
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Endocrinol. , 19 October 2023
Sec. Bone Research
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1281649
This article is part of the Research Topic New Insights into the Relationship between Endocrine Disorders and Dental Diseases View all 5 articles
A correction has been applied to this article in:
Corrigendum: Demineralized dentin matrix promotes gingival healing in alveolar ridge preservation of premolars extracted for orthodontic reason: a split-mouth study
 Xiaofeng Xu1,2,3†
Xiaofeng Xu1,2,3† Dongsheng Peng1,2,4,5†
Dongsheng Peng1,2,4,5† Bowei Zhou1,2†
Bowei Zhou1,2† Kaijin Lin1,2†
Kaijin Lin1,2† Siyi Wang1,2
Siyi Wang1,2 Wei Zhao1,2
Wei Zhao1,2 Minqian Zheng1,2,6*
Minqian Zheng1,2,6* Jin Yang1,2,6*
Jin Yang1,2,6* Jianbin Guo1,2,6*
Jianbin Guo1,2,6*Objective: The purpose of this study was to prospectively evaluate the efficacy of a demineralized dentin matrix (DDM) in decreasing the initial inflammatory response of the gingiva and facilitating the repair and regeneration of soft tissue in alveolar ridge preservation.
Methods: This clinical study employed a split-mouth design. Fourteen patients with a total of forty-four sites underwent extraction and alveolar ridge preservation (ARP) procedures. A Bilaterally symmetrical extraction operation were conducted on the premolars of each patient. The experimental group received DDM as a graft material for ARP, while the control group underwent natural healing. Within the first month postoperatively, the pain condition, color, and swelling status of the extraction sites were initially assessed at different time points Subsequently, measurements were taken for buccal gingival margin height, buccal-lingual width, extraction socket contour, and the extraction socket area and healing rate were digitally measured. Additionally, Alcian Blue staining was used for histological evaluation of the content during alveolar socket healing.
Results: Both groups experienced uneventful healing, with no adverse reactions observed at any of the extraction sites. The differences in VAS pain scores between the two groups postoperatively were not statistically significant. In the early stage of gingival tissue healing (3 days postoperatively), there were statistically significant differences in gingival condition and buccal gingival margin height between the two groups. In the later stage of gingival tissue healing (7, 14, and 30 days postoperatively), there were statistically significant differences in buccal-lingual width, extraction socket healing area, and healing rate between the two groups. Furthermore, the histological results from Alcian Blue staining suggested that the experimental group may play a significant role in promoting gingival tissue healing, possibly by regulating inflammatory responses when compared to the control group.
Conclusion: The application of DDM in alveolar ridge preservation has been found to diminish initial gingival inflammation after tooth extraction. Additionally, it has shown the ability to accelerate early gingival soft tissue healing and preserve its anatomical contour.
Clinical trial registration: chictr.org.cn, identifier ChiCTR2100050650.
After tooth extraction, the loss of both soft and hard tissue contours at the extraction site can occur due to normal physiological remodeling and the absence of stimuli from chewing function (1, 2), This can have negative impacts on subsequent treatments such as implant therapy (3), orthodontic treatment (4, 5), and removable denture restoration (6, 7). Alveolar ridge preservation (ARP) refers to a series of treatment methods wherein, immediately after tooth extraction, bone grafting or the use of biological materials is performed within the extraction socket. This approach aims to slow down alveolar bone resorption, thereby effectively maintaining the existing soft and hard tissue contours to the maximum extent possible (8).
In previous studies of ARP, a wide range of biological materials were employed, including autogenous bone, allografts, xenografts, autologous blood-derived products, and bioactive agents (9, 10). Although autogenous bone possesses osteogenic, osteoinductive, and osteoconductive characteristics and is considered the “gold standard” of bone grafting materials (11), obtaining autogenous bone requires creating a secondary surgical site, which may lead to complications at the donor site (12). Allogeneic bone grafts can result in immune rejection reactions and the spread of diseases (13); furthermore, xenogeneic bone grafts have disadvantages such as high costs and a lack of capacity to promote early ossification and bone induction (14). Therefore, the search for cost-effective bone graft materials with superior material properties remains a focal point of concern in the current field of oral medicine.
Currently, demineralized dentin matrix (DDM) derived from extracted teeth is an emerging bone graft material with high biocompatibility (15). Since its first systematic report in 2008, the effectiveness and safety of DDM in bone augmentation procedures have been demonstrated through numerous animal experiments and clinical studies (16), Preliminary clinical research conducted by our research group has also confirmed the efficacy of DDM in bone regeneration (15). The inorganic components in DDM can serve as a scaffold to maintain space and volume, promoting donor cell attachment and proliferation, thus conferring osteoconductive properties (17). The organic components, on the other hand, supply a variety of growth factors that facilitate bone reconstruction and repair, creating an ideal environment for cellular differentiation and proliferation, thereby imparting osteoinductive properties (18).
During the initial stage of tooth extraction wound healing, the migration of fibroblasts is a fundamental component of wound contraction, and the expression of myofibroblast-related genes plays a crucial role in the early stages of healing (19). It is worth noting that recent studies by Bernardi (20) and Bianchi (21) have further revealed a positive response of DDM in inducing proliferation, adhesion, and migration of human periodontal ligament fibroblasts, indicating that DDM has the potential to promote the growth of human periodontal ligament fibroblasts cells. Therefore, DDM is not only effective in bone augmentation but also may have a potential promoting effect on soft tissue healing. However, in previous studies utilizing DDM for ARP, most of the research has focused on evaluating its effectiveness in bone augmentation, with a lack of comprehensive systematic reports on soft tissue healing aspects.
Based on previous research, this study hypothesizes that the application of DDM in ARP can promote early healing of the gingival tissue at the extracted tooth site. This split-mouth study involves the extraction of premolars required for orthodontic treatment. Immediately adjacent to the chairside, DDM is prepared and promptly filled into the extraction socket. The aim is to observe the potential promoting effects of DDM in ARP on gingival healing at the extracted tooth site.
This was a single-center, parallel-group, split-mouth design trial with balanced randomization (1:1) conducted at the Department of Oral and Maxillofacial Surgery, Affiliated Stomatological Hospital of Fujian Medical University from September 2020 to March 2022.
Recruiting patients from orthodontics requiring extraction of premolars to participate in this study. The primary researcher screened and recruited the patients. In the clinical research process, uninvolved researchers were tasked with using a coin-tossing method to select the experimental and control groups, where “heads” represented the experimental group and “tails” represented the control group.
The study followed the principles of the Declaration of Helsinki for research involving human subjects, and all participants provided written informed consent. Approval was obtained from the institutional review board of the School and Hospital of Stomatology, Fujian Medical University (Fujian, China). The trial was registered in the Chinese Clinical Trial Registry on 01/09/2021, which is a member of the World Health Organization’s international clinical trials registry, under the Registry Number ChiCTR2100050650. No significant changes were made to the trial design after the study had commenced.
Inclusion criteria (1): Orthodontic patients (over 12 years old) who need symmetrical extraction of homonymous maxillary premolars (2); The patients had symmetrical left and right occlusion and alveolar bone (3); There is no bone metabolism disease such as diabetes and osteoporosis (4); Healthy periodontal tissue, no bone destruction such as cysts and tumors, and no history of orthodontic treatment (5); Preoperative blood tests indicated normal coagulation function and platelet count (6); The patient had no smoking history, no pregnancy, and good compliance.
Exclusion criteria (1): Those suffering from any systemic diseases (2); History of trauma or surgery, fracture or even loss of alveolar bone (3); Systemic diseases or drug application that may affect the healing of soft and hard tissues.
The sample size was determined for each of the two groups, with 22 tooth extraction sites in each group. It was determined by ensuring a test power of at least 85% and a significance level of no more than 5% (using G*Power 3.1.9.2 software from Dusseldorf University). This calculation of sample size was based on the effect size estimation derived from a previously published research study (22).
All patients underwent tooth extractions performed by the same surgeon, who used minimally invasive tooth forceps and administered local anesthesia with 4% articaine. The dental professional meticulously removed soft tissues and foreign objects, including dental calculus and restorative materials. The crown enamel and cementum were subsequently removed using a turbine, while the remaining tooth tissues were crushed and filtered through a 1 mm sieve to obtain dentin particles. Dentin particles were then processed using the VacuaSonic® System equipment (CosmoBioMedicare, Korea) to obtain DDM (Figure 1). In the experimental group, the extraction socket was solely filled with DDM, elevating it by 1 mm above the alveolar crest, while the control side was allowed to heal naturally (Figure 2).
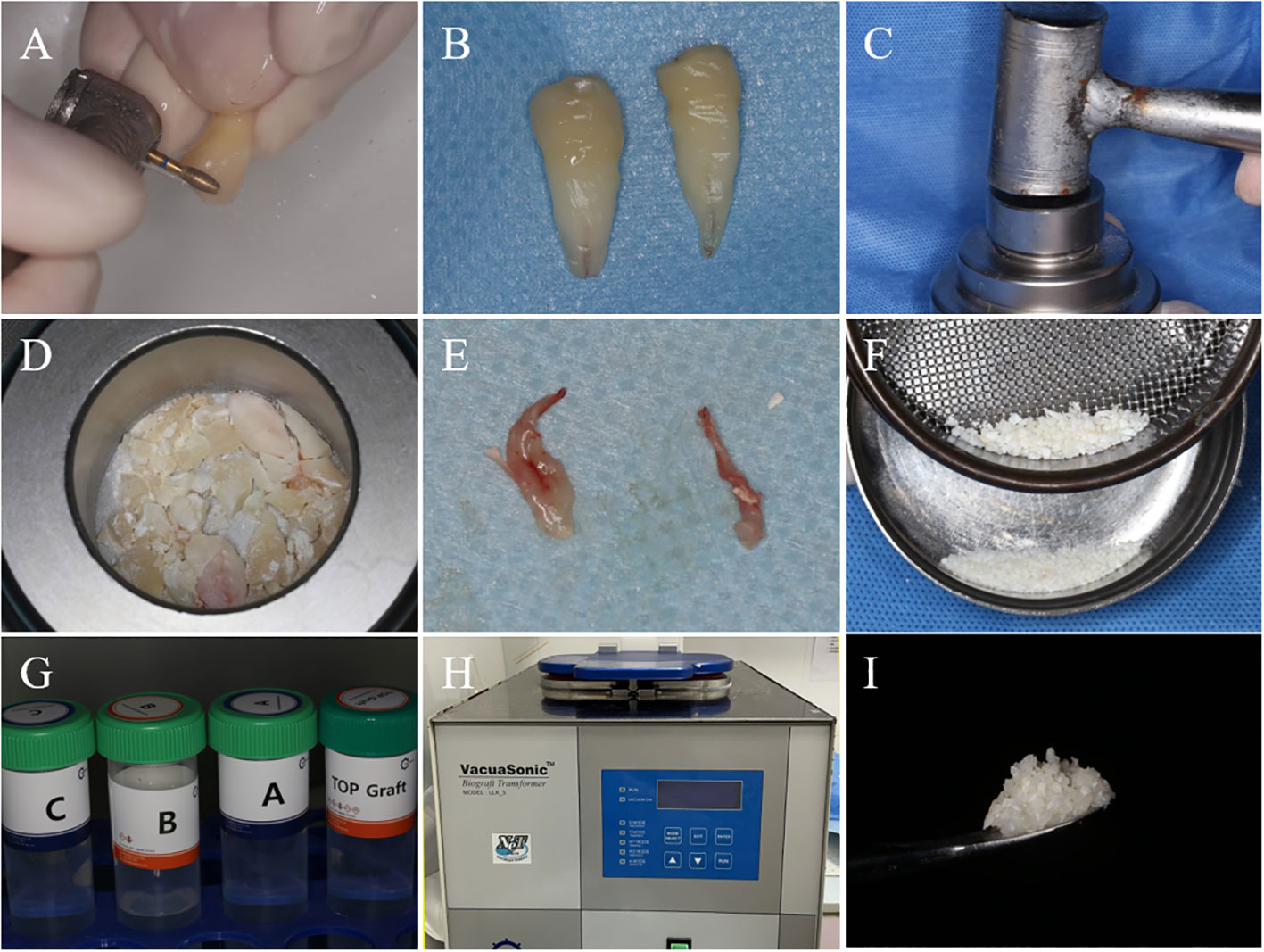
Figure 1 The process of DDM preparation. (A) After tooth extraction, the enamel and cementum of the teeth are removed using a high-speed handpiece. (B) Teeth after the removal of enamel and cementum. (C) Powder Kit tool for grinding dental tissues. (D) Dental tissue after initial grinding. (E) Removed dental pulp tissue. (F) After grinding dental tissue, filter it through a 1mm sieve to obtain dentin particles with a diameter smaller than 1mm. (G) The DecalSi® PDM reagent used for demineralization, washing, and sterilization of dentin particles. (H) Using the VacuaSonic® System device for programmed treatment of dentin particles. (I) DDM obtained after processing.
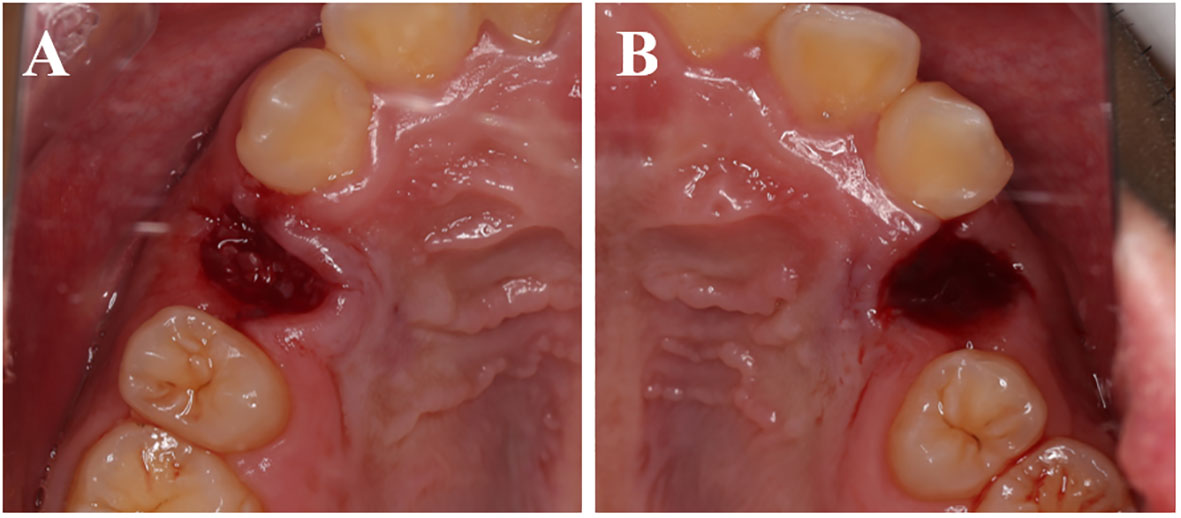
Figure 2 Immediate postoperative. (A) The experimental group shows the DDM filling the tooth extraction socket. (B) The control group shows a blood clot filling the tooth extraction socket.
Following the operation, it is advisable to apply a cold compress to both sides of the surgical area for 24 to 48 hours. Additionally, it is recommended to consume warm or cool foods two hours after the operation and adhere to the required precautions for post-tooth extraction care. Starting from the second day post-operation, it is suggested to rinse the mouth with diluted tinidazole (10-15 ml Tid) for f three days.
During the postoperative follow-up, measurements of the parameters were conducted by two experienced doctors, one being an oral and maxillofacial surgeon, and the other being a periodontist.
Pain intensity of patients on the 1st day postoperatively was assessed using the Visual Analogue Scale (VAS) method (Table 1) (23). Gingival congestion and swelling scores at the surgical site were evaluated and documented using the gingival condition classification during follow-up visits at 3 and 7 days postoperatively (Table 2) (24).
Immediately after the operation, photographs were taken of the buccal plane of the vertical extraction socket. Additional photographs were captured during the follow-up visits on the 3rd and 30th days (Figure 3A). The height of the buccal gingival margin was measured using Image J software (National Institutes of Health, American).
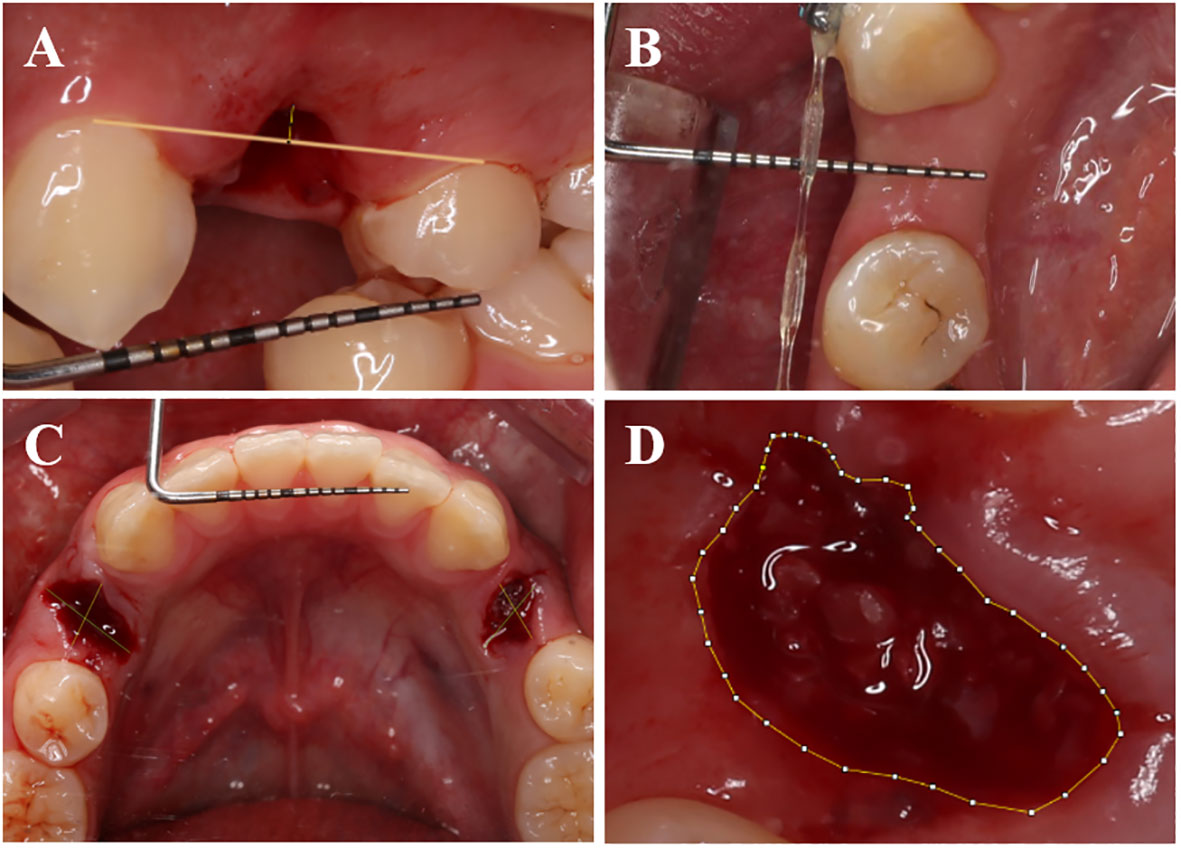
Figure 3 Measurement methods for certain parameters. (A) Capture photographs vertically aligned with the buccal side of the tooth extraction socket, and in Image J software, measure the postoperative buccal gingival margin height using the periodontal probe scale as a reference. (B) Utilize a periodontal probe to measure the buccal-lingual (palatal) width of the soft tissue on the alveolar crest postoperatively. (C) Capture photographs vertically aligned with the occlusal surface of the extraction socket, and in ImageJ software, use the periodontal probe scale as a reference to measure the postoperative extraction socket contour. (D) Use Image J software to trace the outline of the tooth extraction socket and calculate the healing area of the socket.
During the 30-day follow-up visit after operation, a periodontal probe was employed to measure and document the buccal-lingual (palate) dimension of the alveolar ridge’s soft tissue (Figure 3B).
Images were captured of the occlusal surface of the extraction socket right after the operation, as well as on the 7th, 14th, and 30th days. The approach employed to assess the dimensions of the mesial-distal (M-D) and buccal-lingual (B-L) extraction sockets post-operation was adopted from Suttapreyasri et al. (25) (Figure 3C).
Researchers employed a CEREC chairside system scanner (Sirona, Germany) to capture intraoral digital optical impressions right after the operation, as well as at postoperative days, 7, 14, and 30. The extraction socket’s area was quantified utilizing Image J software, with scale adjustments applied (Figure 3D). Subsequently, the healing rate of the extraction socket was computed employing a specific formula (1) (26) (Figure 4).
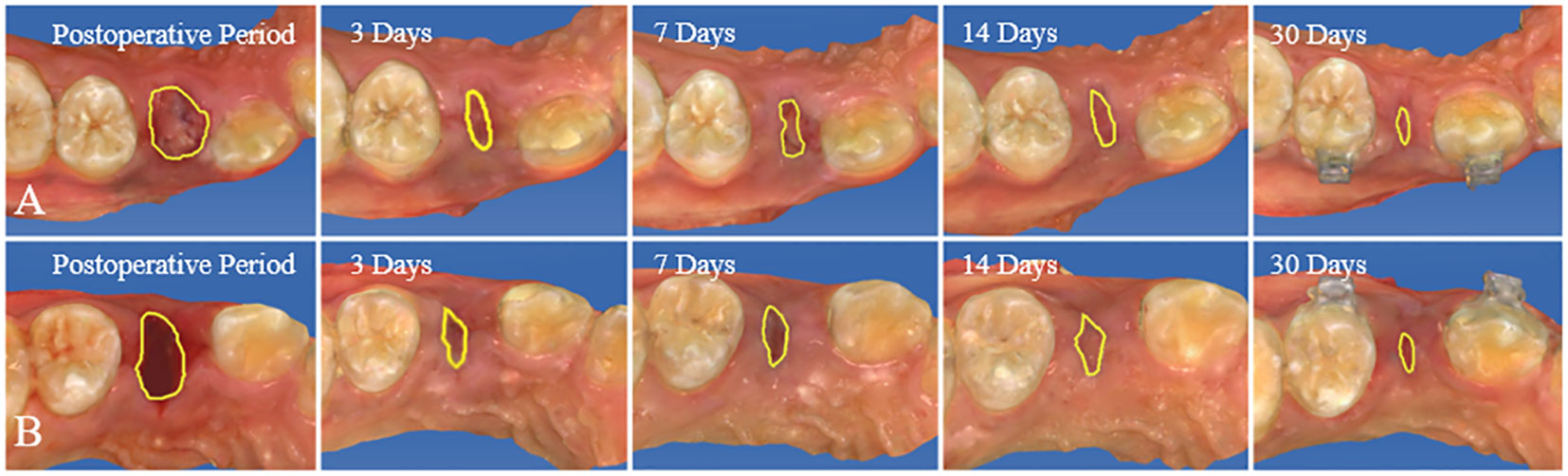
Figure 4 Utilize Image J software to digitally outline the extraction socket in intraoral optical impression images and assess the postoperative healing rate of the extraction socket. (A) Control group. (B) Experiment group.
Using forceps, fibrous tissue samples measuring approximately 1mm × 1mm were taken from the surface of the extraction sockets of some patients in the experimental group 3 days after the operation. In contrast, blood clots from the extraction sockets were obtained for the control group and subsequently placed separately in 10% formaldehyde fixative solution for fixation, with a fixation time of 24 hours. Following specimen fixation, a stepwise process was employed including rinsing in running water, automated dehydration using a tissue specimen dehydrator, xylene-based transparency, wax immersion, paraffin embedding, and tissue sectioning using a rotary microtome (with an average thickness of 3μm). Subsequently, Alcian Blue staining was conducted followed by microscopic observation.
All collected data underwent statistical analysis using SPSS 26.0 software (SPSS, USA). Normality and variance homogeneity of the data were assessed using the Shapiro-Wilk and Levene tests. In cases of ordinal data, the nonparametric rank-sum test for two independent samples was applied. If the assumptions of a normal distribution and homogeneity of variances are met, a paired t-test will be used. In cases where the data does not follow a normal distribution, the nonparametric Wilcoxon signed-rank test will be employed. A P-value below 0.05 denoted statistical significance in the observed discrepancies.
This study included 14 patients requiring bilateral removal of maxillary or mandibular premolars for orthodontic treatment, involving a total of 44 premolars. Among participants, there were 4 males and 10 females, with an average age of 22.1 ± 8.1 years. All patients received regular follow-up, and postoperative healing of the extraction sockets was excellent, with no complications or significant bone graft material loss observed (Figure 5). In the radiographic images at 30 days postoperative, DDM within the extraction sockets of the experimental group can be observed, preserving the outline of hard tissues (Figure 6).

Figure 5 From immediately postoperative to 30 days postoperative, both the experimental and control groups show good healing of the extraction sockets, without any complications or evident bone graft material loss. (A) Control group. (B) Experiment group.
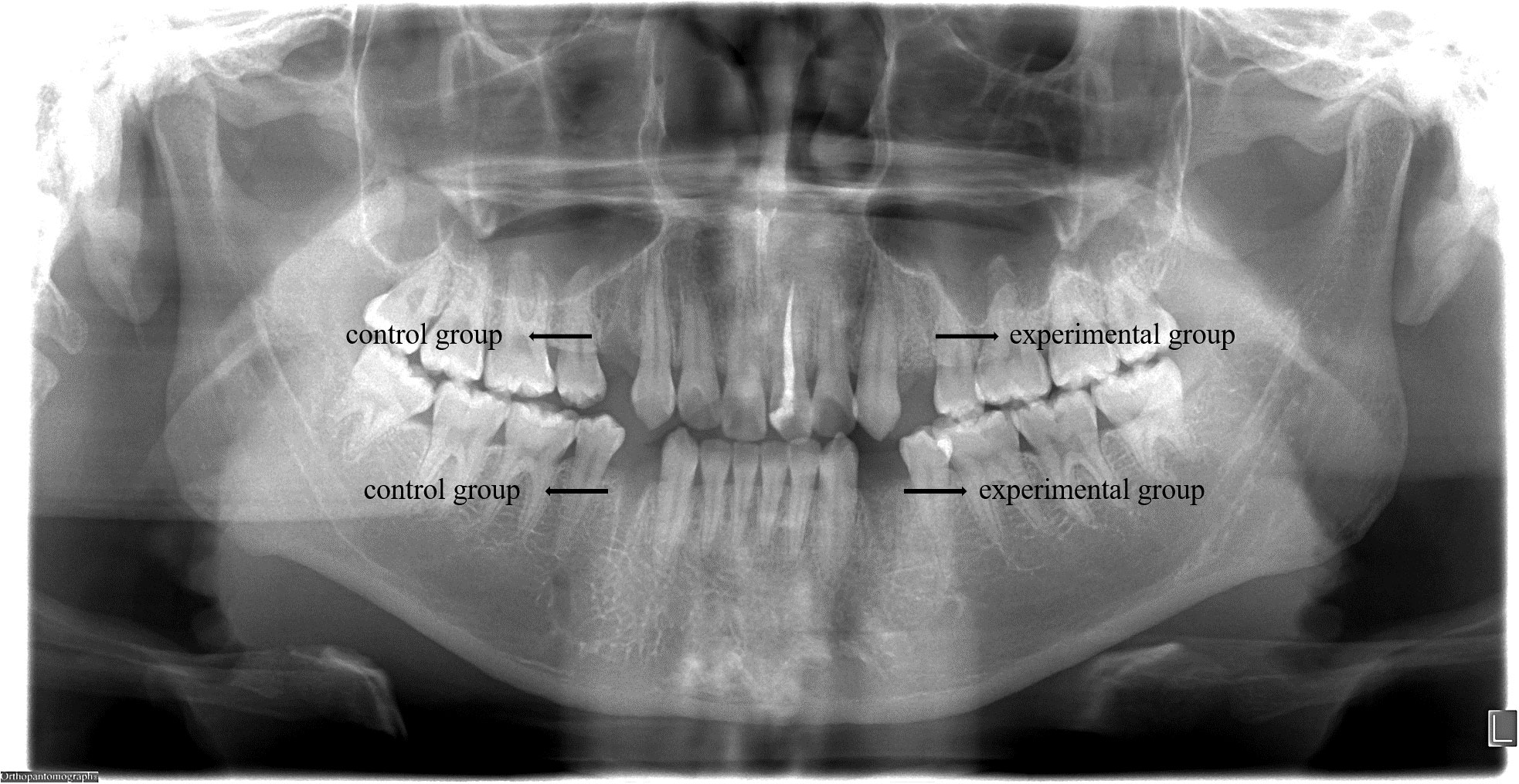
Figure 6 At 30 days postoperative, radiographic images reveal the presence of DDM in the extraction sockets of the experimental group, preserving the outline of hard tissues, while the control group shows signs of bone resorption.
One day post-operation, the experimental group reported 9 painless sites, 11 sites with mild pain, 2 sites with moderate pain, and no sites with severe pain. In contrast, the control group displayed 5 painless sites, 12 sites with mild pain, 4 sites with moderate pain, and 1 site with severe pain. Despite these differences in pain levels, statistical analysis utilizing Table 3 revealed no significant variation in pain levels between the experimental and control groups (P>0.05).
After tooth extraction, the gingival color was evaluated at 3 days post-operation in both the experimental and control groups. Within the experimental group, 2 sites exhibited consistent gingival color with the surrounding area, while 18 sites displayed redness and 2 sites appeared slightly dull in color. In contrast, within the control group, 8 sites were red and 14 sites were slightly dull in color. The disparity between the two groups was found to be statistically significant (P<0.05). After a week of extraction, among the experimental group, 18 sites demonstrated gingival color matching the surrounding area, and 4 sites exhibited a reddish hue. Meanwhile, in the control group, 14 sites showcased harmonized color with the surrounding gingiva, and 8 sites appeared reddish. Nevertheless, the variation between the two groups lacked statistical significance (P>0.05) (Figure 7A).
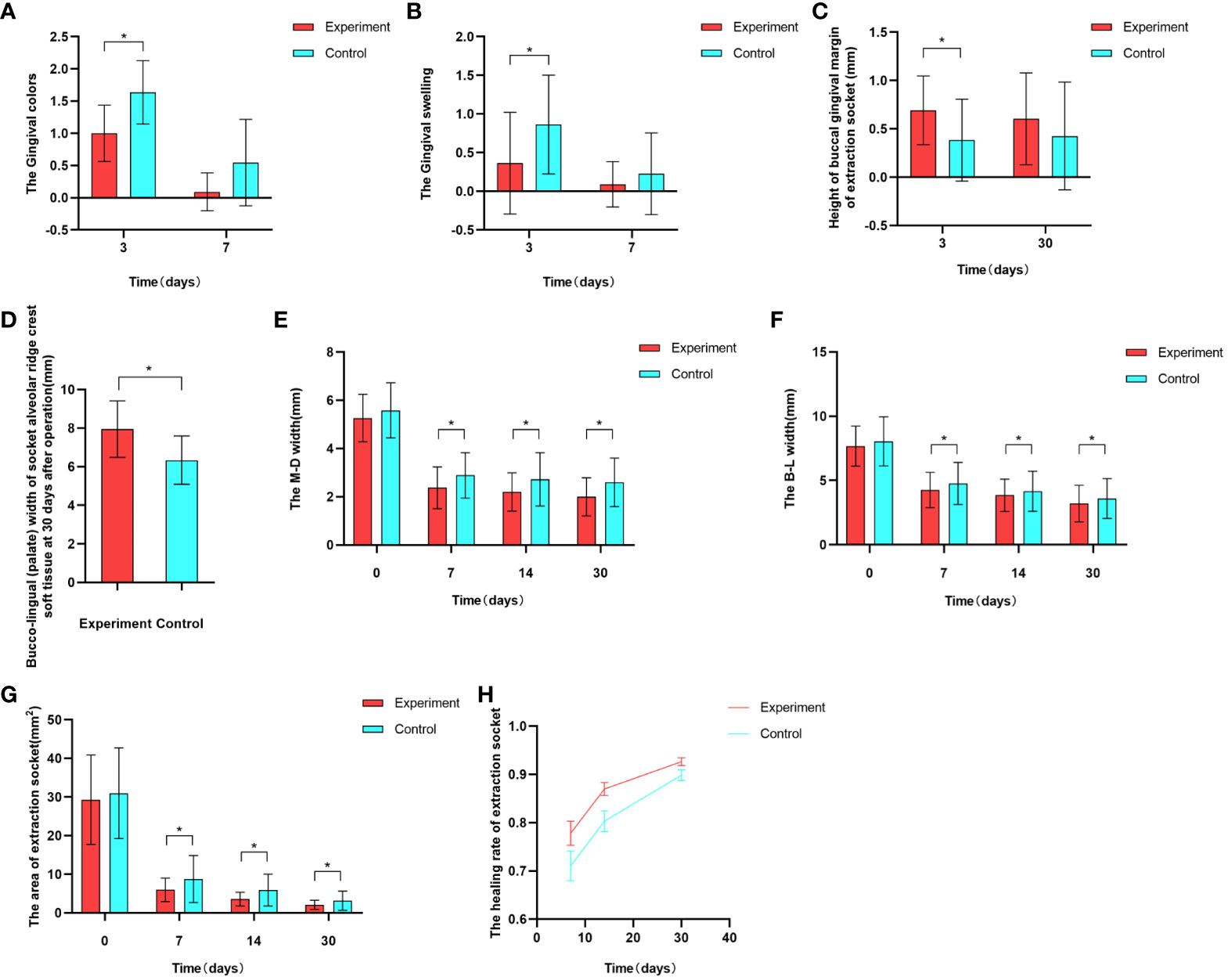
Figure 7 Graphical representation of some experimental results. (A) Comparison of gingival color between the experimental and control groups at 3 and 7 days postoperative . (B) Comparison of gingival swelling between the experimental and control groups at 3 and 7 days postoperative. (C) Comparison of buccal gingival margin height in the experimental and control groups at 3 and 30 days postoperative. (D) Comparison of buccal-lingual (palate) width of soft tissue on the alveolar crest in the experimental and control groups at 30 days postoperative. (E, F) Comparison of extraction socket contours between the experimental and control groups at immediately postoperative, 7 days, 14 days, and 30 days postoperative. (G, H) Comparison of extraction socket healing area and healing rate between the experimental and control groups at immediately postoperative, 7 days, 14 days, and 30 days postoperative.
At 3 days postoperative, both experimental and control groups exhibited gingival swelling. In the experimental group, 16 sites displayed no notable swelling, while 4 sites showed slight swelling and 2 sites exhibited evident swelling. Conversely, the control group had 6 sites without notable swelling, 13 sites with slight swelling, and 3 sites with evident swelling. The disparity between the groups was statistically significant (P<0.05). After a week, within the experimental group, 20 sites showed no apparent swelling and slight swelling was observed in 2 sites. In contrast, the control group had 18 sites without apparent swelling, 3 sites with slight swelling, and 1 site with evident swelling. However, the distinction between the two groups lacked statistical significance (P>0.05) (Figure 7B).
Three days postoperative, the gingival margin height on the buccal side of the extraction socket was 0.696 ± 0.345 in the experimental group, and 0.384 ± 0.425 in the control group, with a statistically significant difference between the two groups (P<0.05)(Figure 7C).
At 30 days postoperative, measurements of the buccal gingival margin were taken in both the experimental group (0.604 ± 0.475) and the control group (0.427 ± 0.558). While the experimental group displayed a slightly higher measurement, the disparity between the two groups lacked statistical significance (P>0.05)(Figure 7C).
At 30 days postoperative, the buccal-lingual (palatal) width of the alveolar crest soft tissue in the experimental group was 7.949 ± 1.460, while in the control group, it was 6.341 ± 1.257. The difference between the two groups was statistically significant(Figure 7D).
Initially, there was no notable difference between the experimental and control groups following the operation (P>0.05). However, after 7, 14, and 30 days, the M-D and B-L widths of the experimental group were significantly lower than those of the control group.The difference was statistically significant (P<0.05) (Table 4, Figures 7E, F).
Immediately after operation, the healing area of the extraction socket in the experimental group was 29.297 ± 11.586, while in the control group, it was 30.977 ± 11.746. The difference between the two groups had no statistical significance (P>0.05). However, on postoperative days 7, 14, and 30, the extraction socket areas of the experimental group were 5.352 (3.720, 7.792), 3.451 (2.308, 4.095), and 1.826 (1.005, 2.930), respectively, while those of the control group were 5.650 (4.673, 12.691), 3.873 (2.997, 7.977), and 2.472 (1.777, 4.021). The differences between the two groups were statistically significant in all cases (P>0.05)(Figure 7G).
At postoperative days 7, 14, and 30, the healing rates of the extraction sockets in the experimental group were 77.82 ± 11.81, 86.98 ± 6.29, and 92.66 ± 3.73, respectively, while in the control group, they were 71.05 ± 14.47, 80.33 ± 10.12, and 89.86 ± 5.13. The differences between the two groups were statistically significant in all cases (P<0.05) (Figure 7H).
At 3 days postoperative, the results of Alcian Blue-hematoxylin and eosin staining, as shown in Figure 8, revealed that in the experimental group under the microscope, loose porous structures of DDM particles could be observed, with significant infiltration of neutrophils in the vicinity, and neutrophil infiltration was also observed inside dentinal tubules; while in the control group, only a small amount of neutrophil infiltration was observed.
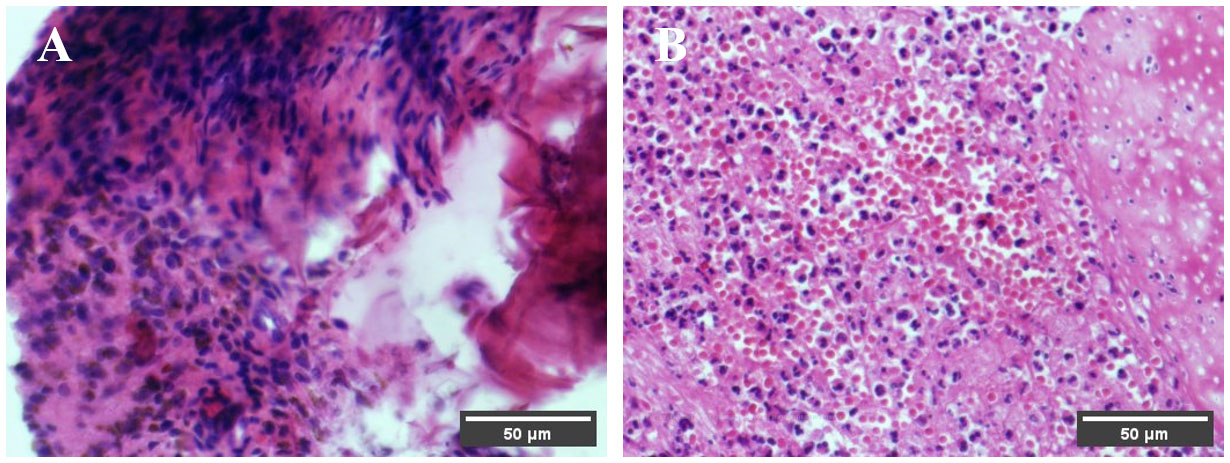
Figure 8 Pathological findings at 3 days postoperative. Under the microscope, the DDM particles in the experimental group exhibit a loose porous structure, and neutrophil infiltration is observed around both the DDM particles in the experimental group and the blood clots in the control group. (A) Control group. (B) Experiment group.
After a tooth extraction, the alveolar bone experiences a lack of typical physiological stimulation. Consequently, the residual alveolar ridge undergoes irreversible resorption, potentially resulting in bone loss and recession of soft tissue at the extraction site (1). This phenomenon is notably pronounced within orthodontic treatment, where tooth extractions commonly become necessary for addressing concerns such as crowded dentition, protrusion, and severe caries (27). Such effects might potentially influence the pace of tooth movement in orthodontic treatment and the extent of root resorption observed in adjacent teeth, along with the emergence of gingival clefts in subsequent orthodontic interventions (5, 28–30).
Previous research has employed diverse bone substitute materials such as deproteinized bovine bone mineral, nanocrystalline hydroxyapatite, allograft bone, bone ceramic, and BMP2-functionalized biomimetic calcium phosphate grafts. These materials aim to mitigate the effects of tooth extraction on subsequent orthodontic procedures (30–35). However, the predominant focus of research in this domain lies in areas such as tooth movement, space closure, root resorption, and gingival clefts. Regrettably, only a limited array of studies had delved into the ramifications of these procedures on the healing of soft tissue.
In 1967, Urist postulated that rabbit dentin possesses the capability to initiate bone formation by orchestrating the conversion of connective tissue into bone via the process of endochondral osteogenesis (36, 37). Since then, a multitude of animal and clinical studies have been undertaken to validate the biocompatibility, biodegradability, osteoinductive, and osteoconductive properties of DDM (18, 38–41).
The present study utilized DDM for alveolar ridge preservation after orthodontic extraction. Initially, the focus centered on observing its promotional effect on gingival soft tissue. Employing a split-mouth design, premolars from a single patient’s dental arch were randomly assigned to either an experimental or control group. This design proves advantageous, facilitating equitable inter-group comparisons and mitigating the influence of external variables, such as diet and oral hygiene, on experimental outcomes. The experiment divulged no statistically significant variation in socket width and the area between the experimental and control groups post tooth extraction, signifying parity in initial conditions and the elimination of selection bias. Consequently, subsequent comparisons can be executed with a high degree of confidence.
This study excluded methods like employing a collagen membrane, connective tissue graft, and sutures to close the socket when applying DDM to ARP following orthodontic extractions. This decision was rooted in the research’s objective to directly observe the impact of DDM on gingival soft tissue, unobstructed by external variables. Consequently, the alveolar socket was left unsealed. Nevertheless, a panoramic radiograph captured 30 days post-operation depicted the persistent high-density filling image of DDM at the alveolar ridge level (Figure 5B). This observation implies that DDM can be effectively retained within ARP even in the absence of alveolar socket sealing. This finding is in line with the research conducted by Lim (22), Brkovic (42), and Saito (43), indicating that the open healing method of ARP, which involves no initial wound closure, can successfully preserve bone substitute materials and facilitate the healing of both soft and hard tissues.
Complications such as wound bleeding, surgical site swelling, and pain are common after tooth extraction (44). The study utilized a minimally invasive approach for extracting premolar teeth. On the third day post-operation, the experimental group exhibited lighter gingival color and reduced swelling in contrast to the control group. These findings suggest that DDM can effectively mitigate the inflammatory response during the initial stages of soft tissue healing. This effect is likely attributed to the presence of growth factors, such as TGF-β and VEGF, within DDM, which have the potential to suppress the local inflammatory response (40, 45, 46). The pathological sections confirmed that the inflammatory response process in the experiment extraction socket healing was similar to that of the control group 3 days after operation. This finding is consistent with Morikawa (47), Pikuła (48), and Zarei (49) research, which suggests that growth factors can accelerate soft tissue healing. On the 7th day post-operation, there was no notable contrast in gingival color and swelling between the experimental and control groups. This could be attributed to the gradual reduction of gingival soft tissue inflammation on the 7th-day post-operation, indicating that both groups had achieved secondary healing (50).
In this study, socket preservation was performed using DDM grafts, and the results were evaluated on the 3rd and 30th day. The findings indicate that the experimental group was able to maintain the buccal gingival margin height better than the control group, suggesting that DDM applied in ARP can reduce gingival recession. Other studies conducted by scholars have also demonstrated that DDM implantation in the socket can help maintain the height of the buccal bone plate, thereby preserving the width of the keratinized gingiva (51). After 30 days of socket preservation using DDM grafts, the experimental group showed a larger width of the soft tissue buccal-lingual (palate) of the alveolar ridge crest compared to the control group. This finding is consistent with previous studies by Del and Elfana (52, 53). DDM is an effective bone graft material for ARP as it helps maintain the width of the buccal bone plate of the extraction socket, which in turn preserves the fullness of the gingival and prevents the collapse of the buccal soft tissue.
The study findings reveal that at immediate, 7-day, 14-day, and 30-day post-operation intervals, the mesial-distal and buccal-lingual dimensions of the extraction socket were narrower in the experimental group compared to the control group. Furthermore, the healing rate of extraction sockets was notably greater in the experimental group, and the difference was statistically significant. This outcome can be ascribed to the presence of pivotal growth factors such as TGF-β, FGF, VEGF, EGF, and PDGF within DDM, which wield a pivotal role in the initial healing of soft tissues. The presence of these growth factors stimulates the recruitment of fibroblasts to the site of injury, accelerating their proliferation and expediting the deposition of the extracellular matrix (15, 49). Rinastiti (54) demonstrated the histological impact of transplanting the human amniotic membrane onto rabbit gingival wounds. This study revealed that the amniotic membrane effectively accelerates granulation tissue formation in gingival wounds through a rapid increase in fibroblast population and vascularization. The presence of bFGF, EGF, TGF-β, IL-1, and other growth factors within the amniotic membrane likely contributes to the expedited healing of gingival wounds. These growth factors possess the capacity to initiate fibroblast proliferation and promote neovascularization in gingival wounds. Stephan’s systematic review further substantiates the crucial role of growth factors in the wound-healing process (55). As bone graft materials play a role in the immune microenvironment during osteogenesis, the immune microenvironment is a complex environment composed of various immune cells, growth factors, extracellular matrix, and related signaling molecules, and these components play an important role in tissue regeneration and repair (56–58).
Several limitations of this study merit attention. Firstly, potential patient-related factors influencing wound healing were not accounted for. Subsequent research endeavors will delve into assessing the influence of DDM on gingival healing across diverse age groups. Secondly, the scope of histological analysis encompassed merely 4 of the 22 sites, potentially necessitating a broader sample and extended observation duration in future investigations. Thirdly, the specific demineralization rate and particle size of DDM were unreported. Therefore, a more comprehensive exploration of the physical and chemical attributes of DDM is imperative for optimizing its clinical efficacy. Furthermore, future investigations will compare the impact of various graft materials with DDM on gingival healing.
This study demonstrates that the use of DDM in alveolar ridge preservation following orthodontic extraction can decrease the early inflammatory response of gingival tissue healing, accelerate the healing of gingival tissue, and maintain the contour of the tissue.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by institutional review board of School and Hospital of Stomatology, Fujian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
JG: Writing – original draft, Writing – review & editing. XX: Writing – original draft, Writing – review & editing. DP: Writing – original draft. BZ: Writing – original draft. KL: Writing – original draft. SW: Writing – original draft. WZ: Writing – review & editing. MZ: Writing – review & editing. JY: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Project was supported by the Natural Science Foundation of Fujian Province, China (Grant No. 2020J01646, 2022J01764), Science and Technology Innovation Joint Foundation of Fujian Province, China (Grant No. 2020Y9031), Science and technology planning project of Fujian Provincial Health Commission (Grant No. 2022GGA041, Grant No. 2021GGB016), Startup Fund for scientific research, Fujian Medical University(Grant No.2021QH1133).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1281649/full#supplementary-material
DDM, Demineralized dentin matrix; ARP, Alveolar ridge preserve; VAS, Visual Analogue Scale; M-D, Mesial-distal; B-L, buccal-lingual.
1. Tan WL, Wong TL, Wong MC, Lang NP. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral implants Res (2012) 23 Suppl 5:1–21. doi: 10.1111/j.1600-0501.2011.02375.x
2. Araújo MG, Silva CO, Misawa M, Sukekava F. Alveolar socket healing: what can we learn? Periodontol 2000 (2015) 68(1):122–34. doi: 10.1111/prd.12082
3. Buser D, Chappuis V, Belser UC, Chen S. Implant placement post extraction in esthetic single tooth sites: when immediate, when early, when late? Periodontol 2000 (2017) 73(1):84–102. doi: 10.1111/prd.12170
4. Bertl K, Neuner H, Meran A, Bertl MH, Reich I, Nemec M, et al. Does the time-point of orthodontic space closure initiation after tooth extraction affect the incidence of gingival cleft development? A randomized controlled clinical trial. J periodontol (2020) 91(5):572–81. doi: 10.1002/jper.19-0376
5. Stappert D, Geiman R, Zadi ZH, Reynolds MA. Gingival clefts revisited: evaluation of the characteristics that make one more susceptible to gingival clefts. Am J orthodontics dentofacial orthopedics (2018) 154(5):677–82. doi: 10.1016/j.ajodo.2018.01.018
6. Kumagai H, Fueki K, Yoshida-Kohno E, Wakabayashi N. Factors associated with mucosal pain in patients with partial removable dental prostheses. J Oral Rehabil (2016) 43(9):683–91. doi: 10.1111/joor.12417
7. Huumonen S, Haikola B, Oikarinen K, Söderholm AL, Remes-Lyly T, Sipilä K. Residual ridge resorption, lower denture stability and subjective complaints among edentulous individuals. J Oral Rehabil (2012) 39(5):384–90. doi: 10.1111/j.1365-2842.2011.02284.x
8. Avila-Ortiz G, Elangovan S, Kramer KW, Blanchette D, Dawson DV. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J Dental Res (2014) 93(10):950–8. doi: 10.1177/0022034514541127
9. Avila-Ortiz G, Chambrone L, Vignoletti F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J Clin periodontol (2019) 46 Suppl 21:195–223. doi: 10.1111/jcpe.13057
10. Canullo L, Del Fabbro M, Khijmatgar S, Panda S, Ravidà A, Tommasato G, et al. Dimensional and histomorphometric evaluation of biomaterials used for alveolar ridge preservation: A systematic review and network meta-analysis. Clin Oral investigations (2022) 26(1):141–58. doi: 10.1007/s00784-021-04248-1
11. Fillingham Y, Jacobs J. Bone grafts and their substitutes. Bone Joint J (2016) 98-b(1 Suppl A):6–9. doi: 10.1302/0301-620x.98b.36350
12. Zhao R, Yang R, Cooper PR, Khurshid Z, Shavandi A, Ratnayake J. Bone grafts and substitutes in dentistry: A review of current trends and developments. Molecules (Basel Switzerland) (2021) 26(10):3007. doi: 10.3390/molecules26103007
13. Zimmermann G, Moghaddam A. Allograft bone matrix versus synthetic bone graft substitutes. Injury (2011) 42 Suppl 2:S16–21. doi: 10.1016/j.injury.2011.06.199
14. Haugen HJ, Lyngstadaas SP, Rossi F, Perale G. Bone grafts: which is the ideal biomaterial? J Clin periodontol (2019) 46 Suppl 21:92–102. doi: 10.1111/jcpe.13058
15. Kim YK, Lee J, Um IW, Kim KW, Murata M, Akazawa T, et al. Tooth-derived bone graft material. J Korean Assoc Oral Maxillofac Surgeons (2013) 39(3):103–11. doi: 10.5125/jkaoms.2013.39.3.103
16. Grawish ME, Grawish LM, Grawish HM, Grawish MM, Holiel AA, Sultan N, et al. Demineralized dentin matrix for dental and alveolar bone tissues regeneration: an innovative scope review. Tissue Eng regenerative Med (2022) 19(4):687–701. doi: 10.1007/s13770-022-00438-4
17. Kim MG, Lee JH, Kim GC, Hwang DS, Kim CH, Kim BJ, et al. The effect of autogenous tooth bone graft material without organic matter and type I collagen treatment on bone regeneration. Maxillofac Plast reconstructive Surg (2021) 43(1):17. doi: 10.1186/s40902-021-00302-w
18. Zhang S, Li X, Qi Y, Ma X, Qiao S, Cai H, et al. Comparison of autogenous tooth materials and other bone grafts. Tissue Eng regenerative Med (2021) 18(3):327–41. doi: 10.1007/s13770-021-00333-4
19. Marconcini S, Denaro M, Cosola S, Gabriele M, Toti P, Mijiritsky E, et al. Myofibroblast gene expression profile after tooth extraction in the rabbit. Materials (Basel Switzerland) (2019) 12(22):3697. doi: 10.3390/ma12223697
20. Bernardi S, Mummolo S, Varvara G, Marchetti E, Continenza MA, Marzo G, et al. Bio-morphological evaluation of periodontal ligament fibroblasts on mineralized dentin graft: an in vitro study. J Biol regulators homeostatic Agents (2019) 33(1):275–80.
21. Bianchi S, Mancini L, Torge D, Cristiano L, Mattei A, Varvara G, et al. Bio-morphological reaction of human periodontal ligament fibroblasts to different types of dentinal derivates: in vitro study. Int J Mol Sci (2021) 22(16):8681. doi: 10.3390/ijms22168681
22. Lim HC, Shin HS, Cho IW, Koo KT, Park JC. Ridge preservation in molar extraction sites with an open-healing approach: A randomized controlled clinical trial. J Clin periodontol (2019) 46(11):1144–54. doi: 10.1111/jcpe.13184
23. Karcioglu O, Topacoglu H, Dikme O, Dikme O. A systematic review of the pain scales in adults: which to use? Am J Emergency Med (2018) 36(4):707–14. doi: 10.1016/j.ajem.2018.01.008
24. Wessels R, De Roose S, De Bruyckere T, Eghbali A, Jacquet W, De Rouck T, et al. The mucosal scarring index: reliability of a new composite index for assessing scarring following oral surgery. Clin Oral investigations (2019) 23(3):1209–15. doi: 10.1007/s00784-018-2535-6
25. Suttapreyasri S, Leepong N. Influence of platelet-rich fibrin on alveolar ridge preservation. J craniofacial Surg (2013) 24(4):1088–94. doi: 10.1097/SCS.0b013e31828b6dc3
26. Nagelschmidt M, Becker D, Bönninghoff N, Engelhardt GH. Effect of fibronectin therapy and fibronectin deficiency on wound healing: A study in rats. J Trauma (1987) 27(11):1267–71. doi: 10.1097/00005373-198711000-00011
27. Peck S. Extractions, retention and stability: the search for orthodontic truth. Eur J orthodontics (2017) 39(2):109–15. doi: 10.1093/ejo/cjx004
28. Lindskog-Stokland B, Hansen K, Ekestubbe A, Wennström JL. Orthodontic tooth movement into edentulous ridge areas–a case series. Eur J orthodontics (2013) 35(3):277–85. doi: 10.1093/ejo/cjr029
29. Oltramari PV, de Lima Navarro R, Henriques JF, Taga R, Cestari TM, Ceolin DS, et al. Orthodontic movement in bone defects filled with xenogenic graft: an experimental study in minipigs. Am J orthodontics dentofacial orthopedics (2007) 131(3):302. doi: 10.1016/j.ajodo.2006.07.020
30. Seifi M, Arayesh A, Shamloo N, Hamedi R. Effect of nanocrystalline hydroxyapatite socket preservation on orthodontically induced inflammatory root resorption. Cell J (2015) 16(4):514–27. doi: 10.22074/cellj.2015.496
31. Araújo MG, Carmagnola D, Berglundh T, Thilander B, Lindhe J. Orthodontic movement in bone defects augmented with bio-oss. An experimental study in dogs. J Clin periodontol (2001) 28(1):73–80. doi: 10.1034/j.1600-051x.2001.280111.x
32. Reichert C, Wenghöfer M, Götz W, Jäger A. Pilot study on orthodontic space closure after guided bone regeneration. J orofacial orthopedics = Fortschr der Kieferorthopadie (2011) 72(1):45–50. doi: 10.1007/s00056-010-0006-z
33. Reichert C, Wenghoefer M, Kutschera E, Götz W, Jäger A. [Ridge preservation with synthetic nanocrystalline hydroxyapatite reduces the severity of gingival invaginations-a prospective clinical study]. J orofacial orthopedics = Fortschr der Kieferorthopadie (2014) 75(1):7–15. doi: 10.1007/s00056-013-0175-7
34. Ru N, Liu SS, Bai Y, Li S, Liu Y, Wei X. Boneceramic graft regenerates alveolar defects but slows orthodontic tooth movement with less root resorption. Am J orthodontics dentofacial orthopedics (2016) 149(4):523–32. doi: 10.1016/j.ajodo.2015.09.027
35. Jiang S, Liu T, Wu G, Li W, Feng X, Pathak JL, et al. Bmp2-functionalized biomimetic calcium phosphate graft promotes alveolar defect healing during orthodontic tooth movement in beagle dogs. Front bioengineering Biotechnol (2020) 8:517. doi: 10.3389/fbioe.2020.00517
36. Urist MR. Bone: formation by autoinduction. Sci (New York NY) (1965) 150(3698):893–9. doi: 10.1126/science.150.3698.893
37. Bang G, Urist MR. Bone induction in excavation chambers in matrix of decalcified dentin. Arch Surg (Chicago Ill: 1960) (1967) 94(6):781–9. doi: 10.1001/archsurg.1967.01330120035008
38. Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral surgery Oral medicine Oral pathology Oral radiology endodontics (2010) 109(4):496–503. doi: 10.1016/j.tripleo.2009.10.017
39. Jeong KI, Kim SG, Kim YK, Oh JS, Jeong MA, Park JJ. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant dentistry (2011) 20(6):471–5. doi: 10.1097/ID.0b013e3182386d74
40. Reis-Filho CR, Silva ER, Martins AB, Pessoa FF, Gomes PV, de Araújo MS, et al. Demineralised human dentine matrix stimulates the expression of vegf and accelerates the bone repair in tooth sockets of rats. Arch Oral Biol (2012) 57(5):469–76. doi: 10.1016/j.archoralbio.2011.10.011
41. Kim YK, Kim SG, Yun PY, Yeo IS, Jin SC, Oh JS, et al. Autogenous teeth used for bone grafting: A comparison with traditional grafting materials. Oral surgery Oral medicine Oral Pathol Oral Radiol (2014) 117(1):e39–45. doi: 10.1016/j.oooo.2012.04.018
42. Brkovic BM, Prasad HS, Rohrer MD, Konandreas G, Agrogiannis G, Antunovic D, et al. Beta-tricalcium phosphate/type I collagen cones with or without a barrier membrane in human extraction socket healing: clinical, histologic, histomorphometric, and immunohistochemical evaluation. Clin Oral investigations (2012) 16(2):581–90. doi: 10.1007/s00784-011-0531-1
43. Saito H, Couso-Queiruga E, Shiau HJ, Stuhr S, Prasad H, Allareddy TV, et al. Evaluation of poly lactic-co-glycolic acid-coated B-tricalcium phosphate for alveolar ridge preservation: A multicenter randomized controlled trial. J periodontol (2021) 92(4):524–35. doi: 10.1002/jper.20-0360
44. Lodi G, Azzi L, Varoni EM, Pentenero M, Del Fabbro M, Carrassi A, et al. Antibiotics to prevent complications following tooth extractions. Cochrane Database systematic Rev (2021) 2(2):Cd003811. doi: 10.1002/14651858.CD003811.pub3
45. Schmidt-Schultz TH, Schultz M. Intact growth factors are conserved in the extracellular matrix of ancient human bone and teeth: A storehouse for the study of human evolution in health and disease. Biol Chem (2005) 386(8):767–76. doi: 10.1515/bc.2005.090
46. Gao J, Symons AL, Bartold PM. Expression of transforming growth factor-beta 1 (Tgf-beta1) in the developing periodontium of rats. J Dental Res (1998) 77(9):1708–16. doi: 10.1177/00220345980770090701
47. Morikawa M, Derynck R, Miyazono K. Tgf-B and the tgf-B Family: context-dependent roles in cell and tissue physiology. Cold Spring Harbor Perspect Biol (2016) 8(5):a021873. doi: 10.1101/cshperspect.a021873
48. Pikuła M, Langa P, Kosikowska P, Trzonkowski P. Stem cells and growth factors in wound healing. Postepy higieny i medycyny doswiadczalnej (Online) (2015) 69:874–85. doi: 10.5604/17322693.1162989
49. Zarei F, Soleimaninejad M. Role of growth factors and biomaterials in wound healing. Artif cells nanomed Biotechnol (2018) 46(sup1):906–11. doi: 10.1080/21691401.2018.1439836
50. Sculean A, Gruber R, Bosshardt DD. Soft tissue wound healing around teeth and dental implants. J Clin periodontol (2014) 41 Suppl 15:S6–22. doi: 10.1111/jcpe.12206
51. Kim YK, Lee JH, Um IW, Cho WJ. Guided bone regeneration using demineralized dentin matrix: long-term follow-up. J Oral Maxillofac Surg (2016) 74(3):515. doi: 10.1016/j.joms.2015.10.030
52. Del Canto-Díaz A, de Elío-Oliveros J, Del Canto-Díaz M, Alobera-Gracia MA, Del Canto-Pingarrón M, Martínez-González JM. Use of autologous tooth-derived graft material in the post-extraction dental socket. Pilot study. Medicina oral patologia Oral y cirugia bucal (2019) 24(1):e53–60. doi: 10.4317/medoral.22536
53. Elfana A, El-Kholy S, Saleh HA, Fawzy El-Sayed K. Alveolar ridge preservation using autogenous whole-tooth versus demineralized dentin grafts: A randomized controlled clinical trial. Clin Oral implants Res (2021) 32(5):539–48. doi: 10.1111/clr.13722
54. Rinastiti M, Harijadi, Santoso AL, Sosroseno W. Histological evaluation of rabbit gingival wound healing transplanted with human amniotic membrane. Int J Oral Maxillofac Surg (2006) 35(3):247–51. doi: 10.1016/j.ijom.2005.09.012
55. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair regeneration (2008) 16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x
56. Geng Z, Cheng Y, Ma L, Li Z, Cui Z, Zhu S, et al. Nanosized strontium substituted hydroxyapatite prepared from egg shell for enhanced biological properties. J biomaterials Appl (2018) 32(7):896–905. doi: 10.1177/0885328217748124
57. Wang Y, Zhang H, Hu Y, Jing Y, Geng Z, Su J. Bone repair biomaterials: A perspective from immunomodulation. Advanced Funct Materials (2022) 32(51):2208639. doi: 10.1002/adfm.202208639
Keywords: demineralized dentin matrix, alveolar ridge preservation, gingival healing, bone graft materials, orthodontics
Citation: Xu X, Peng D, Zhou B, Lin K, Wang S, Zhao W, Zheng M, Yang J and Guo J (2023) Demineralized dentin matrix promotes gingival healing in alveolar ridge preservation of premolars extracted for orthodontic reason: a split-mouth study. Front. Endocrinol. 14:1281649. doi: 10.3389/fendo.2023.1281649
Received: 22 August 2023; Accepted: 03 October 2023;
Published: 19 October 2023.
Edited by:
Nianye Zheng, Washington University in St. Louis, United StatesReviewed by:
Zhen Geng, Shanghai University, ChinaCopyright © 2023 Xu, Peng, Zhou, Lin, Wang, Zhao, Zheng, Yang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minqian Zheng, em1xMjIxMDkyQHNpbmEuY29t; Jin Yang, eS1qaW5AMTYzLmNvbQ==; Jianbin Guo, amlhbmJpbkBmam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.