
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 27 November 2023
Sec. Obesity
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1281203
This article is part of the Research TopicObesity and Type 2 Diabetes Mellitus: Novel and Alternative Functional Bioactive Nutritional InterventionsView all 12 articles
 Wei Fang1†
Wei Fang1† Xiaojie Yuan2†
Xiaojie Yuan2† Weijian Li3,4
Weijian Li3,4 Samuel Seery5
Samuel Seery5 Guanzhi Chen6
Guanzhi Chen6 Zefeng Cai4
Zefeng Cai4 Zegui Huang3,4
Zegui Huang3,4 Xianxuan Wang3,4
Xianxuan Wang3,4 Weiqiang Wu4
Weiqiang Wu4 Zhichao Chen4
Zhichao Chen4 Yan Li1*
Yan Li1* Shouling Wu7*
Shouling Wu7* Youren Chen4*
Youren Chen4*Background: Excessive weight gain and obesity are widely accepted as risk factors for diabetes mellitus, and the age at which obesity onsets may be related to the development of cardiovascular diseases and certain cancers. Here, we aimed to investigate associations between the onset-age of overweight/obesity and risk of developing diabetes mellitus in China.
Methods: 42,144 people with the normal weight range and without diabetes at baseline, were enrolled from the Kailuan cohort which began on the 1st June 2006. All participants were followed-up, biennially, until 31st December 2017. During follow-up, 11,220 participants had become overweight/obese. For each case, one normal-weight control was matched according to age ( ± 1 year) and sex. Our final analysis included 10,858 case-control pairs. An age-scaled Cox model was implemented to estimate hazard ratios (HR) with corresponding 95% confidence intervals (CI) for diabetes mellitus incidence across age-groups.
Results: At a median follow-up of 5.46 years, 1,403 cases of diabetes mellitus were identified. After multivariate adjustments, age-scaled Cox modelling suggested that risk gradually attenuated with every 10 year increase in age of onset of overweight/obesity. Diabetes mellitus adjusted HRs (aHRs) for new-onset overweight/obesity at <45years, 45-54 years, and 55-64 years were 1.47 (95%CI, 1.12-1.93), 1.38 (95%CI, 1.13-1.68), 1.32 (95%CI, 1.09-1.59), respectively. However, new-onset of overweight/obesity at ≥65 years did not relate to diabetes mellitus (aHR, 1.20; 95%CI, 0.92-1.57). This trend was not observed in women or the new-onset obesity subgroup but was evident in men and the new overweight onset subgroup.
Conclusion: Participants with early onset of excessive weight gain issues are at considerably higher risk of developing diabetes mellitus compared to those who maintain a normal weight.
Lifestyles change substantially with increased economic prosperity although these changes are not always positive. We are witnessing a type 2 diabetes mellitus pandemic which is closely linked to sedentary lifestyles and weight-gain; however, the prevalence of type 1 is also rising. Diabetes has therefore become a major public health concern in both India and China, where there has been substantial economic development and almost one 5th of the world’s population reside. In China alone, the prevalence of overweight/obese adults is approximately 34.3% and 16.4%, respectively (1, 2). Perhaps even more alarming is the prevalence of obesity in children which is rapidly rising (3, 4).
Coincidentally, the prevalence of diabetes has rapidly increased from 9.7% in 2007 to 11.2% in 2017 among Chinese adults (5). Therefore, special attention must be paid to excessive weight gain and obese individuals in China because diabetes mellitus creates a huge economic burden for governments and for individuals, who not only encounter well-known macro and micro-vascular complications, but also encounter depression, anxiety, and all too frequently, die early (6, 7). In more developed societies, excessive weight gain and obesity are widely accepted as risk factors for diabetes mellitus. However, there are genetic differences and lifestyle factors which contribute to insulin resistance and therefore the prevalence of diabetes varies between nationalities and within ethnicities (8). Chinese researchers have postulated that multisectoral efforts are required to address the diabetes epidemic in China; however, these efforts must not be entirely reactive. We need to develop evidence-based preventive strategies to tackle this growing problem.
Demarcation between pre-symptomatic diabetic cases and those who encounter symptoms remains unclear, especially for the public. For example, people may attribute fatigue and macro and micro-vascular issues to ageing rather than being signs of diabetes which should initiate health seeking behaviors. Given the magnitude of the clinical iceberg in China, this is not always the case and so we, as a global community must learn about the differences between and within nationalities in order to identify (and intervene) pre-symptomatic cases. Two studies from Kailuan cohort found that hypertension and diabetes mellitus are risk factors for developing cardiovascular diseases (CVD), which were different across different onset ages in China (9, 10). Further research has suggested that the age at which obesity onsets may be related to the development of cardiovascular diseases and certain cancers (11, 12). However, little is known about correlations between the age of onset of overweight/obesity and risk of developing diabetes mellitus, especially across the mainland Chinese population. It is also hoped that by studying a large, Chinese cohort, we will add to the comparative evidence-base to ensure public health interventions are more highly specified. Therefore, we aimed to investigate associations between the onset-age of overweight/obesity and risk of developing diabetes mellitus in China.
This is an exposure-control matched cohort study based on Kailuan cohort in Tangshan, Hebei province. From June 2006 to October 2007, participants from the Kailuan community completed questionnaires and a first survey in Kailuan General Hospital and 10 affiliated hospitals. Subsequent surveys including questionnaires and blood tests were provided every two years, in 2008 to 2009, 2010 to 2011, 2012 to 2013, 2014 to 2015, and 2016 to 2017. All questionnaires were completed by trained nurses and blood tests were taken by laboratory technicians. Data entry was performed by double entry using Epidata, and those with more than half the required data missing were excluded.
A total of 101,510 participants aged between 18-98 years were recruited between 2006 to 2007. After excluding 59,366 participants who did not have baseline body mass index (BMI) or fasting plasma glucose (FBG) information (n = 313), overweight/obese participants (n = 42,586), 3,144 with low-weight, had been diagnosed with diabetes mellitus (n = 9,489) at baseline, or 3,834 lost to follow-up and there were 42,144 participants available for matching (Figure S1A). From 2008 to 2015, 11,220 were considered new-onset excessive weight gain, which included overweight cases and those considered obese. After randomly matching participants with overweight or obese participants with those who maintained a normal BMI across the follow-up period according to age (+/- 1 year), sex and visitations, a total of 21,716 participants (overweight/obese, n=10,858; normal-weight, n=10,858) were finally included. The study was based on The Kailuan Study (trial identification: ChiCTR-TNC-11001489), approved by ethics committee of Kailuan General Hospital. Informed consent was required before individuals were granted access to participate.
During each examination, weight and height were recorded using calibrated RGZ-120 scales with participants removed footwear and over-clothes. Measures were rounded to the nearest 0.1 kg for weight and 0.1 cm for height. Weight status was ascertained according to BMI, which was calculated by dividing body weight (kg) by height squared (m2). Cut-off points were predetermined according to the World Health Organization’s standard ranges (i.e. underweight, BMI < 18.5 kg/m2; normal-weight, 18.5 ≤ BMI <25.0 kg/m2; overweight, 25.0 ≤ BMI < 30.0 kg/m2; and obesity, BMI ≥ 30.0 kg/m2) (13).
New-onset weight gain was defined according to BMI changes from normal-weight at baseline to overweight or obese recorded prior to 31st December 2015 or the point of diabetes mellitus diagnosis. Every case was matched with a control who maintained normal-weight across the follow-up period, according age (+/-1 year), sex and visit time (Figure S1B).
Diabetes mellitus incidence was determined according to FBG ≥7.00 mmol/L (126 mg/dL) and/or treatment with anti-hyperglycemic drugs, and/or self-reported physician-diagnosed diabetes mellitus during the follow-up period according to American Diabetes Association guidelines (14). Blood samples from each participant were collected in the morning of each survey after at least a 12-hour fast. FBG was tested using hexokinase method by automatic biochemical analyzer (Hitachi 747; Hitachi, Tokyo, Japan). Diabetes histories and related treatments were collected by trained nurses through structured questionnaires (details are provided in the Supplementary Materials). We did not further distinguish type 1 or type 2 diabetes mellitus. The baseline for this study was defined according to the onset time of excessive weight gain or the time that participants with normal-weight were matched. All participants were followed-up until the date of diabetes mellitus diagnosis or until the final visit on the 31st December 2017, whichever came first.
Data around other related variables were also collected and updated through questionnaires and blood tests every two year. Covariates were derived from the examination year at which each matched pair was confirmed. Family history of diabetes, education level, physical activity, cigarette smoking and alcohol drinking status were obtained through self-reported questionnaires. Education level was defined as “less than high school”, “high school”, or “university degree or higher”. Active physical activity was defined as aerobic exercise ≥ 3 times per week. Smoking and alcohol drinking status were stratified into three levels: “current”, “former” and “never”. Total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C) and low-density lipoprotein-cholesterol (LDL-C) were also tested using an automatic biochemical analyzer (Hitachi 747; Hitachi, Tokyo, Japan). Blood pressure was measured three times, and mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) was used.
New-onset weight gain including participants with overweight or obesity and matched controls were stratified into four groups according to onset age: <45 years, 45-54 years, 55-64 years and ≥65 years. Continuous variables were expressed as means with corresponding standard deviations (SD) and compared using Student’s t test or one-way analysis of variance (ANOVA) analysis. Categorical variables were shown as proportions and compared using a standard Chi-Square test.
An age-scaled Cox regression model, which took age rather than follow-up time as the time scale, was used to calculate the hazard ratios (HR) and 95% confidence intervals (CI) for risk of developing diabetes mellitus at new-onset overweight/obesity, compared with normal-weight participants across age-groups (15). Multivariate adjusted models were implemented for systolic blood pressure (SBP), FBG, TG, HDL-C, LDL-C, cigarette smoking status, alcohol drinking status, physical activity, family history of diabetes and education level, considering a high collinearity between SBP and DBP as well as TC, HDL-C and LDL-C. A further form of subgroup analysis compared by sex and among overweight and obese participants, separately. To evaluate fluctuations in body weight, we conducted subgroup analysis of those who reduced their weight to within the normal BMI range and those who maintained their overweight or obese status.
To test robustness and address the potential for reverse causation, we further performed sensitivity analyses by excluding participants who were diagnosed with malignant tumors during study, and those who encountered diabetes mellitus within the first year of follow-up.
All analyses were performed using SAS (Version 9.4). A fully conditional specification method was used to impute missing values for covariates using multivariate imputation by chained equation (MICE) method (16, 17). Details of missing covariates were presented in Table S1. All statistical tests were two-sided, and p <0.05 set as the threshold for statistical significance.
A total of 21,716 participants (overweight/obese, n=10,858; normal-weight, n=10,858) were finally included. Aggregated participant characteristics are provided in Tables 1, 2. Compared with participants categorized as normal-weight, new-onset overweight/obese subjects had higher FBG, SBP, DBP, TC, TG and LDL-C level, but lower HDL-C levels and a lower prevalence of smokers (Table 1). Among people with new-onset overweight/obesity a younger onset age correlated with lower FBG, SBP, DBP, TC, HDL-C, and LDL-C levels (Table 2). Additionally, these people were more likely to be smokers, alcohol drinkers, and physically inactive. They also had higher levels of TG and education overall but an increased prevalence in the family history of diabetes compared to those with an older onset age.
During a median of 5.46 years (118,381 person-years) follow-up, we identified 1,403 previously undiagnosed diabetes cases. Compared to those who maintained a normal-weight during follow-up, people with new-onset overweight/obesity showed a higher risk of developing diabetes (adjusted HR [aHR], 1.29; 95% CI 1.02-1.63). However, the risk was different across onset ages (P for interaction < 0.05). As shown in Figure 1, the incidence and rate of diabetes were higher in people with new-onset overweight/obesity across all age-groups. In conjunction with increasing age, the number and rate appeared to consistently increase.
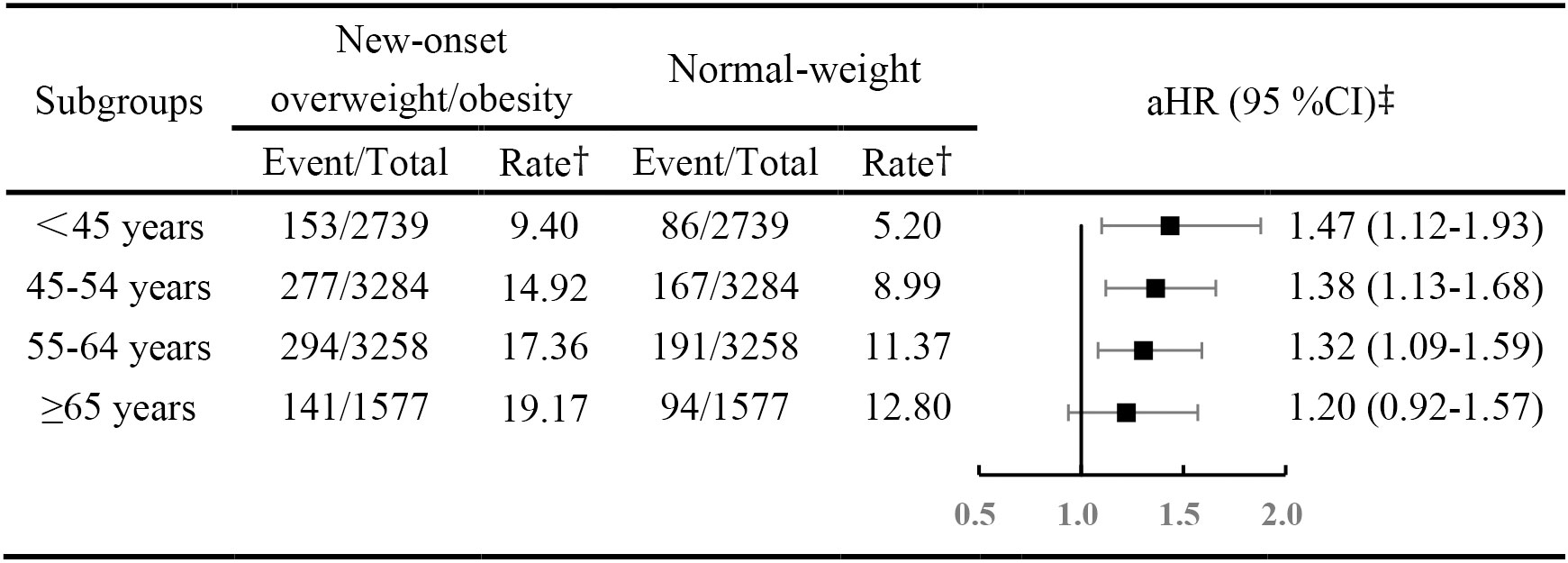
Figure 1 Hazard rations for diabetes mellitus across age-based onset groups, among new onset overweight and obesity versus normal-weight participants. † The rate was per 1,000 person years. ‡ Adjusted for systolic blood pressure, fasting blood glucose, triglyceride, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, cigarette smoking status, alcohol drinking status, physical exercise, family history of diabetes, education degree. Abbreviations: aHR, adjusted hazard ratio; CIs, confidence intervals.
Compared with those considered to have maintained a normal weight, people with overweight/obesity were at a higher risk of developing diabetes, after adjusting for education level, cigarette smoking and alcohol drinking status, physical activity, family history of diabetes, SBP, TG, LDL-C and HDL-C level. The risk of developing diabetes gradually attenuated with every decade increase in age at onset of overweight/obesity, with a aHR of 1.47 (95% CI, 1.12-1.93) in those onset age <45 years, 1.38 (95% CI, 1.13-1.68) in those onset age from 45 to 54 years and 1.32 (95% CI, 1.09-1.59) in those onset age from 55 to 64 years. Although, those whose onset age was 65 years or older did not appear at higher risk (aHR,1.22; 95% CI, 0.94-1.59).
Stratified by sex, results did not change substantially in males, with an aHR of 1.56 (95% CI, 1.16-2.10) in those onset age<45 years, 1.31 (95% CI, 1.04-1.65) in those onset age from 45 to 54 years and 1.30 (95% CI, 1.05-1.62) in those onset age from 55 to 64 years (Table 3). However, a positive correlation was observed in the relationship between women with overweight/obesity whose onset age was between 45 to 54 years (aHR, 1.58; 95% CI, 1.09-2.28). Although this was not considered a significant interaction (P = 0.743).
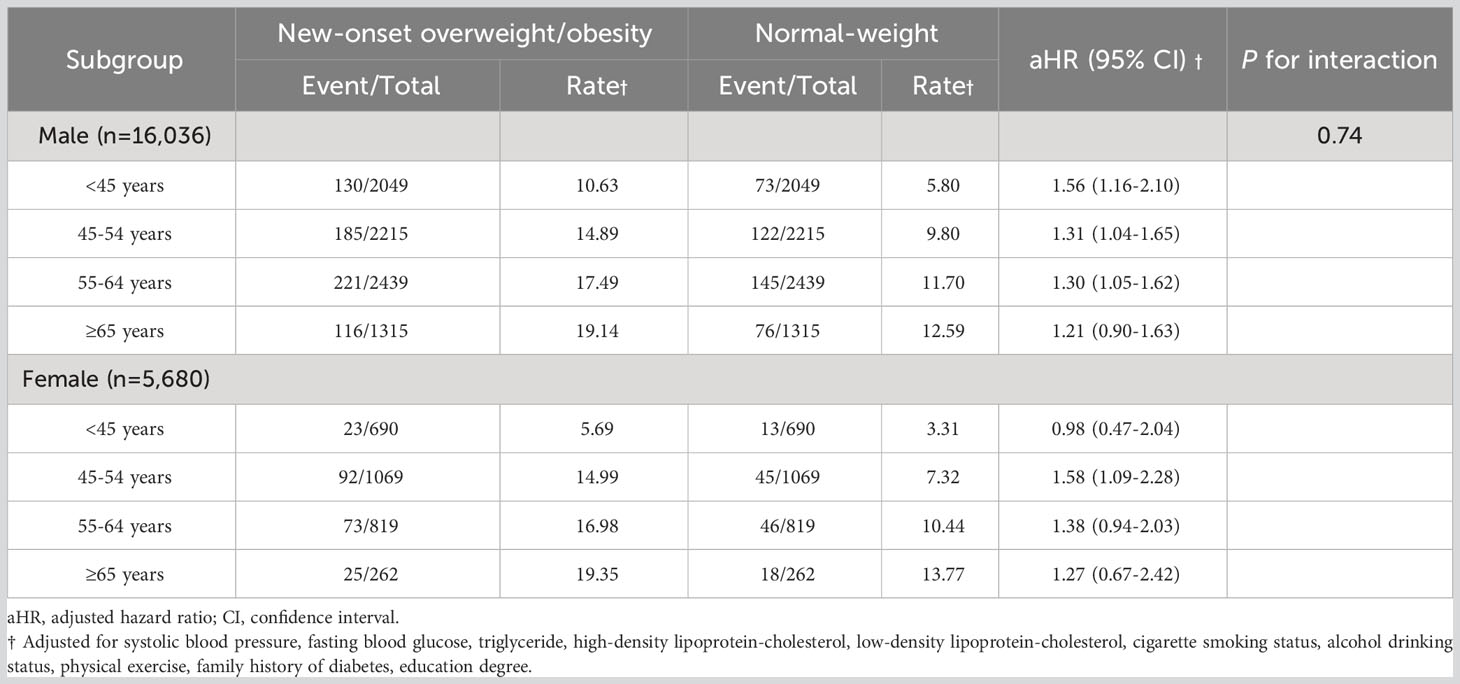
Table 3 Hazard ratios for diabetes mellitus across age-based onset groups, among new-onset overweight and obesity versus normal-weight participants by sex.
We further divided the overweight/obesity group into participants with overweight and participants with obesity in order to compare risks of developing diabetes (Table 4). The overweight group had a significant association with DM occurrence, with an aHR of 1.44 (95% CI, 1.09-1.91) in those onset age<45 years, 1.37 (95% CI, 1.12-1.67) in those onset age from 45 to 54 years and 1.32 (95% CI, 1.09-1.59) in those onset age from 55 to 64 years. However, there was no significant finding in those considered obesity (All P > 0.05).
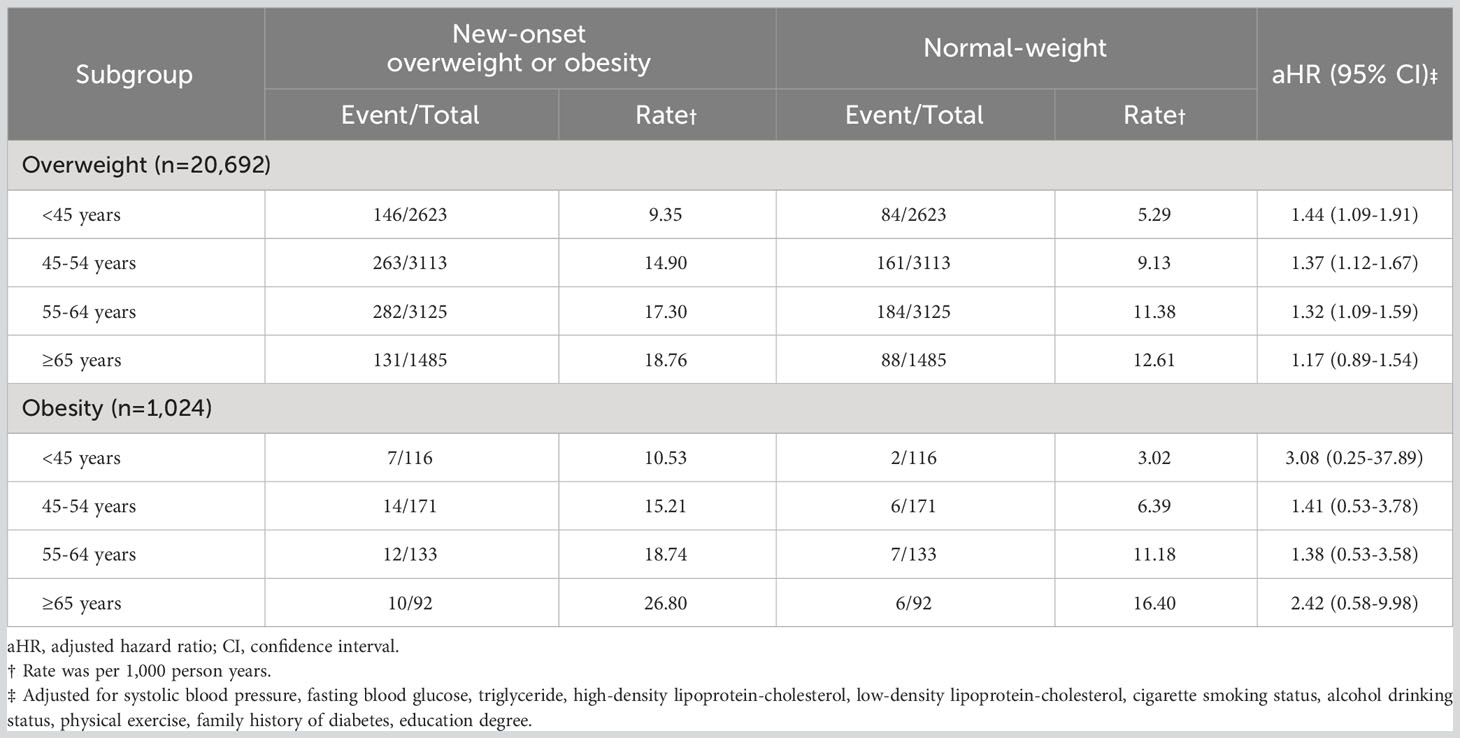
Table 4 Hazard ratios for incident diabetes mellitus among patients with new-onset overweight and obesity versus normal-weight participants, across separate age-groups.
To assess the influence of weight fluctuations, we further divided the overweight/obese group into participants who were initially categorized as overweight or obese but who subsequently achieved normal BMI, and those who remained in the overweight or obese category (Table 5). Results showed that regardless of the onset age reducing weight to within a normal BMI range significantly reduced the risk of developing diabetes. The HR was 0.35 (95% CI, 0.24-0.49) in those with an onset age <45 years, and 0.31 (95% CI, 0.24-0.41) in those onset age from 45 to 54 years, 0.35 (95% CI, 0.27-0.45) in those onset age from 55 to 64 years and 0.30 (95% CI, 0.21-0.44) in those onset age ≥65 years.

Table 5 Hazard ratios for incident diabetes mellitus of changed to a normal BMI among patients with new-onset overweight and obesity, across separate age-groups.
Results remained consistent after excluding participants who were diagnosed with malignant tumors during study and after excluding those with outcome events within the first year of follow-up (Table 6).
Our findings suggest that new-onset overweight/obesity is associated with a higher risk developing diabetes mellitus among Chinese mainlanders, although the magnitude of this effect varied across the lifespan. Participants considered (using BMI) as overweight or obese at age <45 years were at the highest risk of developing diabetes mellitus, compared with age- and sex- matched controls. The aforementioned risk gradually attenuated with each decade increase in excessive weight gain onset age. The results also remained stable in men and when analysis was constricted to overweight participants, but not for those considered obese.
To date, few studies have investigated the relationship between excessive weight gain onset age and risk of developing diabetes. In a related, British birth cohort study, which compared those who had never been obese, childhood obesity and younger-adulthood obesity have a 4.38-fold (95% CI, 1.86–10.31) and 3.96-fold (95%CI, 2.10-7.43) risk of hemoglobin A1c (HbA1c) ≥7%, respectively (18). HbA1c is an indicator for glucose metabolism, where a threshold of 6.5% could also be used to diagnose diabetes mellitus (19). An HbA1c reading of between 6.0–6.9% corresponds with 60% developing diabetes within a 10 years follow-up period (20). In this study, similar trends were found for onset age of overweight/obesity which associations were non-significant at a younger-adulthood and mid-adulthood onset age (18), which is similar with our findings. These studies suggest the impact of excessive weight gain in terms of diabetes across the life course, although single comprehensive studies which investigate these changes across a specific nation are few in number.
Excessive weight gain is associated with an increased risk of developing insulin resistance and type 2 diabetes. In obese individuals, adipose tissue releases increased amounts of non-esterified fatty acids, glycerol, hormones, pro-inflammatory cytokines and other factors that are involved in the development of insulin resistance (21). When insulin resistance is accompanied by dysfunction of pancreatic islet beta-cells, failure to control blood glucose levels results in type 2 diabetes. A previous study reported that in addition to initial BMI, obesity onset at younger ages also means cumulative exposure, which is associated with an increased risk of developing type 2 diabetes (22). This supports our finding that an early onset age of excessive weight gain also means a longer cumulative exposure to detrimental factors. Secondly, evidence suggests that earlier excessive weight gain is closely related to genetic predispositions (23, 24). Previous studies have also identified numerous genetic loci and gene variants such as FTO, MC4R, ADAMTS9 and GRB14/COBLL1, which have been found to be associated with overweight/obesity and diabetes (23, 25, 26). Early-onset overweight/obesity participants are likely to be genetically susceptible and carry the above genes which create a higher risk of diabetes. Thirdly, being overweight or obese could interfere with age induced epigenetic changes including DNA methylation, non-coding RNA (ncRNA) and histone modifications. Altered DNA methylation in different tissues (human pancreatic islets, skeletal muscle and adipose tissue) could reduce insulin secretion and increases insulin resistance via differs patterns. Additionally, early onset overweight/obesity may initiate DNA methylation and gene expression in early adulthood and lead to high risk of diabetes (27–32). It is also reported that changes in BMI are accompanied by widespread metabolic changes in early adult, resulting in chronically increased levels of circulating free fatty acids and adipokines, which is again closely associated with diabetes (33–36). Finally, individuals with young-onset overweight/obesity tended to have an unhealthy lifestyle, which likely contributes to the development of diabetes.
In this study, participants with overweight/obesity whose onset age ≥65 years were not observed to be statistically correlate with a higher risk of incident diabetes, compared with normal-weight participants. We supposed that there were some competing risks in older adults who may develop diabetes and obesity simultaneously, reducing the HR of the association. This phenomenon was also observed in other studies (37, 38).
We conducted gender-specific stratification analysis, only observing a positive correlation among women who were overweight or obese and had an onset age of between 45 to 54 years. In overweight or obese women with an onset age <45 years, there was no correlation (aHR, 0.98; 95% CI, 0.47-2.02), which was different from men who had an aHR = 1.52 (95% CI, 1.13-2.04). In a related longitudinal Australian study of women’s health researchers found that obesity onset age negatively correlates with an increased risk of developing diabetes (aHR, 0.87; 95% CI, 0.79-0.96, per 1 year increment) (22). This may have a biological basis because premenopausal women may have a degree of protection from circulating estrogen (39). Another reason could be differences in fat distribution between men and women, where women have a greater proportion of subcutaneous fat and adipose tissue whereas men tend to harbour visceral fat on the abdomin which is a known driver in the progression of disease (40). Therefore, even though these women who became overweight/obese at an onset age <45 years, the risk of developing diabetes mellitus does not necessarily increase. This assertion is supported by sex differences in the prevalences because there are more men with pre-pubescent diabetes, whereas there are more women with postmenapausal diabetes (41). The development of diabetes mellitus after menopause is thought to occur through alterations in insulin secretion, insulin sensitivity, and glucose effectiveness (42).
We observed that the risk of women at onset age between 45 to 54 years was higher than men at same age (aHR, 1.57 vs aHR, 1.29), which suggests that the risk of developing diabetes mellitus by weight gain may be much higher in women after menopause. Though a positive correlation was observed in women at onset age higher than 55, such association was not statistically significant. This may be due to the comparatively small sample size of these groups (1,638 at an onset age of between 55 to 64 years and 524 at an onset age higher than 65 years). On the basis of the incidence of 7.26% and 8.20%, the power to find a relationship in these two groups was 0.60 and 0.26 (expected HR = 1.5).
According to previous reports, the risk of obesity may be higher than being overweight in a similar age group (18), but we did not observe that in this study. Hypothetically, this may be due to the comparatively low prevalence of obesity in China when we defined obesity according to World Health Organization (WHO) cutoff (BMI ≥ 30 kg/m2). On the other hand, because of the incidence of 6.25% and sample size of 1024, the power to find a relationship in women was only 0.37 (expected HR = 1.5). Therefore, a larger sample is required to explore this further.
Considering the influence of weight loss, we conducted a separate subgroup analysis of overweight or obese category between those who changed to a normal BMI and those maintained in the overweight or obese category. We observed a steady risk reduction in those who changed to a normal BMI, which was consistent with a retrospective cohort study of U.S. adults (HR, 0.33; 95% CI, 0.14-0.76) (43). Surprisingly, the risk reduction effect we observed was steady in all age group. That is to say, weight loss at any age to reduce the risk of developing diabetes.
Apart from diabetes mellitus, researchers have also found weight gain positively correlates to mortality and some cancers, and the younger the onset age of weight gain, the higher the risk (37, 44, 45). A typical study from the United States reported that weight gain at all ages positively relate to mortality, but with stronger associations for weight gain between ages 18 and 35 years and ages 35 and 50 years than between ages 50 and 69 years (44). Another study from the United States showed such relationship between onset age of weight gain and pancreatic cancer (37). The risk change from 1.50 (95% 1.26-1.77) from age 14 to 19 to 1.16 (95% 1.02-1.32) from age 50 to 59, and non-significant from age 60 to 69 (37). Also, age of onset of obesity may, at least in part, affect the prevalence of cardiovascular risk factors in severe obesity (46). Therefore, weight gain over different stages of life from early childhood is implicated in the development of diabetes, specific cancer, cardiovascular diseases and even mortality. Uncertainty remains about whether some life stages are more influential than others.
This was a large prospective study investigating the association between onset age of overweight/obesity and the risk of developing diabetes which use age, rather than study time, as the time scale to optimally account for the observational study design. However, this study also has several limitations. The distribution of gender in our study was imbalanced (men 73.8%) and the characteristics and mechanisms of overweight/obesity and diabetes may be different between men and women. Secondly, our definition of overweight/obesity is based on BMI, which is only an indicator of total body adiposity and could not accurately represent the distribution of adipose tissue or bone density. However, BMI is the most commonly used and practical measure of obesity both in children and adults. There are studies which have proved that BMI is generally consistent with indicators of central adiposity such as waist circumference and waist-to-height ratio (47, 48). Thirdly, the diagnosis of diabetes was based on a single measurement of FBG rather than oral glucose tolerance testing or the measurement of HbA1c, and therefore, the incidence of diabetes might be underestimated. Finally, our regression model included adjustments only for a small number of covariates, which means residual confounders may still exist.
Our study found that new-onset overweight/obesity correlates with an increased risk of developing diabetes mellitus among Chinese population <65 years. Participants with early onset excessive weight gain or obesity were at a higher risk of having diabetes. The results highlight the importance of preventing the onset of excessive weight gain as we age. Although, further research is needed to link lifestyles, developmental stages, and physiological changes to understand these interactions more clearly.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the ethics committee in Kailuan General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
WF: Formal Analysis, Software, Writing – original draft. XY: Formal Analysis, Software, Writing – original draft. WL: Data curation, Writing – review & editing. SS: Writing – review & editing. GC: Data curation, Writing – review & editing. ZFC: Data curation, Writing – review & editing. ZH: Data curation, Writing – review & editing. XW: Data curation, Writing – review & editing. WW: Data curation, Writing – review & editing. ZCC: Data curation, Writing – review & editing. YL: Funding acquisition, Methodology, Writing – review & editing. SW: Conceptualization, Project administration, Writing – review & editing. YC: Conceptualization, Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 81870312 and 82070385).
The authors thank the participants who made this cohort study possible and Professor Dianna Magliano from School of Public Health and Preventive Medicine, Monash University, for her insights.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1281203/full#supplementary-material
1. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol (2021) 9(6):373–92. doi: 10.1016/S2213-8587(21)00045-0
2. The State Council Information Office of the People's Republic of China. Press briefing for the Report on Chinese Residents' Chronic Diseases and Nutrition 2020. Available at: http://www.gov.cn/xinwen/2020-12/24/content_5572983.html (Accessed June 21, 2021).
3. Dong Y, Ma J, Song Y, Ma Y, Dong B, Zou Z, et al. Secular trends in blood pressure and overweight and obesity in chinese boys and girls aged 7 to 17 years from 1995 to 2014. Hypertension (Dallas Tex 1979) (2018) 72(2):298–305. doi: 10.1161/HYPERTENSIONAHA.118.11291
4. NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet (London England) (2017) 390(10113):2627–42. doi: 10.1016/S0140-6736(17)32129-3
5. Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ (Clinical Res ed) (2020) 369:m997. doi: 10.1136/bmj.m997
6. Sun X, Yan AF, Shi Z, Zhao B, Yan N, Li K, et al. Health consequences of obesity and projected future obesity health burden in China. Obes (Silver Spring Md) (2022) 30(9):1724–51. doi: 10.1002/oby.23472
7. Ma RCW. Epidemiology of diabetes and diabetic complications in China. Diabetologia (2018) 61(6):1249–60. doi: 10.1007/s00125-018-4557-7
8. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol (2018) 14(2):88–98. doi: 10.1038/nrendo.2017.151
9. Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol (2020) 75(23):2921–30. doi: 10.1016/j.jacc.2020.04.038
10. Zhao M, Song L, Sun L, Wang M, Wang C, Yao S, et al. Associations of type 2 diabetes onset age with cardiovascular disease and mortality: The kailuan study. Diabetes Care (2021) 44(6):1426–32. doi: 10.2337/dc20-2375
11. de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam RM. Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: a cohort study. Am J Epidemiol (2014) 179(11):1353–65. doi: 10.1093/aje/kwu052
12. Hassan MM, Abdel-Wahab R, Kaseb A, Shalaby A, Phan AT, El-Serag HB, et al. Obesity early in adulthood increases risk but does not affect outcomes of hepatocellular carcinoma. Gastroenterology (2015) 149(1):119–29. doi: 10.1053/j.gastro.2015.03.044
13. WHO Consultation on Obesity ( 1999: Geneva, Switzerland) & World Health Organization. (2000). Obesity : preventing and managing the global epidemic : report of a WHO consultation. World Health Organization. Available at: https://iris.who.int/handle/10665/42330
14. Society CD. Guidelines for prevention and treatment of type 2 diabetes in China (2020 version). Zhong Hua Tang Niao Bing Za Zhi (2021) 13(4):315–409. doi: 10.3760/cma.j.cn115791-20210221-00095
15. Cologne J, Hsu WL, Abbott RD, Ohishi W, Grant EJ, Fujiwara S, et al. Proportional hazards regression in epidemiologic follow-up studies: an intuitive consideration of primary time scale. Epidemiol (Cambridge Mass) (2012) 23(4):565–73. doi: 10.1097/EDE.0b013e318253e418
16. Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Method (2017) 17(1):162. doi: 10.1186/s12874-017-0442-1
17. Austin PC, White IR, Lee DS, van Buuren S. Missing data in clinical research: A tutorial on multiple imputation. Can J Cardiol (2021) 37(9):1322–31. doi: 10.1016/j.cjca.2020.11.010
18. Power C, Thomas C. Changes in BMI, duration of overweight and obesity, and glucose metabolism: 45 years of follow-up of a birth cohort. Diabetes Care (2011) 34(9):1986–91. doi: 10.2337/dc10-1482
19. American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S17–s38. doi: 10.2337/dc22-S002
20. Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med (2007) 120(8):720–7. doi: 10.1016/j.amjmed.2007.03.022
21. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature (2006) 444(7121):840–6. doi: 10.1038/nature05482
22. Luo J, Hodge A, Hendryx M, Byles JE. Age of obesity onset, cumulative obesity exposure over early adulthood and risk of type 2 diabetes. Diabetologia (2020) 63(3):519–27. doi: 10.1007/s00125-019-05058-7
23. Ng MC, Tam CH, So WY, Ho JS, Chan AW, Lee HM, et al. Implication of genetic variants near NEGR1, SEC16B, TMEM18, ETV5/DGKG, GNPDA2, LIN7C/BDNF, MTCH2, BCDIN3D/FAIM2, SH2B1, FTO, MC4R, and KCTD15 with obesity and type 2 diabetes in 7705 Chinese. J Clin Endocrinol Metab (2010) 95(5):2418–25. doi: 10.1210/jc.2009-2077
24. Li G, Li Y, Han L, Wang D, Zhang Q, Xiao X, et al. Interaction between early environment and genetic predisposition instigates the metabolically obese, normal weight phenotype in children: findings from the BCAMS study. Eur J Endocrinol (2020) 182(4):393–403. doi: 10.1530/EJE-19-0755
25. Robiou-du-Pont S, Bonnefond A, Yengo L, Vaillant E, Lobbens S, Durand E, et al. Contribution of 24 obesity-associated genetic variants to insulin resistance, pancreatic beta-cell function and type 2 diabetes risk in the French population. Int J Obes (2005) (2013) 37(7):980–5. doi: 10.1038/ijo.2012.175
26. Grarup N, Sandholt CH, Hansen T, Pedersen O. Genetic susceptibility to type 2 diabetes and obesity: from genome-wide association studies to rare variants and beyond. Diabetologia (2014) 57(8):1528–41. doi: 10.1007/s00125-014-3270-4
27. Rönn T, Ling C. DNA methylation as a diagnostic and therapeutic target in the battle against Type 2 diabetes. Epigenomics (2015) 7(3):451–60. doi: 10.2217/epi.15.7
28. Kim K, Joyce BT, Zheng Y, Schreiner PJ, Jacobs DR Jr., Catov JM, et al. DNA methylation grimAge and incident diabetes: the coronary artery risk development in young adults (CARDIA) study. Diabetes (2021) 70(6):1404–13. doi: 10.2337/db20-1167
29. Davegårdh C, García-Calzón S, Bacos K, Ling C. DNA methylation in the pathogenesis of type 2 diabetes in humans. Mol Metab (2018) 14:12–25. doi: 10.1016/j.molmet.2018.01.022
30. Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature (2017) 541(7635):81–6. doi: 10.1038/nature20784
31. Drong AW, Lindgren CM, McCarthy MI. The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther (2012) 92(6):707–15. doi: 10.1038/clpt.2012.149
32. Almén MS, Nilsson EK, Jacobsson JA, Kalnina I, Klovins J, Fredriksson R, et al. Genome-wide analysis reveals DNA methylation markers that vary with both age and obesity. Gene (2014) 548(1):61–7. doi: 10.1016/j.gene.2014.07.009
33. Beyene HB, Olshansky G, Smith AA, Giles C, Huynh K, Cinel M, et al. High-coverage plasma lipidomics reveals novel sex-specific lipidomic fingerprints of age and BMI: Evidence from two large population cohort studies. PloS Biol (2020) 18(9):e3000870. doi: 10.1371/journal.pbio.3000870
34. Mousa A, Naderpoor N, Mellett N, Wilson K, Plebanski M, Meikle PJ, et al. Lipidomic profiling reveals early-stage metabolic dysfunction in overweight or obese humans. Biochim Biophys Acta Mol Cell Biol Lipids (2019) 1864(3):335–43. doi: 10.1016/j.bbalip.2018.12.014
35. Würtz P, Wang Q, Kangas AJ, Richmond RC, Skarp J, Tiainen M, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PloS Med (2014) 11(12):e1001765. doi: 10.1371/journal.pmed.1001765
36. Razquin C, Toledo E, Clish CB, Ruiz-Canela M, Dennis C, Corella D, et al. Plasma lipidomic profiling and risk of type 2 diabetes in the PREDIMED trial. Diabetes Care (2018) 41(12):2617–24. doi: 10.2337/dc18-0840
37. Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. Jama (2009) 301(24):2553–62. doi: 10.1001/jama.2009.886
38. Biggs ML, Mukamal KJ, Luchsinger JA, Ix JH, Carnethon MR, Newman AB, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. Jama (2010) 303(24):2504–12. doi: 10.1001/jama.2010.843
39. De Paoli M, Werstuck GH. Role of estrogen in type 1 and type 2 diabetes mellitus: A review of clinical and preclinical data. Can J Diabetes (2020) 44(5):448–52. doi: 10.1016/j.jcjd.2020.01.003
40. Brettle H, Tran V, Drummond GR, Franks AE, Petrovski S, Vinh A, et al. Sex hormones, intestinal inflammation, and the gut microbiome: Major influencers of the sexual dimorphisms in obesity. Front Immunol (2022) 13:971048. doi: 10.3389/fimmu.2022.971048
41. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care (2004) 27(5):1047–53. doi: 10.2337/diacare.27.5.1047
42. Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav (2018) 187:20–3. doi: 10.1016/j.physbeh.2017.08.016
43. Stokes A, Collins JM, Grant BF, Scamuffa RF, Hsiao CW, Johnston SS, et al. Obesity progression between young adulthood and midlife and incident diabetes: A retrospective cohort study of U.S. Adults. Diabetes Care (2018) 41(5):1025–31. doi: 10.2337/dc17-2336
44. Adams KF, Leitzmann MF, Rachel BB, Demetrius A, Harris nB, Albert H, et al. Body mass and weight change in adults in relation to mortality risk. Am J Epidemiol (2014) 179(2):135. doi: 10.1093/aje/kwt254
45. Chen C, Ye Y, Zhang Y, Pan XF, Pan A. Weight change across adulthood in relation to all cause and cause specific mortality: prospective cohort study. BMJ (Clinical Res ed) (2019) 367:l5584. doi: 10.1136/bmj.l5584
46. Borrelli R, Contaldo F, Reed LA, Isernia C, Di Biase G, Mancini M. Cardiovascular risk factors and age of onset of obesity in severely obese patients. Int J vitamin Nutr Res Internationale Z fur Vitamin- und Ernahrungsforschung J Int vitaminologie Nutr (1988) 58(2):236–40.
47. Wannamethee SG, Papacosta O, Whincup PH, Carson C, Thomas MC, Lawlor DA, et al. Assessing prediction of diabetes in older adults using different adiposity measures: a 7 year prospective study in 6,923 older men and women. Diabetologia (2010) 53(5):890–8. doi: 10.1007/s00125-010-1670-7
Keywords: overweight, obesity, onset age, diabetes mellitus, prospective cohort
Citation: Fang W, Yuan X, Li W, Seery S, Chen G, Cai Z, Huang Z, Wang X, Wu W, Chen Z, Li Y, Wu S and Chen Y (2023) Excessive weight gain onset-age and risk of developing diabetes mellitus: a large, prospective Chinese cohort study. Front. Endocrinol. 14:1281203. doi: 10.3389/fendo.2023.1281203
Received: 22 August 2023; Accepted: 31 October 2023;
Published: 27 November 2023.
Edited by:
Júlio Cezar De Oliveira, Universidade Federal de Mato Grosso, BrazilReviewed by:
Inha Jung, Korea University Ansan Hospital, Republic of KoreaCopyright © 2023 Fang, Yuan, Li, Seery, Chen, Cai, Huang, Wang, Wu, Chen, Li, Wu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youren Chen, eXJjaGVuM0BzdHUuZWR1LmNu; Shouling Wu, ZHJ3dXNsQDE2My5jb20=; Yan Li, cHJvZmxlZXlhbkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.