- 1Department of Obstetrics and Gynecology, Wenzhou Central Hospital, Wenzhou, Zhejiang, China
- 2Reproductive Medicine Center, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 3Department of Endocrinology, The Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
- 4Department of Endocrinology and Metabology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, Shandong, China
- 5Department of Anesthesia, Zhongshan Hospital, Fudan University, Shanghai, China
Objective: To investigate the effects of β-cell dysfunction on IVF outcomes in women with PCOS.
Methods: This retrospective cohort study includes 1,212 women with PCOS undergoing their first IVF cycle between September 2010 and December 2019. Beta-cell dysfunction was measured by homeostasis model assessment of β-cell function (HOMA-β) index.
Results: In quartiles of HOMA-β, the incidence of miscarriage dramatically increased from 10.2% (Q1) to 31.1% (Q4) (P for trend <0.001). Likewise, the incidence of miscarriage in quartiles of HOMA-β also showed a similar trend (P for trend <0.001). After adjusting for confounding factors, logistic regression analyses showed that high HOMA-IR values were independently associated with a high risk of miscarriage, with the odds ratios (OR) and 95% confidence intervals for quartiles 2–4 versus quartile 1 were 1.30 (0.69-2.46), 1.82 (0.97-3.43), and 3.57 (1.86-6.85), respectively (P for trend <0.001). When analyzed jointly, women in the highest HOMA-IR and highest HOMA-β group exhibited the highest risk for miscarriage compared with all other groups. Furthermore, higher HOMA-IR values were associated with higher risks of miscarriage among PCOS women regardless of HOMA-β values.
Conclusions: β-cell dysfunction is independently associated with increased miscarriage rate and decreased live birth rate in women with PCOS. It also plays a synergistic role with IR in terms of the reproductive outcomes, while the influence of IR overweighs that of β-cell dysfunction.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders characterized by oligo-anovulation, hyperandrogenism and polycystic ovarian morphology, affecting 5%–18% women of reproductive age (1). Most women with PCOS may experience irregular menstruation, metabolic disorders, hirsutism and infertility (2). Therefore, subfertility has become a growing problem and there is an increased use of in vitro fertilization (IVF) as a last resort in women with PCOS (3). Moreover, compared to non-PCOS, PCOS is associated with increased risk of adverse pregnancy outcomes, including miscarriage (1.7-fold higher), gestational hypertension (2-fold higher), preeclampsia (4-fold higher), gestational diabetes (3-fold higher) and premature delivery (2-fold higher) (4–7). Hitherto, the pathogenesis of adverse pregnancy outcomes of PCOS has not yet been fully elucidated, making it more difficult to perform interventions and improve pregnancy outcomes.
Approximately 45%-65% of women with PCOS have insulin resistance (IR), which is considered an initiating factor and plays a key role in the development of PCOS (8, 9). It has been confirmed that IR is closely related to adverse pregnancy outcomes (especially increased risk of miscarriage) in women with PCOS undergoing IVF treatment (10, 11). In contrast to IR, relatively fewer studies have explored the effects of β-cell function (insulin secretion) on metabolic and pregnancy outcomes in women with PCOS. Our previous study indicated that both IR and β-cell dysfunction independently affected cardiometabolic abnormalities including obesity, central obesity, dyslipidemia and high blood glucose in women with PCOS. IR was also correlated with a higher prevalence of cardiometabolic abnormalities than β-cell dysfunction (76.7% vs. 61.2%) (12). These results indicate the different roles of IR and β-cell dysfunction in the development of cardiometabolic disorders in PCOS. However, it remains unknown whether these two pathological states exhibit different effects on pregnancy outcomes. Thus, in the present retrospective cohort study, we aimed to investigate the effects of β-cell dysfunction on IVF outcomes in women with PCOS.
Patients and methods
Participants
Initially, a total of 1,515 infertile women with PCOS undergoing their first in-vitro fertilization embryo transfer (IVF-ET) cycle from September 2010 to December 2019 at the Reproductive Center of the First Affiliated Hospital of Wenzhou Medical University were enrolled in this study. The diagnostic criteria included two out of three following features according to the 2003 Rotterdam diagnostic criteria (13): (1) menstrual abnormalities, including oligomenorrhea or amenorrhea; (2) clinical and/or biochemical hyperandrogenism, including hirsutism (Ferriman-Galwey score >6) or testosterone concentration >2.6 nmol/L; and (3) polycystic ovarian morphology under B-ultrasound as indicated by the number of follicles with a diameter of 2-9 mm ≥12 and/or ovarian volume ≥10 ml. The exclusion criteria were as follows: women older than 40 years of age (n=7); women with a history of thyroid dysfunction n=19), hyperprolactinemia (n=33), hydrosalpinx (n=109), endometriosis (n=39), adenomyosis (n=30), chromosome abnormality (n=20), pituitary microadenoma (n=1), recurrent spontaneous abortion (n=24) and uterine malformation (n=10); and women lost to follow-up during pregnancy (n=11). Finally, 1,212 women with PCOS were included in the study analyses. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China) (2021R05), and approved a waiver of patient consent for the reason that all data were deidentified in this retrospective study.
Study procedures
All patients received a standardized ovarian stimulation protocol (GnRH antagonist protocol or long GnRH agonist protocol), oocyte retrieval, fertilization, and embryo transfer. The GnRH antagonist protocol or long GnRH agonist protocol used in our reproductive center has been previously described (14). Good-quality embryos at cleavage stage were defined according to the Istanbul consensus with <10% fragmentation, stage-specific cell size and no multimucleation (15). Due to the elective single-embryo transfer policy, no more than two embryos have been transferred since June 2016. Luteal supportive therapy was administered orally with dydrogesterone (20 mg daily) and vaginally with progesterone (90 mg daily), starting on the day of oocyte retrieval, and was continued until 8 weeks of gestation.
Definitions of β-cell dysfunction and insulin resistance
Beta-cell function was estimated by the homeostasis model assessment of β-cell function (HOMA-β) index as follows: HOMA-β=(20×FINS)/(FBG-3.5) (16). Beta-cell dysfunction was defined as HOMA-β in the top quartile (HOMA-β >186.86). IR was estimated by HOMA-IR index as follows: HOMA-IR=fasting blood glucose (FBG, mmol/L) x fasting insulin (FINS, mIU/L)/22.5 (17). The top quartile of HOMA-IR, which was greater than 3.75 in the present study, was defined as insulin resistance.
Laboratory testing
Blood samples were acquired after overnight fasting for at least 8 hours. Levels of FBG, FINS and gonadal hormones were quantified by chemiluminescence. Concentrations of total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were measured by a dry slide enzymatic colorimetric assay. Serum LH, FSH, E2, testosterone and AMH were measured using an ultrasensitive enzyme-linked immunosorbent assay (ELISA) (Unicel Dxl 800, Beckman Coulter, USA). Fasting plasma glucose, total cholesterol (TC), serum triglycerides (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were quantified by an autoanalyzer (AU 5800, Beckman, USA). The AMH data were limited due to the regular measurement of AMH in infertile women since June 2016 in our center. The intra-assay and inter-assay variations for the testing method were mentioned in our previous study (18). All measurements were performed at the First Affiliated Hospital of Wenzhou Medical University.
Definitions of IVF outcomes
Biochemical pregnancy was defined as the detection of β-hCG in urine or serum after embryo transfer (19). Clinical pregnancy was defined as the presence of a gestational sac with fetal heart activity under ultrasound examination 35 days after embryo transfer (19). Spontaneous loss of an intra-uterine pregnancy prior to 22 completed weeks of gestational age was defined as miscarriage (19). A live birth was defined as baby born after 22 weeks of gestational age (19). The definition of the clinical pregnancy rate was the number of clinical pregnancies per 100 embryo transfer cycles (19). The miscarriage rate was defined as the number of spontaneous fetal loss per 100 clinical pregnancy cycles (19). The live birth rate was defined as the number of deliveries per 100 embryo transfer cycles (19). All IVF outcomes were obtained through electronic medical records.
Statistical analysis
SPSS 23.0 software was used for all statistical analyses in the study. We divided the distribution of HOMA-β values into four groups from the lowest quartile (quartile 1, Q1) to the highest quartile (quartile 4, Q4). Participants were analyzed according to the quartile groups of HOMA-β. Demographic and biochemical variables with a skewed distribution were presented as the medians (interquartile ranges) according to quartiles of HOMA-β. P values for trends across all quartiles were calculated by linear regression analysis for continuous variables. Nonnormal distributed data were logarithmically transformed prior to linear regression analysis. Logistic regression analysis was performed to obtain the odds ratios for IVF outcomes based on quartiles of HOMA-β after adjusting for relevant variables (log-transformed). Meanwhile, P values for trends across quartiles were calculated by the Cochran–Mantel–Haenszel method. Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were calculated in three models for the logistic regression analyses. Adjustments were made for the following variables: no variable was adjusted in model 1; age and BMI were adjusted in model 2; and SBP, HOMA-IR, TC, TG, basal T levels and the number of transferred embryos were further adjusted in model 3 based on model 2. Interaction analysis of HOMA-IR and HOMA-β on IVF outcomes was adjusted for age, BMI, SBP, TG, TC, basal T levels and the number of transferred embryos. ORs (95% CIs) of miscarriage and live birth were used to compare the combined effects of HOMA-IR and HOMA-β between different groups. All P values were two-sided, and P<0.05 was considered statistically significant.
Results
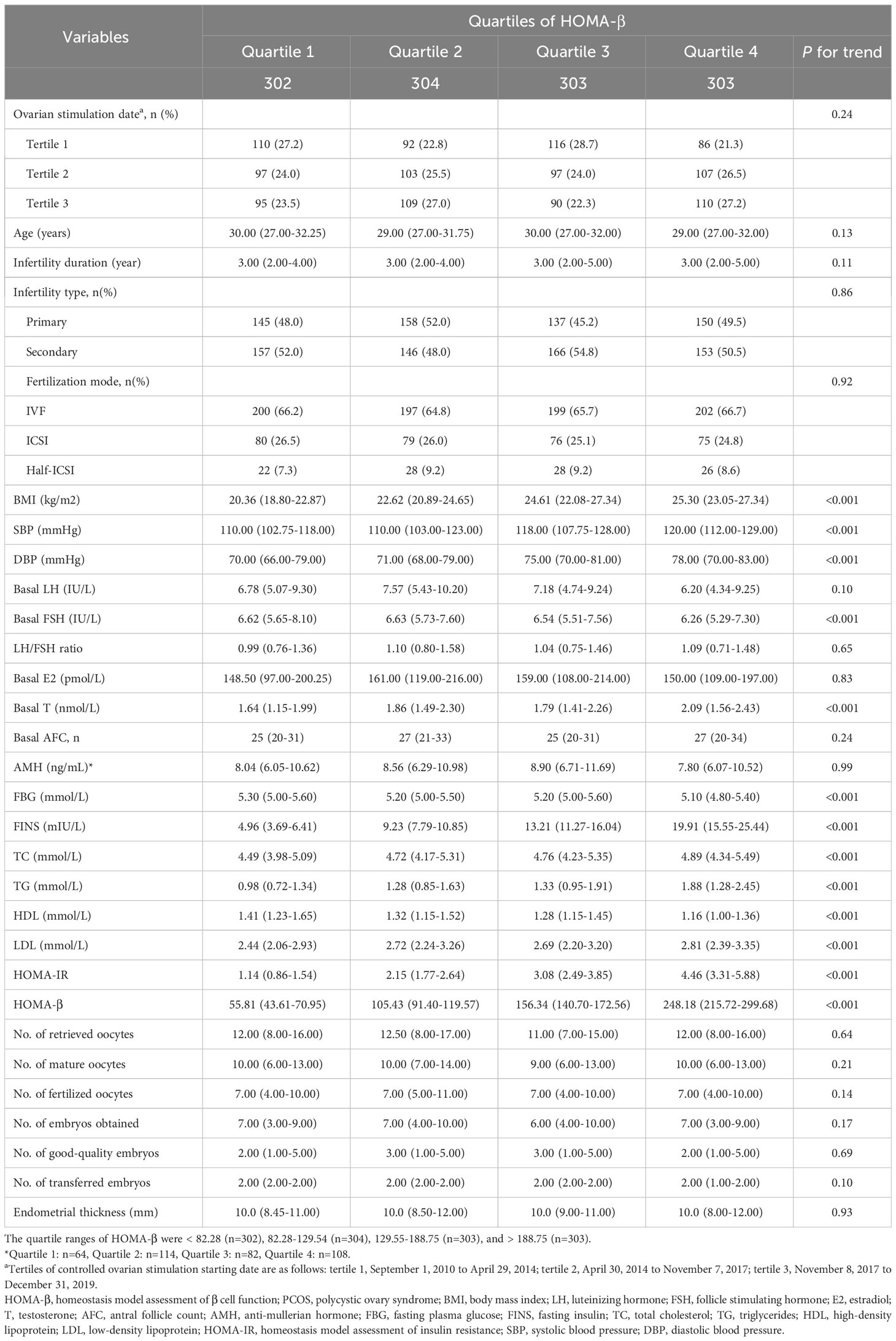
Baseline characteristics according to quartiles of HOMA-β in PCOS
Among the 1,212 infertile women with PCOS in the present study, the average age and the infertility duration was 29.69 and 3.81 years, respectively. There were 590 participants diagnosed with primary infertility with a prevalence rate of 48.7%, while the overall prevalence of secondary infertility was 51.3%.
The baseline characteristics of women with PCOS according to the quartiles of HOMA-β were described in Table 1. Subjects with a lower HOMA-β presented elevation in basal LH, FSH, FBG and HDL, but decreases in BMI, blood pressure, basal T, TC, TG, LDL, HOMA-IR, compared to those with higher HOMA-β (Table 1). The IVF outcomes, including fertilization mode, the number of retrieved oocytes, mature oocytes, fertilized oocytes, embryos obtained, good-quality embryos, transferred embryos and endometrial thickness, showed no significant differences among the quartiles of HOMA-β.
IVF outcomes based on quartiles of HOMA-β and different beta-cell function plus insulin levels in PCOS
Figure 1A showed the IVF outcomes based on quartiles of HOMA-β. In quartiles of HOMA-β, the miscarriage rate in Q4 was significantly higher than that in the other 3 quartiles (all P values <0.05). From the lowest quartile to the highest quartile of HOMA-β, the incidence of the miscarriage rate dramatically increased from 8.5% to 29.0% (P for trend <0.001). However, the live birth rate decreased from Q1 to Q4 (P for trend <0.05). No significant differences were observed in clinical pregnancy rate, biochemical pregnancy rate and ectopic pregnancy rate among the quartiles (P >0.05). The IVF outcomes in women with both IR and β-cell dysfunction (top quartile HOMA-IR and HOMA-beta) versus women without IR and without β-cells dysfunction were shown in Figure 1B. Compared with women without IR and β-cell dysfunction, the miscarriage rate in women with both IR and β-cell dysfunction was significantly higher (29.7% vs. 12.1%, P <0.001). Although the live birth rate in women with both IR and β-cell dysfunction was comparatively lower, no significant difference was found between the two groups (40.5% vs. 46.9%, P >0.05). Furthermore, the reproductive outcomes of women with and without resistance or with and without β-cell dysfunction were shown in Table 2. The miscarriage rate in women with IR or β-cell dysfunction was significantly higher than the controlled groups, while the live birth rate in women with IR was significantly lower than the non-IR group.
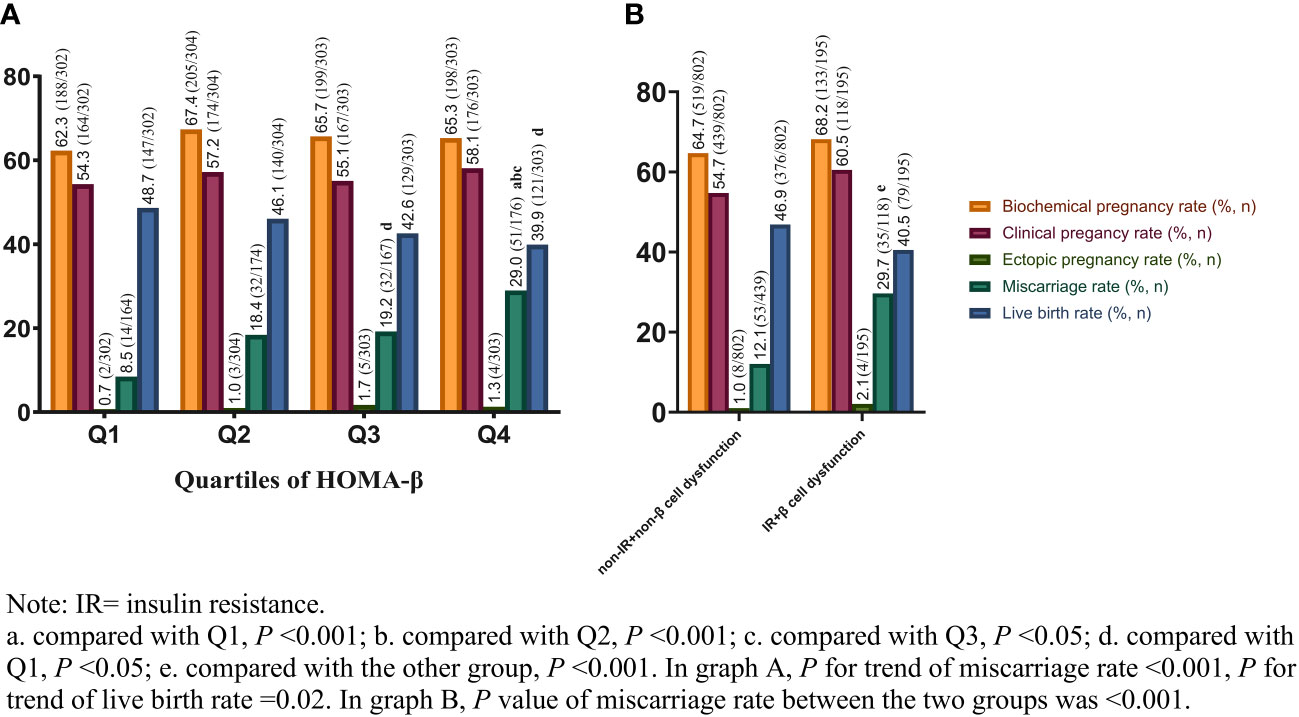
Figure 1 IVF outcomes based on quartiles of HOMA-β (A) and different beta-cell function plus insulin levels (B) in PCOS. IVF, in-vitro fertilization; HOMA-β, homeostasis model assessment of β cell function; PCOS, polycystic ovary syndrome.
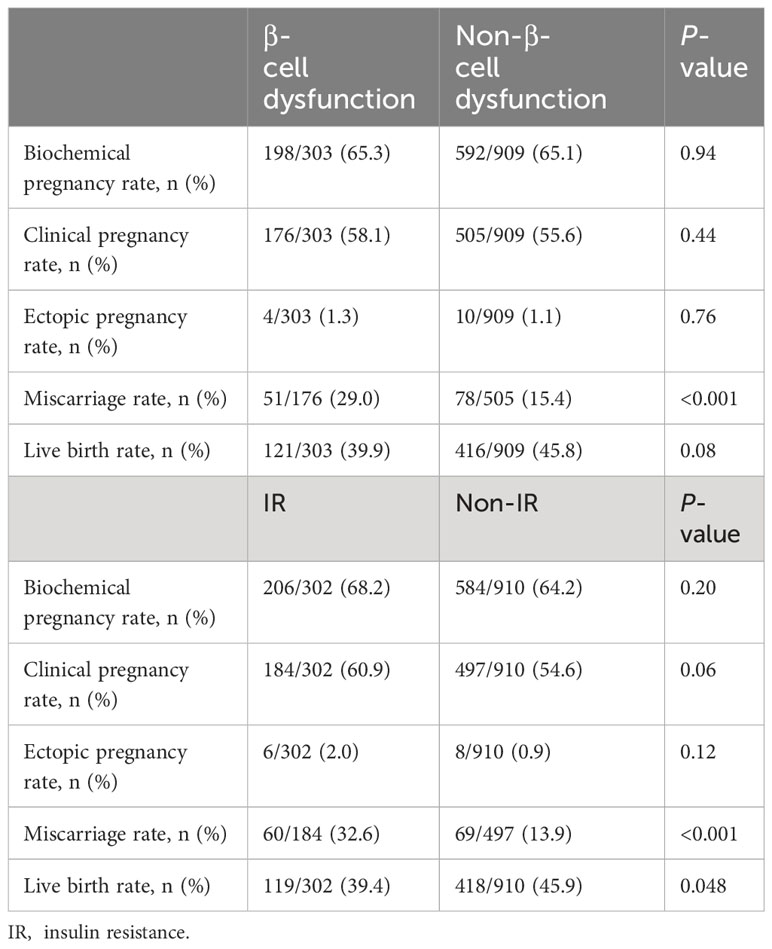
Table 2 Reproductive outcomes in women with and without β-cell dysfunction or with and without insulin resistance.
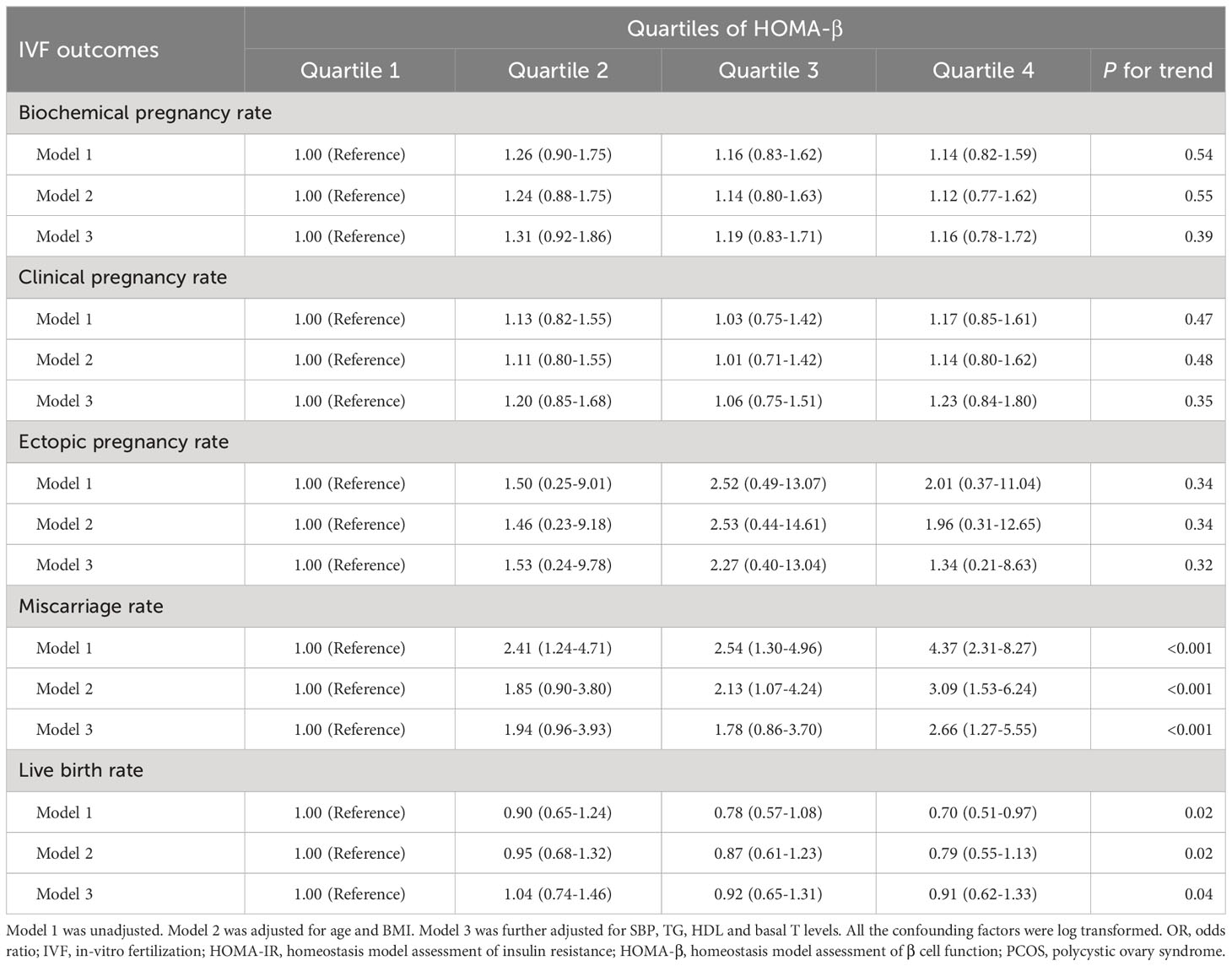
Odds ratios for IVF outcomes based on quartiles of HOMA-β in PCOS
Table 3 lists the prevalence ratios for relevant IVF outcomes, including clinical pregnancy rate, biochemical pregnancy rate, ectopic pregnancy rate, miscarriage rate and live birth rate, according to quartiles of HOMA-β. With the first quartile of HOMA-β as the reference group, univariate logistic regression analysis showed significantly increased ORs for the prevalent miscarriage rate across HOMA-β categories (OR=4.37, 95% CI: 2.31-8.27) and the lowest odds ratio of live birth rate (OR=0.70, 95% CI: 0.51-0.97). After adjustment for traditional confounding factors (model 2), the ORs for the prevalent miscarriage rate, as compared with the lowest quartile, were 1.85 (95% CI, 0.90-3.80) for Q2, 2.13 (95% CI, 1.07-4.24) for Q3, and 3.09 (95% CI, 1.53-6.24) for Q4, respectively (P for trend <0.001). Following further adjustment for SBP, TG, TC, basal T levels and the number of transferred embryos (model 3), a 94%, 78%, and 166% increase in ORs for the risk of the prevalent miscarriage rate was found in the second, third and fourth quartile, respectively, compared with those in the top one (Pfor trend <0.001). In terms of live birth rate in quartiles of HOMA-β, there was significant decreasing trend from Q1 to Q4 in model 1, 2 and 3 (P for trend <0.05). Other IVF outcomes, such as the clinical pregnancy rate, biochemical pregnancy rate and ectopic pregnancy rate, were comparable in all 3 models in quartiles of HOMA-β.
Joint effects of HOMA-IR and HOMA-β on IVF outcomes
Figure 2 showed the unadjusted odds ratios of miscarriage and live birth by comparing nine groups of patients with various combinations of HOMA-β and HOMA-IR values. Women with both high HOMA-β and high HOMA-IR values (group 7) exhibited the highest OR for miscarriage compared to all other groups. Furthermore, a higher HOMA-IR value (groups 7, 8 and 9) was associated with a relatively higher OR of miscarriage among participants regardless of the levels of HOMA-β values. However, the odds ratios of live birth in different groups according to the values of HOMA-IR and HOMA-β showed no significant differences after adjusting for confounding factors.
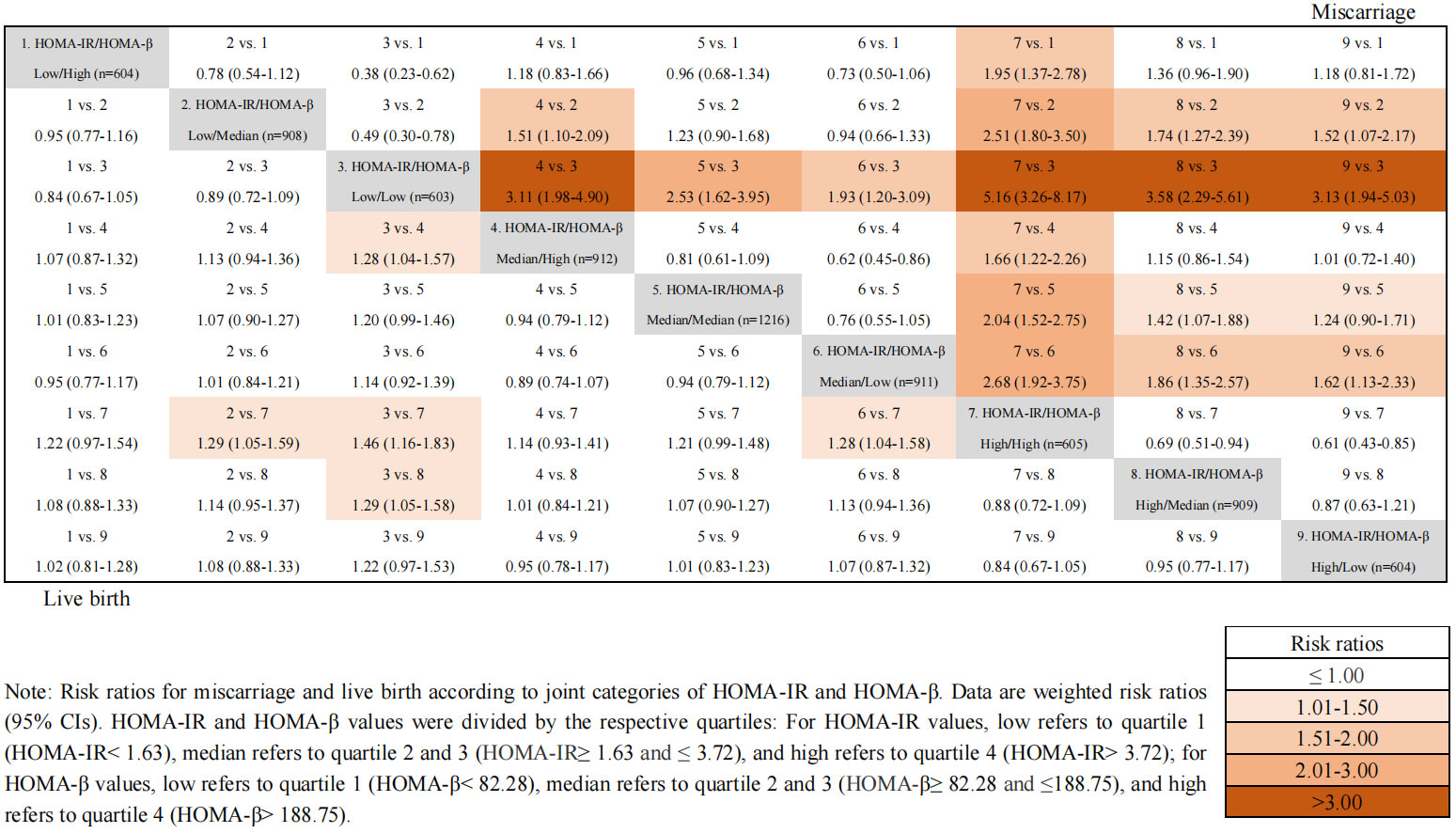
Figure 2 Joint effects of HOMA-IR and HOMA-β on IVF outcomes. Odds ratios for miscarriage and live birth according to joint categories of HOMA-IR and HOMA-β. Data are weighted odds ratios (95% CIs). HOMA-IR and HOMA-β values were divided by the respective quartiles: For HOMA-IR values, low refers to quartile 1 (HOMA-IR< 1.63), median refers to quartile 2 and 3 (HOMA-IR ≥1.63 and ≤ 3.72), and high refers to quartile 4 (HOMA-IR> 3.72); for HOMA-β values, low refers to quartile 1 (HOMA-β < 82.28), median refers to quartile 2 and 3 (HOMA-β≥ 82.28 and ≤188.75), and high refers to quartile 4 (HOMA-β> 188.75).
Discussion
To the best of our knowledge, this is the first cohort study to explore the independent effect of β-cell dysfunction and the interaction effects of β-cell dysfunction and IR on the risks of IVF outcomes in women with PCOS. In the current study, we found that HOMA-β values were independently correlated with an increased risk of miscarriage.
Previous studies mainly have focused on the higher risk of miscarriage in PCOS women with insulin resistance (20, 21), while evidence regarding the effects of β-cell function on IVF outcomes is relatively sparse. Our results showed that high HOMA-β values were associated with an increased risk of miscarriage and a lower incidence of live birth, indicating that β-cell dysfunction (excess insulin secretion) independently exerted adverse reproductive effects on PCOS. The decrease in the HOMA-β values indicated a decrease in the sensitivity of human somatic cells to insulin receptors. Variation in β‐cell capacity is attributed to the growth of the β‐cell pool and insulin secretion ability (22). The balance between such a hyperdynamic β‐cell pool and insulin resistance ensures a steady flow of nutrients in women with PCOS when attempting conception (23). The exacerbation of IR leads to growing demands for insulin secretion by pancreatic β-cells. However, a hyperbolic relationship exists between IR and insulin secretion, that is with continued deterioration of IR, the compensation in insulin secretion could be limited with continued deterioration of IR (24). The resultant unfavorable state uncovers potential defects of β-cell function, thereby precipitating the development of gestational diabetes or type 2 diabetes in women with PCOS (25). Previous studies have demonstrated that the variation trend of HOMA-IR and HOMA-β is consistent in women with PCOS (12, 26), which agree with our findings. Therefore, it could be indicated that PCOS women with β-cell dysfunction are still at higher risks of miscarriage and lower incidence of live birth even without IR.
Furthermore, considering the classic feedback loop between hyperinsulinemia and IR, our study further examined the joint effects of β-cell dysfunction and IR on the miscarriage and live birth in women with PCOS. Our results suggested that women with both high HOMA-β and high HOMA-IR values exhibited the highest odds ratios for miscarriage and the lowest odds ratios for live birth. Additionally, according to various combinations of HOMA-β and HOMA-IR, the association of a lower HOMA-β value with the odds ratio of miscarriage was strengthened by a high HOMA-IR value, indicating that although both insulin resistance and β-cell dysfunction were closely associated with miscarriage, the effect of IR overweighed that of β-cell dysfunction. A recent study indicated that women with PCOS and IR might result in a higher risk of miscarriage, but did not impair live birth rate (27). It has been reported that the variation of HOMA-β is hyperbolic in the progression of diabetes and is highly interfered by insulin resistance, which increases the difficulty of determining whether the value of HOMA-β represents the compensation stage or decompensation stage (28). In this study, we found that women with IR has significantly lower live birth rate, which is not in accordance with previous findings. Moreover, although the live birth rate were comparable between the β-cell dysfunction and non-β-cell dysfunction, women with both high HOMA-β and high HOMA-IR values exhibited the lowest odds ratios for live birth after interactive analysis. This result indicates that the negative association between IR and adverse reproductive outcomes seemed to be amplified with more vulnerable β-cell function in women with PCOS.
When compared with women with both low values of HOMA-IR (HOMA-IR< 1.63) and HOMA-β (HOMA-β<82.28), women with both insulin resistance (HOMA-IR>3.72) and β-cell dysfunction (HOMA-β>188.75) have approximately 5.16-fold higher miscarriage rate, and 1.46-fold lower live birth rate. Therefore, from a clinical point of view, it could be hypothesized that after appropriate pretreatment to lower HOMA-IR and HOMA-β, the reproductive outcomes in women with PCOS might have improved significantly. These insights shine a light on the application of a novel therapy for PCOS with insulin-sensitizing drugs. Metformin, an insulin-sensitizing drug, has been widely applied in the pretreatment of women with PCOS undergoing IVF treatment during the past decades (29). Metformin administration could reduce the risk of ovarian hyperstimulation syndrome and miscarriage in women with PCOS, which provides a new idea to improve the reproductive outcomes in PCOS women with insulin resistance and β-cell dysfunction (30).
Several potential mechanisms account for the negative effects of β-cell dysfunction as well as IR on reproductive outcomes in women with PCOS. First, the high insulin level in peripheral blood leads to the abnormal secretion of insulin-like growth factor (IGF-1) and IGF-2. IGF facilitates the implantation of the human embryo in the endometrium during IVF and plays an important role in trophoblast morphogenesis and placental microvasculature (31, 32). Second, studies on PCOS-like rats indicate that IR causes the activation of ferroptosis in the gravid uterus and placenta. Furthermore, necroptosis and apoptosis might play a role in compensating or coordinating for IR-induced ferroptosis when the gravid uterine and placental dysfunction occur (33). In addition, hyperinsulinemia and IR can lead to increased secretion of reactive oxygen species, which further cause mitochondrial and placental dysfunction after pregnancy, thus increasing the risk of miscarriage and decreasing the live birth rate (34, 35).
To our knowledge, this study was unique in that we included women with PCOS undergoing their first IVF cycle and studied both the independent and combined effects of β-cell dysfunction and insulin resistance on IVF outcomes. The novelty of our study was the independent and joint effect of HOMA-β and HOMA-IR on reproductive effects. Considering that PCOS women with various combinations of β-cell dysfunction and IR might present different odds ratios for miscarriage, early screening and individualized intervention should thus be tailored. Therefore, even in PCOS women with mild hyperglycemia, glucose‐lowering interventions before IVF could improve pregnancy outcomes. In addition, the calculation of HOMA-IR and HOMA-β is easy and has been widely acknowledged, which anticipates a highly practical value of our findings in the clinical practice. However, the present study had several limitations and should be taken into consideration. First, this is a retrospective study that looks back for a long span of time in which clinical practice, biochemical measurements, IVF treatments might have changed dramatically. In order to minimize the potential bias, we analyzed the subjects from Q1 to Q4 according to the tertiles of ovarian stimulation date in Table 1. We found that there were no significant differences in subjects from Q1 to Q4 in terms of the grouping of ovarian stimulation date in both Tables. However, prospectively designed multi-centers studies, follow-up of newborns and the effect of medical pretreatment on reproductive outcomes are still needed for further evaluation. Second, glucose clamp has been undeniably recognized as the gold standard for evaluating insulin metabolism. However, they may be perceived as invasive and expensive for use in clinical studies with large samples. Thus, surrogate markers, such as HOMA, have been proposed as alternative markers for insulin sensitivity and secretion, which can be repeatable and reproducible in the same way as gold standards. Although validation studies have indicated tight correlations between the HOMA models and gold-standard methods, the findings should be interpreted carefully. Third, although carefully adjusted for a set of confounders in the analysis, unmeasured confounders, such as dietary factors and physical activity, may affect the results to some extent. In addition, since the subjects in the present study were Chinese women, these conclusions might not be directly applied to populations of other ethnicities.
Conclusions
In summary, our findings indicate that β-cell dysfunction is independently associated with increased miscarriage rate and decreased live birth rate in women with PCOS. Furthermore, it also plays a synergistic role with IR in terms of the reproductive outcomes, while the influence of IR overweighs that of β-cell dysfunction. Therefore, early screening and interventions for β-cell dysfunction and IR in women with PCOS may be extremely helpful for improving conception opportunities.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The First Affiliated Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because all the data were deidentified in this retrospective study.
Author contributions
WH: Conceptualization, Formal Analysis, Methodology, Writing – original draft. CL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. LD: Data curation, Writing – review & editing. YL: Data curation, Writing – original draft. HZ: Formal Analysis, Software, Writing – original draft. SW: Formal Analysis, Writing – review & editing. HY: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Health Department of Zhejiang province (2021KY785), Wenzhou Municipal Science and Technology Bureau Foundation of Wenzhou, Zhejiang, China (Y2020517, Y20220055), Wenzhou Key Laboratory of Reproduction and Genetics (2022HZSY0051), and Zhejiang Provincial Natural Science Foundation of China (LQ21H040010). No other potential conflicts of interest relevant to this article were reported.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewers YH and JZ shared parent affiliation with the AUTs CL, YL, HZ, HY to the handling editor at time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinology. (2022) 10:668–80. doi: 10.1016/S2213-8587(22)00163-2
2. Mirza FG, Tahlak MA, Rjeili RB, Hazari K, Ennab F, Hodgman C, et al. Polycystic ovarian syndrome (PCOS): does the challenge end at conception? Int J Environ Res Public Health. (2022) 19:14914. doi: 10.3390/ijerph192214914
3. Guan SY, Liu YY, Guo Y, Shen XX, Liu Y, Jin HX. Potential biomarkers for clinical outcomes of IVF cycles in women with/without PCOS: Searching with metabolomics. Front Endocrinology. (2022) 13:982200. doi: 10.3389/fendo.2022.982200
4. Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. (2015) 21:575–92. doi: 10.1093/humupd/dmv029
5. Sha T, Wang X, Cheng W, Yan Y. A meta-analysis of pregnancy-related outcomes and complications in women with polycystic ovary syndrome undergoing IVF. Reprod Biomedicine Online. (2019) 39:281–93. doi: 10.1016/j.rbmo.2019.03.203
6. Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Silagy M, Arora C, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity-A systematic review, meta-analysis, and meta-regression. Obes Rev. (2019) 20:659–74. doi: 10.1111/obr.12829
7. Palomba S, Santagni S, Gibbins K, La Sala GB, Silver RM. Pregnancy complications in spontaneous and assisted conceptions of women with infertility and subfertility factors. A Compr Review. Reprod Biomedicine Online. (2016) 33:612–28. doi: 10.1016/j.rbmo.2016.08.007
8. Moghetti P, Tosi F. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest. (2021) 44:233–44. doi: 10.1007/s40618-020-01351-0
9. Armanini D, Boscaro M, Bordin L, Sabbadin C. Controversies in the pathogenesis, diagnosis and treatment of PCOS: focus on insulin resistance, inflammation, and hyperandrogenism. Int J Mol Sci. (2022) 23:4110. doi: 10.3390/ijms23084110
10. Li Y, Chen C, Ma Y, Xiao J, Luo G, Li Y, et al. Multi-system reproductive metabolic disorder: significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. (2019) 228:167–75. doi: 10.1016/j.lfs.2019.04.046
11. Kampmann U, Knorr S, Fuglsang J, Ovesen P. Determinants of maternal insulin resistance during pregnancy: an updated overview. J Diabetes Res. (2019) 2019:5320156. doi: 10.1155/2019/5320156
12. Mu L, Zhao Y, Lai Y, Li R, Qiao J. Insulin resistance and β-cell dysfunction and the relationship with cardio-metabolic disorders among women with polycystic ovary syndrome. Clin Endocrinol (Oxf). (2018) 89:779–88. doi: 10.1111/cen.13832
13. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility Sterility. (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004
14. Chen Y, Ye B, Yang X, Zheng J, Lin J, Zhao J. Predicting the outcome of different protocols of in vitro fertilization with anti-Muüllerian hormone levels in patients with polycystic ovary syndrome. J Int Med Res. (2017) 45:1138–47. doi: 10.1177/0300060517704140
15. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod (Oxford England). (2011) 26:1270–83. doi: 10.1093/humrep/der037
16. Adamska A, Polak AM, Krentowska A, Łebkowska A, Hryniewicka J, Leśniewska M, et al. Increased serum fetuin-B concentration is associated with HOMA-β and indices of liver steatosis in women with polycystic ovary syndrome: a pilot study. Endocrine Connections. (2019) 8:1159–67. doi: 10.1530/EC-19-0243
17. Song H, Yu Z, Li P, Wang Y, Shi Y. HOMA-IR for predicting clinical pregnancy rate during IVF. Gynecological Endocrinol Off J Int Soc Gynecological Endocrinology. (2022) 38:33–8. doi: 10.1080/09513590.2021.1952976
18. Yang H, Wang G, Liu C, Ding L, Li Y, Chen Y, et al. Elevated serum uric acid level is associated with adverse reproductive outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization or intracytoplasmic sperm injection embryo transfer cycles: a retrospective cohort study. Am J Obstetrics Gynecology. (2023) 228(3):324.e1–10. doi: 10.1016/j.ajog.2022.11.1287
19. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertility Sterility. (2017) 108:393–406. doi: 10.1016/j.fertnstert.2017.06.005
20. Yang T, Yang Y, Zhang Q, Liu D, Liu N, Li Y, et al. Homeostatic model assessment for insulin resistance is associated with late miscarriage in non-dyslipidemic women undergoing fresh IVF/ICSI embryo transfer. Front Endocrinology. (2022) 13:880518. doi: 10.3389/fendo.2022.880518
21. Chen Y, Guo J, Zhang Q, Zhang C. Insulin resistance is a risk factor for early miscarriage and macrosomia in patients with polycystic ovary syndrome from the first embryo transfer cycle: A retrospective cohort study. Front Endocrinology. (2022) 13:853473. doi: 10.3389/fendo.2022.853473
22. Baeyens L, Hindi S, Sorenson RL, German MS. β-Cell adaptation in pregnancy. Diabetes Obes Metab. (2016) 18 Suppl 1:63–70. doi: 10.1111/dom.12716
23. Retnakaran R. Adiponectin and β-cell adaptation in pregnancy. Diabetes. (2017) 66:1121–2. doi: 10.2337/dbi17-0001
24. Turner RC, Holman RR, Matthews D, Hockaday TD, Peto J. Insulin deficiency and insulin resistance interaction in diabetes: estimation of their relative contribution by feedback analysis from basal plasma insulin and glucose concentrations. Metabolism: Clin Experimental. (1979) 28:1086–96. doi: 10.1016/0026-0495(79)90146-X
25. Diamanti-Kandarakis E, Xyrafis X, Boutzios G, Christakou C. Pancreatic beta-cells dysfunction in polycystic ovary syndrome. Panminerva Med. (2008) 50:315–25.
26. Ezeh U, Chen IY, Chen YH, Azziz R. Adipocyte insulin resistance in PCOS: relationship with GLUT-4 expression and whole-body glucose disposal and β-cell function. J Clin Endocrinol Metab. (2020) 105:e2408–20. doi: 10.1210/clinem/dgaa235
27. Luo Z, Wang L, Wang Y, Fan Y, Jiang L, Xu X, et al. Impact of insulin resistance on ovarian sensitivity and pregnancy outcomes in patients with polycystic ovary syndrome undergoing IVF. J Clin Med. (2023) 12:818. doi: 10.3390/jcm12030818
28. Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. (2009) 373:2215–21. doi: 10.1016/S0140-6736(09)60619-X
29. Palomba S, Falbo A, Zullo F, Orio F Jr. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev. (2009) 30:1–50. doi: 10.1210/er.2008-0030
30. Magzoub R, Kheirelseid EAH, Perks C, Lewis S. Does metformin improve reproduction outcomes for non-obese, infertile women with polycystic ovary syndrome? Meta-analysis and systematic review. Eur J Obstet Gynecol Reprod Biol. (2022) 271:38–62. doi: 10.1016/j.ejogrb.2022.01.025
31. Sandovici I, Georgopoulou A, Pérez-García V, Hufnagel A, López-Tello J, Lam BYH, et al. The imprinted Igf2-Igf2r axis is critical for matching placental microvasculature expansion to fetal growth. Dev Cell. (2022) 57:63–79.e8. doi: 10.1016/j.devcel.2021.12.005
32. Druckmann R, Rohr UD. IGF-1 in gynecology and obstetrics: update 2002. Maturitas. (2002) 41 Suppl 1:S65–83. doi: 10.1016/S0378-5122(02)00016-6
33. Zhang Y, Hu M, Jia W, Liu G, Zhang J, Wang B, et al. Hyperandrogenism and insulin resistance modulate gravid uterine and placental ferroptosis in PCOS-like rats. J Endocrinology. (2020) 246:247–63. doi: 10.1530/JOE-20-0155
34. Zhang Y, Zhao W, Xu H, Hu M, Guo X, Jia W, et al. Hyperandrogenism and insulin resistance-induced fetal loss: evidence for placental mitochondrial abnormalities and elevated reactive oxygen species production in pregnant rats that mimic the clinical features of polycystic ovary syndrome. J Physiol. (2019) 597:3927–50. doi: 10.1113/JP277879
Keywords: β-Cell dysfunction, insulin resistance, hyperinsulinemia, polycystic ovary syndrome, IVF outcome
Citation: Huang W, Liu C, Ding L, Li Y, Zhou H, Wang S and Yang H (2024) The effect of β-cell dysfunction on reproductive outcomes of PCOS undergoing IVF or ICSI embryo transfer cycles: a retrospective cohort study. Front. Endocrinol. 15:1327041. doi: 10.3389/fendo.2024.1327041
Received: 24 October 2023; Accepted: 09 February 2024;
Published: 05 March 2024.
Edited by:
Harpal Singh Randeva, University Hospitals Coventry and Warwickshire NHS Trust, United KingdomReviewed by:
Fahiel Casillas, Metropolitan Autonomous University, MexicoJunzhao Zhao, Wenzhou Medical University, China
Ying Hua, Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, China
Copyright © 2024 Huang, Liu, Ding, Li, Zhou, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Yang, MTM5NTc3MjA0OTFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Wenle Huang
Wenle Huang Chang Liu
Chang Liu Lin Ding
Lin Ding Yan Li2
Yan Li2 Haiyan Yang
Haiyan Yang