, studies have revealed that the recurrence rate of PTC within 5 years is a high level, ranging from 2%-13% (3–6), which was difficult in the treatment of PTC. To address this issue, the use of thyroid-stimulating hormone (TSH) suppression has been advocated for postoperative patients to mitigate the growth of tumor cells and reduce the risk of recurrence (7, 8). In addition, the American thyroid association 2016 guidelines recommend radioactive iodine (131I) for patients with a high risk of recurrence, such as those with residual disease or metastasis (7, 9–11). However, patients exposed to a low level of TSH are at risk of developing subclinical hyperthyroidism including cardiovascular disease. For menopausal women, excessive TSH suppression may lead to osteoporosis and increase the risk of fractures (12–15). Patients receiving 131I therapy may experience damage to the parotid gland and an increased risk of secondary tumors, which increases with the radiation dose (16, 17). Currently, decisions regarding the extent of TSH suppression and the dosage of 131I are primarily based on pathological invasion assessments, which vary among physicians and surgeons. Some clinicians favor more aggressive treatments to prevent recurrences (18, 19).
Some studies have indicated that PTC exhibits varying biological behaviors across different age groups and gender demographics (20, 21). Specifically, women were considered to be more prone to developing PTC, yet they typically experienced milder behavior and a more favorable prognosis, particularly among pre-menopausal females. In addition, younger patients display a more positive prognosis but are at an increased risk of recurrence (22, 23). A study on active observation of papillary thyroid microcarcinoma(PTMC) reported that tumors are more likely to progress in younger patients (24). The diverse hormonal and immunological internal environments influenced by gender and age differences could be responsible for these variable behaviors.
Our study integrated the clinical data, preoperative examinations, and postoperative pathology of the PTC patients to delineate the clinical trends within diverse subgroups. Through analyses of the gene expression database, we investigated the gene expression levels across various subgroups to unravel the mechanism underpinning the observed phenotype. This research aimed to provide a more personalized and less harmful postoperative therapeutic regimen.
2 Materials and methods
2.1 Clinical data
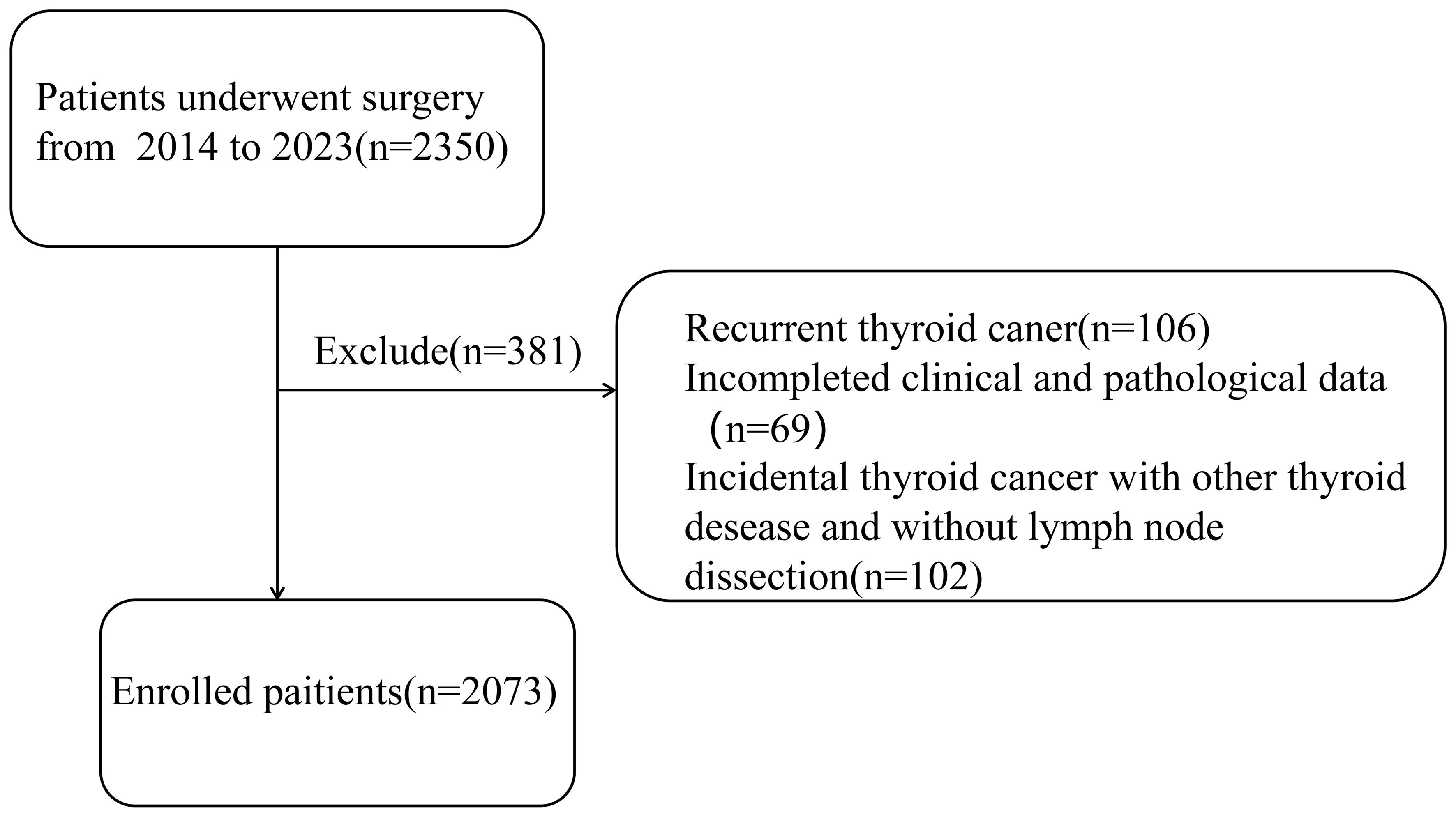
A total of 2073 patients from 2014 to 2023 were selected in this study, and all of them were diagnosed with PTC and underwent surgery by the same doctor. What’s more, in our center, all patients diagnosed with PTC would undergo routine prophylactic central compartment lymph node dissection, and the pathological results were obtained separately by two experienced pathologists. This study passed the ethical review based on the Declaration of Helsinki and obtained informed consent from the patients. Inclusion criteria were: 1) initial thyroid cancer surgery; 2) completed clinical and pathological data; Exclusion criteria were: 1) recurrent thyroid cancer; 2) incidental thyroid cancer without central lymph node dissection (Figure 1).
We set 45 years old as the cutoff age, which was aligned with the mean age of our entire cohort and conformed to the changes in female hormone levels. Based on the character of clinical data, all participants were divided as follows: LNM (absent and present), tumor size(less than 1 cm or more than 1 cm), extrathyroidal extension (limited in capsule or violating surrounding tissues), status of thyroid function (hypothyroidism(TSH>4.94uIU/L or FT3 ≤ 2.43pmol/L or FT4 ≤ 9.01pmol/L), normal thyroid function (TSH:0.35uIU/L-4.94uIU/L, FT3:2.43pmol/L-6.01pmol/L and FT4:9.01pmol/L-19.05) and hyperthyroidism (TSH<0.35uIU/L or FT3>6.01pmol/L or FT4>19.05pmol/L), and the level of TSH(high: TSH > 2uIU/L, low: TSH ≤ 2uIU/L). What’s more, the ratio of TSH/FT3 and TSH/FT4 were used to represent the response of the hypothalamic-pituitary-thyroid axis.
2.2 Public data of gene expression
We downloaded the bulk gene expression data and clinical data of PTC from the TCGA database UCSC (https://xenabrowser.net/) and deleted the cases without LNM information. Further, 497 cases with complete age and sex information were selected, and the included genes were normally detected in at least 75% of participants. scRNA-seq data were extracted from the Gene Expression Omnibus (GEO) dataset (GSE184362), and the quality control was performed according to the standard Seurat process.
2.3 Statistical analysis
The SPSS 22.0 software was used to analyze the statistical data. The continuous measurement data were expressed as ± S and a t-test was performed for statistical analysis. What’s more, categorical variables such as sex, age, tumor size, degree of extrathyroidal extension, and status of thyroid function were counted by their frequency, and binary logistic regression analysis was performed for statistical analysis. Then, multivariate analysis was performed by multivariate logistic regression analysis. A P value <0.05 indicated a statistically significant difference. The different expressed genes (DEGs) from the TCGA database were screened using the “DESeq2” package in R 4.2.2 software. The DEGs were defined as the average gene expression ratio and two times the standard deviation, with P <0.05.
3 Results
3.1 Clinical data and single-factor analysis of LNM
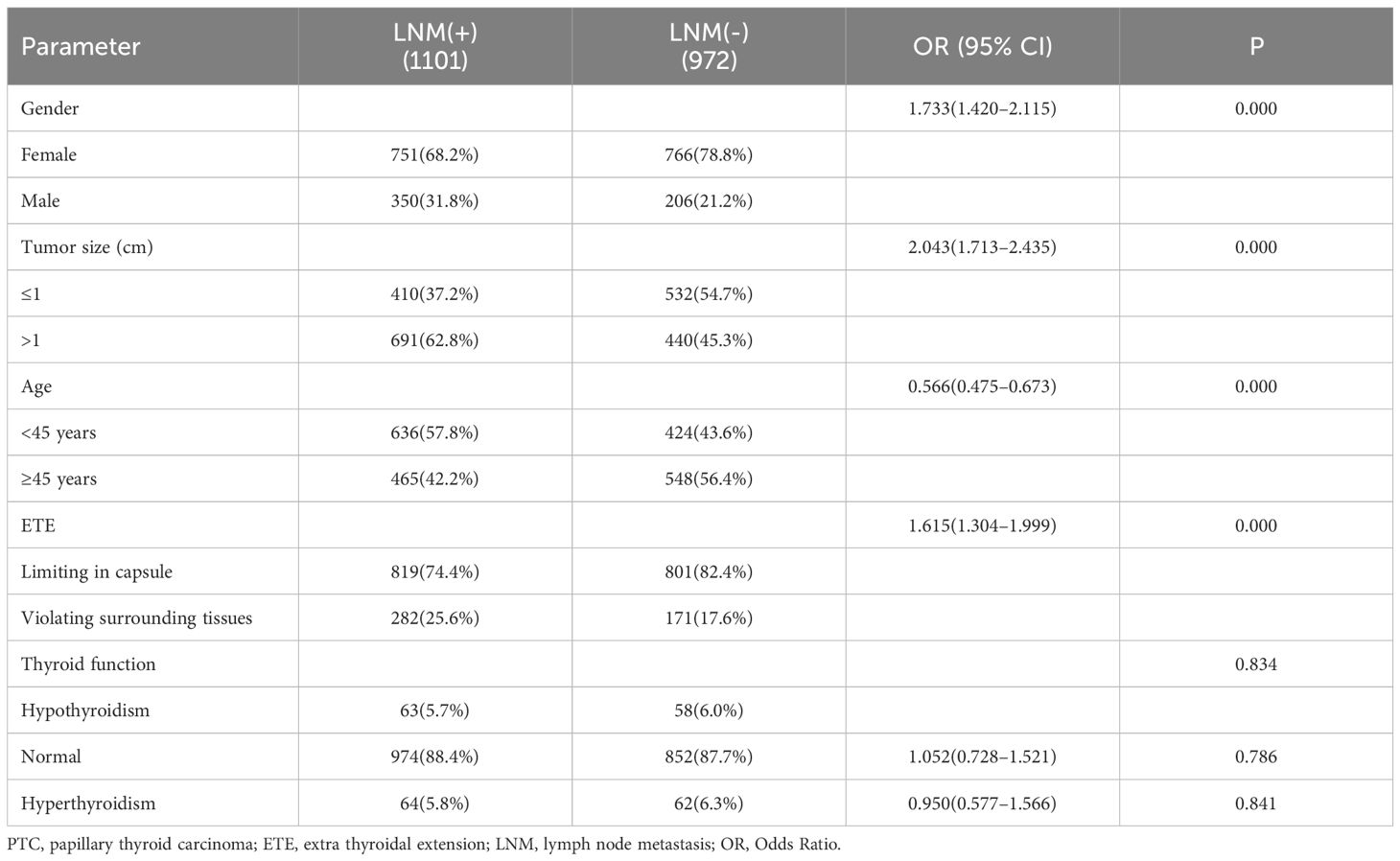
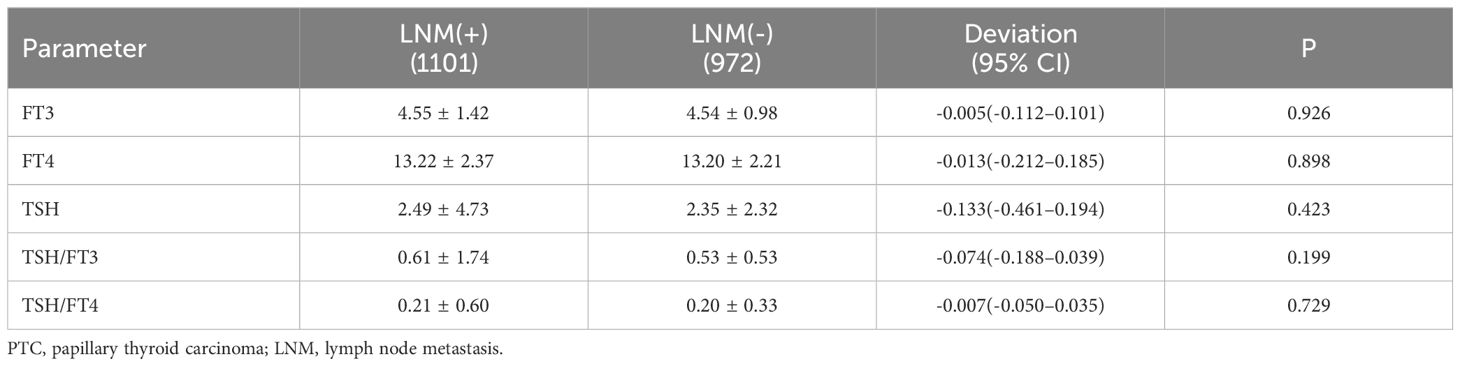
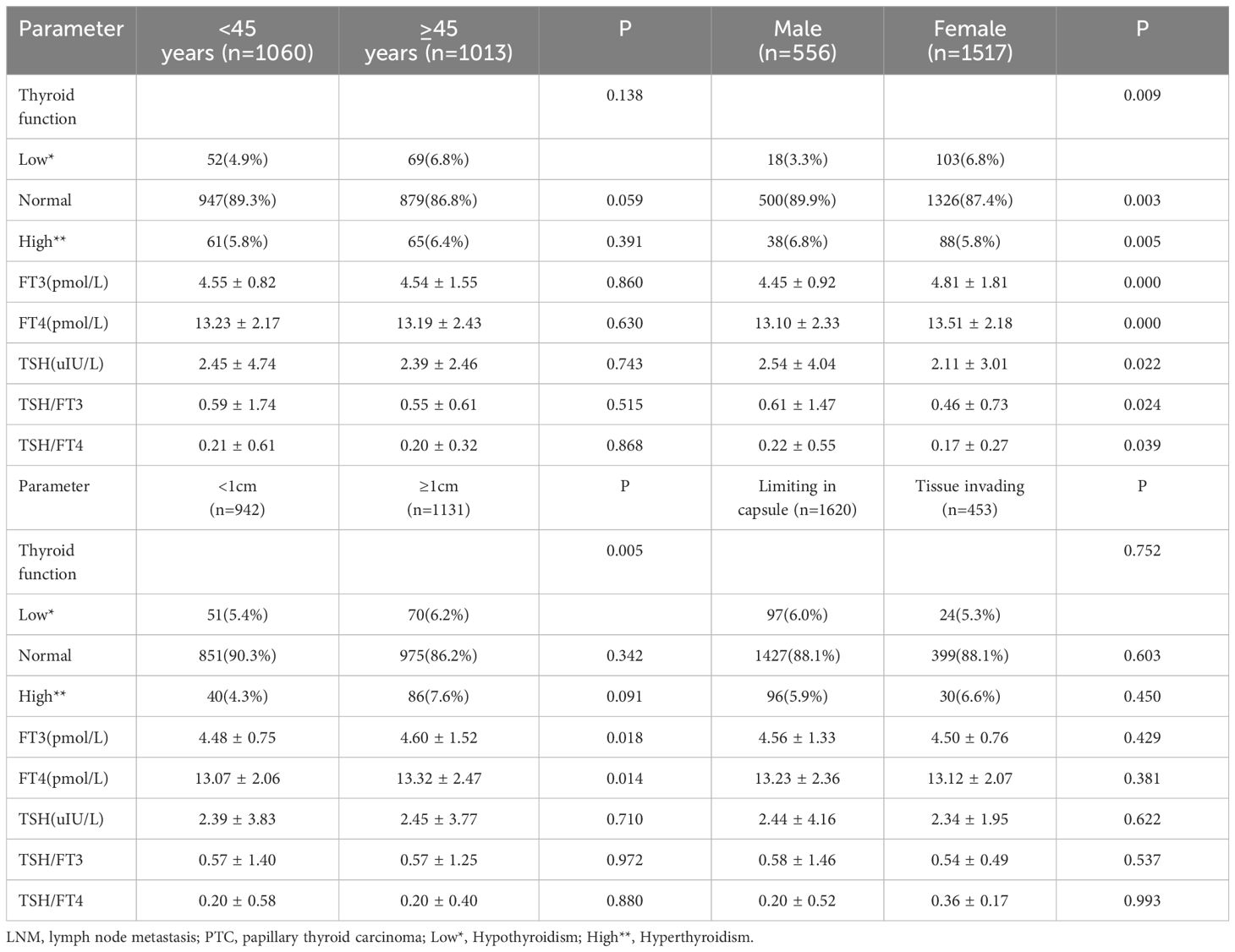
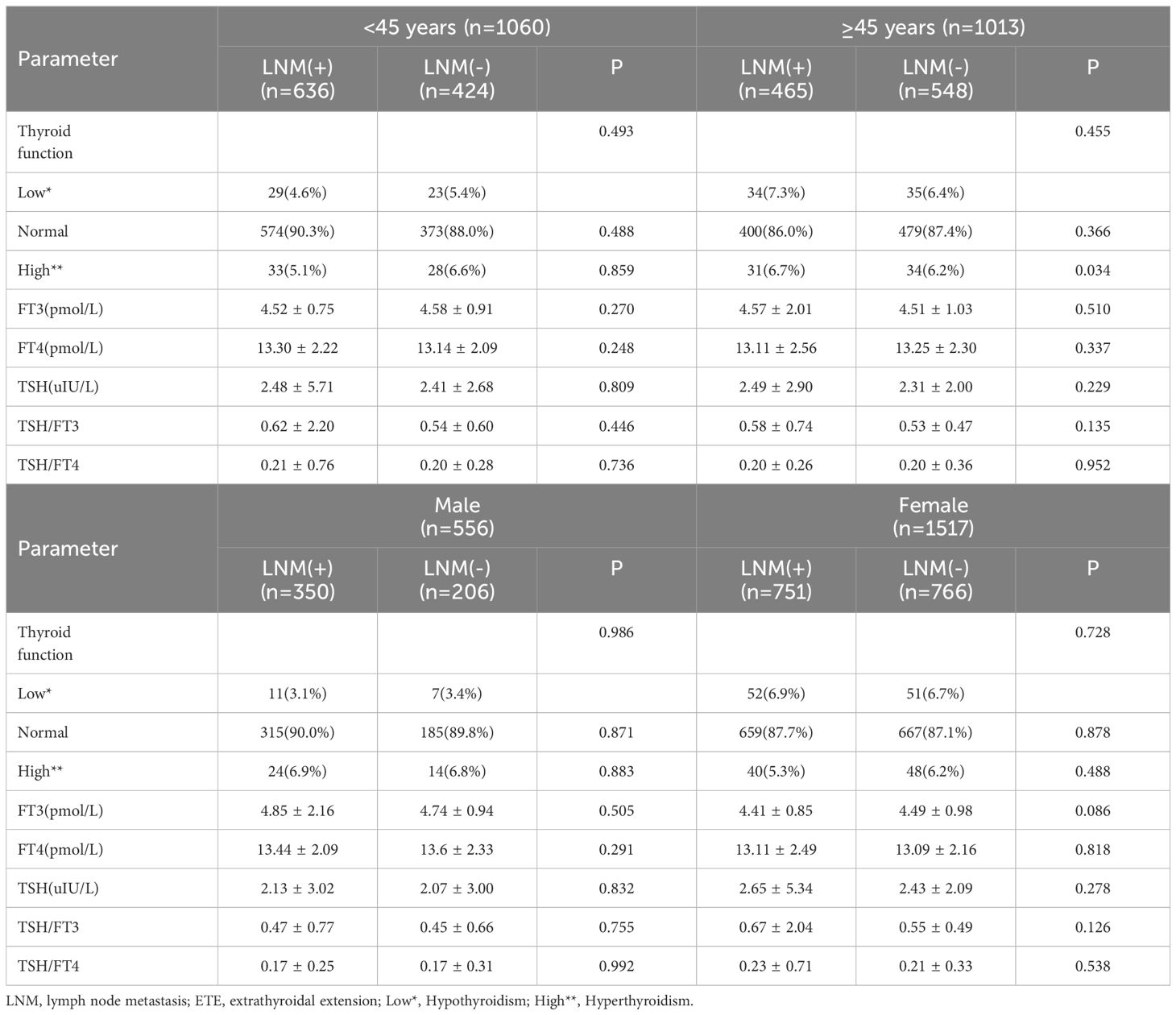
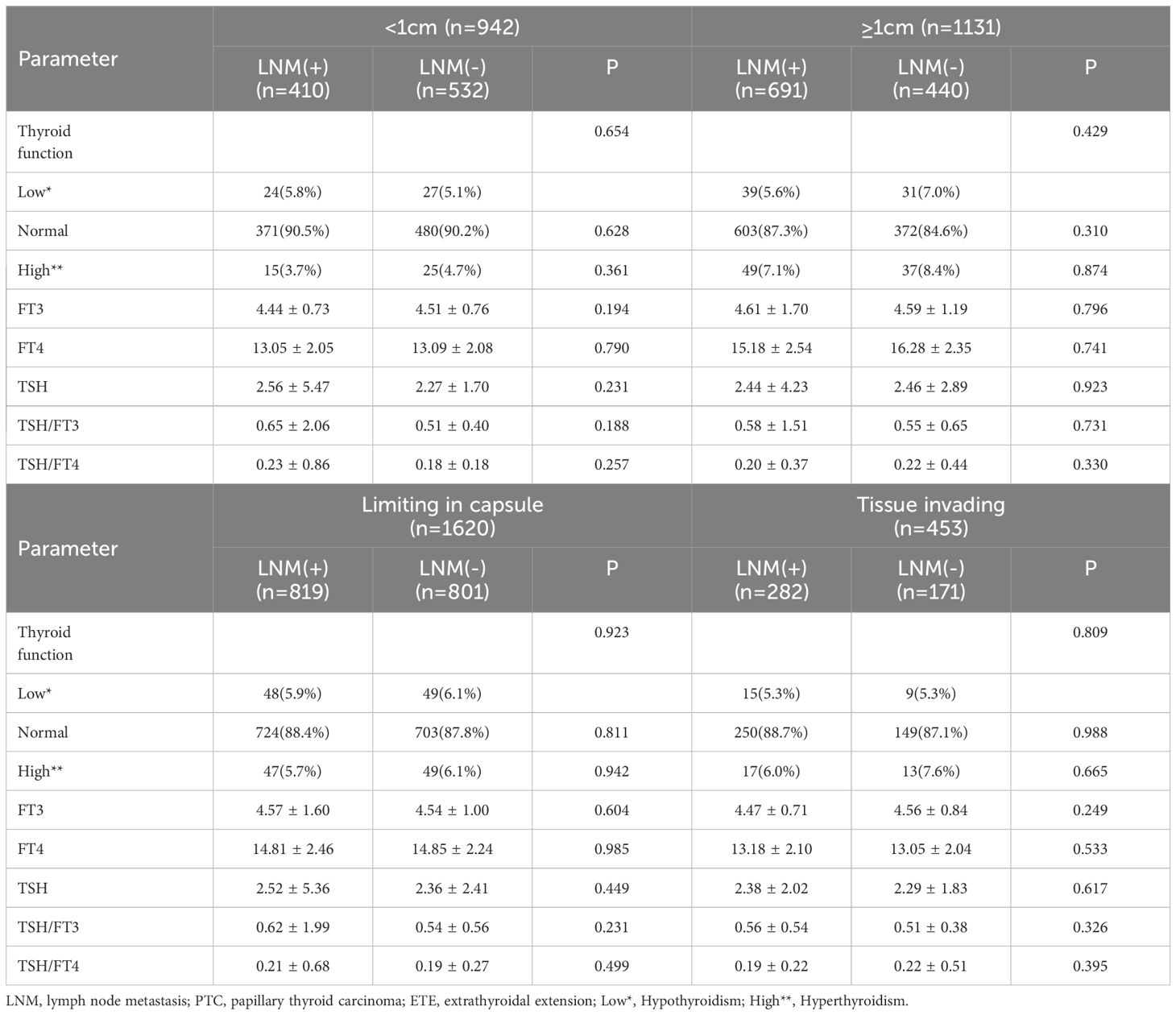
According to the characteristics of clinical data, the age range was 13 to 89 years, and with 1060 patients under 45 years old,1013 were older than 45 years. All patients were divided into 556 males and 1517 females, and the ratio of males to females was 1:2.73. The average age of males was 44.2, and 45.3 for females. Among them, 942 were PTMC with a diameter less than 1cm, and 1131 carcinomas with a diameter larger than 1cm. In 1169 cases, the tumor was limited to the capsule, but in 454 cases invaded surrounding tissues. According to the definition of thyroid function, there were 121 with hypothyroidism, 1826 with normal thyroid function, and 126 patients with hyperthyroidism. Among all patients, 1101(53.1%) cases occurred lymph node metastasis, and 972(46.9%) without. The univariate analysis performed by binary logistic regression analysis showed the younger age, male gender, larger tumor size, and ETE were risk factors for LNM (P<0.05), but the factors of thyroid function weren’t(P>0.05) (Table 1). Then, We showed interest in the levels of FT3, FT4, TSH, TSH/FT3, and TSH/FT4 in different lymph node metastatic status, but the results of the t-test showed no significant difference here (P>0.05) (Table 2). What’s more, we conducted analyses to compare the thyroid function and the levels of FT3, FT4, TSH, TSH, TSH/FT3, and TSH/FT4 in different statuses of age, sex, tumor size, and capsule invasion (Table 3). The study’s findings revealed no significant differences in thyroid function status or the levels of FT3, FT4, TSH, TSH, TSH/FT3, and TSH/FT4 when comparing different age groups and varying degrees of capsule invasion(P>0.05). However, upon conducting t-tests and binary logistic regression analyses, it was observed that within subgroups of larger sizes, there was a statistically significant increase in the levels of FT3 and FT4 (P<0.05), whereas the levels of TSH, TSH/FT3, and TSH/FT4 did not exhibit any significant differences (P>0.05). Additionally, gender-based comparisons indicated notable variations in thyroid function, as well as the levels of FT3, FT4, TSH, TSH/FT3, and TSH/FT4, between females and males (P<0.05). Women presented with a higher prevalence of hypothyroidism; the mean TSH level among women was 2.54 uIU/L, compared to 2.11 uIU/L in men. Moreover, the mean TSH/FT3 ratio was significantly higher in women at 0.61, contrasting with 0.46 in men.
We conducted a further investigation to elucidate the influence of thyroid function and the concentrations of FT3, FT4, TSH, TSH/FT3, and TSH/FT4 on lymph node metastasis (LNM) across various patient subgroups (Tables 4, 5). Employing chi-square and t-test analyses within subgroups stratified by sex and age, our findings revealed no significant association between thyroid function and the levels of FT3, FT4, TSH, TSH/FT3, and TSH/FT4 with the incidence of LNM in these demographic subgroups (P>0.05). Additionally, acknowledging that larger tumor size and extrathyroidal extension are established risk factors for lymph node metastasis, we aimed to investigate the relevance of thyroid function parameters, including serum levels of FT3, FT4, TSH, TSH/FT3, and TSH/FT4, concerning LNM across various tumor sizes and invasive states, and the results indicated that these thyroid function-related factors did not exert a significant influence on the incidence of LNM (P > 0.05).
3.2 Multi-factor analysis and nomogram of LNM
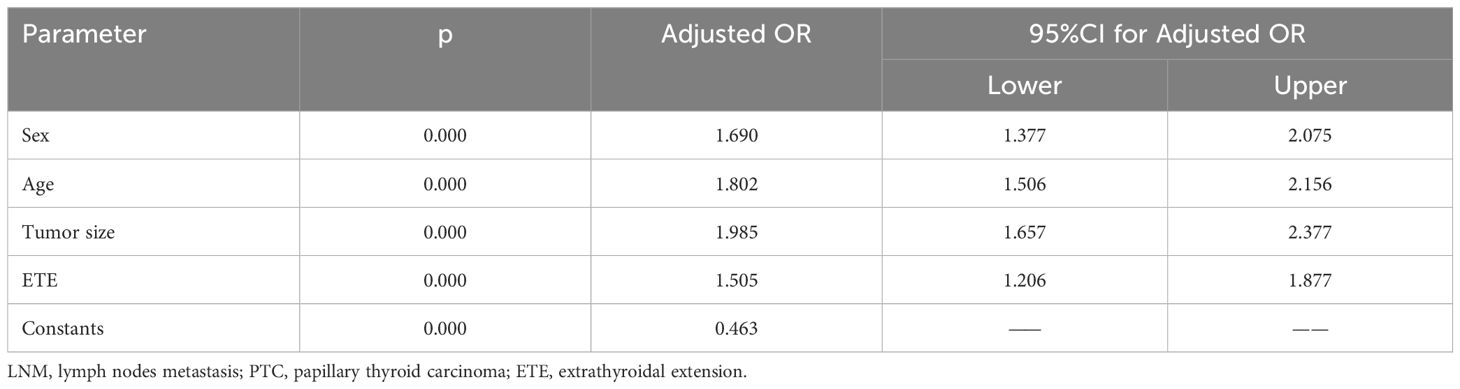
Based on the above univariate analysis, factors that may have associations with LNM (P<0.05), including sex, age, tumor size, and degree of capsule invasion were included in the multivariate logistic regression model. The results showed that the larger size (OR=1.985, 95% CI 1.657–2.377; P<0.05), extrathyroidal extension (OR=1.505, 95% CI 1.206–1.877; P<0.05), male gender (OR=1.690, 95% CI 1.377–2.075; P<0.05) and younger age (OR=1.802, 95% CI 1.506–2.156; P<0.05) were independent risk factors for LNM (Table 6). Based on the results of multivariate logistic regression analysis, a nomogram predictive model was created to show the weight of each factor in this model (Figure 2A). To explore the impact of TSH on lymph node metastasis in each subgroup, a forest map was drawn, and the results showed that TSH levels had no significant effect on each subgroup(P>0.05) (Figure 2B). After 1000 internal verifications, an internal alignment curve was plotted and showed the average absolute error of the actual risk probability and the predicted risk probability of the model was 0.023 (P<0.05) (Figure 2C). The area under the receiver operating characteristic(ROC) curve was 0.647 (95% CI 0.624–0.671; P<0.05), indicating that the diagnostic efficiency of this model was superior to that of a single factor model (Figure 2D).
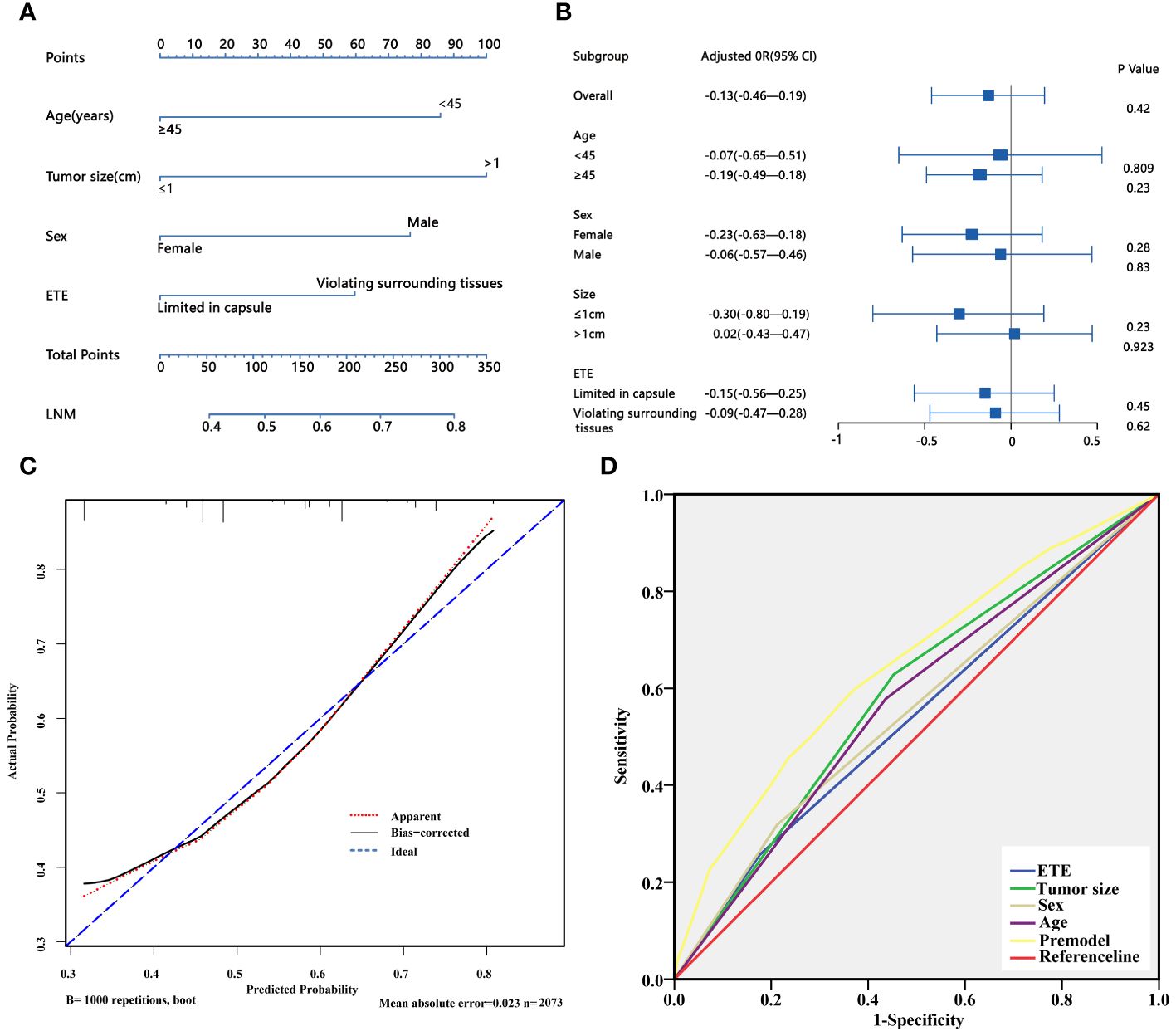
Figure 2 (A) Nomogram for predicting LNM in PTC patients; (B) The forest plot shows the impact of TSH on LNM in different subgroups. (C) Discrimination plot of the LNM predict mode. (D) ROC curve of the LNM predict model. LNM, lymph node metastasis; ETE, extrathyroidal extension.
3.3 TCGA bulk gene expression and DEGs
Among the 497 samples, 444 were tumor tissues and 53 were para-tumor tissues. All participants were divided into subgroups according to the tissue sources, LNM status, sex, and age. The baseline was females, and older age, which conformed to the status of LNM. Genes were defined as upregulated, downregulated, and unchanged in different groups according to the definition of DEGs. The intersection DEGs of subgroups were constituted into Venn maps (Figures 3A–D). We discovered a common trend among three intersecting genes across four subgroups: SLC5A5(solute carrier family5), AVPR1A(arginine vasopressin receptor 1A), and CBLN1(cerebellin 1 precursor protein). Specifically, both SLC5A5 and AVPR1A were downregulated in tumor and LNM tissues. Subsequently, we conducted survival analyses on these three genes and found that SLC5A5 and AVPR1A were associated with disease-free survival and that patients with a higher level of SLC5A5 and AVPR1A exhibited increased disease-free survival rates. However, at the same time, the higher level of SLC5A5 indicated poorer overall survival (Figures 4A–F).
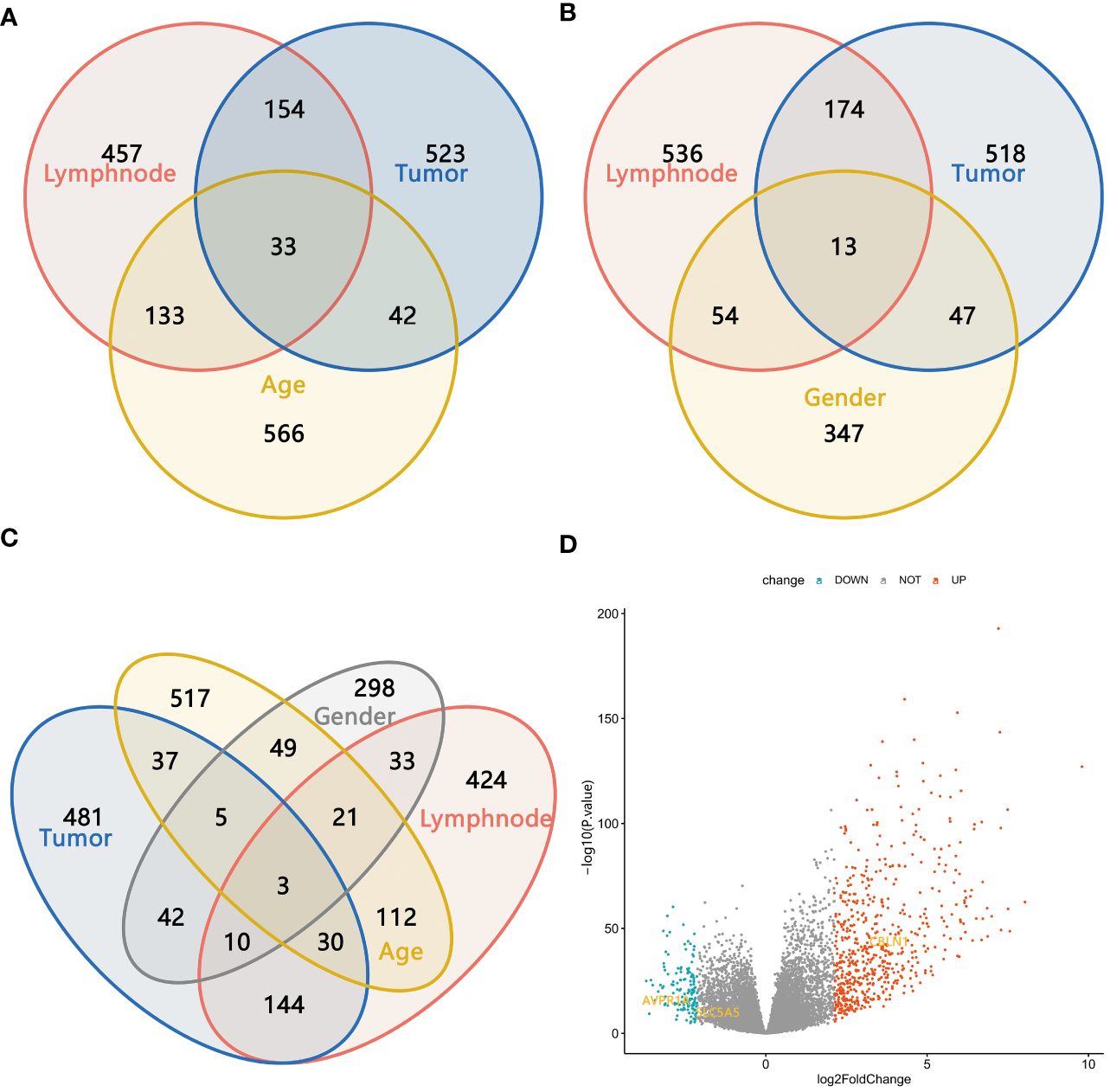
Figure 3 (A–C) The Venn maps based on the intersection DEGs of different subgroups; (D) Three intersection genes with the same trend in four subgroups.
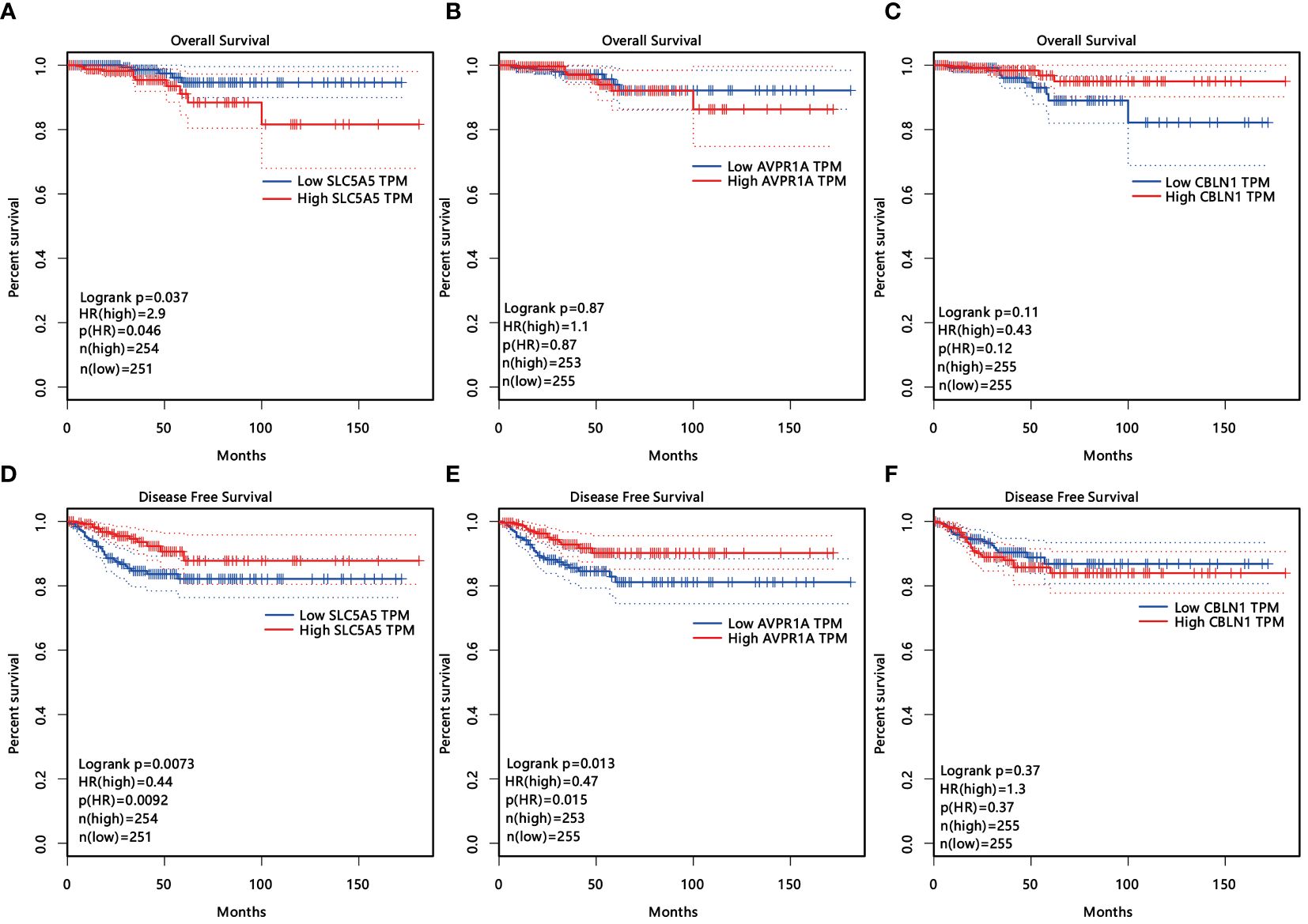
Figure 4 (A–F) The influence of three age- and sex-related DEGs (SLC5A5, AVPR1A, CLNB1) on overall survival (A–C) and disease-free survival (D-F).
3.4 GEO database of single-cell RNA sequencing
As members of the sodium iodide transport family, we show a strong interest in SLC5A5. By analyzing the expression of SLC5A5 across various tissues through the single-cell database, we discovered that SLC5A5 was downregulated in tumors and tissues with LNM. Furthermore, we observed a positive correlation between SLC5A5 expression and TSHR, while it appears that there is no clear association between SLC5A5 and ESR1(Figures 5A–D).
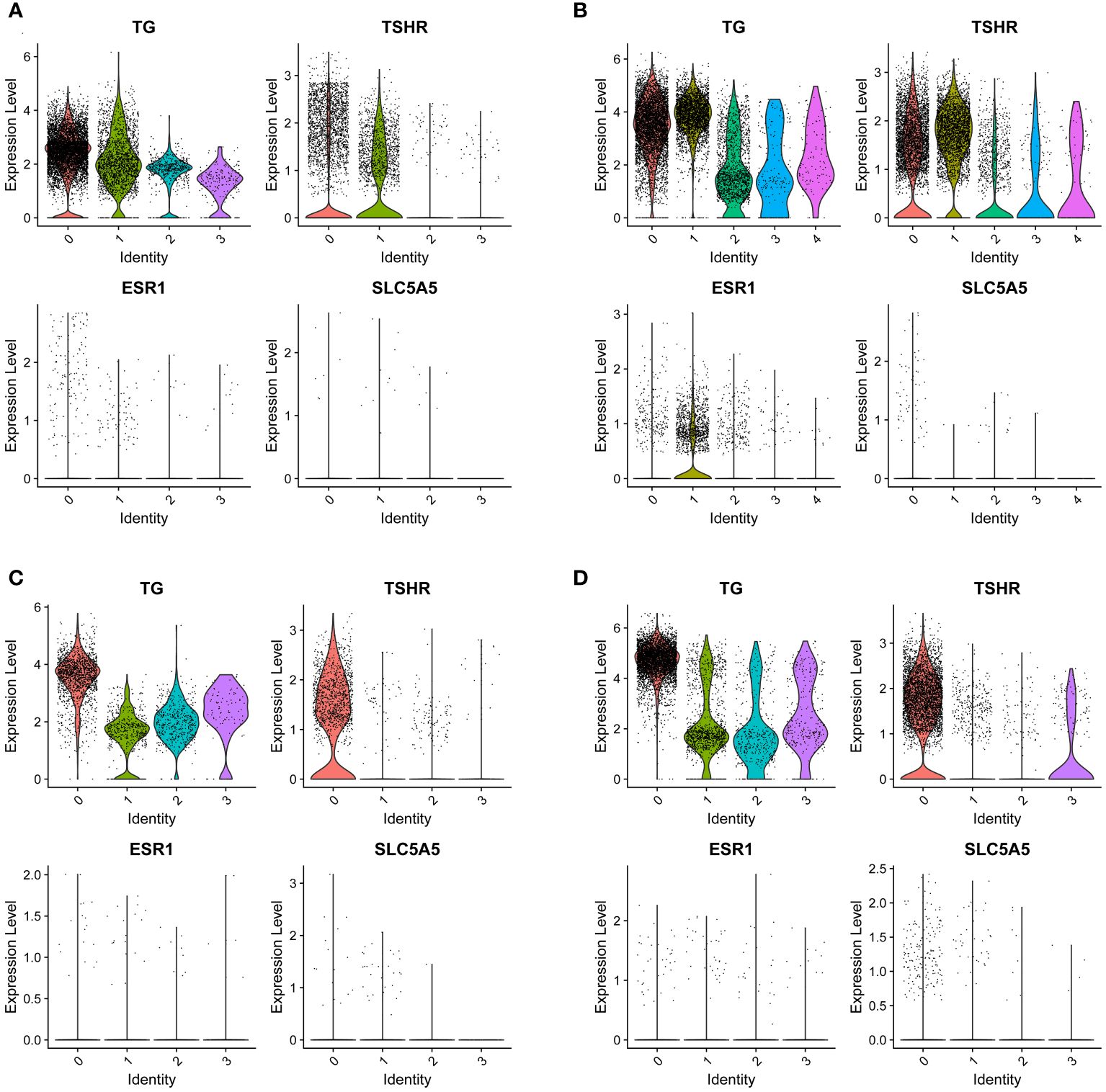
Figure 5 The expression of SLC5A5 and its correlation genes in four different tissues. (A) Tumor with lymph node metastasis; (B) Para-tumor tissue with lymph node metastasis (C) Tumor without lymph node metastasis (D) Para-tumor tissue without lymph node metastasis. TG, thyroglobulin; TSHR, thyrotropin receptor; SLC5A5, solute carrier family5; ESR1, estrogen receptor. The number of lateral axes represents different cell subgroups.
4 Discussion
LNM can serve as an indication of aggressive behavior and signs of recurrence potential in PTC (25, 26). In our study, women exhibited a greater propensity towards hypothyroidism and presented with elevated serum TSH levels, which may result from an increased likelihood of Hashimoto’s thyroiditis in women, which may contribute to the destruction of thyroid tissue during the chronic phase. It’s widely accepted that a higher TSH level promotes the proliferation of thyroid cells, leading to lymph node metastasis and invasion of surrounding tissues. Consequently, TSH suppression was theoretically an effective method to prevent the recurrence of PTC and is considered viable for low-risk PTMC patients who opt to actively monitor their condition (7). Despite some studies suggesting that TSH suppression could be beneficial for patients at high risk of recurrence, nevertheless, it was useless for low-risk patients (27). Furthermore, endogenous TSH didn’t affect the outcomes of 131I ablation therapy for the residual lesions (28). In our study, we found that thyroid function and the level of TSH didn’t affect the status of LNM in patients with different ages, genders, tumor size, and extrathyroidal extension. On the contrary, our research found that females had a higher level of TSH but a lower risk of LNM. For these findings, we suspected that the level of TSH wasn’t the exclusive factor that affects the aggressive behavior of PTC.
In addition to our findings, we discovered that in male and younger patients, the expression of SLC5A5 is downregulated, mirroring its expression in tissue with lymph node metastasis. As a sodium iodide transporter-related gene, the low expression of SLC5A5 was a manifestation of dedifferentiation in cells (29). Furthermore, our research indicated that a decreased expression of SLC5A5 correlated with a shorter disease-free survival time, which means male and younger patients are easier to recurrence. Individuals with a low level of SLC5A5 were less responsive to 131I treatment and more prone to encounter LNM and postoperative recurrences (30). In addition, our study discovered a positive correction between the expression of TSHR and ESR1 to SLC5A5, which explained why the expression of SLC5A5 is higher in females. In reality, males were more likely to be resistant to the therapy of 131I and experience recurrence. Consequently, we propose that patients should receive individualized doses of 131I, with men requiring a higher dose. As a sodium iodide transporter-related gene, SLC5A5 was regulated by upstream gene mutations and epigenetic changes, therefore drugs targeting SLC5A5 can be studied for use in 131I-resistant patients (31–33).
As a conclusion of our single-center study, it becomes imperative to collaborate with other centers to verify the reliability of the results. Additionally, our study merely captured the preoperative examinations; hence, we want to aim to accumulate more detailed insights into the postoperative follow-up to dynamically assess the intricacies of the treatment process. Lastly, we require additional evidence based on the molecular evidence to reinforce our conclusions.
5 Summary
Regarding the uncertain influence of TSH on the behavior of PTC, a flexible approach to TSH suppression indications for patients at risk of cardiovascular disease or those experiencing menopausal women was needed. Additionally, variations in 131I absorption capabilities due to SLC5A5 expression levels across different genders and ages suggest that postoperative PTC therapies should take into account these demographic differences to minimize patient harm.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Second Hospital of Tianjin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
CY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. J-JS: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – review & editing. XH: Conceptualization, Funding acquisition, Project administration, Resources, Visualization, Writing – review & editing. LJ: Data curation, Formal analysis, Methodology, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
I would like to express my special thanks to my partners for the encouragement and support they gave me during my studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1339191/full#supplementary-material
References
1. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. (2016) 12:646–53. doi: 10.1038/nrendo.2016.110
2. Banerjee M, Muenz DG, Worden FP, Wong SL, Haymart MR. Conditional survival in patients with thyroid cancer. Thyroid. (2014) 24:1784–9. doi: 10.1089/thy.2014.0264
3. Mercante G, Frasoldati A, Pedroni C, Formisano D, Renna L, Piana S, et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid. (2009) 19:707–16. doi: 10.1089/thy.2008.0270
4. van Gerwen M, Alsen M, Lee E, Sinclair C, Genden E, Taioli E. Recurrence-free survival after total thyroidectomy and lobectomy in patients with papillary thyroid microcarcinoma. J Endocrinol Invest. (2021) 44:725–34. doi: 10.1007/s40618-020-01342-1
5. Kim H, Kim TH, Choe JH, Kim JH, Kim JS, Oh YL, et al. Patterns of initial recurrence in completely resected papillary thyroid carcinoma. Thyroid. (2017) 27:908–14. doi: 10.1089/thy.2016.0648
6. Kim SK, Park I, Woo JW, Lee JH, Choe JH, Kim JH, et al. Total thyroidectomy versus lobectomy in conventional papillary thyroid microcarcinoma: Analysis of 8,676 patients at a single institution. Surgery. (2017) 161:485–92. doi: 10.1016/j.surg.2016.07.037
7. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
8. Biondi B, Cooper DS. Thyroid hormone suppression therapy. Endocrinol Metab Clinics North America. (2019) 48:227–+. doi: 10.1016/j.ecl.2018.10.008
9. Hackshaw A, Kadalayil L, Mallick U. Well-being after radiation therapy in thyroid cancer. New Engl J Med. (2013) 368:685–6. doi: 10.1056/NEJMc1212592
10. Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. New Engl J Med. (2012) 366:1674–85. doi: 10.1056/NEJMoa1109589
11. Schlumberger M, Leboulleux S, Catargi B, Deandreis D, Zerdoud S, Bardet S, et al. Outcome after ablation in patients with low-risk thyroid cancer (ESTIMABL1): 5-year follow-up results of a randomised, phase 3, equivalence trial. Lancet Diabetes Endocrinol. (2018) 6:618–26. doi: 10.1016/S2213-8587(18)30113-X
12. Wang LY, Smith AW, Palmer FL, Tuttle RM, Mahrous A, Nixon IJ, et al. Thyrotropin suppression increases the risk of osteoporosis without decreasing recurrence in ATA low- and intermediate-risk patients with differentiated thyroid carcinoma. Thyroid. (2015) 25:300–7. doi: 10.1089/thy.2014.0287
13. Pajamäki N, Metso S, Hakala T, Ebeling T, Huhtala H, Ryödi E, et al. Long-term cardiovascular morbidity and mortality in patients treated for differentiated thyroid cancer. Clin Endocrinol. (2018) 88:303–10. doi: 10.1111/cen.13519
14. Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab. (2010) 95:186–93. doi: 10.1210/jc.2009-1625
15. Sugitani I, Fujimoto Y. Effect of postoperative thyrotropin suppressive therapy on bone mineral density in patients with papillary thyroid carcinoma: a prospective controlled study. Surgery. (2011) 150:1250–7. doi: 10.1016/j.surg.2011.09.013
16. Fard-Esfahani A, Emami-Ardekani A, Fallahi B, Fard-Esfahani P, Beiki D, Hassanzadeh-Rad A, et al. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl Med Commun. (2014) 35:808–17. doi: 10.1097/MNM.0000000000000132
17. Wu JQ, Feng HJ, Ouyang W, Sun YG, Chen P, Wang J, et al. Systematic evaluation of salivary gland damage following I-131 therapy in differentiated thyroid cancer patients by quantitative scintigraphy and clinical follow-up. Nucl Med Commun. (2015) 36:819–26. doi: 10.1097/MNM.0000000000000325
18. Papaleontiou M, Chen DW, Banerjee M, Reyes-Gastelum D, Hamilton AS, Ward KC, et al. Thyrotropin suppression for papillary thyroid cancer: A physician survey study. Thyroid. (2021) 31:1383–90. doi: 10.1089/thy.2021.0033
19. Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the use of (131)I therapy in differentiated thyroid cancer: A joint statement from the american thyroid association, the european association of nuclear medicine, the society of nuclear medicine and molecular imaging, and the european thyroid association. Thyroid. (2019) 29:461–70. doi: 10.1089/thy.2018.0597
20. Suteau V, Munier M, Briet C, Rodien P. Sex bias in differentiated thyroid cancer. Int J Mol Sci. (2021) 22(23). doi: 10.3390/ijms222312992
21. Rahbari R, Zhang LS, Kebebew E. Thyroid cancer gender disparity. Future Oncol. (2010) 6:1771–9. doi: 10.2217/fon.10.127
22. Zhang D, Tang JN, Kong DG, Cui QX, Wang K, Gong Y, et al. Impact of gender and age on the prognosis of differentiated thyroid carcinoma: a retrospective analysis based on SEER. Hormones Cancer. (2018) 9:361–70. doi: 10.1007/s12672-018-0340-y
23. Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, et al. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab. (2012) 97:E878–87. doi: 10.1210/jc.2011-2864
24. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. (2014) 24:27–34. doi: 10.1089/thy.2013.0367
25. Ho AS, Luu M, Shafqat I, Mallen-St Clair J, Chen MM, Chen YF, et al. Predictive impact of metastatic lymph node burden on distant metastasis across papillary thyroid cancer variants. Thyroid. (2021) 31:1549–57. doi: 10.1089/thy.2021.0131
26. Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol. (2015) 33:2370–U66. doi: 10.1200/JCO.2014.59.8391
27. Lee MC, Kim MJ, Choi HS, Cho SW, Lee GH, Park YJ, et al. Postoperative thyroid-stimulating hormone levels did not affect recurrence after thyroid lobectomy in patients with papillary thyroid cancer. Endocrinol Metab (Seoul). (2019) 34:150–7. doi: 10.3803/EnM.2019.34.2.150
28. Vrachimis A, Riemann B, Mader U, Reiners C, Verburg FA. Endogenous TSH levels at the time of (131)I ablation do not influence ablation success, recurrence-free survival or differentiated thyroid cancer-related mortality. Eur J Nucl Med Mol Imaging. (2016) 43:224–31. doi: 10.1007/s00259-015-3223-2
29. de Morais RM, Sobrinho AB, de Souza Silva CM, de Oliveira JR, da Silva ICR, de Toledo Nóbrega O, et al. The role of the NIS (SLC5A5) gene in papillary thyroid cancer: A systematic review. Int J Endocrinol. (2018) 2018:1–11. doi: 10.1155/2018/9128754
30. Plantinga TS, Tesselaar MH, Morreau H, Corssmit EP, Willemsen BK, Kusters B, et al. Autophagy activity is associated with membranous sodium iodide symporter expression and clinical response to radioiodine therapy in non-medullary thyroid cancer. Autophagy. (2016) 12:1195–205. doi: 10.1080/15548627.2016.1174802
31. Gierlikowski W, Broniarek K, Cheda L, Rogulski Z, Kotlarek-Lysakowska M. MiR-181a-5p regulates NIS expression in papillary thyroid carcinoma. Int J Mol Sci. (2021) 22(11). doi: 10.3390/ijms22116067
32. Zhang ZJ, Liu DX, Murugan AK, Liu ZM, Xing MZ. Histone deacetylation of NIS promoter underlies BRAF V600E-promoted NIS silencing in thyroid cancer. Endocrine-Related Cancer. (2014) 21:161–73. doi: 10.1530/ERC-13-0399
Keywords: papillary thyroid carcinoma, lymph node metastasis, TSH, 131I, SLC5A5
Citation: Yan C, Sun J, He X and Jia L (2024) An age-and sex-matched postoperative therapy should be required in thyroid papillary carcinoma. Front. Endocrinol. 15:1339191. doi: 10.3389/fendo.2024.1339191
Received: 15 November 2023; Accepted: 29 May 2024;
Published: 21 June 2024.
Edited by:
Malgorzata Gabriela Wasniewska, University of Messina, ItalyReviewed by:
Cristina Martín-Arriscado Arroba, Fundación Investigación Biomédica Hospital 12 de Octubre, SpainRaisa Ghosh, National Institutes of Health (NIH), United States
Copyright © 2024 Yan, Sun, He and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinjin Sun, anN1bjAyQHRtdS5lZHUuY24=; Xianghui He, aGV4aDg4QHRtdS5lZHUuY24=
 Caigu Yan
Caigu Yan Jinjin Sun
Jinjin Sun Xianghui He
Xianghui He Lanning Jia2
Lanning Jia2