- 1Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Fangshan Hospital of Beijing University of Chinese Medicine, Beijing, China
Background: Reducing the occurrence of diabetes is considered a primary criterion for evaluating the effectiveness of interventions for prediabetes. There is existing evidence that early lifestyle-based interventions can significantly decrease the incidence of diabetes. However, whether effective interventions can reduce long-term outcomes in patients, including all-cause mortality, cardiovascular risks, and the occurrence of microvascular complications, which are the most concerning issues for both patients and clinicians, remains a subject of inconsistent research findings. And there is no direct evidence to answer whether effective intervention has long-term benefits for prediabetic patients. Therefore, we conducted a systematic review and meta-analysis to assess the relationship between early effective intervention and macrovascular and microvascular complications in prediabetic patients.
Methods: PubMed, Embase, and Cochrane Central Register of Controlled Trials were searched for the randomized controlled trials of lifestyle or/and drugs intervention in prediabetes from inception to 2023.9.15. Two investigators independently reviewed the included studies and extracted relevant data. Random or fixed effects model meta-analysis to derive overall relative risk (RR) with 95% CI for all-cause mortality, cardiovascular events, and microvascular complications.
Results: As of September 15, 2023, a total of 7 effective intervention studies were included, comprising 26 articles out of 25,671 articles. These studies involved 26,389 patients with a total follow-up duration of 178,038.6 person-years. The results indicate that effective intervention can significantly reduce all-cause mortality in prediabetic patients without a history of cardiovascular disease by 17% (RR 0.83, 95% CI 0.70-0.98). Additionally, effective intervention reduced the incidence of retinopathy by 38% (RR 0.62, 95% CI 0.70-0.98). Furthermore, the study results suggest that women and younger individuals have lower all-cause mortality and cardiovascular mortality. Subsequently, we conducted an in-depth analysis of patients without a history of cardiovascular disease. The results revealed that prediabetic patients with a 10-year cardiovascular risk >10% experienced more significant benefits in terms of all-cause mortality (P=0.01). When comparing the results of all-cause mortality and cardiovascular mortality from the Da Qing Diabetes Prevention Outcome Study longitudinally, it was evident that the duration of follow-up is a key factor influencing long-term benefits. In other words, the beneficial effects become more pronounced as the intervention duration reaches a certain threshold.
Conclusion: Early effective intervention, which significantly reduces the incidence of diabetes, can effectively lower all-cause mortality in prediabetic patients without a history of cardiovascular disease (especially those with a 10-year cardiovascular risk >10%), with women and younger individuals benefiting more significantly. Additionally, the duration of follow-up is a key factor influencing outcomes. The conclusions of this study can provide evidence-based guidance for the clinical treatment of prediabetic patients to prevent cardiovascular and microvascular complications.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42020160985.
Introduction
Prediabetes is defined as the intermediate metabolic state between normal blood glucose levels and diabetes, including impaired glucose tolerance (IGT) and impaired fasting glucose (IFG). According to the latest estimates of the International Diabetes Federation (IDF) (2017), 352 million adults (7.3%) can be classified as prediabetes (1). Prediabetes is the risk factor for type 2 diabetes and its microvascular complications (2–4). Multiple large sample meta-analyses have shown that prediabetes is also risk factor for macroangiopathy (5, 6) and is associated with increased risk of liver cancer, endometrial cancer, and gastric/colorectal cancer (7).
Therefore, timely identification of prediabetic individuals and effective management are key to preventing the onset of diabetes. American Diabetes Association (ADA) suggests that lifestyle intervention is the basic management for prediabetes. The recommended drug intervention may consider IGT and IFG, age <60 years, body mass index (BMI) >=35kg/m2, family history of first-degree related diabetes, high concentration individuals with triglycerides proceed (8). Studies have shown that early intervention can significantly reduce the incidence of diabetes in patients with prediabetes (9, 10). Such as insulin-sensitizing agents and treatment that reduces postprandial hyperglycemia can reduce the risk of progression to T2DM in high-risk prediabetes subjects (11), and lifestyle intervention is the cornerstone for preventing progression to diabetes (12).
However, for prediabetes, just like diabetes, the primary focus of early intervention should be on preventing the occurrence of macrovascular and microvascular complications. So, can interventions that effectively reduce the incidence of diabetes in prediabetic patients also effectively reduce the occurrence of microvascular and macrovascular complications? Based on a comprehensive review of current evidence, the impact of effective intervention on long-term outcome measures, including all-cause mortality and cardiovascular events, remains inconsistent. For example, the Da Qing Diabetes Prevention Outcome Study (DQ) demonstrated that lifestyle intervention could effectively reduce the incidence of diabetes and suggest a reduction in all-cause mortality (13–17). The STOP-Non-insulin dependent diabetes mellitus trial (STOP), which combined lifestyle and acarbose, showed a significant reduction in cardiovascular events (18–21). However, the Acarbose Cardiovascular Evaluation Study (ACE), which applied acarbose in combination with lifestyle intervention, was effective in reducing diabetes incidence but had no effect on cardiovascular events (22, 23). Therefore, the current evidence does not provide clear guidance for clinicians regarding the long-term benefits of prediabetic patients. Hence, we aim to assess the impact of effective intervention on the long-term outcomes of prediabetic patients through a systematic review and meta-analysis.
Methods
This study was implemented and reported in accordance with the guidelines for systematic review and meta-analysis of the preferred reporting project (PRISMA) (24). This analysis does not involve personal information, ethical approval or patient consent is exempted, and has been prospectively registered in PROSPERO: CRD42020160985.
Data sources and searches
Pubmed, Embase and Cochrane Central Register of Controlled Trials for randomized controlled studies of prediabetes interventions were searched from inception to 2023.9.15. At the same time, references and related systematic reviews are included in the literature to help complete the search. A manual search of the references of included trials supplemented the electronic search.
Study selection
Studies were included if they met the following PICO(S) (participants, intervention, comparators, outcomes (study designs)) criteria:
1. Participants: The participants were prediabetes (IGT and/or IFG) patients, no gender or racial restrictions, including with or without a history of cardiovascular disease.
2. Intervention: Interventions included lifestyle (including dietary and exercise recommendations) or drugs (including acarbose, metformin)。
3. Comparator: The intervention measures in the control group are either a blank control, a placebo, or lifestyle guidance.
4. Outcome: main outcome is all-cause mortality, secondary outcomes include composite cardiovascular outcomes (including cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke, heart failure hospitalization, arterial revascularization or hospitalization of unstable angina), core cardiovascular outcomes (including cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke), cardiovascular mortality, microvascular complications (retinopathy, renal disease, peripheral neuropathy).
5. Study designs: The study type was randomized controlled trial (blind or not); JADAD score between 5 and 7 points.
Studies were excluded for: duplicate publications, only the abstract or lack of data and cannot obtain full-text articles, unable to extract data for research, and non-English literature. Studies included less than 100 participants.
Definitions of different indicators/standards
Effective Intervention: In the RCTs we included in the analysis, the intervention that can significantly reduce the occurrence of diabetes is referred to as an effective intervention, and when compared to the control group, it achieves statistical significance (P<0.05).
Cardiovascular History: Patients without a history of cardiovascular disease, meaning individuals included in the study who do not have obvious coronary heart disease. Studies specifically including patients with a history of coronary heart disease are classified as patients with cardiovascular history.
Age Classification: The “Report on the Nutrition and Chronic Diseases Status of Chinese Residents (2020)” indicates that being over 50 is a significant risk factor for the progression from prediabetes to diabetes. Additionally, individuals over the age of 50 tend to exhibit more abnormalities in glucose and lipid metabolism, along with a higher incidence of coronary heart disease. So, we using 50 years as the cutoff, individuals aged 50 or older are categorized as older patients, while others are considered general patients, including those younger than 50 and those older than 50.
Weight Classification: According to the World Health Organization (WHO) classification criteria for obesity, a BMI greater than 25 is considered obese. Therefore, based on a body mass index (BMI) of 25 as the threshold, individuals with a BMI greater than or equal to 25 are classified as overweight patients, while others are considered general patients, including those with a BMI less than 25 and those with a BMI greater than 25.
10-Year Cardiovascular Risk: The 10-year cardiovascular risk of the composite cardiovascular outcome was calculated by multiplying the annualized rate of cardiovascular outcome in the control group by 10 years. High cardiovascular risk is defined as the 10-year cardiovascular risk > = 10%, and low cardiovascular risk is defined as 10-year cardiovascular risk <10%.
Data extraction and quality assessment
Screening out duplicate articles with EndNote X9, two authors (XA and YZ) extracted and checked the data with the Office form separately, included the basic characteristics of the included study (including the country where the study was carried out, the number of patients included, the basic characteristics of the included patients, study design methods, interventions, intervention time, follow-up time, main outcomes, JADAD score) and the basic characteristics of the included patients (age, gender, race, body mass index (BMI), smoking history, hypertension, dyslipidemia), if there is any objection, it would be resolved through consultation. If the result data is reported at multiple follow-up points, the data from the longest follow-up would be selected.
I2 and Cochran’s Q test were used to evaluate the heterogeneity of treatment effects between trials. P <0.05 and I2> 50% of the Cochran’s Q test indicated significant heterogeneity. For studies with low heterogeneity, fixed-effect model is used. Studies with greater heterogeneity, the source of heterogeneity is first searched, and a random-effects model is used for analysis. For studies with no source of heterogeneity, only descriptive analysis.
Grading of the evidence
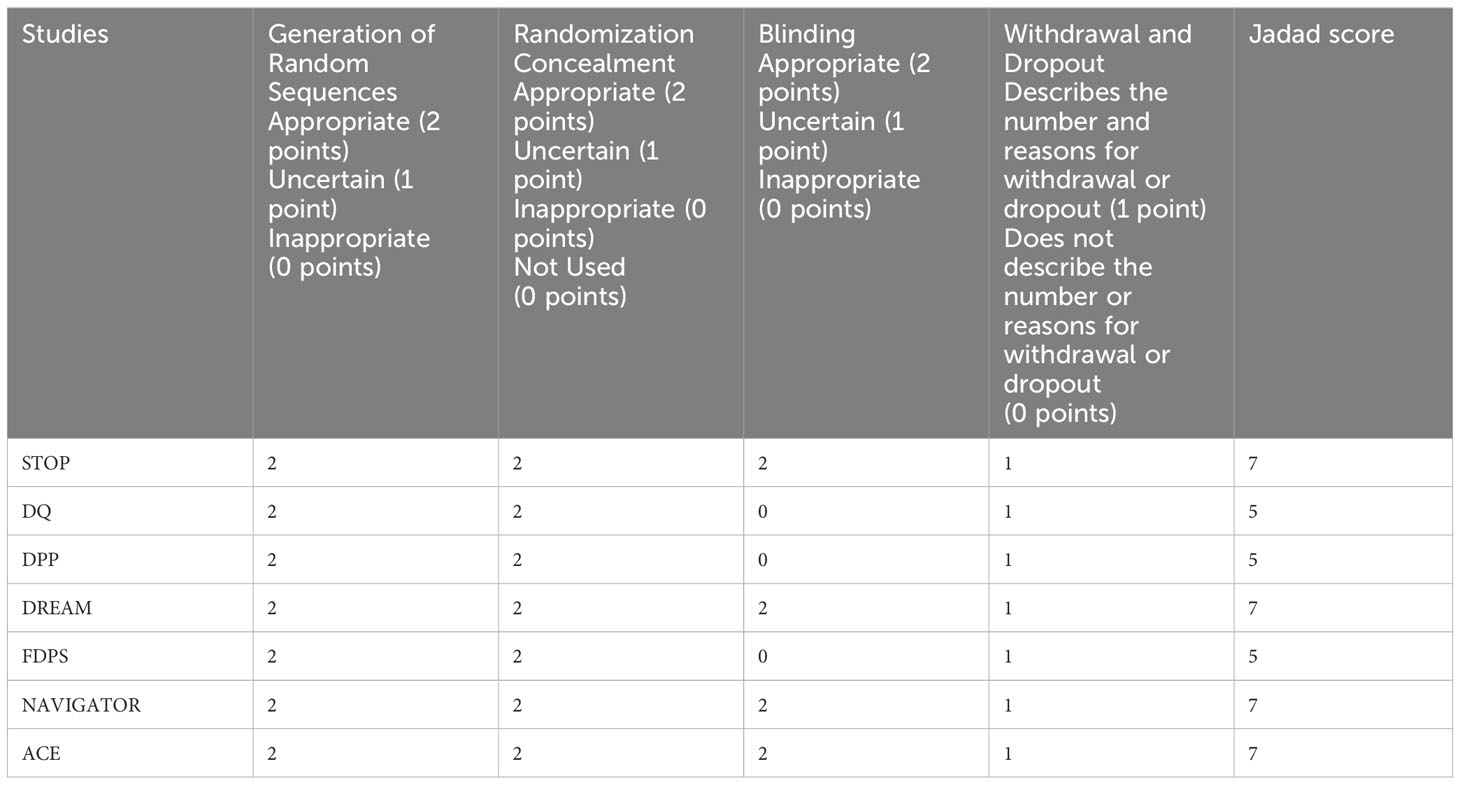
The quality of the studies was evaluated by JADAD 7 points (25), including the generation of random sequence, randomization hiding, blind method, withdrawal and exit. 1 to 3 is divided into low-quality study, 4 to 7 is divided into high-quality study. The two authors independently assessed the risk of publication bias in each study, and disputes were resolved through negotiation.
Data analysis
The results were analyzed with RevMan 5.2 provided by Cochrane Collaboration (26) and Prism 9.5.1. The secondary classification results were analyzed by relative risk (RR) and 95% confidence interval (CI). According to the characteristics of the patients, including the history of cardiovascular disease, age, obesity, gender, 10-year cardiovascular risk factors, outcomes were evaluated by subgroup analysis. According to the Cochrane manual, funnel plots were used to assess potential publication bias (27). Heterogeneity was evaluated using I2 statistics. I2 <25% represents low heterogeneity, 25-50% represents medium heterogeneity, and> 50% represents high heterogeneity, considering the heterogeneity difference P value, if I2 <50% and P> 0.05 then Fixed effect model was used, if I2 > 50% and P <0.05, random effect model was used. For studies with large heterogeneity that cannot find the source of heterogeneity, only descriptive analysis is made.
Results
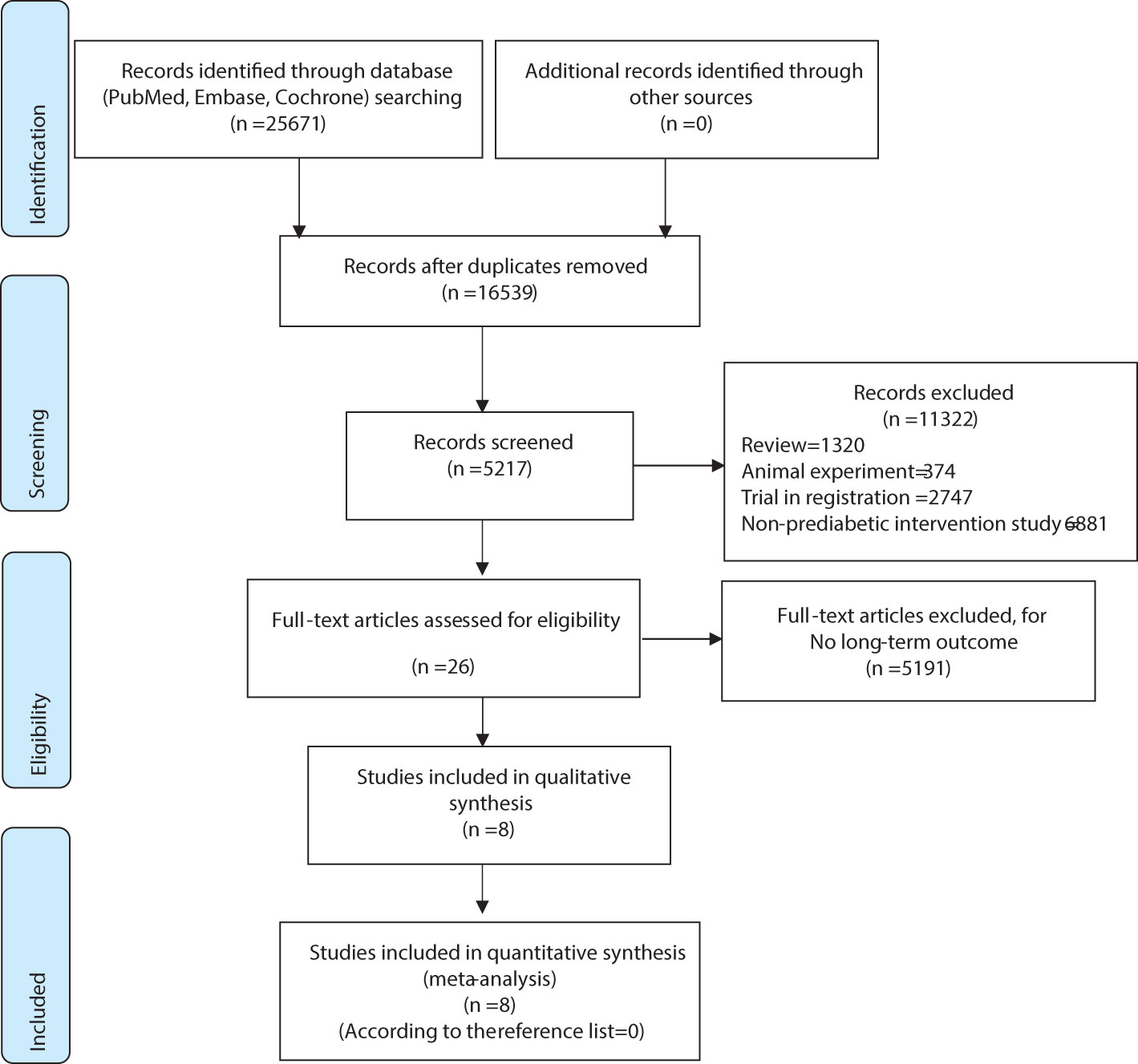
As shown in Figure 1 in the flowchart, we retrieved a total of 25,671 articles from three databases. First, we excluded duplicate articles, leaving us with 16,539 articles. Next, we reviewed titles and abstracts and excluded 11,322 unrelated articles, including 1,320 reviews, 374 animal experiments, 2,747 registered studies, and 6,881 articles without interventions. After a full-text review, we excluded 5,191 articles that lacked long-term outcome measures, leaving us with 26. No additional articles were added from the reference lists. In the end, a total of 8 studies were included for analysis, and two of these studies utilized a 2x2 factorial design. Simultaneously, we further analyzed seven studies based on the effectiveness of the intervention measures employed in the research (Figures 1, 2).
Basic characteristics of the including studies and quality evaluation
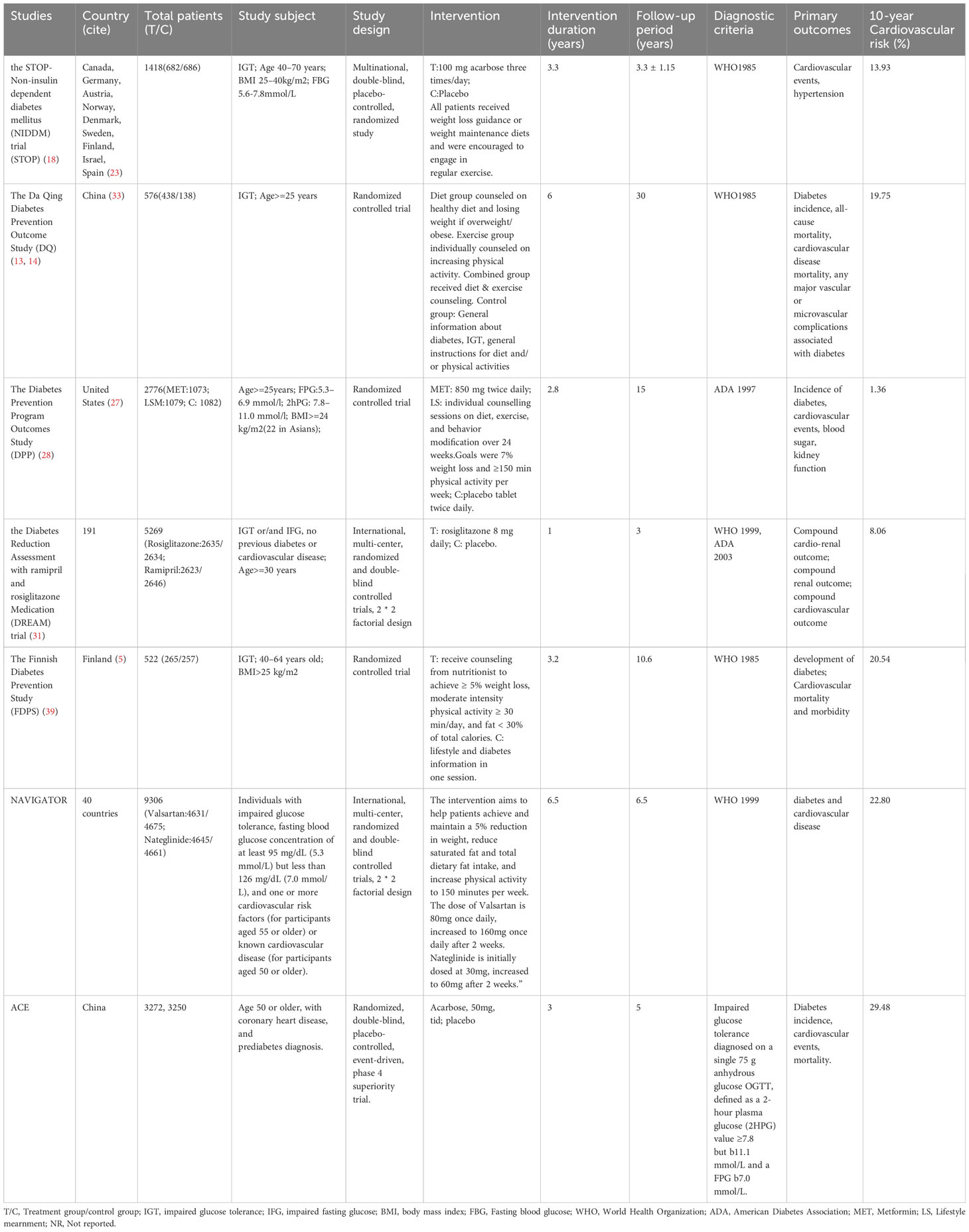
We conducted a statistical analysis of the 8 studies included, and the results indicated that, by analyzing the diabetes outcomes of prediabetic patients through early effective intervention, we initially included 7 studies. These studies encompassed, The Acarbose Cardiovascular Evaluation (ACE) (22, 23), the Da Qing Diabetes Prevention Outcome Study (DQ) (13–17), the Diabetes Prevention Program Outcomes Study (DPP) (28–30), the Diabetes Reduction Assessment with ramipril and rosiglitazone Medication (DREAM- Rosiglitazone) trial (31–34), Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR- Valsartan) (35–38), the STOP-Non-insulin dependent diabetes mellitus trial (STOP) (18–21), The Finnish Diabetes Prevention Study (FDPS) (39–42) (Table 1).
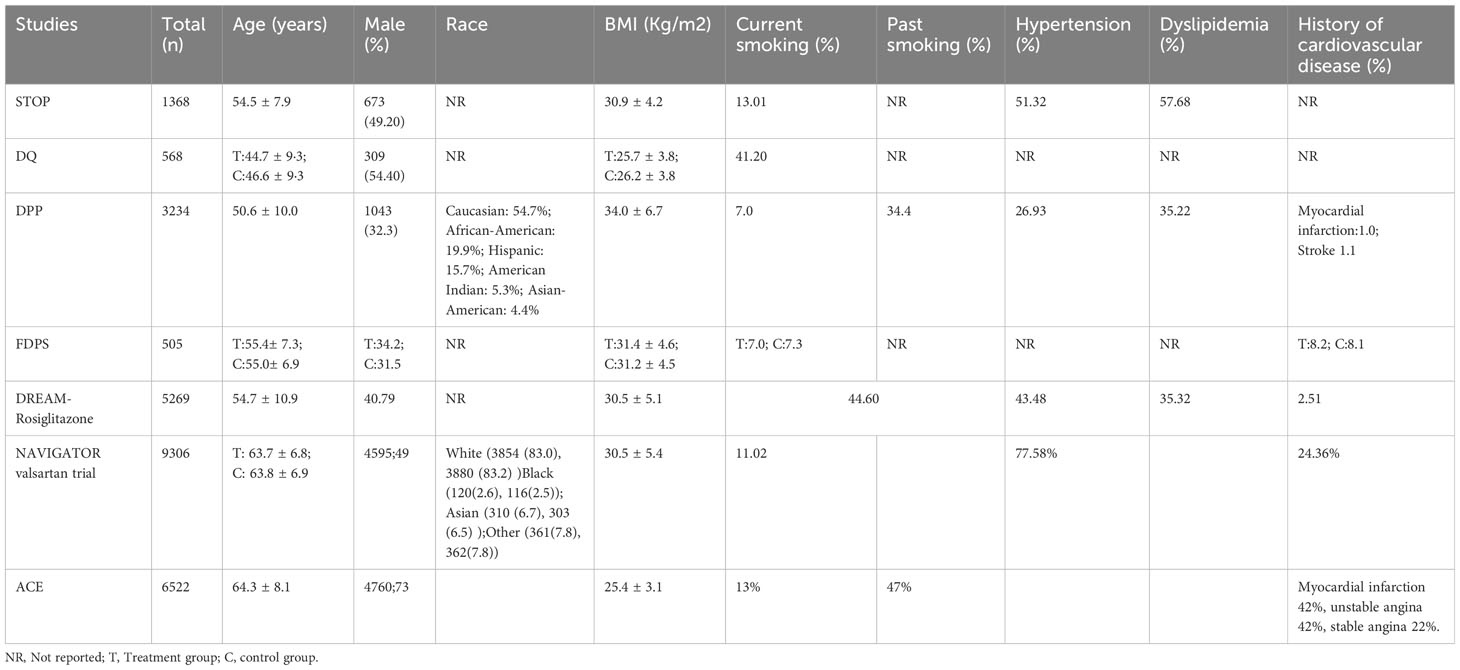
Included in the studies, the STOP study, DREAM study, NAVIGATOR study, and ACE study were randomized double-blind placebo-controlled studies, while the DQ study, DPP study, FDPS study were randomized controlled studies. Regarding the intervention measures in different studies, the DQ study and FDPS study solely involved lifestyle interventions. Studies involving drug interventions were all based on lifestyle interventions, including the STOP study (acarbose, 100mg, three times/day), DPP study (metformin, 850mg, twice/day), DREAM study (rosiglitazone 8 mg daily), NAVIGATOR study (dose of valsartan started at 80mg daily and increased to 160mg daily after 2 weeks), and ACE study (acarbose, 50mg, three times/day). We also summarized the baseline characteristics of the included studies, including age, gender ratio, ethnicity, BMI, smoking history, history of hypertension, history of lipid abnormalities, and history of cardiovascular disease (Table 2).
For the studies we included, the JADAD scores were 7 for the STOP, DREAM, NAVIGATOR, and ACE studies, and 5 for the DQ, DPP, and FDPS studies, all indicating high-quality research (Table 3).
Main outcome
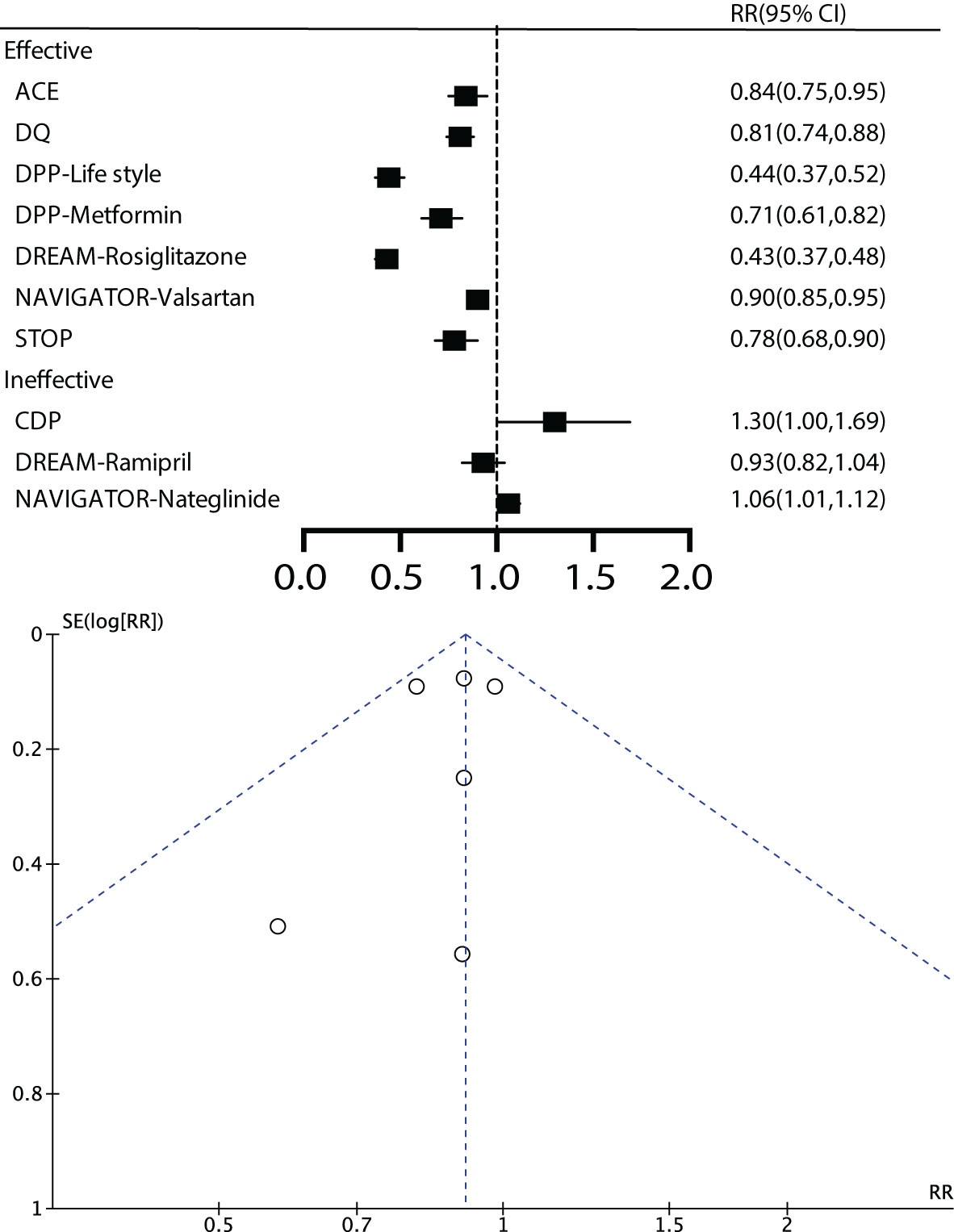
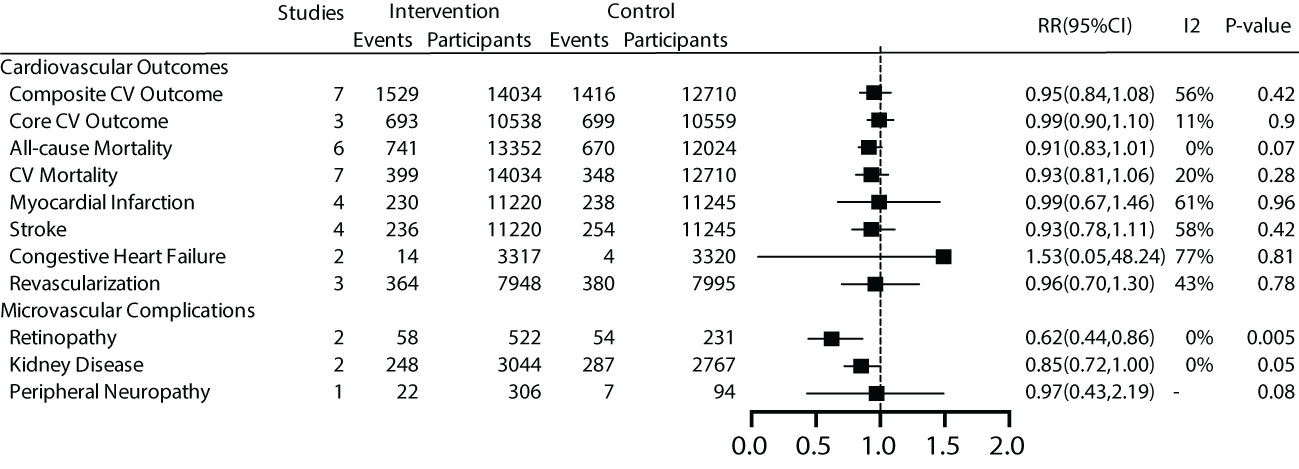
For the main outcome, namely all-cause mortality, a total of 6 studies were included, comprising 25,867 patients with a total follow-up duration of 172,505.4 person-years. The results indicated low heterogeneity among the various studies (I2 = 0%, P=0.40). Using a fixed-effect model for analysis, the results showed that effective intervention could reduce all-cause mortality by 9% (RR 0.91, 95% CI 0.83-1.01), but it was not statistically significant (P=0.07). The funnel plot exhibited a symmetrical shape, suggesting the absence of publication bias (Figure 3).
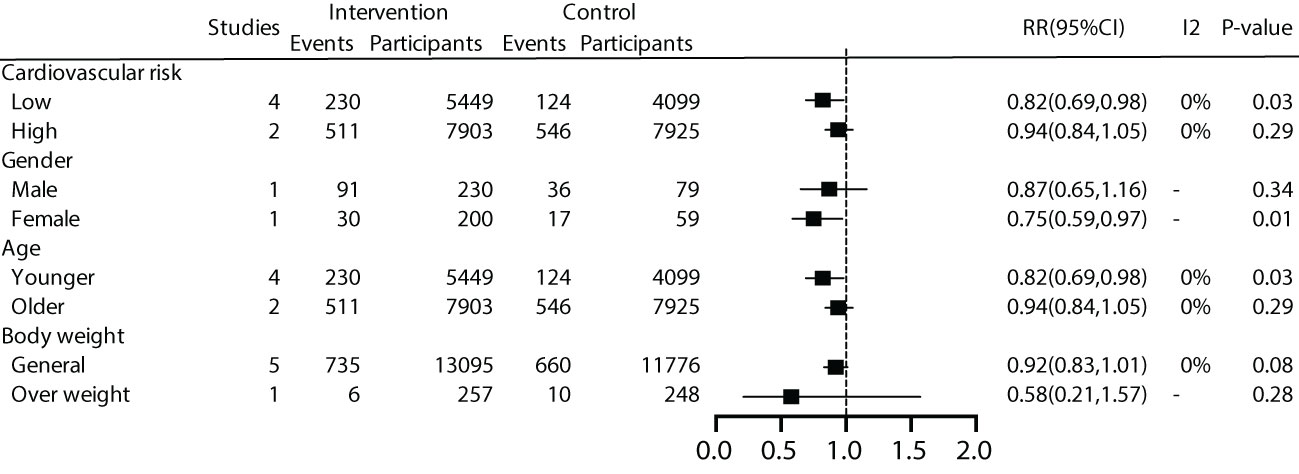
We further conducted subgroup analyses on patient characteristics, including a history of cardiovascular disease, gender, age, and BMI. The results revealed that early effective intervention significantly reduced all-cause mortality by 18% in prediabetic patients without a history of cardiovascular disease (RR=0.82, 95% CI=0.69~0.98, P=0.03, I2 = 0%), but not in patients with a history of cardiovascular disease (P=0.29). Moreover, women (P=0.01) and younger individuals (P=0.01) exhibited lower all-cause mortality rates. Subgroup analysis based on BMI showed no significant benefit in all-cause mortality for general patients (P=0.08) or overweight patients (P=0.28) (Figure 4).
For patients without a history of cardiovascular disease, we conducted a more in-depth analysis based on a 10-year cardiovascular risk. The results revealed that early intervention effectively reduced all-cause mortality by 21% for patients with a 10-year cardiovascular risk >10% (RR=0.79, 95% CI=0.66~0.95, P=0.01, I2 = 0%), while the effect was not significant for studies with a 10-year cardiovascular risk <10% (P=0.67). (Supplementary Appendix).
Secondary outcomes
The results indicated that early effective intervention did not significantly reduce composite cardiovascular events (RR=0.95, 95% CI=0.84~1.08, P=0.42, I2 = 56%), core cardiovascular events (RR=0.99, 95% CI=0.90~1.10, P=0.9, I2 = 11%), cardiovascular death (RR=0.93, 95% CI=0.81~1.06, P=0.28, I2 = 20%), occurrence of myocardial infarction events (RR=0.99, 95%, CI=0.67~1.46, P=0.96, I2 = 61%), stroke events (RR=0.93, 95% CI=0.78~1.11, P=0.42, I2 = 58%), congestive heart failure (RR=1.53, 95% CI=0.05~48.24, P=0.81, I2 = 77%), and revascularization (RR=0.96, 95% CI=0.70~1.30, P=0.78, I2 = 43%) (Figure 3).
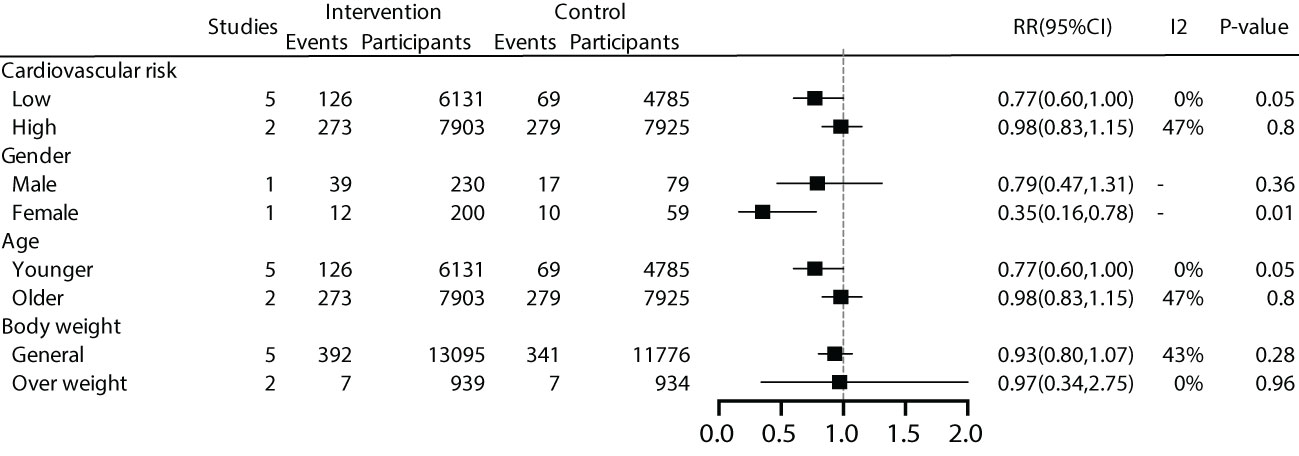
We conducted subgroup analyses based on patient characteristics, including a history of cardiovascular disease, gender, age, and BMI. The results showed that early effective intervention could significantly reduce cardiovascular mortality by 23% in patients without a history of cardiovascular disease (RR 0.77, 95% CI 0.60-1.00), although it was not statistically significant (P=0.05). Women exhibited lower cardiovascular mortality rates. A 23% reduction in cardiovascular mortality was also observed in younger individuals (RR 0.77, 95% CI 0.60-1.00), but it lacked statistical significance (P=0.05). No significant benefits were demonstrated for overweight and general populations (Figure 5).
For patients without a history of cardiovascular disease, we conducted a deeper analysis based on a 10-year cardiovascular risk. The results showed that effective intervention could reduce cardiovascular mortality by 26% for patients with a 10-year cardiovascular risk >10% (RR=0.74, 95% CI=0.57~0.98, P=0.03, I2 = 0%), while the effect was not significant for patients with a 10-year cardiovascular risk <10% (P=0.80) (Supplementary Appendix).
Regarding microvascular complications, the number of included studies was limited. For microvascular events, subgroup analysis was not conducted due to the limited number of studies. The overall results showed that early effective intervention could reduce retinopathy by 38% (RR=0.62, 95% CI=0.44~0.86, P=0.005, I2 = 0%), although there was a trend towards reducing kidney disease by 15%, it was not significant (P =0.05). Peripheral neuropathy did not demonstrate significant benefits (P =0.08) (Figure 3).
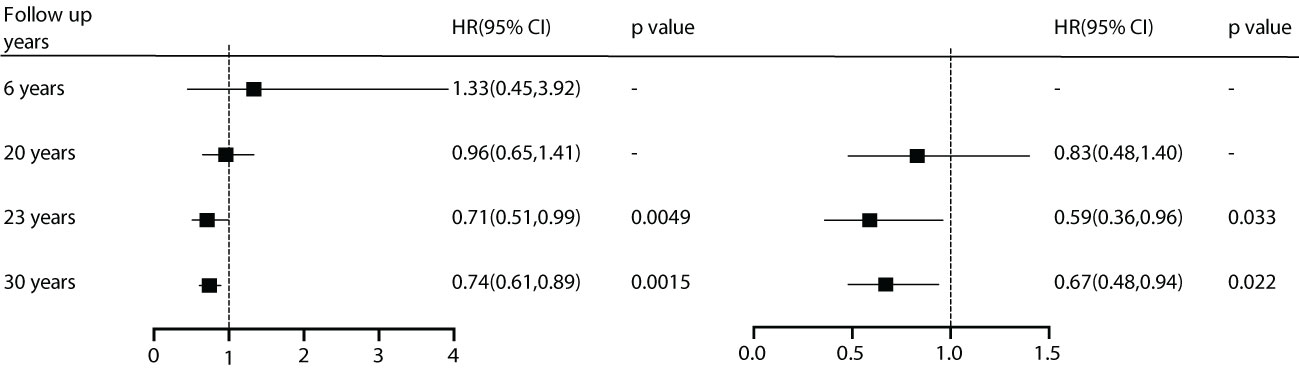
During the analysis of the included studies, we noted that the DQ study conducted data summarization and analysis at 6, 20, 23, and 30 years. Although diabetes incidence was reduced with effective intervention at all time points, microvascular events showed benefits early on. However, for cardiovascular events, there were no advantages observed in the first 20 years. Starting at 23 years, the benefits in all-cause mortality (HR) and cardiovascular mortality became significantly prominent. This suggests that besides patient characteristics, follow-up duration is also a crucial factor for effective intervention (Figure 6).
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to assess the benefits of effective intervention on microvascular and macrovascular events in prediabetic patients. Through standardized research methods, we found that, for patients without a history of cardiovascular disease, women, and younger individuals, there were lower all-cause mortality rates. In patients without a history of cardiovascular disease, for those with a 10-year cardiovascular risk >10%, there were significant reductions in all-cause mortality and cardiovascular mortality. Additionally, through longitudinal comparisons in the DQ study, we found that follow-up duration may be a critical factor in assessing effectiveness. In light of these findings, we provide evidence to guide clinical practitioners in long-term interventions for prediabetic patients.
Combining prior relevant research with our study results, it is evident that prediabetic patients require attention and early management. Some now consider prediabetes as a suboptimal health condition, and prediabetes and diabetes are seen as a continuum of elevated blood glucose and cardiovascular risk. This suggests that the principles applicable to the treatment of type 2 diabetes should also be applied to prediabetes (43, 44). Firstly, prediabetes shares the same vascular risk factors as diabetes, such as glucose abnormalities, hypertension, lipid abnormalities, obesity, and insulin resistance (44). These factors may also accelerate the development of atherosclerosis (45–47), and for prediabetic patients with comorbidities such as atrial fibrillation, there is also an increased likelihood of major cardiovascular and cerebrovascular events (48). Additionally, prediabetic patients may exhibit physiological and pathological abnormalities similar to insulin resistance (44, 49), which appears to be a significant factor in the development of cardiovascular diseases (50). For diabetic patients, the risk of cardiovascular events is 2-4 times higher than that of non-diabetic individuals (51, 52), and these risks are nearly equivalent to those of prediabetic patients (53–55).
Considering the current evidence on prediabetes management, lifestyle intervention forms the foundation. These studies collectively suggest that regardless of medication intervention, lifestyle intervention should be maintained in the long term for individuals with prediabetes. The ‘Expert Consensus on the Prevention of Type 2 Diabetes in Chinese Adults’ states that when intensive lifestyle intervention persists for more than 6 months and blood glucose control remains unsatisfactory, medication treatment may be considered for young individuals with good economic conditions, higher health needs, and access to medical services. The consensus identifies three classes of medications for prediabetes intervention: metformin, acarbose, and thiazolidinediones (TZDs). However, it emphasizes that lifestyle intervention primarily aids in weight loss, and obesity is a strong risk factor for cardiovascular diseases (56, 57), of course, this also includes type 2 diabetes (58). This is because weight loss can reduce or even reverse ectopic deposition, improve chronic inflammation, cardiovascular risk factors, and achieve the delay of disease progression (59). A 4-year follow-up observational longitudinal study indicated that weight loss surgery can significantly increase the remission rate of prediabetes (60).
The progression of prediabetes is reversible, and effective intervention is crucial for the prognosis of individuals with prediabetes (61). Combining diet and physical exercise is more effective in reducing diabetes development than any single strategy, as calorie intake and physical activity are independently associated with reducing diabetes risk, and their combination may produce an additive effect (62–64). The DQ study indicated that the reduction in diabetes incidence in the intervention group was mainly due to changes in dietary structure, increased physical activity, or improved physical fitness (13). Recent cost-effectiveness analyses have shown that lifestyle intervention is the most cost-effective approach (65). The DPP study demonstrated that intensive lifestyle intervention could reduce cardiovascular risk factors, including high blood pressure, high triglyceride levels, and low high-density lipoprotein levels (66). The effectiveness of lifestyle intervention diminishes over time, indicating the need for long-term adherence to realize significant benefits (62) However, the DQ study showed that the advantages of lifestyle intervention in reducing diabetes incidence persisted for 30 years after intensive lifestyle intervention. The DQ study provides three potential explanations for the long-term benefits of lifestyle intervention. Firstly, lifestyle intervention may lead to sustained changes in normal behavior beyond the study period. Secondly, these interventions may lead to changes in preventive care and health promotion efforts provided by community clinics, with effects observed beyond the study period. Finally, lifestyle intervention may result in a type of metabolic memory, where motivation for long-term lifestyle changes is crucial for improving intervention compliance (67). In this regard, Penn et al. suggested that an expectation of one year or longer is a necessary condition for establishing long-term behavioral changes (68). For high-risk populations with prediabetes, especially those with additional cardiovascular risk factors, medication treatment is recommended (6). Medication intervention can more rapidly reduce and stabilize risk factors in prediabetic patients. The DPP study showed that metformin did not have a treatment advantage over lifestyle intervention (29), but metformin’s effectiveness in reducing the conversion rate of prediabetes still exists (69). Changes in lifestyle may be the most effective, and the addition of pharmacological drugs does not necessarily increase the benefits. However, the STOP study also showed that early intervention with acarbose could significantly reduce cardiovascular outcomes. This could be related to the multiple benefits, including weight reduction, decreased BMI, waist circumference, blood pressure, postprandial 2-hour blood glucose, and triglyceride levels associated with acarbose. Similarly, the ACE study, which also used acarbose, did not demonstrate significant benefits, possibly due to the lower acarbose dosage and the study population’s high prevalence of coronary heart disease (22). Population-specific factors and gastrointestinal side effects should also be considered in practical application. Research indicates that acarbose appears to be more effective in preventing and reversing prediabetes in Eastern populations compared to Western populations (70). Of course, there are currently numerous clinical studies underway, including the CINEMA study which focuses on comprehensive, patient-centered, team-based interventions (71). These efforts contribute to further enriching comprehensive management strategies for prediabetes.
Our study demonstrates that long-term benefits of early effective intervention are more significant for patients without a history of coronary heart disease. This could be attributed to the fact that patients with a history of cardiovascular disease receive more combined treatments, which may not allow the advantages of intervention to be well highlighted. In the ACE study, for instance, 93% of the patients took statins, 98% took antiplatelet drugs, and 66% used beta-blockers. In the NAVIGATOR study, 39.39% of the patients used beta-blockers (which increased to 41.26% by the end of follow-up), 73.24% used antihypertensive drugs (76.35% by the end of follow-up), 38.44% used lipid-lowering medications (50.06% by the end of follow-up), and 36.80% used antiplatelet drugs (45.49% by the end of follow-up). This is significantly higher compared to studies involving individuals without a history of cardiovascular disease, such as the DREAM study, where 12.94% of the patients took statins, 14.31% took antiplatelet drugs, and 17.31% used beta-blockers. Another possible reason could be the insufficient dosage of intervention medications. The ACE study may reflect lower acarbose dosage (50 mg vs. 100 mg three times a day) compared to the STOP-NIDDM study. In the NAVIGATOR study, the most convincing evidence for improved cardiovascular outcomes with higher dosage was seen with valsartan, where patients received twice the daily dose.
Apart from lifestyle modifications, medications, and surgical interventions, it is crucial for clinical physicians to develop more rational, precise, and effective comprehensive management strategies for prediabetic patients, especially if high-risk populations, including those at risk for diabetes and cardiovascular diseases, can be accurately identified. Of course, we can refer to currently recognized relevant risk factors, including age, body mass index, family history of diabetes, history of hypertension, and physical activity level. However, more precise prediction models or scoring systems can better quantify the risks associated with prediabetic patients. For example, a recent study in China, based on data from 184,188 prediabetic patients, developed and validated personalized prediabetes prediction charts for Chinese adults, aiding in the identification of high-risk populations (72). Nevertheless, overall, there is currently no widely recommended optimal model available. Therefore, future research should focus on improving the clinical relevance and predictive performance of existing models (73, 74).
In our study, due to data limitations, there was relatively less data available for gender, age, and weight. Gender data were only available from the DQ study, and although age was categorized with a cutoff at 50 years, the NAVIGATOR and ACE studies included patients aged 50 and above. However, for the STOP study, DPP, FDPS, and DREAM, as well as NAVIGATOR and ACE, there were significant age differences. Nevertheless, we did not observe additional benefits in older individuals. It is possible that both studies included patients with a history of cardiovascular disease. Regarding gender, subgroup analysis was conducted only in the DQ study, revealing that although a reduction in mortality was primarily seen in female patients, early effective intervention did not significantly benefit males. This could be attributed to a higher number of male smokers, which may have attenuated the benefits of early intervention. Subgroup analysis based on weight revealed that for overweight patients, cardiovascular benefits were not significant. Further analysis based on an average BMI greater than 30 showed that even after excluding the DQ and ACE studies, all-cause mortality remained non-significant. Follow-up time is a key factor to consider for assessing long-term benefits. The DQ study indicated that differences in cardiovascular disease mortality between the intervention and control groups began to appear 12 years after the start of the study and slowly increased to 17% by the 20-year follow-up, reaching statistical significance only after 23 years (15). The WCK study also suggested that at the end of 10 years of treatment, there was no significant impact on mortality (75), but significant differences started to emerge when the follow-up period exceeded 22 years (76). Although the FDPS study did not show a reduced risk of cardiovascular disease over the 10-year follow-up, the incidence of type 2 diabetes in the intervention group remained significantly lower than the control group (40). This was due to a lower complication rate in the intervention group, primarily occurring over a longer time after randomization, possibly indicating a delayed onset of diabetes. This also suggests that follow-up time is a crucial factor for assessing the long-term benefits of intervention.
Our study, based on the synthesis of current high-quality research, indicates that effective intervention, especially lifestyle intervention, significantly benefits individuals with prediabetes, particularly those without a history of coronary heart disease. Subgroup analysis of the data suggests that individuals at high cardiovascular risk for 10 years, as well as women, exhibit more pronounced benefits. At present, the evidence does not clearly define age or BMI as factors influencing the effectiveness of long-term intervention. Lastly, considering the DQ study, follow-up time appears to be a crucial factor for evaluating long-term benefits of intervention. Therefore, patients should adhere to interventions in the long term, and lifestyle intervention is the most cost-effective and easily accepted approach. Given the current data limitations, detailed stratification of patients based on baseline levels such as age, weight, family history, blood pressure, and lipid profiles, which are risk factors for both macrovascular and microvascular complications, cannot be performed. Follow-up time is a critical factor, but the studies included in the analysis had varying follow-up durations, ranging from 3 to 30 years. Consequently, achieving balanced data among the groups based on current evidence may pose some challenges and potential biases. Therefore, we hope that further research will continue to address the disparity in follow-up times to obtain more reliable evidence.
It is estimated that by 2025, the global population defined as having IFG and/or IGT as prediabetes will reach 472 million (77). Individuals with prediabetes should be aware of their increased risk of future vascular complications (78). However, the vast majority of prediabetic individuals may not even be aware of their risk of developing diabetes (79). Based on the current high-quality evidence, our results indicate that early effective intervention can be used as an effective primary prevention strategy for diabetes, reducing all-cause mortality. This benefit is particularly notable in individuals without a history of cardiovascular disease, those at high cardiovascular risk for 10 years, and women. This provides a basis for clinical guidance in the intervention of individuals with prediabetes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XA: Data curation, Formal analysis, Writing – original draft. YZ: Data curation, Formal analysis, Writing – review & editing. WS: Methodology, Writing – review & editing. XK: Data curation, Methodology, Writing – review & editing. HJ: Investigation, Methodology, Writing – review & editing. YS: Data curation, Methodology, Writing – review & editing. LJ: Data curation, Writing – review & editing. XZ: Data curation, Writing – review & editing. QG: Data curation, Writing – review & editing. FL: Conceptualization, Writing – review & editing. XT: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This is work was supported by the National Natural Science Foundation of China (82305205 to XA).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1294819/full#supplementary-material
References
1. International diabetes federation IDA. 8th ed. Brussels, Belgium: International Diabetes Federation (2017).
2. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol. (2011) 8:228–36. doi: 10.1038/nrendo.2011.183
3. Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. (2014) 2:56–64. doi: 10.1016/S2213-8587(13)70112-8
4. Ferrannini E. Definition of intervention points in prediabetes. Lancet Diabetes Endocrinol. (2014) 2:667–75. doi: 10.1016/S2213-8587(13)70175-X
5. Lim SC, Tai ES, Tan BY, Chew SK, Tan CE. Cardiovascular risk profile in individuals with borderline glycemia: the effect of the 1997 American Diabetes Association diagnostic criteria and the 1998 World Health Organization Provisional Report. Diabetes Care. (2000) 23:278–82. doi: 10.2337/diacare.23.3.278
6. Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. Bmj. (2016) 355:i5953. doi: 10.1136/bmj.i5953
7. Huang Y, Cai X, Qiu M, Chen P, Tang H, Hu Y, et al. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia. (2014) 57:2261–9. doi: 10.1007/s00125-014-3361-2
8. Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. (2007) 30:753–9. doi: 10.2337/dc07-9920
9. Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK. Global diabetes prevention interventions: A systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care. (2018) 41:1526–34. doi: 10.2337/dc17-2222
10. Norris SL, Zhang X, Avenell A, Gregg E, Brown TJ, Schmid CH, et al. Long-term non-pharmacological weight loss interventions for adults with prediabetes. Cochrane Database systemat Rev. (2005) 2005:CD005270. doi: 10.1002/14651858
11. Del Prato S, Bianchi C, Miccoli R, Penno G. Pharmacological intervention in prediabetes: considering the risks and benefits. Diabetes Obes Metab. (2007) 9 Suppl 1:17–22. doi: 10.1111/j.1463-1326.2007.00766.x
12. Mangan A, Docherty NG, Le Roux CW, Al-Najim W. Current and emerging pharmacotherapy for prediabetes: are we moving forward? Expert Opin Pharmacother. (2018) 19:1663–73. doi: 10.1080/14656566.2018.1517155
13. Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. (2019) 7:452–61. doi: 10.1016/S2213-8587(19)30093-2
14. Gong Q, Gregg EW, Wang J, An Y, Zhang P, Yang W, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia. (2011) 54:300–7. doi: 10.1007/s00125-010-1948-9
15. Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. (2014) 2:474–80. doi: 10.1016/S2213-8587(14)70057-9
16. Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. (2008) 371:1783–9. doi: 10.1016/S0140-6736(08)60766-7
17. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. (1997) 20:537–44. doi: 10.2337/diacare.20.4.537
18. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. Jama. (2003) 290:486–94. doi: 10.1001/jama.290.4.486
19. Zeymer U, Schwarzmaier-D’assie A, Petzinna D, Chiasson JL. Effect of acarbose treatment on the risk of silent myocardial infarctions in patients with impaired glucose tolerance: results of the randomised STOP-NIDDM trial electrocardiography substudy. Eur J Cardiovasc Prev Rehabil. (2004) 11:412–5. doi: 10.1097/00149831-200410000-00009
20. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. (2002) 359:2072–7. doi: 10.1016/S0140-6736(02)08905-5
21. Chiasson JL, Gomis R, Hanefeld M, Josse RG, Karasik A, Laakso M. The STOP-NIDDM Trial: an international study on the efficacy of an alpha-glucosidase inhibitor to prevent type 2 diabetes in a population with impaired glucose tolerance: rationale, design, and preliminary screening data. Study to Prevent Non-Insulin-Dependent Diabetes Mellitus. Diabetes Care. (1998) 21:1720–5. doi: 10.2337/diacare.21.10.1720
22. Holman RR, Coleman RL, Chan JCN, Chiasson JL, Feng H, Ge J, et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. (2017) 5:877–86. doi: 10.1016/S2213-8587(17)30309-1
23. Holman RR, Bethel MA, Chan JC, Chiasson JL, Doran Z, Ge J, et al. Rationale for and design of the Acarbose Cardiovascular Evaluation (ACE) trial. Am Heart J. (2014) 168:23–9.e2. doi: 10.1016/j.ahj.2014.03.021
24. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. Jama. (2015) 313:1657–65. doi: 10.1001/jama.2015.3656
25. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
26. Godlee F. The cochrane collaboration. BMJ (Clinical Res ed). (1994) 309:969–70. doi: 10.1136/bmj.309.6960.969
27. Furlan AD, Pennick V, Bombardier C, van Tulder M. 2019 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine. (2009) 34:1929–41. doi: 10.1097/BRS.0b013e3181b1c99f
28. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. (2015) 3:866–75. doi: 10.1016/S2213-8587(15)00291-0
29. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. (2002) 346:393–403. doi: 10.1056/NEJMoa012512
30. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. (2009) 374:1677–86. doi: 10.1016/S0140-6736(09)61457-4
31. Dagenais GR, Gerstein HC, Holman R, Budaj A, Escalante A, Hedner T, et al. Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care. (2008) 31:1007–14. doi: 10.2337/dc07-1868
32. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. (2006) 368:1096–105. doi: 10.1016/S0140-6736(06)69420-8
33. Gerstein HC, Yusuf S, Holman R, Bosch J, Pogue J. Rationale, design and recruitment characteristics of a large, simple international trial of diabetes prevention: the DREAM trial. Diabetologia. (2004) 47:1519–27. doi: 10.1007/s00125-004-1485-5
34. Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. (2006) 355:1551–62. doi: 10.1056/NEJMoa065061
35. Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. (2010) 362:1463–76. doi: 10.1056/NEJMoa1001122
36. Currie G, Bethel MA, Holzhauer B, Haffner SM, Holman RR, McMurray JJV. Effect of valsartan on kidney outcomes in people with impaired glucose tolerance. Diabetes Obes Metab. (2017) 19:791–9. doi: 10.1111/dom.12877
37. McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. (2010) 362:1477–90. doi: 10.1056/NEJMoa1001121
38. Califf RM, Boolell M, Haffner SM, Bethel M, McMurray J, Duggal A, et al. Prevention of diabetes and cardiovascular disease in patients with impaired glucose tolerance: rationale and design of the Nateglinide And Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) Trial. Am Heart J. (2008) 156:623–32. doi: 10.1016/j.ahj.2008.05.017
39. Aro A, Kauppinen A, Kivinen N, Selander T, Kinnunen K, Tuomilehto J, et al. Life style intervention improves retinopathy status-the finnish diabetes prevention study. Nutrients. (2019) 11:1691. doi: 10.3390/nu11071691
40. Uusitupa M, Peltonen M, Lindström J, Aunola S, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, et al. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study–secondary analysis of the randomized trial. PloS One. (2009) 4:e5656. doi: 10.1371/journal.pone.0005656
41. Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. (2001) 344:1343–50. doi: 10.1056/NEJM200105033441801
42. Eriksson J, Lindström J, Valle T, Aunola S, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of Type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia. (1999) 42:793–801. doi: 10.1007/s001250051229
43. Faerch K, Borch-Johnsen K, Holst JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia. (2009) 52:1714–23. doi: 10.1007/s00125-009-1443-3
44. DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol. (2011) 108:3b–24b. doi: 10.1016/j.amjcard.2011.03.013
45. Howard BV, Robbins DC, Sievers ML, Lee ET, Rhoades D, Devereux RB, et al. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: The Strong Heart Study. Arterioscler Thromb Vasc Biol. (2000) 20:830–5. doi: 10.1161/01.ATV.20.3.830
46. Hsu JC, Yang YY, Chuang SL, Lee JK, Lin LY. Prediabetes increases the risk of major limb and cardiovascular events. Cardiovasc Diabetol. (2023) 22:348. doi: 10.1186/s12933-023-02085-y
47. Gadde KM, Yin X, Goldberg RB, Orchard TJ, Schlögl M, Dabelea D, et al. Coronary artery calcium and cognitive decline in the diabetes prevention program outcomes study. J Am Heart Assoc. (2023) 12:e029671. doi: 10.1161/JAHA.123.029671
48. Desai R, Katukuri N, Goguri SR, Kothawala A, Alle NR, Bellamkonda MK, et al. Prediabetes: An overlooked risk factor for major adverse cardiac and cerebrovascular events in atrial fibrillation patients. World J Diabetes. (2024) 15:24–33. doi: 10.4239/wjd.v15.i1.24
49. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. (2010) 53:1270–87. doi: 10.1007/s00125-010-1684-1
50. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. (2001) 414:799–806. doi: 10.1038/414799a
51. Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. (2007) 115:114–26. doi: 10.1161/CIRCULATIONAHA.106.179294
52. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. (1998) 339:229–34. doi: 10.1056/NEJM199807233390404
53. Bansal N. Prediabetes diagnosis and treatment: A review. World J Diabetes. (2015) 6:296–303. doi: 10.4239/wjd.v6.i2.296
54. Maschirow L, Khalaf K, Al-Aubaidy HA, Jelinek HF. Inflammation, coagulation, endothelial dysfunction and oxidative stress in prediabetes–Biomarkers as a possible tool for early disease detection for rural screening. Clin Biochem. (2015) 48:581–5. doi: 10.1016/j.clinbiochem.2015.02.015
55. Bahar A, Makhlough A, Yousefi A, Kashi Z, Abediankenari S. Correlation between prediabetes conditions and microalbuminuria. Nephrourol Mon. (2013) 5:741–4. doi: 10.5812/numonthly
56. Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. (2010) 89:309–19. doi: 10.1016/j.diabres.2010.04.012
57. Cloostermans L, Wendel-Vos W, Doornbos G, Howard B, Craig CL, Kivimaki M, et al. Independent and combined effects of physical activity and body mass index on the development of Type 2 Diabetes - a meta-analysis of 9 prospective cohort studies. Int J Behav Nutr Phys Act. (2015) 12:147. doi: 10.1186/s12966-015-0304-3
58. Li YY, Tong LP, Wu XD, Lin D, Lin Y, Lin XY. Analysis of influencing factors and interaction of body weight and disease outcome in patients with prediabetes. World J Diabetes. (2023) 14:1551–61. doi: 10.4239/wjd.v14.i10.1551
59. Churuangsuk C, Hall J, Reynolds A, Griffin SJ, Combet E, Lean MEJ. Diets for weight management in adults with type 2 diabetes: an umbrella review of published meta-analyses and systematic review of trials of diets for diabetes remission. Diabetologia. (2022) 65:14–36. doi: 10.1007/s00125-021-05577-2
60. Borges-Canha M, Neves JS, Silva MM, Mendonça F, Moreno T, Ribeiro S, et al. Prediabetes remission after bariatric surgery: a 4-years follow-up study. BMC Endocr Disord. (2024) 24:7. doi: 10.1530/endoabs.90.EP286
61. Farag HFM, Elrewany E, Abdel-Aziz BF, Sultan EA. Prevalence and predictors of undiagnosed type 2 diabetes and pre-diabetes among adult Egyptians: a community-based survey. BMC Public Health. (2023) 23:949. doi: 10.1186/s12889-023-15819-0
62. Haw JS, Galaviz KI, Straus AN, Kowalski AJ, Magee MJ, Weber MB, et al. Long-term sustainability of diabetes prevention approaches: A systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. (2017) 177:1808–17. doi: 10.1001/jamainternmed.2017.6040
63. Harris E. Lifestyle changes lowered blood glucose in people with prediabetes. Jama. (2023) 330:2241. doi: 10.1001/jama.2023.23506
64. Meripour M, Mohamadian H, Khafaie MA. Effect of Lifestyle Promotion in the PRECEDE-PROCEED model among pre-diabetic adults based on PERSIAN cohort study: a randomized controlled trial study. J Diabetes Metab Disord. (2023) 22:1499–509. doi: 10.1007/s40200-023-01273-7
65. Herman WH, Edelstein SL, Ratner RE, Montez MG, Ackermann RT, Orchard TJ, et al. Effectiveness and cost-effectiveness of diabetes prevention among adherent participants. Am J Manag Care. (2013) 19:194–202.
66. Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, et al. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. (2005) 28:888–94. doi: 10.2337/diacare.28.4.888
67. Gillett M, Royle P, Snaith A, Scotland G, Poobalan A, Imamura M, et al. Non-pharmacological interventions to reduce the risk of diabetes in people with impaired glucose regulation: a systematic review and economic evaluation. Health Technol Assess. (2012) 16:1–236, iii-iv. doi: 10.3310/hta16330
68. Penn L, Dombrowski SU, Sniehotta FF, White M. Participants’ perspectives on making and maintaining behavioural changes in a lifestyle intervention for type 2 diabetes prevention: a qualitative study using the theory domain framework. BMJ Open. (2013) 3:e002949. doi: 10.1136/bmjopen-2013-002949
69. Lily M, Godwin M. Treating prediabetes with metformin: systematic review and meta-analysis. Can Fam Physician. (2009) 55:363–9.
70. Hu R, Li Y, Lv Q, Wu T, Tong N. Acarbose monotherapy and type 2 diabetes prevention in eastern and western prediabetes: an ethnicity-specific meta-analysis. Clin Ther. (2015) 37:1798–812. doi: 10.1016/j.clinthera.2015.05.504
71. Neeland IJ, Arafah A, Bourges-Sevenier B, Dazard JE, Albar Z, Landskroner Z, et al. Second-year results from CINEMA: A novel, patient-centered, team-based intervention for patients with Type 2 diabetes or prediabetes at high cardiovascular risk. Am J Prev Cardiol. (2024) 17:100630. doi: 10.1016/j.ajpc.2023.100630
72. Hu Y, Han Y, Liu Y, Cui Y, Ni Z, Wei L, et al. A nomogram model for predicting 5-year risk of prediabetes in Chinese adults. Sci Rep. (2023) 13:22523. doi: 10.1038/s41598-023-50122-3
73. Liu Y, Yu S, Feng W, Mo H, Hua Y, Zhang M, et al. A meta-analysis of diabetes risk prediction models applied to prediabetes screening. Diabetes Obes Metab. (2024). doi: 10.1111/dom.15457
74. Zhang J, Zhang Z, Zhang K, Ge X, Sun R, Zhai X. Early detection of type 2 diabetes risk: limitations of current diagnostic criteria. Front Endocrinol (Lausanne). (2023) 14:1260623. doi: 10.3389/fendo.2023.1260623
75. Sartor G, Schersten B, Carlstrom S, Melander A, Norden A, Persson G. Ten-year follow-up of subjects with impaired glucose tolerance: prevention of diabetes by tolbutamide and diet regulation. Diabetes. (1980) 29:41–9. doi: 10.2337/diabetes.29.1.41
76. Knowler WC, Sartor G, Melander A, Schersten B. Glucose tolerance and mortality, including a substudy of tolbutamide treatment. Diabetologia. (1997) 40:680–6. doi: 10.1007/s001250050734
77. Magalhaes ME, Cavalcanti BA, Cavalcanti S. Could pre-diabetes be considered a clinical condition? opinions from an endocrinologist and a cardiologist. Diabetol Metab Syndr. (2010) 2:2. doi: 10.1186/1758-5996-2-2
78. Lee M, Saver JL, Hong KS, Song S, Chang KH, Ovbiagele B. Effect of pre-diabetes on future risk of stroke: meta-analysis. Bmj. (2012) 344:e3564. doi: 10.1136/bmj.e3564
Keywords: prediabetes, early intervention, cardiovascular disease, meta-analysis, lifestyle
Citation: An X, Zhang Y, Sun W, Kang X, Ji H, Sun Y, Jiang L, Zhao X, Gao Q, Lian F and Tong X (2024) Early effective intervention can significantly reduce all-cause mortality in prediabetic patients: a systematic review and meta-analysis based on high-quality clinical studies. Front. Endocrinol. 15:1294819. doi: 10.3389/fendo.2024.1294819
Received: 15 September 2023; Accepted: 19 February 2024;
Published: 01 March 2024.
Edited by:
Christian Göbl, Medical University of Vienna, AustriaReviewed by:
Godfrey Mutashambara Rwegerera, University of Botswana, BotswanaSudhanshu Kumar Bharti, Patna University, India
Copyright © 2024 An, Zhang, Sun, Kang, Ji, Sun, Jiang, Zhao, Gao, Lian and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolin Tong, dG9uZ3hpYW9saW5AdmlwLjE2My5jb20=; Fengmei Lian, TGZtNTY1QHNvaHUuY29t
†These authors have contributed equally to this work and share first authorship
 Xuedong An
Xuedong An Yuehong Zhang
Yuehong Zhang Wenjie Sun
Wenjie Sun Xiaomin Kang
Xiaomin Kang Hangyu Ji
Hangyu Ji Yuting Sun
Yuting Sun Linlin Jiang
Linlin Jiang Xuefei Zhao
Xuefei Zhao Qing Gao1
Qing Gao1 Fengmei Lian
Fengmei Lian Xiaolin Tong
Xiaolin Tong