- Department of Oncology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Objective: Evidence has been increasingly pointing towards a potential link between phenotypes related to obesity and the incidence of colorectal cancer. However, confirming this as a direct causal connection remains elusive. This investigation aims to elucidate the causative links between obesity-associated phenotypes and the incidence of colorectal cancer.
Methods: Employing the Two Sample Mendelian Randomization (TwoSampleMR) R package, analyses were conducted using Mendelian randomization (MR) to discern potential causative links between obesity categories sourced from both the Institute for Education and University (IEU) Open GWAS Project and Zenodo, and colorectal tumors (data obtained from IEU Open GWAS and FinnGen). For primary evaluations, the study utilized the Wald ratio and the Inverse Variance Weighting (IVW) methods, while the MR-Egger approach was integrated for sensitivity assessment. Bidirectional Mendelian Randomization (Bidirectional MR), as well as Linkage Disequilibrium (LD) Score Regression with well-imputed HapMap3 single nucleotide polymorphisms (SNPs), were additionally executed. Sensitivity assessments entailed IVW, MR-Egger methodologies to assess heterogeneity and pleiotropy, along with a leave-one-out strategy. Instrumental variables were chosen judiciously based on predetermined P-value thresholds and F-statistics.
Results: Results from MR evaluations did not identify a clear causative link between BMI and colorectal malignancy. Conversely, both measures of obesity, the Waist-Hip Ratio (WHR) and its adjusted form for BMI (WHRadjBMI), displayed a connection to increased risk of colorectal cancer, especially prominent among female subjects. Reverse MR analyses dismissed potential reverse causality between colorectal malignancies and obesity. A significant genetic interplay was observed between WHR, WHRadjBMI, and colorectal cancer instances. Ensuing MR probes spotlighted inflammatory bowel ailment as a protective factor, while salad intake was indicated as a potential risk concerning colorectal malignancies. Sensitivity reviews, which included tests for both pleiotropy and heterogeneity, validated the robustness of the MR findings.
Conclusion: Findings from this research indicate that specific obesity-related parameters, notably WHR and WHRadjBMI, carry a causal relationship with an elevated colorectal cancer risk. The impact is distinctly more evident among females. Such insights might be pivotal for public health deliberations, hinting that individuals boasting a high WHR might necessitate intensified colorectal cancer screenings.
Introduction
Worldwide, colorectal tumors (CRC) stand out as ranking third in cancer prevalence and occupying the position of the second most frequent driver behind cancer-related deaths (1). Even with advancements in both diagnosis and treatment methodologies, a significant surge in CRC occurrences was observed (2, 3), reinforcing the urgency of potent preventive approaches. Simultaneously, the worldwide increase in obesity, exemplified by a rising Body Mass Index (BMI) among adults, became a pivotal research focus (4). Statistics from the World Health Organization (WHO) highlighted an alarming trend, with approximately 1.5 billion adults worldwide classified as overweight and a significant 500 million deemed obese (5). Notably, a concurrent trend associated rising obesity levels with a heightened incidence of CRC (2, 6).
In obesity evaluation, both BMI and Waist-Hip Ratio (WHR) were highlighted as crucial modifiable determinants associated with CRC (7–9). Notably, obesity accounted for an estimated 4.6% of cancer-related mortalities worldwide (10). Furthermore, weight loss appeared to confer some protective benefits, especially among postmenopausal women (11). Associations between obesity and CRC emerged as intricate, shaped by several intertwined determinants. These encompassed elevations in insulin and IGF-1 pathways, growth factors, persistent inflammation, disruptions in hormonal equilibrium, adipokines, and fluctuations in sex hormone concentrations (12–14). Although these connections were reported, variations existed across different scientific publications. Distinct studies emphasized an elevated mortality risk among CRC patients possessing elevated BMI relative to those with a standard BMI (15, 16). Particularly, studies presented by Baade et al. (17) in conjunction with Kuiper et al. (18) demonstrated an augmented rate of deaths related to CRC in individuals with excessive weight, with recorded increases of 25% and 55% respectively. Yet, contrasting studies suggested that elevated BMI was not linked with increased mortality and could even be associated with reduced mortality risks in CRC patients (19, 20). These inconsistent results illuminated the intricacies and challenges in understanding the obesity-CRC relationship. Epidemiological data were often compromised by confounding variables, and their interpretive scope was additionally narrowed by issues of reverse causality (21). Furthermore, the study of other obesity-related metrics, like WHR, within the CRC context remained insufficient (22). These limitations underscored the pressing need for comprehensive, well-designed prospective studies to unravel the complex relationship between obesity and CRC.
In epidemiological research, Mendelian Randomization (MR) is esteemed as an invaluable tool, leveraging genetic markers to determine causative relationships between risk determinants and health outcomes (23). Within this framework, causal inferences are further refined by phenotype-specific genetic variants (24). Crucially, MR overcomes challenges frequently associated with observational analysis, including confounding and issues tied to causality reversal, thus facilitating the assessment of enduring risk factors through a wide range of health outcomes (25, 26).
For the study at hand, exhaustive evaluations were undertaken using two-sample MR and Genetic Correlation methodologies to determine the causative ties between obesity indicators—specifically BMI and WHR—and the susceptibility to CRC. Aggregate data was sourced from comprehensive Genome-Wide Association Studies (GWAS) focusing on obesity and CRC. Particular attention was given to the implications of BMI and WHR adjusted for BMI (WHRadjBMI) in cancer vulnerability. This consideration stemmed from previous claims positing that genetic markers associated with WHR might delineate more obscure biological pathways than those linked to BMI or WHRadjBMI (27). Consistent with prevailing research conclusions, it was hypothesized that obesity metrics, particularly BMI and WHR, had a causative relationship with CRC risk. Employing MR as an analytical approach in this study provided a method less prone to the biases observed in traditional observational research. The research focused on deriving insights vital for the development of future health prevention approaches and the definition of public health guidelines.
Materials and methods
Obesity-related GWAS information acquisition
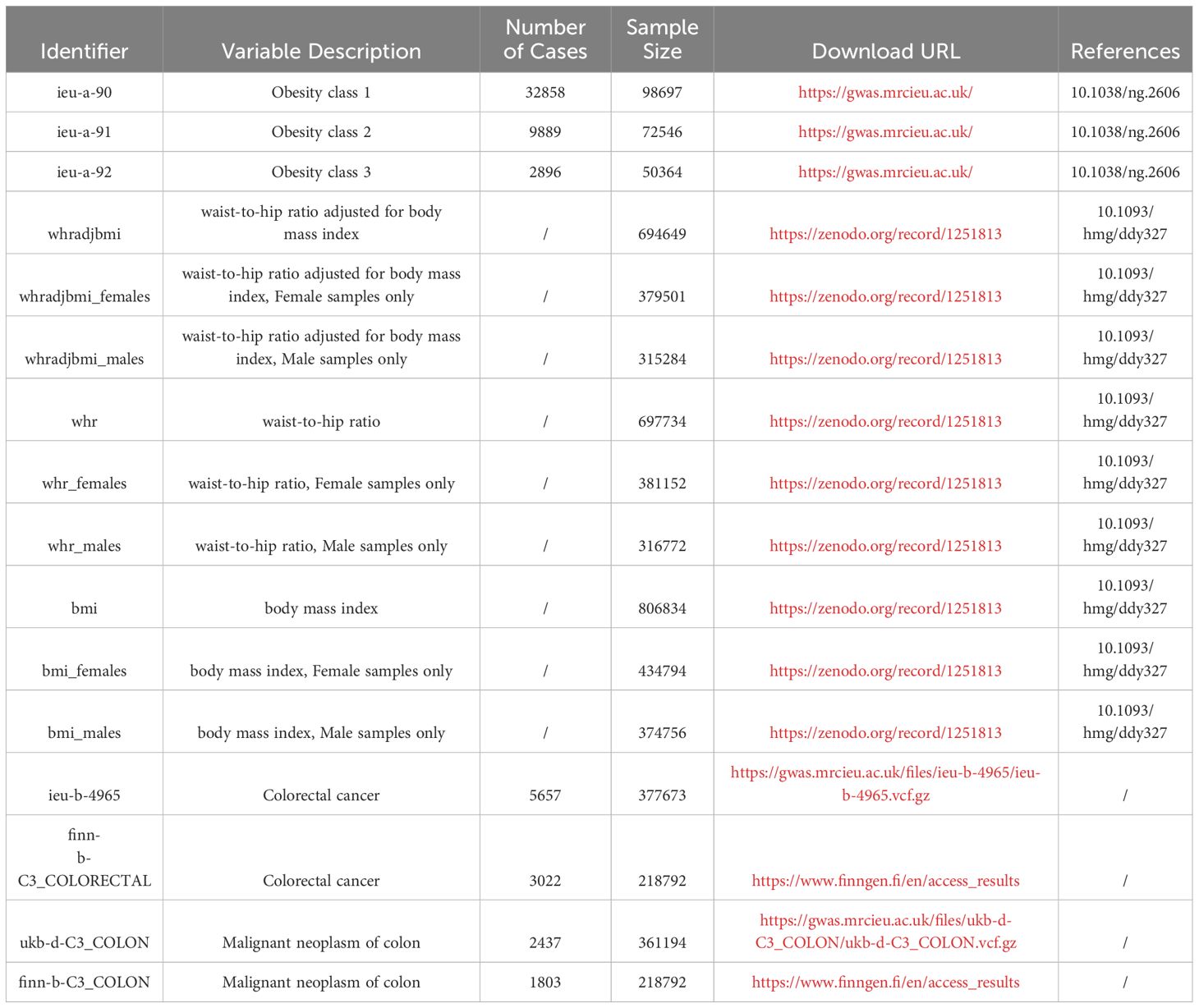
Data, sorted based on obesity classification, was sourced from the IEU Open GWAS Project platform at https://gwas.mrcieu.ac.uk/. Distinct codes for these datasets include: ieu-a-90 for Obesity class 1, ieu-a-91 indicating Obesity class 2, and ieu-a-92 marking Obesity class 3. Within Obesity class 1, a count of 32,858 cases was observed, with the total sample reaching 98,697. For Obesity class 2, 9,889 cases were identified within a 72,546 sample. Obesity class 3 had 2,896 cases among a sample of 50,364 (28). Other datasets pertaining to obesity metrics were derived from https://zenodo.org/record/1251813. This array encompasses: Waist-Hip Ratio adjusted for Body Mass Index (WHRadjBMI), WHRadjBMI for females (WHRadjBMI_females), WHRadjBMI for males (WHRadjBMI_males); The raw Waist-Hip Ratio (WHR), WHR limited to females (WHR_females), and WHR restricted to males (WHR_males); Standard Body Mass Index (BMI) values, female-specific BMI (BMI_females), and male-specific BMI (BMI_males) (29). Details alongside the requisite download links have been itemized in Table 1.
Colorectal cancer outcome data
Information related to malignant colorectal tumors was sourced from two distinct platforms: the IEU Open GWAS Project and the FinnGen platform. Within these platforms, data were drawn from four specific cohorts. These are labeled as ukb-d-C3_COLON and ieu-b-4965 within the UK Biobank database, and finn-b-C3_COLON and finn-b-C3_COLORECTAL in the FinnGen database. It was noted that there was no observed sample overlap between the datasets from UK Biobank and the FinnGen database. A comprehensive description and relevant download links can be found in Table 1.
Additional data
A literature review was conducted to identify risk factors related to colorectal cancer, excluding genetic factors, race, and gender. Identified were twenty probable risk determinants: habits like smoking; dietary choices such as consuming processed meat, red meat, and alcohol; a scarcity in consuming fruits and vegetables; the presence of obesity; levels of physical exertion; consuming whole grains, dietary fiber, dairy items, fish, and tree nuts; specific vitamins such as D and C; utilization of calcium enhancers, non-steroidal anti-inflammatory medications, or aspirin; undergoing hormone replacement during the menopausal phase; administering statins; existence of type 2 diabetes; and conditions like inflammatory bowel disease (30). Subsequently, GWAS data for thirteen of these risk factors were retrieved from the OpenGWAS database: smoking (ukb-b-223), processed meat (ukb-b-6324), frequency of alcohol consumption (ukb-b-5779), salad/raw vegetable intake (ukb-b-1996), fresh fruit intake (ukb-b-3881), whole grains (ukb-d-1448_3), dietary fiber (ukb-b-19085), vitamin C (ukb-b-19390), vitamin D (ukb-b-18593), calcium supplements (ukb-b-7043), hormone replacement therapy during menopause (ukb-b-18541), type 2 diabetes (ebi-a-GCST006867), and inflammatory bowel disease (ieu-a-294).
Conducting the Mendelian randomization assessment
Analyses rooted in Mendelian randomization were carried out on datasets relating to obesity and colorectal cancer using the TwoSampleMR R package (Version 0.5.7, TwoSampleMR documentation) (31). During the Mendelian randomization, the choice of linear model computation hinged upon the count of instrumental variables retained for each obesity-linked trait. For cases utilizing a single tool variable, the method involving Wald ratios was deemed suitable. Conversely, when confronted with 2-3 such variables, preference was given to the model with Inverse Variance Weighted (IVW; employing fixed effects). In cases with greater than three instrumental factors, the IVW model incorporating multiplicative effects is preferred. Standard methods, such as MR Egger and Maximum Likelihood, alongside weight-focused evaluations, are factored into the assessment.
Inverse Mendelian randomization analysis
An effort was undertaken to discern whether obesity serves as a cause or consequence of colorectal cancer, or if a genuine bidirectional causal relationship between the two variables exists. Genetic variations pertaining to both obesity and colorectal cancer were employed to rigorously assess the three putative causal scenarios: 1) obesity precipitates colorectal cancer; 2) colorectal cancer induces obesity; or 3) there exists a true bidirectional causative link connecting obesity and colorectal cancer. When evaluating outcomes from both perspectives, disparities in statistical power associated with instrumental variables (IVs) were meticulously factored in.
Assessing genetic interplay in colorectal cancer instances
Utilizing Linkage Disequilibrium Score Regression (LDSC), a recognized instrument for ascertaining heritability and genetic correlation through genome-wide association scrutiny, the differentiation of genuine polygenic signals from potential confounders like population stratification and cryptic relatedness becomes feasible. Information on genome-wide association analysis linked to obesity has been acquired, along with data on colorectal cancer from distinctive sources, namely FinnGen and the UK Biobank. The genomic association of obesity with colorectal malignancies got determined using the LDSC software (Version 1.0.1, accessible via an online repository https://github.com/bulik/ldsc/wiki/LD-Score-Estimation-Tutorial) (32). Originating from the third cycle of the 1000 Genomes Project, pertinent LD scores related to European heritage have been gathered. In order to counteract any reduced statistical potency stemming from subpar imputation accuracy, the scrutiny was confined to well-imputed HapMap3 single nucleotide polymorphisms (SNPs). A significance determination for the correlation adopted a P-value cutoff of 0.05.
Sensitivity analyses
Within this evaluation, heterogeneity and pleiotropy levels underwent meticulous scrutiny using MR. Employing both the IVW approach and the MR Egger method, heterogeneity was accurately gauged. In contrast, pleiotropy was exclusively assessed through the MR Egger method. Leave-one-out analyses were carried out, omitting individual SNPs to discern any key SNPs that might alter the general outcomes.
Criteria for instrumental variable selection
In the initial phase of the present investigation, instrumental variables associated with the exposure variable were meticulously screened based on the subsequent criteria:
A P-value threshold of less than 5e-8 was imposed,
An F-statistic exceeding 10 was required,
Linkage disequilibrium was eradicated within a 10,000kb window and an r2 value of 0.001,
A minimum allele frequency exceeding 0.01 was mandated.
Results
Mendelian randomization outcomes concerning obesity and colorectal cancer
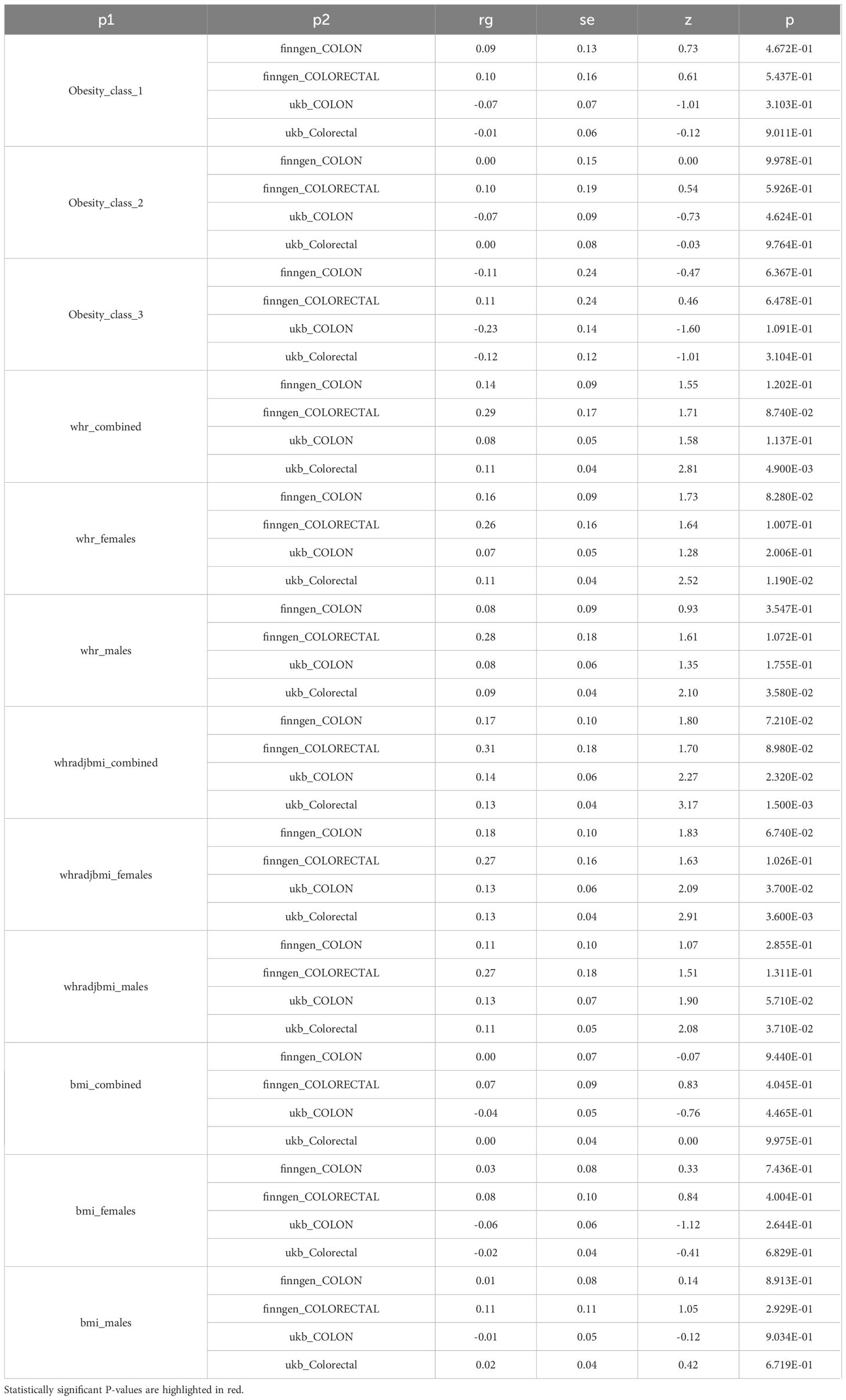
The study’s schematic representation (Figure 1) and its elaboration within the Methods segment indicate that chosen SNPs tied to obesity phenotypes were employed as exposure determinants. In tandem, four colorectal cancer-associated cohorts served as the outcome determinants for Mendelian randomization. Relevant P-values for these models were determined. From the outcomes procured via IVW strategies, no marked causal linkage was discerned between BMI and colorectal cancer. Yet, a notable link was identified between WHR and its adjusted variant (WHRadjBMI) and colorectal cancer, implying WHR’s potential as a colorectal cancer risk determinant. Remarkably, gender-specific findings revealed this significant association only in the female segment, absent in males, suggesting an amplified colorectal cancer risk for females exhibiting a raised WHR (Figure 2 and Table 2, noteworthy outcomes emphasized in red). Scatter diagrams further attested to the risk that heightened WHR imposes concerning colorectal cancer’s initiation and advancement (Figure 3). Additional Mendelian randomization analyses for heterogeneity and pleiotropy were also performed. It was observed that no pleiotropic effects were present in the data (intercept_pval > 0.05). While potential heterogeneity could be noted in some data sets for WHR and WHRadjBMI in relation to colorectal cancer (Q_pval < 0.05), such heterogeneity did not significantly impact the results, as evidenced by the IVW random-effects model.
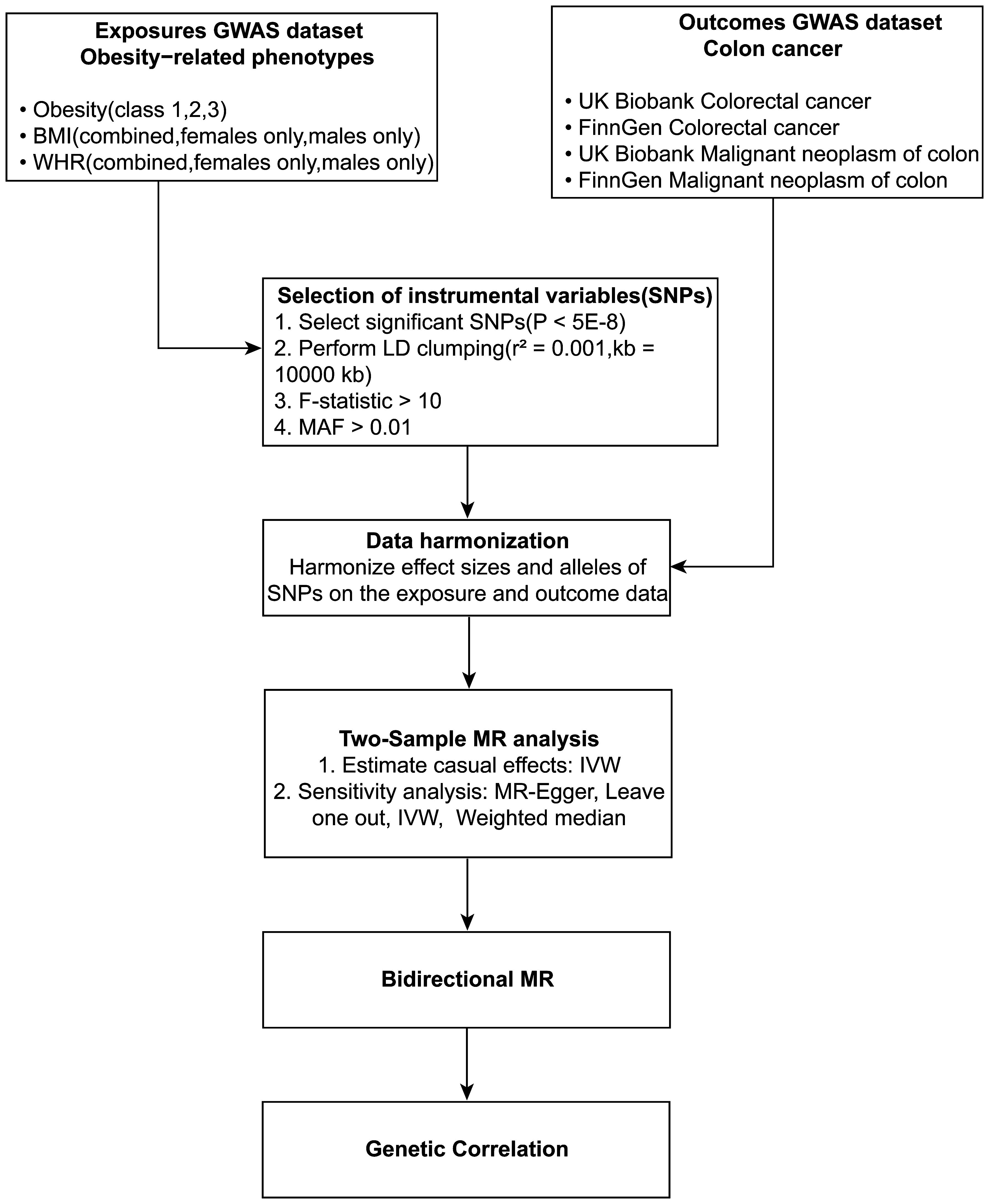
Figure 1 Schematic diagram of the study design. Depicted in the flowchart is the incorporation of obesity-linked SNPs as determinant variables and colorectal cancer sets as consequential variables in the MR analysis.
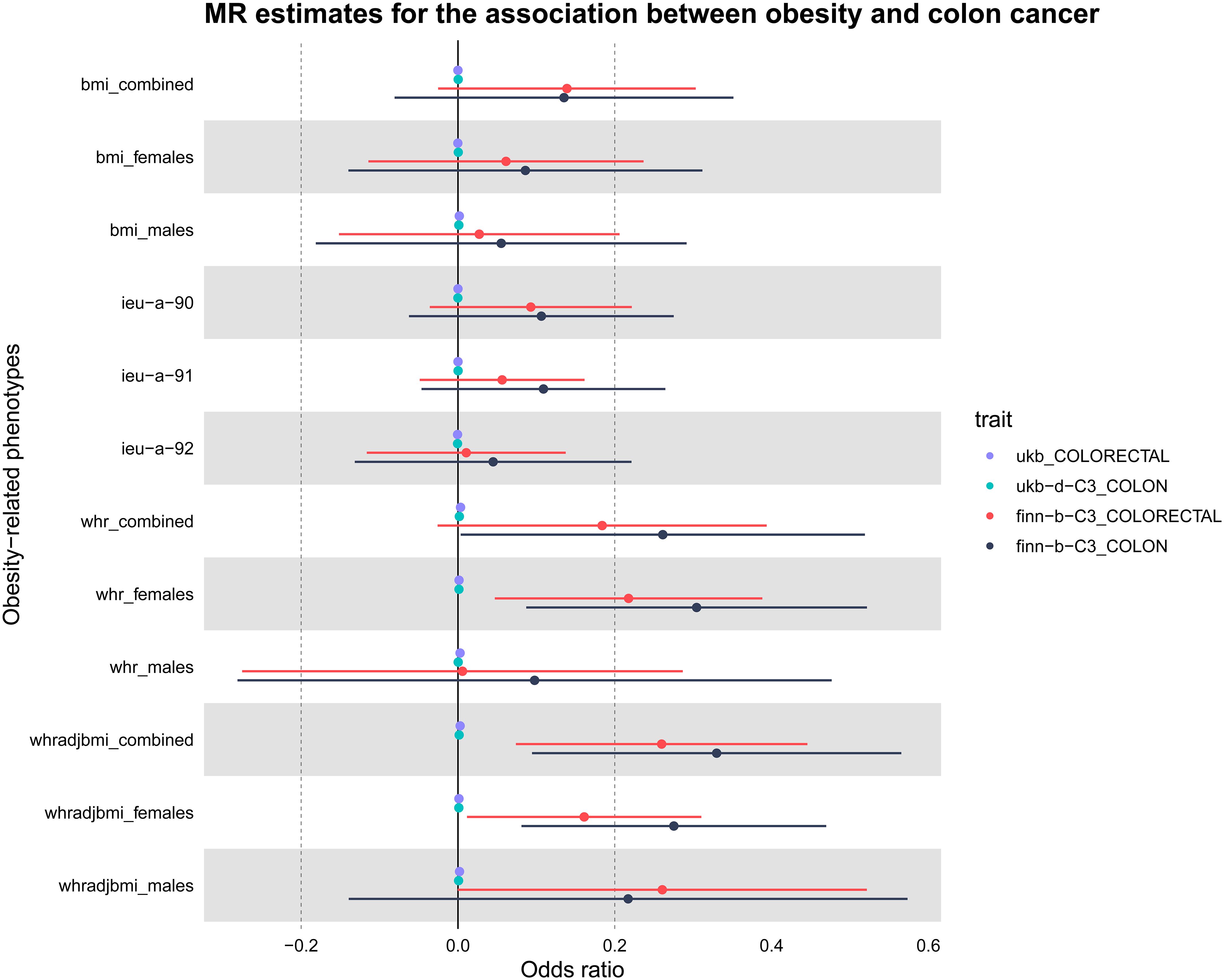
Figure 2 Presentation of MR estimations in a Forest plot concerning obesity-related determinants and colorectal cancer. The forest plot depicts both sex-aggregated and sex-stratified IVW estimates. Data were sourced from four independent cohorts. Notably, significant associations with colorectal cancer risk were identified exclusively for WHR, particularly in the female subgroup.
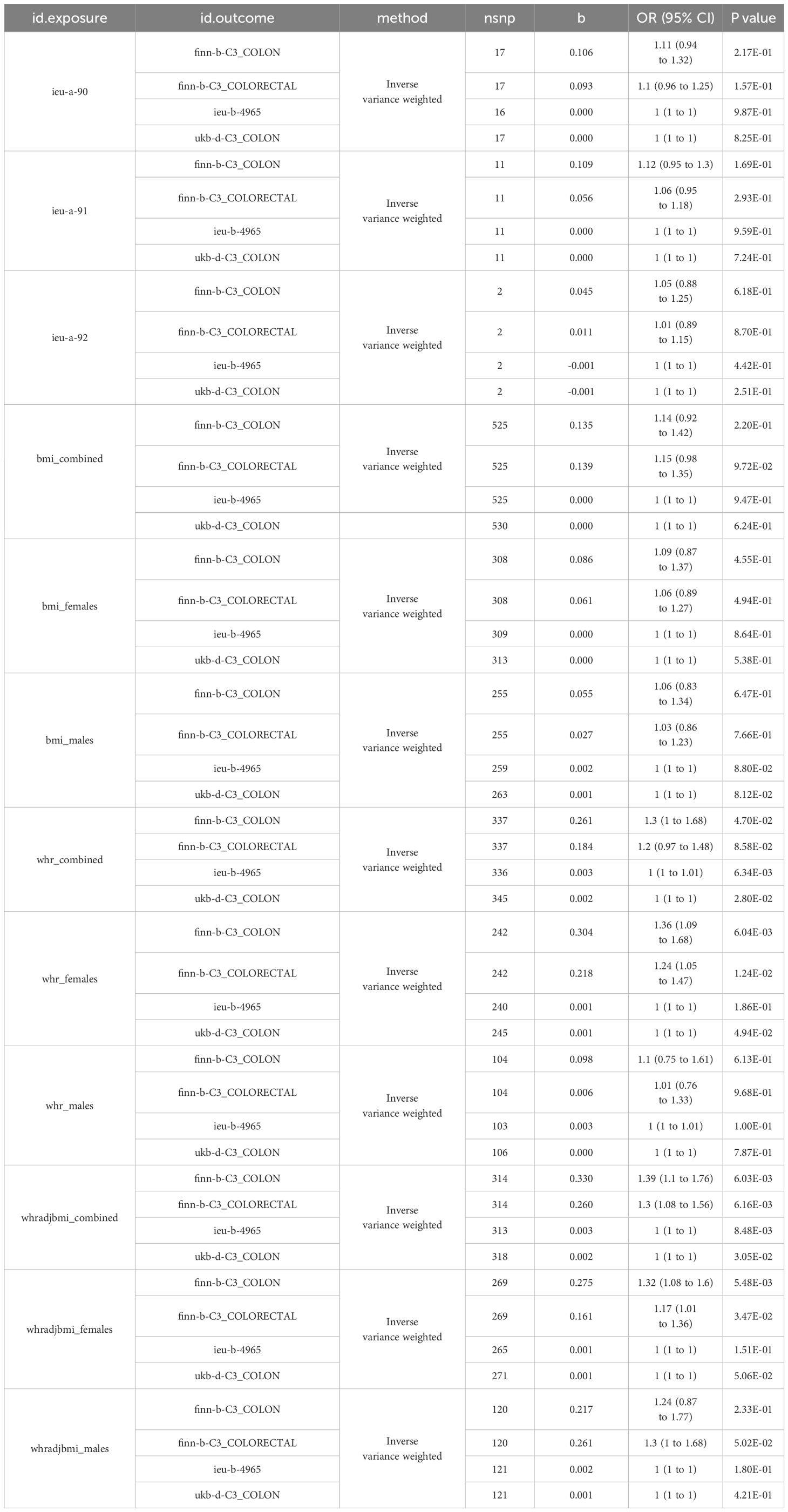
Table 2 Mendelian randomization analysis results for obesity-related risk factors in colorectal cancer across different cohorts.
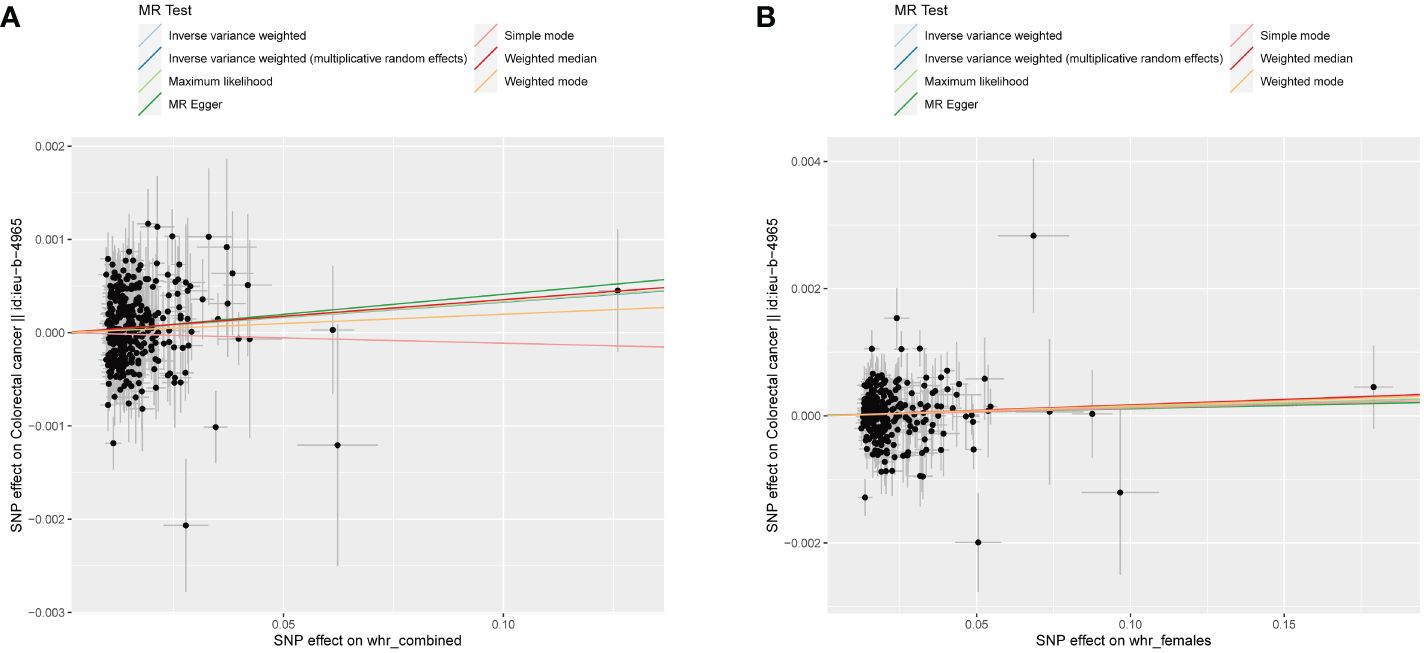
Figure 3 WHR scatter diagrams concerning colorectal malignancy susceptibility. (A) Combined-sex WHR scatter plot reveals a direct positive correlation with the incidence of colorectal malignancy. (B) Female-specific WHR scatter plot revealing a significant association with colorectal cancer. Both plots were generated using an IVW random-effects model and showed no significant pleiotropic effects (intercept_pval > 0.05). Potential dataset heterogeneity (Q_pval < 0.05) did not significantly alter the results.
Reverse Mendelian randomization
In order to evaluate the causal relationship linking obesity-linked phenotypes and colorectal cancer, critical genetic loci from genome-wide association research focused on colorectal cancer served as exposure benchmarks. On the flip side, phenotypes associated with obesity acted as the outcome variables in a dual-sample Mendelian analysis. Considering there were no significant loci at a P-value cutoff of 5e-8, this cutoff got shifted to 5e-6, keeping the rest of the conditions unchanged (33). Through the application of the IVW approach, it was determined that there was no statistical significance in all reverse Mendelian randomization evaluations. Consequently, a reverse causal relationship was negated, as depicted in Figure 4. Consequently, the onset of colorectal cancer does not contribute to obesity or related phenotypes (Table 3).
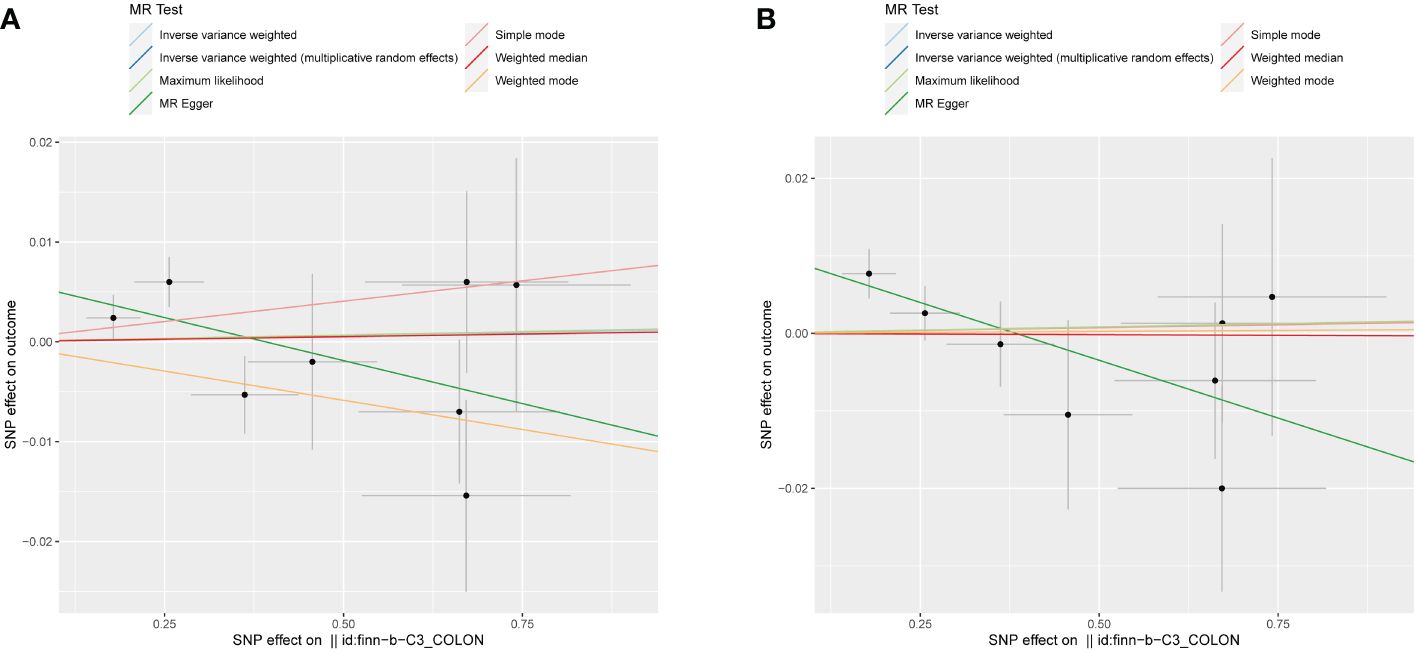
Figure 4 Scatter plots illustrating the absence of a reverse causal relationship between colorectal cancer and obesity-related phenotypes. (A) Combined-sex scatter plot of BMI showing no significant relationship with colorectal cancer. (B) Female-specific scatter plot of WHR also revealing no significant association with colorectal cancer. Both plots were generated using an IVW method and showed no statistical significance, thereby refuting a reverse causal link between colorectal cancer and obesity-related phenotypes.
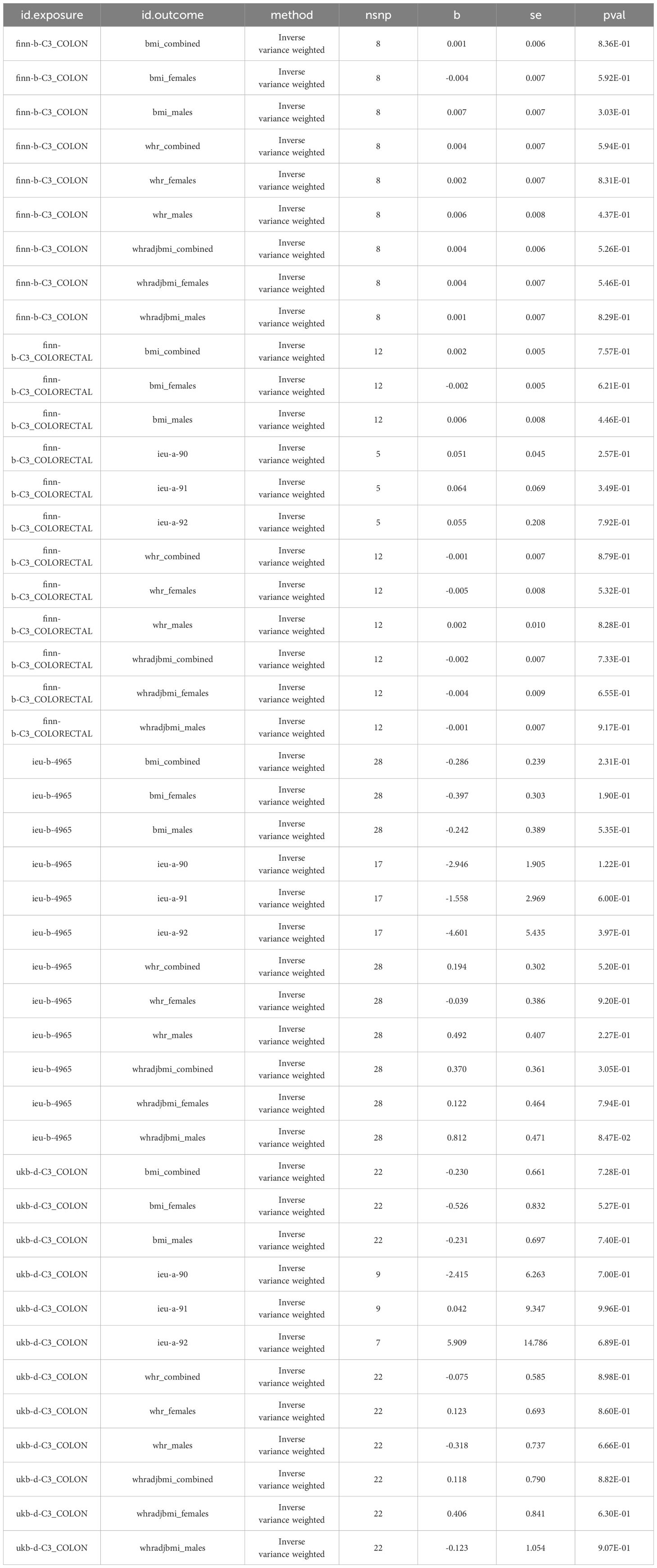
Table 3 Summary of reverse Mendelian randomization analyses investigating the potential causal link between colorectal cancer and obesity-related phenotypes.
Genetic correlation between colorectal cancer and obesity-related phenotypes
Using the provided methodologies, a study was carried out to explore the genetic connection of colorectal cancer to obesity-related phenotypes. A genetic correlation analysis (rg) was conducted on the aforementioned datasets using LDSC. The results indicated a notable genetic correlation between colorectal cancer data from the UK Biobank and WHR measurements, achieving statistical significance (P < 0.05, denoted with an asterisk). Furthermore, an elevated genetic correlation (rg) was observed overall between WHR and colorectal cancer, as illustrated in Figure 5. Upon examination, a genetic correlation of 0.13 emerged between colorectal cancer and WHR, factoring in BMI adjustments (WHRadjBMI); the significance of this correlation is statistically supported (P = 1.500E-03) in Table 4.
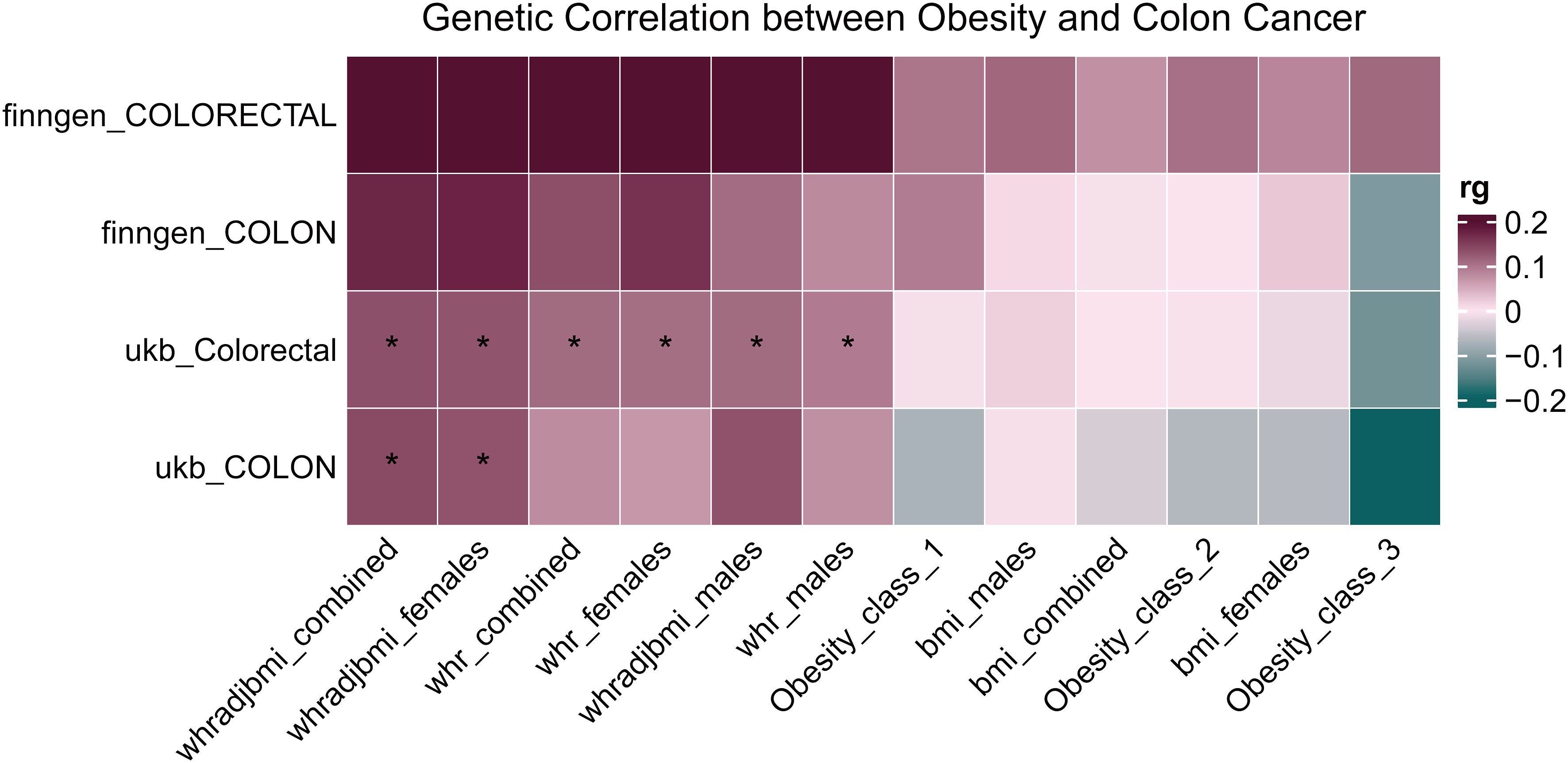
Figure 5 Heatmap of genetic correlation between colorectal cancer and obesity-related phenotypes. The heatmap represents the genetic correlation assessed through LDSC between colorectal cancer data sourced from the UK Biobank and various obesity-related phenotypes. Statistically significant correlations are denoted with an asterisk. *P < 0.05.
Mendelian analysis concerning risk determinants for colorectal cancer
In alignment with the delineated procedure, a study was undertaken to elucidate the correlation between certain risk determinants and colorectal cancer prevalence. These determinants were utilized as exposure indices, with colorectal cancer identified as the outcome index. Based on the quantity of instrumental variables, suitable linear models were determined, producing the MR outcomes illustrated in Figure 6. Upon setting a P-value threshold at < 0.05, inflammatory bowel disease was identified as a potential protective mechanism against colorectal cancer. Conversely, consumption of salads and raw vegetables is identified as a potential hazard. Statistical significance was not found for the other risk determinants analyzed.

Figure 6 MR results for risk factors associated with colorectal cancer. MR results identify inflammatory bowel disease as a protective factor and salad/raw vegetable intake as a risk factor.
Discussion
The global ascent of obesity and its prospective consequences for various malignancies, including CRC, has emerged as a focal point of research. Over recent decades, there has been a marked escalation in the BMI of adults worldwide (4). The WHO statistics has substantiated this upward trajectory, indicating that close to 1.5 billion adults globally were overweight, of which a concerning 500 million were categorized as obese (5). Notably, a parallel trend has been observed between the rise in obesity rates and the increased incidence of CRC (2, 6). Numerous studies have further substantiated a definitive relationship between obesity and CRC risk (8, 34, 35). Consistent findings indicating a decreased risk of CRC following weight loss surgeries have been delineated in studies conducted in Italy (36), England (37), and the United States (38). Furthermore, of clinical significance, the research direction has shifted recently, with studies beginning to examine the prognostic value of obesity on post-diagnosis survival rates for CRC patients (39, 40). Through genetic predictive investigations, Papadimitriou et al. discerned a relationship between weight during early childhood (around 10 years of age) and elevated risks of CRC, notably with a significant rise in distal colon cancer risks (41). In obese East Asian populations, studies spearheaded by Kwon et al. highlighted that overweight status and obesity emerge as prominent determinants in the progression of colorectal tumors (42). Similar to findings in European populations, Suzuki et al. identified in Asians a discernible positive correlation between elevated BMI and CRC risk (43, 44). Current dominant research findings, despite discrepancies, have consistently associated elevated BMI with poorer survival outcomes (16, 45, 46).
BMI, traditionally viewed as a cornerstone in the evaluation of obesity-cancer correlations, failed to display a causative link with colorectal cancer in our MR analysis. This result aligned with previous studies, notably by Yamamoto-Honda et al., that found no significant association between BMI and CRC, especially within Asian groups (47). Moreover, research conducted by Zhai et al. suggested that in colorectal cancer patients, it is visceral obesity, not BMI, that provided a more precise evaluation of obesity’s influence on metabolic and postoperative infectious complications (48). To delve into other obesity-related risk indicators, our two-sample MR approach sourced data from the IEU Open GWAS Project, Zenodo, and FinnGen. Interestingly, central obesity metrics, specifically WHR and its adjusted variant (WHRadjBMI), demonstrated pronounced causal relationships with CRC, suggesting their potential significance in evaluating risk. In line with this perspective, Hashemi Madani et al. found that there was a notable link between WHR and elevated risks of cancers in the colon and stomach among females. This highlights the pivotal importance of central obesity, particularly the distribution of abdominal fat, in influencing certain cancer susceptibilities (49). Similarly, studies by Mili et al. and Safizadeh et al. substantiated those measures of central obesity, including waist circumference and WHR, provided more robust and consistent predictions of CRC incidence compared to BMI (50, 51). The link between central obesity and CRC incidence highlights the importance of considering gender differences. Prior investigations underscored notable gender discrepancies within the genetic mechanisms governing body metrics, particularly BMI and WHR, likely modulated by reproductive hormones (52, 53). Notably, research by Mureșan et al. indicated that women exhibit increased oxidative stress markers and reduced homocysteine levels during the later stages of pregnancy, suggesting that these gender-specific metabolic changes during critical periods could influence long-term cancer risks (54). Distinctive findings of this study, utilizing the IVW method, demonstrated a marked relationship between increased WHR and susceptibility to colorectal cancer, notably in females, without any evident connection in males. Observational studies consistently revealed an association between augmented BMI and CRC risk, with gender serving as a potential modulator (9). With a spotlight on gender discrepancies, Ortega et al. found CRC risk tethered to both overall and abdominal fat in men, whereas in women, the risk was solely attributed to WHR (55). Consistently, employing Mendelian randomization, Bull et al. established a direct correlation of heightened BMI with male colon cancer risk, contrasting with a stronger WHR association in females (56). Hashemi Madani et al. also observed that WHR significantly correlated with colon and gastric cancer risks in females, unlike in males, suggesting a gender-specific interplay between obesity metrics and cancer risks (49). Interestingly, Thrift et al., via Mendelian randomization, pinpointed an elevated BMI as a potent risk for colon cancer in females, but such an association was elusive in males when BMI was genetically determined (44). Similarly, Loh et al. brought attention to the WHRadjBMI, discerning a notable relationship between an increase in WHRadjBMI and augmented colorectal cancer potential in females; such a connection proved elusive for males (57).
The observed gender-specific associations necessitated further exploration of the underlying biological mechanisms. Elevated abdominal adiposity, displaying strong associations with elements including hyperinsulinemia, anomalies in insulin function, and modifications in systems of the IGF realm, might have influenced the probability of colorectal cancer development and the resulting fatality rates (58, 59). Interestingly, according to Trevisan et al., there exists a distinct gender-based variation, with males showing a more pronounced association, when connecting elevated body mass to colorectal cancer deaths, influenced by conditions such as hyperinsulinemia and insulin resistance (60). The discerned correlation aligned with the prevailing scientific understanding that links obesity-induced colorectal cancer susceptibility to processes such as disruptions in glucose metabolism, insulin-like growth factors, gender-related hormones, peptides derived from adipose tissue, markers of inflammatory responses, and oxidative stress (61, 62). Interestingly, a Swedish clinical research highlighted a connection between leptin metrics and CRC likelihood in males; however, such a connection wasn’t evident among females (63). Additionally, certain leptin gene SNPs increased CRC susceptibility in females regardless of obesity, whereas a specific ADIPOQ SNP manifested only in obese males, suggesting gender-specific CRC predispositions (64). Furthermore, the occurrence of such gender-based variations might have been associated with sex hormone fluctuations. According to Slattery et al., estrogen exposure was protective against MSI+ colon tumors in women; however, its insufficiency in the elderly female population increased tumor susceptibility (65). Concurrently, Bell et al. reported that elevated triglyceride levels correlated with increased BMI in males but with heightened WHR in females, highlighting gender-specific metabolic implications influenced by adiposity patterns and hormonal profiles (66). Moreover, elevated serum markers such as CRP-1, TNF-α, and IL-6, indicative of persistent inflammation, have been linked to CRC progression, with this association appearing stronger in males (67, 68). Overall, gender differences appeared to influence the link between obesity indicators and a predisposition to CRC, underscoring the need for targeted research.
The research brought to light correlations between obesity-driven phenotypes and the predisposition to colorectal cancer, most notably emphasizing the relationships concerning WHR, WHRadjBMI, and female incidences. These findings emphasized the need for strategic screening among individuals with elevated WHR and underscored the value of diversified datasets in subsequent research endeavors. However, certain constraints in this investigation warranted attention. Primary data were sourced from the IEU Open GWAS Project and Zenodo. Despite their comprehensiveness, it remains plausible that these repositories do not fully capture global genetic variations, thus posing challenges to the universal applicability of the results. Efforts were made to control for recognized confounding factors; however, the possibility of unaccounted variables remains. The validity of the instrumental variables selected depended on the strength and consistency of the primary GWAS studies, which introduces the possibility of biases influencing the MR findings. Significant heterogeneity was identified in the datasets related to WHR and WHRadjBMI, introducing additional complexity without substantially altering the primary findings. Owing to the inherent observational character of MR analyses, the research could be vulnerable to certain limitations, including pleiotropy. Possibilities of dataset intersection or undisclosed associations, particularly in the context of the UK Biobank and FinnGen samples, could have existed. Moreover, while extensive GWAS datasets were considered, the unavailability of key datasets could have impeded the detailed evaluation of colorectal cancer attributes. Nevertheless, the study demonstrated a significant association between WHR, WHRadjBMI, and the incidence of colorectal cancer, predominantly in females. Based on these findings, there is a pronounced need for enhanced screening procedures for individuals with elevated WHR values. Moreover, the results underscore the imperative of incorporating broader and more diverse datasets in subsequent research.
Conclusion
In a bidirectional MR examination focused on obesity-related phenotypes pertaining to colorectal cancer, complemented by genetic correlation data, a causal connection between obesity’s influence and colorectal cancer was delineated. There wasn’t any pronounced association involving grade 3 obesity parameters and BMI when considering colorectal cancer. Nonetheless, a notable causal relationship existed between WHR and WHRadjBMI, emphasizing a distinct gender variation, with a higher prevalence in females. Furthermore, a potent genetic correlation between both WHR metrics and colorectal cancer was corroborated, reinforcing the causal inference. Collectively, these findings suggest that an increased WHR acts as a significant predictor, enhancing the risk for colorectal cancer development and progression. Clinically, attention is focused on the critical importance WHR measurements play in assessing CRC vulnerability, notably significant among females. Integration of WHR evaluations into standard clinical practice is recommended, enabling prompt interventions and providing guidance on behavioral modifications. In summary, recognizing the significance of increased WHR offers a vital perspective for both clinical considerations and early interventions in colorectal cancer.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://gwas.mrcieu.ac.uk/, ieu-a-90, ieu-a-91, ieu-a-92, ieu-b-4965, ukb-d-C3_COLON, ukb-b-223, ukb-b-6324, ukb-b-5779, ukb-b-1996, ukb-b-3881, ukb-d-1448_3, ukb-b-19085, ukb-b-19390, Dukb-b-18593, ukb-b-7043, ukb-b-18541, ebi-a-GCST006867, ieu-a-294, https://www.finngen.fi/en, finn-b-C3_COLON, finn-b-C3_COLORECTAL.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
XC: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. MY: Data curation, Supervision, Writing – review & editing. WZ: Investigation, Resources, Writing – review & editing. JT: Investigation, Resources, Writing – review & editing. QL: Data curation, Resources, Supervision, Writing – review & editing. XY: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding Support for this research was derived from the State Key Program of the National Natural Science Foundation of China (Ref.: 82130092). Further backing originated from the Innovative Capacity Building Project of the Hubei Engineering Research Center for Radiotherapy and Radiation Protection at Tongji Hospital, affiliated with Tongji Medical College, Huazhong University of Science and Technology (Ref.: 2018-420114-35-03-071705).
Acknowledgments
The authors extend their heartfelt gratitude to the IEU Open GWAS Project and Zenodo platforms for making the crucial genetic data accessible. Special thanks go to the UK Biobank study and FinnGen consortium for their invaluable contributions to this research. We appreciate the collaborative spirit of the scientific community in promoting open data sharing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MR, Mendelian Randomization; GWAS, Genome-Wide Association Studies; BMI, Body Mass Index; CRC, Colorectal Cancer; WHR, Waist-Hip Ratio; WHRadjBMI, Waist-Hip Ratio adjusted for BMI; WHO, World Health Organization; IVW, Inverse Variance Weighted; IVs, Instrumental Variables; IGF, Insulin-like Growth Factor; SNPs, Single Nucleotide Polymorphisms; LDSC, Linkage Disequilibrium Score Regression.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. (2017) 66:683–91. doi: 10.1136/gutjnl-2015-310912
3. Marmol I, Sanchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int J Mol Sci. (2017) 18:197. doi: 10.3390/ijms18010197
4. Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. (2011) 377:557–67. doi: 10.1016/S0140-6736(10)62037-5
5. Whitlock K, Gill RS, Birch DW, Karmali S. The association between obesity and colorectal cancer. Gastroenterol Res Pract. (2012) 2012:768247. doi: 10.1155/2012/768247
6. Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. (2018) 6:95–104. doi: 10.1016/S2213-8587(17)30366-2
7. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
8. Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. (2010) 11:19–30. doi: 10.1111/j.1467-789X.2009.00613.x
9. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. (2013) 62:933–47. doi: 10.1136/gutjnl-2013-304701
10. Zhi X, Kuang XH, Liu K, Li J. The global burden and temporal trend of cancer attributable to high body mass index: Estimates from the Global Burden of Disease Study 2019. Front Nutr. (2022) 9:918330. doi: 10.3389/fnut.2022.918330
11. Luo J, Hendryx M, Manson JE, Figueiredo JC, LeBlanc ES, Barrington W, et al. Intentional weight loss and obesity-related cancer risk. JNCI Cancer Spectr. (2019) 3:pkz054. doi: 10.1093/jncics/pkz054
12. Song N, Huang D, Jang D, Kim MJ, Jeong SY, Shin A, et al. Optimal body mass index cut-off point for predicting colorectal cancer survival in an Asian population: A national health information database analysis. Cancers (Basel). (2020) 12:830. doi: 10.3390/cancers12040830
13. Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. (2015) 15:484–98. doi: 10.1038/nrc3967
14. Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. (2014) 10:455–65. doi: 10.1038/nrendo.2014.94
15. Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O'Connell MJ, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. (2006) 98:1647–54. doi: 10.1093/jnci/djj442
16. Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. (2012) 30:42–52. doi: 10.1200/JCO.2011.38.0287
17. Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev. (2011) 20:1410–20. doi: 10.1158/1055-9965.EPI-11-0079
18. Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. (2012) 23:1939–48. doi: 10.1007/s10552-012-0071-2
19. Ballian N, Yamane B, Leverson G, Harms B, Heise CP, Foley EF, et al. Body mass index does not affect postoperative morbidity and oncologic outcomes of total mesorectal excision for rectal adenocarcinoma. Ann Surg Oncol. (2010) 17:1606–13. doi: 10.1245/s10434-010-0908-4
20. Simkens LH, Koopman M, Mol L, Veldhuis GJ, Ten Bokkel Huinink D, Muller EW, et al. Influence of body mass index on outcome in advanced colorectal cancer patients receiving chemotherapy with or without targeted therapy. Eur J Cancer. (2011) 47:2560–7. doi: 10.1016/j.ejca.2011.06.038
21. Mentxaka A, Gomez-Ambrosi J, Neira G, Ramirez B, Becerril S, Rodriguez A, et al. Increased expression levels of netrin-1 in visceral adipose tissue during obesity favour colon cancer cell migration. Cancers (Basel). (2023) 15:1038. doi: 10.3390/cancers15041038
22. Gunter MJ, Riboli E. Obesity and gastrointestinal cancers - where do we go from here? Nat Rev Gastroenterol Hepatol. (2018) 15:651–2. doi: 10.1038/s41575-018-0073-y
23. Evans DM, Davey Smith G. Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu Rev Genomics Hum Genet. (2015) 16:327–50. doi: 10.1146/annurev-genom-090314-050016
24. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
25. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
26. Sanderson E. Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med. (2021) 11:a038984. doi: 10.1101/cshperspect.a038984
27. Winkler TW, Gunther F, Hollerer S, Zimmermann M, Loos RJ, Kutalik Z, et al. A joint view on genetic variants for adiposity differentiates subtypes with distinct metabolic implications. Nat Commun. (2018) 9:1946. doi: 10.1038/s41467-018-04124-9
28. Berndt SI, Gustafsson S, Magi R, Ganna A, Wheeler E, Feitosa MF, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. (2013) 45:501–12. doi: 10.1038/ng.2606
29. Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. (2019) 28:166–74. doi: 10.1093/hmg/ddy327
30. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. (2019) 394:1467–80. doi: 10.1016/S0140-6736(19)32319-0
31. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
32. Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. (2015) 47:1236–41. doi: 10.1038/ng.3406
33. Luo J, le Cessie S, Blauw GJ, Franceschi C, Noordam R, van Heemst D. Systemic inflammatory markers in relation to cognitive function and measures of brain atrophy: a Mendelian randomization study. Geroscience. (2022) 44:2259–70. doi: 10.1007/s11357-022-00602-7
34. Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. (2007) 16:2533–47. doi: 10.1158/1055-9965.EPI-07-0708
35. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. (2007) 86:556–65. doi: 10.1093/ajcn/86.3.556
36. Ciccioriccio MC, Iossa A, Boru CE, De Angelis F, Termine P, Giuffre M, et al. Colorectal cancer after bariatric surgery (Cric-Abs 2020): Sicob (Italian society of obesity surgery) endorsed national survey. Int J Obes (Lond). (2021) 45:2527–31. doi: 10.1038/s41366-021-00910-6
37. Aravani A, Downing A, Thomas JD, Lagergren J, Morris EJA, Hull MA. Obesity surgery and risk of colorectal and other obesity-related cancers: An English population-based cohort study. Cancer Epidemiol. (2018) 53:99–104. doi: 10.1016/j.canep.2018.01.002
38. Kwak M, Mehaffey JH, Hawkins RB, Hedrick TL, Slingluff CL Jr., Schirmer B, et al. Bariatric surgery is independently associated with a decrease in the development of colorectal lesions. Surgery. (2019) 166:322–6. doi: 10.1016/j.surg.2019.03.013
39. Hines RB, Shanmugam C, Waterbor JW, McGwin G Jr., Funkhouser E, Coffey CS, et al. Effect of comorbidity and body mass index on the survival of African-American and Caucasian patients with colon cancer. Cancer. (2009) 115:5798–806. doi: 10.1002/cncr.24598
40. Sinicrope FA, Foster NR, Sargent DJ, O'Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. (2010) 16:1884–93. doi: 10.1158/1078-0432.CCR-09-2636
41. Papadimitriou N, Bull CJ, Jenab M, Hughes DJ, Bell JA, Sanderson E, et al. Separating the effects of early and later life adiposity on colorectal cancer risk: a Mendelian randomization study. BMC Med. (2023) 21:5. doi: 10.1186/s12916-022-02702-9
42. Kwon HJ, Kim HJ, Park YS, Lim JH, Park KJ, Shin CM, et al. Body mass index as a predictor of advanced colorectal neoplasia. J Cancer Prev. (2013) 18:144–8. doi: 10.15430/JCP.2013.18.2.144
43. Suzuki S, Goto A, Nakatochi M, Narita A, Yamaji T, Sawada N, et al. Body mass index and colorectal cancer risk: A Mendelian randomization study. Cancer Sci. (2021) 112:1579–88. doi: 10.1111/cas.14824
44. Thrift AP, Gong J, Peters U, Chang-Claude J, Rudolph A, Slattery ML, et al. Mendelian randomization study of body mass index and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. (2015) 24:1024–31. doi: 10.1158/1055-9965.EPI-14-1309
45. Doria-Rose VP, Newcomb PA, Morimoto LM, Hampton JM, Trentham-Dietz A. Body mass index and the risk of death following the diagnosis of colorectal cancer in postmenopausal women (United States). Cancer Causes Control. (2006) 17:63–70. doi: 10.1007/s10552-005-0360-0
46. Parkin E, O'Reilly DA, Sherlock DJ, Manoharan P, Renehan AG. Excess adiposity and survival in patients with colorectal cancer: a systematic review. Obes Rev. (2014) 15:434–51. doi: 10.1111/obr.12140
47. Yamamoto-Honda R, Takahashi Y, Yoshida Y, Kwazu S, Iwamoto Y, Kajio H, et al. Body mass index and the risk of cancer incidence in patients with type 2 diabetes in Japan: Results from the National Center Diabetes Database. J Diabetes Investig. (2016) 7:908–14. doi: 10.1111/jdi.12522
48. Zhai W, Yang Y, Zhang K, Sun L, Luo M, Han X, et al. Impact of visceral obesity on infectious complications after resection for colorectal cancer: a retrospective cohort study. Lipids Health Dis. (2023) 22:139. doi: 10.1186/s12944-023-01890-4
49. Hashemi Madani N, Etemadi A, Nalini M, Poustchi H, Khajavi A, Mirzazade E, et al. Obesity and incident gastrointestinal cancers: overall body size or central obesity measures, which factor matters? Eur J Cancer Prev. (2021) 30:267–74. doi: 10.1097/CEJ.0000000000000657
50. Mili N, Paschou SA, Goulis DG, Dimopoulos MA, Lambrinoudaki I, Psaltopoulou T. Obesity, metabolic syndrome, and cancer: pathophysiological and therapeutic associations. Endocrine. (2021) 74:478–97. doi: 10.1007/s12020-021-02884-x
51. Safizadeh F, Mandic M, Pulte D, Niedermaier T, Hoffmeister M, Brenner H. The underestimated impact of excess body weight on colorectal cancer risk: Evidence from the UK Biobank cohort. Br J Cancer. (2023) 129:829–37. doi: 10.1038/s41416-023-02351-6
52. Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. (2007) 21:415–30. doi: 10.1016/j.beem.2007.04.007
53. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). (2008) 32:949–58. doi: 10.1038/ijo.2008.25
54. Mariana Muresan OM, Antal L, Dobjanschi L, Antonescu A, Vicas L, Bodog F, et al. Correlation between reactive oxygen species and homocysteine levels in normal pregnancy. Farmacia. (2011) 59:179–90.
55. Ortega LS, Bradbury KE, Cross AJ, Morris JS, Gunter MJ, Murphy N. A prospective investigation of body size, body fat composition and colorectal cancer risk in the UK biobank. Sci Rep. (2017) 7:17807. doi: 10.1038/s41598-017-17997-5
56. Bull CJ, Bell JA, Murphy N, Sanderson E, Davey Smith G, Timpson NJ, et al. Adiposity, metabolites, and colorectal cancer risk: Mendelian randomization study. BMC Med. (2020) 18:396. doi: 10.1186/s12916-020-01855-9
57. Loh NY, Wang W, Noordam R, Christodoulides C. Obesity, fat distribution and risk of cancer in women and men: A Mendelian randomisation study. Nutrients. (2022) 14:5259. doi: 10.3390/nu14245259
58. Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. (2007) 86:s836–42. doi: 10.1093/ajcn/86.3.836S
59. Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. (2009) 27:176–85. doi: 10.1200/JCO.2008.17.9945
60. Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F, et al. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev. (2001) 10:937–41.
61. Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. (2008) 114:71–83. doi: 10.1080/13813450801954303
62. Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. (2005) 97:1679–87. doi: 10.1093/jnci/dji375
63. Stattin P, Palmqvist R, Soderberg S, Biessy C, Ardnor B, Hallmans G, et al. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol Rep. (2003) 10:2015–21. doi: 10.3892/or
64. Chun KA, Kocarnik JM, Hardikar SS, Robinson JR, Berndt SI, Chan AT, et al. Leptin gene variants and colorectal cancer risk: Sex-specific associations. PloS One. (2018) 13:e0206519. doi: 10.1371/journal.pone.0206519
65. Slattery ML, Potter JD, Curtin K, Edwards S, Ma KN, Anderson K, et al. Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer. Cancer Res. (2001) 61:126–30.
66. Bell JA, Richardson TG, Wang Q, Sanderson E, Palmer T, Walker V, et al. Effects of general and central adiposity on circulating lipoprotein, lipid, and metabolite levels in UK Biobank: A multivariable Mendelian randomization study. Lancet Reg Health Eur. (2022) 21:100457. doi: 10.1016/j.lanepe.2022.100457
67. Song M, Wu K, Ogino S, Fuchs CS, Giovannucci EL, Chan AT. A prospective study of plasma inflammatory markers and risk of colorectal cancer in men. Br J Cancer. (2013) 108:1891–8. doi: 10.1038/bjc.2013.172
Keywords: obesity-related phenotypes, Mendelian randomization, colorectal cancer, waist-hip ratio, WHRadjBMI, causal relationship
Citation: Chen X, Yang M, Zhao W, Tu J, Liu Q and Yuan X (2024) Mendelian randomization unraveled: gender-specific insights into obesity-related phenotypes and colorectal cancer susceptibility. Front. Endocrinol. 15:1322253. doi: 10.3389/fendo.2024.1322253
Received: 16 October 2023; Accepted: 24 May 2024;
Published: 06 June 2024.
Edited by:
Claire Perks, University of Bristol, United KingdomReviewed by:
Marcos Edgar Herkenhoff, University of São Paulo, BrazilCosmin Mihai Vesa, University of Oradea, Romania
Copyright © 2024 Chen, Yang, Zhao, Tu, Liu and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianglin Yuan, eXVhbnhpYW5nbGluQGh1c3QuZWR1LmNu; Qingxu Liu, bGl1cWluZ3h1QHRqaC50am11LmVkdS5jbg==; Jingyao Tu, dHVqaW5neWFvMDcwMkAxNjMuY29t
†ORCID: Xinyi Chen, orcid.org/0000-0002-0638-1380
Xianglin Yuan, orcid.org/0000-0003-4653-5388
 Xinyi Chen
Xinyi Chen Mu Yang
Mu Yang Weiheng Zhao
Weiheng Zhao Xianglin Yuan
Xianglin Yuan