- Department of Hepatology, Shuguang Hospital Attached to Shanghai Chinese Medicine University, Shanghai, China
Background: There has been controversy and uncertainty regarding the causal relationship between type 1 diabetes, its consequences, liver fibrosis, and cirrhosis. In order to determine the causal relationship, we conducted a Mendelian randomization study (MR).
Methods: For the first time, we subjected multiple diabetes data to analyze its relationship with the progression of liver fibrosis. Once the instrumental variables had been extracted, we assessed them employing Cochran’s Q multi-analysis, inverse variance weighted, MR-Egger, MR-PRESSO, weighted mode, and weighted median.
Results: Genetically predicted type 1 diabetes (OR = 1.13, 95% CI: 1.04–1.23, **P = 3.42 × 10−3), type 1 diabetes without complications (OR = 1.12, 95% CI: 1.03–1.23, *P = 1.26 × 10−2), type 1 diabetes with coma (OR = 1.09, 95% CI: 1–1.18, *P = 4.74 × 10−2), type 1 diabetes with ketoacidosis (OR = 1.07, 95% CI: 1.01–1.13, *P = 1.3 × 10−2), type 1 diabetes with neurological complications (OR = 1.18, 95% CI: 1.11–1.26, ***P = 4.05 × 10−7), type 1 diabetes with ophthalmic complications (OR = 1.16, 95% CI: 1.05–1.28, **P = 3.06 × 10−3), type 1 diabetes with renal complications (OR = 1.07, 95% CI: 1–1.13, *P = 3.45 × 10−2), type 1 diabetes with other specified/multiple/unspecified complications (OR = 1.12, 95% CI: 1.02–1.23, *P = 1.41 × 10−2) were all associated with an increased risk of liver fibrosis progression.
Conclusions: According to our MR investigation, type 1 diabetes and both its acute and chronic implications may increase the likelihood that liver fibrosis could continue to develop. Additionally, type 1 diabetes with neurological and ocular problems is more likely to accelerate the development of liver fibrosis and inflammation, which offers new insights for genetic investigations.
1 Introduction
A global health priority (1), type 1 diabetes (T1D) has witnessed a 3%–4% increase in prevalence over the past three decades (2). The risk of multiorgan complications, including acute complications [diabetic ketoacidosis, coma, etc. (3)] and chronic complications [ophthalmopathy, neurological, renal disease, etc. (4)], is increased by the imbalance in glycemic control triggered by insulin deficiency (5) brought on by pancreatic beta-cell autoimmunity. In recent years, we have witnessed an abundance of research on the liver, one of the organs related to type 1 diabetes (6).
A significant worldwide health issue is liver fibrosis induced by either viral or metabolic liver disease (7). Cirrhosis with structural loss, ensuing functional failure, and the emergence of life-threatening consequences have been defined as advanced chronic fibrosis (8). Liver fibrosis is a potentially reversible pathophysiological event (9, 10). Consequently, preventing the development of liver fibrosis to the point of cirrhosis is a potential way to prevent liver-related deaths (7). T2D boosts the risk of cirrhosis and liver-related mortality, particularly in people with NAFLD (11). Nevertheless, the link between liver fibrosis and the less prevalent type of diabetes, T1D, is rather uncommon and has generated debate. According to certain research, it results in little to no progression of liver fibrosis (12, 13), and according to two mate analysis studies, NAFLD patients with T1D experienced little to no progression of liver fibrosis (14, 15). According to certain observational studies, it may result in liver fibrosis (16, 17), which heightens liver stiffness (18, 19). One study revealed that 1 in 20 T1D patients had increased liver stiffness (12) or a 2.0% frequency of advanced fibrosis (15), whereas a Brazilian cross-sectional investigation indicated that 8.4% of T1D people had liver fibrosis (20). The most current MATE observational study (21) comprised just three investigations of T1D patients. However, virtually nothing has been established regarding the association between type 1 diabetes complications and the risk of liver fibrosis in the general population. Possible explanations include the low proportion of T1D patients recruited compared with T2D patients, the rarity of patients with comorbidities, and the length of certain studies, which makes it more challenging to perform research since patients are more likely to drop out. On the other hand, conclusions regarding the causal relationship between T1D, comorbid multisystem complications, and the degree of risk of liver fibrosis progression are hindered by the residual confounding of observational studies and the inherent difficulties of potential reverse causality, a challenge that may be overcome using MR methods.
Similar to RCT research, Mendelian randomization is an efficient statistical technique for determining causation based on whole-genome sequencing data (22). Public big data analysis is more effective and possesses a greater degree of proof as opposed to randomized controlled research, and it is capable of preventing bias and reverse causality. Previous MR research has investigated the risk factors for liver fibrosis and even cirrhosis, and MR studies have also discovered an association between the comorbidity of type 1 diabetes and NAFLD (23). However, neither of these two has previously been the subject of Mendelian randomization experiments.
In this study, we performed the first MR analysis of type 1 diabetes, its acute and chronic complications, liver fibrosis, and cirrhosis. Additionally, we investigated the possible causal relationship between these, as well as offered new suggestions for the early prevention and treatment of liver fibrosis progression and cirrhosis in patients with type 1 diabetes, especially in combination with acute and chronic complications.
2 Methods
2.1 Study design
This is a two-way two-sample MR and MVMR study, and an overview of the study design is presented in Figure 1.
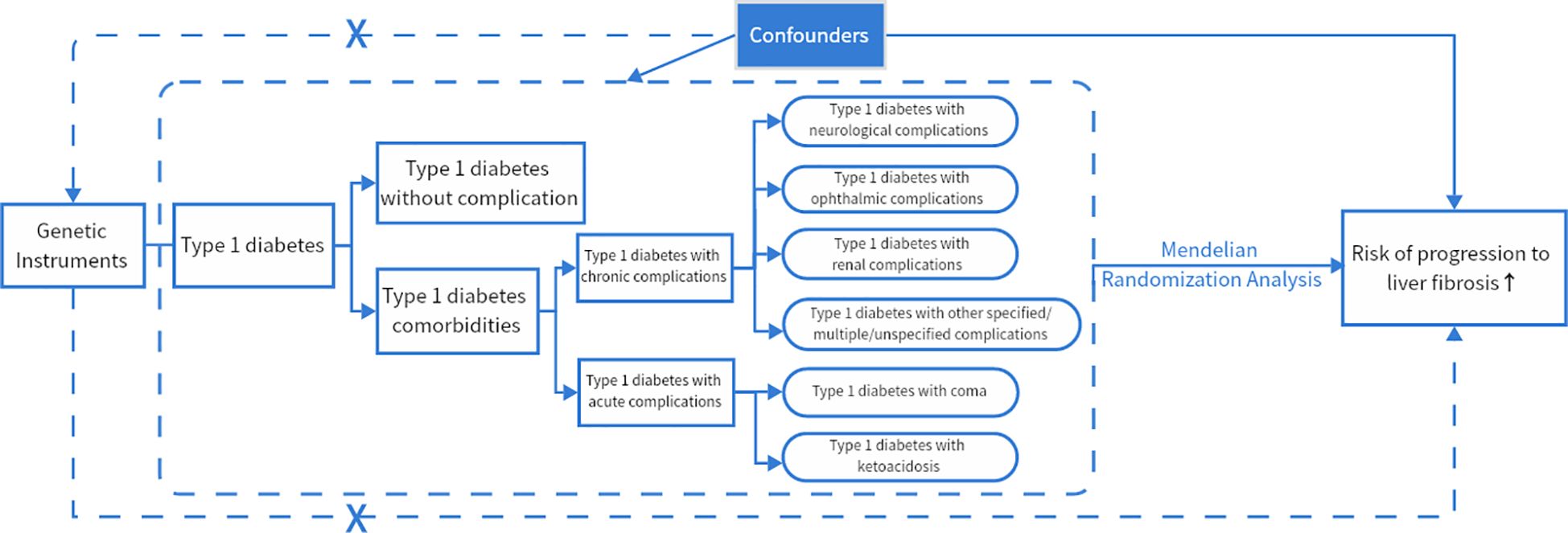
Figure 1 MR framework overview. If genetic variants (SNPs) can reliably control for disease risk factors (type 1 diabetes and its acute and chronic complications), and if there is a causal association between risk factors (type 1 diabetes and its acute and chronic complications) and disease endpoints (cirrhosis, liver fibrosis), then the genetic variants (SNPs) themselves should be associated with disease endpoints (cirrhosis, liver fibrosis). The assumptions required to produce unbiased estimates of causal effects as well as the rules of Mendelian randomization analysis of risk variables and risk of advancement of liver fibrosis can be modified. Potential pleiotropy or direct causality between variables is indicated by collapsed lines, which would go against the Mendelian randomization assumption.
2.2 Genetic epidemiology data sources
In the discovery phase, we utilized association statistics from databases and data from the FinnGen (https://www.finngen.fi/en/node/17) database based on gene-wide investigations. Type 1 diabetes (total), type 1 diabetes without complications, type 1 diabetes with coma, type 1 diabetes with ketoacidosis, type 1 diabetes with neurological complications, type 1 diabetes with ophthalmic complications, type 1 diabetes with renal complications, and type 1 diabetes with other specified/multiple/unspecified complications were among the variables selected for the genetic factor construction. The data were of European ancestry, and each cohort underwent GWAS, genotype estimation, and quality control pr (QC). At the same time, the initial GWAS was authorized by the appropriate institutional review board.
2.2.1 Type 1 diabetes data sources
As the exposure factors, we specifically included type 1 diabetes (total) and type 1 diabetes without complications. Firstly, 188,103 European patients with type 1 diabetes without complications and 186,323 patients with type 1 diabetes had been selected based on phenotypic data as genetic instrumental variables for T1D. The enrolled patients were diagnosed with type 1 diabetes, both with and without complications, and the average age of first onset was 20.6 years. The absence of complications in type 1 diabetes diagnosis excluded complications associated with type 1 diabetes, diabetes caused by starvation, diabetes during pregnancy and delivery, neonatal diabetes, simple glycosuria, decreased glucose tolerance, and postoperative hypoinsulinemia. The mean age of first onset was 29.26 years.
2.2.2 Type 1 diabetes with acute-complication data sources
As one of the exposure factors, we included type 1 diabetes with coma and type 1 diabetes with ketoacidosis. As genetic instrumental variables for type 1 diabetes paired with acute complications, 184,423 European patients with type 11 diabetes complicated by coma and 184,512 European patients with type 1 diabetes and ketoacidosis were identified. Type 1 diabetes-related comas with or without ketoacidosis, hypoglycemia coma, hyperosmolar coma, and hyperglycemic coma were all diagnosed. Patients experiencing both type 1 diabetic acidosis and type 1 diabetic ketoacidosis were diagnosed as type 1 diabetes with ketoacidosis.
2.2.3 Type 1 diabetes with chronic complications data sources
The following complications were listed as one of the exposure factors: type 1 diabetes with neurological complications, type 1 diabetes with ophthalmic complications, type 1 diabetes with renal complications, and type 1 diabetes with other specified/multiple/unspecified complications. As genetic instrumental variables for chronic complications of T1D, 183,763 European patients with type 1 diabetes with neurological complications, 186,062 type 1 diabetes with ophthalmic complications, 184,148 type 1 diabetes with renal complications, and 186,601 type 1 diabetes with other specified/multiple/unspecified complications were selected. The diagnosis of type 1 diabetes with neurological complications includes type 1 diabetes with autonomic neuropathy, type 1 diabetes with mononeuropathy, type 1 diabetes with polyneuropathy, and type 1 diabetes with myasthenia gravis. The diagnosis of type 1 diabetes’ ocular complications includes type 1 diabetes with retinopathy and type 1 diabetes with cataracts. Type 1 diabetes with diabetic nephropathy, type 1 diabetes with intracapillary glomerulonephropathy, and type 1 diabetes with Kimmelstiel-Wilson syndrome were all diagnosed as having renal consequences. Type 1 diabetes with arthropathy, type 1 diabetes with neurologic diabetic arthropathy, type 1 diabetes with numerous complications, and type 1 diabetes with undefined complications were all diagnosed as type 1 diabetes with other specified/multiple/unspecified complications.
2.2.4 Liver fibrosis and cirrhosis data sources
Cirrhosis and liver fibrosis were included as outcomes. The FinnGen database’s “K11_FIBROCHIRLIV” code was employed to select 214,403 European individuals with liver fibrosis and cirrhosis. The diagnosis excluded other liver disorders and included liver fibrosis, cirrhosis, liver fibrosis with cirrhosis, primary biliary cirrhosis, secondary biliary cirrhosis, biliary cirrhosis, and other undefined cirrhosis (e.g., cryptogenic, macronodular, mixed type). The mean age at the first onset of 63.88 years concentrated was between 50 and 80 years. The combined final report served as a genetic instrumental variable for cirrhosis and liver fibrosis. All of the above groups were not notably sex-specific.
2.3 Tool variable selection
To guarantee that there was an effect, three fundamental model assumptions of the MR analysis were satisfied. In order to prove that genetic variations (liver fibrosis, cirrhosis) and exposure (type 1 diabetes and its acute and chronic consequences) must be related, an association assumption was first created. The genetic variable P < 5e−08, and we expanded the study to set P < 5e−06 because there were too few genetic variables for type 1 diabetes with ketoacidosis, type 1 diabetes with neurological complications, and type 1 diabetes with renal complications. LD refers to non-random (nonrandom) associations between alleles (alleles) of different loci (loci) and is measured using two parameters r² and kb. We set the window to 10,000 kb and r² < 0.01 to ensure the independence of the selected genetic variants. Second, by employing the Phenoscan website, we eliminated confounding sociodemographic and disease state factors, such as age, obesity, heavy alcohol use, non-alcoholic fatty liver disease, hepatitis B or C infection, autoimmune disease, cholestatic disease, and iron or copper overload, which may contribute to liver fibrosis and cirrhosis. Finally, SNPs associated with outcomes were excluded and P2 > 5 × 10−5 was set to satisfy the exclusivity assumption. In addition, F-value calculation was performed and SNPs with F <10 were excluded.
2.4 Statistical methods
2.4.1 MR analysis method
For the two-sample MR analysis between exposure and outcome, the inverse variance weighted (IVW, random effects) method was employed. We first evaluated the causative association between liver fibrosis and cirrhosis and type 1 diabetes and its acute and chronic consequences using IVW as the major approach of analysis. It is a method for combining two or more random variables in order to reduce the aggregate variance, where the weight of each random variable in the sum is inversely proportional to its variance. Subsequently, as additional analysis approaches, we employed MR-Egger, weighted median (24), weighted mode, MR-PRESSO (25), and simple median. If the presumption that all contained SNPs may be utilized as genuine IVs is met, the IVW technique yields precise estimates (26).
2.4.2 Sensitivity analysis to assess genetic markers
Employing MR-Egger, we failed to discover any evidence to support the null hypothesis of pleiotropy for genetic markers (P-value for pleiotropy > 0.05). We took into account the existence of an intercept term and utilized it to evaluate pleiotropy in the MR-Egger hypothesis. When the proportion of invalid instrumental variables is as high as 50% and the precision of the estimates varies substantially among instrumental variables, the weighted median method (27), defined as the median of the weighted empirical density function of the ratio estimates, continues to generate consistent effect estimates. Despite the fact that it is not as effective as IVW, the simple median nevertheless supports multiplicity (28). We utilized the “MR-PRESSO global test,” “MR-PRESSO outlier test,” and “MR-PRESSO distortion test” to eliminate anomalous SNPs (outliers) and estimate adjusted results. MR-PRESSO is a frequently employed method for testing horizontal multiplicity. To eliminate anomalous SNPs (outliers) and estimate the corrected results, researchers employed the “PRESSO outlier test” and the “MR-PRESSO distortion test”. The absence of horizontal pleiotropy effects for both variables was our final consideration. A prevalent test for heterogeneity is the Cochran Q statistic (29). The Cochran Q statistic is a standardized weighted sum of squares of the variances across studies, with smaller P values (usually at the level of α <0.10), indicating the presence of heterogeneity. the Q statistic usually exhibits a high statistical power when the number of included studies is large. Ultimately, we considered the absence of heterogeneity effects.
R (version 5.0.26) was utilized to analyze all of the aforementioned statistical analyses. The weighted median method, weighted mode method, and IVW method are all implemented in the TwoSampleMR R package. The MR-Egger analysis is performed by the MendelianRandomizationR program. The MR-PRESSO analysis is carried out through the MR-PRESSO R package.
3 Results
SNPs with large outliers and those that departed from the symmetric midline utilizing scatter plots and funnel plots were initially omitted since they might be confounding factors in liver fibrosis and cirrhosis. We obtained eight separate sets of IVs without linkage disequilibrium (Supplementary Material). The bias of weak IVs was eliminated by the F statistics of these eight groups of IVs, which were all more than 1,000. Finally, for these eight groups, we created scatter plots (Figure 2) and leave-one-out plots (Figure 3).
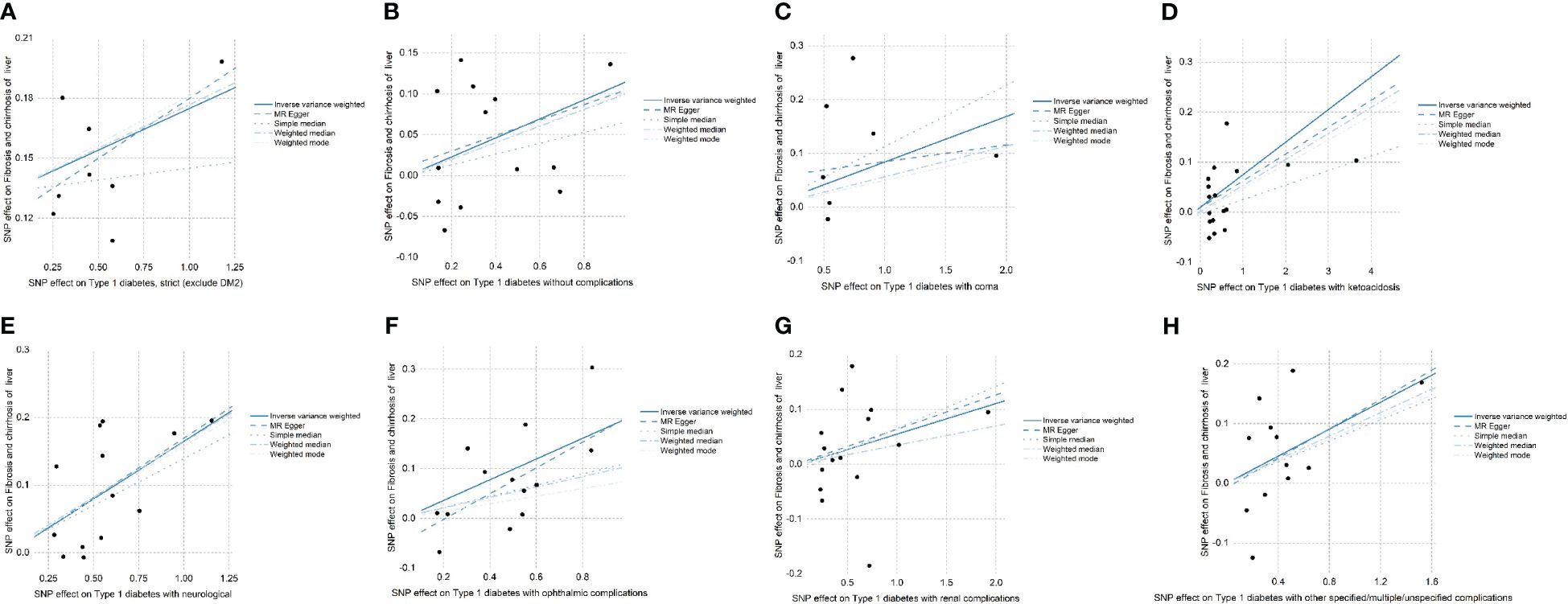
Figure 2 (A–H) The effects of SNPs on type 1 diabetes, type 1 diabetes with complications, liver fibrosis, and cirrhosis are displayed in a scatter plot. (A) Strict type 1 diabetes in patients with liver fibrosis and cirrhosis (exclude DM2). (B) Type 1 diabetes without complications in patients with liver fibrosis and cirrhosis. (C) Type 1 diabetes in patients with coma. (D) Type 1 diabetes in patients with ketoacidosis. (E) Type 1 diabetes in patients with neurological complications. (F) Type 1 diabetes in patients with ophthalmic complications. (G) Type 1 diabetes. (H) Type 1 diabetes with other specified/multiple/unspecified complications in liver fibrosis and cirrhosis. Mendelian randomization is referred to as MR and inverse variance weighted is referred to as IVW. The outcomes of these regression studies are represented by the IVW, MR-Egger, weighted median, weighted mode, and simple median slopes.
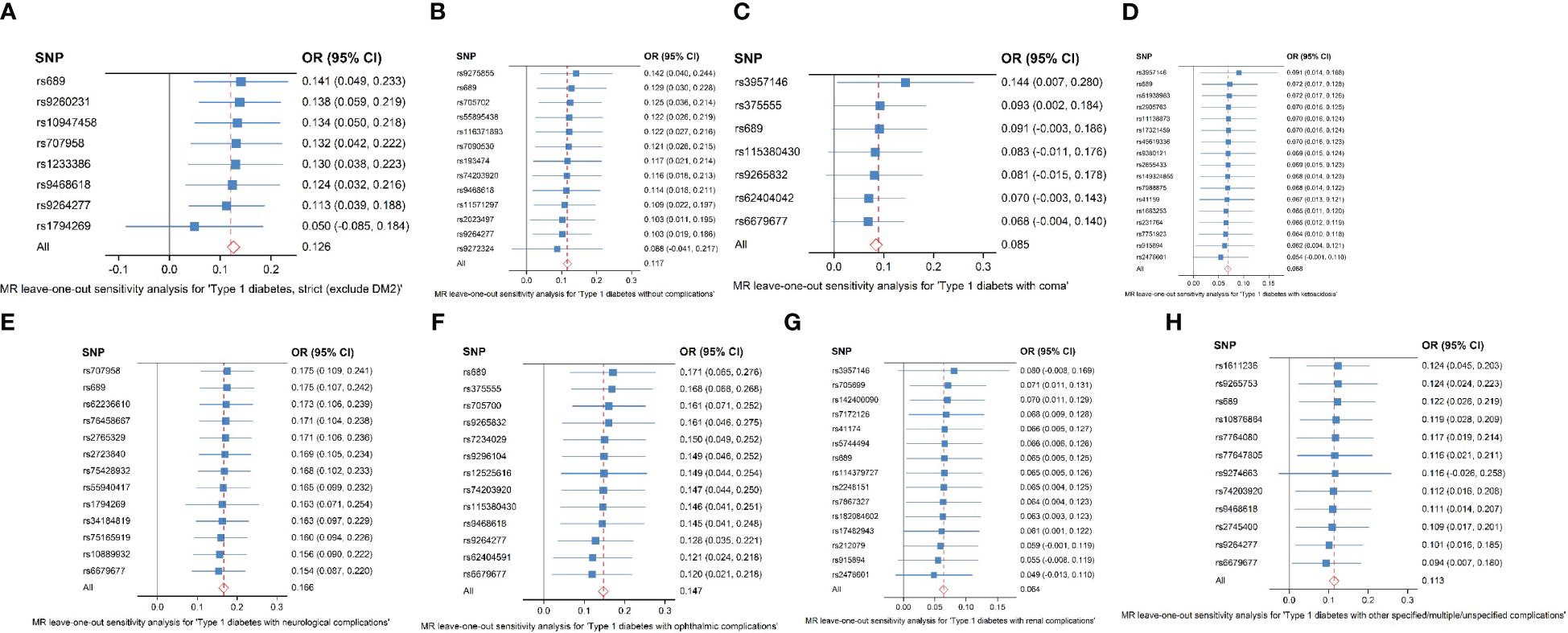
Figure 3 (A–H) Analysis of the “leave-one-out” causal association between type 1 diabetes and its consequences, including liver fibrosis and cirrhosis. (A) Strict type 1 diabetes in patients with liver fibrosis and cirrhosis (exclude DM2). (B) Type 1 diabetes without complications in patients with liver fibrosis and cirrhosis. (C) Type 1 diabetes in patients with coma. (D) Type 1 diabetes in patients with ketoacidosis. (E) Type 1 diabetes in patients with neurological complications. (F) Type 1 diabetes in patients with ophthalmic complications. (G) Type 1 diabetes with renal complications in liver fibrosis and cirrhosis. (H) Type 1 diabetes with other specified/multiple/unspecified complications in liver fibrosis and cirrhosis. The causal estimate and 95% confidence interval from the MR analysis after each SNP was removed are represented by the black dots and bars in the leave-one-out forest plot. The total MR estimate and 95% CI obtained using fixed-effect IVW approaches are represented by the red dots and bars.
The results of the forest plot (Figure 4) presented a positive causal relationship between both type 1 diabetes and its complications and liver fibrosis and cirrhosis. Firstly, we performed IVW analysis, type 1 diabetes (exclude DM2): OR = 1.13, 95% CI: 1.04–1.23, **P = 3.42 × 10−3. Type 1 diabetes without complications: OR = 1.12, 95% CI: 1.03–1.23, *P = 1.26 × 10−2. Type 1 diabetes with coma: OR = 1.09, 95% CI: 1–1.18, *P = 4.74 × 10−2. Type 1 diabetes with ketoacidosis: OR = 1.07, 95% CI: 1.01–1.13, *P = 1.3 × 10−2. Type 1 diabetes with neurological complications: OR = 1.18, 95% CI: 1.11–1.26, ***P = 4.05 × 10−7. Type 1 diabetes with ophthalmic complications: OR = 1.16, 95% CI: 1.05–1.28, **P = 3.06 × 10−3. Type 1 diabetes with renal complications: OR = 1.07, 95% CI: 1–1.13, *P = 3.45 × 10−2. Type 1 diabetes with other specified/multiple/unspecified complications: OR = 1.12, 95% CI: 1.02–1.23, *P = 1.41 × 10−2. In addition, MR-Egger’ analysis portrayed that type 1 diabetes with ophthalmic complications: OR = 1.3, 95% CI: 1.04–1.62, *P = 4.43 × 10−2. Type 1 diabetes with neurological complications: OR = 1.19, 95% CI: 1.04–1.36, *P = 2.68 × 10−2. Weighted median analysis shows that type 1 diabetes (exclude DM2): OR = 1.14, 95% CI: 1.05–1.24, **P = 1.86 × 10−3. Type 1 diabetes with neurological complications: OR = 1.18, 95% CI: 1.08–1.28, ***P = 1.48 × 10−4. Type 1 diabetes with other specified/multiple/unspecified complications: OR = 1.1, 95% CI: 1.01–1.2, *P = 2.37 × 10−2. Weighted mode analysis shows that type 1 diabetes (exclude DM2): OR = 1.15, 95% CI: 1.06–1.25, *P = 1.03 × 10−2. Type 1 diabetes with neurological complications: OR = 1.17, 95% CI: 1.07–1.28, **P = 5.07 × 10−3. Simple median analysis showed that type 1 diabetes with neurological complications: OR = 1.15, 95% CI: 1.03–1.28, *P = 1.26 × 10−2. The scatter plot (Figure 2) demonstrated that the five lines of “inverse variance weighted,” “MR-Egger,” “weighed median,” “weighed mode,” and “simple mode” all had consistent slope beta values and P values. Taking these findings together, we concluded that type 1 diabetes and complications had significant differences in liver fibrosis and cirrhosis and that they all satisfied the positive relationship, i.e., the higher the risk of having type 1 diabetes and complications. Cochran’s IVWQ test results revealed that there was no discernible heterogeneity in these IVs (Table 1). According to the outcomes of the MR-Egger regression intercept analysis, there was also no discernible directional horizontal pleiotropy (Table 1). However additional MR-PRESSO analysis likewise failed to detect any appreciable horizontal pleiotropy (Table 1).

Figure 4 Type 1 diabetes and type 1 diabetes with acute and chronic complications on liver fibrosis and cirrhosis forest chart.
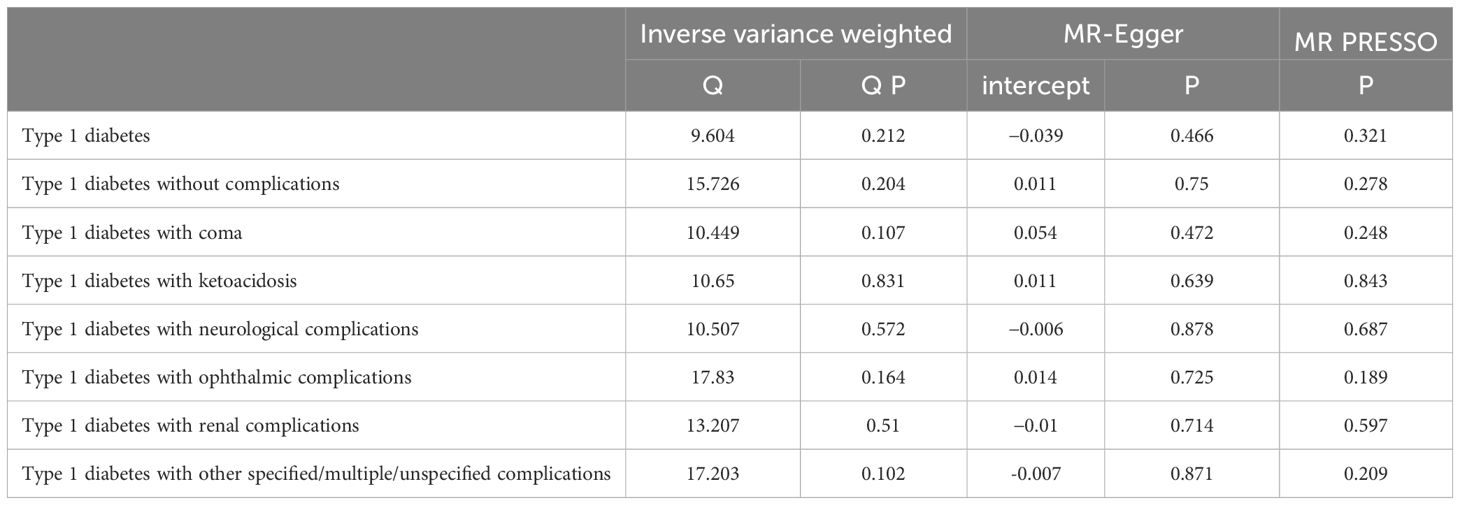
Table 1 Trilinear table of type 1 diabetes and type 1 diabetes combined with acute and chronic complications and liver fibrosis and sclerosis levels of pleiotropy and heterogeneity analysis.
4 Discussion
To determine the causal link between type 1 diabetes, complications, and liver fibrosis and cirrhosis, we conducted the first thorough bivariate multivariate MR analysis of type 1 diabetes, its acute and chronic consequences (exposure), and liver fibrosis and cirrhosis (outcome). According to our research, there is a causal association between type 1 diabetes, its acute and chronic consequences, and liver fibrosis and cirrhosis, implying that these conditions raise the likelihood of liver fibrosis advancement.
As a result, we surmised that T1D might hasten the development of liver fibrosis. Millions of people throughout the world suffer from type 1 diabetes (30), an inflammatory illness, and one of its complications is liver alterations (18, 31). We are aware that the long-term exposure of hepatocytes to glucose levels above the physiological range is a factor in the development of fibrosis (32), looking back at earlier investigations into type 1 diabetes and liver fibrosis. Patients with advanced fibrosis are more likely to suffer from metabolic syndrome, particularly if they have unfavorable lipid profiles, or are less sensitive to insulin compared with people without progressive fibrosis (15). Recent research reveals that sedentary lifestyles and high-calorie consumption are contributing factors in a rising proportion of T1D patients becoming overweight and developing insulin resistance (IR) features (33). Adipose tissue that is metabolically harmful is produced by insulin resistance in the liver and the periphery, which is increasingly thought to be associated with T1D (34). Adipose tissue inflammation is one of the factors contributing to steatohepatitis and fibrosis, especially in NAFLD (35), when type 1 diabetes is more severe or combined with complications manifested by elevated liver enzymes, which are typically linked to increased liver fat (6, 36). A similar relevance to glycogen deposition in hepatocytes has also been demonstrated in other studies (37). Hyperglycemia or insulin resistance, increased glycogen synthesis, stimulation of immunological cells, fibrogenic-programmed cells in the vasculature, or organ-specific parenchymal cells are all effects of inadequately managed T1D hyperglycemia (17). Therefore, we hypothesize that variations in insulinemia and hyperglycemia could possess a significant role in T1D-related liver fibrosis.
In addition, this study also discovered that patients with type 1 diabetes who experienced neurological and ophthalmic complications possessed ORs that were higher than the mean OR value for people with type 1 diabetes, whereas patients who experienced ketoacidosis and coma, renal complications, and other specified/multiple/unspecified complications experienced ORs that were lower than the mean OR value for people with type 1 diabetes, which is more in line with observational studies. The first observational study discovered an association between ALT and GGT activity and type 1 diabetes along with joint restriction and neuropathy, and GGT activity was also discovered to be associated with the severity of retinopathy (38). Another Dutch cross-sectional study revealed that concomitant neuropathy and ophthalmopathy were considerably more prevalent in the 150 patients with type 1 diabetes than coexisting renal and cardiovascular illness, but unfortunately, the number of participants was insufficient to draw any conclusions. Unfortunately, a correlation factor analysis (15) was not possible due to the small number of patients with type 1 diabetes and its comorbidities. Recent studies (39, 40) have additionally demonstrated that people with severe insulin-deficient diabetes are more likely to develop retinopathy and neuropathy. In this context, we hypothesize that type 1 diabetes with neurological and ocular problems is more likely to encourage the development of liver inflammation and fibrosis in comparison with type 1 diabetes with other comorbidities. Meanwhile, the OR of type 1 diabetes with chronic complications was slightly greater than the OR of type 1 diabetes without complications and acute complications. However, despite the fact that the data were high and the ORs and 95% CI of type 1 diabetes patients without complications could not significantly differ from those of patients with combined complications, it is possible that this is because the patients included in the study did not have enough combined multisystem complications to be separated, the complications were not identified, or the lesions were mild but not to the point where other complications could be diagnosed, etc., or the difference between the progression of liver fibrosis solely and the presence or absence of combination problems in type 1 diabetes was not significant.
Unquestionably, this study discovered that type 1 diabetes and its consequences, to a greater or lesser extent, speed up the progression of liver fibrosis, which has ramifications for both current and future generations’ clinical and public health. In this regard, our recommendation is to make an effort to manage blood glucose in type 1 diabetes patients in order to lessen the progression of multiple diseases such as liver fibrosis and to slow down oscillations in glucose and insulinemia. Future research is required in order to better comprehend the mechanisms behind the association between type 1 diabetes, its concomitant conditions, and the risk of cirrhosis and liver fibrosis.
5 Strengths and limitations
5.1 Strengths
We conducted the first Mendelian randomization study to effectively examine the causal association between type 1 diabetes and its acute and chronic consequences, including liver fibrosis and cirrhosis. The implementation of this strategy contains various benefits. First, we were able to lessen the potential effects of confounding variables and the reverse causality demonstrated in observational research by employing randomly assigned variations as instrumental variables. Secondly, to increase the accuracy of the estimations, we included data from studies with larger sample sizes (>18,000 for type 1 diabetes and both its acute and chronic sequelae, and up to 214,403 for liver fibrosis and cirrhosis). Following that, we combined data from more recent investigations with the FinnGen database in our analysis. Finally, a variety of techniques (MR-Egger, weighted median, MR-PRESSO, weighted mode, simple median, and elimination of outlier pleiotropic SNPs) were employed to identify and compensate for any pleiotropy bias.
5.2 Limitations
First off, as genetic data were lacking, we were only able to do analyses on Europeans for type 1 diabetes, its acute and chronic consequences, and liver fibrosis and sclerosis. Genetic variety makes it challenging to make firm conclusions about the causes of racial differences, so our findings might not be generalizable to other racial populations. However, we have no knowledge of any information indicating that the majority of ethnic groups should not be included in the current study. Secondly, it was impossible to separate the enormous number of patients with concomitant multisystem illnesses who were included in the study, and we did not have enough confidence that each complication was a different illness; thus, the ORs did not significantly differ across groups.
6 Conclusions
According to our MR investigation, type 1 diabetes and both its acute and chronic consequences might increase the likelihood that liver fibrosis could develop. The development of liver inflammation and fibrosis is more likely to be promoted by type 1 diabetes with neurological and ocular problems, which offers fresh insights into the genetic and clinical research of liver fibrosis and cirrhosis. Future research is required to clarify the probable processes underlying how type 1 diabetes, as well as its acute and chronic consequences, contribute to liver fibrosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
This research has been conducted using published studies and consortia providing publicly available summary statistics. All original studies have been approved by the corresponding ethical review board, and the participants have provided informed consent. In addition, no individual-level data were utilized in this study. Therefore, no new ethical review board approval was required. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
GH: Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. YG: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Shanghai Municipal Health Commission (ZY(2021–2023)-0302, shslczdzk01201, SHGZS-202246); Shanghai Municipal Education Commission (2022076–11); National Administration of Traditional Chinese Medicine (Qihuang scholar in the National Support Program for Leading Talents of Traditional Chinese Medicine).
Acknowledgments
The authors would like to express their appreciation to the participants and researchers of the FinnGen study. The datasets generated during and/or analyzed in the current study are available in the FinnGen repository (https://www.finngen.fi/en/node/17).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1302611/full#supplementary-material
References
1. Hou Y, Ding W, Wu P, Liu C, Ding L, Liu J, et al. Adipose-derived stem cells alleviate liver injury induced by type 1 diabetes mellitus by inhibiting mitochondrial stress and attenuating inflammation. Stem Cell Res Ther. (2022) 13:132. doi: 10.1186/s13287-022-02760-z
2. Norris JM, Johnson RK, Stene LC. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. (2020) 8:226–38. doi: 10.1016/S2213-8587(19)30412-7
3. Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. (2010) 39:481–97. doi: 10.1016/j.ecl.2010.05.011
4. Northam EA, Rankins D, Lin A, Wellard RM, Pell GS, Finch SJ, et al. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care. (2009) 32:445–50. doi: 10.2337/dc08-1657
5. Barnett R. Type 1 diabetes. Lancet (London England). (2018) 391:195. doi: 10.1016/S0140-6736(18)30024-2
6. Mertens J, De Block C, Spinhoven M, Driessen A, Francque SM, Kwanten WJ. Hepatopathy associated with type 1 diabetes: distinguishing non-alcoholic fatty liver disease from glycogenic hepatopathy. Front Pharmacol. (2021) 12:768576. doi: 10.3389/fphar.2021.768576
7. Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. (2020) 9(4):875. doi: 10.3390/cells9040875
8. Campana L, Iredale JP. Regression of liver fibrosis. Semin Liver Dis. (2017) 37:1–10. doi: 10.1055/s-0036-1597816
9. Foglia B, Novo E, Protopapa F, Maggiora M, Bocca C, Cannito S, et al. Hypoxia, hypoxia-inducible factors and liver fibrosis. Cells. (2021) 10(7):1764. doi: 10.3390/cells10071764
10. Smith A, Baumgartner K, Bositis C. Cirrhosis: diagnosis and management. Am Fam Physician. (2019) 10012:759–70.
11. Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat Rev Endocrinol. (2011) 7:456–65. doi: 10.1038/nrendo.2011.72
12. de Vries M, Westerink J, El-Morabit F, Kaasjager H, de Valk HW. Prevalence of non-alcoholic fatty liver disease (NAFLD) and its association with surrogate markers of insulin resistance in patients with type 1 diabetes. Diabetes Res Clin Pract. (2022) 186:109827. doi: 10.1016/j.diabres.2022.109827
13. Lundholm MD, Bena J, Zhou K, Tsushima Y, Kashyap SR. Prevalence and clinical determinants of non-alcoholic fatty liver disease by liver scores in adults with type 1 diabetes. J Diabetes Complications. (2023) 37:108405. doi: 10.1016/j.jdiacomp.2023.108405
14. de Vries M, Westerink J, Kaasjager K, de Valk HW. Prevalence of nonalcoholic fatty liver disease (NAFLD) in patients with type 1 diabetes mellitus: A systematic review and meta-analysis. J Clin Endocrinol Metab. (2020) 105:3842–53. doi: 10.1210/clinem/dgaa575
15. de Vries M, El-Morabit F, van Erpecum KJ, Westerink J, Bac ST, Kaasjager H, et al. Non-alcoholic fatty liver disease: identical etiologic factors in patients with type 1 and type 2 diabetes. Eur J Intern Med. (2022) 100:77–82. doi: 10.1016/j.ejim.2022.03.025
16. Harman DJ, Kaye PV, Harris R, Suzuki A, Gazis A, Aithal GP. Prevalence and natural history of histologically proven chronic liver disease in a longitudinal cohort of patients with type 1 diabetes. Hepatology. (2014) 60:158–68. doi: 10.1002/hep.v60.1
17. Tuleta I, Frangogiannis NG. Diabetic fibrosis. Biochim Biophys Acta Mol Basis Dis. (2021) 1867:166044. doi: 10.1016/j.bbadis.2020.166044
18. Elkabbany ZA, Elbarbary NS, Ismail EA, Mohamed NA, Ragab D, Abdel Alem S, et al. Transient elastography as a noninvasive assessment tool for hepatopathies of different etiology in pediatric type 1 diabetes mellitus. J Diabetes Complications. (2017) 31:186–94. doi: 10.1016/j.jdiacomp.2016.09.009
19. El-Sayed MH, Thabet RA, Hamza MT, Hussein MS, El Saeed MM. Liver disease in children and adolescents with type 1 diabetes mellitus: A link between glycemic control and hepatopathy. Diabetes Res Clin Pract. (2020) 170:108458. doi: 10.1016/j.diabres.2020.108458
20. Barros BSV, Monteiro FC, Terra C, Gomes MB. Prevalence of non-alcoholic fatty liver disease and its associated factors in individuals with type 1 diabetes: a cross-sectional study in a tertiary care center in Brazil. Diabetol Metab Syndrome. (2021) 13:33. doi: 10.1186/s13098-021-00649-0
21. Ciardullo S, Perseghin G. Prevalence of elevated liver stiffness in patients with type 1 and type 2 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. (2022) 190:109981. doi: 10.1016/j.diabres.2022.109981
22. Sekula P, Del Greco MF, Pattaro C, Kottgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
23. Liu N, Wang G, Liu C, Liu J, Huang S, Zhou Y, et al. Non-alcoholic fatty liver disease and complications in type 1 and type 2 diabetes: A Mendelian randomization study. Diabetes Obes Metab. (2023) 25:365–76. doi: 10.1111/dom.14877
24. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
25. McElroy LM, Likhitsup A, Scott Winder G, Saeed N, Hassan A, Sonnenday CJ, et al. Gender disparities in patients with alcoholic liver disease evaluated for liver transplantation. Transplantation. (2020) 104:293–8. doi: 10.1097/TP.0000000000002843
26. Yuan S, Larsson S. Causal associations of iron status with gout and rheumatoid arthritis, but not with inflammatory bowel disease. Clin Nutr. (2020) 39:3119–24. doi: 10.1016/j.clnu.2020.01.019
27. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
28. Milne RL, Kuchenbaecker KB, Michailidou K, Beesley J, Kar S, Lindstrom S, et al. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat Genet. (2017) 49:1767–78. doi: 10.1038/ng.3785
29. Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. (2006) 11(2):193–206. doi: 10.1037/1082-989X.11.2.193
30. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. (2010) 464:1293–300. doi: 10.1038/nature08933
31. Stadler M, Bollow E, Fritsch M, Kerner W, Schuetz-Fuhrmann I, Krakow D, et al. Prevalence of elevated liver enzymes in adults with type 1 diabetes: A multicentre analysis of the German/Austrian DPV database. Diabetes Obes Metab. (2017) 19:1171–8. doi: 10.1111/dom.12929
32. Meyer G, Dauth N, Grimm M, Herrmann E, Bojunga J, Friedrich-Rust M. Shear wave elastography reveals a high prevalence of NAFLD-related fibrosis even in type 1 diabetes. Exp Clin Endocrinol Diabetes. (2022) 130:532–8. doi: 10.1055/a-1666-0431
33. Priya G, Kalra S. A review of insulin resistance in type 1 diabetes: is there a place for adjunctive metformin? Diabetes Ther. (2018) 9:349–61. doi: 10.1007/s13300-017-0333-9
34. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. (2020) 8:616–27. doi: 10.1016/S2213-8587(20)30110-8
35. Mertens J, Van Gaal LF, Francque SM, De Block C. NAFLD in type 1 diabetes: overrated or underappreciated? Ther Adv Endocrinol Metab. (2021) 12:20420188211055557. doi: 10.1177/20420188211055557
36. Glushko T, Kushchayev SV, Trifanov D, Salei A, Morales D, Berry G, et al. Focal hepatic glycogenosis in a patient with uncontrolled diabetes mellitus type 1. J Comput Assist Tomogr. (2018) 42:230–5. doi: 10.1097/RCT.0000000000000673
37. Khoury J, Zohar Y, Shehadeh N, Saadi T. Glycogenic hepatopathy. Hepatobiliary Pancreat Dis Int. (2018) 17:113–8. doi: 10.1016/j.hbpd.2018.02.006
38. Arkkila PE, Koskinen PJ, Kantola IM, Ronnemaa T, Seppanen E, Viikari JS. Diabetic complications are associated with liver enzyme activities in people with type 1 diabetes. Diabetes Res Clin Pract. (2001) 52:113–8. doi: 10.1016/S0168-8227(00)00241-2
39. Ahlqvist E, Prasad RB, Groop L. 100 YEARS OF INSULIN: Towards improved precision and a new classification of diabetes mellitus. J endocrinology. (2021) 252(3):R59–70. doi: 10.1530/JOE-20-0596
Keywords: type 1 diabetes, type 1 diabetes with acute complications, type 1 diabetes with chronic complications, risk of liver fibrosis progression, Mendelian randomization study
Citation: Huo G and Gao Y (2024) Type 1 diabetes and combined acute and chronic complications are associated with risk of progression of liver fibrosis: a Mendelian randomization study. Front. Endocrinol. 15:1302611. doi: 10.3389/fendo.2024.1302611
Received: 26 September 2023; Accepted: 10 July 2024;
Published: 05 August 2024.
Edited by:
Ildiko Szanto, Hôpitaux Universitaires de Genève (HUG), SwitzerlandReviewed by:
Fusong Jiang, Shanghai Jiao Tong University, ChinaHaiping Duan, Qingdao Municipal Center for Disease Control and Prevention, China
Copyright © 2024 Huo and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yueqiu Gao, Z2FveXVlcWl1QHNodXRjbS5lZHUuY24=
 Guangyuan Huo
Guangyuan Huo Yueqiu Gao
Yueqiu Gao