
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 03 January 2024
Sec. Obesity
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1308254
This article is part of the Research Topic Insights in Obesity: 2023 View all 9 articles
Background: The relationship between weight change patterns and arthritis onset, specifically rheumatoid arthritis (RA) and osteoarthritis (OA), is unclear. We examined the association between weight changes from young adulthood to midlife and arthritis onset.
Methods: Using data from NHANES 1999–2018, participants with self-reported arthritis were selected. Age at diagnosis determined arthritis onset. Weight change patterns were based on BMI at age 25 and 10 years before the survey. Patterns were categorized as stable non-obese, non-obese to obese, obese to non-obese, and stable obese. Cox regression models and restricted cubic spline (RCS) analysis were employed, calculating hazard ratios (HRs) and 95% confidence intervals (CIs) considering covariates.
Results: Out of 20,859 participants (male 11,017, 52.82%), 4922 developed arthritis over a mean 8.66-year follow-up. Compared to stable non-obese individuals, the HRs for arthritis were 1.55 (95% CI=1.45 to 1.66, P < 0.0001) for non-obese to obese and 1.74 (95% CI=1.56 to 1.95, P < 0.0001) for stable obese. Those gaining 10-20 kg had a HR of 1.33 (95% CI=1.22 to 1.46, P < 0.0001), and gains >20 kg had a HR of 1.56 (95% CI=1.42 to 1.71, P < 0.0001), compared to stable weight (change within 2.5 kg). Identical results observed for OA and RA. RCS showed a nonlinear relationship between weight change and arthritis (all P < 0.01).
Conclusions: Stable obesity and weight gain during adulthood increase arthritis risk. Maintaining a non-obese weight throughout adult years might reduce arthritis risk in later life.
Arthritis is a medical condition that involves infections within and around the joints. It is a prevalent chronic systemic inflammatory disease, characterized by symptoms such as redness, swelling, heat, and pain (1). Currently, there are 355 million individuals worldwide who are affected by arthritis (2). It is estimated that by 2040, approximately 25.9% of all American adults will be diagnosed with arthritis (3). This often results in limited movement and a consequent decrease in the quality of life for affected individuals. Its etiology is multifactorial, encompassing genetic, environmental, and lifestyle factors (4). Among these, body weight, particularly as measured by the body mass index (BMI), has emerged as a significant modifiable risk factor for the development of certain types of arthritis, notably osteoarthritis (OA) (5). The Global Burden of Disease study in 2017 highlighted the relevance of high BMI as a primary risk factor for the burden of OA in Brazil (5). This association is not limited to static measures of weight but extends to dynamic changes in weight over time. For instance, an increasing trend of knee radiographs with OA-like features has been observed in rheumatoid arthritis (RA) patients in recent decades, with a higher BMI identified as an independent factor for such manifestations (6).
Of the many types of arthritis, OA and RA are the two most common forms, each affecting millions worldwide and presenting distinct challenges in healthcare (7). While they both share the general term ‘arthritis’, OA and RA have distinct pathophysiological mechanisms, clinical manifestations, and therapeutic approaches (4, 8). OA is a degenerative joint disease primarily affecting the articular cartilage and subchondral bone, often related to aging, mechanical stress, or metabolic disturbances (9). OA is now understood as a disease affecting the entire joint, involving not just the cartilage, but also the subchondral bone, meniscus, synovial membrane, and infrapatellar fat pad (10). It is marked by joint pain, stiffness, and reduced function, predominantly affecting the knees, hips, and hands (11). Conversely, RA is an autoimmune disease leading to chronic inflammation in joints. This systemic disorder is characterized by symmetric polyarthritis, typically affecting the small joints of the hands and feet, and can result in significant joint damage and deformity if not managed properly (4, 12). Additionally, RA is associated with extra-articular manifestations and systemic inflammation, contributing to increased cardiovascular risk and mortality (13). Furthermore, body composition differences between RA and OA patients have been noted, with OA patients typically presenting a higher total fat mass compared to their RA counterparts (14). Besides the substantial physical impact on patients, OA contributes significantly to escalating healthcare costs in the US (15). While there is emerging evidence suggesting that weight loss from young adulthood to midlife can substantially reduce arthritis risk (16), recent studies indicate that the differential effects of weight changes on the onset and progression of OA and RA are complex and warrant further investigation (17).
Thus, understanding these weight change patterns is crucial, as weight trajectories during these life stages can have long-term implications for joint health and overall well-being. Given the profound implications of arthritis and its associated limited activity, addressing this condition remains a pressing concern for both clinical and public health systems. This study aims to shed light on this relationship, offering insights that could inform preventive strategies and interventions.
The National Health and Nutrition Examination Survey (NHANES) is a research project conducted by the National Center for Health Statistics (NCHS) to gather health and nutrition data on the population of the United States. Each year, approximately 5,000 individuals, selected through a nationally representative sample, are included in the survey. To ensure representative results, the organization employs a stratified, multistage, and clustered probability sampling design. Furthermore, all participants provide written informed consent prior to data collection. The study is conducted by qualified medical professionals and staff, who utilize various methods such as questionnaires, physical examinations, and laboratory data available through open online sources (https://www.cdc.gov/nchs/nhanes).
In this study, data from 10 consecutive NHANES cycles from 1999 to 2018 were used. A total of 101,316 participants were included in the analysis, with exclusions for individuals younger than 40 years or older than 75 years, underweight individuals (BMI 10 years ago or at 25 years < 18.5 kg/m2), and those with missing BMI data at 10 years before baseline or at both age 25 years and 10 years before baseline. Moreover, subjects with an unclear onset of arthritis, those who had prevalent arthritis before the beginning of our follow-up study, and individuals with missing information on their arthritis status were excluded. Finally, a total of 20,859 participants were eligible for further analyses. The detailed study flowchart is depicted in Figure 1.
The NHANES Anthropometry section utilized standardized examination procedures to measure participants’ standing height and weight at baseline. Participants were asked to recall their weight at age 25 and 10 years prior to the survey, while baseline weight and height were measured during the physical examination. BMI is calculated by taking a person’s weight in kilograms and dividing it by their height in meters squared. Obesity is defined as a BMI of 30.0 kg/m² or higher. BMI was computed based on the measured height during the examination, except for participants aged 50 years and above during the survey. For those aged 50 years or older, their reported height at age 25 was used to determine BMI at that age to account for the possibility of height decline with age, while their measured height during the examination was used to calculate BMI ten years prior to the exam (18). We categorized weight change patterns based on BMI status at the previous two time points, specifically BMI at age 25 and 10 years prior to the survey. These patterns included stable non-obese (BMI < 30 at both times), non-obese to obese (BMI < 30 at age 25 and ≥ 30 ten years before the survey), obesity to non-obesity (BMI ≥ 30 at age 25 and < 30 ten years before the survey), and stable obesity (BMI ≥ 30 at both times). We determined absolute weight change by subtracting weight at age 25 from ten years before the survey. We determined absolute weight change by subtracting weight at age 25 from ten years before the survey. Subsequently, we classified the absolute weight change into five groups: weight loss group (weight loss ≥2.5 kg), stable weight group (weight change within 2.5 kg, reference group), mild weight gain (2.5 kg ≤ weight gain <10.0 kg), moderate weight gain (10 kg ≤ weight gain <20.0 kg), and severe weight gain (weight gain ≥20.0 kg) (19, 20). Covariate data were collected through demographic and questionnaire surveys, which included information on gender, baseline age, ethnicity (Mexican American, non-Hispanic Black, non-Hispanic White, and other race), baseline smoking status (never, former, now), marital status (married, unmarried), history of malignancy (yes or no), baseline education level (less than high school, high school or equivalent, college or above) and baseline family poverty income ratio. Poverty income ratio (0–1.0, 1.1–3.0, > 3.0) was used to reflect family income. Smoking status is divided into three categories: current (individuals who have smoked more than 100 cigarettes in their lifetime and have smoked within the past 2 years at the time of the interview), former (individuals who have smoked more than 100 cigarettes in their lifetime but quit more than 2 years before the interview), and never (individuals who have not smoked 100 cigarettes in their lifetime).
Information on arthritis diagnosis is collected from personal interviews where participants self-report their health history. Participants were questioned whether a doctor or other healthcare professional had ever diagnosed them with arthritis. If they responded affirmatively, they were prompted to specify the type of arthritis they had been diagnosed with, such as osteoarthritis (OA), rheumatoid arthritis (RA), psoriatic arthritis, or other forms. We utilized the age participants reported being diagnosed to determine the onset of arthritis. The methodology for this retrospective cohort study, using NHANES data, has been comprehensively described in detail in prior publications (16, 18, 20, 21). The design of the current study is illustrated in Supplementary Figure S1.
Following the guidelines set by the Centers for Disease Control and Prevention (CDC), we employed complex sample analyses to explore the relationship between weight change patterns and the likelihood of US adults developing arthritis. The NHANES uses design weighting to produce accurate national estimates. Survey-weighted means (95% CI) were used to represent continuous variables between groups, while survey-weighted percentages (95% CI) were used for categorical variables. We used Cox proportional hazards models to determine the hazard ratios (HRs) and their 95% CIs for incident arthritis in relation to weight change patterns from age 25 years to 10 years before survey. For the main analyses, we examined the associations between the four weight change trajectories and developing OA or RA.
The stable non-obesity pattern was used as the reference to which all other weight change patterns were compared. The basic model did not adjust for any variables. In model 1, adjustments were made for baseline age, gender, education status, and race/ethnicity. We further adjusted for marital status, family income-poverty ratio level, smoking status, and history of malignancy in model 2. Using the survival curves corresponding to weight change patterns, we assessed the proportional hazards assumption and found no notable divergence in the proportionality of hazards over time. Subsequent subgroup analyses were then performed based on baseline age (< 50 and≥50 years), gender (male and female), race (Mexican American, non-Hispanic Black, and non-Hispanic White), baseline education level (less than high school, high school or equivalent, college or above), and history of malignancy (yes or no).
We also investigated the associations between absolute weight change groups and arthritis risk. The stable weight group was used as the reference to which all other weight change patterns were compared. Additionally, we examined the dose-response correlation through a restricted cubic spline using four knots at the 5th, 35th, 65th, and 95th. In the restricted cubic spline, the absolute weight changes were also treated as continuous variables. The covariates adjusted within the restricted cubic spline were the same as the covariates adjusted in model 2 of the Cox proportional hazards regression model.
Two sensitivity analyses were performed to test the robustness of the results. These analyses included two key adjustments. First, we excluded participants with a history of malignancy. Recognizing that malignancies can significantly influence an individual’s BMI, either through weight loss (cachexia) or weight gain, depending on the type, location, and stage of the cancer (22, 23), we excluded participants with a history of malignancy. This step aimed to minimize potential confounding effects that these conditions might have on the relationship between BMI changes and arthritis risk. Second, we excluded participants diagnosed with arthritis within the first two years of follow-up to address potential reverse causation bias. This approach helped ensure that the observed associations were not due to pre-existing, undiagnosed arthritis influencing weight changes rather than weight changes influencing the risk of developing arthritis. These sensitivity analyses were critical to validate the stability of our findings and to ensure that the observed associations were not artifacts of specific subgroups or biased by reverse causation. All data processing and analysis were conducted using the R statistical software version 4.1.2 (R Core Team, Vienna, Austria). A p-value of less than 0.05 (two-tailed) was deemed to indicate statistical significance.
A total of 20,859 participants were included in the study. The prevalence of arthritis was 23.59% (4922/20859) in the overall population. Table 1 reported weighted characteristics of study participants across weight change patterns from early to middle adulthood. The mean age of the sample was 53.74 years at baseline, and 48.49% were female. The range of age at 10 years before baseline was 30–65 years. The mean BMI was 23.92 kg/m2 at age 25, 27.49 kg/m2 10 years before survey, and 29.35 kg/m2 at baseline. On average, participants gained 4.93 kg weight from 10 years before survey to baseline. We observed that the distribution of baseline age, gender, race, family poverty income ratio, education level, marital status, BMI at baseline, and BMI 10 years ago were statistically significant (all P < 0.05) across weight change patterns. Nevertheless, no significant difference (P > 0.05) was observed for smoke status and history of malignancy.
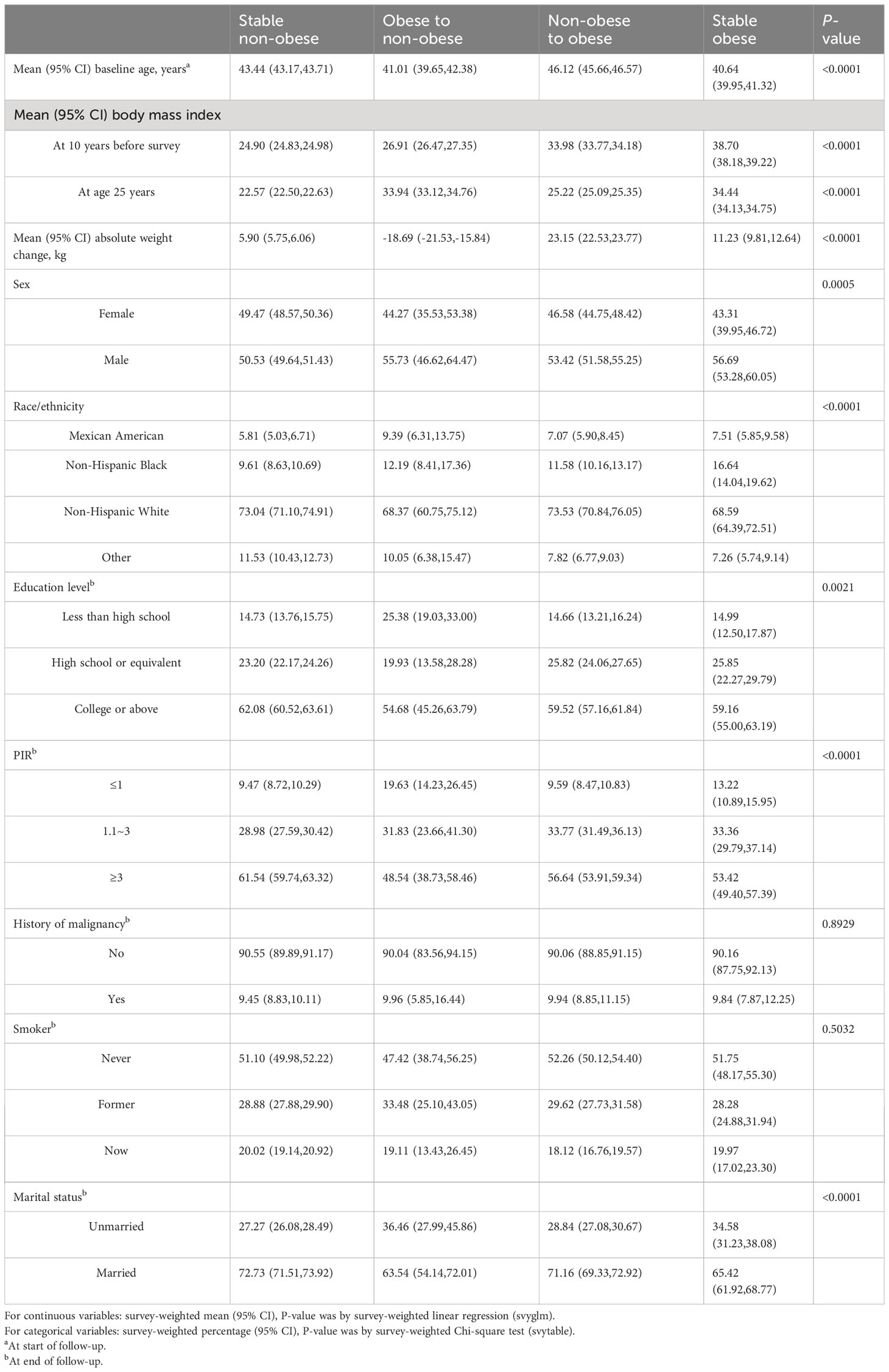
Table 1 Characteristics of NHANES 1999–2018 participants based on their weight change trajectories from age 25 to 10 years before survey.
Among 20,859 participants, 4922 had a diagnosis of arthritis, yielding an overall prevalence rate of 23.59%. Figure 2 presented cumulative incidence curves by time in study for each weight change group. The cumulative incidence of arthritis, OA, and RA varied significantly among the four weight change patterns, as shown in Figures 2A–C (all P < 0.01).

Figure 2 Cumulative incidence curve of arthritis, rheumatoid arthritis, and osteoarthritis for weight change patterns. Arthritis (A), rheumatoid arthritis (B), and osteoarthritis (C).
Compared with stable non-obesity individuals, non-obese to obese participants had an increased risk of developing arthritis over a 10-year period, with a HR of 1.55 (95% confidence interval [CI]=1.45 to 1.66, P < 0.001). Participants who remained obese from young adulthood to midlife had 1.74 times (95% CI: 1.56–1.95) higher risk of developing arthritis during the 10-year follow-up. However, there was no significant difference in arthritis risk (HR 1.02; 95% CI 0.75–1.39) between those who transitioned from an obese BMI to a non-obese BMI during the study period. When considering absolute weight changes, the HRs (95% CI) for arthritis in the moderate weight gain group (10 kg ≤ weight gain < 20.0 kg) were 1.33 (1.22 to 1.46) compared to the stable weight group (weight change within 2.5 kg) from 10 years before the survey to baseline. The HRs (95% CI) for arthritis in the severe weight gain group (weight gain ≥ 20.0 kg) were 1.56 (1.42 to 1.71).
Compared with stable non-obesity individuals, non-obese to obese participants had an increased risk of developing RA over a 10-year period, with a HR of 1.46 (95% CI =1.25 to 1.70, P < 0.001). Participants who remained obese from young adulthood to midlife had 1.58 times (95% CI: 1.24–2.00) higher risk of developing RA during the 10-year follow-up. However, the obesity to non-obesity group was not significantly associated with incident RA risk (HR 1.17; 95% CI 0.71–1.93, P = 0.5360). When considering absolute weight changes, the HRs (95% CI) for RA in the moderate weight gain group (10 kg ≤ weight gain < 20.0 kg) were 1.32 (1.09 to 1.60) compared to the stable weight group (weight change within 2.5 kg) from 10 years before the survey to baseline. The HRs (95% CI) for RA in the severe weight gain group (weight gain ≥ 20.0 kg) were 1.32 (1.08 to 1.62).
Compared with stable non-obesity individuals, non-obese to obese participants had an increased risk of developing OA over a 10-year period, with a HR of 1.66 (95% CI =1.49 to 1.85, P < 0.001). Participants who remained obese from young adulthood to midlife had 1.80 times (95% CI: 1.52–2.13) higher risk of developing OA during the 10-year follow-up. However, the obesity to non-obesity group was not significantly associated with incident OA risk (HR 1.20; 95% CI 0.80–1.79, P = 0.3882). When considering absolute weight changes, the HRs (95% CI) for OA in the moderate weight gain group (10 kg ≤ weight gain < 20.0 kg) were 1.32 (1.15 to 1.53) compared to the stable weight group (weight change within 2.5 kg) from 10 years before the survey to baseline. The HRs (95% CI) for OA in the severe weight gain group (weight gain ≥ 20.0 kg) were 1.50 (1.30 to 1.74).
When evaluating the absolute weight change, there was a U-shaped association between absolute weight change and the risk of incident arthritis, RA and OA (Figure 3). After multivariable adjustment, restricted cubic spline showed a U-shaped association between absolute weight change with arthritis (Figure 3A), OA (Figure 3B), and RA (Figure 3C), respectively.
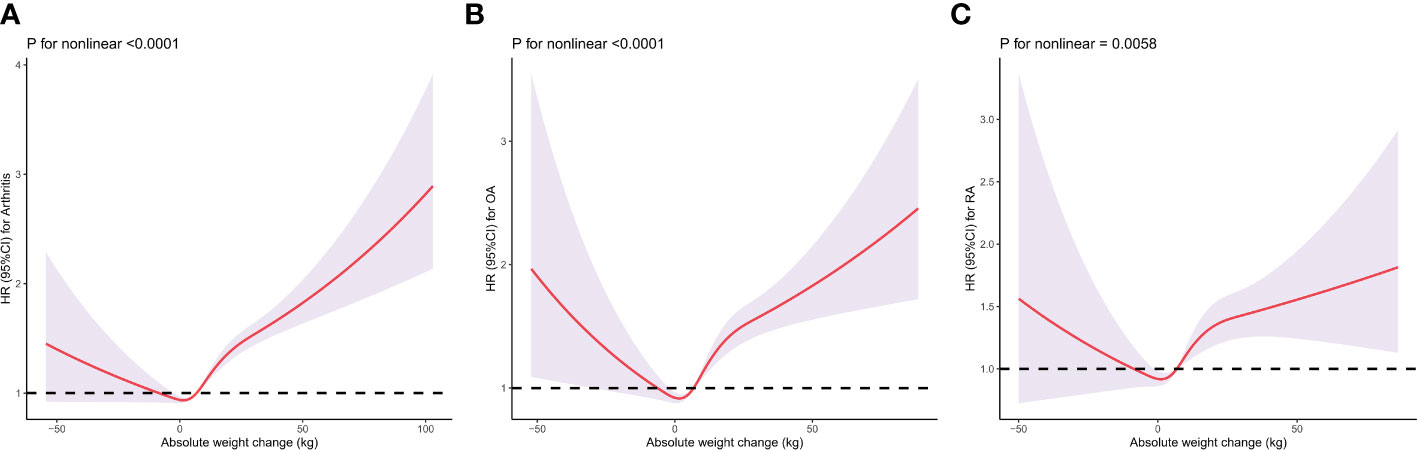
Figure 3 Dose–response association between absolute weight change across adulthood and risk of arthritis (A), rheumatoid arthritis (B), and osteoarthritis (C). Multivariable Cox regression model adjusted for gender, baseline age, race/ethnicity, marital status, education level, family income-poverty ratio level, smoking status, and history of malignancy. Each panel represents a different aspect of arthritis risk: (A) for overall arthritis, "(B) for OA, and (C) for RA. The y-axis indicates the Hazard Ratio (HR) for arthritis, while the x-axis shows the absolute weight change in kilograms. The spline curves demonstrate that the relationship between weight change and arthritis risk is not linear. For example, in (A), there is a notable increase in arthritis risk with both weight gain and weight loss beyond certain thresholds. Similarly, (B, C) show distinct patterns for OA and RA, respectively. The ‘P for nonlinear’ values beneath each graph indicates the statistical significance of the nonlinear relationship, with lower values suggesting a stronger departure from linearity.
When stratified by baseline age (< 50 and≥50 years), gender (male and female), race (Mexican American, non-Hispanic Black, and non-Hispanic White), baseline education level (less than high school, high school or equivalent, college or above), and history of malignancy (yes or no), the relationships were consistent with our primary findings regarding arthritis, OA, and RA (Figures 4A–C).
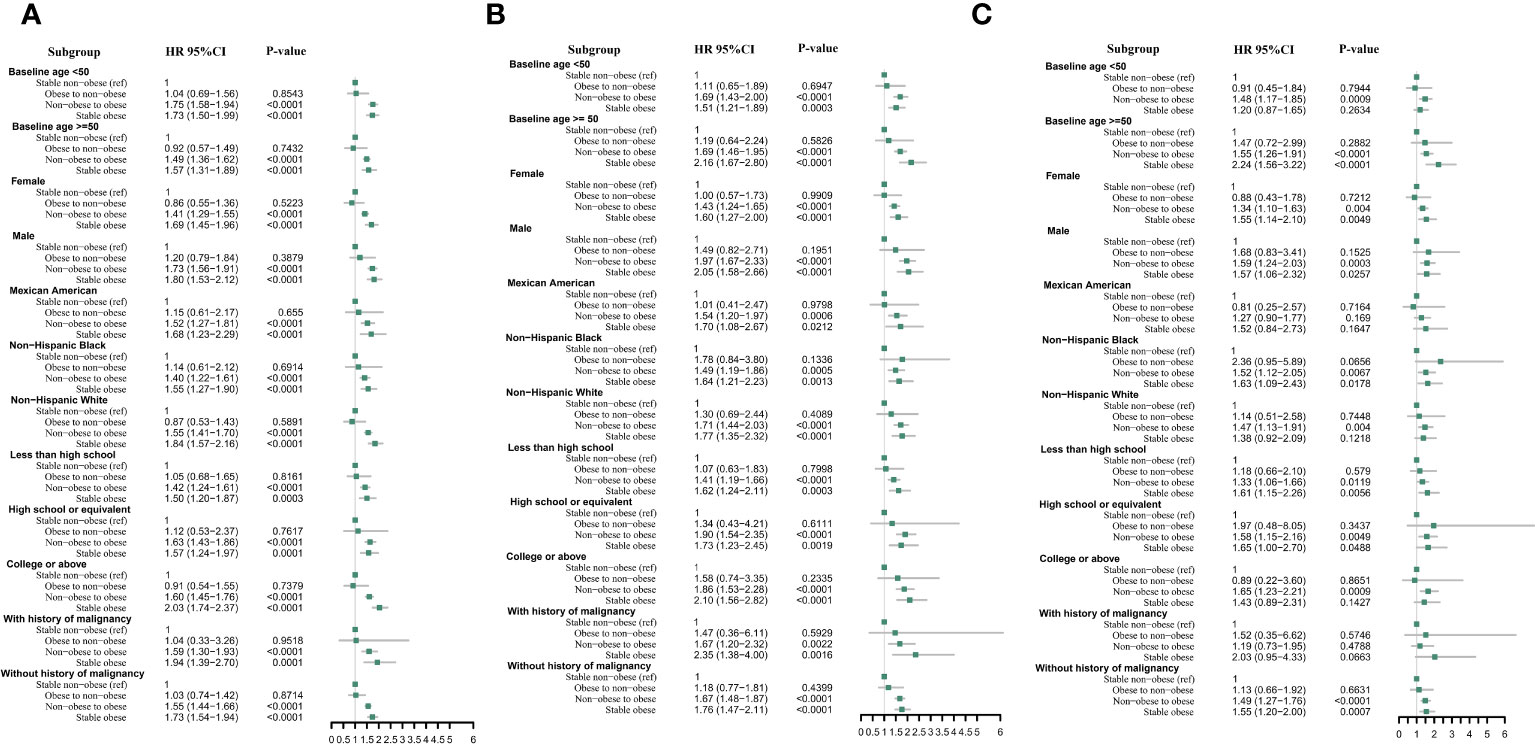
Figure 4 Multivariable cox proportional hazard ratios for arthritis (A), rheumatoid arthritis (B), and osteoarthritis (C) with weight change patterns across adulthood in subgroups stratified by patients’ characteristics.
We conducted additional sensitivity analyses to assess the robustness of our findings. After excluding participants with a history of malignancy, we found that individuals in the non-obese to obese, stable obese, moderate weight gain, and severe weight gain groups had a significantly higher risk of arthritis, OA, and RA even after adjusting for all potential confounders (Supplementary Table S1, all P < 0.01). Furthermore, when we excluded participants who were diagnosed with arthritis within the first 2 years of follow-up, we still observed a significantly increased risk of arthritis, OA, and RA in the non-obese to obese, stable obese, moderate weight gain, and severe weight gain groups after adjusting for all cofounders (Supplementary Table S2, all P < 0.01). These results were consistent with the results in Table 2.
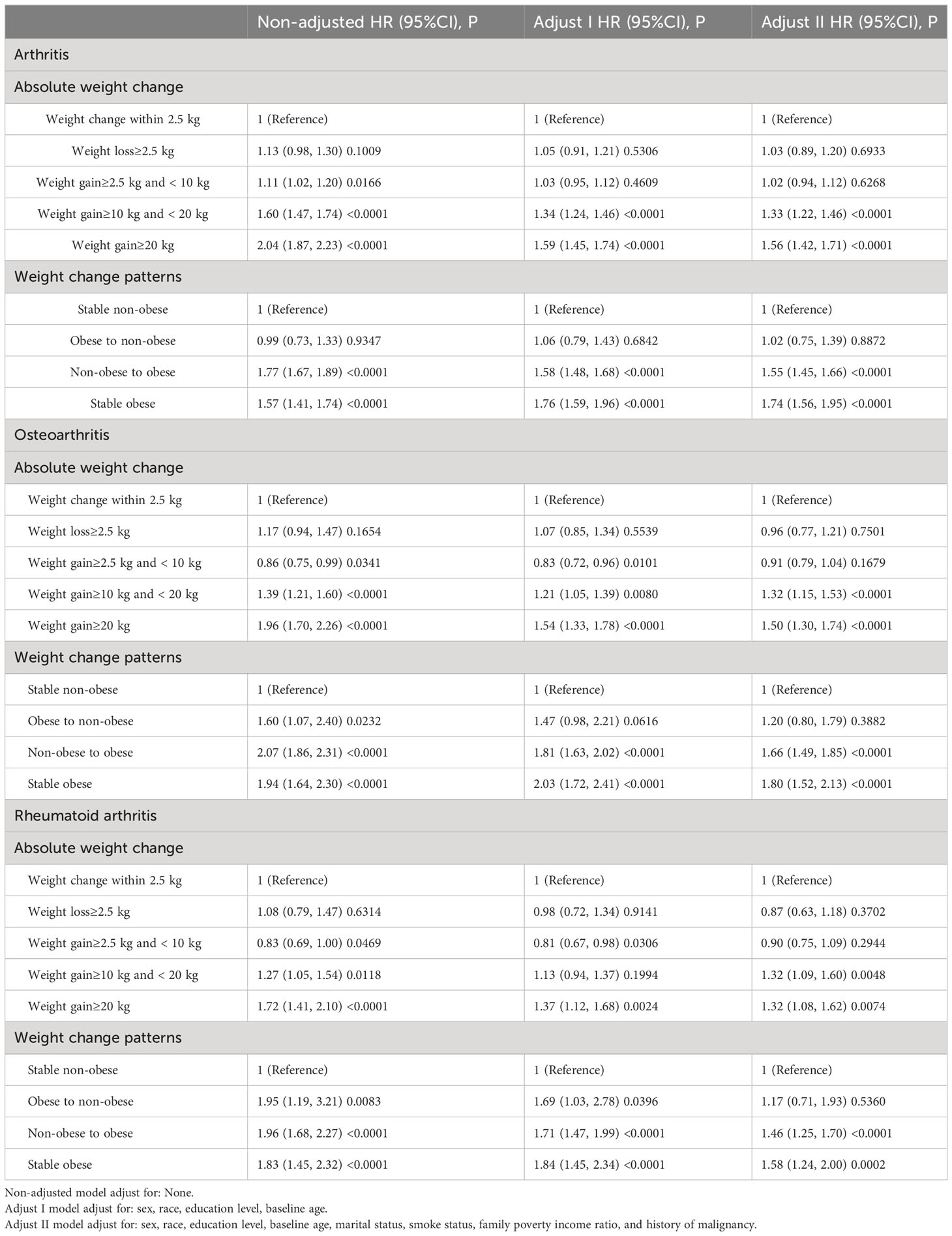
Table 2 Hazard ratio (HR) and 95% confidence intervals (CIs) of arthritis with weight change patterns across adulthood in the NHANES 1999–2018.
Based on the nationally representative US adults’ cohort, we revealed the association between long-term weight change patterns across adulthood and arthritis, OA, and RA. Our study, which utilizes the NHANES database, provides insight into the intricate pathogenesis of arthritis by exploring the relationship between weight fluctuations during various life stages and the subsequent risk of joint diseases. Our findings highlight a significant correlation between changes in weight from early adulthood to middle age and the likelihood of developing both OA and RA.
This interplay between weight dynamics and arthritis risk can be understood better through several underlying mechanisms. Excess weight places excessive stress on weight-bearing joints, making individuals more susceptible to OA (24). This increased mechanical stress, combined with changes in gait and biomechanics, can accelerate joint degeneration (25). In addition to these mechanical factors, systemic inflammation plays a crucial role in the development of both RA and OA. Adipose tissue actively produces inflammatory mediators such as TNF-alpha and IL-6 (26). Additionally, the role of the infrapatellar fat pad (IPFP) in OA deserves attention. Recent studies have identified the IPFP as an active mediator in OA pathogenesis, with its inflammation and hypertrophy linked to increased OA severity (27). This highlights the complex interplay between adipose tissue within the joint and OA development. Leptin, an adipokine, has implications beyond regulating appetite, contributing to cartilage degradation and inflammation (28). Furthermore, changes in diet and weight can affect the gut microbiome, which may impact systemic inflammation and conditions like RA (29). Disparities in gut microbial equilibrium can potentiate systemic inflammation, affecting distal organs, including the joints (30).
Perspectives on BMI and arthritis vary. Some cohort studies deduced childhood overweight metrics to associate with adult knee pain, specifically among men, irrespective of adult weight (31). On the other hand, some studies have shown that there is no clear association between adolescent BMI and the risk of knee osteoarthritis when adult BMI is taken into account (32). Our analysis also supports these findings by demonstrating that individuals who undergo weight loss over the course of their lifetime have similar risks of developing arthritis as those who maintain a consistently lower weight. Our findings concur with previous epidemiological studies that have linked obesity with the onset of arthritis (33). Obesity has long been recognized as a major risk factor for OA, particularly of the knee (34). The mechanical strain exerted by excess weight on the weight-bearing joints is thought to contribute to cartilage breakdown, the hallmark of OA (35). Advancements in biomechanics highlight the crucial role of mechanical strain in OA pathogenesis, particularly in cartilage breakdown. Chondrocytes, central to articular cartilage, respond significantly to mechanical loading within the extracellular matrix. This response influences cartilage integrity and OA progression. Studies emphasize that understanding chondrocyte biomechanics is key to comprehending OA development, linking mechanical properties and cellular responses to OA’s onset and severity (36, 37). However, the relationship between obesity and RA has been less consistently established. A meta-analysis found a modestly increased risk of RA in obese individuals, suggesting that adipose tissue might play a role in RA’s pathogenesis by producing pro-inflammatory cytokines (38). Our study provides further insight into this topic by examining the risk of arthritis based on patterns of weight change. We found that individuals who transitioned from a non-obese state to an obese state had a higher hazard ratio for developing arthritis than those who maintained a stable non-obese weight. This progression towards obesity over time may expose individuals to prolonged periods of systemic inflammation, thereby increasing their risk of developing both RA and OA (39). It’s interesting that participants in the “obese to non-obese” category were not significantly associated with an increased risk in our study. This underscores the potential reversibility of some obesity-related risks with weight reduction. To clarify, this observation indicates that individuals who were initially obese but later transitioned to a non-obese status did not demonstrate the same elevated risk for arthritis as those who remained obese or those who transitioned from non-obese to obese. This finding suggests a notable aspect of our study: the potential for mitigating obesity-related arthritis risks through weight loss. It implies that individuals who successfully reduce their weight from an obese to a non-obese BMI might lower or even negate the heightened arthritis risk typically associated with obesity. This could be due to improvements in systemic inflammation, mechanical stress on joints, or other factors related to weight change. Our analysis thus highlights the importance of weight management and its possible protective effects against arthritis risk. Although weight loss interventions have been shown to reduce the symptoms and progression of established OA (40), our findings suggest that weight loss could also have preventive implications for those at risk. Furthermore, the observed nonlinear relationship between absolute weight change and arthritis risk is of interest. This could indicate that small amounts of weight gain might not significantly influence arthritis risk, but more pronounced weight changes have substantial impacts.
The strengths of our study include the utilization of a nationally representative database, which enhances the generalizability of our findings. Furthermore, by categorizing participants based on weight change patterns, we could investigate the risk associated with dynamic weight changes, which is more representative of real-life scenarios than static BMI measurements at a single point in time. Our findings indicate significant associations between weight change patterns and arthritis risk. However, it is essential to acknowledge that these correlations do not inherently imply causation. The observational nature of our study limits our ability to establish direct causal relationships. We recognize potential confounding factors, such as lifestyle behaviors, genetic predispositions, and other comorbidities, which were not fully controlled in our analysis. Additionally, the reliance on self-reported data in NHANES might introduce recall bias, particularly regarding arthritis diagnosis and historical weight information. These considerations highlight the need for cautious interpretation of our results and underscore the importance of further research to elucidate the complex interplay between weight dynamics and arthritis risk.
Our study highlights the importance of weight management throughout adulthood as a potentially effective strategy for preventing the onset of arthritis, including its major subtypes, osteoarthritis and rheumatoid arthritis. As obesity and arthritis continue to increase in prevalence globally, our findings highlight the need for public health initiatives that prioritize weight stability and reduction from early adulthood onwards. Future prospective studies are needed to further investigate the mechanisms underlying the relationship between weight change patterns and arthritis risk, and to explore potential interventions that could mitigate this risk.
Publicly available datasets were analyzed in this study. This data can be found here: Publicly available dataset was analyzed in this study. The National Health and Nutrition Examination Survey dataset are publicly available at https://www.cdc.gov/nchs/nhanes/index.htm.
KN: Data curation, Formal Analysis, Validation, Writing – original draft, Writing – review & editing. MZ: Conceptualization, Data curation, Formal Analysis, Software, Validation, Writing – original draft, Writing – review & editing. SH: Data curation, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft. XS: Conceptualization, Formal analysis, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. LL: Data curation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing. YZ: Data curation, Funding acquisition, Resources, Software, Supervision, Visualization, Writing – original draft. PX: Data curation, Funding acquisition, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank the National Center for Health Statistics of the Centers for Disease Control and Prevention for sharing the National Health and Nutrition Examination Survey (NHANES) data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1308254/full#supplementary-material
Supplementary Figure 1 | Survival analysis study design: weight change and arthritis onset.
1. Sparks JA. Rheumatoid arthritis. Ann Intern Med (2019) 170(1):ITC1–ITC16. doi: 10.7326/AITC201901010
2. Cai Y, Zhang J, Liang J, Xiao M, Zhang G, Jing Z, et al. The burden of rheumatoid arthritis: findings from the 2019 global burden of diseases study and forecasts for 2030 by bayesian age-period-cohort analysis. J Clin Med (2023) 12(4):1291. doi: 10.3390/jcm12041291
3. Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015-2040. Arthritis Rheumatol (2016) 68(7):1582–7. doi: 10.1002/art.39692
4. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet (2016) 388(10055):2023–38. doi: 10.1016/S0140-6736(16)30173-8
5. Wolf J, França EB, Assunção A. The burden of low back pain, rheumatoid arthritis, osteoarthritis, and gout and their respective attributable risk factors in Brazil: results of the GBD 2017 study. Rev Soc Bras Med Trop (2022) 55(suppl 1):e0285. doi: 10.1590/0037-8682-0285-2021
6. Takeda R, Matsumoto T, Maenohara Y, Omata Y, Inui H, Nagase Y, et al. Increasing trend of radiographic features of knee osteoarthritis in rheumatoid arthritis patients before total knee arthroplasty. Sci Rep (2022) 12(1):10452. doi: 10.1038/s41598-022-14440-2
7. Eakin GS, Amodeo KL, Kahlon RS. Arthritis and its public health burden. Dela J Public Health (2017) 3(1):36–44. doi: 10.32481/djph.2017.03.006
8. Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) (2012) 64(4):465–74. doi: 10.1002/acr.21596
9. Loeser RF, Goldring SR, Scanzello CR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum (2012) 64(6):1697–707. doi: 10.1002/art.34453
10. Zeng N, Yan ZP, Chen XY, Ni GX. Infrapatellar fat pad and knee osteoarthritis. Aging Dis (2020) 11(5):1317–28. doi: 10.14336/AD.2019.1116
11. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet (2019) 393(10182):1745–59. doi: 10.1016/S0140-6736(19)30417-9
12. McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet (2017) 389(10086):2328–37. doi: 10.1016/S0140-6736(17)31472-1
13. Chen X, Zhang M, Wang T, Li Y, Wei M. Influence factors of extra-articular manifestations in rheumatoid arthritis. Open Med (Wars) (2020) 15(1):787–95. doi: 10.1515/med-2020-0217
14. Dobrovolskaya O, Feklistov A, Demin N, Sorokina A, Nikitinskaya O, Toroptsova N. AB0312 BODY COMPOSITION AND BONE MINERAL DENSITY IN PATIENTS WITH RHEUMATOID ARTHRITIS AND OSTEOARTHRITIS. Ann Rheumatic Dis (2022) 81:1281–1. doi: 10.1136/annrheumdis-2022-eular.4096
15. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage (2013) 21(9):1145–53. doi: 10.1016/j.joca.2013.03.018
16. Berry KM, Neogi T, Baker JF, Collins JM, Waggoner JR, Hsiao CW, et al. Obesity progression between young adulthood and midlife and incident arthritis: A retrospective cohort study of US adults. Arthritis Care Res (Hoboken) (2021) 73(3):318–27. doi: 10.1002/acr.24252
17. Christensen R, Henriksen M Fau - Leeds AR, Leeds AR, Gudbergsen H, Gudbergsen H, Christensen P, et al. Effect of weight maintenance on symptoms of knee osteoarthritis in obese patients: a twelve-month randomized controlled trial. Arthritis Care Res (Hoboken) (2015) 67(5):640–50. doi: 10.1002/acr.22504
18. Stokes A, Collins JM, Grant BF, Scamuffa RF, Hsiao CW, Johnston SS, et al. Obesity progression between young adulthood and midlife and incident diabetes: A retrospective cohort study of U.S. Adults. Diabetes Care (2018) 41(5):1025–31. doi: 10.2337/dc17-2336
19. Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. Jama (2017) 318(3):255–69. doi: 10.1001/jama.2017.7092
20. Chen C, Ye Y, Zhang Y, Pan XF, Pan A. Weight change across adulthood in relation to all cause and cause specific mortality: prospective cohort study. Bmj (2019) 367:l5584. doi: 10.1136/bmj.l5584
21. Zhou Y, Wang T, Yin X, Sun Y, Seow WJ. Association of weight loss from early to middle adulthood and incident hypertension risk later in life. Nutrients (2020) 12(9). doi: 10.3390/nu12092622
22. Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr (2006) 83(6):1345–50. doi: 10.1093/ajcn/83.6.1345
23. Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut (2012) 61(1):6–32. doi: 10.1136/gutjnl-2011-300831
24. Vincent HK, Heywood K Fau - Connelly J, Connelly J Fau - Hurley RW, Hurley RW. Obesity and weight loss in the treatment and prevention of osteoarthritis. PM R (2012) 4(5 Suppl):S59–67. doi: 10.1016/j.pmrj.2012.01.005
25. Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol (2011) 25(6):815–23. doi: 10.1016/j.berh.2011.11.013
26. Scotece M, Conde J, Vuolteenaho K, Koskinen A, López V, Gómez-Reino J, et al. Adipokines as drug targets in joint and bone disease. Drug Discovery Today (2014) 19(3):241–58. doi: 10.1016/j.drudis.2013.07.012
27. Jiang LF, Fang JH, Wu LD. Role of infrapatellar fat pad in pathological process of knee osteoarthritis: Future applications in treatment. World J Clin cases (2019) 7(16):2134–42. doi: 10.12998/wjcc.v7.i16.2134
28. Monteiro L, Pereira J, Palhinha L, Moraes-Vieira PMM. Leptin in the regulation of the immunometabolism of adipose tissue-macrophages. J Leukoc Biol (2019) 106(3):703–16. doi: 10.1002/JLB.MR1218-478R
29. Zhao T, Wei Y, Zhu Y, Xie Z, Hai Q, Li Z, et al. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front Immunol (2022) 13:1007165. doi: 10.3389/fimmu.2022.1007165
30. Ramires LA-O, Santos GA-O, Ramires RA-O, da Fonseca LA-OX, Jeyaraman MA-O, Muthu SA-O, et al. The association between gut microbiota and osteoarthritis: does the disease begin in the gut? Int J Mol Sci (2022) 23(3):1494. doi: 10.3390/ijms23031494
31. Antony B, Jones G, Venn A, Cicuttini F, March L, Blizzard L, et al. Association between childhood overweight measures and adulthood knee pain, stiffness and dysfunction: a 25-year cohort study. Ann Rheum Dis (2015) 74(4):711–7. doi: 10.1136/annrheumdis-2013-204161
32. Manek NJ, Hart D, Spector TD, MacGregor AJ. The association of body mass index and osteoarthritis of the knee joint: an examination of genetic and environmental influences. Arthritis Rheum (2003) 48(4):1024–9. doi: 10.1002/art.10884
33. Messier SP. Obesity and osteoarthritis: disease genesis and nonpharmacologic weight management. Rheum Dis Clin North Am (2008) 34(3):713–29. doi: 10.1016/j.rdc.2008.04.007
34. King LK, March L, Anandacoomarasamy A, Anandacoomarasamy A. Obesity & osteoarthritis. Indian J Med Res (2013) 138(2):185–93.
35. Carman WJ, Sowers M Fau - Hawthorne VM, Hawthorne VM, Weissfeld LA, Weissfeld LA. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol (1994) 139(2):119–29. doi: 10.1093/oxfordjournals.aje.a116974
36. Pettenuzzo S, Arduino A, Belluzzi EA-O, Pozzuoli AA-O, Fontanella CA-O, Ruggieri PA-O, et al. Biomechanics of chondrocytes and chondrons in healthy conditions and osteoarthritis: A review of the mechanical characterisations at the microscale. Biomedicines (2023) 11(7):1942. doi: 10.3390/biomedicines11071942
37. Belluzzi E, Todros S, Pozzuoli A, Ruggieri P, Carniel EL, Berardo A. Human cartilage biomechanics: experimental and theoretical approaches towards the identification of mechanical properties in healthy and osteoarthritic conditions. Processes (2023) 11(4):1014. doi: 10.3390/pr11041014
38. Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther (2015) 17(1):86. doi: 10.1186/s13075-015-0601-x
39. Zen M, Salmaso L, Amidei CB, Giollo A, Fedeli U, Bellio S, et al. AB1628 THE INCIDENCE AND PREVALENCE OF RHEUMATOID ARTHRITIS IN ITALY IN THE LAST DECADE. Ann Rheumatic Dis (2023) 82(Suppl 1):2048–8. doi: 10.1136/annrheumdis-2023-eular.1432
Keywords: weight change, arthritis, rheumatoid arthritis, osteoarthritis, cohort study
Citation: Nan K, Zhang M, Hu S, Shao X, Liu L, Zhi Y and Xu P (2024) Relationship of weight change patterns from young to middle adulthood with incident rheumatoid arthritis and osteoarthritis: a retrospective cohort study. Front. Endocrinol. 14:1308254. doi: 10.3389/fendo.2023.1308254
Received: 06 October 2023; Accepted: 08 December 2023;
Published: 03 January 2024.
Edited by:
Katsunori Nonogaki, Tohoku University, JapanReviewed by:
Assunta Pozzuoli, University of Padua, ItalyCopyright © 2024 Nan, Zhang, Hu, Shao, Liu, Zhi and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Xu, c291c291MzY5QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.