- 1Division of Bariatric and Metabolic Surgery, Department of General Surgery, Qilu Hospital of Shandong University, Jinan, China
- 2Department of Surgery, First Clinical College, Shandong University, Jinan, China
Background: No sex-specific guidelines for surgical anti-obesity strategies have been proposed, partially due to the controversy regarding sex-related differences in weight loss after bariatric metabolic surgery.
Objectives: To explore sex dimorphism in the effect and predictors of weight loss after sleeve gastrectomy (SG), thereby providing clinical evidence for the sex-specific surgical treatment strategy.
Methods: In a prospective cohort design, participants scheduled for SG at an affiliated hospital between November 2020 and January 2022 were assessed for eligibility and allocated to the Male or Female group with a 1-year follow-up after surgery. The primary outcome was the sex difference in the weight-loss effect after SG indicated by both percentage of total weight loss (TWL%) and excess weight loss (EWL%). The secondary outcome was the analysis of sex-specific preoperative predictors of weight loss after SG based on univariate and multivariate analyses. Independent predictors were obtained to construct a nomogram model. The discrimination, calibration, and clinical utility of the nomogram were based on receiver operating characteristic curve, concordance index, calibration curve, and decision curve analysis, respectively.
Results: Ninety-five male and 226 female patients were initially included. After propensity score matching by baseline body mass index (BMI), 85 male and 143 female patients achieved comparable TWL% and EWL% for 1 year after SG. For male patients, baseline BMI, area under the curve for insulin during oral glucose tolerance test, and progesterone were independent predictors of weight loss after SG. Baseline BMI, age, thyroid stimulating hormone, and Self-Rating Anxiety Scale score were independent predictors for female patients.
Conclusion: No obvious sex difference is detected in the weight-loss effect after SG. Sex dimorphism exists in the predictors of weight loss after SG. Further research with long-term and a multicenter design is needed to confirm the predictive model.
1 Introduction
Obesity has become a global epidemic affecting more than 988 million people worldwide by 2020 (1). Bariatric metabolic surgery has been recognized as an effective and evidence-based surgical treatment for morbid obesity (2). Sleeve gastrectomy (SG) has become the most common bariatric metabolic procedure worldwide (3). However, the weight-loss effect after SG varied among patients. Insufficient weight loss and weight regain have become challenging issues (4).
Body weight can be affected by biologic, psychosocial, and behavioral factors (5). Given sex-related differences in psychosocial status, hormonal homeostasis, and body fat distribution (6, 7), responses to weight-management strategies are likely to differ by sex. For example, males were reported to get more health benefits from moderate-intensity exercise (8). However, females are significantly more successful on pharmacotherapy for weight loss (9). Moreover, there is no consensus in clinic in terms of the sex-related differences in the weight-loss effect after SG (10, 11). As mentioned above, further research is needed to illustrate the sex differences in the weight-loss effect after SG.
Preoperative prediction of weight loss is helpful not only for defining realistic expectations and maintaining motivation for patients but also for surgeons to select good candidates and reduce failures (12). However, the factors that predict weight loss following SG cannot be conclusively determined (13). Moreover, no sex-specific guidelines for surgical anti-obesity strategies have yet been proposed. Thus, it is of great importance to identify the sex-specific preoperative predictors of weight loss after SG.
Based on a prospective cohort, the present study aims to determine the sex difference in the weight-loss effect after SG indicated by both percentage of total weight loss (TWL%) and excess weight loss (EWL%), and further construct the sex-specific nomograms based on analysis of the sex-specific preoperative predictors of weight loss after SG, providing clinical evidence for the surgical treatment strategy to help achieve better weight loss.
2 Materials and methods
2.1 Study design and patients
The protocol of this study was approved by the Ethics Committee on Scientific Research of Shandong University Qilu Hospital on February 24, 2017. All data were retrieved from a prospectively collected database (SDBMSR, https://sdbmsr.yiducloud.com.cn). The conduction of this study conformed to the principles outlined in the Declaration of Helsinki. Each participant was informed in detail about the purpose, process, potential risks and benefits of the research on the day of admission. All participants signed written informed consent forms before assessment.
2.2 Patients and follow-up
Participants were assessed for eligibility if they were scheduled for SG between November 2020 and January 2022 at University hospital. The exclusion criteria were as follows: (1) uncontrolled mental illness such as schizophrenia, bipolar affective disorder or severe organ dysfunction such as heart failure, respiratory failure; (2) treatment with weight-loss medications; (3) SG as a revision surgery; (4) pregnancy during follow-up; and (5) incomplete follow-up data. Patients were assigned into Male and Female groups according to sex.
All patients underwent follow-up at 1, 3, 6, and 12 months after surgery. The primary outcome was weight loss after surgery evaluated by the TWL% and EWL%. The secondary outcome was the analysis of predictors of weight loss after SG in male and female patients. TWL% was calculated as weight loss/baseline weight × 100%; EWL% was calculated as [preoperative weight - postoperative weight]/[preoperative weight - 23×(body length)2)] × 100% (14, 15).
2.3 Procedure
All SG operations were performed laparoscopically by the same experienced team as reported before (16). The first step is the greater curvature was dissected free from the omentum starting 2-4 cm from the pylorus and up to the angle of His. After exposure of the left diaphragmatic crus and adequate clearance of the posterior stomach, a vertical gastrectomy was initiated from 4-6 cm proximal to the pylorus with the use of a 36-Fr bougie to create a tubular stomach. Upon discharge, the patient was given suggestions for dietary and physical activities. Dietitians recommend gradually transitioning from liquid to solid foods and achieving energy balance through higher protein, lower fat and lower carbohydrate intake. The exercise prescriber will develop an individualized exercise prescription including training frequency, intensity, time, and type.
2.4 Propensity score matching
Nearest-neighbor matching with caliper was used to balance baseline body mass index (BMI) between the Male and Female groups. Matching was performed with the use of a 1:2 protocol without replacement, with a caliper width equal to 0.2 (17).
2.5 Psychological assessment
The Chinese versions of the Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) were used to evaluate the psychological situation of anxiety and depression of patients the day before surgery (18, 19). A score above 50 or 53 was defined as anxiety or depression in the SAS and SDS. The levels of anxiety or depression were further classified according to the score.
2.6 Oral glucose tolerance test
Participants underwent a 2-hour oral glucose tolerance test (OGTT) (75 g of glucose in 250 ml water) on the second day of admission after an overnight fast. Blood samples were collected at 0, 30, 60, and 120 minutes after glucose intake. The plasma levels of glucose and insulin were determined at each time point.
2.7 Body composition by dual-energy X-ray absorptiometry
Visceral fat area, and body fat percentage were determined with dual-energy X-ray absorptiometry using the HOLOGIC DELPHI system with QDR software, v.11.1 (Hologic Bedford, MA, USA). The exams were whole-body scans, all of which were performed in a temperature-controlled laboratory. All operations were carried out by trained researchers according to the manufacturer’s instructional protocols. The very few cases with missing data were excluded from the analyses.
2.8 Biochemical analysis
Blood lipid analysis, index of thyroid function, sex hormone, liver and renal function, and the levels of plasma glucose were measured using a Roche Cobas 8000 modular analyzer system (Roche Diagnostics, IN, USA). Plasma insulin was determined by a two-site enzymatic assay using a Tosoh 2000 autoanalyzer (Tosoh Corp., Tokyo, Japan). The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated as fasting insulin (mU/mL) ×fasting plasma glucose (mmol/L)/22.5.
2.9 Nomogram-based prediction
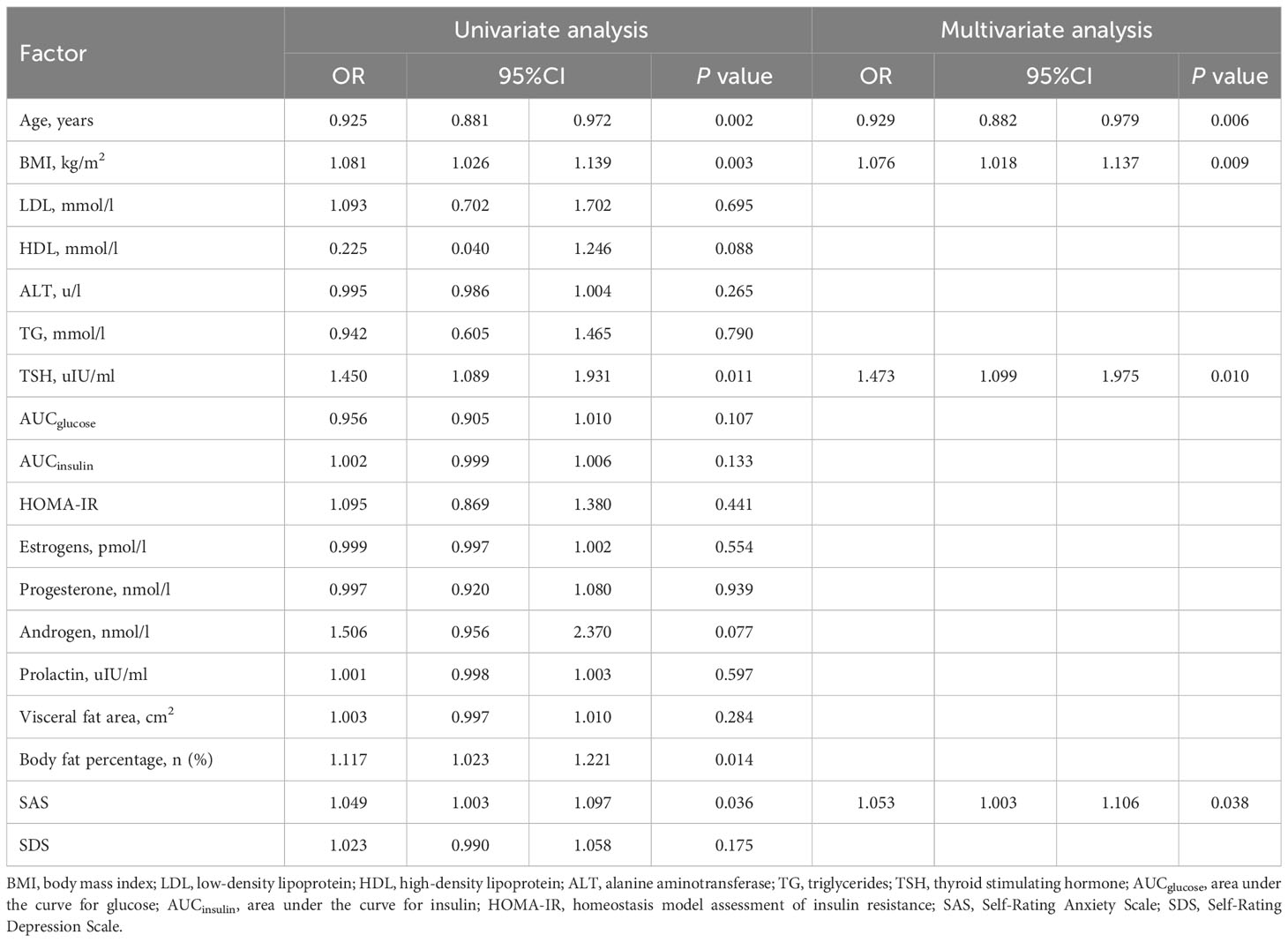
Candidate clinical predictors included age, BMI, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides (TG), alanine aminotransferase (ALT), thyroid stimulating hormone (TSH), area under the curve for glucose (AUCglucose), area under the curve for insulin (AUCinsulin), HOMA-IR, estrogen, progesterone, androgen, prolactin, visceral fat area, body fat percentage, SAS score, and SDS score.
There is currently no clear standard for judging postoperative weight loss with respect to TWL%. In order to make more convincing predictions, patients in the Male and Female groups were ranked in descending order separately and further divided into three equal parts according to the 1-year TWL%. Univariate regression analysis was conducted between the one-third of patients with the highest TWL% and the one-third with the lowest TWL% for potential predictive factors. Those with P < 0.1 were further analyzed by multivariate regression. The results are shown as odds ratios (ORs) with 95% confidence intervals (CIs). Significant factors (P < 0.05) according to the results of the multivariate regression were considered independent predictors and used to establish the nomogram.
The area under the receiver operating characteristic (ROC) curve (AUC) and concordance index (C-index) were used to evaluate the predictive accuracy of the nomogram model. A calibration curve was used to evaluate the accuracy of the prediction. A decision curve analysis (DCA) was performed to assess the clinical usefulness of the nomogram. The nomogram was internally validated for discrimination and calibration by bootstrapping (1000 resamples).
2.10 Statistical analysis
Statistical analysis was performed using SPSS Statistics version 25.0 (SPSS Inc., Chicago, Illinois, US) and R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables that conformed to normal distribution were presented as the means ± SDs and compared using the independent t test; variables without normal distribution were given as median with interquartile range and compared by the Mann−Whitney U test. Categorical variables were shown as numbers with percentages and compared with the chi-squared test or Fisher’s exact test. Two-way ANOVA followed by Bonferroni’s multiple comparisons was performed to analyze weight loss over time after surgery, and the results were reported as the AP by group, BP over time, and CP due to the interaction of the two factors. The AUC of glucose and insulin during OGTT was calculated by the trapezoidal method. A P value <0.05 was considered to indicate significance.
3 Results
3.1 Baseline characteristics of participants
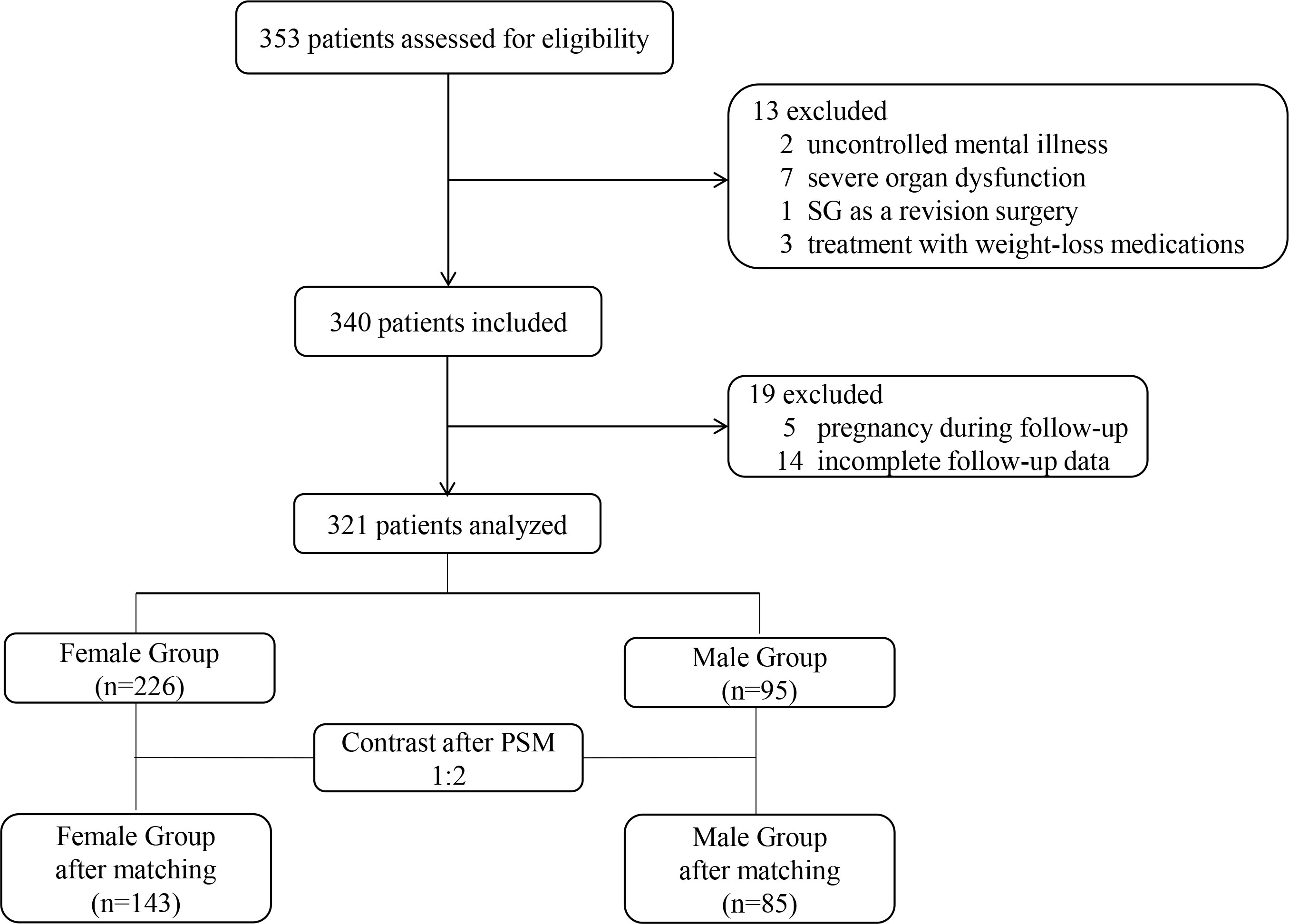
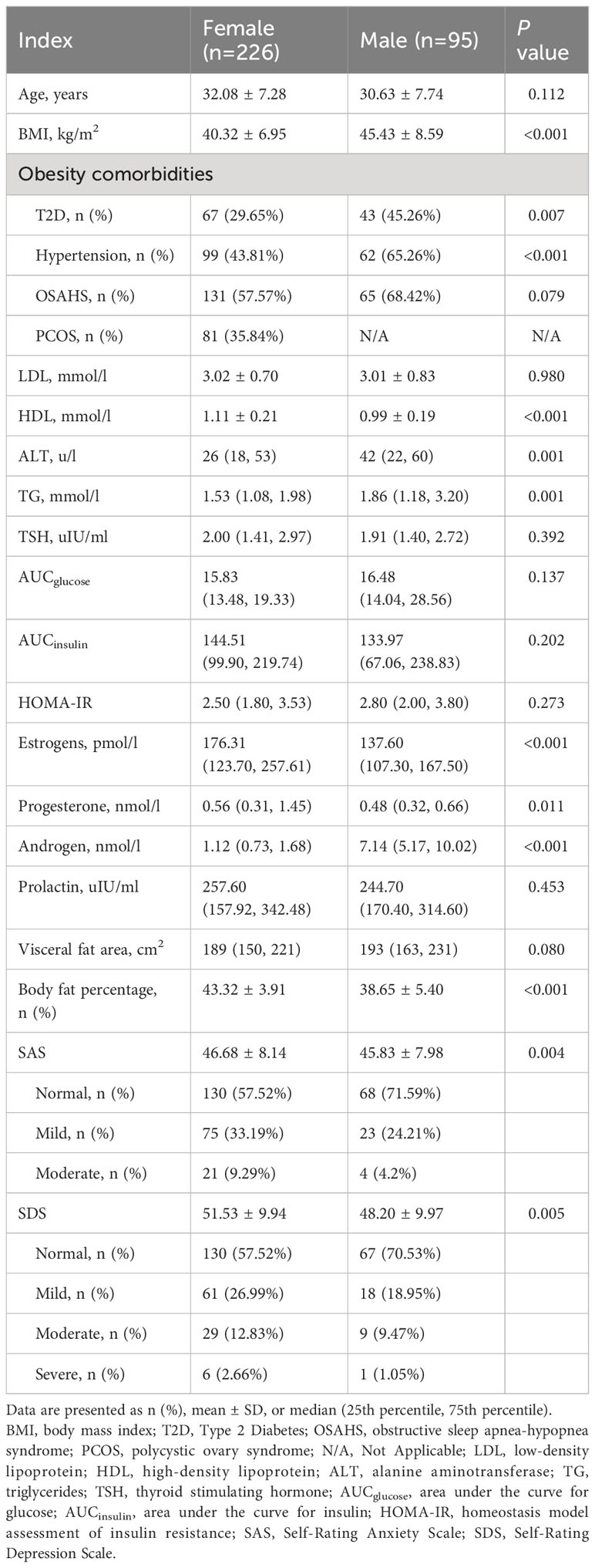
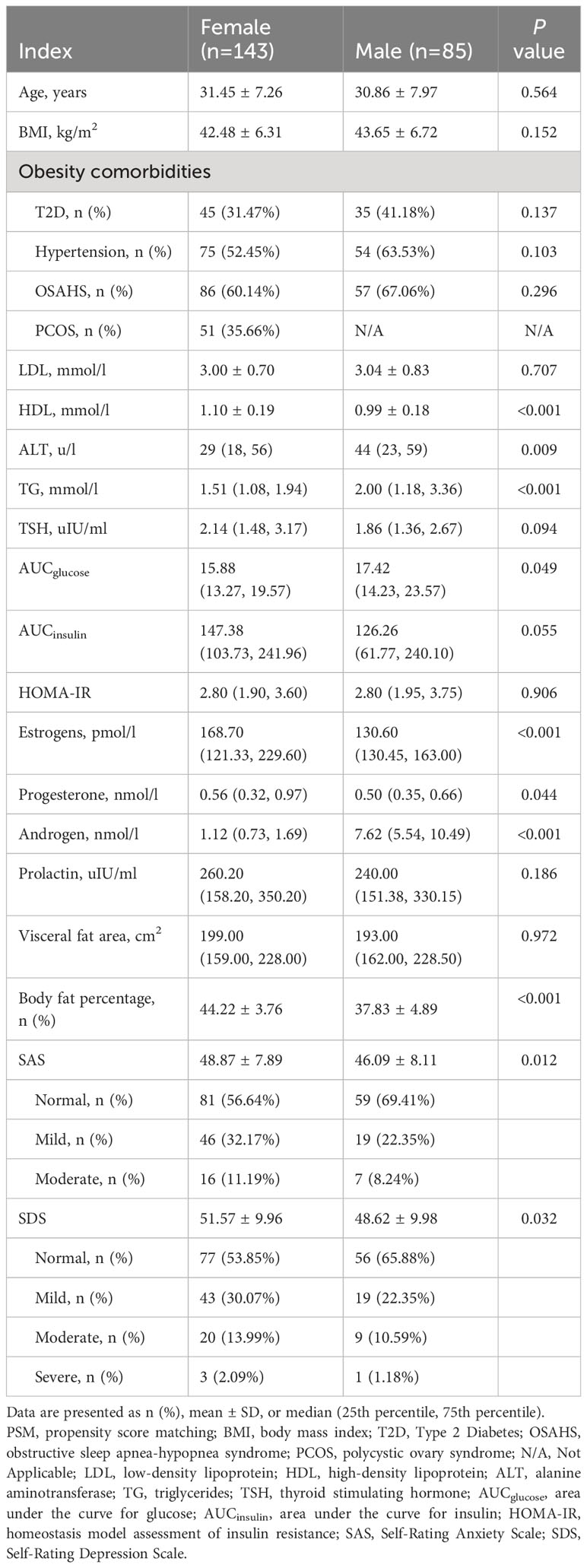
From the 353 patients assessed for eligibility, 321 patients (95 male and 226 female) were included (Figure 1). The male patients had a significantly higher BMI (45.43 ± 8.59 vs. 40.32 ± 6.95 kg/m2; P<0.001; Table 1). After propensity score matching (PSM), 85 patients in the Male group and 143 patients in the Female group were preserved with balanced baseline BMI (42.48 ± 6.31 vs. 43.65 ± 6.72 kg/m2; P=0.152; Table 2). The obesity comorbidities were paired between the two groups. The Male group had higher levels of ALT, TG, AUCglucose, and androgen, while having lower levels of HDL, estrogen, progesterone, body fat percentage, SAS score and SDS score (Table 2).
3.2 Weight loss after SG
Along with the decrease in BMI, the TWL% and EWL% continued to increase for up to 1 year after SG in both the Male and Female groups (Figures 2A–C). For the original 321 patients before PSM, although no significant between-group difference was found in TWL% after SG (AP =0.097), the Female group showed a higher EWL% at 6 and 12 months after SG than the Male group (Figure 2C). However, this difference disappeared after PSM by baseline BMI. The Male and Female groups showed comparable BMI, TWL% and EWL% after SG (Figures 2D–F). These results suggest that for selective patients with comparable BMI, sex was not an influencing factor of weight loss for up to 1 year after SG.
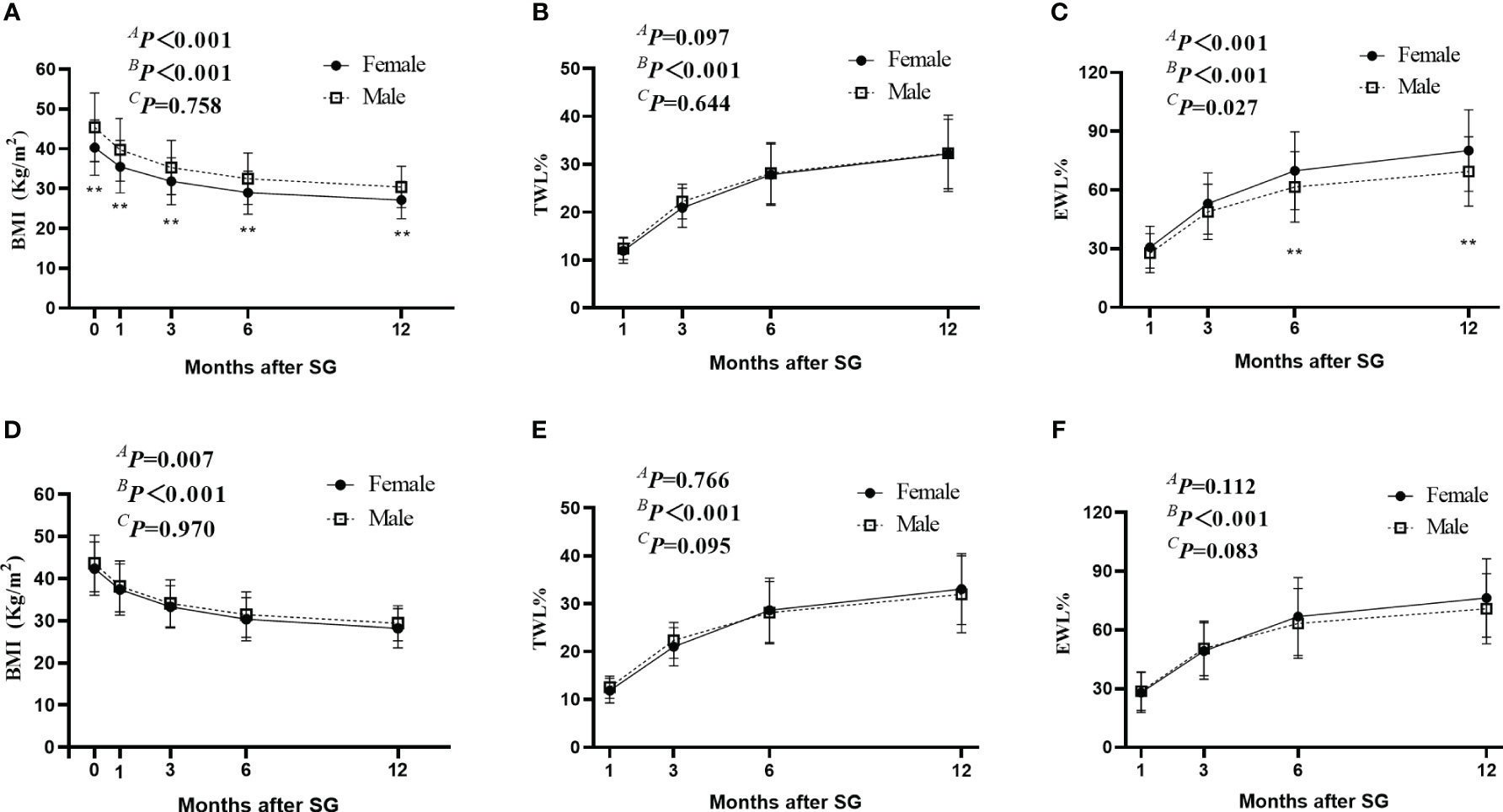
Figure 2 Weight-loss effect after SG. (A–C) indicated the change of BMI (A), TWL% (B), and EWL% (C) after SG in the original cohort before PSM. D-F indicated the change of BMI (D), TWL% (E), and EWL% (F) after SG in the Male group and Female group after PSM. AP by group, BP over time, and CP due to the interaction of the two factors. ** P <0.05 SG, sleeve gastrectomy; PSM, propensity score matching; BMI, body mass index; TWL%, percentage of total weight loss; EWL%, percentage of excess weight loss.
3.3 Predictor selection and development of the nomogram
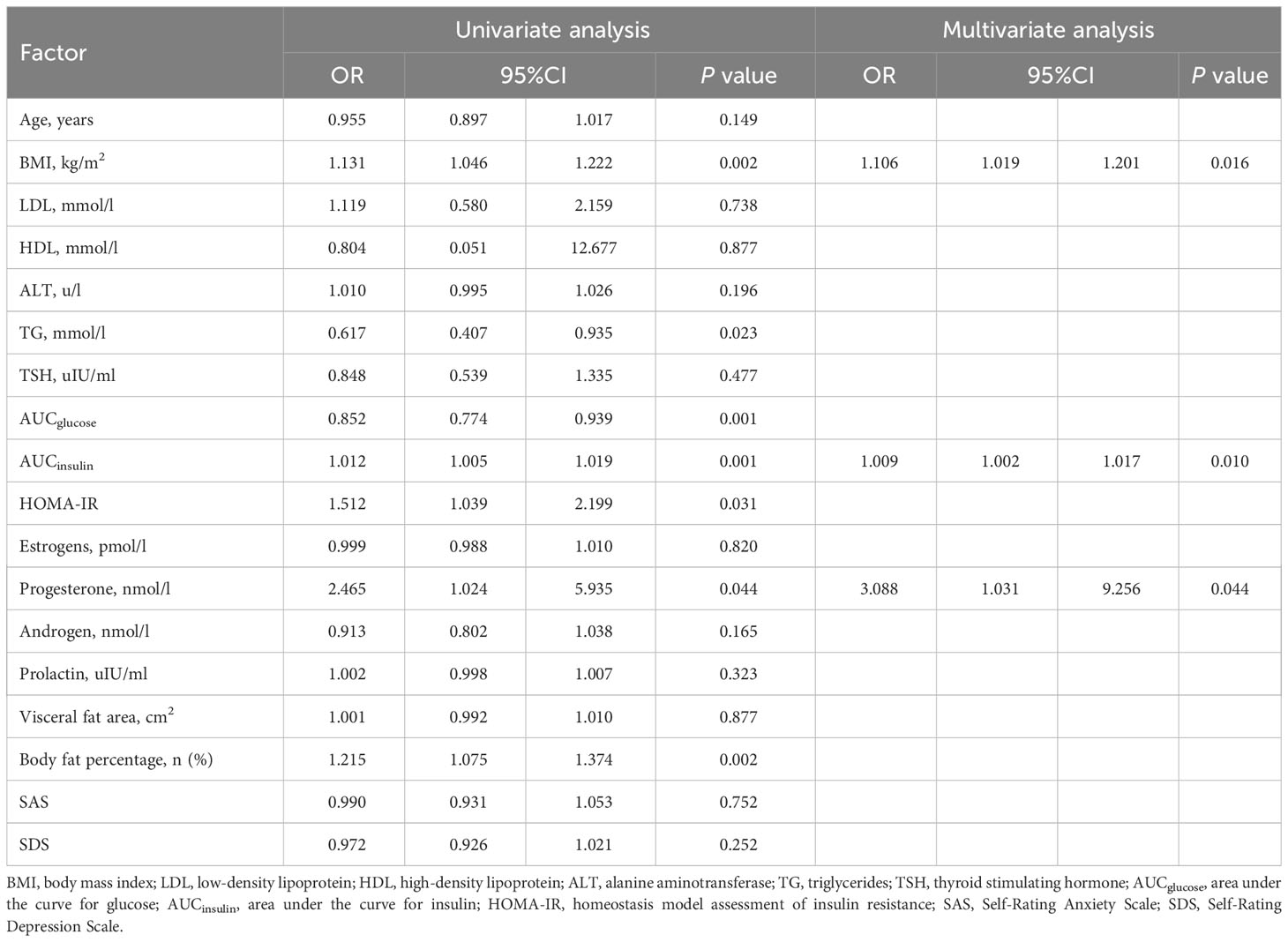
Patients with TWL% <27.65% and >35.29% in the Male group as well as those with TWL% <29.26% and >35.49% in the Female group were included in the analysis of predictors. In the multivariate analysis, factors significantly and independently associated with the weight-loss effect in the Male group were baseline BMI [odds ratio (OR) 1.106, 95% confidence interval (CI) 1.019-1.201, P=0.016], AUCinsulin [OR 1.009, 95% CI 1.002-1.017, P=0.010], and progesterone [OR 3.088, 95% CI 1.031-9.256, P=0.044]. In the Female group, age [OR 0.929, 95% CI 0.882-0.979, P=0.006], baseline BMI [OR 1.076, 95% CI 1.018-1.147, P=0.009], TSH [OR 1.473, 95% CI 1.009-1.975, P=0.010], and SAS score [OR 1.053, 95% CI 1.003-1.106, P=0.038] were associated with the weight-loss effect after SG (Tables 3, 4). These predictor factors were incorporated into the nomogram (Figures 3, 4). Also, the visualization of sex-specific nomogram model of weight loss effect was illustrated in Figure 5.
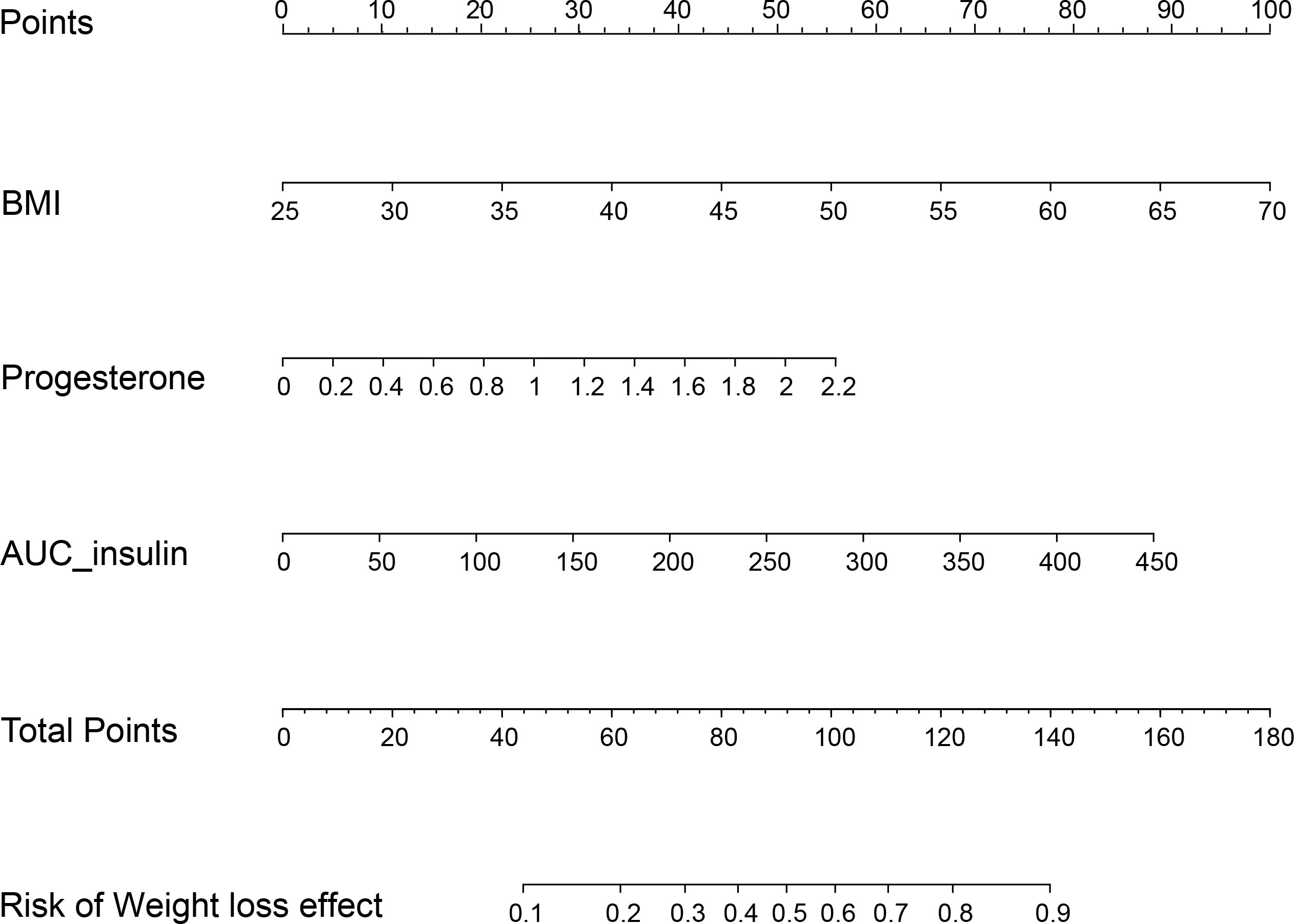
Figure 3 Nomogram for prediction of weight loss effect in the Male group. BMI, body mass index; AUCinsulin, area under the curve for insulin.
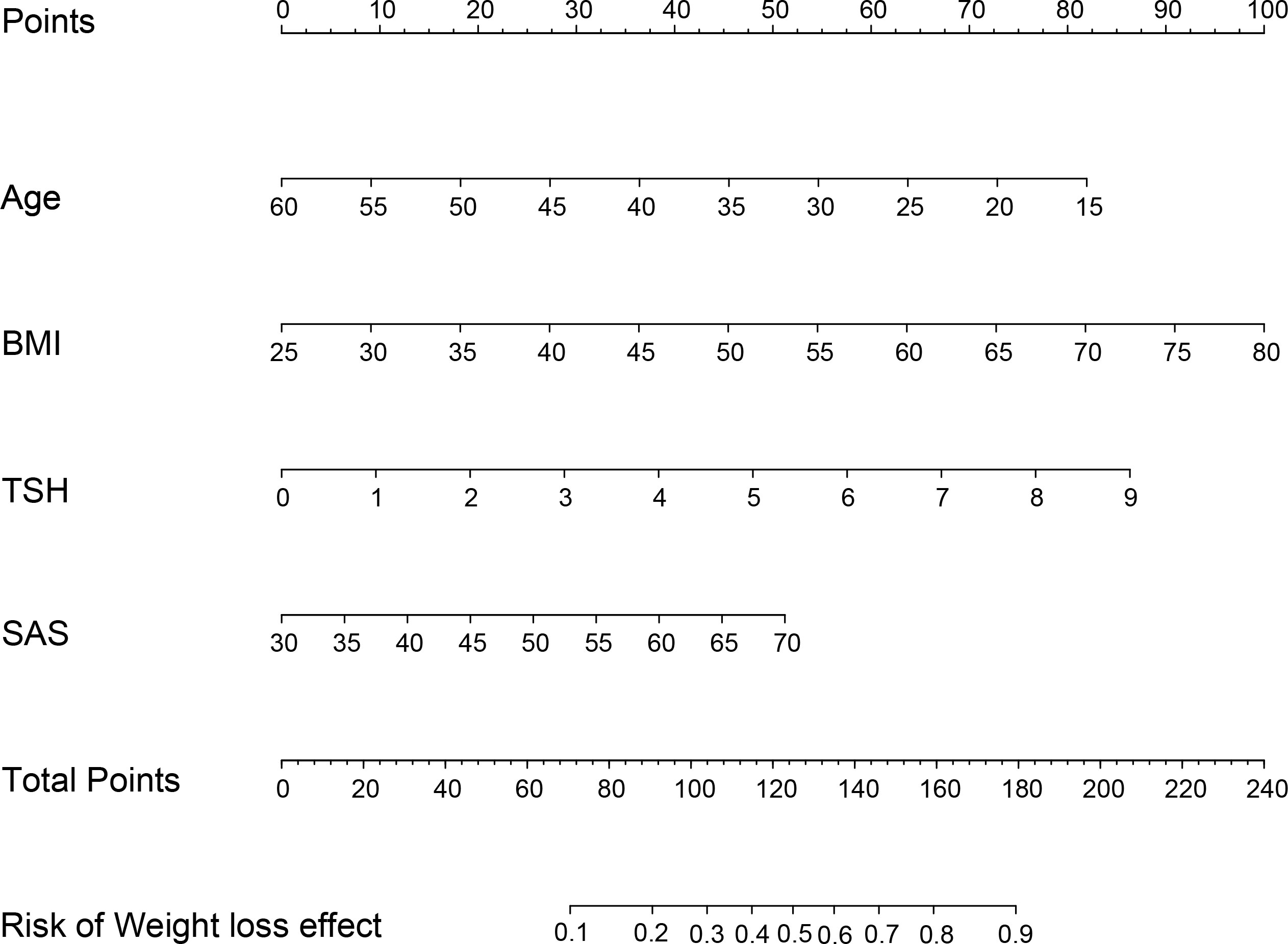
Figure 4 Nomogram for prediction of weight loss effect in Female group. BMI, body mass index; TSH, thyroid stimulating hormone; SAS, Self-Rating Anxiety Scale.

Figure 5 Visualization of sex-specific nomogram model of weight loss effect. (+) indicates positive correlation with weight loss effect; (-) indicates negative correlation with weight loss effect. BMI, body mass index; AUCinsulin, area under the curve for insulin; TSH, thyroid stimulating hormone; SAS, Self-Rating Anxiety Scale.
3.4 Validation and clinical utility of the nomogram for predicting
The AUC of the predicted nomogram was 0.844 (95% CI 0.745-0.943) in the Male group and 0.761 (95% CI 0.685-0.837) in the Female group (Figures 6A, D). The corrected C-index after bootstrapping was 0.818 and 0.738 in the Male and Female groups, respectively. The calibration curves of the nomogram for the predicted weight-loss effect in both groups showed good agreement between prediction and observation (Figures 6B, E). The DCA curve shows the obvious net benefits of the nomogram in both the Male and Female groups (Figures 6C, F).
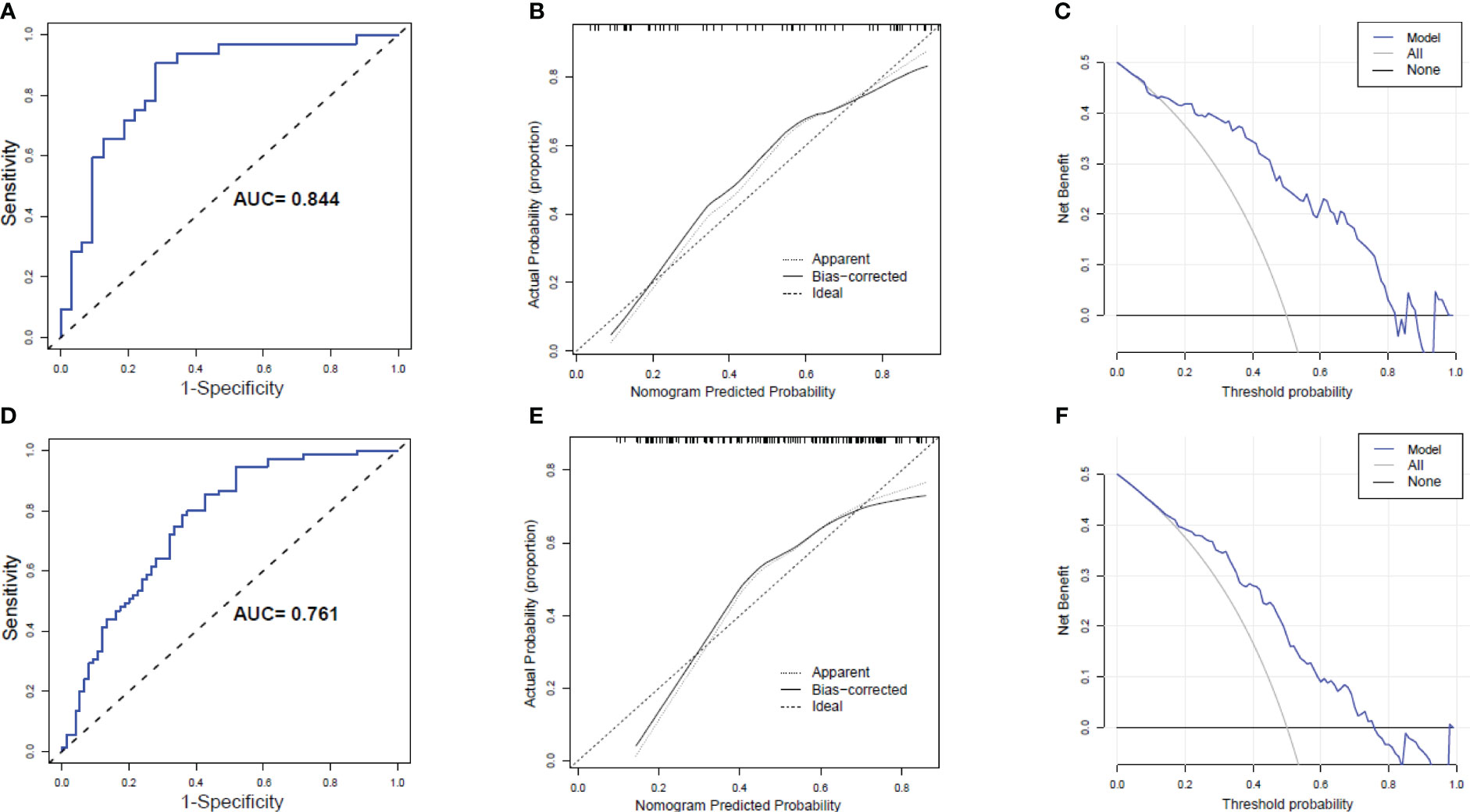
Figure 6 Receiver operating characteristic (ROC) curves, Calibration curves and Decision curve analysis (DCA) of the nomogram prediction in the Male group and Female group. (A) ROC curves in the Male group; (B) Calibration curves of in the Male group. (C) DCA in the Male group. (D) ROC curves in the Female group; (E) Calibration curves of in the Female group. (F) DCA in the Female group.
4 Discussion
The principal findings of the current study were that although there was no sex difference in the weight-loss effect after SG, sex dimorphism exists in predictors of weight loss after SG. For male patients, baseline BMI, AUCinsulin, and progesterone were independent predictors of weight loss after surgery. For female patients, baseline BMI, age, TSH, and SAS were independent predictors of weight loss after SG.
A marked sex disproportion exists in patients undergoing bariatric metabolic procedures. According to the Fourth IFSO Global Registry Report 2018, female patients was 73.7% among those who underwent the primary procedure (20). Controversy existed for a long time regarding whether male patients could achieve better weight-loss outcomes after bariatric surgery. A recent systematic review collected evidence on this issue and suggested that sex did not have a clear effect on the weight loss efficiency of SG (6). However, that conclusion was weakened by the high heterogeneity and low comparability of the study design, subject characteristics, and follow-up time in the 5 included studies. More specifically, 2 studies favored females, 1 favored males, and 2 showed similar weight loss between sex groups. Moreover, most of the 5 included studies did not have a baseline BMI that was balanced between the Male and Female groups. In the present study, male patients accounted for 29.56% of the entire cohort with higher BMI before surgery. Our results confirmed that for patients with comparable BMI, no sex difference was detected in the weight-loss effect after SG.
While SG is a well-established and widely performed procedure, there are still concerns that the weight-loss effect of SG cannot catch up with that of Roux-en-Y gastric bypass. The weight-loss effect of SG varied among patients and was affected by a series of factors. Identifying such factors can help surgeons make better care plans for the specific patient and decrease the numbers of revisional patients and nonresponders. Although studies are currently exploring predictors of weight loss after SG, there is no consensus among investigators (21). More importantly, there is a particular lack of sex-specific prediction models. The present study aimed to address this issue based on a prospective cohort.
The present study showed that higher baseline BMI predicted better postoperative weight loss in both sex groups. However, some studies have suggested that patients with a lower BMI tended to have more weight loss after SG (22, 23). This inconsistency could be explained by the discrepancy in the weight-loss metric used. Specifically, those other studies used EWL% or percentage of excess BMI loss (EBMIL%), whereas TWL% was used in the present study. Researchers have referred to the issue as the “Double Booby-Trap” of EWL%/EBMIL% to indicate that the conclusion would be overturned when EWL%/EBMIL% was used instead of TWL% (24). The “Double Booby-Trap” effect worsens with lower baseline BMI due to the algebraic construction of the EWL%/EBMIL%. In fact, many official academic organizations have recommended using TWL% as an alternative to EWL% (or EBMIL%) as the primary measure of weight loss (25). Studies are needed to provide more evidence on the correlation between baseline BMI and TWL% after SG. Furthermore, the necessity of PSM for baseline BMI was further demonstrated given that baseline BMI was an important predictor of weight loss in both sex groups.
In female patients, age is negatively associated with weight-loss outcomes, as has been demonstrated in a large number of studies (26–29). The basal energy expenditure has a tendency to decrease tremendously with age, switching from 60 to 70% of total metabolism around the age of 20–30 to 40% at the age of 50 (30). In addition, younger female obese patients usually have higher motivation for weight loss, probably because of their expectation of regaining self-confidence (31). Our study suggests the necessity for female patients to undergo surgery without delay because older patients need to make more efforts to achieve better weight-loss results.
Female patients with higher preoperative TSH levels achieved better weight-loss outcomes. Clinical studies have demonstrated a positive correlation between obesity and plasma TSH levels (32, 33). A previous study initially reported that the TSH level decreased significantly after SG in euthyroid patients. However, the TSH decrease was not associated with EWL% (34). The exact mechanism leading to a decrease in TSH following SG is not clear. The main explanation, suggested by several studies, is related to a decrease in leptin levels following surgery, which was produced by adipocytes and was shown to have a stimulatory effect on thyroid activity. Muraca et al. reported that baseline TSH levels had no association with weight loss after SG (35). However, as most studies assessing the efficiency of SG failed to report results by sex, there is still a lack of direct evidence in terms of the correlation between baseline TSH level and the weight-loss effect after SG in female patients. The predictive value of baseline TSH needs to be confirmed by further studies.
The rate of psychological behavior abnormalities among obese people who are willing to undergo bariatric surgery is as high as 70% (36). In the present study, preoperative anxiety was another positive predictor of weight loss in female patients. These results are believed to be related to the increased adherence to the postoperative instructions and were consistent with those of previous studies (37, 38). A study published in 2022 revealed an interesting trajectory in which patients with higher levels of anxiety lost the most weight 12 months after bariatric surgery but tended to regain more weight 30 months after surgery (39). The mechanisms by which anxiety contributes to this trajectory should be examined in future research. Our study suggested that female patients are more susceptible to the impact of anxiety. For female patients, the identification and intervention of psychological disorders, especially those associated with anxiety, is of vital importance.
In the present study, higher levels of progesterone predicted better weight-loss outcomes in male patients. Although little is known about the physiology, endocrinology, and pharmacology of progesterone in male, it has been found that progesterone can bind to certain receptors in adipose tissue and regulate lipoprotein lipase, thereby increasing fat accumulation (40). In addition, progesterone can synergize with estrogen to reduce lipolysis and promote fat accumulation (41). Unfortunately, there is limited evidence on the alteration of progesterone after SG in male patients, let alone its effect on or correlation with weight loss. A meta-analysis published in 2019 reported the impact of bariatric surgery on male sex hormones (42). However, no progesterone data were included. Based on our prediction model, progesterone may play specific physiological and pathophysiological roles in the weight-loss effect after SG in male. Further studies are needed.
Insulin resistance is the fundamental pathophysiological change in obesity. An increase in body weight induces insulin resistance and compensates for hyperinsulinemia, resulting in fat accumulation and metabolic disorders (43). AUCinsulin represents the total amount of insulin secretion after oral glucose load and can indicate insulin resistance to some extent (44). Our previous study confirmed the great effect of SG on the remission of insulin resistance (16), and the decrease in AUCinsulin after SG was related to weight loss after surgery. Therefore, patients with higher levels of AUCinsulin are likely to benefit more from surgery to achieve better weight loss. The present study confirmed that AUCinsulin was another positive predictor of weight loss after SG in male patients.
The major strength of the present study is that the sex-specific preoperative predictors of weight loss after SG were explored from a comprehensive set of clinical variables based on a prospective cohort. Our study has several limitations. First, the 1-year follow-up time was not long enough. However, the period of weight loss after SG is approximately 1 year, and the longer-term outcome may be affected by more factors in addition to surgery. Second, due to the single-center design and limited sample size, the predictive model was validated internally by bootstrapping and needs to be further confirmed by external validation.
5 Conclusion
The sex was not an influencing factor of weight loss for up to 1 year after SG. However, sex dimorphism still exists in terms of the preoperative predictors of weight loss after SG. For different sex groups, there could be differences in the focus of preoperative evaluation in regard to the weight-loss effect. Research with multi-centered design and long-term follow-up as well as the mechanism of TSH affecting the weight loss effect are of great significance in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee on Scientific Research of Shandong University Qilu Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JS: Data curation, Formal Analysis, Writing – original draft. TZ: Data curation, Formal Analysis, Writing – original draft. SX: Data curation, Formal Analysis, Writing – original draft. TL: Supervision, Validation, Writing – original draft. YZ: Supervision, Validation, Writing – original draft. XH: Funding acquisition, Writing – review & editing, Writing – original draft. SL: Funding acquisition, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by grants from the National Natural Science Foundation of China (82100853), the Natural Science Foundation of Shandong Province of China (ZR2021QH028), and the Clinical Research Project of Shandong University (2020SDUCRCC024).
Acknowledgments
The authors thank the nursing staff of the Department of General Surgery, Qilu Hospital of Shandong University, for their expert assistance in performing the studies, and all the patients included and their families for their cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. World Obesity Atlas (2023). Available at: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023.
2. Testa G, Granero R, Siragusa C, Belligoli A, Sanna M, Rusconi ML, et al. Psychological predictors of poor weight loss following LSG: relevance of general psychopathology and impulsivity. Eat Weight Disord (2020) 25(6):1621–9. doi: 10.1007/s40519-019-00800-x
3. Chung AY, Thompson R, Overby DW, Duke MC, Farrell TM. Sleeve gastrectomy: surgical tips. J Laparoendosc Adv Surg Tech A. (2018) 28(8):930–7. doi: 10.1089/lap.2018.0392
4. Franken RJ, Sluiter NR, Franken J, de Vries R, Souverein D, Gerdes VEA, et al. Treatment options for weight regain or insufficient weight loss after sleeve gastrectomy: a systematic review and meta-analysis. Obes Surg (2022) 32(6):2035–46. doi: 10.1007/s11695-022-06020-0
5. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care (2016) 22(7 Suppl):s176–85.
6. Risi R, Rossini G, Tozzi R, Pieralice S, Monte L, Masi D, et al. Sex difference in the safety and efficacy of bariatric procedures: a systematic review and meta-analysis. Surg Obes Relat Dis (2022) 18(7):983–96. doi: 10.1016/j.soard.2022.03.022
7. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol (2015) 402:113–9. doi: 10.1016/j.mce.2014.11.029
8. Bhogal MS, Langford R. Gender differences in weight loss; evidence from a NHS weight management service. Public Health (2014) 128(9):811–3. doi: 10.1016/j.puhe.2014.06.019
9. Cooper AJ, Gupta SR, Moustafa AF, Chao AM. Sex/Gender Differences in Obesity Prevalence, Comorbidities, and Treatment. Curr Obes Rep. (2001) 10(4):458–66. doi: 10.1007/s13679-021-00453-x
10. Groth D, Woźniewska P, Olszewska M, Zabielski P, Ładny JR, Dadan J, et al. Gender-related metabolic outcomes of laparoscopic sleeve gastrectomy in 6-month follow-up. Wideochir Inne Tech Maloinwazyjne. (2020) 15(1):148–56. doi: 10.5114/wiitm.2019.86800
11. Perrone F, Bianciardi E, Benavoli D, Tognoni V, Niolu C, Siracusano A, et al. Gender influence on long-term weight loss and comorbidities after laparoscopic sleeve gastrectomy and roux-en-Y gastric bypass: a prospective study with a 5-year follow-up. Obes Surg (2016) 26(2):276–81. doi: 10.1007/s11695-015-1746-z
12. Seyssel K, Suter M, Pattou F, Caiazzo R, Verkindt H, Raverdy V, et al. A predictive model of weight loss after roux-en-Y gastric bypass up to 5 years after surgery: a useful tool to select and manage candidates to bariatric surgery. Obes Surg (2018) 28(11):3393–9. doi: 10.1007/s11695-018-3355-0
13. Andersen JR, Aadland E, Nilsen RM, Våge V. Predictors of weight loss are different in men and women after sleeve gastrectomy. Obes Surg (2014) 24(4):594–8. doi: 10.1007/s11695-013-1124-7
14. Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in asian Indian adults. Diabetes Care (2003) 26(5):1380–4. doi: 10.2337/diacare.26.5.1380
15. van de Laar AW, van Rijswijk AS, Kakar H, Bruin SC. Sensitivity and specificity of 50% Excess weight loss (50%EWL) and twelve other bariatric criteria for weight loss success. Obes Surg (2018) 28(8):2297–304. doi: 10.1007/s11695-018-3173-4
16. Huang X, Zhao Y, Liu T, Wu D, Shu J, Yue W, et al. β-cell function and insulin dynamics in obese patients with and without diabetes after sleeve gastrectomy. Diabetes. doi: 10.2337/db22-1048
17. Witzel S, Maier A, Steinbach R, Grosskreutz J, Koch JC, Sarikidi A, et al. Safety and Effectiveness of Long-term Intravenous Administration of Edaravone for Treatment of Patients With Amyotrophic Lateral Sclerosis [published correction appears in JAMA Neurol. JAMA Neurol (2022) 79(2):121–30. doi: 10.1001/jamaneurol.2021.4893
18. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. (1971) 12(6):371–9. doi: 10.1016/S0033-3182(71)71479-0
19. ZUNG WW. A SELF-RATING DEPRESSION SCALE. Arch Gen Psychiatry (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
20. Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO global registry report 2018. Obes Surg (2019) 29(3):782–95. doi: 10.1007/s11695-018-3593-1
21. Cottam S, Cottam D, Cottam A. Sleeve gastrectomy weight loss and the preoperative and postoperative predictors: a systematic review. Obes Surg (2019) 29(4):1388–96. doi: 10.1007/s11695-018-03666-7
22. Martin DJ, Lee CM, Rigas G, Tam CS. Predictors of weight loss 2 years after laparoscopic sleeve gastrectomy. Asian J Endosc Surg (2015) 8(3):328–32. doi: 10.1111/ases.12193
23. Binda A, Jaworski P, Kudlicka E, Ciesielski A, Cabaj H, Tarnowski W. The impact of selected factors on parameters of weight loss after sleeve gastrectomy. Wideochir Inne Tech Maloinwazyjne. (2016) 11(4):288–94. doi: 10.5114/wiitm.2016.64999
24. van de Laar AW. The %EBMIL/%EWL double booby-trap. A comment on studies that compare the effect of bariatric surgery between heavier and lighter patients. Obes Surg (2016) 26(3):612–3. doi: 10.1007/s11695-015-1967-1
25. van de Laar AW, Emous M, Hazebroek EJ, Boerma EJ, Faneyte IF, Nienhuijs SW. Reporting weight loss 2021: position statement of the dutch society for metabolic and bariatric surgery (DSMBS). Obes Surg (2021) 31(10):4607–11. doi: 10.1007/s11695-021-05580-x
26. Akhtar SJ, Helmer SD, Quinn KR, Lancaster BA, Howes JL, Brown NM. The effect of insurance status on bariatric surgery outcomes: A retrospective chart review study. Am Surg (2023) 89(5):1887–92. doi: 10.1177/00031348221074245
27. El Ansari W, Elhag W. Preoperative prediction of body mass index of patients with type 2 diabetes at 1 year after laparoscopic sleeve gastrectomy: cross-sectional study. Metab Syndr Relat Disord (2022) 20(6):360–6. doi: 10.1089/met.2021.0153
28. Janik MR, Rogula TG, Mustafa RR, Saleh AA, Abbas M, Khaitan L. Setting realistic expectations for weight loss after laparoscopic sleeve gastrectomy. Wideochir Inne Tech Maloinwazyjne. (2019) 14(3):415–9. doi: 10.5114/wiitm.2019.81661
29. Nagao Y, Diana M, Vix M, D'Urso A, Mutter D, Marescaux J. Age impact on weight loss and glycolipid profile after laparoscopic sleeve gastrectomy: experience with 308 consecutive patients. Surg Endosc. (2014) 28(3):803–10. doi: 10.1007/s00464-013-3261-4
30. Ferrucci L, Schrack JA, Knuth ND, Simonsick EM. Aging and the energetic cost of life. J Am Geriatr Soc (2012) 60(9):1768–9. doi: 10.1111/j.1532-5415.2012.04102.x
31. Theunissen CMJ, van Vlijmen A, Tak DJAM, Nyklíček I, de Jongh MAC, Langenhoff BS. Motivation and weight loss expectations in bariatric surgery candidates: association with 1- and 2-year results after bariatric surgery. Obes Surg (2020) 30(11):4411–21. doi: 10.1007/s11695-020-04811-x
32. Aykota MR, Atabey M. Effect of sleeve gastrectomy on thyroid-stimulating hormone levels in morbidly obese patients with normal thyroid function. Eur Rev Med Pharmacol Sci (2021) 25(1):233–40. doi: 10.26355/eurrev_202101_24389
33. Salman MA, Rabiee A, Salman A, Qassem MG, Ameen MA, Hassan AM, et al. Laparoscopic sleeve gastrectomy has A positive impact on subclinical hypothyroidism among obese patients: A prospective study. World J Surg (2021) 45(10):3130–7. doi: 10.1007/s00268-021-06201-5
34. Abu-Ghanem Y, Inbar R, Tyomkin V, Kent I, Berkovich L, Ghinea R, et al. Effect of sleeve gastrectomy on thyroid hormone levels. Obes Surg (2015) 25(3):452–6. doi: 10.1007/s11695-014-1415-7
35. Muraca E, Oltolini A, Pizzi M, Villa M, Manzoni G, Perra S, et al. Baseline TSH levels and short-term weight loss after different procedures of bariatric surgery. Int J Obes (Lond). (2021) 45(2):326–30. doi: 10.1038/s41366-020-00665-6
36. Fuchs HF, Laughter V, Harnsberger CR, Broderick RC, Berducci M, DuCoin C, et al. Patients with psychiatric comorbidity can safely undergo bariatric surgery with equivalent success. Surg Endosc. (2016) 30(1):251–8. doi: 10.1007/s00464-015-4196-8
37. Agüera Z, García-Ruiz-de-Gordejuela A, Vilarrasa N, Sanchez I, Baño M, Camacho L, et al. Psychological and personality predictors of weight loss and comorbid metabolic changes after bariatric surgery. Eur Eat Disord Rev (2015) 23(6):509–16. doi: 10.1002/erv.2404
38. Kinzl JF, Schrattenecker M, Traweger C, Mattesich M, Fiala M, Biebl W. Psychosocial predictors of weight loss after bariatric surgery. Obes Surg (2006) 16(12):1609–14. doi: 10.1381/096089206779319301
39. Aylward L, Lilly C, Tabone L, Szoka N, Abunnaja S, Cox S. Anxiety predicts reduced weight loss 30 months after bariatric surgery. Surg Obes Relat Dis (2022) 18(7):919–27. doi: 10.1016/j.soard.2022.04.007
40. Hatta H, Atomi Y, Shinohara S, Yamamoto Y, Yamada S. The effects of ovarian hormones on glucose and fatty acid oxidation during exercise in female ovariectomized rats. Horm Metab Res (1988) 20(10):609–11. doi: 10.1055/s-2007-1010897
41. Campbell SE, Febbraio MA. Effects of ovarian hormones on exercise metabolism. Curr Opin Clin Nutr Metab Care (2001) 4(6):515–20. doi: 10.1097/00075197-200111000-00009
42. Lee Y, Dang JT, Switzer N, Yu J, Tian C, Birch DW, et al. Impact of bariatric surgery on male sex hormones and sperm quality: a systematic review and meta-analysis. Obes Surg (2019) 29(1):334–46. doi: 10.1007/s11695-018-3557-5
43. Barazzoni R, Gortan Cappellari G, Ragni M, Nisoli E. Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord (2018) 23(2):149–57. doi: 10.1007/s40519-018-0481-6
Keywords: sleeve gastrectomy, weight loss, sex dimorphism, predictor, nomogram
Citation: Shu J, Zhu T, Xiong S, Liu T, Zhao Y, Huang X and Liu S (2024) Sex dimorphism in the effect and predictors of weight loss after sleeve gastrectomy. Front. Endocrinol. 14:1333051. doi: 10.3389/fendo.2023.1333051
Received: 04 November 2023; Accepted: 18 December 2023;
Published: 10 January 2024.
Edited by:
Yayun Wang, Air Force Medical University, ChinaReviewed by:
Mohamad Mokadem, The University of Iowa, United StatesSabrina Oussaada, Academic Medical Center, Netherlands
Copyright © 2024 Shu, Zhu, Xiong, Liu, Zhao, Huang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaozhuang Liu, bGl1c2hhb3podWFuZ0BzZHUuZWR1LmNu; Xin Huang, aHVhbmd4aW5zZHVAMTYzLmNvbQ==
 Jiaxin Shu
Jiaxin Shu Tao Zhu1,2
Tao Zhu1,2 Teng Liu
Teng Liu Xin Huang
Xin Huang Shaozhuang Liu
Shaozhuang Liu