- Department of Endemic Diseases, Fujian Center for Disease Control and Prevention, Fuzhou, China
Background: Iodine deficiency is a major public health problem in pregnant women. Serum iodine (SI) may represent a useful biomarker for iodine nutrition evaluation. We aimed to assess the relationship between serum iodine concentration (SIC) and urinary iodine concentration (UIC), dietary iodine, thyroid function, and thyroid diseases in pregnant women in the southeast coast of China, and to provide a normal reference range of SIC for pregnant women.
Methods: A multistage random sampling method was used to select the study population. We collected urine and blood samples from pregnant women and determined UIC and SIC as well as thyroid function using Arsenic-Cerium Catalytic Spectrophotometry, inductively coupled plasma mass spectrometry, and Beckman Coulter Access2 chemiluminescent immunoanalyzer and kit, respectively, and administered a questionnaire on dietary iodine intake in pregnant women.
Results: There was a significant negative correlation between SI and thyroid-stimulating hormone (TSH) (r = −0.141) and a significant positive correlation between SI and free triiodothyronine (FT3) (r = 0.106), free thyroxine (FT4) (r = 0.236), triiodothyronine (TT3) (r = 0.229), total thyroxine (TT4) (r = 0.433), and dietary iodine intake (r = 0.068). There was a significant difference in SI levels of pregnancy between the second (78.13 μg/L) and third trimester (75.37 μg/L) (p = 0.018). SI levels between inadequate intake (74.58 μg/L) and appropriate intake (77.92 μg/L) groups were statistically different (p = 0.036). Low SIC was a risk factor for the development of hypothyroxinemia (adjusted OR = 3.14, 95% confidence interval: 1.75–5.66). The reference range for SIC in normal pregnant women is 45.03–112.44 μg/L.
Conclusion: SI may be a composite indicator of iodine nutritional status and thyroid function.
1 Introduction
Iodine is one of the essential trace elements in the human body and is the main component in the synthesis of thyroid hormones. Deficiency or excess of iodine can be harmful to human health (1). Nearly 2 billion people worldwide still suffer from inadequate iodine intake (2). China used to have a high prevalence of iodine deficiency disorders (IDDs) (3).
Iodine deficiency, especially in pregnant women, is a major public health problem (4, 5). In the 1990s, China adopted universal salt iodization (USI) as the national strategy (6). After years of mandatory USI, China has virtually eliminated IDDs (7). Therefore, public health concern has shifted toward mild iodine deficiency (median urinary iodine concentration [UIC] was 50–99 μg/L in adults) to moderate iodine deficiency (median UIC was 20–49 μg/L in adults), which remains prevalent in many regions (1, 8, 9), especially among pregnant women. Owing to their special physiological status, pregnant women have a 50% higher iodine requirement than non-pregnant adults and are at increased risk of iodine deficiency (10). IDDs in pregnant women lead to increased miscarriage and infant mortality (11). Adequate dietary iodine intake is therefore particularly important for women during pregnancy, and the need for iodine nutritional assessment of pregnant women is even more urgent.
Because of widespread iodine deficiency or some cases of iodine overexposure, iodine biomonitoring is important, but there is no established biomarker for individual iodine status (12). Considering that serum iodine (SI) levels exhibited much less individual variation (13) and it is the biomonitoring approach being closest to the bioavailable I− supply for the thyroid gland (14), SI may, therefore, represent a better and important biomarker for iodine nutrition evaluation (13). SI has been reported to be valuable in the diagnosis of thyroid diseases (15). Therefore, the timely detection of abnormal SI levels may predict the risk of clinical or subclinical thyroid diseases. However, there are fewer studies on the relationship between SI and dietary iodine, thyroid function, and disease in pregnant women. There are no standards for reference ranges for normal human SI in China; only some reference ranges have been proposed by renowned foreign laboratories Mayo Clinic, Quest Diagnostics, and World Health Organization (WHO), and they do not differentiate between different groups of people, so there is still a lack of uniform standards for normal reference ranges in China (16).
Fujian Province is on the southeast coast of China, and previous studies have shown that coastal pregnant women are still mildly deficient in iodine nutrition (17). This study aims to evaluate the relationship between SI and urinary iodine (UI), dietary iodine intake, thyroid function, and thyroid diseases in pregnant women, and to provide a normal reference range for SI in pregnant women in the southeast coastal region. The study will provide more evidence for the evaluation of iodine nutrition status and iodine metabolism processes in pregnant women, and provide further scientific reference for the current USI policy in China.
2 Subjects and methods
2.1 Study population
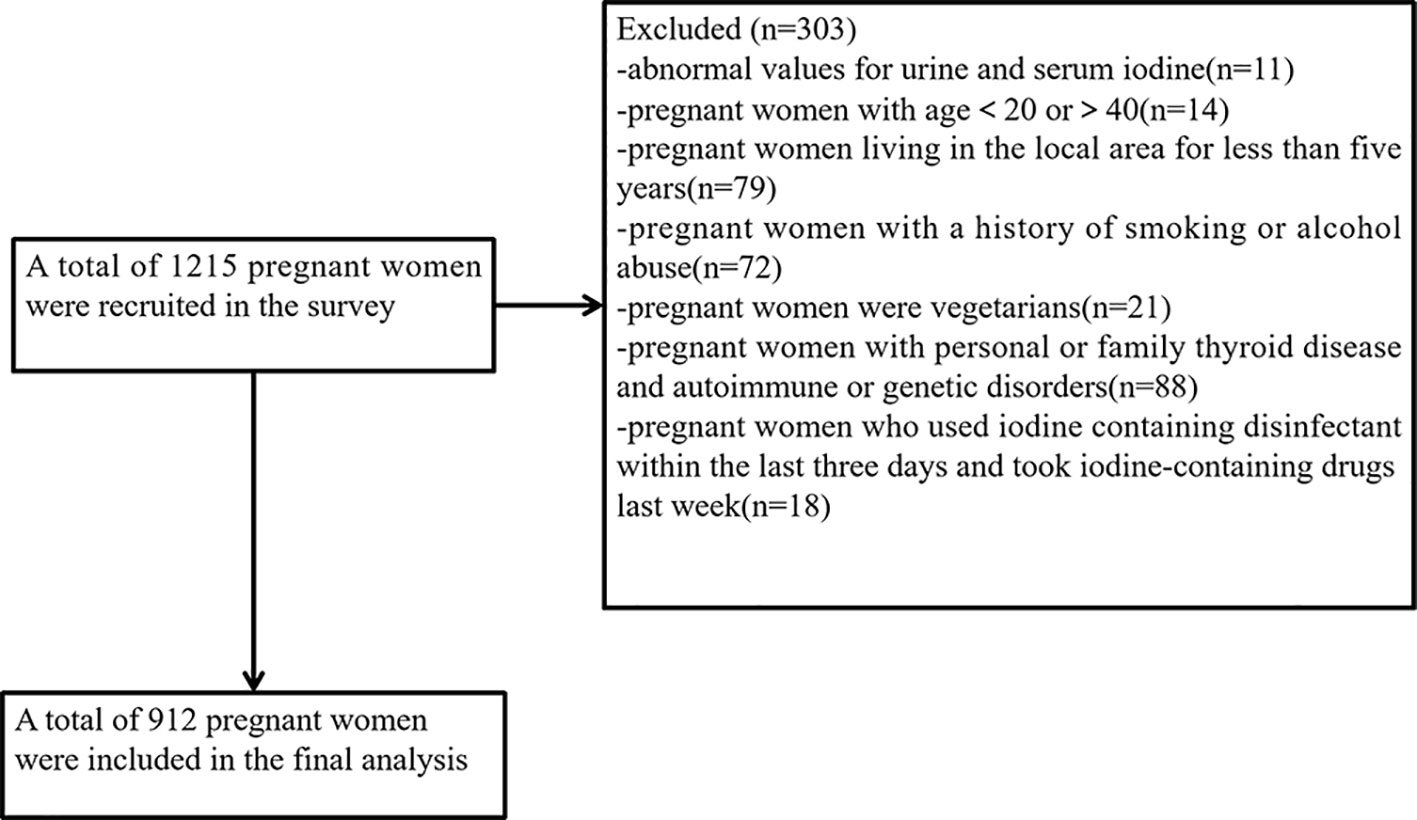
This cross-sectional study employed the multistage cluster sampling method in 2020. Data collection was conducted from 1 June 2020 to 30 September 2020. The selection of survey sites was described in detail in our previous study (17). One urban and one rural survey site were selected from each of the nine administrative divisions in Fujian Province, making a total of 18 survey sites. In each monitoring county, five towns were randomly selected from five different directions (east, west, south, north, and west). A certain number of pregnant women were selected from each township (insufficient numbers could be filled in neighboring towns). All subjects were asked to complete questionnaires that included demographic information (e.g., name, age, gestational week, height, weight, etc.), medical history (e.g., endocrine system diseases), smoking, alcohol abuse, and family history of thyroid and other endocrine disorders. The participants from the villages were recruited, and the inclusion criteria were as follows: (1) previously healthy, no history of thyroid diseases, no history of autoimmune disease, no history of endocrine disease, no family genetic disorders, etc.; (2) age between 20 and 40 years old and have lived in the local area for 5 years or more; and (3) no special dietary habits, e.g., vegetarian diet. The exclusion criteria were as follows: (1) those with a history of smoking or alcohol abuse; (2) those who have used iodine-containing lotions in the last 3 days, those who have taken cydiodine tablets, amiodarone hydrochloride, or those who have had recent imaging tests. A total of 1,215 pregnant women were surveyed in this study. Based on the inclusion and exclusion criteria and the exclusion of 9 abnormal values for urine samples and 2 abnormal values for blood samples, a total of 912 pregnant women were eventually included in the statistical analysis. The flowchart is presented in Figure 1.
2.2 Sample collection
No less than 5 mL of fasting spot-urine sample, 5 mL of fasting venous blood, and 50 g of edible salt were collected from each participant in the morning (between 8:00 and 11:00) to test iodine levels and thyroid function without determining creatinine. Urine samples from all subjects were kept in clean plastic tubes at 4°C. Blood samples are stored at a low temperature (−20°C). Salt samples should be sealed in plastic bags and protected from sunlight. Urine and salt samples should not be mixed. Water samples were collected on a township basis and stored in iodine-free treated polyethylene plastic bottles at 4°C. All samples collected will be sent to the Centre for Disease Control and Prevention for testing. Dietary surveys and biological sample collection were carried out simultaneously. Food Frequency Questionnaire (FFQ) was used to investigate the dietary iodine intake of pregnant women. We evaluated the iodine concentration in food with reference to the China Food Composition Tables (Standard Edition 6th edition) (18). We selected foods whose iodine content is more than 10 μg/100 g, and dietary iodine contribution is more than 1% (19). Food items surveyed included staple food groups, soya bean products, meat, eggs, dairy products, aquatic products, fruits and vegetables, offal, snacks, nuts, beverages, and condiments. The food frequency (times) was divided into ≥1 time/day, 1–6 times/week, 1–3 times/month, 1–5 times/half-year, and not eat (19). With reference to the color charts of foods in China Food Composition Tables (18), the professional investigators asked the participants about the frequency and food consumption. Drinking water intake was recorded. Dietary iodine intake (μg/day) = Σ (intake of each type of food × iodine content of each type of food) + amount of drinking water × iodine content of water + amount of iodinated vitamins × iodine content of vitamins + salt intake × salt iodine content × (1–20%), 20% being the cooking loss rate of iodized salt as defined by WHO (20).
2.3 Determination methods
The samples were processed in the laboratory of Fujian Provincial Center for Disease Control and Prevention and their UIC was determined by Arsenic-Cerium Catalytic Spectrophotometry (21), salt samples were tested by the general test method of the salt industry (22), water iodine was detected by the method recommended by the National Reference Laboratory of Iodine Deficiency Disorders (23), and serum iodine concentration (SIC) was detected by inductively coupled plasma mass spectrometry (24). A Beckman Coulter Access2 chemiluminescent immunoassay analyzer and kit were used to measure eight indicators of serum thyroid function: thyroid-stimulating hormone (TSH), thyroglobulin (Tg), free triiodothyronine (FT3), free thyroxine (FT4), total triiodothyronine (TT3), total thyroxine (TT4), thyroid peroxidase antibody (TPOAb), and thyroglobulin antibody (TGAb). Specially trained technicians performed thyroid ultrasonography. We used GE LOGIQ BOOK XP manufactured by General Electric Medical Systems (China) with a 7.5-MHz probe to measure the thyroid volume, nodule diameter. The thyroid volume was calculated according to 0.479 × (left lobe: length ×width × thickness + right lobe: length × width × thickness) (length, width, and thickness in the equation used the unit of cm, and thyroid volume used the unit of mL).
2.4 Chemicals and instrumentation
Principles, instruments, and chemicals for iodine measurement are described detailly in the Supplementary File entitled “Chemicals and instrumentation for iodine measurement”. Iodine measurements of all samples were performed at Fujian Province Center for Disease Control and Prevention and met the quality control requirements of the National Reference Laboratory for Iodine Deficiency Disorders. We use nationally certified reference Standard Substances for quality control. Only when all measured values of the reference substance are controlled can the test results be accepted. The standard curve’s correlation coefficient had to be greater than 0.999 (25).
2.5 Diagnostic criteria
The gestational week was determined based on the time of the last menstruation (<13 weeks was defined as the first trimester (T1), 13–27 weeks was the second trimester (T2), and ≥28 weeks was the third trimester (T3) (26). UI in pregnant women <150 µg/L is considered insufficient, 150–249 µg/L is considered adequate, 250–499 µg/L is considered above requirements, and ≥500 µg/L is considered excessive (1). According to the Reference Intake of Dietary Nutrients for China Residents (27), iodine intake less than 160 μg/day is defined as insufficient iodine intake, 160–600 μg/day is defined as appropriate iodine intake, and more than 600 μg/day is defined as excessive iodine intake. The recommended iodine intake for pregnant women is 230 μg/day. TSH and FT4 were categorized according to gestation period (T1–T3). The normal reference ranges (with 95% confidence interval [CI]) for the test kits (Beckman) used for TSH (in mIU/L) and FT4 (in pmol/L) according to trimester (T1, T2, and T3) were as follows: 0.03–4.00, 0.35–3.86, and 0.46–4.82 mIU/L for TSH and 9.54–16.09, 7.33–12.07, and 6.40–11.21 pmol/L for FT4, respectively. Thyroid diseases were diagnosed according to the Guidelines for the Diagnosis and Management of Thyroid Disease during Pregnancy and the Postpartum (28). Diagnostic criteria for thyroid disorders in pregnant women are summarized in Supplementary Table 1. TPOAb was considered positive with values >9 IU/mL and TGAb with values >4 IU/mL by the test kits (Beckman). Thyroid ultrasonography was performed according to Chinese health standards and the normal female thyroid volume is ≤18 mL (29) and thyroid nodule is one or more nodule (>5 mm) without goiter (30).
2.6 Statistical analysis
WPS (Beijing and Zhuhai Kingsoft Software Company) and SPSS version 24 (IBM, Armonk, NY, USA) were used to collate and analyze the data. The Kolmogorov–Smirnov test was used to test whether the data conformed to a normal distribution. Non-normally distributed data were reported as median (M) and interquartile range (IQR, P25–P75). We calculate P2.5 to P97.5 as the 95% medical reference range for the non-normal data. The correlation between the non-normally distributed two sets of variables was analyzed using Spearman’s correlation. We used Curve Estimation fitting model to test a trend between the two sets of data and chose the most appropriate model based on the R2. The Mann–Whitney U test was used for comparison between two non-normal data sets, and the Kruskal–Wallis H test was used for comparison between multiple non-normal data sets. Normally distributed data were expressed as mean ± standard deviation (SD), and 95% medical reference range of information was calculated using mean ±1.96×SD. Analyses of variance (ANOVAs) were used for comparisons between multiple groups of normal data, and LSD tests were used for two-way comparisons. Maternal SIC less than the 10th percentile is defined as low SIC and that greater than the 90th percentile is defined as high SIC (31). We used receiver operating characteristic (ROC) curves to determine the diagnostic value of SIC and UIC. The closer the area under the ROC curve is to 1, the higher the diagnostic value. Set p < 0.05 as statistically significant.
2.7 Ethics approval and consent to participate
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Medical Ethics Committee of Fujian Provincial Center for Disease Control and Prevention (No. 2020032). Written informed consent was obtained from all subjects/patients.
3 Results
3.1 Description of the basic characteristics
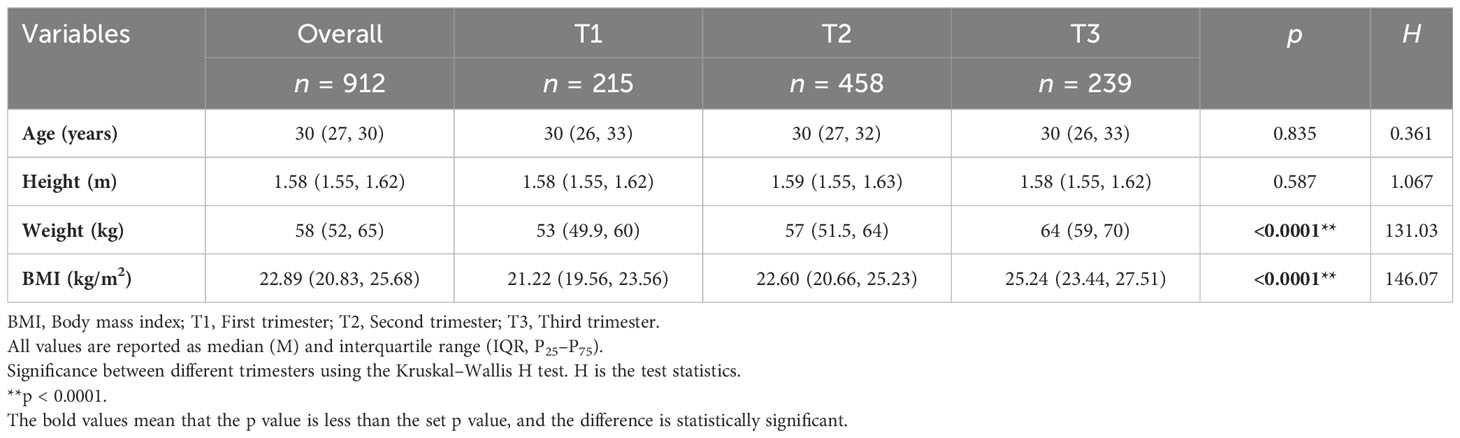
Basic characteristics of pregnant women at different trimesters are shown in Table 1. This included a total of 215 (23.6%) women in the first trimester, 458 (50.2%) in the second trimester, and 239 (26.2%) in the third trimester. After testing for normality, age, height, weight, and BMI were non-normally distributed and the results were expressed as medians and quartiles; the Kruskal–Wallis H test was used for comparison between groups. Differences in age and height between trimester groups were not statistically significant. Weight and BMI were highest in the third trimester. There were significant differences in body weight and BMI between trimester groups (p < 0.0001).
3.2 Iodine nutrition index
SIC, UIC, and iodine intake in pregnant women at different trimesters are provided in Table 2. After the normality test, UIC, SIC, and iodine intake showed non-normal distribution, the results were expressed by median and quartile, and the Kruskal–Wallis H test was used for comparison among groups. The difference in UIC between trimester groups was not statistically significant (H = 1.299, p = 0.522). The median value of SIC in pregnant women was the lowest in the third trimester and the highest in the second trimester. Median UIC of pregnant women in all trimesters was less than 150 μg/L, which can be classified as iodine insufficiency according to the WHO criteria. We found a significant difference in SI levels of pregnancy between the second (78.13 μg/L) and third trimester (75.37 μg/L) (p = 0.018). Iodine intake during pregnancy is less than the recommended iodine intake of 230 μg/day. There was no significant difference in iodine intake between trimester groups (H = 0.017, p = 0.992).
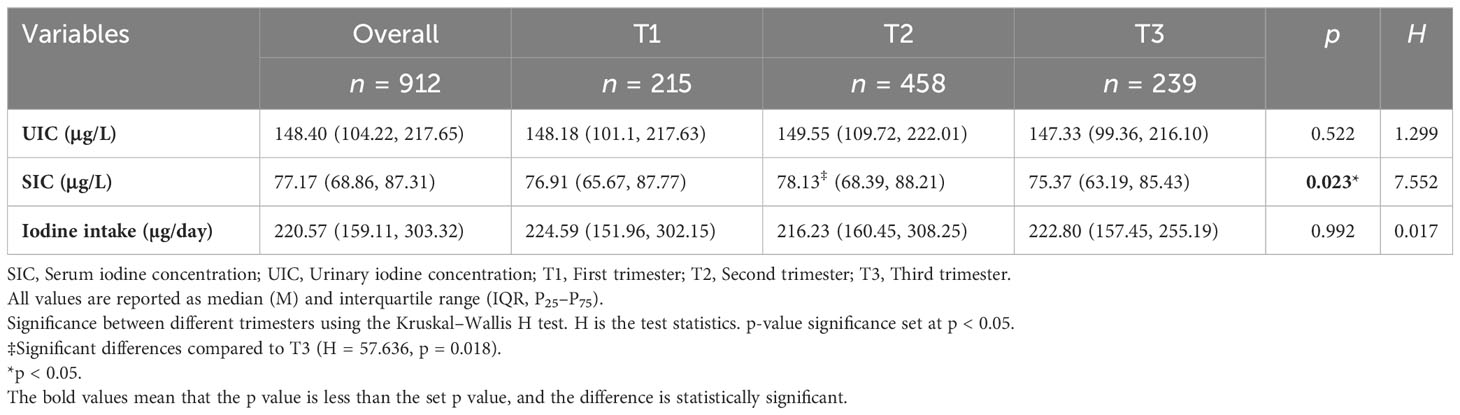
Table 2 Serum iodine concentration (SIC), urinary iodine concentration (UIC), and iodine intake in pregnant women at different trimesters.
3.3 Correlation analysis of factors influencing SI
The correlation between maternal SIC and basic characteristics, UIC, thyroid function, and iodine nutrition variables is shown in Supplementary Table 2. As shown in Figure 2, SIC was nonlinearly correlated with TSH, FT3, FT4, TT3, TT4, and dietary iodine intake. The SIC was statistically significantly correlated with TSH (r = −0.141, p < 0.001), FT3 (r = 0.106, p = 0.001), FT4 (r = 0.236, p < 0.001), TT3 (r = 0.229, p < 0.001), TT4 (r = 0.433, p < 0.001), and dietary iodine intake (r = 0.068, p = 0.041). SIC showed strong positive correlations with TT4. In contrast, SIC was not significantly correlated with UIC.
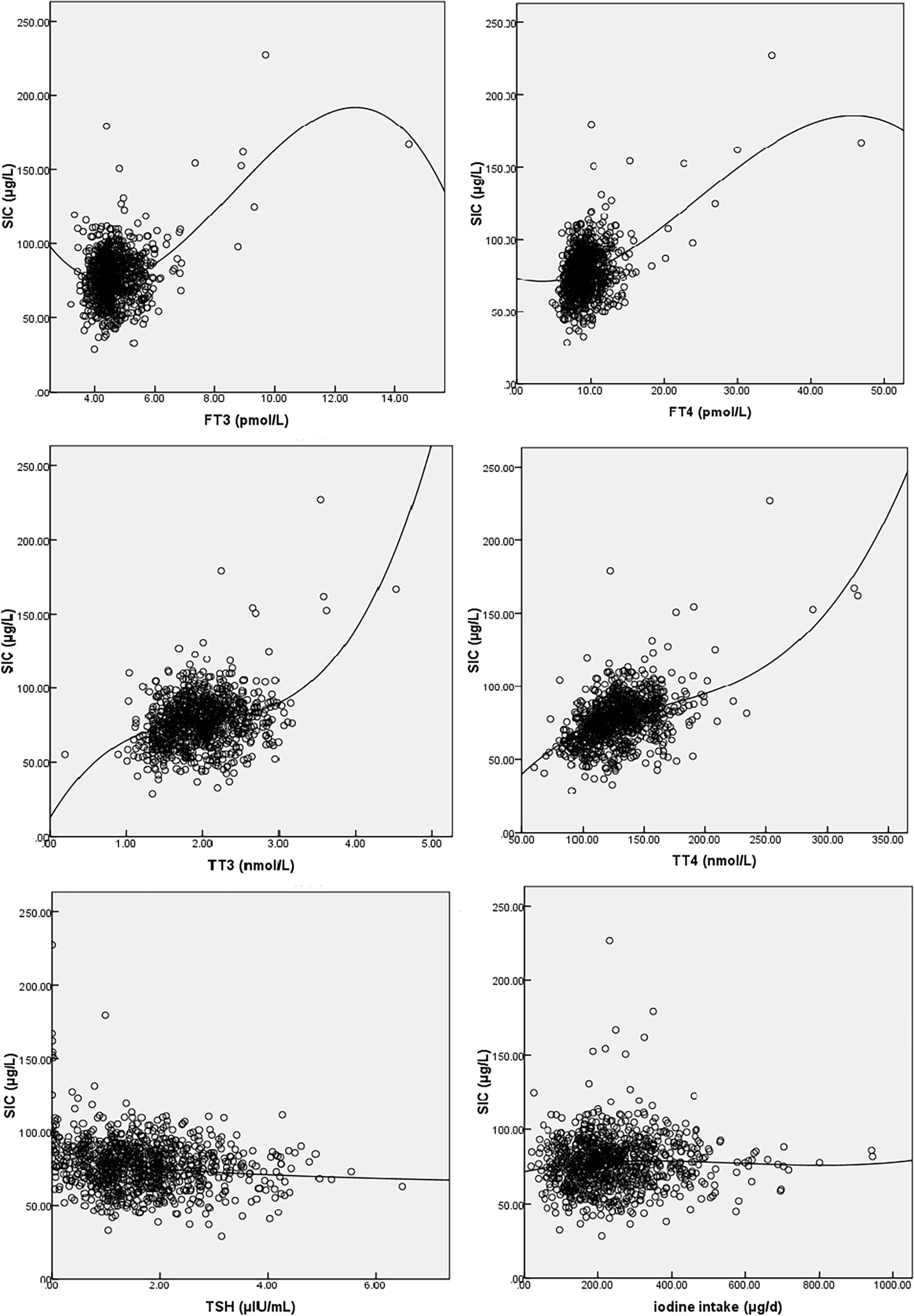
Figure 2 Fitted graphs for serum iodine (SI) and free triiodothyronine (FT3), free thyroxine (FT4), triiodothyronine (TT3), total thyroxine (TT4), thyroid-stimulating hormone (TSH), and iodine intake. The line represents regression trend. SI, serum iodine; FT3, free triiodothyronine; FT4, free thyroxine; TT3, triiodothyronine; TT4, total thyroxine; TSH, thyroid-stimulating hormone.
3.4 Relationship between SI and iodine nutritional status
Characteristics of SIC in pregnant women with different iodine nutritional status in different trimesters are shown in Table 3. For all pregnant women surveyed, the median UIC was calculated on a district basis to determine the iodine nutrition level of pregnant women in that area. In 10 districts (counties), the median UIC of pregnant women was <150 μg/L, and they belonged to iodine-deficient areas; in 8 districts (counties), the median UI level of pregnant women was 150–249 μg/L, and they belonged to iodine-suitable areas. There was no statistical difference between groups (Z = −1.608, p = 0.108).
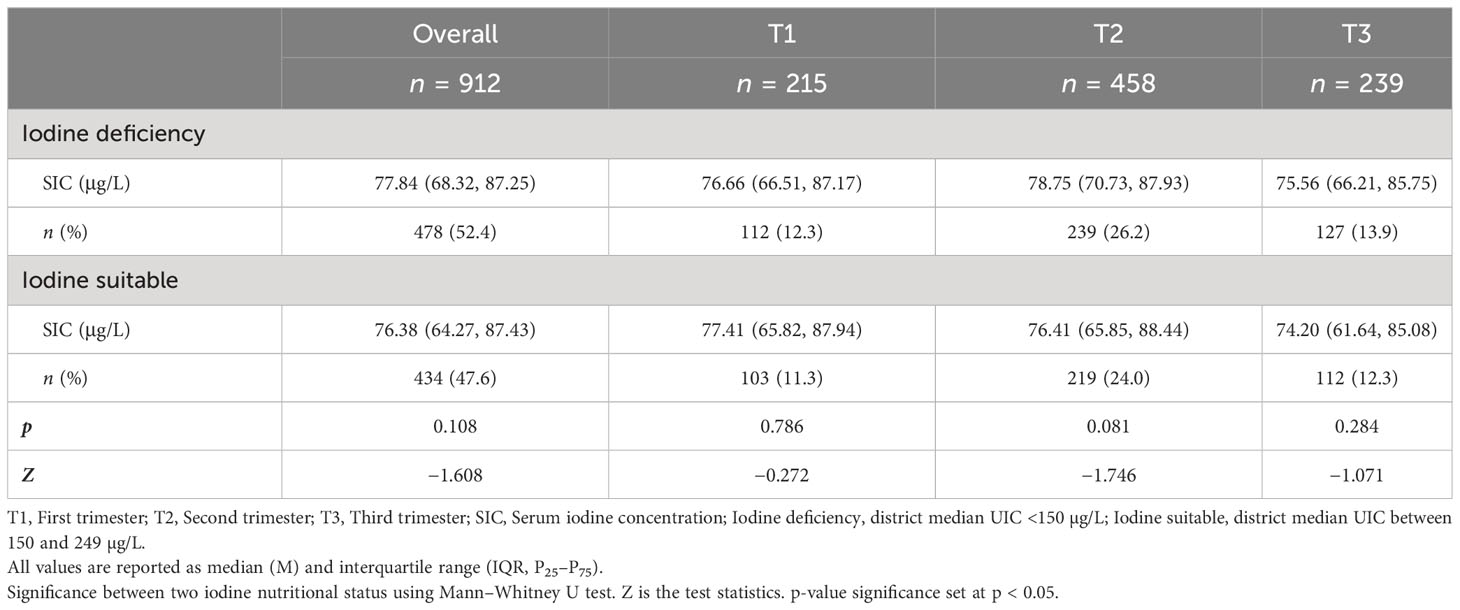
Table 3 Characteristics of SIC in pregnant women with different biological iodine nutritional status in different trimesters.
3.5 Relationship between SI and iodine intake
Characteristics of maternal SIC at different levels of iodine intake in different trimesters are shown in Table 4. The highest proportion of pregnant women had appropriate iodine intake (72.59%), followed by iodine inadequate intake (25.66%). The results showed that there was a significant difference in SIC between inadequate intake (74.58 μg/L) and appropriate intake (77.92 μg/L) groups in pregnant women (p = 0.036).
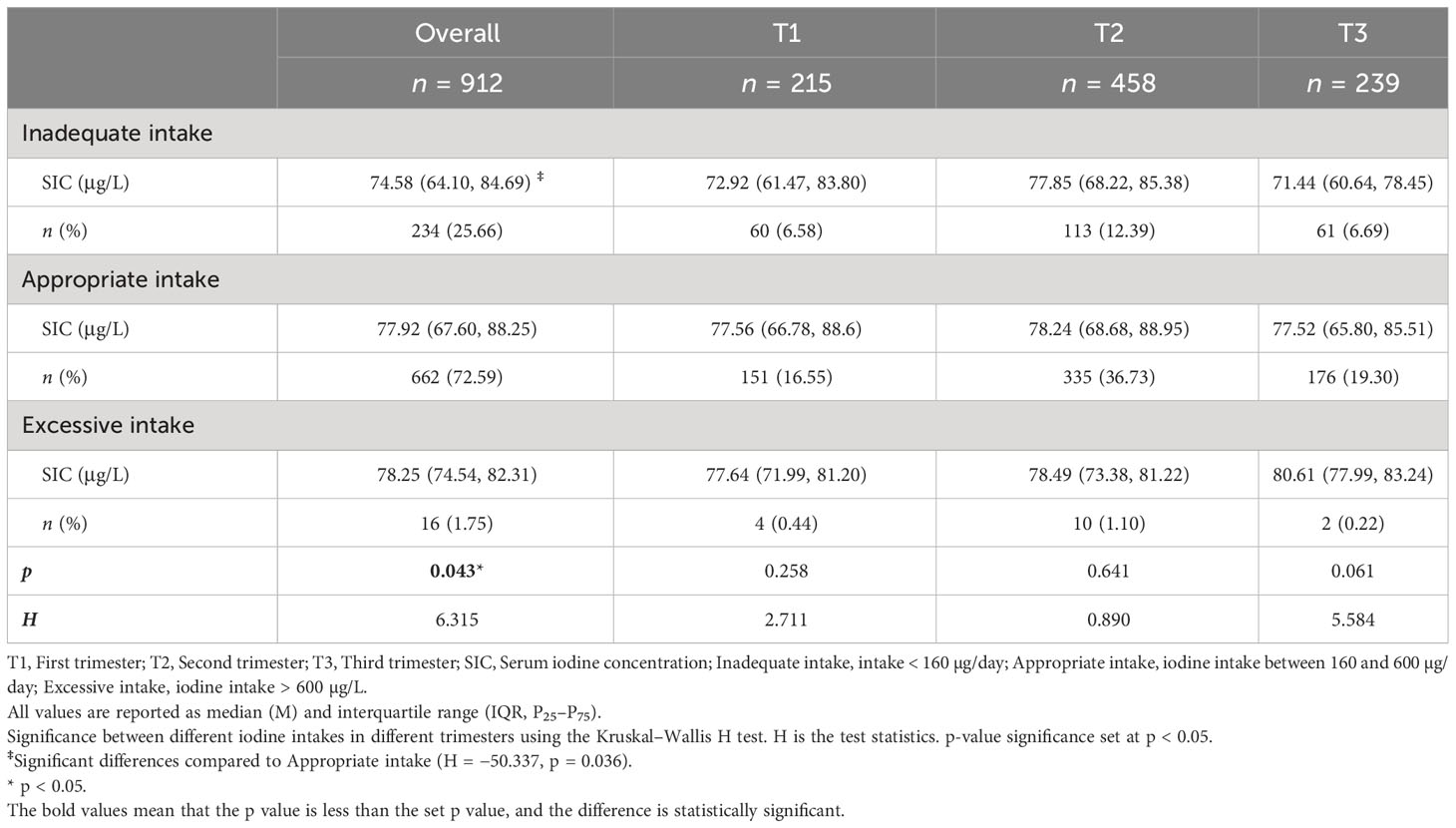
Table 4 Characteristics of maternal SIC at different levels of iodine intake in different trimesters.
3.6 Analysis of low or high SIC as a risk factor for thyroid diseases
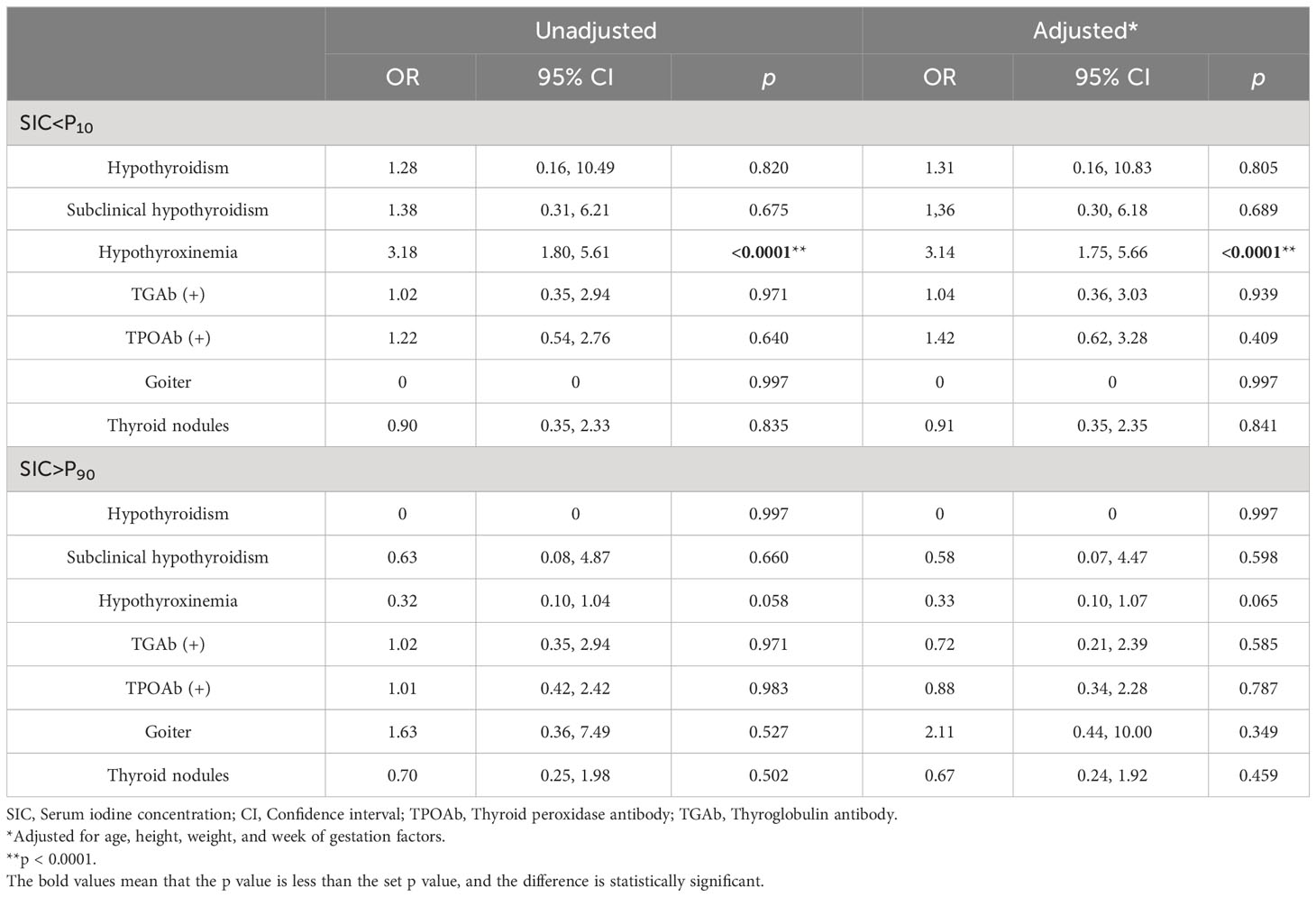
The number of pregnant women with thyroid diseases at different gestation periods is shown in Supplementary Table 3, which shows the prevalence of thyroid disorders in pregnant women during different trimesters. Analyses of SIC <10% or >90% as a risk factor for thyroid diseases are shown in Table 5. When total SIC <10% or >90% was analyzed as risk factors for thyroid diseases, the 10th percentile of SI in pregnant women was 56.59 μg/L and the 90th percentile was 96.52 μg/L. Logistic regression results showed that low SIC was associated with hypothyroxinemia. OR for hypothyroxinemia is 3.18 (95% CI: 1.80–5.61) when SIC was less than the 10th percentile of the study sample. After adjusting for age, height, weight,and gestational factors, the OR remained statistically significant (adjusted OR = 3.14, p = 0.000 < 0.0001).
3.7 Comparison of the diagnostic value of SI and UI
ROC curves for the diagnosis of hypothyroxinemia with SI and UI are shown in Figure 3. The area under the ROC curve for the diagnosis of hypothyroxinemia using serum and urine iodine was 0.66 and 0.57, respectively. In the diagnosis of thyroid diseases, the diagnostic value of SI was significant (p < 0.0001). SI had a higher diagnostic value than urine iodine (Z = 2.110, p = 0.035).

Figure 3 Receiver operating characteristic (ROC) curves for the diagnosis of hypothyroxinemia with serum and urinary iodine. CI, confidence interval; UIC, urinary iodine concentration; SIC, serum iodine concentration. The area under the ROC curve for SI was 0.66 (95% CI: 0.60–0.72, p < 0.0001) and UI was 0.57 (95% CI: 0.50–0.63, p = 0.053); SI had a higher diagnostic value than UI (Z = 2.110, p = 0.035).
3.8 Establishment of reference range of SIC
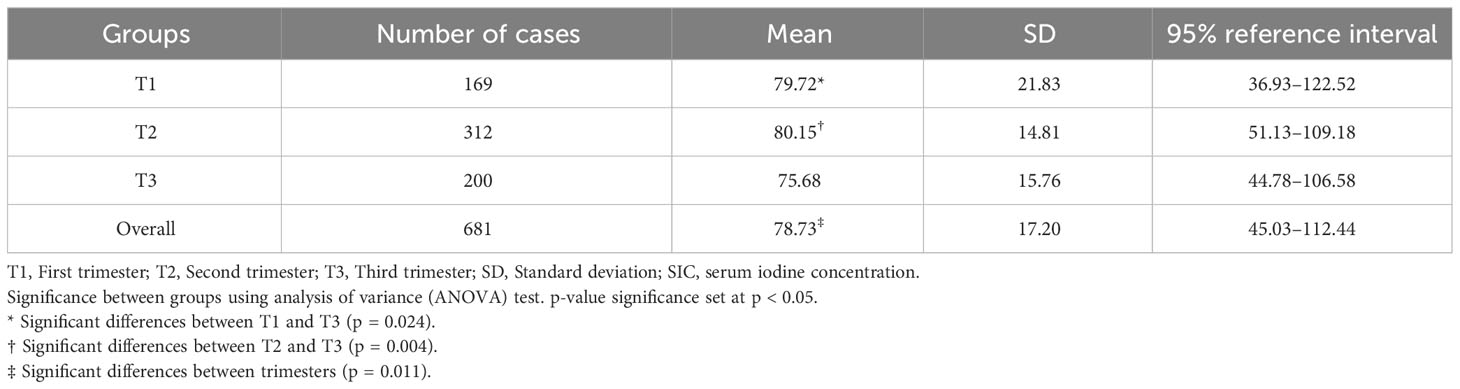
Characteristics of SIC in normal pregnant women by trimester are shown in Table 6. After excluding thyroid diseases, thyroid nodule, and goiter, 681 normal pregnant women were identified. The Kolmogorov–Smirnov test showed that the SI data were normally distributed (Z = 1.28, p = 0.074), so the 95% reference interval was determined using the normal distribution method. The reference range for SI in normal pregnant women is 45.03–112.44 µg/L. SI ranges from 36.93 to 122.52 µg/L in early pregnancy, 51.13–109.18 µg/L in mid-pregnancy, and 44.78–106.58 µg/L in late pregnancy. Comparisons between SI at different trimesters were analyzed by ANOVA. The mean SIC of pregnant women in the first (79.72 µg/L), second (80.15 µg/L), and third (75.68 µg/L) trimester was statistically different (F = 4.54, p = 0.011). The differences in SI between early and late pregnancy and between mid- and late pregnancy were statistically significant.
4 Discussion
Indicators and methods for assessing the iodine nutritional status of individuals are a controversial issue. The most commonly used tool is the UIC, which is influenced by a number of factors and has inconsistent results. Some studies have measured UIC by collecting 24-h urine samples to improve the accuracy of iodine nutrition assessment, but these methods are not applicable to large-scale epidemiological surveys (12). In addition, some studies have attempted to mitigate the effect of urine output on UIC by adjusting for urinary creatinine concentration (5, 32). However, factors such as age, ethnicity, skeletal muscle content, and protein intake can affect urinary creatinine concentration (33, 34). Therefore, the use of creatinine-adjusted UIC to assess individual iodine nutritional status remains highly controversial. In this context, the search for new individual iodine nutritional markers is crucial to accurately assess the iodine nutritional status of pregnant women and may provide a new scientific basis for personalized iodine supplementation in high-risk groups.
SI, as an important link of iodine metabolism in the body, is affected by iodine intake on the one hand, and on the other hand, thyroid dysfunction and thyroid disorders may be associated with SIC abnormalities. In this study, the applicability of SI as an indicator of individual iodine nutritional status was investigated from these two perspectives, which provides a basis for further evaluation of individual iodine nutritional status. In this study, pregnant women were found to be mildly deficient in iodine. There is a correlation between maternal SIC, thyroid function, and dietary iodine. When SICs were low, the percentage of hypothyroxinemia increased. Reference ranges during normal pregnancies were derived. SI appeared to be an indicator of a combination of iodine nutritional status and thyroid function.
In this study, the median UI of pregnant women was 148.40 μg/L < 150 μg/L, indicating mild iodine deficiency in pregnant women in Fujian Province, which was similar to our previous study and the results in coastal China (3, 17, 35, 36). Similar to provinces like Fujian, pregnant women in some developed countries also face iodine deficiency, such as those in Austria, Norway, and Sweden (37, 38). The causes of iodine deficiency in pregnant women are as follows: (1) a 50% increase in thyroid hormone secretion during pregnancy (10); (2) preferential administration of fetal iodine for fetal neurological development (39); (3) increased renal clearance of iodine during pregnancy (39); and (4) increased activity of the T3 deiodinase (39). In addition, vomiting during pregnancy may partially contribute to iodine loss. In addition, some pregnant women have opted for a low-salt diet to prevent edema and gestational hypertension (17). The UI of pregnant women in this study did not vary significantly, in contrast with the downward trend of SI. SIC was the lowest in the third trimester of pregnancy and lower than in the second trimester, which may be due to the continuous transport of large amounts of iodine ions from the pregnant woman to the fetus during pregnancy (31). The trend of changes in UIC in pregnant women is controversial: some studies suggest a decrease with increasing gestational weeks (40), some reports suggest an increase (41), and others report no change throughout pregnancy (42). The UIC of pregnant women in different trimesters did not vary significantly while SIC was significant, which match those observed in another study (36). Foreign studies have shown that iodine supplementation during pregnancy can prevent UIC from decreasing during pregnancy (43). Dietary iodine intake of pregnant women in this study was lower than the recommended intake (RNI, 230 μg/day), which is similar to the results of previous studies (19). This may be due to the fact that vomiting reaction affects the appetite of pregnant women. For pregnant women with mild iodine deficiency, iodine supplements are recommended. Iodine nutrition monitoring during pregnancy should be emphasized and iodine supplement is recommended according to local conditions.
This study showed that SI was positively correlated with TT3, TT4, and FT4, similar to the results of Songlin Yu et al. (16, 36), which can explain that SI is closely related to thyroid bioavailable iodine (12–15). SI is negatively correlated with TSH, the most sensitive indicator of abnormal thyroid function (44), indicating that SI may be a good biomarker for evaluating thyroid function. The increase in TSH is due to depletion of iodine stores in the thyroid, which may lead to hypothyroidism and elevated TSH levels (31, 45). Elevated TSH due to decreased SI has been reported to be associated with an increased risk of pre-term birth, placental abruption, fetal death, and neurodevelopmental impairment (46). SIC was associated with serum FT4 levels, but not associated with UIC. In this study, mean SIC in normal pregnant women increased in the second trimester of pregnancy, and then decreased in the third trimester of pregnancy. The difference between the SIC in normal pregnant women in the second trimester and the third trimester of pregnancy was statistically significant, which was similar to the results reported by Li et al. who reported that the SIC of pregnant women increased continuously from the 8th week of pregnancy to the 20th week of pregnancy and then decreased (5). This result also supports the speculation that the elevated SIC in pregnant women may be due to increased synthesis and secretion of thyroid hormones.
There is no significant correlation between SI and UI in our study, which is inconsistent with some research results (31, 47). The reason may be that the one-time UI in our study is not a relevant indicator of individual iodine nutritional status during pregnancy. This may be due to the fact that there are many factors affecting UIC in pregnant women such as water intake and sampling location, which may lead to individual UIC levels fluctuating greatly. For example, a Danish study found significant differences in UIC in samples from the same group of pregnant women collected at different locations (48). Another explanation is the modification of glomerular filtration and the ureter dilatation during pregnancy (5). In this study, there was a nonlinear relationship between SI and thyroid function, which was consistent with the study of Jin et al. (47). The study also found that SI was positively correlated with dietary iodine intake. Median SIC increased from the group with insufficient iodine intake to the group with excessive iodine intake, and the SIC among different dietary iodine intake groups was statistically significant. This suggests that SIC is useful in determining dietary intake. Inappropriate iodine intake may lead to abnormal SIC, which can affect thyroid function (36). The results above suggest that SIC is more useful and reliable in assessing the iodine nutritional status of individuals compared to UIC.
Previous studies have shown that thyroid disease can be caused by inadequate or excessive iodine intake (49), while few national and international epidemiological studies explored the effect of different SIC levels on thyroid diseases. Our study found that low SIC was associated with an increased percentage of hypothyroxinemia. Iodine deficiency can lead to maternal hypothyroxinemia (50, 51). Low SICs may be caused by insufficient iodine intake, which, in turn, leads to insufficient synthesis and secretion of thyroid hormones and ultimately lower serum thyroid hormone concentrations, which is in agreement with recent findings (31, 36). These results highlight the potential use of SIC as a biomarker of iodine nutritional status in individual pregnant women. Further studies are needed to confirm the relationship between SI and abnormal thyroid function, and more data are needed to support the interaction between SI and iodine nutritional status and thyroid hormone levels in women during pregnancy.
To further compare the diagnostic value of SIC and UIC for thyroid diseases, we analyzed the area under the curve (AUC) for SIC and UIC. The closer the area under the ROC curve is to 1, the higher the diagnostic value. We found that SIC has a good diagnostic value for thyroid diseases, and its accuracy is higher than that of UI. The results of this study are similar to other studies (47, 52). Therefore, SIC is a reflection of thyroid function, and maintaining SIC within the normal range is beneficial to thyroid health.
This study provides reference ranges for SICs in pregnant women in a mildly iodine-deficient region of southeastern coastal China. SIC levels during pregnancy have also been reported in some previous small studies (5, 13). In our study, the SIC of 78.73 μg/L in normal pregnant women was lower than the SIC in thyroid disorder patients in other iodine excess areas (47). The 95% reference intervals for SIC in the first, second, and third trimester, respectively, in this study were lower than in previous studies conducted in iodine-sufficient and iodine-deficient areas of China (31, 36). The 95% reference ranges for SIC provided by internationally recognized laboratories such as Mayo Clinic, Quest Diagnostics, and the WHO are 40–92 μg/L, 52–109 μg/L, and 45–90 μg/L, respectively (53). The range of SIC in normal pregnant women found in the present study was in agreement with previous works (47, 54) and was higher than the reference range of 36.0–79.3µg/L in non-pregnant women (16). This result can be explained by increased iodine absorption adaptive to an increased iodine demand during pregnancy, as well as higher consumption of iodine-rich foods in coastal areas. The elevated SIC in pregnant women may also be caused by a surge of thyroid hormones with TT4 levels being higher (55). Thus, our findings suggest that SIC may be a useful biomarker for assessing iodine nutritional status in individual pregnant women.
The main strengths of this study are as follows. Multistage sampling was used in this study and the sample was highly representative. The FFQ included the main food groups containing iodine, and the information collected by the questionnaire was adequate. This study, conducted on a large number of pregnant women, demonstrates that SI is an interesting biomarker of iodine status. Indeed, SI, as an important part of iodine metabolism in the body, is influenced by iodine intake on the one hand, and is associated with thyroid function and hypothyroxinemia on the other hand. This study also defines reference ranges of SICs in pregnant women. It is the first time that the above study has been conducted on pregnant women in Fujian Province.
Our study also has the following limitations: first, we only collected spot urine samples from pregnant women and lacked 24-h urine sample data and creatinine data. As our study is based on a large-scale epidemiological survey, these methods present greater challenges in terms of sample collection. Secondly, the study was a cross-sectional study with no repeated measurements in the same participants. In future studies, SIC and iodine intake should be tested throughout pregnancy using prospective study methods, and the reliability and applicability of SIC in assessing individual iodine status in the general population should be verified.
Data availability statement
The data presented in this study are available on reasonable request from the corresponding author. The raw data cannot be shared publicly due to ethical restrictions because they contain potentially sensitive information. Requests to access the datasets should be directed to 18906913056@163.com.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Fujian Provincial Center for Disease Control and Prevention (No.2020032). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SJ: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. XW: Investigation, Writing – review & editing. JW: Investigation, Writing – review & editing. DC: Investigation, Writing – review & editing. ZC: Conceptualization, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was sponsored by the Fujian Provincial Health Technology Project (2020CXA020) and Fujian Provincial Natural Science Funding (2020J01093).
Acknowledgments
The authors would like to express their gratitude to all the pregnant women who participated. The authors also thank the staff at the county or district level CDCs who managed the sample collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1289572/full#supplementary-material
References
1. WHO, UNICEF, ICCIDD. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers. 3rd Ed. Geneva: WHO (2007).
2. Zimmermann MB, Andersson M. Update on iodine status worldwide. Curr Opin endocrinology diabetes Obes (2012) 19(5):382–7. doi: 10.1097/MED.0b013e328357271a
3. Chen X, Wu C, Wang Z, Wu C, Guo Y, Zhu X, et al. Iodine nutrition status and thyroid autoimmunity during pregnancy: A cross-sectional study of 4635 pregnant women. Nutr J (2022) 21(1):7. doi: 10.1186/s12937-022-00760-6
4. Rodriguez-Diaz E, Pearce EN. Iodine Status and Supplementation before, During, and after Pregnancy. Best Pract Res Clin Endocrinol Metab (2020) 34(4):101430. doi: 10.1016/j.beem.2020.101430
5. Li C, Peng S, Zhang X, Xie X, Wang D, Mao J, et al. The urine iodine to creatinine as an optimal index of iodine during pregnancy in an iodine adequate area in China. J Clin Endocrinol Metab (2016) 101(3):1290–8. doi: 10.1210/jc.2015-3519
6. Zhao J, van der Haar F. Progress in salt iodization and improved iodine nutrition in China, 1995–99. Food Nutr Bull (2004) 25(4):337–43. doi: 10.1177/156482650402500403
7. Sun D, Codling K, Chang S, Zhang S, Shen H, Su X, et al. Eliminating iodine deficiency in China: achievements, challenges and global implications. Nutrients (2017) 9(4):361. doi: 10.3390/nu9040361
8. Pearce EN, Lazarus JH, Moreno-Reyes R, Zimmermann MB. Consequences of iodine deficiency and excess in pregnant women: an overview of current knowns and unknowns. Am J Clin Nutr (2016) 104 Suppl 3(Suppl 3):918s–23s. doi: 10.3945/ajcn.115.110429
9. Fan X, Zhao L, Wang S, Song K, Wang B, Xie Y, et al. Relation between iodine nutrition and thyroid diseases in Qinghai, China. Front Endocrinol (Lausanne) (2023) 14:1234482. doi: 10.3389/fendo.2023.1234482
10. Zimmermann MB. Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: A review. Am J Clin Nutr (2009) 89(2):668s–72s. doi: 10.3945/ajcn.2008.26811C
11. Zimmermann MB, Jooste PL, Pandav CS. Iodine-deficiency disorders. Lancet (2008) 372(9645):1251–62. doi: 10.1016/s0140-6736(08)61005-3
12. König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr (2011) 141(11):2049–54. doi: 10.3945/jn.111.144071
13. Yu S, Yin Y, Cheng Q, Han J, Cheng X, Guo Y, et al. Validation of a simple inductively coupled plasma mass spectrometry method for detecting urine and serum iodine and evaluation of iodine status of pregnant women in Beijing. Scandinavian J Clin Lab Invest (2018) 78(6):501–7. doi: 10.1080/00365513.2018.1512150
14. Michalke B, Witte H. Characterization of a rapid and reliable method for iodide biomonitoring in serum and urine based on ion chromatography-icp-mass spectrometry. J Trace elements Med Biol Organ Soc Minerals Trace Elements (GMS) (2015) 29:63–8. doi: 10.1016/j.jtemb.2014.05.002
15. Allain P, Berre S, Krari N, Lainé-Cessac P, Le Bouil A, Barbot N, et al. Use of plasma iodine assay for diagnosing thyroid disorders. J Clin Pathol (1993) 46(5):453–5. doi: 10.1136/jcp.46.5.453
16. Yu S, Wang D, Cheng X, Zhang Q, Wang M, Guo H, et al. Establishing reference intervals for urine and serum iodine levels: A nationwide multicenter study of a euthyroid Chinese population. Clinica chimica acta; Int J Clin Chem (2020) 502:34–40. doi: 10.1016/j.cca.2019.11.038
17. Lin Y, Chen D, Wu J, Chen Z. Iodine status five years after the adjustment of universal salt iodization: A cross-sectional study in Fujian Province, China. Nutr J (2021) 20(1):17. doi: 10.1186/s12937-021-00676-7
18. Yang Y. Chinese Food Composition Standard Edition. 6th Edition. Beijing: Peking University Medical Press (2019).
19. Sun R, Fan L, Du Y, Liu L, Qian T, Zhao M, et al. The relationship between different iodine sources and nutrition in pregnant women and adults. Front Endocrinol (Lausanne) (2022) 13:924990. doi: 10.3389/fendo.2022.924990
20. WHO. Recommended Iodine Levels in Salt and Guidelines for Monitoring Their Adequacy and Effectiveness. Geneva: WHO (1996).
21. National Health and Family Planning Commission of the People's Republic of China. Determination of iodine in urine—As³+-Ce4+catalytic spectrophotometry, WS/T 107.1–2016. Beijing: Standards Press of China (2016).
22. Standardization Administration of China. General Test Method in Salt Industry -Determination of Iodic Ion, GB/T 13025.7–2012. Beijing: Standards Press of China (2012).
23. Wang H, Liu L, Li S, Gu Y, li X, Wang J, et al. Determination of iodine in drinking water by As3+-Ce4+ Catalytic spectrophotometry. Chin J Endemiol (2007) 26(3):4. doi: 10.3760/cma.j.issn.1000-4955.2007.03.035
24. People's Republic of China National Health Commission. Determination of Iodine in Serum-Inductively Coupled Plasma Mass Spectrometry, WS/T 783—2021. Beijing: Standards Press of China (2021).
25. Liu Z, Lin Y, Wu J, Chen D, Wu X, Lan Y, et al. Is the urinary iodine/creatinine ratio applicable to assess short term individual iodine status in Chinese adults? Comparison of iodine estimates from 24-H urine and timed-spot urine samples in different periods of the day. Nutr Metab (2022) 19(1):27. doi: 10.1186/s12986-022-00656-6
27. Chinese Nutrition Society. Dietary nutrient reference intakes for Chinese residents. 2013 Edition. Beijing: Science Press (2014).
28. Li CY. Guidelines for the management of thyroid diseases during pregnancy and postpartum. Chin J Endocrinol Metab (2019) 35(8):636. doi: 10.3760/cma.j.issn.1000-6699.2019.08.003
29. Ministry of Health of the Peoples Republic of China. Diagnostic criteria for endemic goiter, WS276-2007. Beijing: Standards Press of China (2007).
30. Li Y, Teng D, Ba J, Chen B, Du J, He L, et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: epidemiological evidence from 31 provinces of mainland China. Thyroid Off J Am Thyroid Assoc (2020) 30(4):568–79. doi: 10.1089/thy.2019.0067
31. Pan Z, Cui T, Chen W, Gao S, Pearce EN, Wang W, et al. Serum iodine concentration in pregnant women and its association with urinary iodine concentration and thyroid function. Clin Endocrinol (2019) 90(5):711–8. doi: 10.1111/cen.13945
32. Chen W, Li X, Guo X, Shen J, Tan L, Lin L, et al. Urinary iodine excretion (Uie) estimated by iodine/creatinine ratio from spot urine in Chinese school-age children. Clin Endocrinol (2017) 86(4):628–33. doi: 10.1111/cen.13282
33. Iacone R, D'Elia L, Guida B, Barbato A, Scanzano C, Strazzullo P. Validation of daily urinary creatinine excretion measurement by muscle-creatinine equivalence. J Clin Lab Anal (2018) 32(6):e22407. doi: 10.1002/jcla.22407
34. Hilderink JM, van der Linden N, Kimenai DM, Litjens EJR, Klinkenberg LJJ, Aref BM, et al. Biological variation of creatinine, cystatin C, and Egfr over 24 hours. Clin Chem (2018) 64(5):851–60. doi: 10.1373/clinchem.2017.282517
35. Wang Z, Jin W, Zhu Z, Cui X, Song Q, Shi Z, et al. Relationship of household cooking salt and eating out on iodine status of pregnant women in environmental iodine-deficient coastal areas of China. Br J Nutr (2020) 124(9):971–8. doi: 10.1017/s000711452000207x
36. Li X, Tu P, Gu S, Mo Z, Wu L, Xing M, et al. Serum iodine as a potential individual iodine status biomarker: A cohort study of mild iodine deficient pregnant women in China. Nutrients (2023) 15(16):3555. doi: 10.3390/nu15163555
37. Lindorfer H, Krebs M, Kautzky-Willer A, Bancher-Todesca D, Sager M, Gessl A. Iodine deficiency in pregnant women in Austria. Eur J Clin Nutr (2015) 69(3):349–54. doi: 10.1038/ejcn.2014.253
38. Nyström HF, Brantsæter AL, Erlund I, Gunnarsdottir I, Hulthén L, Laurberg P, et al. Iodine status in the nordic countries - past and present. Food Nutr Res (2016) 60:31969. doi: 10.3402/fnr.v60.31969
39. Alemu A, Terefe B, Abebe M, Biadgo B. Thyroid hormone dysfunction during pregnancy: A review. Int J Reprod biomedicine (2016) 14(11):677–86. doi: 10.29252/ijrm.14.11.677
40. Ainy E, Ordookhani A, Hedayati M, Azizi F. Assessment of intertrimester and seasonal variations of urinary iodine concentration during pregnancy in an iodine-replete area. Clin Endocrinol (2007) 67(4):577–81. doi: 10.1111/j.1365-2265.2007.02928.x
41. Kung AW. Iodine nutrition of pregnant and lactating women in Hong Kong, where intake is of borderline sufficiency. Public Health Nutr (2007) 10(12a):1600–1. doi: 10.1017/s1368980007360989
42. Rezvanian H, Aminorroaya A, Majlesi M, Amini A, Hekmatnia A, Kachoie A, et al. Thyroid size and iodine intake in iodine-repleted pregnant women in Isfahan, Iran. Endocrine Pract (2002) 8(1):23–8. doi: 10.4158/ep.8.1.23
43. Brucker-Davis F, Panaïa-Ferrari P, Gal J, Fénichel P, Hiéronimus S. Iodine supplementation throughout pregnancy does not prevent the drop in Ft4 in the second and third trimesters in women with normal initial thyroid function. Eur Thyroid J (2013) 2(3):187–94. doi: 10.1159/000350882
44. Glinoer D, Spencer CA. Serum Tsh determinations in pregnancy: how, when and why? Nat Rev Endocrinol (2010) 6(9):526–9. doi: 10.1038/nrendo.2010.91
45. Glinoer D. The importance of iodine nutrition during pregnancy. Public Health Nutr (2007) 10(12a):1542–6. doi: 10.1017/s1368980007360886
46. Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screening (2000) 7(3):127–30. doi: 10.1136/jms.7.3.127
47. Jin X, Jiang P, Liu L, Jia Q, Liu P, Meng F, et al. The application of serum iodine in assessing individual iodine status. Clin Endocrinol (2017) 87(6):807–14. doi: 10.1111/cen.13421
48. Andersen SL, Sørensen LK, Krejbjerg A, Møller M, Laurberg P. Challenges in the evaluation of urinary iodine status in pregnancy: the importance of iodine supplement intake and time of sampling. Eur Thyroid J (2014) 3(3):179–88. doi: 10.1159/000365145
49. Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol (2015) 3(4):286–95. doi: 10.1016/s2213-8587(14)70225-6
51. Dosiou C, Medici M. Management of endocrine disease: isolated maternal hypothyroxinemia during pregnancy: knowns and unknowns. Eur J Endocrinol (2017) 176(1):R21–r38. doi: 10.1530/eje-16-0354
52. Xu T, Guo W, Ren Z, Wei H, Tan L, Zhang W. Study on the relationship between serum iodine and thyroid dysfunctions: A cross-sectional study. Biol Trace element Res (2023) 201(8):3613–25. doi: 10.1007/s12011-022-03459-1
53. Han JH, Wu L, Yu SL, Fang HL, Kamg WM, Cheng XQ, et al. Values of iodine metabolism biomarkers in assessing the iodine nutrition status in surgically treated patients with thyroid disease. Zhongguo yi xue ke xue yuan xue bao Acta Academiae Medicinae Sinicae (2015) 37(2):221–5. doi: 10.3881/j.issn.1000-503X.2015.02.014
54. Wu Y, Long HH, Zhang SJ, Li MM, Chen CG, Wang C, et al. Reference intervals of serum iodine concentration in Chinese pregnant women. Biol Trace Element Res (2023). doi: 10.1007/s12011-023-03859-x
55. Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid Off J Am Thyroid Assoc (2004) 14(12):1084–90. doi: 10.1089/thy.2004.14.1084
Keywords: pregnant women, iodine nutrition, serum iodine, urinary iodine, dietary iodine, thyroid diseases, thyroid function
Citation: Ji S, Wu X, Wu J, Chen D and Chen Z (2023) Serum iodine concentration and its associations with thyroid function and dietary iodine in pregnant women in the southeast coast of China: a cross-sectional study. Front. Endocrinol. 14:1289572. doi: 10.3389/fendo.2023.1289572
Received: 06 September 2023; Accepted: 23 October 2023;
Published: 09 November 2023.
Edited by:
Nikolay Solovyev, Atlantic Technological University, IrelandReviewed by:
Josiane Arnaud, Centre Hospitalier Universitaire de Grenoble, FranceTommaso Filippini, University of Modena and Reggio Emilia, Italy
Copyright © 2023 Ji, Wu, Wu, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihui Chen, MTg5MDY5MTMwNTZAMTYzLmNvbQ==
 Shumi Ji
Shumi Ji Xiaoyan Wu
Xiaoyan Wu Jiani Wu
Jiani Wu Diqun Chen
Diqun Chen Zhihui Chen
Zhihui Chen