
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 08 March 2024
Sec. Reproduction
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1274376
 Ahmad Mohd Faizal1
Ahmad Mohd Faizal1 Marjanu Hikmah Elias2
Marjanu Hikmah Elias2 Norazilah Mat Jin3
Norazilah Mat Jin3 Muhammad Azrai Abu1
Muhammad Azrai Abu1 Saiful Effendi Syafruddin4
Saiful Effendi Syafruddin4 Ani Amelia Zainuddin1
Ani Amelia Zainuddin1 Nao Suzuki5
Nao Suzuki5 Abdul Kadir Abdul Karim1*
Abdul Kadir Abdul Karim1*The leading indicator for successful outcomes in in-vitro fertilization (IVF) is the quality of gametes in oocytes and sperm. Thus, advanced research aims to highlight the parameter in assessing these qualities – DNA fragmentation in sperm and oocyte development capacity (ODC) via evaluation of microenvironments involving its maturation process. Regarding oocytes, most evidence reveals the role of cumulus cells as non-invasive methods in assessing their development competency, mainly via gene expression evaluation. Our review aims to consolidate the evidence of GDF-9 derivatives, the HAS2, GREM1, and PTGS2 gene expression in cumulus cells used as ODC markers in relevant publications and tailored to current IVF outcomes. In addition to that, we also added the bioinformatic analysis in our review to strengthen the evidence aiming for a better understanding of the pathways and cluster of the genes of interest - HAS2, GREM1, and PTGS2 in cumulus cell level. Otherwise, the current non-invasive method can be used in exploring various causes of infertility that may affect these gene expressions at the cumulus cell level. Nevertheless, this method can also be used in assessing the ODC in various cohorts of women or as an improvement of markers following targeted tools or procedures by evaluating the advancement of these gene expressions following the targeted intervention.
The success of in-vitro fertilisation (IVF) depends mainly on the quality of gametes, specifically the DNA quality of sperm and the overall quality of the oocytes (1). Most research focuses on enhancing oocyte quality to improve IVF outcomes. Clinically, various control ovarian stimulation protocols are used, targeting different gonadotrophin receptors, and timely oocyte collection is performed, with or without intracytoplasmic sperm injection, aiming for optimum fertilisation and high success rates per cycle (2). However, the developmental potential of oocytes following ovarian stimulation varies amongst women, leading to inconclusive overall IVF outcomes. In addition to research on oocyte quality, the culture media and IVF laboratory protocols evolved. Efforts have been exerted to improve media substances with targeted concentration to minimise stress in cultured embryos and promote better progression, leading to the production of good-quality embryos (3, 4). Despite these strategies, the current worldwide IVF success rate remains at approximately 35%–45% (5–7). To address this issue, researchers are exploring the development of oocyte quality. Various factors have been established to contribute to a better quality of the oocytes – mainly the level of hormones, such as growth hormone (GH) with insulin-like growth factor-1 (IGF-1). A recent study reported that higher follicular fluid levels of GH and IGF1 appear to be associated with better oocyte competency. Otherwise, the same study also reported no association of levels of TSH, fT3, fT4, 25OHD, or antithyroid antibodies in follicular fluid for oocyte quality (8). These hormonal levels can be altered due to dietary habits and the women’s body mass index (BMI). In obese women, ovarian inflammation leads to the production of pro-inflammatory cytokines and activation of cell death mechanism, resulting in ovarian tissue damage, microenvironment alteration via the releasing of oxidation stress, and alteration of microbiome metabolism. Not surprisingly, all these factors negatively impact both meiotic and cytoplasmic oocyte maturation – leading to poor oocyte quality (9). In addition to that, hyperinsulinemia also altered the endogenous pathway of steroidogenesis, which may have contributed to the imbalance of the intrinsic pathway in folliculogenesis, leading to suboptimal oocyte development, thus affecting its quality (10).
At the molecular level, the study of oocyte competency aims to predict embryo outcomes more effectively through the use of various biomarkers and signalling pathways (11). A promising non-invasive method involves analysing the cumulus cells (CC), because they can reflect the competency of developing oocytes. Apart from offering mechanical protection, CC plays a crucial role in providing paracrine signalling, metabolism pathways and overall gene regulation that promote oocyte development and maturation competency (12, 13). The CC differentiation, proliferation and expansion will lead to a better oocytes’ developmental regulation – the signaling pathway influence the oocytes quality (Figure 1). Therefore, promising evidence utilise cumulus-expressed genes as markers for assessing oocyte development competency, because they reflect the fundamental processes in the molecular environment (12–15). To date, several genes have been identified to coordinate the maturation of granulosa cells, leading to specific signalling pathways that contribute to CC expansion and promote oocyte development and maturation, primarily through growth differentiation factor 9 (GDF9) (16, 17). As established, GDF-9 is an oocyte-derived growth factor in the transforming growth factor β (TGF-β) superfamily. It plays a critical role in coordinating the expression of several genes that potentially contribute to oocyte and subsequent embryo quality (18, 19). These genes include Hyaluronan synthase 2 (HAS2), Prostaglandin-endoperoxide synthase 2 (PTGS2), Gremlin 1 (GREM1) and steroidogenic acute regulator protein (STAR) (20, 21). Distinctively, the available evidence strongly supports HAS2 and GREM1 as potential markers for CC in predicting oocyte competence compared with other genes. Additionally, oocyte nuclear maturation correlates with PTGS2, STAR, amphiregulin (AREG) and stearoyl-co-enzyme A desaturase 1 and 5 (SCD1 and SCD5), leading to a good CC expansion and development (22, 23). However, conflicting evidence reports that CC increases the expression of Pentraxin-related protein - PTX3, also known as TNF-inducible gene 14 protein (TSG-14), which is ultimately essential to correlate with oocyte maturation and influence its quality (24, 25). Regarding this matter, various genes and pathways are responsible for CC expansion, supporting oocyte development in animal and human models. However, the most established evidence suggests that specific GDF9 target genes, particularly HAS2, GREM1 and PTGS2, are closely correlated with the microenvironment in human oocytes (11, 12, 16, 23).
Understanding the molecular mechanisms underlying the microenvironment of oocyte development competency is crucial to address the variability in IVF success rates amongst women. Extensive efforts have been made to identify key genes and signaling pathways involved in CC expansion and maturation. Notably, HAS2, a critical enzyme responsible for hyaluronan synthesis, has been associated with increased cumulus expansion, indicating its potential as a biomarker for oocyte quality (26). Similarly, GREM1, an antagonist of bone morphogenetic proteins, plays a vital role in modulating the transforming growth factor-beta (TGF-β) pathway, thereby influencing CC function and oocyte developmental competence (12, 27). Additionally, PTGS2, an enzyme involved in prostaglandin synthesis, has been implicated in regulating ovulation, CC expansion and embryo implantation. Unravelling the interplay between these genes and their regulatory pathways provides valuable insights into the complex molecular environment surrounding oocyte development and may hold the key to accurately predict IVF success (28). In recent years, advancements in molecular techniques have provided researchers with the ability to explore gene expression patterns in CC with great precision and sensitivity (14, 23). Through analysing the gene expression profile of CC, researchers aim to identify novel biomarkers that can reliably predict oocyte developmental competence, potentially revolutionising current IVF practices. Additionally, studying the relationship between CC gene expression and oocyte developmental potential offers a non-invasive approach to assess oocyte quality before fertilisation. This approach spares patients from unnecessary invasive procedures and minimises the risk of embryo wastage. Moreover, gaining insights into the molecular basis of oocyte competence may pave the way for the development of personalised IVF treatment strategies, tailored to each individual’s specific molecular profile.
Therefore, in this review, we have compiled published data to elucidate the role of gene expressions of Hyaluronan synthase 2 (HAS2), Gremlin 1 (GREM1) and Prostaglandin-endoperoxide synthase 2 (PTGS2) in CC and their association with human oocyte development competency, particularly in terms of fertilisation and embryo development capacity (11, 13, 25, 27). Additionally, we will explore the involvement of these genes in relevant pathways using integrated bioinformatic analysis. By examining the constellation of these gene expressions and their correlated importance, we aim to consolidate the evidence supporting CC as an excellent biomarker that reflects oocyte competency. This approach will provide a better understanding of oocyte development competency at the molecular level.
This systematic review adhered to the standard guideline protocol based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (29). It was registered in the international database of prospectively registered systematic reviews known as PROSPERO with the registration number CRD42023413686 (30).
The publications within 20 years (2003-2023) were searched using keywords in PubMed and ScienceDirect [All Fields]: ‘In vitro fertilization’ OR IVF OR ‘in vitro maturation’ OR IVM AND ‘gene expression’ AND HAS2 OR ‘hyaluronan synthase 2’ OR PTGS2 OR ‘prostaglandin-endoperoxide synthase 2’ OR COX2 OR GREM1 OR ‘gremlin 1’ AND ‘cumulus cell’ AND ‘oocytes development competency’ OR fertilization OR blastulation OR biochemical OR ‘clinical pregnancy’ OR ‘live birth’ OR ‘oocyte quality’ OR ‘embryo quality.’ Whereas in SCOPUS, the keywords used – [All Fields]: ‘in vitro fertilization’; OR ivf OR ‘in vitro maturation’ OR ‘ivm’ AND [All Fields]: gene AND expression AND [All Fields]: has2 OR ‘hyaluronan synthase 2’; OR ptgs2 OR ‘prostaglandin-endoperoxide synthase 2’; OR cox2 OR grem1 OR ‘gremlin 1’ AND [All Fields]: ‘cumulus cell’ AND [All Fields]: ‘oocytes development competency’ OR fertilization OR blastulation OR biochemical OR & ‘clinical pregnancy’; OR AND ‘live birth’ OR ‘oocyte quality’; OR ‘embryo quality’ AND [All Fields]: ‘infertile women’. Subsequently, all the included studies references were screened for duplication using EndNote® version 20.0.1. The search was improved by manual search using the reference lists from selected articles.
Based on an initial search, fives authors (A.M.F, A.M.A, M.H.I, M.J.N and A.K.A.K.) screened all titles and abstracts of potential manuscripts. The selection criteria included manuscripts published in English from January 2012 to December 2022, evaluating the expression of three specific genes of interest - Hyaluronan synthase 2 (HAS2), Gremlin 1 (GREM1) and Prostaglandin-endoperoxide synthase 2 (PTGS2) - in CC of human oocytes. Following the title and abstract screening, full-text screening was conducted, excluding manuscripts that used different genes, non-CC as experimental material, non-English language, case reports and review articles involving non-human subjects. The remaining potential manuscripts were then independently reviewed. The final selected manuscripts provided a detailed study design, focusing on the expression of all three genes of interest - Hyaluronan synthase 2 (HAS2), Gremlin 1 (GREM1) and Prostaglandin-endoperoxide synthase 2 (PTGS2) - in human CC and correlated these gene expressions with oocyte development competency as the primary outcome. The conflicts in selection amongst authors were resolved through detailed discussions and opinions provided by the fifth, sixth and seventh authors (S.S.E, N.S. and A.A.Z). Additionally, the National Institutes of Health (N.I.H.) tool for observational studies was employed to assess the quality of the selected manuscripts. This evaluation was based on 14 variables, with a scoring system of 1 for ‘yes,’ 0 for ‘no,’ or ‘non-applicable’ for N.A. The manuscripts were then categorised as poor (0–5), fair (6–9) or good (10–14) based on their total scores (Table 1). Overall, the included studies in our review achieved a minimum fair to good score. Subsequently, the final data were extracted and organised based on the authors’ last names, year of publication, country, study design, type of cohort and number of samples (if applicable). Additionally, each study’s gene expression and its correlation with oocyte development competency were tabulated as the main outcome (Table 1).
To identify the possible pathways related to HAS2, GREM1 and PTGS2, all the proteins interaction with these gene product were identified. The predicted functional partners for HAS2, GREM1 and PTGS2 were identified separately using the STRING software (https://string-db.org/). Subsequently, all the proteins interacting with HAS2, GREM1, and PTGS2 were gathered, and their protein-protein interactions were once again identified using the STRING software. The results from STRING were then exported to the Cytoscape software (http://www.cytoscape.org/) to visualise the molecular interaction networks and integrate gene-expression profiles of the included genes. To analyse the target network and identify protein clusters, the MCODE plug-in was utilised with the following settings: degree cut-off = 2, node density cut-off = 0.1, node score cut-off = 0.2, K-score = 2, and max depth = 100). All the clusters were analysed using the Database for Annotation, Visualisation and Integrated Discovery (DAVID) to explore the gene ontology with significant functional-annotation enrichment related to oocyte development (35). Subsequently, the Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway was employed to reveal the involvement of genes in pathways associated with oocyte development (36) (Figure 2).
A total of 108 studies were retrieved during the primary search (Figure 3). After removing 34 duplicates, the remaining 74 articles were thoroughly screened based on our inclusion criteria. Amongst them, 27 articles were excluded, leaving 47 for full-text evaluation. After a detailed evaluation, 43 articles were further discarded: 24 were deemed unsuitable, and 19 were poster presentations or conference abstracts without detailed results. Subsequently, four studies that focused on oocyte development capacity parameters were selected for this review. To ensure quality and minimize bias, all selected articles were evaluated using the National Institutes of Health (N.I.H.) tool for observational studies. Notably, all four articles obtained a good score, indicating a low risk of bias (Supplementary Table 1).
This review included a total of 210 infertility women who underwent IVF, with studies using at least three genes of interest - HAS2, GREM1 and PTGS2 - to correlate with oocyte development competency (ODC) outcomes. The ODC parameters, mainly oocyte maturation rate, fertilisation rate and embryo developmental rate (at least at the 7-cell stage on Day 3 or blastocyst formation following sperm injection), were recorded in all the included studies. The quality of the embryos was assessed based on the degree of fragmentation rates, if applicable, and the excellent quality of embryos was also considered the main outcome. Amongst the included studies, two focused on standard infertility women cohorts undergoing IVF, whilst one focused on decreased ovarian reserve women (31–33). Additionally, one study focused on ODC in an in-vitro maturation (IVM) cohort, comparing the expression of genes of interest in in-vivo and in-vitro maturation settings (34). All the relevant information from the included studies has been summarized in Table 1.
One study agreed that GREM1 higher expression was correlated with better oocyte maturation compared to the other two genes, with sensitivity and specificity for oocyte maturity at 63% and 93%, respectively (32). The same study found that HAS2 also significantly predicts oocyte maturation (32). However, in the IVM cohort, the HAS2 expression was reduced in CC in hCG-primed IVF cycles (34). Otherwise, PTGS2 expressions were comparable in all the studies for oocyte maturation rate (OMR), except for the IVM cohort, which showed a reduction in IVM-GV and IVM-MII CC (31, 34). The expression of HAS2, PTGS2, and GREM1 was low and similar in both follicular-phase-derived and luteal-phase-derived oocytes treated with double stimulation, and there was no association with the outcome in all parameters of interest - OMR, FR, EDR, and GQE (33).
Similarly, for FR, only one study concurred that GREM1 and PTGS2 expression was correlated with better FR compared with the two other genes, with sensitivity and specificity for fertilisation at 72% and 78%, respectively (32). However, one study found a significant correlation between HAS2 expression and 2PN (two pronuclei) fertilisation outcome (31). Meanwhile, a similar expression of HAS2, PTGS2 and GREM1 was found in both follicular-phase-derived and luteal-phase-derived oocytes treated with double stimulation for FR (33).
Regarding EDR, one study revealed that an increase in 5.2-fold relative expression of GREM1 predicted good embryo quality development, with sensitivity and specificity of 83% and 81%, respectively. Additionally, higher PTGS2 expression also significantly predicts good embryo development, similar to GREM1 (32). However, all studies revealed low expression levels of HAS2 that does not correlate with EDR. Conversely, a similar expression of HAS2, PTGS2 and GREM1 was found in both groups follicular-phase-derived and luteal-phase-derived oocytes treated with double stimulation for EDR (33). As for the degree of fragmentation, only one study included it in their outcome, where they found that the degree of fragmentation does not correlate with HAS2, PTGS2 and GREM1 expression in CC (31).
The genes of interest were tabulated based on their fold expression correlating with the proportion of good-grade embryos (GQE). GREM1 exhibited the highest expression fold, being 15-fold higher, whilst PTGS2 and HAS2 showed at least six-fold higher expression in good-grade embryos (32). Furthermore, similar expression of HAS2, PTGS2 and GREM1 was found in both groups follicular-phase-derived and luteal-phase-derived oocytes treated with double stimulation for GQE (33). However, one study found no correlation between all genes of interest - HAS2, PTGS2 and GREM1 - with GQE (31).
In the bioinformatic analysis, a total of 30 predicted functional partner proteins associated with HAS2, GREM1 and PTGS2 were identified. These genes were then subjected to a protein-protein interaction (PPI) network analysis, resulting in a complex network containing 32 nodes and 139 edges. The PPI network showed a highly significant enrichment with a p-value of <1.0e−16 and an average local clustering coefficient of 0.707. To visualise the molecular interactions, the network data from STRING was transferred to Cytoscape software. Additionally, using the molecular complex detection algorithm (MCODE), two significant modules were identified within the PPI network complex (Figure 2). Functional-annotation clustering revealed that cluster 1 comprised 10 nodes and 25 edges (score = 10), whilst cluster 2 consisted of 18 nodes and 59 edges (score = 6.941). The clustering of all the genes in is based on the gene’s cellular location, biological functions, pathways and molecular functions (Figure 4).
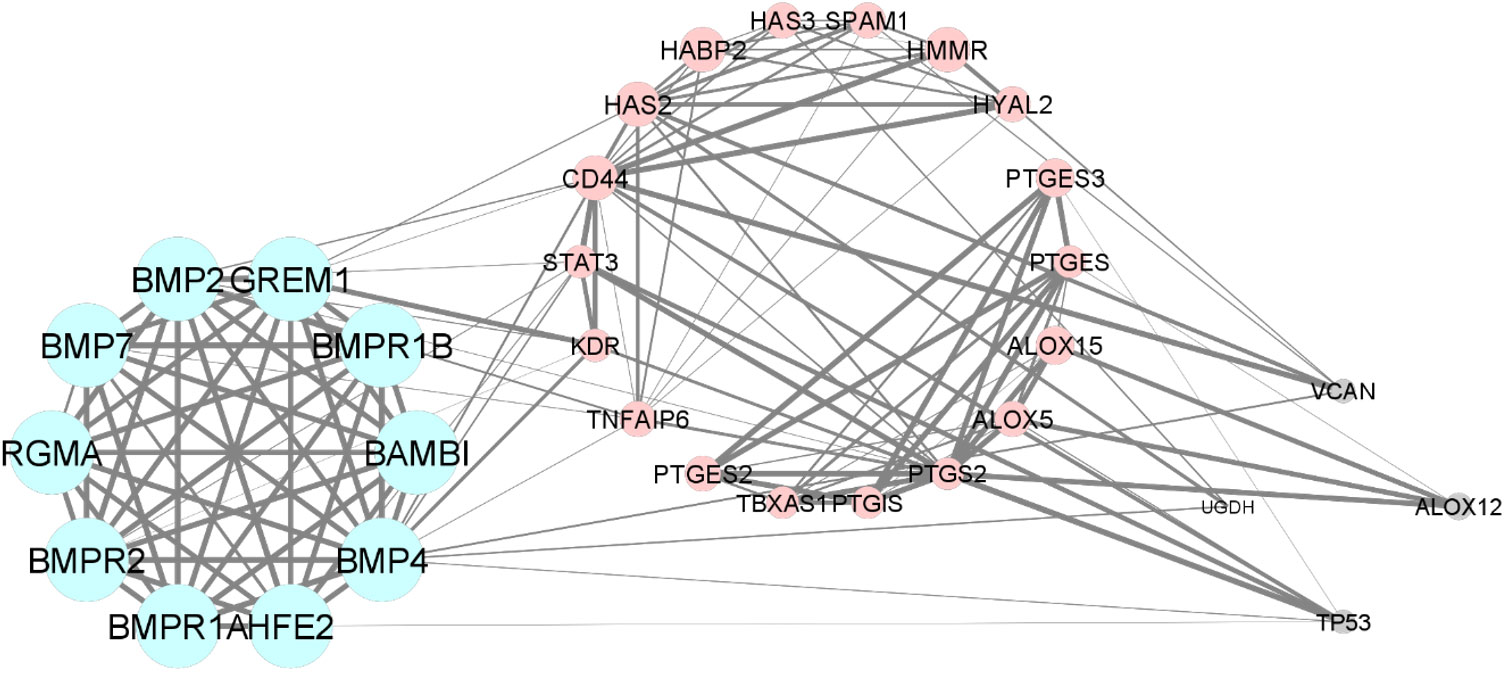
Figure 4 The Protein-protein interaction and clustering of HAS2, GREM1, PTGS2 and their functional partners.
The GO and pathway enrichment analyses revealed that GREM1 is clustered in cluster 1. GREM1 and its functional partner proteins are localised at the cell surface (GO:0009986) and in the extracellular space (GO:0005615). These proteins were found to be involved in the TGF-beta signalling pathway (hsa04350), which includes the bone morphogenic protein (BMP) signalling pathway (GO:0030509), Hippo signalling pathway (hsa04390) and cytokine–cytokine receptor interaction (hsa04060) through BMP binding (GO:0036122). Additionally, the proteins in cluster 1 are also involved in positive regulation of transcription from RNA polymerase II promoter (GO:0045944). HAS2 and PTGS2 are clustered in cluster 2. These proteins and their functional partners are mostly localised at the endoplasmic reticulum membrane (GO:0005789). PTGS2 is involved in arachidonic acid metabolism (hsa00590) and participates in prostaglandin biosynthetic process (GO:0001516). Meanwhile, HAS2 is involved in positive regulation of cell migration (GO:0030335) and response to inflammation through the cyclooxygenase pathway (GO:0019371) and cellular response to interleukin-1 (GO:0071347). Table 2 displays the Functional annotation clustering for clusters 1 and 2, focusing on GREM1, HAS2 and PTGS2 (Table 2).
As established, the critical development of oocyte maturation occurs in the cumulus oocyte complex (37) during the follicular phase (38). Optimal expansion of CC is crucial to ensure better oocyte quality. Therefore, the oocyte developmental competency (ODC) can be assessed at the CC level based on gene expression (12, 13). Numerous studies have utilised GDF-9 derivatives such as Prostaglandin-endoperoxide synthase 2 (PTGS2), gremlin1 (GREM1), hyaluronic acid synthase 2 (HAS2) and pentraxin 3 (PTX3) as markers for ODC, as they reflect the immediate ODC during CC expansion (11, 18, 19). In this review, we found that GREM1 expression is an important marker for oocyte maturation rates (OMR) compared to both HAS2 and PTGS2 (32). Healthy oocytes tend to increase the expression of GREM1 in their CC, promoting folliculogenesis and enhancing spindle activities, ultimately leading to oocyte maturation (39, 40). The majority of the evidence aligns with these findings, consolidating the importance of GREM1 in OMR (12, 13, 17). Our review found that following rescue IVM, PTGS2 expression is reduced, indicating that proper IVM should not be preceded by hCG injection because it may interfere with oocyte maturation rates (34). Therefore, recent evidence recommends pre-IVM culture, as well as standard IVM procedures without hCG, to improve the microenvironment at the CC expansion level, aiming for a better OMR following IVM culture (41). Furthermore, our review revealed that GREM1 is also considered a predictor of good fertilisation rates (FR) and EDR (32). With optimum GREM1 expression following maturation, meiosis II (MII) oocytes can fertilise following insemination by sperm and subsequently undergo embryo development (42). The gathered evidence suggests that a reduction in GREM1 does affect the fertilisation rates and EDR, particularly in suboptimal cohorts, such as decrease ovarian reserved (DOR), or in cases of poor oocyte quality in women with endometriosis or polycystic ovarian syndrome (13, 43, 44).
Besides that, the expression of HAS2 in CC was associated with higher 2PN fertilisation (31). Most evidence postulates that an increase in the fold of HAS2 expression significantly correlates with better oocyte quality, leading to an increase in fertilisation (11, 16, 27, 32). Moreover, the unique expression of HAS2 is synergistically influenced by hCG or LH as maturation triggers (26). Therefore, standard IVM cycles without hCG will incorporate LH in the culture media to enhance HAS2 expression, aiming for better oocyte quality, fertilisation rates (FR) and EDR. Furthermore, our review agrees that the proportion of good quality of embryo (GQE) is influenced by all GDF-9 derivative gene expressions (32, 33). However, a higher fold of GREM1 expression is associated with an increase in the GQE cohort (32). In exploring the GDF-9 derivatives, most publications strongly support the importance of these three genes - HAS2, PTGS2 and GREM1 - in modulating embryo development (45, 46). Numerous pieces of evidence demonstrate that higher median expression of GDF-9 downstream predicts better quality embryo outcomes (11, 15, 19, 27, 40). The regulation of human follicles through GDF-9 has been well-established for decades. Any deviation or mutation in its molecular regulation can lead to possible subfertility, mainly due to a blockage of the early follicular phase, resulting in disruption of the meiosis process. Additionally, subsequent evidence suggests that GDF-9 also influences the later stages of oocyte development (47). Therefore, by dissecting the mechanisms of their regulation, a better understanding of the maturation of ongoing dominant follicles can be achieved. CC expansion is initiated by the secretion of GDF-9, with HAS2 playing a crucial role in promoting expansion through the production of hyaluronic acid. Subsequently, PTGS2 further promotes expansion by secreting prostaglandin E2. Both mechanisms are considered crucial for maturation and ovulation. Defects in these mechanisms, as observed in animal models, result in defects in ovulation, fertilisation, embryo quality and implantation (48). As for GREM1, it acts by inhibiting the signalling for BMP rather than GDF-9. During cumulus expansion, GREM1 promotes mural granulosa cell luteinisation, mostly during the oocyte maturation phase (22). Therefore, all three of these genes influence CC expansion, thus reflecting the ODC in the human reproductive field (49).
From the bioinformatic analysis, GREM1 is clustered in cluster 1, whilst HAS2 and PTGS2 are separated in cluster 2. GREM1, along with its functional partner proteins, such as BMPR2, BMP2 and HFE2, is located in the extracellular space and cell surface, indicating its involvement in interactions with the cellular environment and adjacent cells. Communication is crucial for oocyte development. The cumulus-oocyte complex’s development is primarily driven by short-distance communication through extensive local cell-to-cell interactions, often involving members of the transforming growth factor-beta (TGF-β) family, such as inhibin, activin, anti-Müllerian hormone and growth and differentiation factor 9 (GDF9) (50). GREM1, being a downstream target protein of GDF9, plays a pivotal role in regulating the crosstalk between GDF9 signalling and BMP signalling pathways. It selectively inhibits the differential effect of the BMP signal whilst preserving the GDF9 signal. This unique regulation may promote luteinisation of ovarian granulosa cells whilst supporting CC expansion in the ovarian follicle (51). The proteins in cluster 1 also have roles in positive regulation of transcription from RNA polymerase II promoter and positive regulation of cell proliferation. GREM1, BMP4, BMP2 and BAMBI are associated with promoting cell proliferation, suggesting their potential roles in follicular development and CC expansion.
In contrast to GREM1, the proteins HAS2 and PTGS2 in cluster 2 are localised within the endoplasmic reticulum membrane. This cellular localisation suggests their involvement in cellular compartmentalisation and localisation processes within the oocyte. Both HAS2 and PTGS2 are integral components of key metabolic pathways. In particular, they play crucial roles in the arachidonic acid metabolism pathway, which is important for the production of bioactive lipid mediators, including prostaglandins. The prostaglandin biosynthetic process, enriched by HAS2 and PTGS2, is of particular significance because prostaglandins influence in vitro maturation of oocytes (52). PTGS2’s involvement in the cyclooxygenase pathway further emphasises its significance in prostaglandin synthesis, contributing to various reproductive processes. Moreover, the gene ontology terms associated with HAS2, such as positive regulation of cell migration, indicate its potential involvement in CC expansion, a critical process for successful oocyte maturation. Moreover, the enrichment of cellular response to interleukin-1 by HAS2 suggests its possible role in responding to inflammatory signals during oocyte development. Notably, exposure to high levels of IL-10 and IL-1β has been reported to decrease CC expression of GREM1, indicating that inflammation in the follicular fluid, particularly amongst obese women, could impact the cumulus oocyte complex and alter its microenvironment, significantly influencing GREM1 gene expression (44). Overall, the CC have demonstrated that GDF9 upregulates the expression of essential genes, including HAS2, PTGS2, GREM1 and STAR, whilst concurrently downregulating luteinising hormone receptor, a crucial factor for follicle development and cumulus expansion. The regulation of GDF9 and its downstream factors in CC presents a promising potential as an indicator of oocyte quality (12). Understanding these intricate molecular interactions offers valuable insights into oocyte development and maturation, which could lead to significant advancements in reproductive medicine and fertility treatments.
Our review summarizes the gene expression results of GDF-9 derivatives in CC, which can be reliable predictors in IVF, primarily in OMR, FR, EDR, and GQE. However, CC predictors are constellations of evidence rather than individuals, as different gene expressions represent different IVF parameters based on our review. Our analysis of the literature and bioinformatics provides evidence of a potential link between the expression levels of GREM1, PTGS2, and HAS2 in cumulus cells and the maturation of the oocyte and subsequent fertilization rates and embryo development. However, the link has yet to be consistently confirmed across all studies. Further research is necessary to verify the relevance of this connection. Our study has certain limitations, as the included papers did not correlate the outcomes with live birth rates. Hence, the molecular conclusions are limited to gene expression alone. Nonetheless, these candidate genes’ expression levels can be a valuable indicator correlated with IVF outcomes, predicting good embryo development and quality. These findings can help improve overall IVF outcomes.
AF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. ME: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft. NJ: Conceptualization, Formal analysis, Methodology, Resources, Writing – review & editing. MA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. SS: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing. AZ: Data curation, Formal analysis, Supervision, Writing – review & editing. NS: Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. AK: Project administration, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Fundamental Research Grant Scheme (FRGS), awarded by Ministry of Higher Education of Malaysia with a title of “Elucidating the GREM1, HAS2 and PTGS2 gene expression as oocytes development competency markers among women with poor ovarian reserve following the in vitro maturation (IVM)”. The grant number FRGS/1/2021/SKK01/UKM/02/1. The APC was funded by Innovation & Research Secretariat (SPI) of Faculty of Medicine National University of Malaysia.
We would like to thank all the staff in Advanced Reproductive Centre (ARC) HCTM UKM who contributed to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1274376/full#supplementary-material
1. Dabbagh Rezaeiyeh R, Mehrara A, Mohammad Ali Pour A, Fallahi J, Forouhari S. Impact of various parameters as predictors of the success rate of in vitro fertilization. Int J Fertil Steril. (2022) 16:76–84. doi: 10.22074/IJFS.2021.531672.1134
2. Gallos ID, Eapen A, Price MJ, Sunkara SK, Macklon NS, Bhattacharya S, et al. Controlled ovarian stimulation protocols for assisted reproduction: a network meta-analysis. Cochrane Database Syst Rev. (2017) 2017. doi: 10.1002/14651858.CD012586
3. Gruber I, Klein M. Embryo culture media for human IVF: which possibilities exist? J Turk Ger Gynecol Assoc. (2011) 12:110–7. doi: 10.5152/jtgga
4. Li J, Liu L, Weng J, Yin TL, Yang J, Feng HL. Biological roles of l-carnitine in oocyte and early embryo development. Mol Reprod Dev. (2021) 88:673–85. doi: 10.1002/mrd.23542
5. Zargar M, Dehdashti S, Najafian M, Choghakabodi PM. Pregnancy outcomes following in vitro fertilization using fresh or frozen embryo transfer. JBRA Assist Reprod. (2021) 25:570–4. doi: 10.5935/1518-0557.20210024
6. Wang J, Liu Q, Deng B, Chen F, Liu X, Cheng J. Pregnancy outcomes of Chinese women undergoing IVF with embryonic cryopreservation as compared to natural conception. BMC Pregnancy Childbirth. (2021) 21:39. doi: 10.1186/s12884-020-03486-7
7. Zhu L, Zhang Y, Liu Y, Zhang R, Wu Y, Huang Y, et al. Maternal and live-birth outcomes of pregnancies following assisted reproductive technology: A retrospective cohort study. Sci Rep. (2016) 6:35141. doi: 10.1038/srep35141
8. Scheffler F, Vandecandelaere A, Soyez M, Bosquet D, Lefranc E, Copin H, et al. and IGF1 levels are associated with oocyte cohort quality: A pilot study. Front Endocrinol (Lausanne). (2021) 12:793621. doi: 10.3389/fendo.2021.793621
9. Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. (2019) 158:R79–90. doi: 10.1530/REP-18-0583
10. Lanzone A, Fulghesu AM, Fortini A, Cutillo G, Cucinelli F, Di Simone N, et al. Effect of opiate receptor blockade on the insulin response to oral glucose load in polycystic ovarian disease. Hum Reprod (Oxford England). (1991) 6:1043–9. doi: 10.1093/oxfordjournals.humrep.a137482
11. Sirait B, Wiweko B, Jusuf AA, Iftitah D, Muharam R. Oocyte competence biomarkers associated with oocyte maturation: A review. Front Cell Dev Biol. (2021) 9:710292. doi: 10.3389/fcell.2021.710292
12. Turathum B, Gao EM, Chian RC. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells. (2021) 10. doi: 10.3390/cells10092292
13. Sayutti N, Abu MA, Ahmad MF. PCOS and role of cumulus gene expression in assessing oocytes quality. Front Endocrinol (Lausanne). (2022) 13:843867. doi: 10.3389/fendo.2022.843867
14. El-Maarri O, Jamil MA, Köster M, Nüsgen N, Oldenburg J, Montag M, et al. Stratifying cumulus cell samples based on molecular profiling to help resolve biomarker discrepancies and to predict oocyte developmental competence. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22126377
15. Scarica C, Cimadomo D, Dovere L, Giancani A, Stoppa M, Capalbo A, et al. An integrated investigation of oocyte developmental competence: expression of key genes in human cumulus cells, morphokinetics of early divisions, blastulation, and euploidy. J Assist Reprod Genet. (2019) 36:875–87. doi: 10.1007/s10815-019-01410-3
16. Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. (2008) 14:159–77. doi: 10.1093/humupd/dmm040
17. Hirao Y. Oocyte growth in vitro: potential model for studies of oocyte-granulosa cell interactions. Reprod Med Biol. (2012) 11:1–9. doi: 10.1007/s12522-011-0096-3
18. Kaivo-oja N, Jeffery LA, Ritvos O, Mottershead DG. Smad signalling in the ovary. Reprod Biol Endocrinol. (2006) 4:21. doi: 10.1186/1477-7827-4-21
19. Stocker WA, Walton KL, Richani D, Chan KL, Beilby KH, Finger BJ, et al. A variant of human growth differentiation factor-9 that improves oocyte developmental competence. J Biol Chem. (2020) 295:7981–91. doi: 10.1074/jbc.RA120.013050
20. Assou S, Boumela I, Haouzi D, Anahory T, Dechaud H, De Vos J, et al. Dynamic changes in gene expression during human early embryo development: from fundamental aspects to clinical applications. Hum Reprod Update. (2011) 17:272–90. doi: 10.1093/humupd/dmq036
21. Li Q, McKenzie LJ, Matzuk MM. Revisiting oocyte-somatic cell interactions: in search of novel intrafollicular predictors and regulators of oocyte developmental competence. Mol Hum Reprod. (2008) 14:673–8. doi: 10.1093/molehr/gan064
22. Gao EM, Turathum B, Wang L, Zhang D, Liu YB, Tang RX, et al. The differential metabolomes in cumulus and mural granulosa cells from human preovulatory follicles. Reprod Sci. (2022) 29:1343–56. doi: 10.1007/s43032-021-00691-3
23. Feuerstein P, Cadoret V, Dalbies-Tran R, Guerif F, Bidault R, Royere D. Gene expression in human cumulus cells: one approach to oocyte competence. Hum Reprod. (2007) 22:3069–77. doi: 10.1093/humrep/dem336
24. Camaioni A, Klinger FG, Campagnolo L, Salustri A. The influence of pentraxin 3 on the ovarian function and its impact on fertility. Front Immunol. (2018) 9:2808. doi: 10.3389/fimmu.2018.02808
25. Zhang X, Jafari N, Barnes RB, Confino E, Milad M, Kazer RR. Studies of gene expression in human cumulus cells indicate pentraxin 3 as a possible marker for oocyte quality. Fertil Steril. (2005) 83 Suppl 1:1169–79. doi: 10.1016/j.fertnstert.2004.11.030
26. Yung Y, Ophir L, Yerushalmi GM, Baum M, Hourvitz A, Maman E. HAS2-AS1 is a novel LH/hCG target gene regulating HAS2 expression and enhancing cumulus cells migration. J Ovarian Res. (2019) 12:21. doi: 10.1186/s13048-019-0495-3
27. Monsivais D, Matzuk MM, Pangas SA. The TGF-β Family in the reproductive tract. Cold Spring Harb Perspect Biol. (2017) 9. doi: 10.1101/cshperspect.a022251
28. Anamthathmakula P, Winuthayanon W. Prostaglandin-endoperoxide synthase 2 (PTGS2) in the oviduct: roles in fertilization and early embryo development. Endocrinology. (2021) 162. doi: 10.1210/endocr/bqab025
29. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
30. Pieper D, Rombey T. Where to prospectively register a systematic review. Syst Rev. (2022) 11:8. doi: 10.1186/s13643-021-01877-1
31. Adriaenssens T, Wathlet S, Segers I, Verheyen G, De Vos A, van der Elst J, et al. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod. (2010) 25:1259–70. doi: 10.1093/humrep/deq049
32. McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, et al. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. (2004) 19:2869–74. doi: 10.1093/humrep/deh535
33. Mishieva N, Martazanova B, Bogatyreva K, Korolkova A, Kirillova A, Veyukova M, et al. Cumulus cell gene expression in luteal-phase-derived oocytes after double stimulation in one menstrual cycle. Reprod BioMed Online. (2020) 41:518–26. doi: 10.1016/j.rbmo.2020.05.002
34. Coticchio G, Ophir L, Yung Y, Baum M, Dal Canto M, Mignini-Renzini M, et al. Differential regulation of cumulus cell transcription during oocyte maturation in vivo and in vitro. Int J Dev Biol. (2017) 61:433–7. doi: 10.1387/ijdb.160364gc
35. Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. (2003) 4:R60. doi: 10.1186/gb-2003-4-5-p3
36. Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. (2000) 28:27–30. doi: 10.1093/nar/28.1.27
37. Attanasio M, Cirillo M, Coccia ME, Castaman G, Fatini C, Factors VIII. and von willebrand levels in women undergoing assisted reproduction: are their levels associated with clinical pregnancy outcome? Mediterr J Hematol Infect Dis. (2020) 12:e2020058. doi: 10.4084/mjhid.2020.058
38. Da Broi MG, Giorgi VSI, Wang F, Keefe DL, Albertini D, Navarro PA. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J Assist Reprod Genet. (2018) 35:735–51. doi: 10.1007/s10815-018-1143-3
39. Fragouli E, Lalioti MD, Wells D. The transcriptome of follicular cells: biological insights and clinical implications for the treatment of infertility. Hum Reprod Update. (2014) 20:1–11. doi: 10.1093/humupd/dmt044
40. Sfakianoudis K, Maziotis E, Karantzali E, Kokkini G, Grigoriadis S, Pantou A, et al. Molecular drivers of developmental arrest in the human preimplantation embryo: A systematic review and critical analysis leading to mapping future research. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22158353
41. Ahmad MF, Elias MH, Mat Jin N, Abu MA, Syafruddin SE, Zainuddin AA, et al. The spectrum of in vitro maturation in clinical practice: the current insight. Front Endocrinol (Lausanne). (2023) 14:1192180. doi: 10.3389/fendo.2023.1192180
42. Hatırnaz Ş, Hatırnaz ES, Ellibeş Kaya A, Hatırnaz K, Soyer Çalışkan C, Sezer Ö, et al. Oocyte maturation abnormalities - A systematic review of the evidence and mechanisms in a rare but difficult to manage fertility pheneomina. Turk J Obstet Gynecol. (2022) 19:60–80. doi: 10.4274/tjod.
43. Jindal S, Greenseid K, Berger D, Santoro N, Pal L. Impaired gremlin 1 (GREM1) expression in cumulus cells in young women with diminished ovarian reserve (DOR). J Assist Reprod Genet. (2012) 29:159–62. doi: 10.1007/s10815-011-9684-8
44. Kim T, Kim Y, Lucien F, Zhao Y, Enninga EAL. Decreased gremlin 1 expression in women with BMI ≥35 kg/m(2) is mediated by interleukin 10 and interleukin 1β in the follicular fluid. F S Sci. (2020) 1:16–26. doi: 10.1016/j.xfss.2020.06.003
45. Anderson RA, Sciorio R, Kinnell H, Bayne RAL, Thong KJ, de Sousa PA, et al. Cumulus gene expression as a predictor of human oocyte fertilisation, embryo development and competence to establish a pregnancy. REPRODUCTION. (2009) 138:629–37. doi: 10.1530/REP-09-0144
46. Gebhardt KM, Feil DK, Dunning KR, Lane M, Russell DL. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil Steril. (2011) 96:47–52.e2. doi: 10.1016/j.fertnstert.2011.04.033
47. Sanfins A, Rodrigues P, Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet. (2018) 35:1741–50. doi: 10.1007/s10815-018-1268-4
48. Chen W, Zhai Y, Zhu B, Wu K, Fan Y, Zhou X, et al. Loss of growth differentiation factor 9 causes an arrest of early folliculogenesis in zebrafish-A novel insight into its action mechanism. PloS Genet. (2022) 18:e1010318. doi: 10.1371/journal.pgen.1010318
49. Paulini F, Melo EO. The role of oocyte-secreted factors GDF9 and BMP15 in follicular development and oogenesis. Reprod Domest Anim. (2011) 46:354–61. doi: 10.1111/j.1439-0531.2010.01739.x
50. Marchais M, Gilbert I, Bastien A, Macaulay A, Robert C. Mammalian cumulus-oocyte complex communication: a dialog through long and short distance messaging. J Assist Reprod Genet. (2022) 39:1011–25. doi: 10.1007/s10815-022-02438-8
51. Zhao H, Ge J, Wei J, Liu J, Liu C, Ma C, et al. Effect of FSH on E(2)/GPR30-mediated mouse oocyte maturation in vitro. Cell Signal. (2020) 66:109464. doi: 10.1016/j.cellsig.2019.109464
Keywords: HAS2, PTGS2, grem1, cumulus cells, oocytes development competency, gene expression
Citation: Faizal AM, Elias MH, Jin NM, Abu MA, Syafruddin SE, Zainuddin AA, Suzuki N and Karim AKA (2024) Unravelling the role of HAS2, GREM1, and PTGS2 gene expression in cumulus cells: implications for human oocyte development competency - a systematic review and integrated bioinformatic analysis. Front. Endocrinol. 15:1274376. doi: 10.3389/fendo.2024.1274376
Received: 08 August 2023; Accepted: 26 February 2024;
Published: 08 March 2024.
Edited by:
Etienne Marbaix, Université Catholique de Louvain, BelgiumReviewed by:
Ravikrishna Cheemakurthi, Krishna IVF Clinic, IndiaCopyright © 2024 Faizal, Elias, Jin, Abu, Syafruddin, Zainuddin, Suzuki and Karim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Kadir Abdul Karim, YWJkdWxrYWRpcmFiZHVsa2FyaW1AeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.