- 1College of Medical Technology, Shanghai University of Medicine & Health Sciences, Shanghai, China
- 2Department of Laboratory Medicine, Shanghai University of Medicine & Health Sciences Affiliated Zhoupu Hospital, Shanghai, China
Background: In recent years, the outbreak of COVID-19 caused by SARS-CoV-2 has been witnessed globally. However, the impact of SARS-CoV-2 infection on thyroid dysfunction and subclinical thyroid dysfunction remains unclear. Therefore, this meta-analysis aimed to assess the effects of SARS-CoV-2 infection on thyroid dysfunction and its relationship with the severity of COVID-19.
Methods: We systematically searched databases including PubMed, Willey Library, Embase, Web of Science, CNKI, Wanfang, and VIP. We focused on randomized controlled trials, case-control studies, and cohort studies published between December 2019 and August 2023, examining the association between SARS-CoV-2 infection and hypothyroidism, with a specific emphasis on the severity of the infection. The quality of the research was assessed using the Newcastle-Ottawa Scale (NOS), while statistical analysis was conducted using the meta and metafor packages in R 4.2.1 software.
Results: For the meta-analysis, a total of eight articles were identified based on strict inclusion and exclusion criteria. For the association between SARS-CoV-2 infection and hypothyroidism, three studies (266 samples) comparing TSH levels of COVID-19 and control groups showed no difference in TSH levels [SMD=-0.04,95%CI(-1.22,1.15),P=0.95]. Additionally, two studies examining TT3 (a sample of 176 cases) and two studies examining TT4 (a sample of 176 cases) also showed no difference in TT3 and TT4 between the COVID-19 group and the control group, respectively. However, when evaluating the severity of COVID-19, six studies (565 samples) showed that TSH in the severe group was significantly lower than in the mild group [SMD = -0.55, 95% CI (-0.96, -0.14)], while FT3 was also lower in the severe group [SMD = -0.96, 95% CI (-1.24, -0.67)]. No noticeable differences were observed between the severe and mild groups in their TT3, FT4, and TT4 levels.
Conclusion: SARS-CoV-2 infection may have detrimental effects on thyroid function in individuals with severe symptoms. More research is needed to confirm and explore this relationship.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42023486042.
1 Introduction
Since the initial outbreak of SARS-CoV-2 coronavirus at the end of 2019, the world has been grappling with this unprecedented health crisis, leading to hundreds of millions of infections and innumerable fatalities (1, 2). Early research and clinical observations have focused on how the virus causes direct damage to the respiratory system, especially the lungs (3). However, as time progressed, extensive clinical and laboratory studies have revealed the multifaceted effects of the virus on other organs and physiological systems, with the involvement of the endocrine system receiving particular attention from researchers (4, 5). The thyroid gland serves an essential role in many of the basic physiological processes that occur in the body, such as regulating body temperature, controlling basal metabolic rate, and many other processes related to metabolism, growth and development (6). Moreover, the synthesis, storage, and release of thyroid hormones are also critical factors in maintaining overall homeostasis (7). Therefore, any external agents, particularly novel viruses like SARS-CoV-2 (8, 9), that might impede thyroid function necessitate meticulous and systematic investigation.
Recently, several studies have sought to examine the potential associations between SARS-CoV-2 and thyroid function. Distinct biochemical anomalies in thyroid function have been documented in specific cases, predominantly in the nascent phase of COVID-19 infection or during its more severe manifestations (10–12). For example, certain research noted that a subset of COVID-19 patients had biochemical changes similar to subclinical hypothyroidism after virus invasion, especially the increase of thyroid stimulating hormone (TSH) (10, 11). Subclinical hypothyroidism is defined as elevated serum TSH levels with normal serum thyroid hormones (T4 and T3) in patients with no symptoms of hypothyroidism or only mild symptoms of hypothyroidism. In addition, there are studies suggesting that COVD-19 may trigger an inflammatory response in the thyroid gland, leading to transient hypothyroidism (13). Nevertheless, these studies do not always converge on a shared conclusion (10–16). The inconsistency may be due to a variety of factors, such as the differences in biochemical detection methods used, the population characteristics of the subjects, the geographical environment, and the specific genotypes or variants of SARS-CoV-2 involved. Therefore, any interpretation of these preliminary findings needs to be cautious and take into account these potential confounding factors.
Given that recent studies have drawn divergent conclusions on the association between COVID-19 and thyroid function, a comprehensive and consistent analysis is needed. This can be best captured by a meta-analysis, which systematically integrates studies on thyroid dysfunction associated with SARS-CoV-2 infection, with particular attention to the correlation between COVID severity and hypothyroidism. Through this methodology, we hoped to achieve a clearer and more congruent scientific consensus.
2 Materials and methods
2.1 Literature search strategy
To identify relevant articles on how SARS-CoV-2 infection affects hypothyroidism and subclinical hypothyroidism, computer searches of the Willey Library, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang, and VIP databases were conducted. The search time is from December 2019 to August 2023. Keywords used in the search include “SARS-CoV-2”, ”hypothyroidism”, “thyroid dysfunction”, “subclinical hypothyroidism”, and corresponding Chinese terms.
2.2 Inclusion criteria
Design types included randomized controlled trials, case-control studies, or cohort studies written in English, regardless of sample size. The subjects were patients with hypothyroidism or subclinical hypothyroidism (serum thyroxine (T4) and triiodothyronine (T3) levels were normal, but serum thyroid stimulating hormone (TSH) levels were elevated). The experimental group was infected with SARS-CoV-2 (divided into mild and severe), and the control group was made up of uninfected individuals. The COVID-19 condition severity can be evaluated based on the following indices, including clinical symptoms, laboratory and radiographic abnormalities, hemodynamics and organ function (17). Mild cases refer to individuals with mild clinical symptoms but no shortness of breath or abnormal chest imaging. Severe cases refer to individuals with an oxygen saturation (SpO2) less than 93%, an oxygenation index (PaO2/FiO2) less than 300 mmHg, a respiratory rate greater than 30 breaths/min, or lung infiltrates greater than 50% of the total lung volume. The hypothyroidism patients infected with SARS-CoV-2 were further analyzed according to the severity of COVID-19. The outcome indices included in the study were TSH, TT3, FT3, TT4, and FT4.
2.3 Exclusion criteria
We excluded non-clinical controlled trials (e.g. reviews, guidelines, expert consensus), as well as those with repeated publication, missing data, or statistical errors. Also excluded were studies in which the outcome indices did not include TSH, TT3, FT3, TT4, and FT4.
2.4 Literature screening and data extraction
Preliminary screening was carried out independently by two researchers using the studies’ title and abstract. Both read the full texts of any articles that passed the initial screening to see if these should be included. The two researchers extracted information such as topic, author, publication year, research object, sample size, intervention methods, and important outcome indicators. If there were disagreements throughout the extraction process, an agreement was reached through discussion.
2.5 Literature quality evaluation
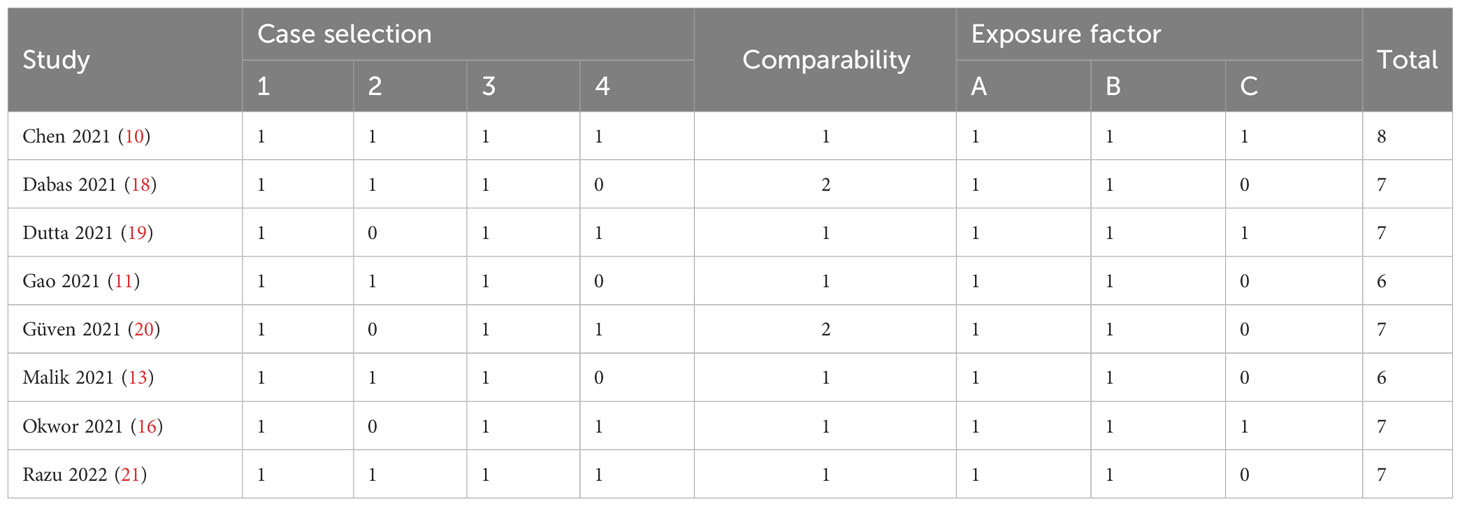
We used the Newcastle-Ottawa Scale (NOS) to rate the quality of each study that was included (14). The NOS scale is a three-part assessment instrument created especially for observational studies to assess study selection, comparison, and outcome. The scale assesses sample comparability, sample selection, and whether each study’s possible confounding variables are deemed adequate. Each article is eligible for a maximum of 9 points under the NOS scoring guidelines. Studies that receive a 7 or more are deemed to be of high literary quality, 4-6 are deemed to be of medium literary quality, and 3 or less are deemed to be of poor literary quality. Two independent reviewers rated each included studies, and when there were disagreements, they discussed them or sought the opinions of other reviewers.
2.6 Statistical analysis
The meta-analysis in this study was carried out employing R 4.2.1 software, using meta and metafor statistical packages. The mean difference value is frequently utilized for calculating the combined effect size in a meta-analysis. For continuous variables, we selected the Standardized Mean Difference (SMD) as the effect size and reported a 95% confidence interval (95% CI). If I^2<50% and P>0.1, the fixed effect model was applied. Otherwise, the random effect model was used, and the risk bias analysis method employed the Egger test. Statistics were judged significant at P<0.05.
3 Results
3.1 Characteristics of included studies
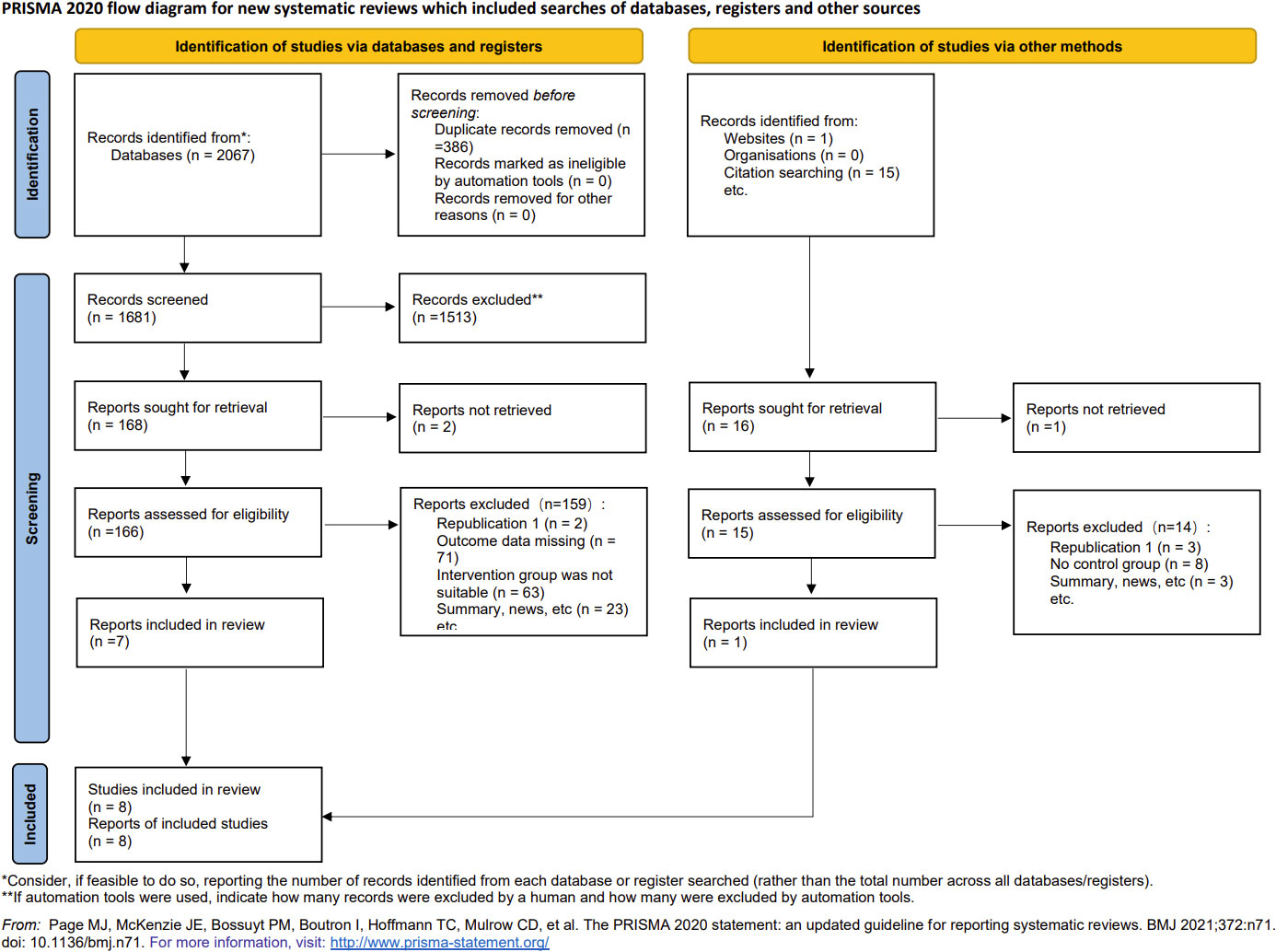
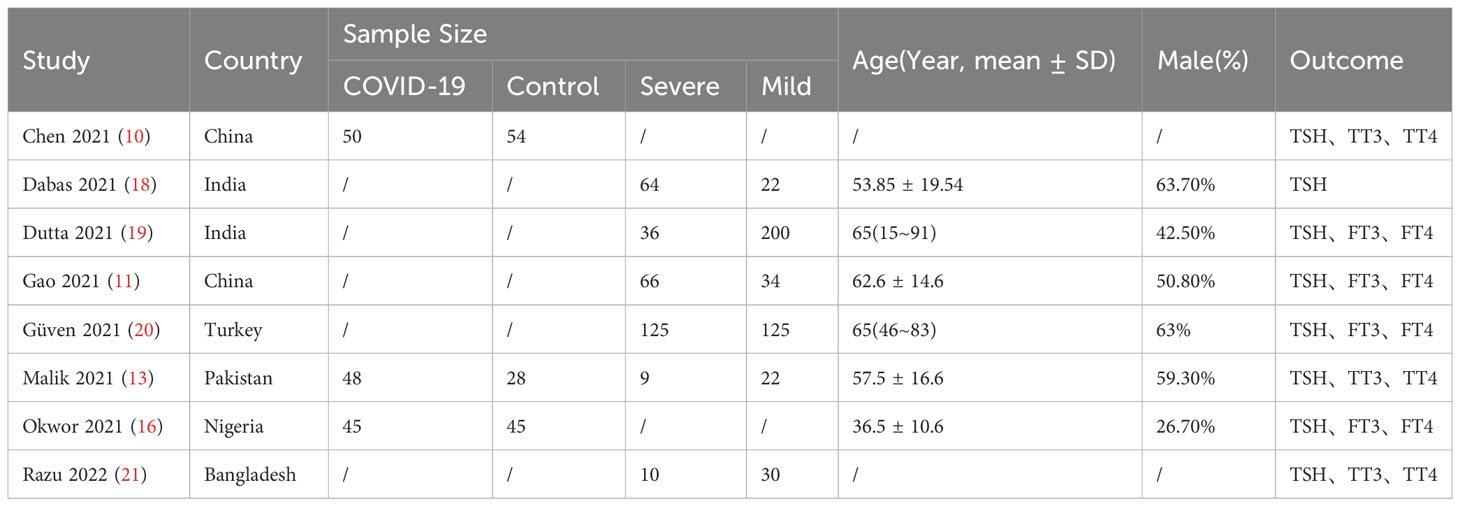
Overall, 2067 articles were retrieved from the database, 386 duplicate articles were excluded, 1513 articles with obvious inconformity were removed through the title and abstract reading, and the remaining 168 articles were downloaded. Seven of the articles were retained after final reading while one addition article was included through web search and literature tracking. Ultimately, a total of 8 articles were included (Figure 1), all of which were cohort or case-control studies involving participants from different nations, such as China, India, Turkey, Pakistan, Nigeria, and Bangladesh. The detailed information is shown in Table 1. The quality of articles is provided in Table 2. The majority of the studies included in this analysis received scores of 6 to 8 points according to the Newcastle-Ottawa Scale (NOS), indicating that they were of medium or high quality. After the quality assessment, no study was disqualified because of lack of reliability.
3.2 Meta-analysis results
3.2.1 The effect of SARS-COV-2 infection on hypothyroidism and subclinical hypothyroidism
3.2.1.1 TSH
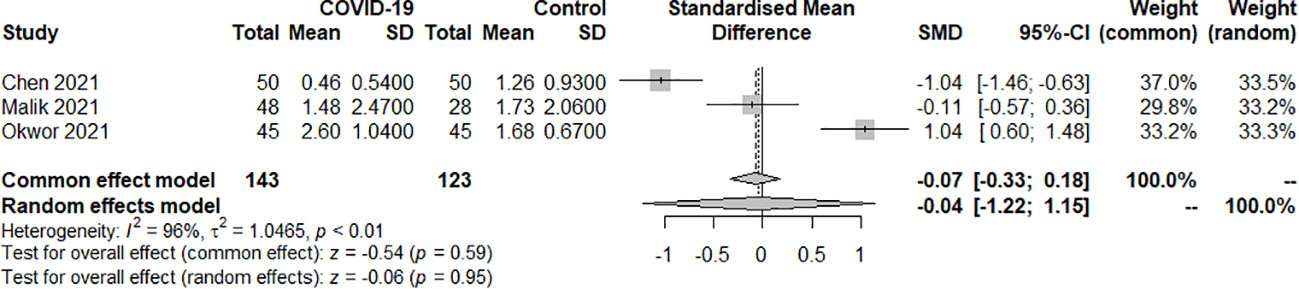
Three studies examined the TSH index of SARS-COV-2 infection in patients with hypothyroidism and subclinical hypothyroidism. A total of 266 samples were included, consisting of 143 patients and 123 controls. The random effect model well explained the heterogeneity test between studies, which had P<0.01, I2 = 96%. The findings revealed no difference in TSH levels between the COVID-19 group and the control group. [SMD=-0.04, 95%CI (-1.22, 1.15), P=0.95] (Figure 2).
3.2.1.2 TT3
Two studies examined the TT3 index of SARS-COV-2 infection in patients with hypothyroidism and subclinical hypothyroidism. A total of 180 samples were included, consisting of 90 patients and 90 controls. The random effect model well explained the heterogeneity test between studies, which had P=0.04, I2 = 77%. The findings revealed no difference in TT3 levels between the COVID-19 group and the control group. [SMD=-0.45, 95%CI (-1.16,0.26), P=0.21] (Figure 3).
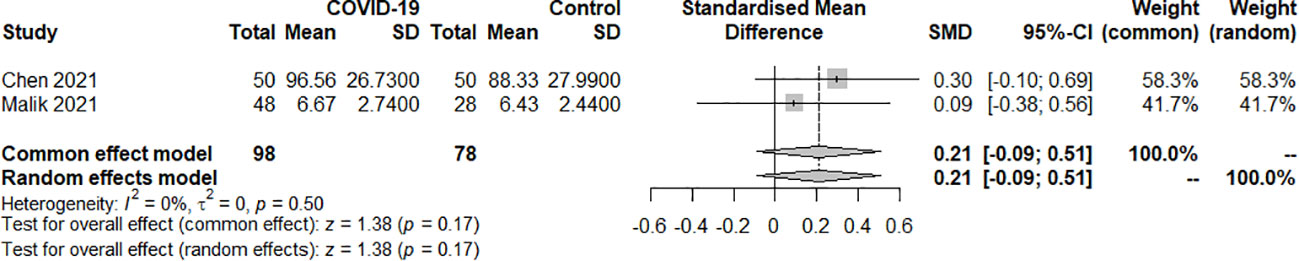
3.2.1.3 TT4
Two studies examined the TT4 index of SARS-COV-2 infection in patients with hypothyroidism and subclinical hypothyroidism. A total of 176 samples were included, consisting of 98 patients and 78 controls. The fixed effect model well explained the heterogeneity test between studies, which had P=0.50, I2 = 0%. The findings revealed no difference in TT4 levels between the COVID-19 group and the control group. [SMD=0.21, 95%CI (-0.09,0.51), P=0.17] (Figure 4).
3.2.2 The effect of COVID-19 severity on hypothyroidism and subclinical hypothyroidism
3.2.2.1 TSH
The effect of the severity of SARS-COV-2 infection on TSH markers in individuals with hypothyroidism and subclinical hypothyroidism was assessed in six studies. A total of 565 samples were included, consisting of 236 severe patients and 329 mild patients. The random effect model explained the heterogeneity test between studies, which was P<0.01, I2 = 70%. The findings indicated that TSH was significantly lower in the severe than the mild group. [SMD=-0.55, 95%CI (-0.96, -0.14), P<0.01] (Figure 5).
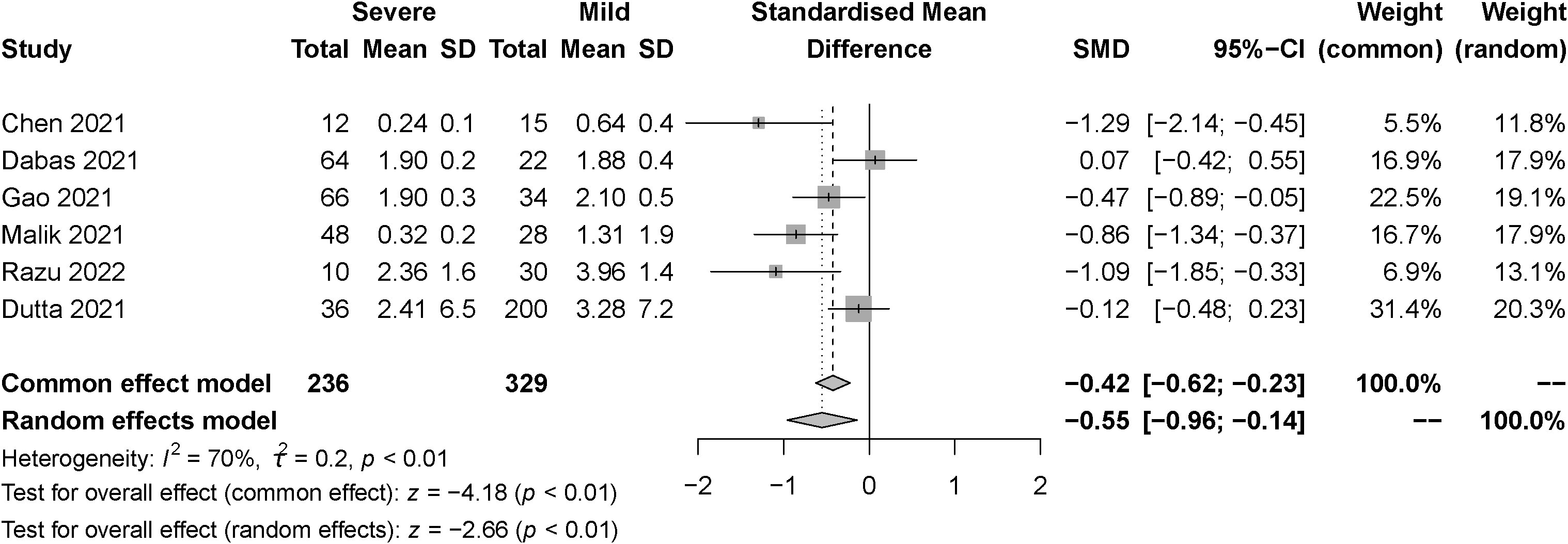
Figure 5 Forest plot of the effect of the severity of SARS-COV-2 infection symptoms on the patient’s TSH.
3.2.2.2 FT3 and TT3
The effect of the severity of SARS-COV-2 infection on FT3 and TT3 markers in individuals with hypothyroidism and subclinical hypothyroidism was assessed in two studies. The random effect model and fixed effect model explained the heterogeneity test results between trials, which were P<0.01, I2 = 98% and P=0.41, I2 = 0%, respectively. According to the findings, FT3 was significantly lower in the severe than the mild group, [SMD=-0.96, 95%CI (-1.24,-0.67), P<0.01] (Figure 6) and TT3 showed no difference. [SMD=5.39, 95%CI (-4.87, 15.66), P=0.03] (Figure 7).
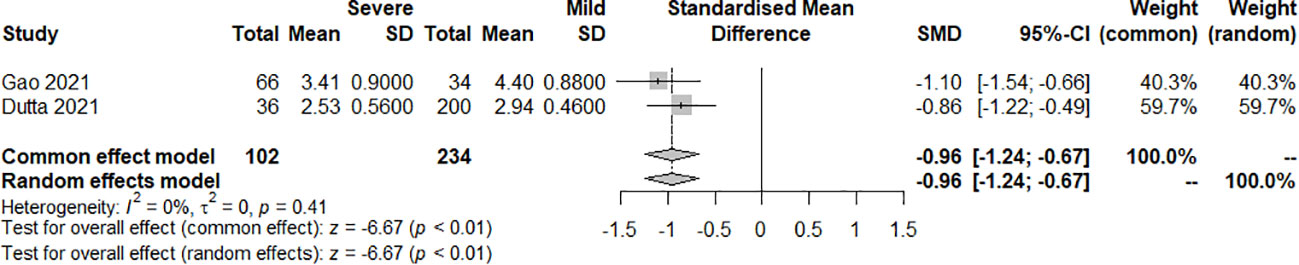
Figure 6 Forest plot of the effect of the severity of SARS-COV-2 infection symptoms on the patient’s FT3.
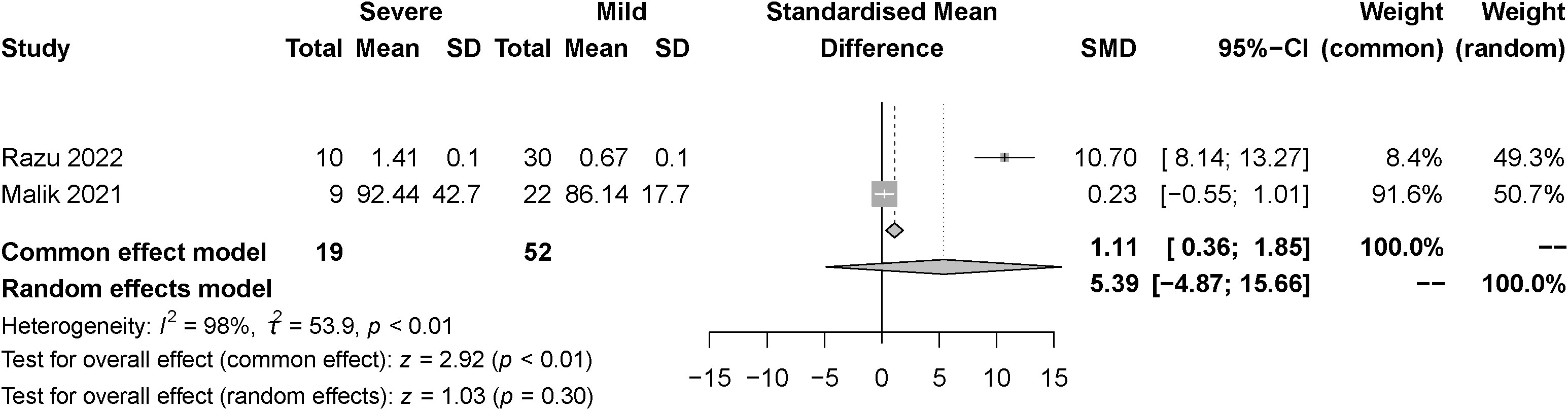
Figure 7 Forest plot of the effect of the severity of SARS-COV-2 infection symptoms on the patient’s TT3.
3.2.2.3 FT4 and TT4
The effect of the severity of SARS-COV-2 infection on FT4 and TT4 markers in individuals with hypothyroidism and subclinical hypothyroidism was assessed in three and two studies respectively. The random effect model explained the heterogeneity test results between trials, which were P<0.01, I2 = 87% and P<0.01, I2 = 98%, respectively. Both studies were described by random effects model. The results indicated no difference between severe and mild groups in FT4. [SMD=-0.00, 95%CI (-0.61, 0.60), P=0.99] (Figure 8) Similarly, there was no difference between groups in TT4. [SMD=5.09, 95%CI (-4.60, 14.79), P=0.30] (Figure 9).
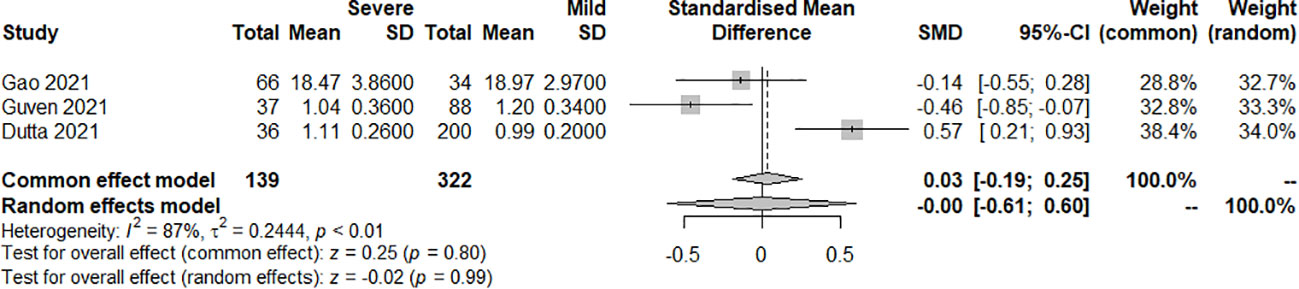
Figure 8 Forest plot of the effect of the severity of SARS-COV-2 infection symptoms on the patient’s FT4.
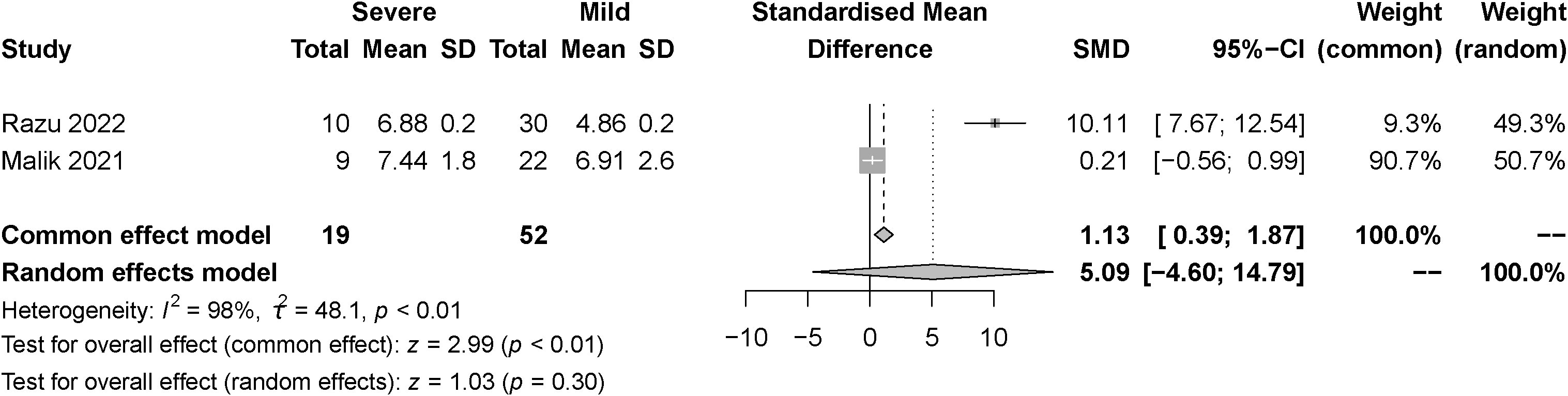
Figure 9 Forest plot of the effect of the severity of SARS-COV-2 infection symptoms on the patient’s TT4.
3.3 Risk of bias assessment
We performed a statistical analysis using the Egger test to determine the degree of bias in the included studies. The findings revealed all indicators had P values higher than 0.05, signifying no bias.
4 Discussion
The global outbreak of SARS-CoV-2 has attracted considerable attention, especially given its potential effects on multiple organs, including the thyroid gland (12, 13). Hypothyroidism and subclinical hypothyroidism are two high-risk endocrine conditions, each presenting with a range of clinical manifestations, symptoms, and biochemical changes. The thyroid gland can be susceptible to a wide range of internal and external factors, which can cause it to behave abnormally in some situations, such as systemic diseases or viral infections (22–24). However, the exact effect of SARS-CoV-2 on thyroid function remains a topic of interest. Therefore, a comprehensive meta-analysis is essential to systematically assess the potential impact of SARS-CoV-2 on thyroid function and provide guidance for future research and treatment.
In this meta-analysis, we systematically examined the effects of SARS-CoV-2 infection on thyroid function. Our overall findings showed that TSH, TT3, and TT4 levels were not significantly affected by SARS-CoV-2 infection, thus aligning with the current popular hypothesis that SARS-CoV-2 infection does not directly induce significant changes in thyroid function (15). However, our findings indicate several relevant effects when we investigated the impact of the severity of COVID-19. Specifically, individuals with severe COVID-19 showed disturbed thyroid function, reflecting lower TSH and FT3 levels compared with those with mild COVID-19.
A number of explanations are plausible for the above observations. Individuals with severe COVID-19 frequently experience a strong systemic inflammatory response, which may have an effect on how thyroid hormones are transformed and released (25). For instance, inflammation may cause cystosulfate deiodinase (DIO2) to function less efficiently, which may influence the conversion of T4 to T3 (26). Also, the virus might directly or indirectly invade thyroid tissues, causing inflammation and cellular damage (27). Furthermore, a variety of pharmacological therapies are frequently administered to critically sick patients, some of which may affect thyroid function (25, 28). For instance, systemic corticosteroids are recommended for use in severe COVID-19 patients while inhibiting serum TSH levels (25). It is worth noting that while these alterations may suggest thyroid dysfunction, it remains uncertain if they correlate directly with clinical outcomes like mortality or hospitalization duration (29, 30). Future studies should further explore these potential mechanisms and their effects on patients’ clinical prognoses.
This meta-analysis has several significant advantages. First, we adopted a systematic methodology and rigorous data analysis process to ensure the accuracy and reliability of the research results. Second, our study is the first systematic assessment of the association between SARS-CoV-2 infection and thyroid function changes, providing new insights into this field. In addition, our study covers data from multiple countries and regions, increasing the breadth and diversity of research. These characteristics make this study have a high reference value in assessing the impact of SARS-CoV-2 on thyroid function.
However, our research also has some limitations. Although we evaluated the quality of the included studies using the NOS scale, there may still be some confounding factors because of the various data sources. Despite our meta-analysis encompassing multiple studies, the sample size remains relatively limited, potentially affecting our statistical robustness and the uniformity of our findings.
5 Conclusion
In conclusion, while SARS-CoV-2 infection itself may not directly induce hypothyroidism, the severity of COVID-19 may contribute to thyroid dysfunction. Specifically, TSH and FT3 levels were significantly lower in individuals with severe COVID-19 than those with mild COVID-19. It is important to explore these findings further in order to understand the potential mechanisms of thyroid dysfunction in the outcome of COVID-19.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JW: Writing – original draft, Writing – review & editing, Data curation, Validation. FZ: Writing – review & editing, Funding acquisition, Project administration, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by General Program of Shanghai Pudong New Area Health Commission (Grant No.PW2022A-19).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Peng M. Outbreak of COVID-19: An emerging global pandemic threat. Biomedicine Pharmacotherapy = Biomedecine Pharmacotherapie (2020) 129:110499. doi: 10.1016/j.biopha.2020.110499
2. Awadasseid A, Wu Y, Tanaka Y, Zhang W. Initial success in the identification and management of the coronavirus disease 2019 (COVID-19) indicates human-to-human transmission in Wuhan, China. Int J Biol Sci (2020) 16(11):1846–60. doi: 10.7150/ijbs.45018
3. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. New Engl J Med (2020) 383(2):120–8. doi: 10.1056/NEJMoa2015432
4. Rabaan AA, Smajlović S, Tombuloglu H, Ćordić S, Hajdarević A, Kudić N, et al. SARS-CoV-2 infection and multi-organ system damage: A review. Biomolecules Biomedicine (2023) 23(1):37–52. doi: 10.17305/BJBMS.2022.7762
5. Rana R, Tripathi A, Kumar N, Ganguly NK. A comprehensive overview on COVID-19: future perspectives. Front Cell Infection Microbiol (2021) 11. doi: 10.3389/fcimb.2021.744903
6. Wang S, Zuo X, Xu B, Yu Q, An Z, Feng D, et al. Exposure to melamine cyanuric acid in adult mice caused motor activity and skeletal muscle energy metabolism disorder. Physiol Behav (2022) 257:113990. doi: 10.1016/j.physbeh.2022.113990
7. Radman M, Portman MA. Thyroid hormone in the pediatric intensive care unit. J Pediatr Intensive Care (2016) 5(4):154–61. doi: 10.1055/s-0036-1583280
8. Bogojevic M, Bansal V, Pattan V, Singh R, Tekin A, Sharma M, et al. Association of hypothyroidism with outcomes in hospitalized adults with COVID-19: Results from the International SCCM Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry. Clin Endocrinol (2022). doi: 10.1111/cen.14699
9. Burekovic A, Halilovic D, Sahbaz A. Hypothyroidism and subclinical hypothyroidism as a consequence of COVID-19 infection. Med Arch (Sarajevo Bosnia Herzegovina) (2022) 76(1):12–6. doi: 10.5455/medarh.2022.76.12-16
10. Chen M, Zhou W, Xu W. Thyroid function analysis in 50 patients with COVID-19: A retrospective study. Thyroid Off J Am Thyroid Assoc (2021) 31(1):8–11. doi: 10.1089/thy.2020.0363
11. Gao W, Guo W, Guo Y, Shi M, Dong G, Wang G, et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinological Invest (2021) 44(5):1031–40. doi: 10.1007/s40618-020-01460-w
12. Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab (2021) 106(2):e926–e35. doi: 10.1210/clinem/dgaa813
13. Malik J, Malik A, Javaid M, Zahid T, Ishaq U, Shoaib M. Thyroid function analysis in COVID-19: A retrospective study from a single center. PloS One (2021) 16(3):e0249421. doi: 10.1371/journal.pone.0249421
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
15. Bagalà V, Sala A, Trevisan C, Okoye C, Incalzi RA, Monzani F, et al. Clinical presentation and prognosis of COVID-19 in older adults with hypothyroidism: data from the GeroCovid observational study. J Endocrinological Invest (2023) 46(9):1891–9. doi: 10.1007/s40618-023-02048-w
16. Okwor CJ, Meka IA, Akinwande KS, Edem VF, Okwor VC. Assessment of thyroid function of newly diagnosed SARS-CoV-2 infected patients in Nigeria. Pan Afr Med J (2021) 40:9. doi: 10.11604/pamj.2021.40.9.26358
17. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2023). Available at: https://www.ncbi.nlm.nih.gov/books/NBK554776/.
18. Dabas A, Singh H, Goswami B, Kumar K, Dubey A, Jhamb U, et al. Thyroid dysfunction in COVID-19. Indian J Endocrinol Metab (2021) 25(3):198–201. doi: 10.4103/IJEM.IJEM_195_21
19. Dutta A, Jevalikar G, Sharma R, Farooqui KJ, Mahendru S, Dewan A, et al. Low FT3 is an independent marker of disease severity in patients hospitalized for COVID-19. Endocrine Connections (2021) 10(11):1455–62. doi: 10.1530/EC-21-0362
20. Güven M, Gültekin H. The prognostic impact of thyroid disorders on the clinical severity of COVID-19: Results of single-centre pandemic hospital. Int J Clin Pract (2021) 75(6):e14129. doi: 10.1111/ijcp.14129
21. Razu MH, Hossain MI, Ahmed ZB, Bhowmik M, Hasan MKE, Kibria MK, et al. Study of thyroid function among COVID-19-affected and non-affected people during pre and post-vaccination. BMC Endocrine Disord (2022) 22(1):309. doi: 10.1186/s12902-022-01187-0
22. Tsivgoulis G, Fragkou PC, Karofylakis E, Paneta M, Papathanasiou K, Palaiodimou L, et al. Hypothyroidism is associated with prolonged COVID-19-induced anosmia: a case-control study. J Neurology Neurosurgery Psychiatry (2021) 92(8): 911–2. doi: 10.1136/jnnp-2021-326587
23. Van Gerwen M, Alsen M, Little C, Barlow J, Naymagon L, Tremblay D, et al. Outcomes of patients with hypothyroidism and COVID-19: a retrospective cohort study. Front Endocrinol (2020) 11:565. doi: 10.3389/fendo.2020.00565
24. Xiong X, Wong CKH, Au ICH, Lai FTT, Li X, Wan EYF, et al. Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: a population-based cohort study. Thyroid Off J Am Thyroid Assoc (2022) 32(5):505–14. doi: 10.1089/thy.2021.0684
25. Chen W, Tian Y, Li Z, Zhu J, Wei T, Lei J. Potential interaction between SARS-CoV-2 and thyroid: a review. Endocrinology (2021) 162(3):bqab004. doi: 10.1210/endocr/bqab004
26. Lee KW, Shin Y, Lee S, et al. Inherited disorders of thyroid hormone metabolism defect caused by the dysregulation of selenoprotein expression. Front Endocrinol (2021) 12:803024. doi: 10.3389/fendo.2021.803024
27. Vahabi M, Ghazanfari T, Sepehrnia S. Molecular mimicry, hyperactive immune system, and SARS-COV-2 are three prerequisites of the autoimmune disease triangle following COVID-19 infection. Int Immunopharmacol (2022) 112:109183. doi: 10.1016/j.intimp.2022.109183
28. Chen W, Lei J, Li Z. Thyroid function changes and COVID-19 severity: egg or chicken? Endocrine (2022) 78(3):436–40. doi: 10.1007/s12020-022-03129-1
29. Xu J, Li Y, Xia Q, Shi Q. Association between thyroid disease and severe coronavirus disease 2019 (COVID-19) infection: a meta-analysis. Iranian J Public Health (2021) 50(8):1517–25. doi: 10.18502/ijph.v50i8.6797
Keywords: SARS-CoV-2, COVID-19, hypothyroidism, subclinical hypothyroidism, meta-analysis
Citation: Wei J and Zhang F (2023) Effects of SARS-CoV-2 infection on hypothyroidism and subclinical hypothyroidism: a meta-analysis. Front. Endocrinol. 14:1291774. doi: 10.3389/fendo.2023.1291774
Received: 10 September 2023; Accepted: 20 November 2023;
Published: 04 December 2023.
Edited by:
Alessandro Antonelli, University of Pisa, ItalyReviewed by:
Mohammad Injamul Hoq, University of Creative Technology Chittagong, BangladeshJoanne Rovet, University of Toronto, Canada
Copyright © 2023 Wei and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenghua Zhang, enB5eXpmaEAxNjMuY29t
 Jiaqi Wei
Jiaqi Wei Fenghua Zhang2*
Fenghua Zhang2*