
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 03 January 2024
Sec. Molecular and Structural Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1307337
Purpose: Polypoidal choroidal vasculopathy (PCV) is an irreversible retinal choroidal disease. Individuals with PCV exhibit diverse baseline characteristics, including systemic characteristics, ocular traits, metabolic factor levels, and different responses to intravitreal anti-VEGF therapy. This study aims to investigate the pathogenesis of PCV by analyzing the systemic characteristics, ocular traits, and cytokine levels at baseline within a cohort of patients who exhibit different responses to anti-VEGF treatment.
Methods: We conducted a retrospective analysis involving 80 eyes diagnosed with PCV. Patients were categorized into two groups based on responses to suboptimal intravitreal ranibizumab injection therapy: those with suboptimal responses and optimal responses. Aqueous humor samples were collected from the experimental eyes, and cytokine expression levels were assessed using cytometric bead array analysis. All subjects were further stratified into two groups according to the median choroidal thickness. Subsequently, logistic regression analysis and the ROC curve were employed to examine the relationship between cytokine expression levels, choroidal thickness, and anti-VEGF response.
Results: The results revealed that compared to the group of optimal anti-VEGF response, the choroid in the suboptimal response group exhibited a significantly greater thickness. Additionally, compared to the suboptimal anti-VEGF response group, the expression levels of VEGF and VCAM-1 were markedly lower observed in the optimal anti-VEGF response group, while TNF-α showed the opposite trend. Logistic regression analysis indicated that VEGF, VCAM-1, and TNF-α in the aqueous humor were independent risk factors for a suboptimal anti-VEGF response. After adjusting other risk factors, the risk of suboptimal anti-VEGF response decreased to 0.998-fold, 0.997-fold, and 1.294-fold. The AUC values for VEGF, VCAM-1, and TNF-α were determined to be 0.805, 0.846, and 0.897, respectively. Furthermore, the risk of VEGF, VCAM-1, and TNF-α were significantly associated with an increased risk of suboptimal anti-VEGF response after correction for risk factors in the thick choroid group.
Conclusions: Our study demonstrated that PCV exhibits systemic and ocular characteristics variations based on different anti-VEGF responses. The levels of cytokines in aqueous humor were found to have a significant correlation with the anti-VEGF response in PCV. VEGF, VCAM-1, and TNF-α are potential targets for assessing treatment response in thick choroidal PCV.
Polypoid choroidal vasculopathy (PCV) is a retinal and choroidal disease characterized by a network of branching choroidal vessels and polypoid focus at the ends of the vessels. It is a major cause of vision impairment in middle-aged and older adults worldwide, with a high prevalence in Asia. The likelihood of macular involvement and a suboptimal prognosis for vision is high (1–3). Recently, PCV has imposed a substantial economic and health burden on the public health system.
Recent studies indicate that PCV, akin to neovascular age-related macular degeneration (AMD), is a systemic multifactorial disease. Both PCV and typical neovascular AMD involve choroidal neovascularization with blood leakage and sub-retinal hemorrhage in the macular region (2, 4, 5). However, evidence suggests that PCV patients with diverse baseline characteristics exhibit different responses to anti-vascular endothelial growth factor (VEGF) therapy than AMD (6–8). Eyes with PCV often have a thicker subfoveal choroidal thickness (SFCT) than age-related and typical non-neovascular and neovascular AMD (9–11). PCV is now categorized as a pachychoroid disease (12). Recent research has linked SFCT to PCV response to anti-VEGF, indicating that patients with thicker choroidal membranes and thicker SFCT demonstrate poor response to 3-month injections of anti-VEGF compared to patients with thinner SFCT (13). Therefore, staging PCV by choroidal thickness has been suggested (14, 15). A study on macular neovascularization (MNV) classified MNV based on the presence or absence of drusen and the presence or absence of thick choroidal features, revealing distinct cytokine profiles among different subgroups and emphasizing the significance of the choroid and cytokine assessment (16). These differences in systemic characteristics and treatment response variations suggest potential differences in the pathogenetic mechanisms of PCV with varying choroidal thicknesses. However, studies analyzing risk factors according to different choroidal thickness subtypes are scarce, necessitating further research. Identifying individuals with suboptimal anti-VEGF responses is critical for enhancing treatment efficacy accurately and efficiently.
This study elucidates the relationship between cytokines expression levels at different choroidal thicknesses and the response to anti-VEGF. It analyzes numerous risk factors associated with PCV, exploring differences among subgroups with distinct choroidal thicknesses, including baseline clinical data and aqueous humor cytokine expression levels. Furthermore, the study aims to identify risk factors for PCV, explore potential therapeutic targets, and contribute to a deeper understanding of PCV pathogenesis.
This retrospective study adhered to the principles outlined in the Declaration of Helsinki and received approval from the Ethics Committee at the Second Affiliated Hospital of Harbin Medical University (KY2020-261). A total of 80 PCV patients admitted to the Second Affiliated Hospital of Harbin Medical University between December 2018 and June 2022 were selected for the study. Informed consent was obtained from all subjects before their inclusion.
The inclusion criteria were as follows:
1. Confirmation of the initial diagnosis as PCV without prior treatment.
2. Receipt of intravitreal ranibizumab injection (IRI) monotherapy with a 3 + PRN regimen at our hospital.
3. Completion of one year of follow-up after commencing treatment, with comprehensive medical records.
The exclusion criteria were as follows:
1. Presence of eye diseases other than PCV.
2. History of intraocular surgery.
3. Inability to tolerate ranibizumab.
4. Failure to comply with protocol rules for treatment.
5. Severe systemic diseases, such as tumors, liver, or kidney dysfunction.
6. Incomplete clinical data in patient records.
“Optimal responders” were characterized as individuals demonstrating the absorption of subretinal fluid and/or intraretinal fluid in the absence of expanding pigment epithelial detachment (PED), or anatomical stabilization (no change in subretinal and/or intraretinal fluid) with an improvement in best-corrected visual acuity (BCVA) of ≥0.1 LogMAR within one year from baseline after initiation of the anti-VEGF treatment. Without meeting these criteria, patients were classified as “suboptimal responders.” The study flow chart is presented in Figure 1.
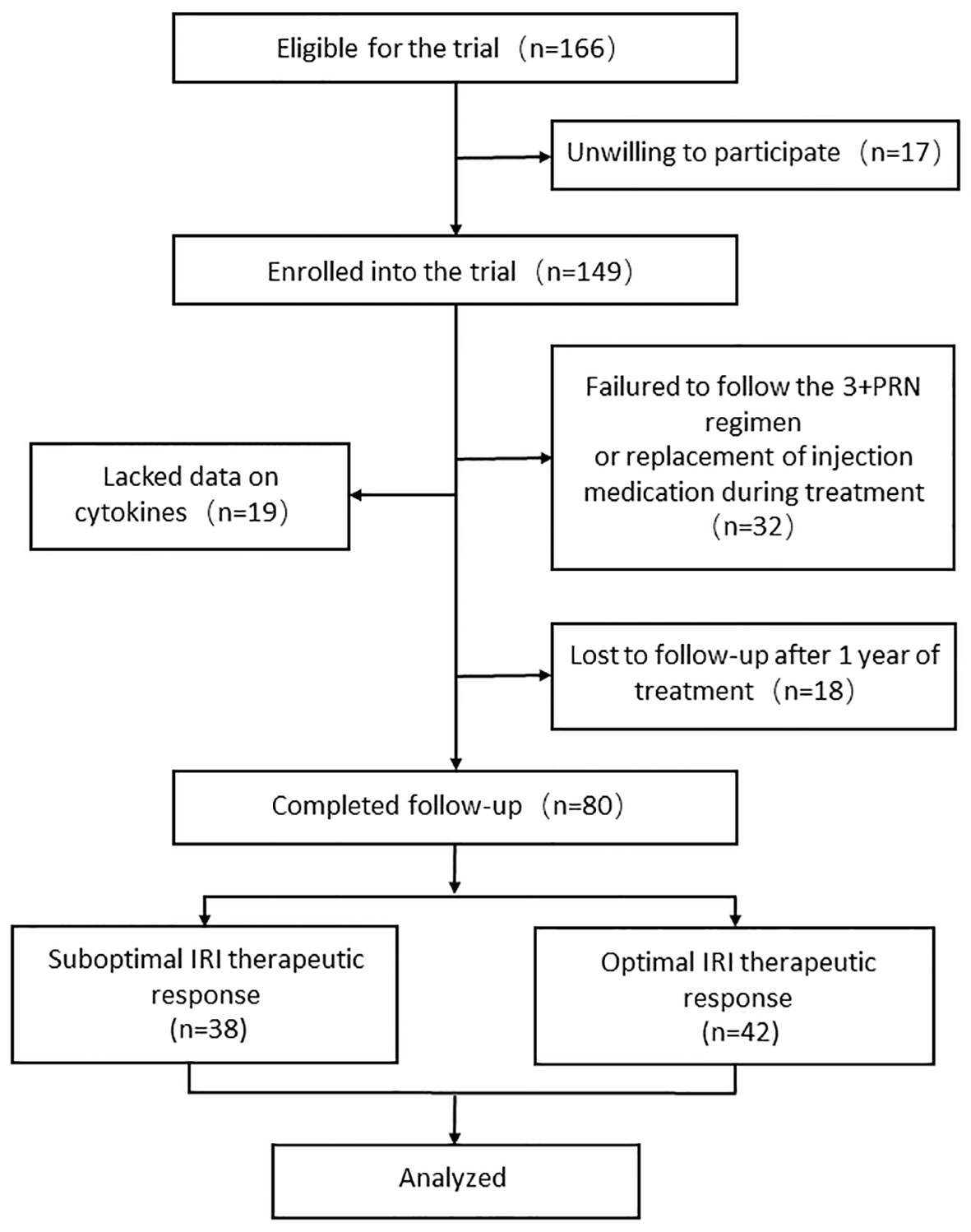
Figure 1 Flow chart of patient recruitment. PRN, pro-re-nata; IRI, intravitreal ranibizumab injection.
The data collection and the definition of sociodemographic characteristics (age, gender, height, weight, smoking status, and alcohol consumption), as well as medical history (hypertension, diabetes, systemic diseases, and other prior medical conditions), were derived from hospital medical records.
BCVA was recorded using LogMAR measurements. The ocular axis length was measured through an A-ultrasound examination (IOL Master 500, Carl Zeiss, Germany), and the average was recorded after three measurements. Before intravitreal anti-VEGF injections, all patients underwent an optical coherence tomography (OCT) examination (Heidelberg Engineering, Heidelberg, Germany). A volume scan comprising 18 horizontal B-scans covering a 6 × 6 mm area of the macula region centered on the fovea was obtained using SD-OCT. Measurements were taken for the greatest linear dimension (GLD), PED types, and central macular thickness (CMT). SFCT was measured in EDI mode and defined as the thickness from the brush membrane to the choroidal-scleral interface. The PCV patients were categorized into thick and thin choroidal groups based on the median SFCT of all patients: SFCT > 243.0 μm was assigned to the thick choroidal group, while SFCT ≤ 243.0 μm was assigned to the thin choroidal group. Before intravitreal anti-VEGF therapy, 100 μL of aqueous humor was collected from all patients and stored in a refrigerator at –80°C. Cytometric bead arrays were used to test the levels of cytokines.
Following the cytometric bead array (CBA) instructions for experimental operations, which utilized the human VEGF flex set (Bead B8) (No. 558336, BD Bioscience, San Jose, CA, USA); human IL-8 flex set (Bead A9) (No. 558277, BD Bioscience, San Jose, CA, USA); human VCAM-1 flex Set (Bead D6) (No. 560427, BD Bioscience, San Jose, CA, USA); human TNF-α flex set (No. 558273, BD Bioscience, San Jose, CA, USA), for standards and samples were experimented with. For each type of bead, 25 μL of supernatant from centrifuged samples/standard samples was added to the beads working solution (23 μL beads diluent + 0.5 for each type of bead). The mixture was left in darkness for one hour, forming the “beads antibody-antigen PE antibodies” complex. Subsequently, 25 μL of prepared phycoerythrin (PE) working solution (23 μL reagent diluent + 0.5 μL of each type of PE) was added to the tube containing the sample, thoroughly mixed, and left in darkness for two hours. The fluorescence intensity of the complex was tested using flow cytometry devices (FACS Canto TMII, BD Bioscience, San Jose, CA, USA) after washing with wash buffer. Standard curves were plotted using FCAP array v3 (BD Bioscience, San Jose, CA, USA) to calculate the concentration values of each indicator.
Continuous variables are presented as mean ± standard deviation (SD) or median and interquartile spacing (IQR). Categorical variable data are expressed in percentages. The comparison of categorical variables was performed using the χ2 test. Continuous variables were compared using the t-test, analysis of variance, Mann-Whitney U test, or Kruskal-Wallis H test, as appropriate. The relationship between aqueous humor cytokine levels and PCV anti-VEGF treatment response was analyzed using logistic regression to calculate the odd ratios (OR) and 95% confidence intervals (CI). Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were employed to determine the sensitivity and specificity of different cytokines expression levels in predicting PCV treatment response. Statistical analysis was conducted using SPSS 26.0 (IBM Corp, New York, NY, USA), and a P-value < 0.05 was considered statistically significant.
This study comprised 80 patients, with an average age of 64 ± 7 years and 53.8% male. Based on the response to anti-VEGF treatment, 47.50% exhibited suboptimal ranibizumab therapeutic response, while 52.50% showed optimal ranibizumab therapeutic response among PCV patients. The baseline characteristics of the suboptimal and optimal ranibizumab therapeutic response groups are detailed in Table 1. Compared to the optimal therapeutic response group, patients in the suboptimal therapeutic response group were significantly younger and received more injections (P < 0.05). Additionally, the group with optimal therapeutic response had a higher prevalence of hypertension, thinner SFCT, worse BCVA, longer eye axis, greater GLD, and a higher probability of PED occurrence (P < 0.05). However, there was no significant difference between the two groups regarding PED typing (P>0.05). The group with a suboptimal therapeutic response to anti-VEGF treatment exhibited lower levels of VEGF and VCAM-1 and higher levels of TNF-α in the aqueous humor (P < 0.05) (Table 1).
The median SFCT of all patients (243 μm) was taken as the boundary value, dividing the patients into the thick choroid group (48.75%) and the thin choroid group (51.25%). The thin choroidal group exhibited a higher prevalence of hypertensive patients (P < 0.05). Compared to the thick choroidal group, the thin choroidal group had a significant worse BCVA, a higher PED case, and fewer injections administered to patients (P < 0.05). However, the two groups had no significant difference in PED typing. Concerning cytokines, the thick choroidal group showed lower VEGF and VCAM-1 levels and higher TNF-α levels in aqueous humor (Table 2).
The relationship between the response to anti-VEGF therapy with ranibizumab and various risk factors was analyzed using univariate logistic regression, with the reaction to suboptimal ranibizumab therapy as the dependent variable. The analysis revealed that SFCT, the number of injections, and the expression level of TNF-α were positively associated with a suboptimal ranibizumab treatment response. In contrast, age, hypertension, axial length, PED occurrence, GLD, and the expression levels of VEGF and VCAM-1 were negatively associated with the response to suboptimal ranibizumab treatment (P < 0.05) (Table 3).
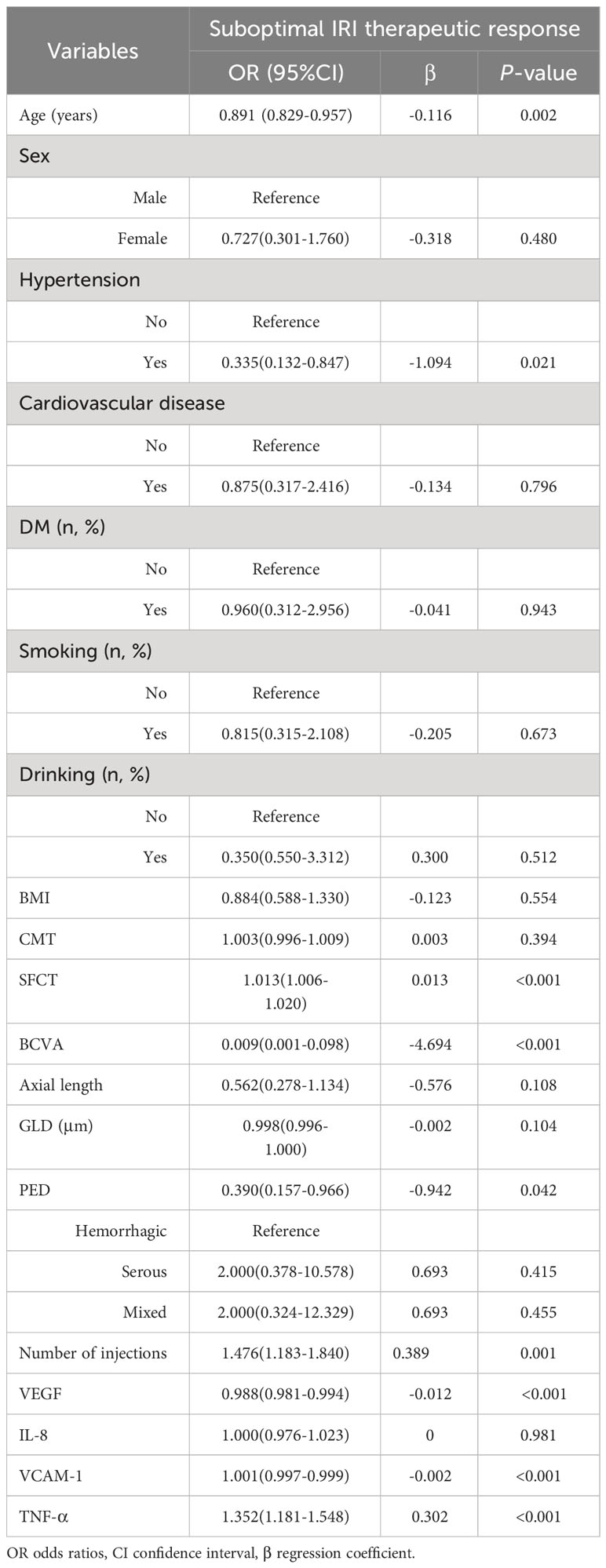
Table 3 Relationship between the therapeutic response and various risk factors after IRI treatment for PCV 1 year.
As presented in Table 4, factors with P ≤ 0.1 were incorporated into the multivariate logistic regression analysis model to analyze the correlation between aqueous humor cytokine levels and the response to ranibizumab anti-VEGF therapy. The suboptimal ranibizumab treatment served as the dependent variable, and there was no evidence of multicollinearity among the independent variables. Confounding factors were not adjusted in Model 1. Model 2 was adjusted for systematic factors such as age, sex, and hypertension. Model 3 was adjusted for sex, age, hypertension, choroidal thickness classification, ocular axis length, PED, and GLD (BCVA and injection numbers were excluded from the adjustment due to being outcomes of the disease). The analysis revealed that VEGF, VCAM-1, and TNF-α were independent risk factors for the response to suboptimal ranibizumab treatment (P < 0.05) (Table 4).
The ROC curves illustrating suboptimal ranibizumab therapeutic response and aqueous humor cytokines levels are depicted in Figure 2. The AUC of suboptimal anti-VEGF treatment response assessed by VEGF was 0.805 (95% CI 0.707-0.902), yielding an optimal cut-off value of 43.900 at a sensitivity of 71.1% and a specificity of 78.6% (P<0.05). VCAM-1 demonstrated an AUC of 0.846 (95% CI: 0.763–0.929) and an optimal cut-off value, sensitivity, and specificity of 862.00, 65.8%, and 88.1%, respectively (P < 0.05). The AUC assessed by TNF-α was 0.897 (95% CI: 0.818–0.976), with the optimal cut-off value, sensitivity, and specificity being 8.450%, 84.2%, and 90.5%, respectively (P < 0.05) (Table 5).
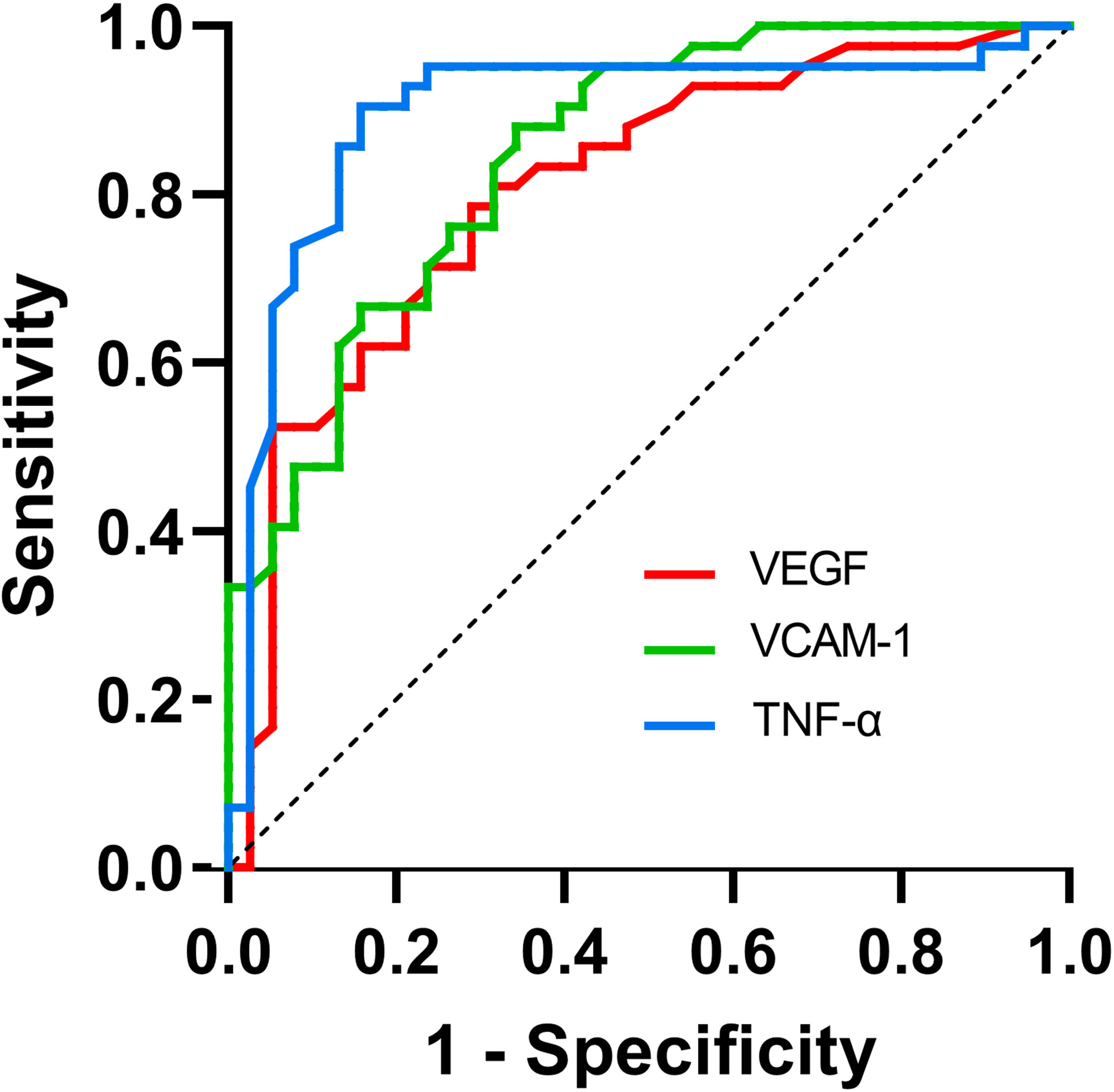
Figure 2 ROC curve for the use of cytokines in the detection of suboptimal IRI therapeutic response.
For further investigation, we included cytokines (VEGF, VCAM-1, and TNF-α) that exhibited differential expression in the two groups. Patients were categorized into thin and thick choroidal groups based on the median SFCT. Logistic regression analysis was conducted on both groups, with the response to suboptimal ranibizumab treatment as the dependent variable. In both the thick and thin choroidal groups, we observed that VEGF, VCAM-1, and TNF-α were identified as risk factors for suboptimal ranibizumab therapeutic response. After adjusting for all confounding risk factors (Model 5, Model 6), VEGF, VCAM-1, and TNF-α remained independent risk factors for obtaining a suboptimal ranibizumab treatment response in the thick choroid group. Only VCAM-1 emerged as an independent risk factor for obtaining a suboptimal ranibizumab treatment response in the thin choroid group (Table 6).
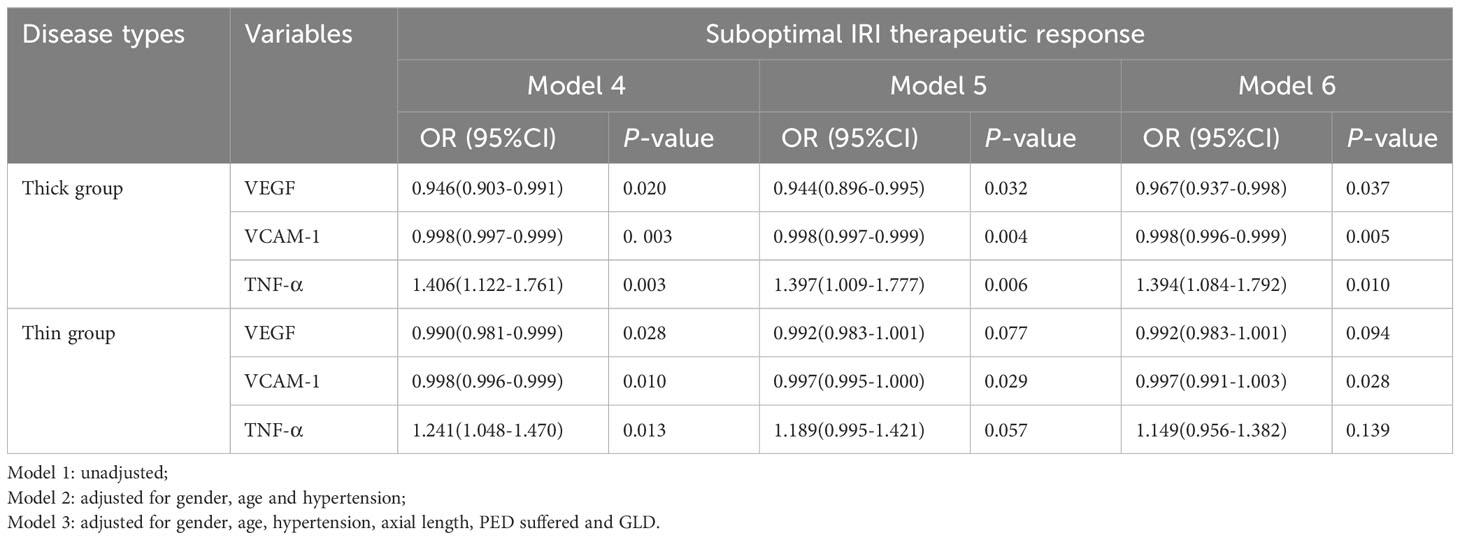
Table 6 Correlations between cytokines and IRI therapeutic response in different choroidal thickness groups.
Our study demonstrated a significant correlation between systemic characteristics and cytokine expression levels in the aqueous humor at baseline with the anti-VEGF treatment response in patients with PCV. The baseline characteristics of PCV varied among subgroups categorized by different choroidal thicknesses. Our study is novel in exploring baseline clinical characteristics in the same cohort of patients grouped according to both parameters.
In this study, we observed that patients with a suboptimal anti-VEGF response had a thicker choroid than those with an optimal anti-VEGF response. Upon observing baseline features, we identified numerous systemic and ocular characteristics similarities between the group with a suboptimal IRI response and the group with a thicker choroid. There were even more similarities between the group with optimal IRI response and the group with a thinner choroid. The group with optimal IRI response and the group with a thin choroid were characterized by older patients and a higher prevalence of hypertension. Regarding ocular characteristics, both groups exhibited worse visual acuity, a higher probability of suffering from PED, a larger GLD, a longer ocular axis length, and fewer injections within a year. The observation of optimal IRI response may result from a floor effect of worse visual acuity, signifying a significant treatment effect. Besides, fewer injections also suggest a favorable responsiveness to anti-VEGF in this patient group. Our aqueous humor cytokine assay in PCV subject eyes revealed higher VEGF and VCAM-1 expression levels in the group with optimal anti-VEGF response and group with a thinner choroid. In contrast, TNF-α expression was lower in the both groups.
Currently, the classification of PCV remains controversial, and whether PCV is considered one of the subtypes of AMD or a separate clinical disease requires further research, notably (10). PCV is presently categorized as a pachychoroid disease, sharing standard features such as a reduced volume of the capillary and Sattler layers, dilation of the large vessel layer, and secondary retinal pigment epithelial dysfunction or neovascularization (12, 17, 18). Not all patients diagnosed with PCV exhibit thick choroid, and our study confirms that PCV with different choroidal thicknesses presents different ocular characteristics (19–21). From a genetic perspective, thick choroid and choroidal vascular hyperpermeability are important features of pachychoroid spectrum diseases. Previous studies have suggested the classification of PCV into two subtypes: pachychoroid neovasculopathy (PNV) and drusen-driven PCV. Examining PCV from a genetic viewpoint may assist in reorganizing the subtypes of drusen-driven AMD and pachychoroid spectrum diseases (22). Recently, the literature has further explored the distribution of choroidal thickness, identifying baseline nasal peripapillary choroidal thickness (nPCT) and SFCT/nPCT ratios as useful as biomarkers reflecting clinical outcomes after anti-VEGF therapy for PCV (23). Our studies revealed that patients with thinner choroidal thickness have a higher incidence of chronic hypertension, a viewpoint supported by numerous studies. Chronic hypertension and heart failure may reduce choroidal thickness, as indicated by a review and meta-analysis examining the relationship between choroidal thickness and cardiovascular disease. Both coronary artery disease and carotid artery stenosis could be linked to the pathology, resulting in decreased blood flow and choroidal thickness. Conversely, acute hypertension increases choroidal thickness, possibly due to the inability of choroidal vasoregulation to withstand acute hypertension-induced choroidal ischemia, leading to altered choroidal permeability and interstitial fluid accumulation, thereby increasing SFCT (24).
Previous genetic studies have identified several causative factors associated with neovascular AMD (nAMD), including VEGF, MCP-1, soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1, IL-6, IL-8, IL-10, C-reactive protein, human growth factor, tumor necrosis factor-α, IL-31, leukemia inhibitory factor, and stromal cell-derived factor-1-α (25–27). Literature has compared the phenotypic/genetic differences between PNV and nAMD, revealing distinct genetic risk scores calculated from AMD susceptibility genes and different etiologies for the two diseases (28). Recent literature has discussed and compared inflammatory cytokine profiles between nAMD and PNV. The study found that VEGF-A was significantly lower in the PNV group than in the nAMD group but almost identical to the control group, affirming differences in cytokine expression between the two diseases (29, 30). IL-8, a chemokine that promotes vascular endothelial proliferation (31–33), has been found to have higher levels in patients with PCV or nAMD in aqueous humor (29, 30). Similarly, our study observed higher overall expression of IL-8 in PCV. However, there was no difference in IL-8 in either subgroup, indicating a minimal correlation between IL-8 and the degree of anti-VEGF response and choroidal thickness. Vitreous anti-VEGF therapy is widely used to treat PCV, helping eliminate retinal fluid and improve BCVA in the short term (34–36). While anti-VEGF is one of the most effective therapies for secondary CNV, the literature reports lower VEGF concentrations in aqueous humor in patients with PNV than those with drusen-associated MNV, and the efficacy of anti-VEGF therapy for PCV is not conclusive (29). Consequently, we hypothesize that thinner choroidal PCV subtypes share similar mechanisms to nAMD regarding cytokine pathogenesis, and elevated VEGF may be reciprocally associated with choroidal ischemia and atrophy of the inner vascular layer. Thick choroidal PCV may have independent pathogenesis and therapeutic targets compared to thin choroidal PCV and nAMD. Improved visual acuity after anti-VEGF therapy has been associated with lower serum levels of TNF-α, and targeting TNF-α may facilitate the treatment of neovascular AMD (37). In our study, TNF-α expression was higher in the thick choroid group and was identified as an independent risk factor for anti-VEGF therapy. Therefore, combined inhibition of VEGF and TNF-α may be a potential option for treating PCV. Our study confirms that low VEGF, VCAM-1, and high expression levels of TNF-α are independent risk factors for suboptimal anti-VEGF response, especially in the thick choroidal group. It demonstrates the relationship between VEGF, VCAM-1 and TNF-α and the anti-VEGF response in PCV with different choroidal thicknesses, providing evidence not documented to date. These results contribute to an improved understanding of anti-VEGF therapy of PCV.
In the future, further research is needed to comprehend the distinctions between typical AMD and PCV and to unravel how and why they differ. Additionally, we need to understand how the immune system in the choroid changes with age and disease. Specific focus should be directed towards the molecular basis of PCV, including a comprehensive understanding of the functionality of genes associated with PCV risk, with a particular emphasis on the choroid. In future clinical practice, evaluating ocular conditions, such as aqueous humor cytokine testing in patients with thick choroidal PCV requiring anti-VEGF therapy, can be instrumental in determining treatment response. This approach can facilitate more timely, accurate, and appropriate treatment based on the obtained results. Moreover, our study contributes valuable insights that can guide strategies for discovering new therapeutic targets for PCV.
Our study provides a foothold in predicting baseline characteristics and diagnosing the anti-VEGF response in PCV with different choroidal thicknesses. However, several limitations should be considered. The study population was derived from a single center, and the sample size was small. Due to the lack of automated choroidal thickness measurement software in current OCT equipment, manual assessment was performed on all SD-OCT images, and repeated measurements were conducted to mitigate potential observational errors associated with manual measurements. Normal subjects and patients with AMD were not included as controls in this experiment, and we solely explored the conditions of patients with PCV of different subtypes. Therefore, the generalizability of our results could be improved, and further studies are needed to validate our findings.
In summary, our study reveals that PCV with different anti-VEGF responses differ in systemic and ocular characteristics. The cytokine levels in the aqueous humor significantly correlate with the anti-VEGF response in PCV. Specifically, VEGF, VCAM-1, and TNF-α are potential targets for assessing treatment response in thick choroidal PCV. In the future, aqueous humor cytokine testing and choroidal thickness assessment hold promise for risk management in PCV patients requiring anti-VEGF therapy.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Review Committee of the Second Affiliated Hospital of Harbin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SD: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. PF: Data curation, Formal Analysis, Writing – original draft. HY: Data curation, Formal Analysis, Writing – original draft. BJ: Conceptualization, Writing – review & editing, Supervision. DS: Conceptualization, Writing – review & editing, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82171103). The journal’s Publication fee and Open Access fees were funded by the authors.
We are grateful to all subjects who participated in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Health (2014) 2:e106–116. doi: 10.1016/S2214-109X(13)70145-1
2. Wong CW, Yanagi Y, Lee WK, Ogura Y, Yeo I, Wong TY, et al. Age-related macular degeneration and polypoidal choroidal vasculopathy in asians. Prog Retin Eye Res (2016) 53:107–39. doi: 10.1016/j.preteyeres.2016.04.002
3. Chaikitmongkol V, Cheung CMG, Koizumi H, Govindahar V, Chhablani J, Lai TYY. Latest developments in polypoidal choroidal vasculopathy: epidemiology, etiology, diagnosis, and treatment. Asia Pac J Ophthalmol (Phila) (2020) 9:260–8. doi: 10.1097/01.APO.0000656992.00746.48
4. Sharma K, Sharma NK, Anand A. Why AMD is a disease of ageing and not of development: mechanisms and insights. Front Aging Neurosci (2014) 10:151. doi: 10.3389/fnagi.2014.00151
5. Fan Q, Cheung CMG, Chen LJ, Yamashiro K, Ahn J, Laude A, et al. Shared genetic variants for polypoidal choroidal vasculopathy and typical neovascular age-related macular degeneration in east asians. J Hum Genet (2017) 62:1049–55. doi: 10.1038/jhg.2017.83
6. Laude A, Cackett PD, Vithana EN, Yeo IY, Wong D, Koh AH, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res (2010) 29:19–29. doi: 10.1016/j.preteyeres.2009.10.001
7. Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, et al. Factors predictive of outcomes 1 year after 3 monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Retina. (2013) 33:1949–58. doi: 10.1097/IAE.0b013e31828bcafa
8. Nowak-Sliwinska P, van den Bergh H, Sickenberg M, Koh AH. Photodynamic therapy for polypoidal choroidal vasculopathy. Prog Retin Eye Res (2013) 37:182–99. doi: 10.1016/j.preteyeres.2013.09.003
9. Ting DS, Ng WY, Ng SR, Tan SP, Yeo IY, Mathur R, et al. Choroidal thickness changes in age-related macular degeneration and polypoidal choroidal vasculopathy: A 12-month prospective study. Am J Ophthalmol (2016) 164:128–36. doi: 10.1016/j.ajo.2015.12.024
10. Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. (2011) 118:840–5. doi: 10.1016/j.ophtha.2010.09.012
11. Lee J, Byeon SH. Prevalence and clinical characteristics of pachydrusen in polypoidal choroidal vasculopathy: multimodal image study. Retina. (2019) 39:670–8. doi: 10.1097/IAE.0000000000002019
12. Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB. Pachychoroid disease. Eye (Lond) (2019) 33:14–33. doi: 10.1038/s41433-018-0158-4
13. Chang YC, Cheng CK. Difference between pachychoroid and nonpachychoroid polypoidal choroidal vasculopathy and their response to anti-vascular endothelial growth factor therapy. Retina. (2020) 40:1403–11. doi: 10.1097/IAE.0000000000002583
14. Gupta P, Ting DSW, Thakku SG, Wong TY, Cheng CY, Wong E, et al. Detailed characterization of choroidal morphologic and vascular features in age-related macular degeneration and polypoidal choroidal vasculopathy. Retina. (2017) 37:2269–80. doi: 10.1097/IAE.0000000000001481
15. Lee WK, Baek J, Dansingani KK, Lee JH, Freund KB. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal subfoveal choroidal thickness. Retina. (2016) 36:S73–82. doi: 10.1097/IAE.0000000000001346
16. Inoda S, Takahashi H, Inoue Y, Tan X, Tampo H, Arai Y, et al. Cytokine profiles of macular neovascularization in the elderly based on a classification from a pachychoroid/drusen perspective. Graefes Arch Clin Exp Ophthalmol (2022) 260:747–58. doi: 10.1007/s00417-021-05445-0
17. Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. (2015) 35:1–9. doi: 10.1097/IAE.0000000000000331
18. Castro-Navarro V, Behar-Cohen F, Chang W, Joussen AM, Lai TYY, Navarro R, et al. Pachychoroid: current concepts on clinical features and pathogenesis. Graefes Arch Clin Exp Ophthalmol (2021) 259:1385–400. doi: 10.1007/s00417-020-04940-0
19. Jordan-Yu JM, Teo KYC, Chakravarthy U, Gan A, Tan ACS, Cheong KX, et al. Polypoidal choroidal vasculopathy features vary according to subfoveal choroidal thickness. Retina. (2021) 41:1084–93. doi: 10.1097/IAE.0000000000002966
20. Dansingani KK, Perlee LT, Hamon S, Lee M, Shah VP, Spaide RF, et al. Risk alleles associated with neovascularization in a pachychoroid phenotype. Ophthalmology. (2016) 123:2628–30. doi: 10.1016/j.ophtha.2016.06.060
21. Yamashiro K, Yanagi Y, Koizumi H, Matsumoto H, Cheung CMG, Gomi F, et al. Relationship between pachychoroid and polypoidal choroidal vasculopathy. J Clin Med (2022) 11:4614. doi: 10.3390/jcm11154614
22. Yamashiro K, Hosoda Y, Miyake M, Ooto S, Tsujikawa A. Characteristics of pachychoroid diseases and age-related macular degeneration: multimodal imaging and genetic backgrounds. J Clin Med (2020) 9:2034. doi: 10.3390/jcm9072034
23. Kim YH, Lee B, Kang E, Oh J. Choroidal thickness profile and clinical outcomes in eyes with polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol (2021) 259:1711–21. doi: 10.1007/s00417-020-05051-6
24. Yeung SC, You Y, Howe KL, Yan P. Choroidal thickness in patients with cardiovascular disease: A review. Surv Ophthalmol (2020) 65:473–86. doi: 10.1016/j.survophthal.2019.12.007
25. Nguyen QD, De Falco S, Behar-Cohen F, Lam WC, Li X, Reichhart N, et al. Placental growth factor and its potential role in diabetic retinopathy and other ocular neovascular diseases. Acta Ophthalmol (2018) 96:e1–9. doi: 10.1111/aos.13325
26. Li X, Cao X, Zhao M, Bao Y. The changes of irisin and inflammatory cytokines in the age-related macular degeneration and retinal vein occlusion. Front Endocrinol (Lausanne) (2022) 13:861757. doi: 10.3389/fendo.2022.861757
27. Fukuda Y, Sakurada Y, Yoneyama S, Kikushima W, Sugiyama A, Matsubara M, et al. Clinical and genetic characteristics of pachydrusen in patients with exudative age-related macular degeneration. Sci Rep (2019) 9:11906. doi: 10.1038/s41598-019-48494-6
28. Miyake M, Ooto S, Yamashiro K, Takahashi A, Yoshikawa M, Akagi-Kurashige Y, et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci Rep (2015) 5:16204. doi: 10.1038/srep16204
29. Terao N, Koizumi H, Kojima K, Yamagishi T, Yamamoto Y, Yoshii K, et al. Distinct aqueous humour cytokine profiles of patients with pachychoroid neovasculopathy and neovascular age-related macular degeneration. Sci Rep (2018) 8:10520. doi: 10.1038/s41598-018-28484-w
30. Hata M, Yamashiro K, Ooto S, Oishi A, Tamura H, Miyata M, et al. Intraocular vascular endothelial growth factor levels in pachychoroid neovasculopathy and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci (2017) 58:292–8. doi: 10.1167/iovs.16-20967
31. Choi SH, Park JY, Kang W, Kim SU, Kim do Y, Ahn SH, et al. Knockdown of HIF-1α and IL-8 induced apoptosis of hepatocellular carcinoma triggers apoptosis of vascular endothelial cells. Apoptosis. (2016) 21:85–95. doi: 10.1007/s10495-015-1185-2
32. Ahuja SK, Ozçelik T, Milatovitch A, Francke U, Murphy PM. Molecular evolution of the human interleukin-8 receptor gene cluster. Nat Genet (1992) 2:31–6. doi: 10.1038/ng0992-31
33. Hautamäki A, Kivioja J, Vavuli S, Kakko S, Savolainen ER, Savolainen MJ, et al. Interleukin 8 promoter polymorphism predicts the initial response to bevacizumab treatment for exudative age-related macular degeneration. Retina. (2013) 33:1815–27. doi: 10.1097/IAE.0b013e318285cf92
34. Lee WK, Iida T, Ogura Y, Chen SJ, Wong TY, Mitchell P, et al. PLANET investigators. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: A randomized clinical trial. JAMA Ophthalmol (2018) 136:786–93. doi: 10.1001/jamaophthalmol.2018.1804
35. Lim TH, Lai TYY, Takahashi K, Wong TY, Chen LJ, Ruamviboonsuk P, et al. EVEREST II study group. Comparison of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: the EVEREST II randomized clinical trial. JAMA Ophthalmol (2020) 138:935–42. doi: 10.1001/jamaophthalmol.2020.2443
36. Miyata M, Ooto S, Yamashiro K, Tamura H, Hata M, Ueda-Arakawa N, et al. Five-year Visual Outcomes after anti-VEGF Therapy with or without Photodynamic Therapy for Polypoidal Choroidal Vasculopathy. Br J Ophthalmol (2019) 103:617–22. doi: 10.1136/bjophthalmol-2018-311963
Keywords: biomarker, polypoidal choroidal vasculopathy, anti-vascular endothelial growth factor, cytokine, choroidal thicknesses, pachychoroid
Citation: Dong S, Fan P, Yu H, Jiang B and Sun D (2024) A study of the relationship between cytokine levels and the response to anti-VEGF therapy in polypoid choroidal vasculopathy with different choroidal thicknesses. Front. Endocrinol. 14:1307337. doi: 10.3389/fendo.2023.1307337
Received: 04 October 2023; Accepted: 07 December 2023;
Published: 03 January 2024.
Edited by:
Anne Fougerat, INRAE Occitanie Toulouse, FranceReviewed by:
Tao Li, Sun Yat-sen University, ChinaCopyright © 2024 Dong, Fan, Yu, Jiang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Jiang, ZXllamlhbmdib0AxMjYuY29t; Dawei Sun, c3VuLmRhd2VpQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.