
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 24 January 2024
Sec. Reproduction
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1320893
This article is part of the Research TopicEndocrine Disrupting Chemicals in Reproductive Health, Fertility, and Early DevelopmentView all 5 articles
 Qing-Chun Guo1
Qing-Chun Guo1 Wen Yao2
Wen Yao2 Chong Liu3
Chong Liu3 Tao-Ran Deng1
Tao-Ran Deng1 Juan Li1
Juan Li1 Hong-Mei Liao1
Hong-Mei Liao1 Wen-Qu Tian1
Wen-Qu Tian1 Yi Wang1
Yi Wang1 Yao-Yao Du1*†
Yao-Yao Du1*† Yu-Feng Li1*†
Yu-Feng Li1*†Introduction: Personal care products (PCPs) contain a number of endocrine-disrupting chemicals (EDCs) that could potentially affect the reproductive function in women of childbearing age. However, studies focused on the effects of PCPs use on reproductive outcomes are very limited. The current study aimed to explore the relationships between PCPs use patterns and reproductive outcomes in women undergoing in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment.
Methods: A total of 1500 women from the Tongji Reproductive and Environmental (TREE) study between December 2018 and January 2020 were included in this study. Participants provided characteristics of PCPs use within the previous three months. Retrieved oocyte number, mature oocyte number, two distinct pronuclei (2PN) zygote number, fertilization rate, cleavage rate, blastocyst formation rate, implantation, clinical pregnancy, miscarriage, and live birth were followed up as reproductive endpoints. Generalized linear regression model was utilized to assess the associations between various categories of PCPs use and reproductive endpoints of IVF/ICSI.
Results: After adjusting for relevant covariates, women who used skin care products ≥14 times per week had a reduction of 22.4% in the maturation rate (95% CI: -39.2%, -1.6%) compared to participants who did not use skin care products. After transferring fresh embryos, women who used cosmetics 1–2 times per week (adjusted OR = 2.2, 95% CI: 1.0, 4.8) or 3–7 times per week (adjusted OR = 2.5, 95% CI: 1.2, 5.2) had a higher possibility of miscarriage than those who did not use cosmetics. There was negative association between the use of gel or soap and the cleavage rate among women aged < 30 years old (P for interaction = 0.01). Among women with BMI ≥ 24 kg/m2, the use of gel or soap was negatively associated with the blastocyst formation rate (P for interaction = 0.04), while cosmetics use was negatively associated with the maturation rate (P for interaction = 0.001).
Conclusion: Our findings suggest that the use of PCPs in women of reproductive age have a potential adverse impact on IVF/ICSI outcomes, particularly skin care and cosmetic products.
Due to increasing environmental pollution and lifestyle changes associated with economic development, couples of childbearing age are facing an growing risk of infertility. Approximately 15% of couples worldwide suffer from infertility (1), which has a negative impact on their psychological and physiological health (2). As effective treatments for infertility, assisted reproductive technologies, especially in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI), have been chosen by many couples. According to the International Committee for Monitoring Assisted Reproductive Technologies World Report, an estimated 2.92 million assisted reproductive technology treatments were performed worldwide in 2014 (3). However, the success rate of ART has not improved. The European IVF–monitoring consortium reports that the overall clinical pregnancy rate of IVF/ICSI treatment is approximately 35% (4). Adverse environmental factors significantly impact female fertility. Therefore, it is imperative to investigate the effects of EDCs on the reproductive outcomes of IVF/ICSI.
With the improvement of sanitary and economic conditions, the use of personal care products (PCPs), including toiletries, cosmetics, and perm and hair coloring products, is gradually increasing worldwide. In 2013, the total value of China’s PCPs industry reached $2.1 billion, accounting for approximately 10% of global production (5). PCPs contain a variety of endocrine-disrupting chemicals (EDCs), including bisphenol A (BPA), phthalates (PAEs), parabens, and triclosan (TCS), which are known to affect endocrine function and impair human fertility (6). A variety of EDCs can enter the body through dermal absorption in the process of direct or indirect contact with PCPs (7). For instance, bisphenol A (BPA) and phthalates (PAEs) are commonly found in body washes and shampoos (8). They have been found to be associated with reduced semen quality, MII oocyte yield, implantation rate, and pregnancy rates (9–12). Triclosan (TCS) and triclocarban (TCC) can be used as antibacterial agent in skin care products (13, 14), and they have been reported to negatively associate with the implantation rate of IVF treatment (15, 16). Benzophenone (BPs), glycol ethers and perfluorooctanoic acid (PFOA) are frequently present in cosmetics (17–19). There’s a growing concern that these compounds might impact the onset of hormone-dependent disorders. Research has associated them with various unfavorable pregnancy outcomes, such as diminished rates of implantation, pregnancy, and live births (20, 21). p–Phenylenediamine (PPD), mainly used in hair coloring products, reduces sex hormone levels and affects oocyte quality in mice, causing abnormalities in early embryonic development (22). These studies shed light on the adverse effects of specific EDCs on reproductive health. However, the ingredients in PCPs are often a mixture of EDCs, and the real situation is more complex in daily life.
Even though the current industry regulations restrict the use of some EDCs to low levels, the possible combined effects (synergistic, inhibitory, etc.) of multiple EDCs still need to be accounted (23). However, only a limited number of studies have assessed the potential influence of PCPs use on reproductive outcomes. Therefore, we collected information on recent PCPs use among women undergoing IVF/ICSI treatment from the Tongji Reproductive and Environmental (TREE) cohort and evaluated the associations between PCPs use and eleven reproductive outcomes.
All participants came from the TREE cohort. Women who were beyond 20 and seeking ART treatment at the Reproductive Medicine Center of Tongji Hospital from December 2018 to January 2020 were recruited (24). Finally, a total of 2057 couples were included in the TREE cohort. Among the participants, 1672 women underwent 1701 IVF/ICSI cycles and 305 intrauterine insemination (IUI) cycles. We further excluded 122 women due to only undergoing IUI treatment, 1 woman due to cancelling oocyte retrieval, 1 woman due to missing information about IVF/ICSI outcomes, and 48 women due to missing information about PCPs characteristics. Finally, 1500 participants were enrolled in the current analysis (Figure 1). Only the first oocyte aspirations were included in this study. This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Each participant signed an informed consent form at enrollment.
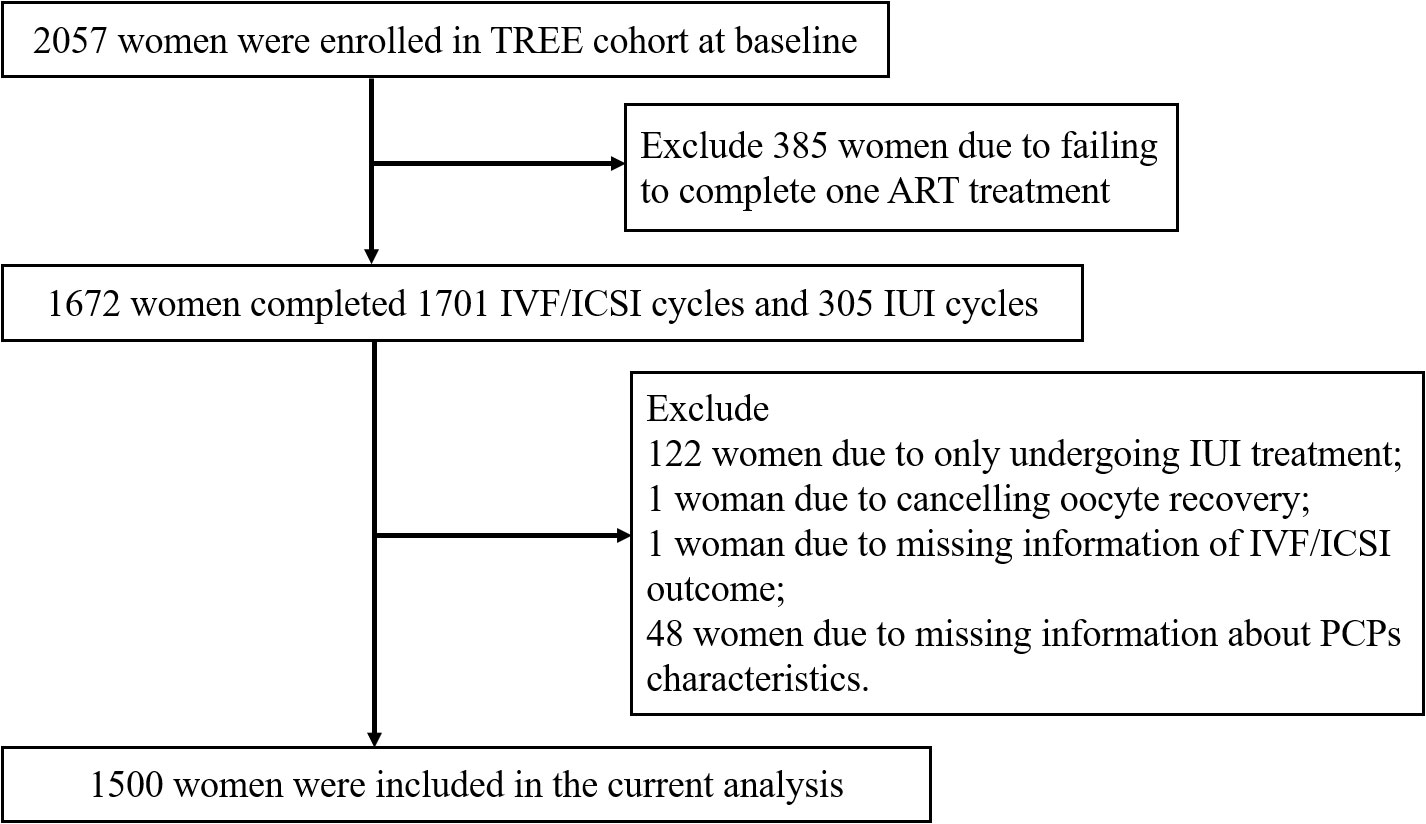
Figure 1 Flow chart for the study population to investigate associations between PCPs characteristics and IVF/ICSI outcomes.
Upon enrollment, each participant completed a baseline questionnaire about their demographics and lifestyle. The use of PCPs was included in the questionnaire. The PCPs characteristics comprised the frequency of weekly use of gel and soap, shampoo, skin care products and cosmetics, as well as whether hair had been colored or permed in the past three months. Skin care products included toner, cream, mask, scrub, sunscreen, etc. Cosmetics included foundation, lipstick, concealer, eyebrow pencil, eye shadow, mascara, nail polish, makeup remover, etc. The frequency of gel or soap was used per week was categorized into four groups: 0, 1 to <3, 3 to <7, and ≥7 times. Shampoo use per week was categorized into four groups: ≤1, 2 to ≤3, 4 to <7 and ≥7 times. Skin care products use per week was classified into four groups: 0, 1 to <7, 7 to <14 and ≥14 times. The use of cosmetics per week was divided into four groups: 0, 1 to < 2, 3 to < 7 and ≥ 7 times.
In the baseline questionnaire, sociodemographic characteristics and lifestyle characteristics were collected. Sociodemographic characteristics included ethnicity, education level, household income, usual residence, and employment status. Lifestyle characteristics included smoking, alcohol consumption, and history of use of PCPs. Smoking status was categorized into active and passive smoking. Smoking more than 100 cigarettes in a lifetime was defined as active smoking. Passive smoking exposure was defined as exposure to others’ tobacco smoke for at least 15 minutes per day on more than 1 day per week (25). Clinical characteristics were extracted from medical records, comprising age, height, weight, gravidity, parity, infertility diagnosis, controlled ovarian hyperstimulation (COH) protocols, fertilization methods, and pregnancy outcomes. Body mass index (BMI) was calculated as weight divided by the square of height. Infertility diagnoses were categorized into four groups: female factor, male factor, mixed factor, and unexplained infertility. Female factors included uterine disease, fallopian tube obstruction and ovulatory dysfunction. Male factors included testicular lesions, poor semen quality and vas deferens obstruction. The ‘mixed factors’ indicates that a couple has both male and female factors (26). Infertility without a definite diagnosis was defined as unexplained infertility. COH protocols consisted of long gonadotropin-releasing hormone (GnRH), antagonist, and other protocols (e.g., nature cycle and mild stimulation protocols).
Potential confounders were selected based on a priori knowledge from previous literatures. The covariates included in the final model were identified by directed acyclic graphs (DAG). In light of the notably low count of active smokers among women (n=85), passive smoking was included as a confounding factor when considering smoking status. Ultimately, all models were adjusted for the following covariates: age, BMI, educational level, household income, passive smoking and alcohol consumption (Supplementary Figures 1, 2). Age and BMI were treated as continuous variables. Educational level (middle school and below, high school and above), income (≤5000 yuan or >5000 yuan), passive smoking (never or ever) and alcohol consumption (never or ever) were retained as dichotomous variables.
IVF/ICSI procedure were conducted as previously described (27). After completion of COH protocols, once transvaginal ultrasound detected two or more follicles with a diameter of ≥ 18mm, or three or more follicles with a diameter of ≥ 17mm, HCG is administered to induce ovulation. Then oocytes and embryos were cultured in sequential embryo culture media. The number of two distinct pronuclei (2PN) zygotes, number of cleavage-stage embryo, and rates of fragmentation were recorded. High-quality embryos were selected to be transferred or cryopreserved, while remaining embryos were cultured to the blastocyst stage before cryopreservation.
The following parameters served as intermediate endpoints for IVF/ICSI procedures: the number of retrieved oocytes, number of mature oocytes, maturation rate, number of normal fertilized oocytes, fertilization rate, cleavage rate and blastocyst formation rate. Mature oocytes were defined as those that had expelled the first polar body and reached the MII stage. The maturation rate was determined by the ratio of mature oocytes to the total number of oocytes retrieved. After fertilization, zygotes with two distinct pronuclei were referred as 2PN zygotes. The fertilization rate was calculated by dividing the number of 2PN zygotes by the number of mature oocytes (28). The cleavage rate is the ratio of the number of cleavage-stage embryos divided by the number of 2PN zygotes (29). The blastocyst formation rate was defined as the number of blastocysts on day five divided by the number of embryos in culture (30).
Pregnancy outcomes included implantation, clinical pregnancy, miscarriage, and live birth. Implantation was defined as a serum human chorionic gonadotropin (HCG) level exceeding 10 mIU/mL approximately 14 days after embryo transfer. Clinical pregnancy was identified when the fetal heartbeat and the fetal sac were observed by ultrasound 28 days after embryo transfer. Loss of pregnancy at less than 28 weeks of gestation was termed miscarriage. A live birth was defined as the delivery of a newborn after 28 weeks of gestation (31).
The mean ± standard deviation (SD) or n (%) was used to describe the demographic, clinical, and PCPs characteristics of all participants. Generalized linear models were used to assess the associations between PCPs characteristics and IVF/ICSI outcomes, using PCPs characteristics as categorical variables. The counts of retrieved oocytes, mature oocytes, and 2PN zygotes were analyzed using the quasi-Poisson distribution and the log-link function. Maturation and fertilization rates were assessed using the quasi-binomial distribution and logit function. After obtaining the regression coefficient, the percentage change was calculated using the following formula: [exp () - 1] × 100%. For implantation, clinical pregnancy, miscarriage, and live birth, binomial distribution and logit function were used. Odds ratios (OR) and 95% CI are used to describe the results. Data with missing values were deleted (all < 5%) (32).
Stratified analyses were performed with the PCPs characteristics as continuous variables. In this study, stratified analyses were specifically conducted for age and BMI. Interaction terms involving the use of different PCPs with age and BMI were included in the GLM described above to validate any interaction effects. In addition, sensitivity analyses were performed to test the robustness of the results. First, crude associations between PCPs characteristics and IVF/ICSI endpoints were analyzed. Second, we excluded women with a diagnosis of PCOS or endometriosis and reanalyzed the data. Finally, association analyses were performed for participants without male-factor infertility. R software (version 4.2.1: The R Project for Statistical Computing, Austria) was used to analyze the data. A P-value of less than 0.05 was regarded as statistically significant.
A total of 1500 women were included in this study. Table 1 lists the demographic, clinical, and PCPs characteristics of all participants. The average age of the participants was 30.9 years, and the mean BMI was 22.1 kg/m2. The majority of participants were of Han ethnicity (96.1%). Most women came from rural areas (64.4%) and were unemployed (51.5%). A total of 94.3% of the participants had never smoked, 76.9% had never consumed alcohol, and 55.3% had never been pregnant. A total of 852 participants (56.8%) were diagnosed with infertility due to female factors. Regarding the use of PCPs, more than half of the women used gel or soap >7 times per week (50.3%). A total of 54.7% of participants used shampoo 4 to <7 times a week. Most participants used skin care products 7 to <14 times per week (45.2%) and did not use make-up products (43.2%). In the past three months, 83.7% of the women had not permed or dyed their hair.
Table 1 provides the cycle-specific characteristics and clinical outcomes. The long GnRH protocol was used in 917 (61.1%) of the 1500 IVF/ICSI cycles, and the antagonist protocol was used in 442 cycles (29.5%). For the insemination method, IVF was applied in 1016 cycles (67.7%), and ICSI was applied in 467 cycles (31.1%). In 1470 cycles, embryo transfer was carried out, of which the percentages of implantation, clinical pregnancy and live birth were 74.0%, 63.5% and 58.0%, respectively.
Table 2 illustrates the relationships between PCPs characteristics and IVF/ICSI intermediate endpoints in the first oocyte aspirations. Compared to participants who did not use skin care products, women who used skin care products ≥14 times per week had a 22.4% (95% CI: –39.2%, –1.6%) decrease in maturation rate. In addition, the participants who used gel or soap ≥7 times per week had 3.2% (95% CI: –14.1%, 9.4%), 6.2% (95% CI: –17.1%, 6.5%), 12.3% (95% CI: –34.1%, 15.1%), and 4.7% (95% CI: –17.0%, 10.0%) decreases in the number of retrieved oocytes, MII oocytes, maturation rate, and 2PN zygotes, respectively. However, these associations were not statistically significant.
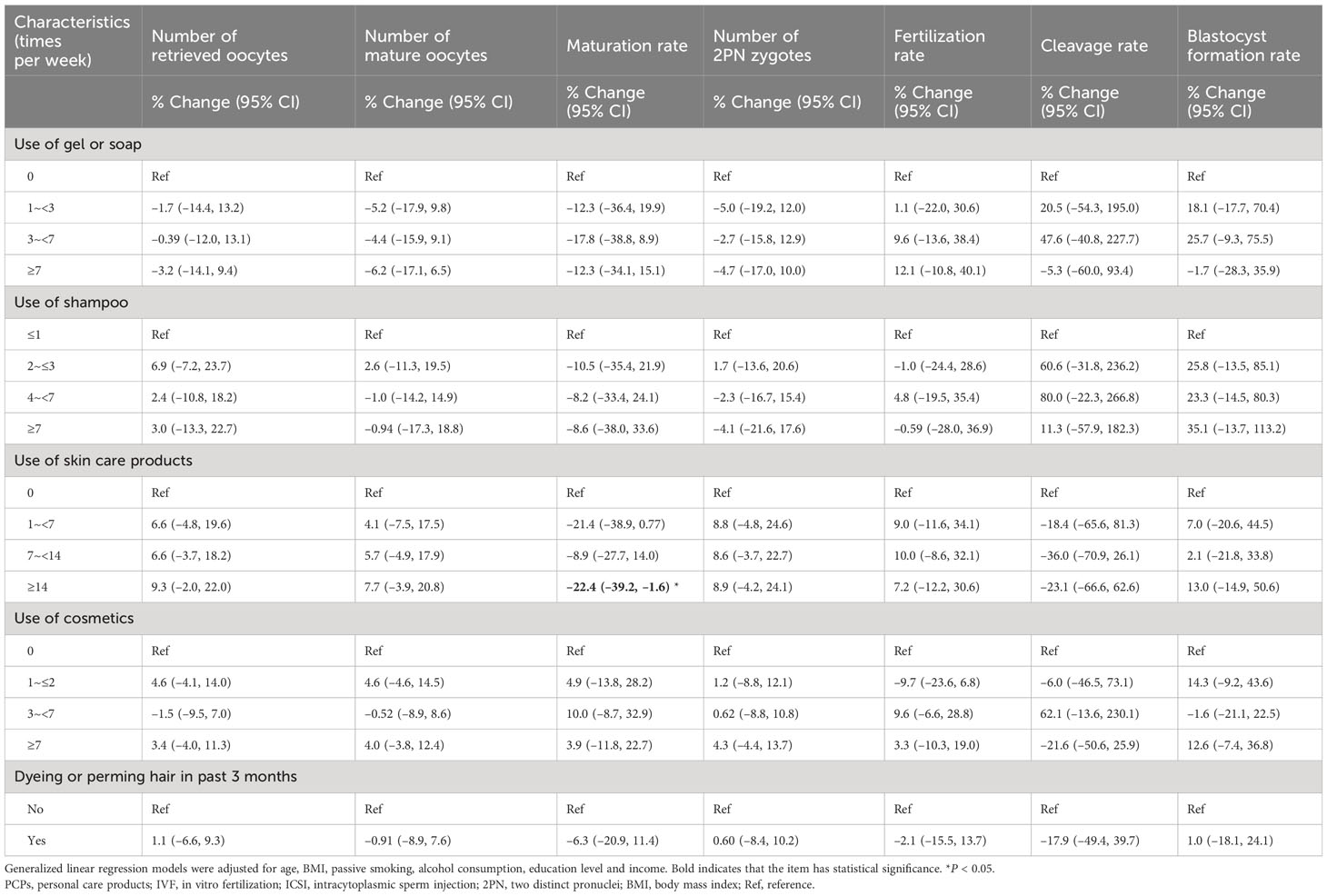
Table 2 Associations between PCPs characteristics and IVF/ICSI parameters in the TREE study (n=1500).
Embryo transfer was performed in 1,364 cycles. Table 3 illustrates the associations between PCPs characteristics and pregnancy outcomes. A null association was found between PCPs use and implantation, clinical pregnancy, miscarriage, and live birth.
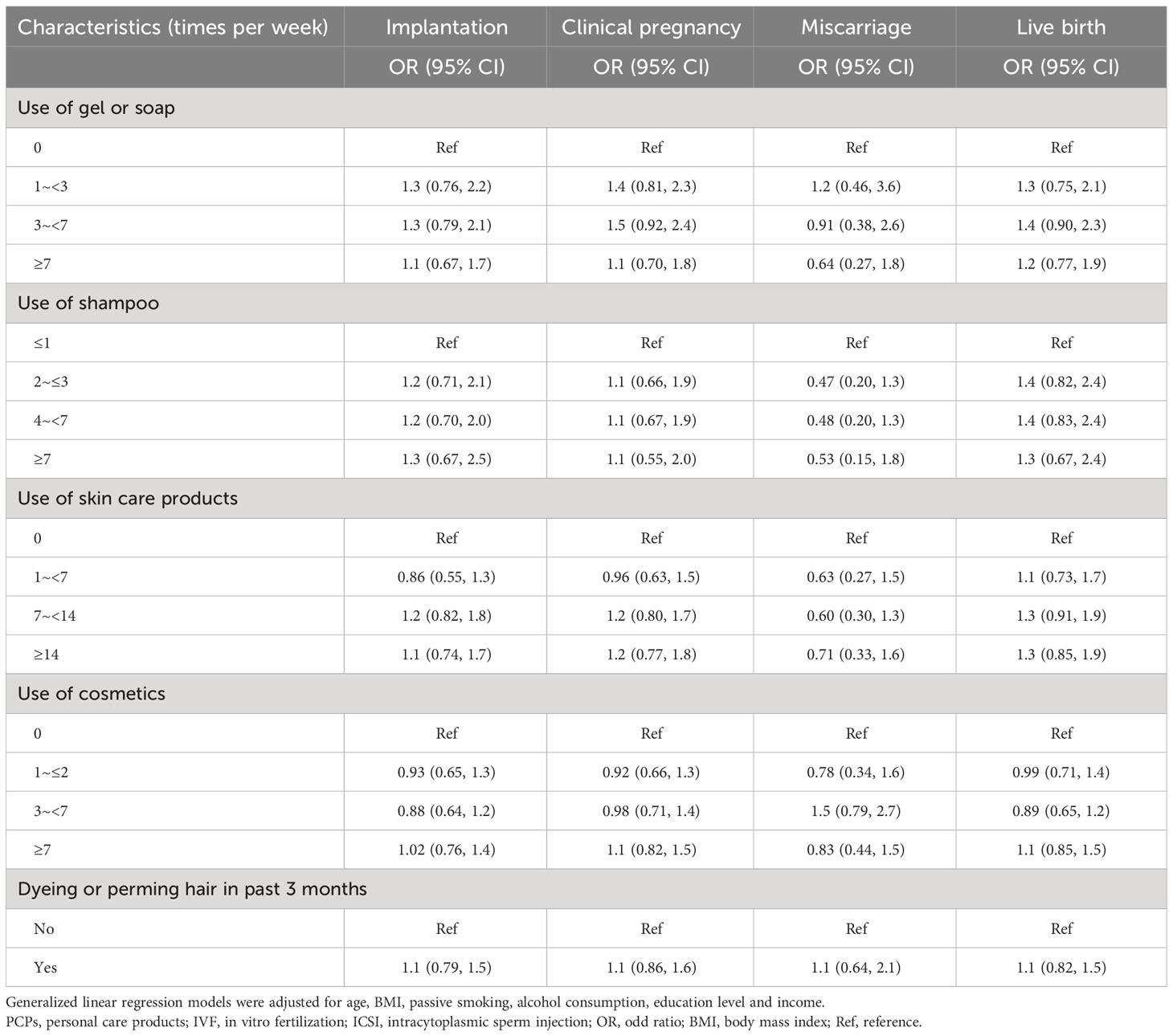
Table 3 Associations between PCPs characteristics and IVF/ICSI pregnancy outcomes among 1364 cycles with embryos transferred.
Age and BMI were stratified to further analyze the interaction effects. The results are presented in Supplementary Tables 1, 2. Age was found to modify the associations of the use of gel or soap with the cleavage rate (β: −0.16, 95% CI: −0.27, −0.06, P = 0.004 for women younger than 30 years; β: 0.07, 95% CI: −0.05, 0.20, P = 0.23 for women aged over 30 years; P for interaction = 0.01) and the blastocyst formation rate (β: −0.04, 95% CI: −0.08, −0.002, P = 0.07 for women younger than 30 years; β: 0.02, 95% CI: −0.03, 0.07, P = 0.39 for women aged over 30 years; P for interaction = 0.04). Age also modified the association of the use of skin care products with the retrieved oocyte number (β: −0.002, 95% CI: −0.01, 0.01, P = 0.70 for women younger than 30 years; β: 0.01, 95% CI: 0.003, 0.02, P = 0.01 for women aged over 30 years; P for interaction = 0.02) and the number of MII oocytes (β: −0.001, 95% CI: −0.01, −0.01, P = 0.78 for women younger than 30 years; β: 0.01, 95% CI: 0.002, 0.02, P = 0.02 for women aged over 30 years; P for interaction = 0.04) (Figure 2).
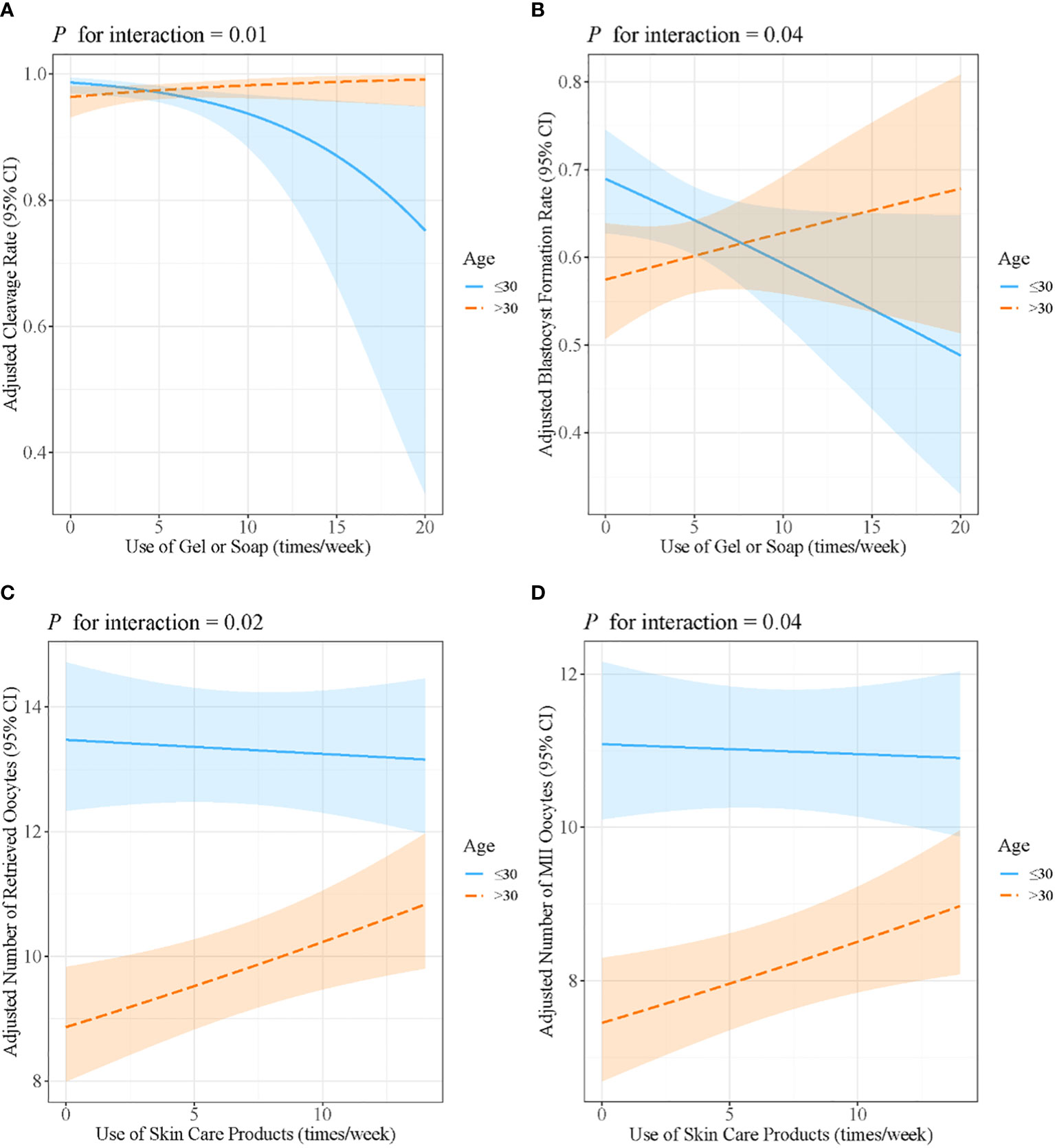
Figure 2 Associations between the use of gel or soap and (A) cleavage rate and (B) blastocyst formation rate, stratified by maternal age. Associations between the use of skin care products and (C) the number of retrieved oocytes and (D) the number of MII oocytes, stratified by maternal age. Models were adjusted for age, BMI, passive smoking, alcohol consumption, education level and income. MII, metaphase II; BMI, body mass index.
The association between the use of gel or soap and the blastocyst formation rate was only significant among women with BMI ≥24 kg/m2 (β: 0.002, 95% CI: −0.03, 0.04, P = 0.92 for women <24 kg/m2; β: −0.08, 95% CI: −0.14, −0.01, P = 0.02 for women ≥24 kg/m2; P for interaction = 0.04). In addition, BMI modified the association between the use of shampoo and cleavage rate (β: −0.08, 95% CI: −0.21, 0.07, P = 0.27 for women <24 kg/m2; β: 0.36, 95% CI: 0.03, 0.75, P = 0.05 for women ≥24 kg/m2; P for interaction = 0.047), as well as the association between the use of skin care products and the blastocyst formation rate (β: 0.03, 95% CI: 0.01, 0.05, P = 0.01 for women <24 kg/m2; β: −0.02, 95% CI: −0.05, 0.04, P = 0.28 for women ≥24 kg/m2; P for interaction = 0.04). The positive association of the use of cosmetics with the maturation rate only existed in women with BMI ≥24 kg/m2 (β: 0.01, 95% CI: −0.004, 0.04, P = 0.16 for women <24 kg/m2; β: −0.05, 95% CI: −0.09, 0.02, P = 0.001 for women ≥24 kg/m2; P for interaction = 0.001) (Figure 3).
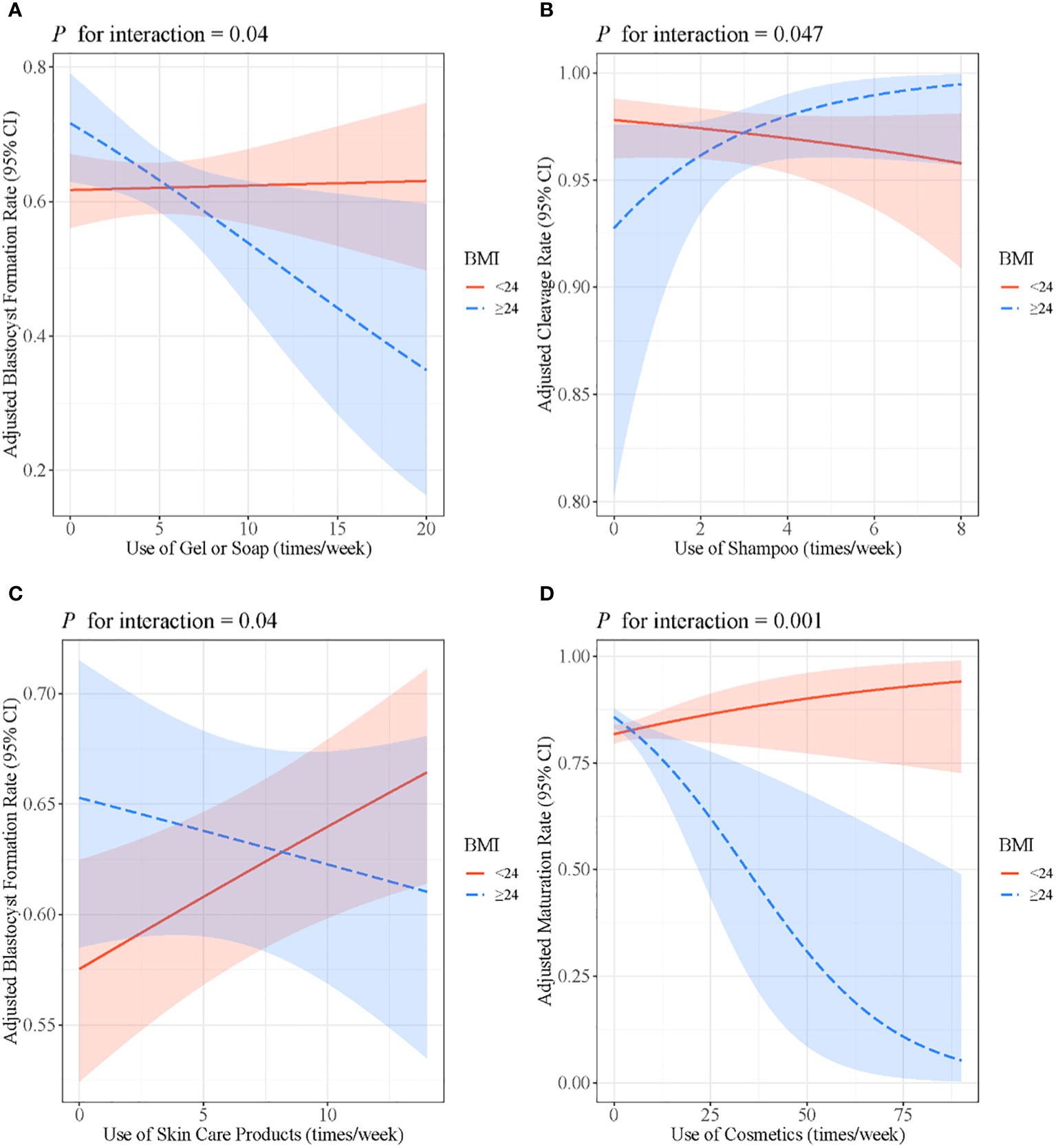
Figure 3 (A) Associations between the use of gel or soap and blastocyst formation rate stratified by BMI. (B) Associations between the use of shampoo and cleavage rate stratified by BMI. (C) Associations between the use of skin care products and blastocyst formation rate stratified by BMI. (D) Associations between the use of cosmetics and maturation rate stratified by BMI. Models were adjusted for age, BMI, passive smoking, alcohol consumption, education level and income. BMI, body mass index.
The results of the crude associations between the use of PCPs and IVF/ICSI outcomes were consistent with the adjusted models (Supplementary Tables 3, 4). The association between PCPs characteristics and pregnancy outcomes in fresh cycles revealed that women who used cosmetics 1–2 times per week (adjusted OR = 2.2, 95% CI: 1.0, 4.8) or 3–7 times per week (adjusted OR = 2.5, 95% CI: 1.2, 5.2) had a higher possibility of miscarriage (Table 4). When we further restricted the analysis to participants without PCOS (Supplementary Tables 5–7), to participants without endometriosis (Supplementary Tables 8–10), and to the participants without male–factor infertility, the results were still stable (Supplementary Tables 11–13).
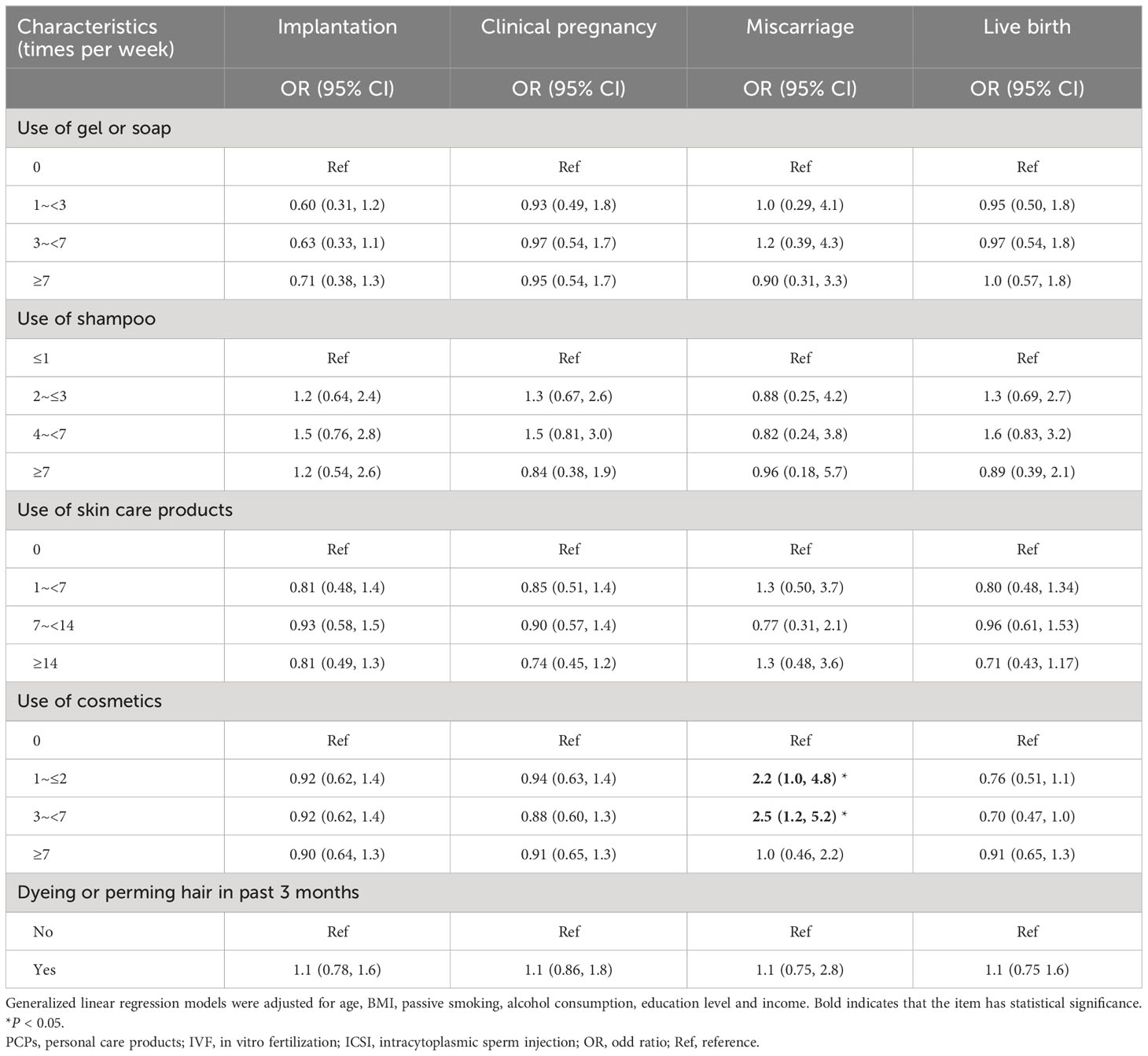
Table 4 Associations between PCPs characteristics and IVF/ICSI pregnancy outcomes among 947 cycles with fresh embryos transferred.
Based on the TREE cohort, we analyzed the association between PCPs use and the reproductive outcomes of IVF/ICSI treatment. The results indicated that among women undergoing IVF/ICSI treatment, women who used skin care products more frequently (≥14 times per week) had lower oocyte maturation rates than those who did not use skin care products. After the transfer of fresh embryos, cosmetic users (1~2 and 3~<7 times per week) had a higher risk of miscarriage than non-users.
As previously mentioned, limited research exists on the association between PCPs use and reproductive outcomes. A recent study showed that women who use soap during pregnancy had a longer mean birth length and a longer mean gestational age at delivery compared to non-users (33). A case-control study from the United States found null association between perinatal use of chemical curl products and preterm birth (34). Another prospective cohort study involving 9710 pregnant women reported an increased risk of small-for-gestational-age infants among those with a high frequency of cosmetics use (≥5 times per week) during pregnancy (35). In addition, studies on hairdressers revealed that they have an increased risk of spontaneous abortion compared to the normal women (36, 37). Although these studies share some similarities with ours, none have reported on the association between PCPs use and oocyte maturation.
There is increasing evidence that the PCPs use patterns influence the levels of exposure markers to EDCs in human specimens. A Korean study involving 5,962 participants showed that urinary concentrations of methylparaben (MeP), ethylparaben (EtP) and propylparaben (PrP) are directly correlated with the use of PCPs (13). PCPs were also the major source of PAE exposure in women (38), and urinary concentrations of MeP, EtP and PrP were notably higher in females than in males (13, 39). Moreover, in a Taiwan birth cohort of 281 pregnant women, the use patterns of skin care products and cosmetics were positively associated with urinary PAEs concentrations. Notably, leave–on PCPs (e.g., toners, lipsticks, essential oils) had stronger associations than rinse–off PCPs (e.g., shampoo, face wash) (40).
Previous studies have reported the associations between internal exposure levels of single or mixed EDCs and female reproductive function, but these have overlooked the potential correlation between the use of PCPs and female fertility. The current study found that the use of skin care products was negatively associated with oocyte maturation in IVF/ICSI treatments. In prior research, urinary concentrations of phthalates, parabens and glycol ethers in women were associated with time to pregnancy, which represents a quantification of the ability to conceive (41). Moreover, among women undergoing IVF treatment in Poland, the urinary concentration of butylparaben (BuP) was also associated with reduced numbers of MII oocytes but had a null association with the rates of implantation, clinical pregnancy, and live birth (42). Animal studies have also found that several ingredients in sunscreens, such as BPs and nanoparticles (NPs), to diminished oocyte developmental potential. For instance, exposure to UV filters 3-benzylidene camphor or BP-2 resulted in decreased ovulation, fewer mature oocytes, and more atretic follicles in Pimephales promelas (43, 44). NPs were shown to cause increased follicular atresia and disrupt follicular maturation in mice (45). ZnO can impair mouse oocyte meiosis and early embryonic development by promoting mitochondrial stress and endoplasmic reticulum stress, activating autophagy and apoptosis (46). TiO2NPs were associated with reduced oocyte number, fertilization rate, cleaved-embryo and blastocyst counts (47). Additionally, components like PPD, ethylene glycol butyl ester, and octocrylene found in hair dyes or cosmetics were associated with mitochondrial dysfunction, leading to impaired oocyte quality (48–50).
Although it is recommended in clinical practice that pregnant women reduce the use of PCPs, Lang et al. (51) found consistent use patterns of hygiene and skincare products throughout pregnancy, whereas the use of cosmetics and hair styling products decreased during this period. Since a variety of BPs and parabens have been found in umbilical cord blood (52) and the concentrations of TCS and TCC in umbilical cord blood are positively correlated with those in maternal serum (53), the fetus may be affected by maternal exposure to PCPs during growth and development. Our study showed that cosmetic use increased the risk of miscarriage. Furthermore, urinary concentrations of bisphenol analogs and phthalates were also reported to be associated with recurrent miscarriage in case–control studies (54–56). A birth cohort from New York reported that butylparaben concentrations in cord blood were associated with an increased risk of preterm birth and decreased gestational age at birth (57). Occupational exposure populations, such as cosmetologists, demonstrated a higher risk of pregnancy complications, like fetal growth restriction, compared to the general population (58, 59). In animal studies, BPA exposure was associated with reduced embryo implantation (60). Benzophenone–3 may induce placental thrombosis and miscarriage (61). Maternal exposure to TiO2NPs was associated with increased atretic follicles, decreased mating and pregnancy rates (62), increased the number of stunted fetuses, and increased fetal mortality (63).
The association between PCPs use and female reproductive function can potentially be explained through several biological mechanisms. Oocyte maturation is susceptible to oxidative stress. For instance, propyl gallate, an ingredient present in cosmetics, may induce oxidative stress and DNA damage, potentially affecting methylation levels and influencing the meiotic maturation of oocytes (64). Moreover, PFOA and PAEs can promote oxidative stress level, inducing apoptosis and necrosis in oocyte (6, 65). During pregnancy, increased oxidative stress, impaired placental function and luteal function, and autoimmune disease may impact embryonic development and even lead to miscarriage. For instance, di-(2–ethylhexyl) phthalate (DEHP) and mono(2–ethylhexyl) phthalate have been reported to affect placental function by altering angiogenesis and promoting oxidative stress (66). In early pregnant mice, exposure to ZnO NP promotes oxidative stress and mitochondrial apoptosis in utero, leading to increased rates of miscarriage (67). In addition, prenatal PFOA exposure inhibited ovarian luteal function in mice, leading to increased embryonic resorption (68). In a prospective study involving patients with recurrent miscarriage, serum BPA levels were significantly higher in antinuclear antibody positive patients than in negative patients (69).
In stratified analyses, we observed that the association of PCPs use with IVF/ICSI parameters varied with age and BMI. It has been shown that adult urinary paraben concentrations are negatively associated with age (70) and that younger women tend to use more toiletries (71). Our results align with these observations, particularly noting the negative association between the use of gel or soap and the cleavage rate observed specifically in women under 30 years of age. Moreover, Wenzel et al. (72) found a positive association between BMI and urinary phthalate concentration. Similarly, our study observed that the use of cosmetics was negatively associated with the maturation rate exclusively in women with BMI ≥ 24 kg/m2. These findings suggest that there might be interaction effects between age, BMI, and PCPs use in women. However, further research into the underlying mechanisms driving these interactions is necessary for a comprehensive understanding of their implications.
According to our knowledge, this study is the first to examine the associations between habitual PCPs usage and IVF/ICSI outcomes. Our strengths lie in the prospective study design, a substantial sample size, and comprehensive data on PCPs use and clinical information. In addition, the current study investigates the direct use of PCPs within infertile patients, which is closer to daily life, offering valuable implications for women planning pregnancy or undergoing fertility treatments. However, certain limitations need to be acknowledged. Firstly, we used the frequency of PCPs use but not the concentrations of EDCs within PCPs for subsequent analysis. Secondly, the PCPs characteristics gathered from questionnaires represent external exposures, potentially leading to inaccuracies due to individual variations in absorption, metabolism, and exposure pathways. Thirdly, the questionnaire did not include information on brands and dosages of PCPs. Fourthly, the information obtained from the questionnaire in a prospective cohort might not entirely represent the PCPs use patterns during pregnancy, possibly introducing bias into the results. Finally, the participants in the current study were recruited from an infertility cohort undergoing IVF/ICSI treatment, potentially limiting the generalizability of the findings to the broader population. Despite not measuring internal exposure levels, this study offers valuable insights into the association between PCPs use and pregnancy outcomes.
In conclusion, our results indicate that the use of PCPs in women undergoing IVF/ICSI treatment was associated with a decreased oocyte maturation rate and an increased miscarriage rate. This hints at the potential impact of exposure to a mixture of EDCs found in PCPs on female fertility. The current study provides information on the associations between PCPs and adverse reproductive outcomes, recommending caution regarding PCPs use during pregnancy preparation. However, further epidemiologic studies are needed for further validation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. This study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Q-CG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. WY: Formal Analysis, Investigation, Writing – review & editing. CL: Methodology, Investigation, Validation. T-RD: Investigation, Writing – review & editing. JL: Investigation, Methodology, Writing – review & editing. H-ML: Investigation, Methodology, Writing – review & editing. W-QT: Investigation, Methodology, Writing – review & editing. YW: Investigation, Methodology, Writing – review & editing. Y-YD: Formal Analysis, Methodology, Supervision, Writing – review & editing. Y-FL: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China [Award number: 82371666].
The authors sincerely thank all the doctors, nurses and embryologists at the Reproductive Center of Tongji Hospital for their hard work, and all the participants of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1320893/full#supplementary-material.
1. Sun H, Gong T-T, Jiang Y-T, Zhang S, Zhao Y-H, Wu Q-J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories 1990-2017: results from a global burden of disease stud. Aging (Albany NY) (2019) 11:10952–91. doi: 10.18632/aging.102497
2. Luk BH-K, Loke AY. The impact of infertility on the psychological well-being, marital relationships, sexual relationships, and quality of life of couples: A systematic review. J Sex Marital Ther (2015) 41:610–25. doi: 10.1080/0092623X.2014.958789
3. Chambers GM, Dyer S, Zegers-Hochschild F, de Mouzon J, Ishihara O, Banker M, et al. International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology 2014†. Hum Reprod (2021) 36:2921–34. doi: 10.1093/humrep/deab198
4. De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2015: results generated from European registries by ESHRE. Hum Reprod Open (2020) 2020(1):hoz038. doi: 10.1093/hropen/hoz038
5. Liu J-L, Wong M-H. Pharmaceuticals and personal care products (PPCPs): a review on environmental contamination in China. Environ Int (2013) 59:208–24. doi: 10.1016/j.envint.2013.06.012
6. Gonsioroski A, Mourikes VE, Flaws JA. Endocrine disruptors in water and their effects on the reproductive system. Int J Mol Sci (2020) 21:1929. doi: 10.3390/ijms21061929
7. Yilmaz B, Terekeci H, Sandal S, Kelestimur F. Endocrine disrupting chemicals: exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev Endocr Metab Disord (2020) 21:127–47. doi: 10.1007/s11154-019-09521-z
8. Bhatnagar A, Anastopoulos I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere (2017) 168:885–902. doi: 10.1016/j.chemosphere.2016.10.121
9. Tavares RS, Martins FC, Oliveira PJ, Ramalho-Santos J, Peixoto FP. Parabens in male infertility-is there a mitochondrial connection? Reprod Toxicol (2009) 27:1–7. doi: 10.1016/j.reprotox.2008.10.002
10. Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect (2012) 120:978–83. doi: 10.1289/ehp.1104307
11. Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, et al. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: results from the EARTH study. Environ Health Perspect (2016) 124:831–9. doi: 10.1289/ehp.1509760
12. Mínguez-Alarcón L, Hauser R, Gaskins AJ. Effects of bisphenol A on male and couple reproductive health: a review. Fertil Steril (2016) 106:864–70. doi: 10.1016/j.fertnstert.2016.07.1118
13. Lim S. The associations between personal care products use and urinary concentrations of phthalates, parabens, and triclosan in various age groups: The Korean National Environmental Health Survey Cycle 3 2015-2017. Sci Total Environ (2020) 742:140640. doi: 10.1016/j.scitotenv.2020.140640
14. Li C, Zhao Y, Liu S, Yang D, Ma H, Zhu Z, et al. Exposure of Chinese adult females to parabens from personal care products: Estimation of intake via dermal contact and health risks. Environ pollut (2021) 272:116043. doi: 10.1016/j.envpol.2020.116043
15. Hua R, Zhou Y, Wu B, Huang Z, Zhu Y, Song Y, et al. Urinary triclosan concentrations and early outcomes of in vitro fertilization-embryo transfer. Reproduction (2017) 153:319–25. doi: 10.1530/REP-16-0501
16. Radwan P, Wielgomas B, Radwan M, Krasiński R, Klimowska A, Zajdel R, et al. Triclosan exposure and in vitro fertilization treatment outcomes in women undergoing in vitro fertilization. Environ Sci pollut Res Int (2021) 28:12993–9. doi: 10.1007/s11356-020-11287-w
17. Cicolella A. Glycol ethers: a ubiquitous family of toxic chemicals: a plea for REACH regulation. Ann N Y Acad Sci (2006) 1076:784–9. doi: 10.1196/annals.1371.049
18. Fujii Y, Harada KH, Koizumi A. Occurrence of perfluorinated carboxylic acids (PFCAs) in personal care products and compounding agents. Chemosphere (2013) 93:538–44. doi: 10.1016/j.chemosphere.2013.06.049
19. Lu S, Long F, Lu P, Lei B, Jiang Z, Liu G, et al. Benzophenone-UV filters in personal care products and urine of schoolchildren from Shenzhen, China: Exposure assessment and possible source. Sci Total Environ (2018) 640–641:1214–20. doi: 10.1016/j.scitotenv.2018.06.015
20. Kunisue T, Chen Z, Buck Louis GM, Sundaram R, Hediger ML, Sun L, et al. Urinary concentrations of benzophenone-type UV filters in U.S. women and their association with endometriosis. Environ Sci Technol (2012) 46:4624–32. doi: 10.1021/es204415a
21. Mínguez-Alarcón L, Chiu Y-H, Nassan FL, Williams PL, Petrozza J, Ford JB, et al. Urinary concentrations of benzophenone-3 and reproductive outcomes among women undergoing infertility treatment with assisted reproductive technologies. Sci Total Environ (2019) 678:390–8. doi: 10.1016/j.scitotenv.2019.04.452
22. Yu L, Zhai J, Wang Y, Geng Y, Chen X, Wen Y, et al. Exposure to N-monoacetyl-p-phenylenediamine impaired ovarian function in mice. J Appl Toxicol (2021) 41:2031–41. doi: 10.1002/jat.4183
23. Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect (2007) 115 Suppl 1:98–105. doi: 10.1289/ehp.9357
24. Deng Y-L, Luo Q, Liu C, Zeng J-Y, Lu T-T, Shi T, et al. Urinary biomarkers of exposure to drinking water disinfection byproducts and ovarian reserve: A cross-sectional study in China. J Hazard Mater (2022) 421:126683. doi: 10.1016/j.jhazmat.2021.126683
25. Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet JM, et al. Smoking in China: findings of the 1996 national prevalence survey. JAMA (1999) 282:1247–53. doi: 10.1001/jama.282.13.1247
26. Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem (2018) 62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012
27. Zhu L, Xi Q, Zhang H, Li Y, Ai J, Jin L. Blastocyst culture and cryopreservation to optimize clinical outcomes of warming cycles. Reprod BioMed Online (2013) 27:154–60. doi: 10.1016/j.rbmo.2013.04.006
28. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care 2017. Hum Reprod (2017) 32:1786–801. doi: 10.1093/humrep/dex234
29. Grøndahl ML, Christiansen SL, Kesmodel US, Agerholm IE, Lemmen JG, Lundstrøm P, et al. Effect of women’s age on embryo morphology, cleavage rate and competence—A multicenter cohort study. PloS One (2017) 12:e0172456. doi: 10.1371/journal.pone.0172456
30. Kermack AJ, Fesenko I, Christensen DR, Parry KL, Lowen P, Wellstead SJ, et al. Incubator type affects human blastocyst formation and embryo metabolism: a randomized controlled trial. Hum Reprod (2022) 37:2757–67. doi: 10.1093/humrep/deac233
31. Yao Q-Y, Yuan X-Q, Liu C, Du Y-Y, Yao Y-C, Wu L-J, et al. Associations of sleep characteristics with outcomes of IVF/ICSI treatment: a prospective cohort study. Hum Reprod (2022) 37:1297–310. doi: 10.1093/humrep/deac040
32. Mirzaei A, Carter SR, Patanwala AE, Schneider CR. Missing data in surveys: Key concepts, approaches, and applications. Res Soc Adm Pharm (2022) 18:2308–16. doi: 10.1016/j.sapharm.2021.03.009
33. Chan M, Preston EV, Fruh V, Quinn MR, Hacker MR, Wylie BJ, et al. Use of personal care products during pregnancy and birth outcomes - A pilot study. Environ Res (2023) 225:115583. doi: 10.1016/j.envres.2023.115583
34. Blackmore-Prince C, Harlow SD, Gargiullo P, Lee MA, Savitz DA. Chemical hair treatments and adverse pregnancy outcome among Black women in central North Carolina. Am J Epidemiol (1999) 149:712–6. doi: 10.1093/oxfordjournals.aje.a009879
35. Li H, Zheng J, Wang H, Huang G, Huang Q, Feng N, et al. Maternal cosmetics use during pregnancy and risks of adverse outcomes: a prospective cohort study. Sci Rep (2019) 9:8030. doi: 10.1038/s41598-019-44546-z
36. Kersemaekers WM, Roeleveld N, Zielhuis GA. Reproductive disorders among hairdressers. Epidemiology (1997) 8:396–401. doi: 10.1097/00001648-199707000-00008
37. Baste V, Moen BE, Riise T, Hollund BE, Øyen N. Infertility and spontaneous abortion among female hairdressers: the Hordaland Health Study. J Occup Environ Med (2008) 50:1371–7. doi: 10.1097/JOM.0b013e3181858723
38. Begum TF, Gerona R, Melamed J, McGough A, Lenhart N, Wong R, et al. Sources of exposure to urinary phthalates among couples undergoing infertility treatment. Int J Hyg Environ Health (2020) 229:113567. doi: 10.1016/j.ijheh.2020.113567
39. Wang L, Wu Y, Zhang W, Kannan K. Characteristic profiles of urinary p-hydroxybenzoic acid and its esters (parabens) in children and adults from the United States and China. Environ Sci Technol (2013) 47:2069–76. doi: 10.1021/es304659r
40. Hsieh C-J, Chang Y-H, Hu A, Chen M-L, Sun C-W, Situmorang RF, et al. Personal care products use and phthalate exposure levels among pregnant women. Sci Total Environ (2019) 648:135–43. doi: 10.1016/j.scitotenv.2018.08.149
41. Hipwell AE, Kahn LG, Factor-Litvak P, Porucznik CA, Siegel EL, Fichorova RN, et al. Exposure to non-persistent chemicals in consumer products and fecundability: a systematic review. Hum Reprod Update (2019) 25:51–71. doi: 10.1093/humupd/dmy032
42. Radwan P, Wielgomas B, Radwan M, Krasiński R, Bujak-Pietrek S, Polańska K, et al. Urinary concentration of selected nonpersistent endocrine disrupting chemicals-reproductive outcomes among women from a fertility clinic. Environ Sci pollut Res Int (2023) 30:45088–96. doi: 10.1007/s11356-023-25355-4
43. Kunz PY, Gries T, Fent K. The ultraviolet filter 3-benzylidene camphor adversely affects reproduction in fathead minnow (Pimephales promelas). Toxicol Sci (2006) 93:311–21. doi: 10.1093/toxsci/kfl070
44. Weisbrod CJ, Kunz PY, Zenker AK, Fent K. Effects of the UV filter benzophenone-2 on reproduction in fish. Toxicol Appl Pharmacol (2007) 225:255–66. doi: 10.1016/j.taap.2007.08.004
45. Liu JA, Yang M, Jing JJK, Ren L, Wei J, Zhang J. Silica nanoparticle exposure inducing granulosa cell apoptosis and follicular atresia in female Balb/c mice. Environ Sci Pollut Res (2018) 25:3423–34. doi: 10.1007/s11356-017-0724-5
46. Huang C, Wu D, Khan FA, Wang Y, Xu J, Luo C, et al. Zinc oxide nanoparticle causes toxicity to the development of mouse oocyte and early embryo. Toxicol Lett (2022) 358:48–58. doi: 10.1016/j.toxlet.2022.01.010
47. Karimipour M, Zirak Javanmard M, Ahmadi A, Jafari A. Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. Int J Reprod BioMed (2018) 16:397–404. doi: 10.29252/ijrm.16.6.397
48. Shi X, Miao Y, Zhang K, Gong S, Xiong B. Ethylene glycol butyl ether deteriorates oocyte quality via impairing mitochondrial function. FASEB J (2021) 35:e21280. doi: 10.1096/fj.202002157R
49. Chang H, Li J, Zhang C, Qian W. Octocrylene exposure impairs mouse oocyte quality by inducing spindle defects and mitochondria dysfunction. Toxicology (2022) 479:153306. doi: 10.1016/j.tox.2022.153306
50. Wang X, Zhao X, Chen Y, Wang Q, Yang H, Xia F. Para-phenylenediamine deteriorates oocyte quality by impairing mitochondrial function. Environ Toxicol (2022) 37:1803–13. doi: 10.1002/tox.23528
51. Lang C, Fisher M, Neisa A, MacKinnon L, Kuchta S, MacPherson S, et al. Personal care product use in pregnancy and the postpartum period: implications for exposure assessment. Int J Environ Res Public Health (2016) 13:105. doi: 10.3390/ijerph13010105
52. Sunyer-Caldú A, Peiró A, Díaz M, Ibáñez L, Gil-Solsona R, Gago-Ferrero P, et al. Target analysis and suspect screening of UV filters, parabens and other chemicals used in personal care products in human cord blood: Prenatal exposure by mother-fetus transfer. Environ Int (2023) 173:107834. doi: 10.1016/j.envint.2023.107834
53. Bai X, Zhang B, He Y, Hong D, Song S, Huang Y, et al. Triclosan and triclocarbon in maternal-fetal serum, urine, and amniotic fluid samples and their implication for prenatal exposure. Environ pollut (2020) 266:115117. doi: 10.1016/j.envpol.2020.115117
54. Chang W-H, Chou W-C, Waits A, Liao K-W, Kuo P-L, Huang P-C. Cumulative risk assessment of phthalates exposure for recurrent pregnancy loss in reproductive-aged women population using multiple hazard indices approaches. Environ Int (2021) 154:106657. doi: 10.1016/j.envint.2021.106657
55. Ao J, Huo X, Zhang J, Mao Y, Li G, Ye J, et al. Environmental exposure to bisphenol analogues and unexplained recurrent miscarriage: A case-control study. Environ Res (2022) 204:112293. doi: 10.1016/j.envres.2021.112293
56. Ji H, Wu Z, Chen D, Miao M, Chen H, Shuai W, et al. Individual and joint effects of phthalates exposure on the risk of early miscarriage. J Expo Sci Environ Epidemiol. (2023). doi: 10.1038/s41370-023-00533-1
57. Geer LA, Pycke BFG, Waxenbaum J, Sherer DM, Abulafia O, Halden RU. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater (2017) 323:177–83. doi: 10.1016/j.jhazmat.2016.03.028
58. Quach T, Von Behren J, Goldberg D, Layefsky M, Reynolds P. Adverse birth outcomes and maternal complications in licensed cosmetologists and manicurists in California. Int Arch Occup Environ Health (2015) 88:823–33. doi: 10.1007/s00420-014-1011-0
59. Halliday-Bell JA, Gissler M, Jaakkola JJK. Work as a hairdresser and cosmetologist and adverse pregnancy outcomes. Occup Med (Lond) (2009) 59:180–4. doi: 10.1093/occmed/kqp017
60. Berger RG, Hancock T, deCatanzaro D. Influence of oral and subcutaneous bisphenol-A on intrauterine implantation of fertilized ova in inseminated female mice. Reprod Toxicol (2007) 23:138–44. doi: 10.1016/j.reprotox.2006.09.005
61. Han X, Lu T, Hu Y, Duan J, Guan Y, Huang X, et al. A metabolomic study on the effect of prenatal exposure to Benzophenone-3 on spontaneous fetal loss in mice. Ecotoxicol Environ Saf (2022) 233:113347. doi: 10.1016/j.ecoenv.2022.113347
62. Hong F, Wang L. Nanosized titanium dioxide-induced premature ovarian failure is associated with abnormalities in serum parameters in female mice. Int J Nanomedicine (2018) 13:2543–9. doi: 10.2147/IJN.S151215
63. Hong F, Zhou Y, Zhao X, Sheng L, Wang L. Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int J Nanomedicine (2017) 12:6197–204. doi: 10.2147/IJN.S143598
64. Yang S-J, Wang Y-S, Zhang L-D, Ding Z-M, Zhou X, Duan Z-Q, et al. High-dose synthetic phenolic antioxidant propyl gallate impairs mouse oocyte meiotic maturation through inducing mitochondrial dysfunction and DNA damage. Environ Toxicol (2023) 38:1800–10. doi: 10.1002/tox.23807
65. Panagiotou EM, Ojasalo V, Damdimopoulou P. Phthalates, ovarian function and fertility in adulthood. Best Pract Res Clin Endocrinol Metab (2021) 35:101552. doi: 10.1016/j.beem.2021.101552
66. Martínez-Razo LD, Martínez-Ibarra A, Vázquez-Martínez ER, Cerbón M. The impact of Di-(2-ethylhexyl) Phthalate and Mono(2-ethylhexyl) Phthalate in placental development, function, and pathophysiology. Environ Int (2021) 146:106228. doi: 10.1016/j.envint.2020.106228
67. Chen L, Wu H, Hong W, Aguilar ZP, Fu F, Xu H. The effect of reproductive toxicity induced by ZnO NPs in mice during early pregnancy through mitochondrial apoptotic pathway. Environ Toxicol (2021) 36:1143–51. doi: 10.1002/tox.23113
68. Chen Y, Zhou L, Xu J, Zhang L, Li M, Xie X, et al. Maternal exposure to perfluorooctanoic acid inhibits luteal function via oxidative stress and apoptosis in pregnant mice. Reprod Toxicol (2017) 69:159–66. doi: 10.1016/j.reprotox.2017.02.010
69. Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod (2005) 20:2325–9. doi: 10.1093/humrep/deh888
70. Kiani Feizabadi G, Hajizadeh Y, Feizi A, Ebrahimpour K. Urinary concentrations of parabens amongst Iranian adults and their associations with socio-demographic factors. J Environ Health Sci Eng (2020) 18:1227–38. doi: 10.1007/s40201-020-00540-6
71. Rivera-Núñez Z, Ashrap P, Barrett ES, Llanos AAM, Watkins DJ, Cathey AL, et al. Personal care products: Demographic characteristics and maternal hormones in pregnant women from Puerto Rico. Environ Res (2022) 206:112376. doi: 10.1016/j.envres.2021.112376
Keywords: personal care products, cosmetics, IVF/ICSI treatment, oocyte quality, reproductive outcomes
Citation: Guo Q-C, Yao W, Liu C, Deng T-R, Li J, Liao H-M, Tian W-Q, Wang Y, Du Y-Y and Li Y-F (2024) Associations of personal care products use with reproductive outcomes of IVF/ICSI treatment. Front. Endocrinol. 14:1320893. doi: 10.3389/fendo.2023.1320893
Received: 13 October 2023; Accepted: 28 December 2023;
Published: 24 January 2024.
Edited by:
Terje Svingen, Technical University of Denmark, DenmarkReviewed by:
Marianna Santonastaso, Università degli Studi della Campania “Luigi Vanvitelli”, ItalyCopyright © 2024 Guo, Yao, Liu, Deng, Li, Liao, Tian, Wang, Du and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao-Yao Du, RFlZQHRqaC50am11LmVkdS5jbg==; Yu-Feng Li, dGpseWY2NkAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.