- 1Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
- 2Department of Medicine, John H. Stroger Jr. Hospital of Cook County, Chicago, IL, United States
Treating advanced thyroid cancer presents challenges due to its resistance to various treatment modalities, thereby limiting therapeutic options. To our knowledge, this study is the first to report the efficacy of temsirolimus in conjunction with dual immunotherapy of nivolumab/ipilimumab to treat heavily treated advanced PDTC. A 50-year-old female initially presented with a rapidly enlarging mass on her right neck. Subsequent diagnosis revealed poorly differentiated thyroid carcinoma, leading to a total thyroidectomy followed by post-operative radioablation therapy. After four years, an examination for persistent cough revealed a recurrence of the disease within multiple mediastinal nodes. Genetic analysis of blood samples uncovered somatic mutations in the tumor, specifically involving PTEN and TP53. The disease progressed despite palliative radiation, lenvatinib, and nivolumab/ipilimumab therapy. Consequently, temsirolimus, functioning as an mTOR inhibitor, was introduced as an adjunct to the nivolumab/ipilimumab regimen. This combination approach yielded remarkable clinical improvement and disease control for a duration of approximately six months. Temsirolimus likely suppressed the aberrantly activated PI3K/AKT/mTOR signaling pathway, facilitated by the PTEN genetic alteration, thus engendering an effective treatment response. This synergy between targeted agents and immunotherapy presents a promising therapeutic strategy for advanced PDTC patients with limited treatment alternatives. In previous clinical trials, mTOR inhibitors have demonstrated the ability to maintain stable disease (SD) in 65% to 74% for advanced thyroid cancer patients, including those with PDTC. When combined with other targeted therapies, the observed SD or partial response rates range from 80% to 97%. Many of these trials primarily involved differentiated thyroid carcinoma, with diverse genetic mutations. Thyroid cancer patients with alterations in the PI3K/mTOR/Akt appeared to benefit most from mTOR inhibitors. However, no clear association between the efficacy of mTOR inhibitors and specific histologies or genetic mutations has been established. Future studies are warranted to elucidate these associations.
Introduction
Poorly differentiated thyroid carcinoma (PDTC), a rare histologic type of malignant thyroid tumor comprising 2 to 13% of all thyroid cancers, features an extremely aggressive disease course (1). The reported survival data varies with a five-year overall survival (OS) of 60-85% to a median survival of 3.7-5 years (2–4). An unfavorable prognosis is associated with the pathological tumor size greater than 4cm, extrathyroidal metastases at diagnosis, refractoriness to radioiodine therapy, and lack of effective treatment strategies (4, 5).
The majority of cases are known to be refractory to radioactive iodine (RAI) therapy, traditional chemotherapy, and radiotherapy (6). Multimodal therapy, including surgery and RAI, can be beneficial in prolonging survival in patients with locoregional disease (7). However, few therapeutic options are available for patients with advanced PDTC who are not eligible for surgical resection or RAI therapy.
Accordingly, immunotherapy or targeted therapy based on up-front genetic information is being examined. The most frequently altered genes in PDTC are TERT and TP53 - others being BRAF, RAS, AKT, PTEN, and PIK3CA - and are associated with pathways like MAPK and PI3K-AKT (8, 9).
We describe the first case of combination therapy with temsirolimus and dual immunotherapy of nivolumab/ipilimumab in PDTC with PTEN mutation. This is the first evidence that illustrates targeting PTEN with temsirolimus can induce a favorable treatment response among PDTC patients. In particular, temsirolimus added to immunotherapy showed a synergistic effect, eliciting a favorable response, which was not achieved with only immunotherapy. Given the lack of literature on the outcomes and management of PDTC, this case can contribute to managing PDTC patients harboring a specific mutation with targeted therapy.
Case presentation
The patient is a 50-year-old female, a former smoker, who had noted a rapidly enlarging mass on the right side of the neck. Fine needle aspiration (FNA) revealed poorly differentiated thyroid cancer. She underwent total thyroidectomy, which revealed a 4.0cm long axis and confirmed poorly differentiated thyroid cancer on the right thyroid. Vascular and capsular invasion were present, but resection margins were clear, and no lymph node dissection was performed. Her left thyroid was benign. She received post-surgical RAI treatment with 153 mCi I-131 and was on levothyroxine post-treatment. Serum thyroglobulin (Tg) level was low before RAI and undetectable post-therapy. Surveillance thyroid sonography showed stable bilateral thyroid bed nodules with sizes below 0.5 cm. No recurrence was found on PET-CT at 18 months and chest CT at 30 months after diagnosis.
Four years after total thyroidectomy, she presented with persistent cough, shortness of breath, and wheezing. Chest CT revealed numerous large lymph nodes, 5.7 cm in the mediastinum, 1.5cm in the left supraclavicular, and multiple 3.0 cm nodules in the right middle and lower lobes. Endobronchial ultrasound bronchoscopy biopsy (EBUS) confirmed poorly differentiated thyroid carcinoma with necrosis, which was clinically suspected as metastasis or recurrence. A positron emission tomography (PET) scan confirmed multiple fluorodeoxyglucose (FDG) avid lesions, suggesting nodal, pulmonary, hepatic, and osseous metastatic disease. The baseline sum of the target lesions was 18.1 cm, with the liver lesion measuring 3.8 cm (Figure 1A). Serum Tg was 0.11ng/mL, thyroid-stimulating hormone (TSH) 0.01μIU/mL, and free thyroxine (fT4) 1.2ng/dL.
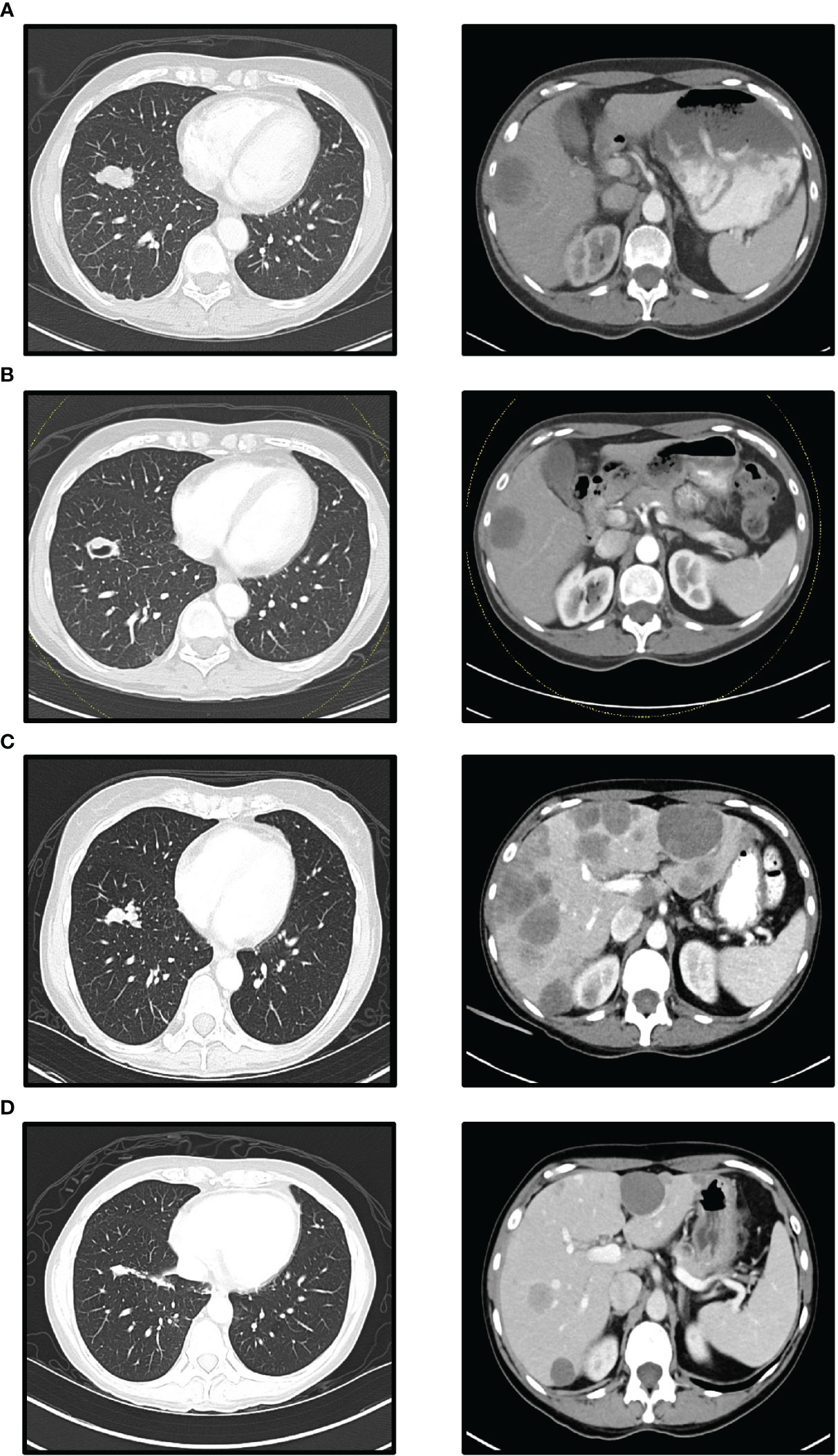
Figure 1 Computed Tomographic (CT) scans (left lower lobe mass and liver metastases): (A) Before nivolumab/ipilimumab treatment. (B) After 42 days of nivolumab/ipilimumab treatment. (C) Before temsirolimus add-on. (D) After 90 days temsirolimus add-on (A, B) show when nivolumab/ipilimumab treatment resulted in a stable disease response for 7 weeks. (C) shows PD response 4 months after starting nivolumab/ipilimumab treatment. The largest liver lesion in (C) measured 88 mm in diameter at its longest. (D) shows significantly reduced lung and liver metastases 3 months after treatment with temsirolimus and nivolumab/ipilimumab.
Circulating tumor DNA (ctDNA) was analyzed using two different assays (Figure 2). The tumor-informed ctDNA assay (assay-t) measures ctDNA molecules detected per mL of the patient’s plasma based on 16 somatic variants selected from whole-genome sequencing of the cancer sample. A 234.21/mL of MTM was detected in our patient at the time of recurrence. The plasma-only ctDNA assay (assay-p) detecting somatic alterations in the circulating cell-free DNA isolated from the patient’s blood specimen demonstrated alterations in PTEN L194fs and TP53 F270S with undetectable microsatellite instability (MSI). She received palliative radiation for impending airway compromise due to metastatic lymphadenopathy and was started on lenvatinib 20 mg daily. Patient had some insomnia, voice changes, and mild fatigue, but tolerated the treatment well. However, the regimen was discontinued due to the progression of the disease after a month.
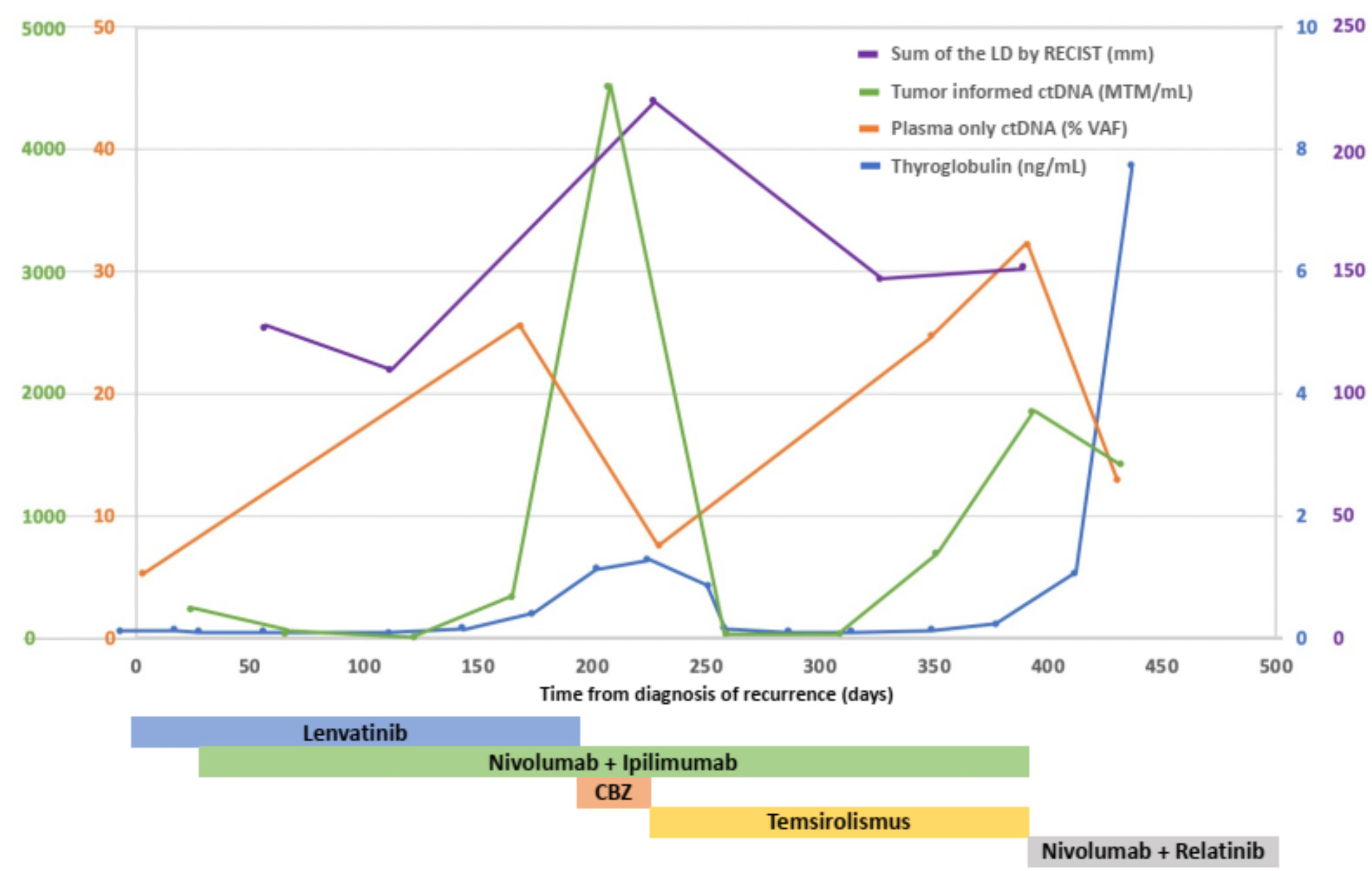
Figure 2 Sum of the LD of target lesions by RECIST (mm), serum thyroglobulin levels (ng/mL), mean ctDNA molecules (MTM) per mL of patient’s plasma measured by tumor-informed circulating tumor DNA (ctDNA), variant allele fraction (VAF) of somatic mutations measured by plasma-only circulating tumor DNA (ctDNA) in the treatment course. After adding temsirolimus to nivolumab and ipilimumab, the patient’s sum of the LD of target lesions, serum thyroglobulin level, and ctDNA measured by tumor informed ctDNA assay decreased remarkably. CBZ, cabozantinib; LD, longest diameters.
A new regimen of nivolumab (3 mg/kg every two weeks) and ipilimumab (1 mg/kg every six weeks) was started (10). Follow-up CT scan after two months showed stable disease with a sum of target lesion of 15 cm, and -17.1% change from baseline (RECIST 1.1; Figures 1A, B). Lenvatinib was continued with the regimen for a synergistic effect. Lenvatinib was discontinued for two weeks due to toxicities, including mouth sores, hand and foot syndrome, and fatigue.
Four months after combination therapy of lenvatinib and nivolumab/ipilimumab, a hard and painful bump appeared on the right forehead. MRI showed extensive osseous metastatic disease in the thoracolumbar spine and bony pelvis. The patient reported excruciating pain in her back and hip, for which she was admitted for pain control. Assay-t showed elevated MTM to 334.72/mL. Tg levels were elevated to 0.40 ng/mL. Progression and toxicity led to the discontinuation of lenvatinib, and cabozantinib 60 mg was added the following month. Follow-up scans showed progression, with the sum of target lesions at 23.3 cm, a 55.3% increase from the previous CT (Figure 1C). The caudate lobe lesion of the liver showed greatest enlargement from 2.1 to 8.8 cm. Tg was elevated to 1.27 ng/mL, and cabozantinib was discontinued due to toxicity.
Thus, temsirolimus at a dosage of 25 mg per week was incorporated into the nivolumab/ipilimumab treatment regimen. The patient reported regaining energy and appetite. Her right forehead nodule disappeared on exam. A follow-up assay-p conducted the following week also showed a 70% decrease in variant allele fraction (VAF), from 25.6% to 7.5% for TP53 F270S and 19.8% to 6.3% for PTEN L194fs. In addition, her Tg had decreased to 0.84 ng/mL, then to 0.16 ng/mL in a month. A follow-up assay-t showed a decrease in MTM from 4526.97/mL to 29.41/mL at three months. Subsequent 1-month follow-up showed stable MTM at 26.00/mL. CT scan also showed partial response with the sum of the target lesion of 15.1cm and a -35.2% change from previous CT (Figure 1D). Tg levels remained in the low range.
The patient has demonstrated stable disease throughout treatment, exceeding six months. During the administration of temsirolimus, the patient initially encountered pancytopenia (hemoglobin 7.1, platelet 318k), a side effect that improved after a dose adjustment to 15mg weekly. Additionally, the patient reported intermittent ankle swelling and facial acne, however these side effects were well-tolerated, and treatment was never suspended. In particular, the patient’s clinical recovery enabled her to regain an independent lifestyle while undergoing temsirolimus treatment. This resurgence in well-being facilitated meaningful life experiences, including attending her child’s high school graduation—a testament to the qualitative impact of the treatment.
The patient experienced a relapse after six months of receiving the combination of temsirolimus and nivolumab/ipilimumab. Subsequently, a new course of treatment was initiated, combining nivolumab with relatinib. However, patient did not improve. Her symptoms worsened rapidly, and she was placed in hospice care, where she died about three months after discontinuing temsirolimus.
Methods
Research and literature were searched through the PubMed database by using the following keywords: thyroid cancer, thyroid neoplasm, mTOR inhibitor, case report, case series, observational study, prospective study, retrospective study, intervention study, and clinical trial. We summarized the case reports, observational studies, and clinical trials where mTOR inhibitors alone or in combination with other treatments were exploited as a treatment for thyroid cancer.
Discussion
We report the first case of effective treatment with temsirolimus combined with dual immunotherapy of nivolumab/ipilimumab in RAI refractory PDTC with multiple metastases. The effectiveness of mTOR inhibitors among other types of thyroid cancers, including anaplastic thyroid cancers (ATC) and medullary thyroid cancer (MTC) have been reported. However, this is the first report that PTEN was targeted with mTOR inhibitors like temsirolimus combined with immunotherapy in PDTC.
The recent advancements of targeted therapies, particularly multi-targeted tyrosine kinase inhibitors (TKIs) have significantly impacted the treatment of metastatic thyroid cancer. Despite this progress, the response to TKIs varies widely among patients with thyroid cancer. For differentiated thyroid cancer (DTC), OS was reported to be 22.2 years in the TKI group compared to 5.6 in the untreated group (11). In the DECISION trial, which led to the FDA approval of sorafenib for treating RAI refractory thyroid cancer, 207 of DTC patients with 11.6% (n = 24) PDTC, showed 12.2% of overall response rate, with further analysis showing poorer PFS in PDTC compared to PTC (NCT00984282) (12). In the SELECT trial, lenvatinib achieved 18.3 months of median PFS compared to 3.6 months for placebo in 261 advanced thyroid cancer patients refractory to I-131including 10.7% (n=28) of PDTC (NCT01321554) (13). The COSMIC 311 trial demonstrated that cabozantinib significantly prolonged PFS among patients with radioiodine-refractory DTC, without PDTC, who lack a standard of care, leading to the FDA approval (NCT03690388) (14). However, for ATC, a phase II trial with lenvatinib was halted due to futility, as the minimum objective response rate threshold of 15% was not met in the interim analysis of 20 patients (15). Moreover, almost all patients eventually showing progression on TKIs (13, 16). The resistance mechanism involves the acquisition of new mutations triggering overactivation of pathways or evoking alternate pathways to bypass the drug’s action (17). Additionally, not all patients with thyroid cancer harbor actionable genetic aberrations. For instance, BRAF V600E mutations were found in only 33% of PDTCs, limiting the efficacy of BRAF-targeted therapies (18).
The immune checkpoint inhibitor therapy, either in combination with TKIs or as a single agent, is also actively underway among aggressive thyroid cancers. In a phase II study with nivolumab and ipilimumab, one out of four PDTCs and three out of ten ATCs achieved PR (NCT03246958) (19). The phase II ATLEP trial showed that the combination of lenvatinib/pembrolizumab yielded a median PFS of 20 months in 8 metastasized PDTC patients and 9.5 months among 27 metastasized ATC patients (20). However, in the phase II national cancer institute trial combining cabozantinib with nivolumab and ipilimumab, interim results did not indicate an efficacy advantage over the currently approved cabozantinib monotherapy in RAI refractory DTC with progression on one prior vascular endothelial growth factor receptor targeted therapy. The objective response rate within 6 months was 10% among 11 patients, including 2 PDTC (NCI#10240) (21).
Given the limited evidence regarding the efficacy of targeted therapies and immunotherapy for PDTC and the aggressive nature of the disease, there is a clinical unmet need for new therapeutic options catering to patients with PDTC refractory to conventional therapies.
We systematically searched clinical trials investigating mTOR inhibitors combined with or without other agents or not among advanced thyroid cancer patients. A total of 7 clinical trials, three retrospectives, one case report, and one preliminary report were found through a literature search using PubMed (National Library of Medicine, Bethesda, MD, USA). In total, eight clinical trials and one retrospective study are analyzed and described in Table 1 (23–31). One case report and one retrospective study were excluded from the table as their patients overlapped with the clinical trials (32, 33). The other retrospective study and preliminary report were excluded from the review as the data on the efficacy of mTOR inhibitor alone or combined with other treatments were not retrievable (34, 35). One clinical trial was excluded as it was reported as an abstract only (36).
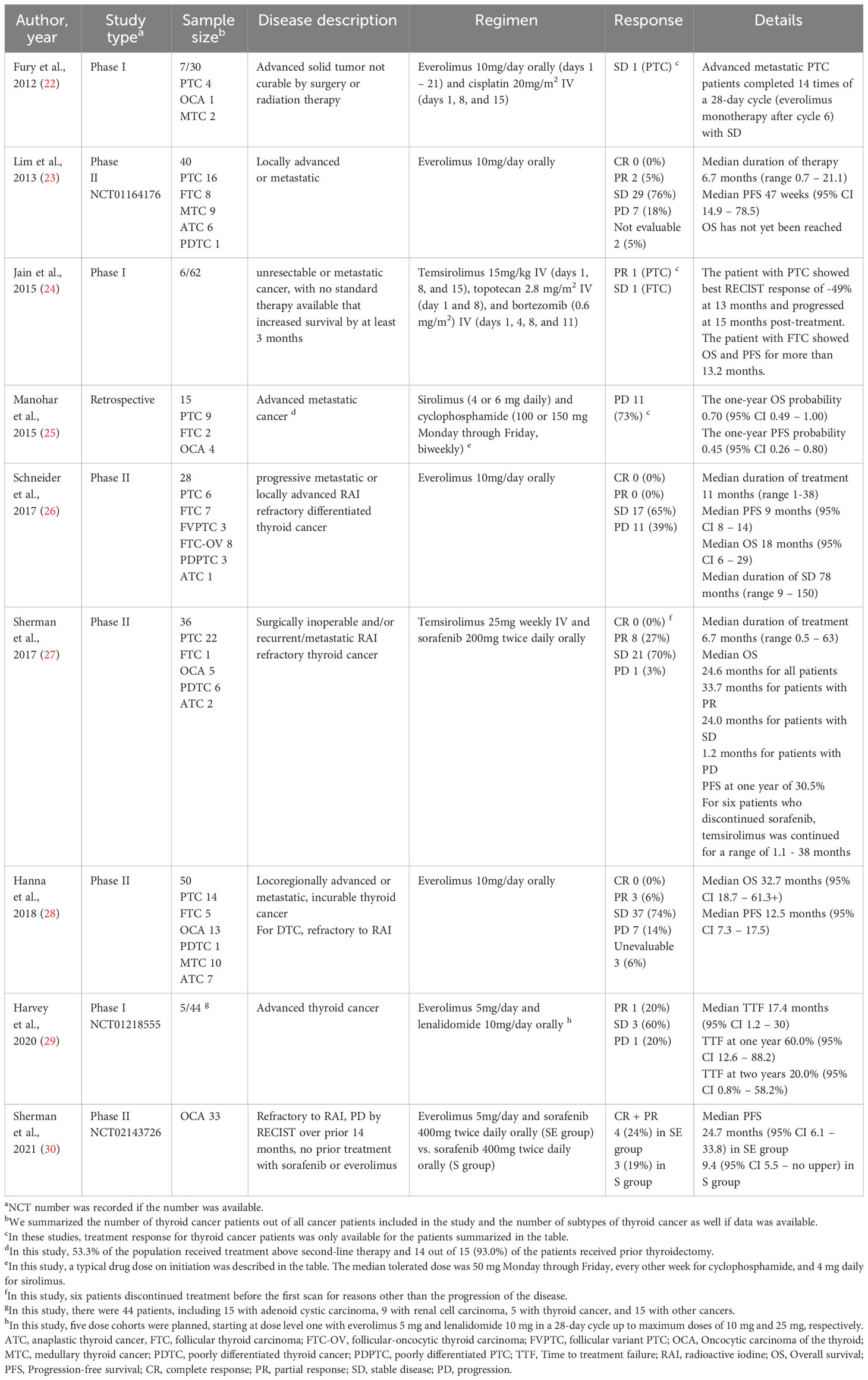
Table 1 Summarization of clinical trials evaluating the efficacy of mTOR inhibitor in thyroid cancer patients.
mTOR inhibition has been reported as having promising results in thyroid cancer. Three phase II clinical trials have reported a stable disease rate of 65–76% in those treated with everolimus across different histologies of thyroid cancer (23, 26, 28). The median PFS and OS ranged from 9 to 12.5 months and 18 to 32.7 months, respectively (23, 26, 28). Median time to treatment failure (TTF) of everolimus with lenalidomide was 17.1 months among advanced thyroid cancer (29). In OCA, everolimus combined with sorafenib elicited longer PFS than sorafenib monotherapy (30). Unlike everolimus, temsirolimus has been studied in combination with other therapeutic agents. In a phase II trial, all patients with thyroid cancer, except one with ATC, experienced stable disease, or partial response on the combination of temsirolimus and sorafenib (27). In another phase I trial, patients with PTC and FTC remained without progression longer than a year with temsirolimus, topotecan, and bortezomib treatment (24). A recent study using patient-derived xenograft mouse models of oncocytic carcinoma of the thyroid (OCA) with upregulated mTOR signaling showed tumor suppressing effect of mTOR inhibitors for both primary tumor and metastasis (37).
The association between the efficacy of mTOR inhibitors and histology is unclear. In a study, no significant difference was noticed according to histology, but a durable clinical response was noticed in a high proportion of DTC and MTC patients (23). In another study, the median PFS of patients with PDTC/ATC was the lowest of any subgroup in one study (28). None of the patients with ATC showed a response on everolimus in the other study (26). For PDTC, the PFS was reported for two patients being 6-15 months and 46 weeks from two studies, respectively (23, 28). Among six patients with PDTC treated with temsirolimus with sorafenib, four patients showed partial response and two patients demonstrated stable disease with 8 months of median duration on treatment (27). Collectively, patients with ATC seem to show a relatively less favorable response to mTOR inhibitors compared to the other histologies.
Thyroid cancer patients with PI3K/mTOR/Akt mutations, like our case, showed favorable responses to mTOR inhibitors, consistent with others (28). For everolimus, the median PFS of ATC/DTC patients was 15.2 months for those who had PI3K/mTOR/Akt alterations (TSC2, FLCN, or NF1), compared to 2.8 months for all PDTC/ATC patients (28). One PDTC patient with TSC2 missense mutation achieved up to 15 months of PFS and more than 21 months of OS (28). One patient with follicular thyroid cancer (FTC) harbored PTEN (c.404T>A, p.(Ile135Lys)) and HRAS (c.182A>G, p.(Gln61Arg)) mutations had PFS of 41 weeks and OS of 113 weeks for everolimus (26). For temsirolimus and sorafenib combination therapy, one patient with PTEN mutation showed a PFS of 22.5 months with maximum disease shrinkage of -51% (27).
TP53 and PTEN mutations are reported in 8% and 4% of PDTC, respectively (18, 38). Phosphatase and tensin homology encoding chromosome 10 (PTEN) is a tumor suppressor gene that encodes a submolecule in the signaling pathway of PIK3CA-PTEN-Akt-mTOR. Currently, there is no approved targeted therapy for PTEN mutation for any solid tumors, including thyroid cancer. However, temsirolimus inhibiting mTOR (a serine-threonine kinase), a downstream effector of PTEN, showed efficacy combined with dual immunotherapy in our patient. In a study involving another mTOR inhibitor, everolimus, the median PFS was 2.8 months in PDTC/ATC patients, but 15.2 months in patients with PI3K/mTOR/Akt-mutation, highlighting that an optimal therapeutic response of mTOR inhibitor correlates with the targetable mutations (28).
However, it should be noted that the impact of other mutations than PI3K/mTOR/Akt pathway on the efficacy of mTOR inhibitors remains unclear. We summarized the genetic alterations and treatment response to everolimus among thyroid cancer patients in Supplementary Table 1. In one study, two patients with a BRAF c.1799T>A mutation for PTC had an approximately 11-month difference in PFS on everolimus (26). In another study with temsirolimus with sorafenib, 3 out of 10 PTC patients with only BRAF mutations showed the longest PFS/OS of more than 21 months, while other seven patients with multiple gene mutations, even those in the PI3K/mTOR/Akt pathway, had a shorter PFS/OS (28). Similarly, two patients with OCA without any mutation showed the longest PFS/OS of more than 21 months compared to temsirolimus and sorafenib (28). This suggests that further research is needed to further evaluate the association of genetic mutation with the effectiveness of mTOR inhibitors to select patients who may benefit from mTOR inhibitors.
Common adverse events associated with mTOR inhibitors were hematologic, metabolic, pulmonary, and cutaneous side effects (39). In one clinical trial, the dosage of everolimus was reduced among 45% of the patients mostly due to fatigue, pneumonitis, and stomatitis (NCT01118065) (40). Dose reduction for temsirolimus was also necessary among patients with renal cell carcinoma to control thrombocytopenia, neutropenia, elevated triglycerides, stomatitis, hyperglycemia, rash, elevated liver aminotransferases, and pneumonia (39). Our patient also suffered pancytopenia from temsirolimus, which resolved after a dose reduction from 25mg weekly to 15mg weekly.
Furthermore, diagnosis based on ctDNA information can directly guides selecting targetable mutations. ctDNA is known to be detected in more than two-thirds of thyroid cancer patients and it has been speculated to be reflective of disease burden, allowing rapid assessment of response to targeted therapies (41). Utilizing two different ctDNA assays, tumor-informed and plasma-only, to monitor the molecular landscape dynamics of the patient’s tumor revealed targeted mutations and showed treatment response. ctDNA measured by MTM (/mL) and VAF (%) of targeted mutation demonstrated a significant drop after initiating temsirolimus, consistent with radiographic responses. Considering that thyroid cancer is known to recur within five years where high-risk features were present, molecular assays may potentially have a role in guiding innovative treatments and benefiting patients (42).
Generalizing the results of our study presents challenges due to its nature as a single case report. Furthermore, determining the precise contribution of each drug to the treatment response is complex, given the combination therapy involved an mTOR inhibitor and dual immunotherapy. Given disease progression after six months in our case, the absence of a further analysis our understanding of mTOR inhibitors remain limited. Further analysis investigating resistance mechanisms along with changes in genomic alterations are warranted. These limitations highlight the need for larger-scale studies encompassing diverse cases and in-depth analyses to discern the nuanced dynamics at play in this therapeutic approach.
Conclusion
We report the first case of a patient with recurrent metastatic PDTC with PTEN mutation who responded well to combination therapy of mTOR inhibitor (temsirolimus) and dual immunotherapy (nivolumab/ipilimumab), who was previously refractory to radiotherapy and other medical therapies, including lenvatinib and cabozantinib. This represents a novel treatment approach for patients with PDTC. Our patient showed a durable response to treatment for over 6 months. This aligns with previous research showing the promising efficacy of mTOR inhibitors among advanced thyroid cancer patients across different histologies and various genetic mutations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Institutional Review Board Committee of Northwestern University, Chicago, IL, USA (STU00207117). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YO: Data curation, Project administration, Writing – original draft, Writing – review & editing, Formal analysis, Investigation, Visualization. JP: Data curation, Validation, Writing – original draft, Writing – review & editing, Investigation. TD: Data curation, Visualization, Writing – original draft, Writing – review & editing, Formal analysis. ZS: Writing – original draft, Writing – review & editing. L-YC: Data curation, Writing – review & editing. YC: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are highly thankful to the patient for providing consent for this case report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1304188/full#supplementary-material
Supplementary Table 1 | Summarization of tumor mutation and treatment response to everolimus among thyroid cancer patients. a In this study, survival data was expressed with the unit of the week and converted into the month. ATC, anaplastic thyroid cancer; FTC, follicular thyroid carcinoma; FTC-OV, follicular-oncocytic thyroid carcinoma; FVPTC, follicular variant PTC; OCA, Oncocytic carcinoma of the thyroid; MTC; medullary thyroid cancer, PDTC, poorly differentiated thyroid cancer; OS, Overall survival; PFS, Progression-free survival.
References
1. Teshima J, Doi H, Fujimori K, Watanabe M, Nakajima N, Nakano T, et al. A human thyroid cancer cell line, DH-14-3, newly established from poorly differentiated thyroid carcinoma. Tohoku J Exp Med (2013) 230(2):75–82. doi: 10.1620/tjem.230.75
2. Siironen P, Hagström J, Mäenpää HO, Louhimo J, Heikkilä A, Heiskanen I, et al. Anaplastic and poorly differentiated thyroid carcinoma: therapeutic strategies and treatment outcome of 52 consecutive patients. Oncology (2010) 79(5-6):400–8. doi: 10.1159/000322640
3. Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly differentiated carcinoma of the thyroid gland: current status and future prospects. Thyroid (2019) 29(3):311–21. doi: 10.1089/thy.2018.0509
4. Ibrahimpasic T, Ghossein R, Carlson DL, Nixon I, Palmer FL, Shaha AR, et al. Outcomes in patients with poorly differentiated thyroid carcinoma. J Clin Endocrinol Metab (2014) 99(4):1245–52. doi: 10.1210/jc.2013-3842
5. Wächter S, Wunderlich A, Roth S, Mintziras I, Maurer E, Hoffmann S, et al. Individualised multimodal treatment strategies for anaplastic and poorly differentiated thyroid cancer. J Clin Med (2018) 7(5):115. doi: 10.3390/jcm7050115
6. Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol (2014) 2(5):356–8. doi: 10.1016/S2213-8587(13)70215-8
7. Hannallah J, Rose J, Guerrero MA. Comprehensive literature review: recent advances in diagnosing and managing patients with poorly differentiated thyroid carcinoma. Int J Endocrinol (2013) 2013:317487. doi: 10.1155/2013/317487
8. Romei C, Elisei R. A narrative review of genetic alterations in primary thyroid epithelial cancer. Int J Mol Sci (2021) 22(4):1726. doi: 10.3390/ijms22041726
9. Romei C, Tacito A, Molinaro E, Piaggi P, Cappagli V, Pieruzzi L, et al. Clinical, pathological and genetic features of anaplastic and poorly differentiated thyroid cancer: A single institute experience. Oncol Lett (2018) 15(6):9174–82. doi: 10.3892/ol.2018.8470
10. Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med (2018) 378(22):2093–104. doi: 10.1056/NEJMoa1801946
11. Iwasaki H, Yamazaki H, Takasaki H, Suganuma N, Sakai R, Nakayama H, et al. Treatment outcomes of differentiated thyroid cancer with distant metastasis improve by tyrosine kinase inhibitors. Oncol Lett (2019) 17(6):5292–300. doi: 10.3892/ol.2019.10180
12. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet (2014) 384(9940):319–28. doi: 10.1016/S0140-6736(14)60421-9
13. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med (2015) 372(7):621–30. doi: 10.1056/NEJMoa1406470
14. Brose MS, Robinson B, Sherman SI, Krajewska J, Lin CC, Vaisman F, et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2021) 22(8):1126–38. doi: 10.1016/S1470-2045(21)00332-6
15. Wirth LJ, Brose MS, Sherman EJ, Licitra L, Schlumberger M, Sherman SI, et al. Open-label, single-arm, multicenter, phase II trial of lenvatinib for the treatment of patients with anaplastic thyroid cancer. J Clin Oncol (2021) 39(21):2359–66. doi: 10.1200/JCO.20.03093
16. Khan HY, Ge J, Nagasaka M, Aboukameel A, Mpilla G, Muqbil I, et al. Targeting XPO1 and PAK4 in 8505C anaplastic thyroid cancer cells: putative implications for overcoming lenvatinib therapy resistance. Int J Mol Sci (2019) 21(1):237. doi: 10.3390/ijms21010237
17. Viola D, Valerio L, Molinaro E, Agate L, Bottici V, Biagini A, et al. Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr Relat Cancer (2016) 23(4):R185–205. doi: 10.1530/ERC-15-0555
18. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest (2016) 126(3):1052–66. doi: 10.1172/JCI85271
19. Lorch JH, Barletta JA, Nehs M, Uppaluri R, Alexander EK, Haddad RI, et al. A phase II study of nivolumab (N) plus ipilimumab (I) in radioidine refractory differentiated thyroid cancer (RAIR DTC) with exploratory cohorts in anaplastic (ATC) and medullary thyroid cancer (MTC). J Clin Oncol (2020) 38(15_suppl):6513. doi: 10.1200/JCO.2020.38.15_suppl.6513
20. Dierks C, Ruf J, Seufert J, Kreissl M, Klein C, Spitzweg C, et al. 1646MO Phase II ATLEP trial: Final results for lenvatinib/pembrolizumab in metastasized anaplastic and poorly differentiated thyroid carcinoma. Ann Oncol (2022) 33:S1295. doi: 10.1016/j.annonc.2022.07.1726
21. Konda B, Sherman E, Massarelli E, Xia B, Muzaffar J, Morris J, et al. Cabozantinib in combination with nivolumab and ipilimumab in patients with radioiodine (RAI)-refractory differentiated thyroid cancer (DTC) whose cancer progressed after one prior vascular endothelial growth factor receptor (VEGFR)-targeted therapy: Interim results of a multicenter phase 2 national cancer institute (NCI)-international thyroid oncology group (ITOG) trial (NCI#10240). Thyroid® (2022) 32(S1):A–98. doi: 10.1089/thy.2022.29137.abstracts
22. Fury MG, Sherman E, Haque S, Korte S, Lisa D, Shen R, et al. A phase I study of daily everolimus plus low-dose weekly cisplatin for patients with advanced solid tumors. Cancer Chemother Pharmacol (2012) 69(3):591–8. doi: 10.1007/s00280-011-1734-5
23. Lim SM, Chang H, Yoon MJ, Hong YK, Kim H, Chung WY, et al. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann Oncol (2013) 24(12):3089–94. doi: 10.1093/annonc/mdt379
24. Jain RK, Hong DS, Naing A, Wheler J, Helgason T, Shi NY, et al. Novel phase I study combining G1 phase, S phase, and G2/M phase cell cycle inhibitors in patients with advanced Malignancies. Cell Cycle (2015) 14(21):3434–40. doi: 10.1080/15384101.2015.1090065
25. Manohar PM, Beesley LJ, Taylor JM, Hesseltine E, Haymart MR, Esfandiari NH, et al. Retrospective study of sirolimus and cyclophosphamide in patients with advanced differentiated thyroid cancers. J Thyroid Disord Ther (2015) 4(3):188. doi: 10.4172/2167-7948.1000188
26. Schneider TC, de Wit D, Links TP, van Erp NP, van der Hoeven JJ, Gelderblom H, et al. Everolimus in patients with advanced follicular-derived thyroid cancer: results of a phase II clinical trial. J Clin Endocrinol Metab (2017) 102(2):698–707. doi: 10.1210/jc.2016-2025
27. Sherman EJ, Dunn LA, Ho AL, Baxi SS, Ghossein RA, Fury MG, et al. Phase 2 study evaluating the combination of sorafenib and temsirolimus in the treatment of radioactive iodine-refractory thyroid cancer. Cancer (2017) 123(21):4114–21. doi: 10.1002/cncr.30861
28. Hanna GJ, Busaidy NL, Chau NG, Wirth LJ, Barletta JA, Calles A, et al. Genomic correlates of response to everolimus in aggressive radioiodine-refractory thyroid cancer: A phase II study. Clin Cancer Res (2018) 24(7):1546–53. doi: 10.1158/1078-0432.CCR-17-2297
29. Harvey RD, Carthon BC, Lewis C, Hossain MS, Zhang C, Chen Z, et al. Phase 1 safety and pharmacodynamic study of lenalidomide combined with everolimus in patients with advanced solid Malignancies with efficacy signal in adenoid cystic carcinoma. Br J Cancer (2020) 123(8):1228–34. doi: 10.1038/s41416-020-0988-2
30. Sorafenib Tosylate With or Without Everolimus in Treating Patients With Advanced, Radioactive Iodine Refractory Hurthle Cell Thyroid Cancer. Available at: https://clinicaltrials.gov/study/NCT02143726.
31. Faggiano A, Modica R, Severino R, Camera L, Fonti R, Del Prete M, et al. The antiproliferative effect of pasireotide LAR alone and in combination with everolimus in patients with medullary thyroid cancer: a single-center, open-label, phase II, proof-of-concept study. Endocrine (2018) 62(1):46–56. doi: 10.1007/s12020-018-1583-7
32. Wagle N, Grabiner BC, Van Allen EM, Amin-Mansour A, Taylor-Weiner A, Rosenberg M, et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med (2014) 371(15):1426–33. doi: 10.1056/NEJMoa1403352
33. Lim SM, Park HS, Kim S, Kim S, Ali SM, Greenbowe JR, et al. Next-generation sequencing reveals somatic mutations that confer exceptional response to everolimus. Oncotarget (2016) 7(9):10547–56. doi: 10.18632/oncotarget.7234
34. Druce M, Chung TT, Grozinsky-Glasberg S, Gross DJ, Grossman AB. Preliminary report of the use of everolimus in a patient with progressive medullary thyroid carcinoma. Clin Endocrinol (Oxf) (2012) 77(1):154–5. doi: 10.1111/j.1365-2265.2011.04296.x
35. Owonikoko TK, Chowdry RP, Chen Z, Kim S, Saba NF, Shin DM, et al. Clinical efficacy of targeted biologic agents as second-line therapy of advanced thyroid cancer. Oncologist (2013) 18(12):1262–9. doi: 10.1634/theoncologist.2013-0250
36. Lorch JH, Busaidy N, Ruan DT, Janne PA, Limaye SA, Wirth LJ, et al. A phase II study of everolimus in patients with aggressive RAI refractory (RAIR) thyroid cancer (TC). J Clin Oncol (2013) 31(15_suppl):6023. doi: 10.1200/jco.2013.31.15_suppl.6023
37. Dong Y, Gong Y, Kuo F, Makarov V, Reznik E, Nanjangud GJ, et al. Targeting the mTOR pathway in hurthle cell carcinoma results in potent antitumor activity. Mol Cancer Ther (2022) 21(2):382–94. doi: 10.1158/1535-7163.MCT-21-0224
38. Sykorova V, Dvorakova S, Vcelak J, Vaclavikova E, Halkova T, Kodetova D, et al. Search for new genetic biomarkers in poorly differentiated and anaplastic thyroid carcinomas using next generation sequencing. Anticancer Res (2015) 35(4):2029–36.
39. Zanardi E, Verzoni E, Grassi P, Necchi A, Giannatempo P, Raggi D, et al. Clinical experience with temsirolimus in the treatment of advanced renal cell carcinoma. Ther Adv Urol (2015) 7(3):152–61. doi: 10.1177/1756287215574457
40. de Wit D, Schneider TC, Moes DJ, Roozen CF, den Hartigh J, Gelderblom H, et al. Everolimus pharmacokinetics and its exposure-toxicity relationship in patients with thyroid cancer. Cancer Chemother Pharmacol (2016) 78(1):63–71. doi: 10.1007/s00280-016-3050-6
41. Allin DM, Shaikh R, Carter P, Thway K, Sharabiani MTA, Gonzales-de-Castro D, et al. Circulating tumour DNA is a potential biomarker for disease progression and response to targeted therapy in advanced thyroid cancer. Eur J Cancer (2018) 103:165–75. doi: 10.1016/j.ejca.2018.08.013
42. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
Keywords: poorly differentiated thyroid carcinomas, mTOR inhibitors, immunotherapy, treatment outcomes, case report
Citation: Oh Y, Park JH, Djunadi TA, Shah Z, Chung LI-Y and Chae YK (2024) Deep response to a combination of mTOR inhibitor temsirolimus and dual immunotherapy of nivolumab/ipilimumab in poorly differentiated thyroid carcinoma with PTEN mutation: a case report and literature review. Front. Endocrinol. 15:1304188. doi: 10.3389/fendo.2024.1304188
Received: 29 September 2023; Accepted: 15 January 2024;
Published: 31 January 2024.
Edited by:
Vladimir Neychev, University of Central Florida, United StatesReviewed by:
Jeffrey A. Knauf, Cleveland Clinic, United StatesAthanasios Bikas, Brigham and Women’s Hospital and Harvard Medical School, United States
Bhavana Konda, The Ohio State University, United States
Copyright © 2024 Oh, Park, Djunadi, Shah, Chung and Chae. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Young Kwang Chae, WUNIQUVAbm0ub3Jn
†These authors have contributed equally to this work
 Youjin Oh
Youjin Oh Joo Hee Park1†
Joo Hee Park1† Trie Arni Djunadi
Trie Arni Djunadi Zunairah Shah
Zunairah Shah Young Kwang Chae
Young Kwang Chae