- 1Department of Breast Surgery, Xingtai City People’s Hospital, Xingtai, Hebei, China
- 2Department of General Surgery, Aerospace Center Hospital, Beijing, China
In the modern era, the escalating global prevalence of obesity has profound implications on female reproductive health. Obesity, transcending mere lifestyle choices, has evolved into a complex disorder affecting physiological and metabolic functions. Concurrently, female infertility is rising as a significant global health issue. Obesity, with its extensive systemic effects, is pinpointed as a major disruptor. The convergence of these health challenges reveals a multifaceted scenario: on one hand, obesity directly impacts female reproductive health, particularly in the context of conditions like polycystic ovary syndrome (PCOS) and menstrual disturbances; on the other, the psychosocial consequences of infertility might intensify weight-gain patterns, forming a challenging cycle. Additionally, the economic implications of treating obesity-related infertility are considerable. This review delves into the myriad ways obesity affects female reproductive health, drawing insights from epidemiological, clinical, and molecular studies. It explores the epidemiological relationship between obesity and PCOS, the influence of obesity on menstrual disturbances, and the broader impact of obesity on female infertility. Weight loss, through pharmacological interventions, surgical methods, or lifestyle adjustments, emerges as a promising strategy. Lastly, the efficacy of assisted reproductive technologies, such as IVF, is influenced by obesity, underscoring the importance of an optimal body mass index. The review also highlights the molecular and physiological mechanisms underlying the impact of obesity on female reproductive health, including the disruption of the hypothalamic-pituitary-ovary axis, altered adipokine secretion, and the role of chronic inflammation and oxidative stress.
Introduction
In the contemporary epoch, the burgeoning prevalence of obesity has emerged as a global health conundrum, casting a shadow over myriad facets of human well-being (1).Defined primarily by an excessive accumulation of adipose tissue, obesity is no longer merely a reflection of lifestyle choices; it has metamorphosed into a multifaceted disorder with profound implications on physiological and metabolic functions (2, 3). According to the World Health Organization, in 2011, more than 1.6 billion adults were overweight, and 400 million were obese highlighting the severity of this global issue (4).
Alongside the rising obesity issue, female infertility, defined as the inability to attain clinical pregnancy following 12 months of consistent unprotected intercourse, is increasingly becoming a major global health concern. The intricate dance of hormones, cellular processes, and anatomical structures that underpin female fertility is susceptible to perturbations, and obesity, with its widespread systemic effects, has been identified as a significant disruptor (5). The intersection of these two health challenges paints a complex picture. On one hand, obesity, with its associated metabolic and endocrine aberrations, can directly impinge on the reproductive health of women (6, 7). On the other, the psychosocial ramifications of infertility can exacerbate lifestyle patterns conducive to weight gain, creating a vicious cycle that is challenging to disrupt. The significance of understanding this relationship is manifold. Beyond the immediate health implications for the affected individuals, there are broader societal and economic repercussions. Infertility can be a source of profound psychological distress, impacting relationships, mental health, and overall quality of life (8). Moreover, in many cultures, childbearing is intricately linked with societal roles and expectations, and infertility can lead to stigmatization and marginalization.
Furthermore, the economic burden associated with treating infertility, particularly in the context of obesity, is substantial (9). From diagnostic procedures to therapeutic interventions, the costs can be prohibitive, placing additional strain on already stretched healthcare systems. In this review, we endeavor to elucidate the multifarious ways in which obesity impinges upon female reproductive health. Drawing from a rich tapestry of epidemiological, clinical, and molecular studies, we aim to provide a comprehensive overview of this critical intersection of metabolic and reproductive health.
Impact of obesity on polycystic ovary syndrome
The epidemiological relationship between obesity and polycystic ovary syndrome (PCOS) has been extensively researched (10–12). PCOS, a hormonal disorder with irregular menstrual cycles and hyperandrogenism, is often linked with metabolic issues like insulin resistance. The complex interaction between obesity and PCOS influences both its etiology and management. Visceral adiposity, more than subcutaneous fat, is notably associated with the hormonal imbalances in PCOS, impacting female fertility (13). Obesity, particularly central obesity, exacerbates the metabolic and reproductive abnormalities associated with PCOS (14). Women with PCOS and obesity are at a heightened risk for insulin resistance, hyperinsulinemia, and type 2 diabetes (15). The hyperinsulinemia, in turn, can lead to increased ovarian androgen production, further aggravating the symptoms of PCOS (15). Additionally, obesity influences the secretion of various adipokines, such as leptin and adiponectin, which are known to play significant roles in reproductive health. Elevated leptin levels, often found in obese individuals, can disrupt normal ovarian function and are associated with the pathophysiology of PCOS (16). Conversely, adiponectin, known for its anti-inflammatory and insulin-sensitizing properties, is typically reduced in obesity and may contribute to the reproductive dysfunctions seen in PCOS (17). This vicious cycle underscores the importance of weight management in women with PCOS. Furthermore, the prevalence of PCOS varies across different populations and is influenced by diagnostic criteria and study design. However, studies clearly indicate a higher prevalence of PCOS among overweight and obese individuals. A study highlighted a 28.3% prevalence of PCOS in overweight and obese women, emphasizing the strong association between these conditions. Notably, the PCOS patients, with an average age of 26 ± 7 years, were significantly younger than the nonhyperandrogenic controls, who had an average age of 32 ± 8 years (18). In women with PCOS, cardiovascular risk factors such as hypertension and dyslipidemia are intensified by obesity. A previous study reported an adverse cardiovascular risk profile in women aged 25-34 years old diagnosed with PCOS, with obesity playing a significant role in this risk elevation (19) (Table 1).
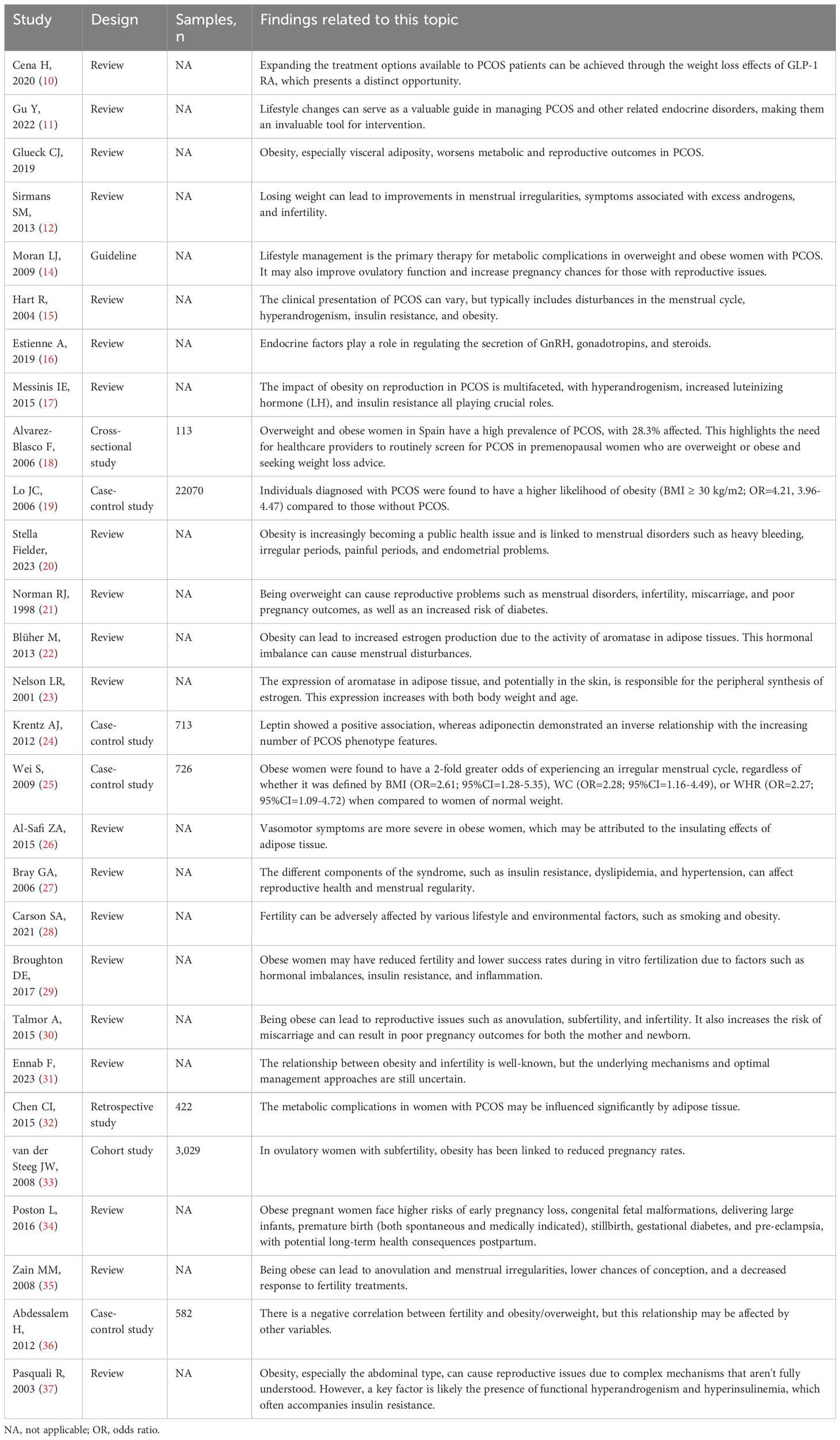
Table 1 The relevant studies linking the obesity for PCOS, menstrual disturbances and female infertility.
Impact of obesity on menstrual disturbances
Obesity’s influence on menstrual disturbances is multifaceted (20, 21). The adipose tissue, abundant in obese individuals, plays a significant role in steroid metabolism, leading to increased estrogen production. This increase is largely due to the activity of aromatase in adipose tissues, which converts androgens to estrogens. The elevated estrogen levels associated with obesity can disrupt the hormonal balance, potentially contributing to menstrual disturbances (22, 23). Obesity also alters the levels of adipokines like leptin and adiponectin, which are crucial in regulating reproductive hormones and menstrual cycles. High leptin levels in obese women can lead to menstrual irregularities and anovulation (24). Elevated estrogen levels can disrupt the regular menstrual cycle, leading to early menarche in adolescents and potentially early menopause in older women (25). Research has delved into the intricate relationship between obesity and the menopausal transition, exploring the impact of obesity on the timing of menopause and its symptoms (26). Furthermore, the metabolic syndrome, which is closely associated with obesity, has been linked to menstrual disturbances. The syndrome’s components, including insulin resistance, dyslipidemia, and hypertension, can influence reproductive health and menstrual regularity (27) (Table 1).
Impact of obesity on female infertility
Obesity’s influence on female infertility is a topic of significant concern in reproductive medicine (28–31). The altered levels of adipokines in obesity, particularly the decrease in adiponectin and increase in leptin, are implicated in the pathogenesis of infertility. These changes can affect ovarian function, disrupt the hormonal balance necessary for ovulation, and impair endometrial receptivity (32). Several studies have delved into the intricate relationship between obesity and female infertility. In a prospective cohort study of 3,029 subfertile couples, a linear decline in spontaneous pregnancy rates was observed with each increase in body mass index (BMI) over 29 kg/m^2, showing a 4% decrease in pregnancy rates per kg/m^2 increase in BMI (33). One study discussed the epidemiological aspects and health consequences of obesity-related infertility, highlighting that assisted reproductive technology does not provide a straightforward solution to obesity-related infertility, as a high BMI also reduces the success rates of these treatments (34). Another research underscored the impact of obesity on female fertility and fertility treatments, emphasizing that the treatment of obesity should be the initial aim in obese infertile women before embarking on fertility treatments (35). A case-control study including 582 women found a significant negative association between obesity and female infertility, with an odds ratio of 3.26 for women with a BMI over 30 kg/m^2 (36). Furthermore, a comprehensive review provided insights into the epidemiology and pathophysiology of obesity in women and its implications for fertility (37) (Table 1).
Impact of age and obesity on female reproductive health
The interplay between age and obesity significantly influences female reproductive health. In women of reproductive age, obesity is associated with various reproductive challenges, including impaired ovulatory function, reduced implantation and pregnancy rates, and increased miscarriage rates. These issues become more pronounced with advancing age, particularly in women approaching the upper limits of their reproductive years. For instance, women aged 38 years and older with obesity experience sub-optimal reproductive performance, impacting fertilization rates, embryo development, and pregnancy outcomes (38, 39). Additionally, overweight and obesity in early adulthood are linked to an increased risk of menstrual irregularities and hypertension in pregnancy (40). This evidence highlights the compounded effects of age and obesity on female fertility, emphasizing the importance of addressing these factors in reproductive health management.
Molecular and physiological mechanisms
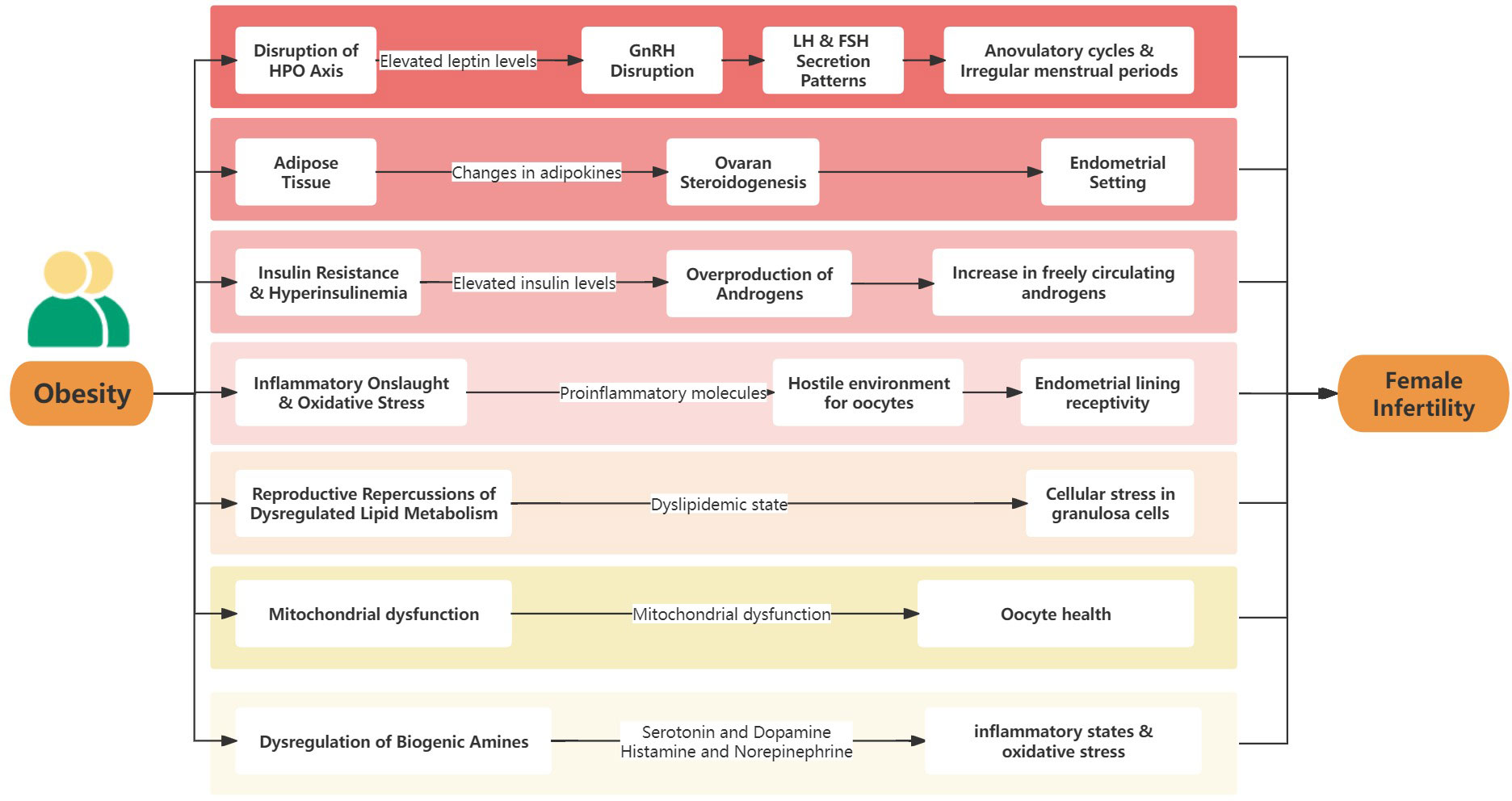
The complex relationship between obesity and female infertility is driven by numerous molecular and physiological mechanisms. Understanding these mechanisms is crucial to fully grasp this intricate association. At the cellular level, obesity induces a series of disruptions that significantly impact the reproductive system (Figure 1).
Disruption of the hypothalamic-pituitary-ovary axis
The hypothalamic-pituitary-ovary (HPO) axis, which orchestrates a delicate balance of hormonal interactions, is fundamental to female reproductive physiology. It regulates the cyclical patterns of menstruation and the intricate process of ovulation (41). However, obesity disrupts this harmonious system, primarily through elevated leptin levels (42). Leptin, reactive oxygen species (ROS), and other adipokines are significantly altered in obesity, contributing to the dysregulation of the HPO axis. Leptin, an adipokine produced by adipose tissue, is significantly increased in obese individuals. This elevation in leptin can interfere with the rhythmic secretion of Gonadotropin-releasing hormone (GnRH) from the hypothalamus. Specifically, high levels of leptin are thought to disrupt the pulsatile nature of GnRH release. This disruption can lead to altered secretion patterns of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which are crucial for the normal menstrual cycle and ovulation (24). In obesity, the increased leptin levels may desensitize the GnRH neurons to leptin’s regulatory effects, leading to a dysregulation in the release of GnRH. This dysregulation can result in either an increase or decrease in the frequency and amplitude of LH and FSH pulses. The altered LH and FSH pulses can then impact follicular development, leading to menstrual irregularities and ovulatory dysfunction (43). Additionally, chemerin, another adipokine, is often elevated in obesity and metabolic syndromes, contributing to the disruption of normal reproductive functions. Chemerin has been implicated in the regulation of adipogenesis and inflammation, and its elevated levels in obesity are associated with insulin resistance and dysregulated lipid metabolism, further impacting the HPO axis (44). Adiponectin, typically decreased in obesity, plays a role in insulin sensitization and has anti-inflammatory properties. Its reduction in obesity can exacerbate the hormonal imbalances associated with reproductive dysfunctions (32). The decrease in adiponectin in obese individuals may contribute to insulin resistance, which can further impact the HPO axis by affecting the secretion and action of GnRH, LH, and FSH. The consequences are wide-ranging: from anovulatory cycles and irregular menstrual periods to a challenging fertility landscape. This hormonal imbalance not only diminishes natural conception chances but also poses challenges for assisted reproductive treatments.
The endocrinological role of adipose tissue
Once regarded merely as a passive fat store, adipose tissue is now understood to be an active endocrine organ. In the context of obesity, the secretion patterns of adipokines, notably leptin and adiponectin, experience significant changes (45). Beyond their central role in metabolic homeostasis, these adipokines also intersect with reproductive functions. Imbalances in adipokine levels can affect ovarian steroidogenesis, resulting in a disrupted hormonal environment. This altered balance, particularly between estrogen and progesterone, can adversely affect the endometrial setting, rendering it less receptive to embryo implantation and early gestation (29).
The dual threat of insulin resistance and hyperinsulinemia
Obesity’s distinct metabolic profile, marked by insulin resistance, has significant repercussions for reproductive health. Elevated insulin levels, which arise as a countermeasure to resistance, trigger a cascade of effects in the ovary. Specifically, they prompt the ovarian theca cells to overproduce androgens, leading to a hyperandrogenic state (46). This scenario, reminiscent of PCOS, is further exacerbated when insulin inhibits the liver’s synthesis of sex hormone-binding globulin (SHBG). The result is an increase in freely circulating androgens, which can interfere with ovulation, causing menstrual irregularities and reducing fertility potential (37).
The inflammatory onslaught and oxidative stress
Obesity is frequently associated with chronic inflammation. In this condition, adipose tissue becomes a major producer of pro-inflammatory molecules, such as TNF-α and IL-6. Accompanying this rise in inflammation, a hallmark of obesity, is an increase in oxidative stress. This oxidative stress in obesity is characterized by an imbalance between the production of ROS and the body’s antioxidant defenses. The excess adipose tissue in obesity contributes to this imbalance, exacerbating inflammation and leading to a cycle of oxidative stress and further inflammatory response, which can disrupt metabolic homeostasis (47, 48). These combined factors create a hostile environment for oocytes, affecting their quality and viability. Furthermore, the inflammatory and oxidative conditions can negatively influence the endometrial lining, reducing its receptivity to embryo implantation and thereby presenting substantial challenges to successful conception (49).
The reproductive repercussions of dysregulated lipid metabolism
The impact of obesity on lipid metabolism is significant. Elevated triglycerides, reduced high-density lipoprotein-cholesterol (HDL-C) levels, and a general dyslipidemic state have implications not only for cardiovascular health but also for reproductive outcomes (50). Lipotoxicity in ovarian granulosa cells, stemming from excessive lipid accumulation, can induce cellular stress through multiple interconnected pathways. The accumulation of lipids leads to increased ROS production, causing oxidative stress (51). Concurrently, this lipid overload disrupts endoplasmic reticulum (ER) function, triggering ER stress and the unfolded protein response, potentially leading to apoptosis if unresolved (52). Additionally, lipid accumulation can provoke an inflammatory response by stimulating pro-inflammatory cytokines, exacerbating cellular stress (53). This scenario is further complicated by impaired mitochondrial function, leading to decreased adenosine triphosphate (ATP) production and further ROS generation, contributing to the overall cellular stress and dysfunction in granulosa cells (54). Such stress can hinder their function, affecting oocyte maturation, follicular development, and overall reproductive capability (55).
The mitochondrial malaise
Mitochondria, cellular powerhouses, play pivotal roles in a plethora of physiological processes, including oocyte maturation and embryonic development (56). However, obesity can lead to mitochondrial dysfunction. This dysfunction, marked by reduced ATP synthesis and increased ROS production, can adversely affect oocyte health, impacting both its cytoplasmic and nuclear maturity. Such challenges to mitochondrial function can limit the developmental potential of embryos, creating significant obstacles to successful conception and subsequent embryonic development (57).
In essence, the link between obesity and female infertility is supported by numerous molecular mechanisms, each adding to the diverse reproductive challenges encountered by obese women. As the obesity pandemic persists, a detailed understanding of these mechanisms becomes crucial in developing targeted therapeutic approaches to mitigate the reproductive issues caused by obesity.
The role of biogenic amines in metabolic disturbances and reproductive health
Biogenic amines, such as serotonin, dopamine, histamine, and norepinephrine, are derived from amino acids and play critical roles in various physiological processes, including mood regulation, appetite control, and cardiovascular function. Recent research indicates a significant link between the dysregulation of these amines and metabolic disturbances associated with obesity, which in turn can have profound effects on female reproductive health (58). For instance, serotonin and dopamine have been implicated in the regulation of energy balance and appetite, processes often altered in obesity. Dysregulation in these neurotransmitters can contribute to the hormonal imbalances seen in obesity, indirectly affecting reproductive health (59). Additionally, histamine and norepinephrine are involved in inflammatory responses and stress regulation. The altered levels of these biogenic amines in obesity can exacerbate inflammatory states and oxidative stress, further impacting oocyte quality and endometrial receptivity (29). Alongside these biogenic amines, polyamines, including spermine, spermidine, and putrescine, are also integral to the discussion of metabolic and reproductive health. These polyamines are involved in cellular and genetic metabolism, aiding in transcription, translation, signaling, and post-translational modifications, which are crucial for maintaining cellular homeostasis and responding to metabolic challenges. Altered polyamine metabolism has been linked to various disease states, including metabolic disorders. Such dysregulation can have implications for reproductive health, as polyamines are known to be involved in cell growth and differentiation, processes that are essential for reproductive function (60–62).
Weight loss as a therapeutic strategy for infertility in obese women
Weight loss emerges as a promising avenue for restoring reproductive health in obese women. A myriad of weight loss strategies, ranging from lifestyle modifications to medical interventions, have been explored for their efficacy in ameliorating obesity-induced infertility.
Pharmacological interventions
Pharmacological interventions are key in addressing the reproductive challenges associated with obesity. Metformin, primarily an anti-diabetic drug, is widely recognized for its insulin-sensitizing properties, which have been beneficial in regulating menstrual cycles and improving ovulation rates in obese women (63). In addition to Metformin, glucagon-like peptide-1 (GLP-1) agonists, originally used for type 2 diabetes, have emerged as a promising option. These agonists have shown potential in improving insulin resistance linked with PCOS, commonly observed in obese women. By enhancing insulin sensitivity, GLP-1 agonists could potentially correct hormonal imbalances and improve fertility outcomes (10, 64). Moreover, antioxidant supplementation has been indicated as a potential strategy for improving reproductive outcomes in obese women. Studies suggest that antioxidants such as α-lipoic acid and myo-inositol may ameliorate oxidative stress in the oocyte environment, potentially contributing to improved fertility (65). Beyond these, the focus is also shifting towards drugs that target adipokines or their receptors. This includes exploring the therapeutic roles of agents that either enhance adiponectin activity or increase its levels, considering adiponectin’s crucial role in ovarian function and hormone production (66). Moreover, ongoing research is investigating molecules that counteract leptin resistance, a prevalent issue in obesity. Restoring leptin sensitivity through such interventions could play a significant role in addressing various reproductive dysfunctions linked to obesity (67).
Surgical interventions
Surgical interventions, particularly bariatric surgery, have been increasingly recognized as a viable approach to address infertility challenges in obese women. Bariatric surgery, which is primarily designed to induce significant weight loss, has been shown to have a positive impact on fertility outcomes. One study found a notable improvement in obesity-related infertility following surgical intervention (29). Another research underscores the link between obesity and infertility, suggesting that weight loss induced by surgical means can be beneficial (35). A systematic review further emphasizes that weight loss in overweight or obese women can significantly enhance fertility treatment outcomes (68). Additionally, research provides insights into the positive outcomes of bariatric surgery in obese infertile women who wish to conceive, emphasizing that the success of these surgical interventions can vary based on factors such as age, the specific surgical procedure, co-morbidities, and BMI before surgery (69).
Lifestyle modifications
Lifestyle modifications have emerged as a pivotal therapeutic strategy for addressing infertility in obese women. Comprehensive programs focusing on weight loss have demonstrated significant improvements in reproductive outcomes across various fertility treatments (70). Particularly in women with PCOS, a condition often associated with obesity and infertility, lifestyle changes have been shown to restore reproductive potential by enhancing insulin sensitivity and regulating luteinizing hormone levels (71). Furthermore, lifestyle interventions targeting central obesity and insulin resistance have been identified as crucial in managing PCOS-related infertility (72). A review on lifestyle factors in individuals seeking infertility treatment emphasized the potential benefits of intensive lifestyle modification programs in aiding weight management and improving fertility outcomes (73). Additionally, structured exercise training programs, when compared to specific dietary interventions, have also shown promise in treating obese PCOS patients with anovulatory infertility (74).
Obesity and its implications on assisted reproductive technologies
Assisted reproductive technologies (ART), such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), have emerged as revolutionary tools in the realm of reproductive medicine, offering hope to countless couples facing infertility challenges. However, the interplay between obesity and the efficacy of these technologies has become a focal point of research and clinical concern. Obesity has been identified as a significant factor that negatively impacts various ART outcomes (75). Specifically, for women undergoing IVF, obesity has been associated with compromised pregnancy outcomes, underscoring the importance of optimal BMI in the context of ART (76). Interestingly, while obesity might diminish clinical pregnancy rates after IVF, its impact on ICSI cycles appears to be less pronounced, suggesting potential intrinsic sperm dysfunctions secondary to obesity might be circumvented in ICSI procedures (77).
Conclusion
The rising global prevalence of obesity and its profound impact on female reproductive health has become a pressing concern. Obesity’s systemic effects, from hormonal imbalances to inflammation, disrupt the intricate processes of female fertility. Molecular mechanisms, including disruptions in the HPO axis and insulin resistance, further elucidate the challenges obese women face in reproductive health. Weight loss, through pharmacological interventions, surgery, or lifestyle changes, offers a promising solution. Additionally, the success of assisted reproductive technologies like IVF is influenced by obesity, highlighting the need for optimal body mass index. Addressing the intersection of obesity and infertility is crucial for improved reproductive outcomes worldwide.
Author contributions
LZ: Writing – original draft. LY: Writing – review & editing. ZG: Writing – review & editing. NY: Visualization, Writing – review & editing. SZ: Methodology, Writing – review & editing. PP: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PCOS, polycystic ovary syndrome; BMI, body mass index; HPO, Hypothalamic-Pituitary-Ovary; GnRH, Gonadotropin-releasing hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone; SHBG, sex hormone-binding globulin; HDL-C, high-density lipoprotein-cholesterol; ATP, adenosine triphosphate; ROS, reactive oxygen species; ART, assisted reproductive technologies; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; BMI, body mass index; ER, endoplasmic reticulum; GLP-1, glucagon-like peptide-1.
References
1. Kappeler L. Role of adipose tissue microRNAs in the onset of metabolic diseases and implications in the context of the DOHaD. Cells (2022) 11(23):3711. doi: 10.3390/cells11233711
2. Bond ST, Calkin AC, Drew BG. Adipose-derived extracellular vesicles: systemic messengers and metabolic regulators in health and disease. Front Physiol (2022) 13:837001. doi: 10.3389/fphys.2022.837001
3. Sharma G, Woods CD, Rajkarnikar R, Hathaway HJ, Prossnitz ER. LBODP005 gper modulates multiple functions in adipose tissue to promote an anti-obese phenotype. J Endocrine Soc (2022) 6(Supplement_1):A3–3. doi: 10.1210/jendso/bvac150.005
4. Al-Hakmani FM, Al-Fadhil FA, Al-Balushi LH, Al-Harthy NA, Al-Bahri ZA, Al-Rawahi NA, et al. The effect of obesity on pregnancy and its outcome in the population of Oman, seeb province. Oman Med J (2016) 31(1):12–7. doi: 10.5001/omj.2016.03
5. Pascuali N, Scotti L, Di Pietro M, Oubiña G, Bas D, May M, et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum Reprod (Oxford England) (2018) 33(5):844–59. doi: 10.1093/humrep/dey045
6. Vilmann LS, Thisted E, Baker JL, Holm JC. Development of obesity and polycystic ovary syndrome in adolescents. Hormone Res paediatrics (2012) 78(5-6):269–78. doi: 10.1159/000345310
7. Barrea L, Muscogiuri G, Pugliese G, de Alteriis G, Colao A, Savastano S. Metabolically healthy obesity (MHO) vs. Metabolically unhealthy obesity (MUO) phenotypes in PCOS: association with endocrine-metabolic profile, adherence to the mediterranean diet, and body composition. Nutrients (2021) 13(11):3925. doi: 10.3390/nu13113925
8. Bright K, Dube L, Hayden KA, Gordon JL. Effectiveness of psychological interventions on mental health, quality of life and relationship satisfaction for individuals and/or couples undergoing fertility treatment: a systematic review and meta-analysis protocol. BMJ Open (2020) 10(7):e036030. doi: 10.1136/bmjopen-2019-036030
9. Bourrion B, Panjo H, Bithorel PL, de la Rochebrochard E, François M, Pelletier-Fleury N. The economic burden of infertility treatment and distribution of expenditures overtime in France: a self-controlled pre-post study. BMC Health Serv Res (2022) 22(1):512. doi: 10.1186/s12913-022-07725-9
10. Cena H, Chiovato L, Nappi RE. Obesity, polycystic ovary syndrome, and infertility: A new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab (2020) 105(8):e2695–2709. doi: 10.1210/clinem/dgaa285
11. Gu Y, Zhou G, Zhou F, Wu Q, Ma C, Zhang Y, et al. Life modifications and PCOS: old story but new tales. Front Endocrinol (2022) 13:808898. doi: 10.3389/fendo.2022.808898
12. Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism: Clin Exp (2019) 92:108–20. doi: 10.1016/j.metabol.2018.11.002
13. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol (2013) 6:1–13. doi: 10.2147/CLEP.S37559
14. Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertility sterility (2009) 92(6):1966–82. doi: 10.1016/j.fertnstert.2008.09.018
15. Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin obstetrics gynaecology (2004) 18(5):671–83. doi: 10.1016/j.bpobgyn.2004.05.001
16. Estienne A, Bongrani A, Reverchon M, Ramé C, Ducluzeau PH, Froment P, et al. Involvement of novel adipokines, chemerin, visfatin, resistin and apelin in reproductive functions in normal and pathological conditions in humans and animal models. Int J Mol Sci (2019) 20(18). doi: 10.3390/ijms20184431
17. Messinis IE, Messini CI, Anifandis G, Dafopoulos K. Polycystic ovaries and obesity. Best Pract Res Clin obstetrics gynaecology (2015) 29(4):479–88. doi: 10.1016/j.bpobgyn.2014.11.001
18. Alvarez-Blasco F, Botella-Carretero JI, San Millán JL, Escobar-Morreale HF. Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch Internal Med (2006) 166(19):2081–6. doi: 10.1001/archinte.166.19.2081
19. Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab (2006) 91(4):1357–63. doi: 10.1210/jc.2005-2430
20. Fielder S, Nickkho-Amiry M, Seif MW. Obesity and menstrual disorders. Best Pract Res Clin obstetrics gynaecology (2023) 89:102343. doi: 10.1016/j.bpobgyn.2023.102343
21. Norman RJ, Clark AM. Obesity and reproductive disorders: a review. Reproduction fertility Dev (1998) 10(1):55–63. doi: 10.1071/R98010
22. Blüher M. Importance of estrogen receptors in adipose tissue function. Mol Metab (2013) 2(3):130–2. doi: 10.1016/j.molmet.2013.07.001
23. Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol (2001) 45(3 Suppl):S116–124. doi: 10.1067/mjd.2001.117432
24. Krentz AJ, von Mühlen D, Barrett-Connor E. Adipocytokine profiles in a putative novel postmenopausal polycystic ovary syndrome (PCOS) phenotype parallel those in premenopausal PCOS: the Rancho Bernardo Study. Metabolism: Clin Exp (2012) 61(9):1238–41. doi: 10.1016/j.metabol.2012.02.001
25. Wei S, Schmidt MD, Dwyer T, Norman RJ, Venn AJ. Obesity and menstrual irregularity: associations with SHBG, testosterone, and insulin. Obes (Silver Spring Md) (2009) 17(5):1070–6. doi: 10.1038/oby.2008.641
26. Al-Safi ZA, Polotsky AJ. Obesity and menopause. Best Pract Res Clin obstetrics gynaecology (2015) 29(4):548–53. doi: 10.1016/j.bpobgyn.2014.12.002
27. Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine (2006) 29(1):109–17. doi: 10.1385/ENDO:29:1:109
28. Carson SA, Kallen AN. Diagnosis and management of infertility: A review. Jama (2021) 326(1):65–76. doi: 10.1001/jama.2021.4788
29. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertility sterility (2017) 107(4):840–7. doi: 10.1016/j.fertnstert.2017.01.017
30. Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin obstetrics gynaecology (2015) 29(4):498–506. doi: 10.1016/j.bpobgyn.2014.10.014
31. Ennab F, Atiomo W. Obesity and female infertility. Best Pract Res Clin obstetrics gynaecology (2023) 89:102336. doi: 10.1016/j.bpobgyn.2023.102336
32. Chen CI, Hsu MI, Lin SH, Chang YC, Hsu CS, Tzeng CR. Adiponectin and leptin in overweight/obese and lean women with polycystic ovary syndrome. Gynecological Endocrinol (2015) 31(4):264–8. doi: 10.3109/09513590.2014.984676
33. van der Steeg JW, Steures P, Eijkemans MJ, Habbema JD, Hompes PG, Burggraaff JM, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod (Oxford England) (2008) 23(2):324–8. doi: 10.1093/humrep/dem371
34. Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol (2016) 4(12):1025–36. doi: 10.1016/S2213-8587(16)30217-0
35. Zain MM, Norman RJ. Impact of obesity on female fertility and fertility treatment. Women's Health (London England) (2008) 4(2):183–94. doi: 10.2217/17455057.4.2.183
36. Abdessalem H, Demmouche A. A case-control study of body mass index and infertility in Algerian women (Sidi Bel Abbes, west of Algeria). Int J Infertility Fetal Med (2012) 6(3):103–7. doi: 10.5005/jp-journals-10016-1110
37. Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update (2003) 9(4):359–72. doi: 10.1093/humupd/dmg024
38. Nair NB, Skaria M, Kumar RS. Female obesity: A probable cause of infertility. J Drug Delivery Ther (2022) 12(4):216–20. doi: 10.22270/jddt.v12i4.5585
39. Zander-Fox DL, Henshaw R, Hamilton H, Lane M. Does obesity really matter? The impact of BMI on embryo quality and pregnancy outcomes after IVF in women aged ≤38 years. Aust New Z J obstetrics gynaecology (2012) 52(3):270–6. doi: 10.1111/j.1479-828X.2012.01453.x
40. Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes related Metab Disord (1997) 21(6):432–8. doi: 10.1038/sj.ijo.0800424
41. Doufas AG, Mastorakos G. The hypothalamic-pituitary-thyroid axis and the female reproductive system. Ann New York Acad Sci (2000) 900:65–76. doi: 10.1111/j.1749-6632.2000.tb06217.x
42. Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (2005) (2009) 33 Suppl 2(Suppl 2):S8–13. doi: 10.1038/ijo.2009.65
43. Bouvattier C, Lahlou N, Roger M, Bougnères P. Hyperleptinaemia is associated with impaired gonadotrophin response to GnRH during late puberty in obese girls, not boys. Eur J Endocrinol (1998) 138(6):653–8. doi: 10.1530/eje.0.1380653
44. Singh A, Choubey M, Bora P, Krishna A. Adiponectin and chemerin: contrary adipokines in regulating reproduction and metabolic disorders. Reprod Sci (Thousand Oaks Calif) (2018) 25(10):1462–73. doi: 10.1177/1933719118770547
45. Blüher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism: Clin Exp (2015) 64(1):131–45. doi: 10.1016/j.metabol.2014.10.016
46. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocrine Rev (2012) 33(6):981–1030. doi: 10.1210/er.2011-1034
47. Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol (2004) 25(1):4–7. doi: 10.1016/j.it.2003.10.013
48. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflammation (2010) 2010:289645. doi: 10.1155/2010/289645
49. Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab syndrome related Disord (2015) 13(10):423–44. doi: 10.1089/met.2015.0095
50. Bitzur R, Cohen H, Kamari Y, Shaish A, Harats D. Triglycerides and HDL cholesterol: stars or second leads in diabetes? Diabetes Care (2009) 32 Suppl 2(Suppl 2):S373–377. doi: 10.2337/dc09-S343
51. Brookheart RT, Michel CI, Listenberger LL, Ory DS, Schaffer JE. The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and endoplasmic reticulum stress. J Biol Chem (2009) 284(12):7446–54. doi: 10.1074/jbc.M806209200
52. Raviv S, Hantisteanu S, Sharon SM, Atzmon Y, Michaeli M, Shalom-Paz E. Lipid droplets in granulosa cells are correlated with reduced pregnancy rates. J Ovarian Res (2020) 13(1):4. doi: 10.1186/s13048-019-0606-1
53. Alves JPM, Fernandes CCL, Lazzaroto CR, Aguiar LH, Calderón CEM, Tavares KCS, et al. Comparative expression profiles of genes related to cellular stress and development in oocyte of goats fed with distinct concentrations of lipids. Acta Scientiae Veterinariae (2015) 43:1296. doi: 10.22456/1679-9216.99981
54. Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, et al. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology (2010) 151(11):5438–45. doi: 10.1210/en.2010-0551
55. Wu LL-Y, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, et al. High-fat diet causes lipotoxicity responses in cumulus–oocyte complexes and decreased fertilization rates. Endocrinology (2010) 151(11):5438–45. doi: 10.1210/en.2010-0551
56. Jeong PS, Yang HJ, Jeon SB, Gwon MA, Kim MJ, Kang HG, et al. Luteolin supplementation during porcine oocyte maturation improves the developmental competence of parthenogenetic activation and cloned embryos. PeerJ (2023) 11:e15618. doi: 10.7717/peerj.15618
57. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA (2012) 307(5):491-7. doi: 10.1001/jama.2012.39
58. Gmoshinski IV, Apryatin SA, Shipelin VA, Nikitjuk DB. Neuromediators and neuropeptides: the biomarkers for metabolic disturbances in obesity. Problems Endocrinol (2018) 64(4):258–69. doi: 10.14341/probl9466
59. Yabut JM, Crane JD, Green AE, Keating DJ, Khan WI, Steinberg GR. Emerging roles for serotonin in regulating metabolism: new implications for an ancient molecule. Endocrine Rev (2019) 40(4):1092–107. doi: 10.1210/er.2018-00283
60. Miller-Fleming L, Olin-Sandoval V, Campbell K, Ralser M. Remaining mysteries of molecular biology: the role of polyamines in the cell. J Mol Biol (2015) 427(21):3389–406. doi: 10.1016/j.jmb.2015.06.020
61. Sagar NA, Tarafdar S, Agarwal S, Tarafdar A, Sharma S. Polyamines: functions, metabolism, and role in human disease management. Med Sci (Basel Switzerland) (2021) 9(2):44. doi: 10.3390/medsci9020044
62. Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life (2009) 61(9):880–94. doi: 10.1002/iub.230
63. Practice Committee of the American Society for Reproductive Medicine. Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): a guideline. Fertility sterility (2017) 108(3):426–41. doi: 10.1016/j.fertnstert.2017.06.026
64. Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod biomedicine Online (2019) 39(2):332–42. doi: 10.1016/j.rbmo.2019.04.017
65. Novielli C, Anelli GM, Lisso F, Marzorati A, Parrilla B, Oneta M, et al. Effects of α-lipoic acid and myo-inositol supplementation on the oocyte environment of infertile obese women: A preliminary study. Reprod Biol (2020) 20(4):541–6. doi: 10.1016/j.repbio.2020.10.002
66. Dimitriadis GK, Kyrou I, Randeva HS. Polycystic ovary syndrome as a proinflammatory state: the role of adipokines. Curr Pharm design (2016) 22(36):5535–46. doi: 10.2174/1381612822666160726103133
67. Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol (2010) 31(3):377–93. doi: 10.1016/j.yfrne.2010.06.002
68. Sim KA, Partridge SR, Sainsbury A. Does weight loss in overweight or obese women improve fertility treatment outcomes? A systematic review. Obes Rev (2014) 15(10):839–50. doi: 10.1111/obr.12217
69. Musella M, Milone M, Bellini M, Sosa Fernandez LM, Leongito M, Milone F. Effect of bariatric surgery on obesity-related infertility. Surg Obes related Dis (2012) 8(4):445–9. doi: 10.1016/j.soard.2011.09.021
70. Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod (Oxford England) (1998) 13(6):1502–5. doi: 10.1093/humrep/13.6.1502
71. Huber-Buchholz MM, Carey DG, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab (1999) 84(4):1470–4. doi: 10.1210/jc.84.4.1470
72. Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol metabolism: TEM (2002) 13(6):251–7. doi: 10.1016/S1043-2760(02)00612-4
73. Anderson K, Nisenblat V, Norman R. Lifestyle factors in people seeking infertility treatment - A review. Aust New Z J obstetrics gynaecology (2010) 50(1):8–20. doi: 10.1111/j.1479-828X.2009.01119.x
74. Palomba S, Giallauria F, Falbo A, Russo T, Oppedisano R, Tolino A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod (Oxford England) (2008) 23(3):642–50. doi: 10.1093/humrep/dem391
75. Dağ Z, Dilbaz B. Impact of obesity on infertility in women. J Turkish German Gynecological Assoc (2015) 16(2):111–7. doi: 10.5152/jtgga.2015.15232.eCollection 2015
76. Kawwass JF, Kulkarni AD, Hipp HS, Crawford S, Kissin DM, Jamieson DJ. Extremities of body mass index and their association with pregnancy outcomes in women undergoing in vitro fertilization in the United States. Fertility sterility (2016) 106(7):1742–50. doi: 10.1016/j.fertnstert.2016.08.028
Keywords: obesity, female infertility, adipose tissue, hormonal profile, reproductive health
Citation: Zheng L, Yang L, Guo Z, Yao N, Zhang S and Pu P (2024) Obesity and its impact on female reproductive health: unraveling the connections. Front. Endocrinol. 14:1326546. doi: 10.3389/fendo.2023.1326546
Received: 23 October 2023; Accepted: 19 December 2023;
Published: 09 January 2024.
Edited by:
Mayank Choubey, NYU Grossman Long Island School of Medicine, United StatesReviewed by:
Arnab Banerjee, Birla Institute of Technology and Science, IndiaCopyright © 2024 Zheng, Yang, Guo, Yao, Zhang and Pu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zheng, eHRybXl5emhlbmdsZWlAMTYzLmNvbQ==
 Lei Zheng
Lei Zheng Lixian Yang1
Lixian Yang1