- 1Department of Obstetrics and Gynecology, Hamamatsu University School of Medicine, Hamamatsu, Japan
- 2National Institutes of Biomedical Innovation, Health and Nutrition, Osaka, Japan
- 3Department of Bioregulatory Science (Physiology), Nippon Medical School, Tokyo, Japan
Introduction
The research reported in ‘The Fetal Origins of Metabolic Disorders Volume II’ is derived from the theory of Developmental Origins of Health and Disease (DOHaD) (1, 2), which connects health disorders with environmental disruptions during early life stage. The DOHaD theory was historically devised from the findings of cohort studies of the long-term health deterioration of certain groups such as those that were born as small neonates or born from mothers experiencing the famine in World War II (1, 2). The findings were supported by animal studies. Epigenetics has provided powerful research tools that allow for the exploration of DNA methylation, histone modifications, and non-coding RNAs for the purpose of searching how adverse exposure in early life results in epigenetic and gene expression changes that contribute to the risk of chronic disease later in life (3). Recently, tauroursodeoxycholic acid (TUDCA), a secondary bile acid, has been used as a therapeutic strategy to minimize adipose tissue dysfunction and metabolic alterations associated with obesity (4). This editorial introduces the hypothesis that TUDCA beneficially remodels the chromatin structure around the genes associated with the developmentally programed obesity-prone phenotype of adults.
TUDCA improves obesity-associated disorders
TUDCA has been used for centuries in Chinese medicine and is approved by the Food and Drug Administration for treatment of primary biliary cholangitis. Furthermore, TUDCA has potential therapeutic benefits in various diseases including diabetes, obesity, and neurodegenerative diseases. TUDCA has cytoprotective activity by alleviation of endoplasmic reticulum (ER) stress as a chemical chaperon-stabilizing unfold protein response (5). Furthermore, TUDCA induces beneficial metabolic effects by activating farnesoid X receptor and G protein-coupled bile acid receptor (4). However, the exact association between its receptor-mediated pathways and its activity as a chemical chaperon remains to be elucidated.
There have been limited numbers of studies that described the favorable effect of TUDCA treatment on DOHaD-associated models. Yung et al. reported the advantageous effect of TUDCA treatment on pregnant women with gestational diabetes (6). Pasha et al. reported that TUDCA treatment improved fetal growth in aged rat dams (7). Our research group reported that TUDCA treatment improved hepatic steatosis (8, 9) and fat pad deposition (10) specifically in adult mice experienced undernourished (UN) in utero.
TUDCA induces beneficial changes of chromatin structure around Cidea and Cidec genes in pups with UN in utero
Previous studies of cell models reported that TUDCA induces epigenetic changes (5); however, few studies have reported advantageous epigenetic effects in experimental DOHaD animal models. Urmi et al. reported that UN in utero caused deteriorated hepatic steatosis of adult pups under obesogenic diet (Figures 1A, B) concomitant with specific augmentation of Cidea gene expression (Figures 1D, E) and suppression of histone modification of H3K27 di-methylation (transcriptional promotion) around Cidea (Figures 1G, H) (9). Both Cidea and Cidec induce the fusion of lipid droplets and augment lipid deposition in hepatocytes. Moreover, TUDCA treatment improved hepatic steatosis in adult pups with UN in utero (Figures 1B, C), but not normally nourished pups (not shown). This occurred with specific inhibition of Cidea gene expression (Figures 1E, F) and upregulation of histone modification of H3K27 di-methylation (transcriptional repression) around Cidea (Figures 1H, I). Similar findings were also observed with Cidec (9). Urmi et al.’s findings suggest that histone remodeling by TUDCA improves hepatic steatosis in a DOHaD animal model; however, further studies using a gene deletion model are necessary to clarify if it is inevitable for the improvement of hepatic steatosis. Nevertheless, TUDCA treatment beneficially remodeled chromatin structures around some key genes of lipid deposition in adult pups with developmental programming by UN in utero, suggesting a possible promising future use of TUDCA as a beneficial modulator of developmentally programmed chromatin structure, which is applicable even in the adult period.
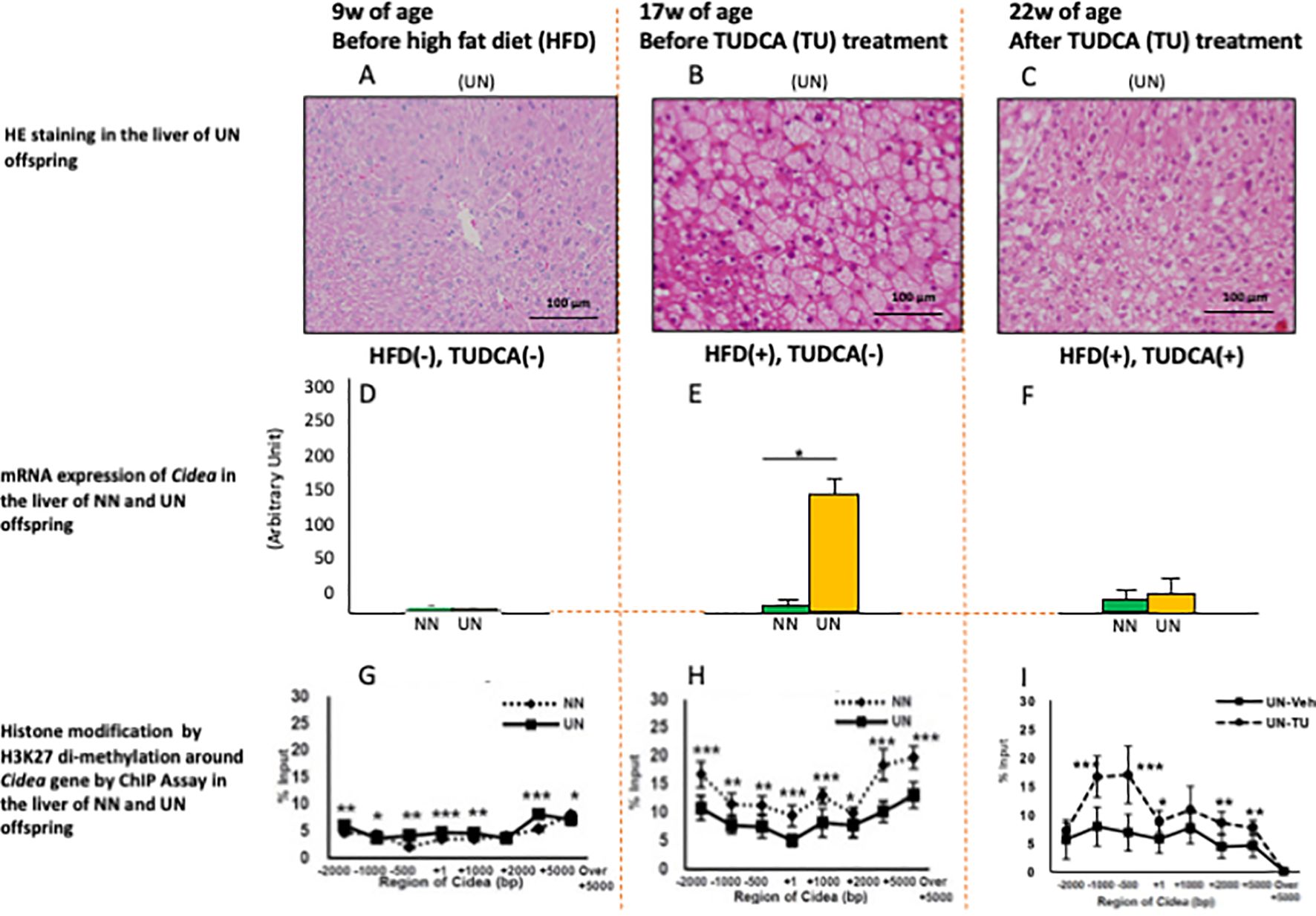
Figure 1. HE staining (A–C), mRNA expression of Cidea gene (D–F), and histone modification by H3K27 di-methylation around Cidea gene by ChIP Assay (G–I) in the liver of the mouse pups at 9w (A, D, G), 17w (B, E, H) and 22w (C, F, I) of age with undernourishment (UN) or normal nourishment (NN) in utero, before or after high fat diet (HFD) and TUDCA (TU) treatment. *P < 0.05. **P < 0.01. ***P < 0.001. Cited from Urmi et al. (9).
Contributions of the articles published in the research topic
The research topics in ‘The Fetal Origins of Metabolic Disorders Volume II’ are: 1) environmental disruption in the early critical period, 2) resultant phenotypic disorders in offspring, and 3) the mechanism of programming. Regarding 1), Doi et al. (hyper link) reported the effects of methamphetamine exposure. Regarding 2), Deer et al. (hyper link) reviewed cardiovascular disease risk and Umeda et al. (hyper link) investigated fetal growth and polyunsaturated fatty acid metabolism. Regarding 3), Olive et al. (hyper link) investigated cortisol regulation and Gracia-Rizo et al. (hyper link) reported the association between glucose metabolism and first-episode psychosis.
Author contributions
All authors contributed to the drafting and editing of this editorial. All have approved the final version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by JSPS KAKENHI Grant Numbers JP20H03823, JP20K09666, and JP20K16886, JP24K0258, AMED under Grant Number JP20gm1310009, and SRF (2024T001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gluckman PD, Hanson MA. Developmental Origins of Health and Disease. Cambridge University Press (2006). Available at: https://www.cambridge.org/core/books/developmental-origins-of-health-and-disease/C06E8EFA67C799F8462B1E1D0B3A5C37.
2. Itoh H, Kanayama N. “Developmental origins of health and diseases (DOHaD); perspective toward preemptive medicine.” In: Konishi I, editor. Precision Medicine in Gynecology and Obstetrics. Springer Nature, Singapore. (2017).
3. Bianco-Miotto T, Craig JM, Gasser YP, van Dijk SJ, Ozanne SE. Epigenetics and DOHaD: from basics to birth and beyond. J Dev Orig Health Dis. (2017) 8:513–9. doi: 10.1017/S2040174417000733
4. Freitas IN, da Silva JA Jr., de Oliveira KM, Lourenconi Alves B, Dos Reis Araujo T, Camporez JP, et al. Insights by which TUDCA is a potential therapy against adiposity. Front Endocrinol (Lausanne). (2023) 14:1090039. doi: 10.3389/fendo.2023.1090039
5. Kusaczuk M. Tauroursodeoxycholate-bile acid with chaperoning activity: Molecular and cellular effects and therapeutic perspectives. Cells. (2019) 8:1471. doi: 10.3390/cells8121471
6. Yung HW, Alnaes-Katjavivi P, Jones CJ, El-Bacha T, Golic M, Staff AC, et al. Placental endoplasmic reticulum stress in gestational diabetes: the potential for therapeutic intervention with chemical chaperones and antioxidants. Diabetologia. (2016) 59:2240–50. doi: 10.1007/s00125-016-4040-2
7. Pasha M, Kirschenman R, Wooldridge A, Spaans F, Cooke CM, Davidge ST. The effect of tauroursodeoxycholic Acid (TUDCA) treatment on placental endoplasmic reticulum (ER) stress in a rat model of advanced maternal age. PLoS One. (2023) 18:e0282442. doi: 10.1371/journal.pone.0282442
8. Muramatsu-Kato K, Itoh H, Kohmura-Kobayashi Y, Ferdous UJ, Tamura N, Yaguchi C, et al. Undernourishment in utero primes hepatic steatosis in adult mice offspring on an obesogenic diet; involvement of endoplasmic reticulum stress. Sci Rep. (2015) 5:16867. doi: 10.1038/srep16867
9. Urmi JF, Itoh H, Muramatsu-Kato K, Kohmura-Kobayashi Y, Hariya N, Jain D, et al. Plasticity of histone modifications around Cidea and Cidec genes with secondary bile in the amelioration of developmentally-programmed hepatic steatosis. Sci Rep. (2019) 9:17100. doi: 10.1038/s41598-019-52943-7
10. Suzuki M, Kohmura-Kobayashi Y, Ueda M, Furuta-Isomura N, Matsumoto M, Oda T, et al. Comparative analysis of gene expression profiles in the adipose tissue of obese adult mice with rapid infantile growth after undernourishment In Utero. Front Endocrinol (Lausanne). (2022) 13:818064. doi: 10.3389/fendo.2022.818064
Keywords: pregnancy, fetus, developmental origins of health and disease (DOHaD), secondary bile acid, histone modifications, epigenome, ER stress
Citation: Itoh H, Aoyama T, Kohmura-Kobayashi Y, Tamura N and Nemoto T (2024) Tauroursodeoxycholic acid as a beneficial modulator for developmentally programed chromatin structure around specific genes. Front. Endocrinol. 15:1211657. doi: 10.3389/fendo.2024.1211657
Received: 25 April 2023; Accepted: 31 July 2024;
Published: 26 August 2024.
Edited by:
Oliana Carnevali, Marche Polytechnic University, ItalyReviewed by:
Dejun Ma, Nankai University, ChinaCopyright © 2024 Itoh, Aoyama, Kohmura-Kobayashi, Tamura and Nemoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroaki Itoh, aWhpcm9ha2lAaGFtYS1tZWQuYWMuanA=
 Hiroaki Itoh
Hiroaki Itoh Tomoko Aoyama
Tomoko Aoyama Yukiko Kohmura-Kobayashi
Yukiko Kohmura-Kobayashi Naoaki Tamura1
Naoaki Tamura1 Takahiro Nemoto
Takahiro Nemoto