- 1Department of Gynecological Endocrinology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, Beijing, China
- 2Department of Women’s Health, University of Tuebingen, University Women’s Hospital and Research Centre for Women’s Health, Tuebingen, Germany
Cervical cancer in pregnancy (CCIP) refers to cervical cancer diagnosed during pregnancy, the most common gynecological malignant tumor. Because of the special physiological changes of CCIP, although preserving ovarian function and fertility is very important, the methods are very limited. There is no guideline or consensus on the preservation methods of ovarian function and fertility in this special period. Therefore, the Committee of Fertility Protection and Preservation of China Association for the Promotion of Health Science and Technology, combined with the Chinese Society of Gynecological Endocrinology affiliated to the International Society of Gynecological Endocrinology, Society Endocrinology Branch of Beijing Institute of Obstetrics & Gynecology, combined with Society on Fertility Preservation affiliated with the Chinese Preventive Medicine Association, organized relevant experts from different disciplines to formulate this consensus, in order to guide ovarian function and fertility preservation of CCIP patients.
1 Introduction
Cervical cancer in pregnancy (CCIP) is the most common gynecological malignant tumor diagnosed during pregnancy, accounting for 71.6% (1, 2). The median age of CCIP diagnosis is about 35 years old, and the most common pathological type is squamous cell carcinoma, with a median gestational age of 18.4 weeks (3–5). Because CCIP patients are all women of reproductive age, pelvic radiotherapy, and neoadjuvant chemotherapy will seriously damage their ovarian function, and preserving ovarian function and fertility is necessary.
However, due to the particularity of CCIP patients during pregnancy, under the action of high estrogen and progesterone during pregnancy, the hypothalamus is strongly negatively fed back, which inhibits the synthesis and secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), and the follicles in the ovary are in the state of primordial or primary follicles, so ovarian stimulation cannot be carried out. Even after the termination of pregnancy, stimulation from the primordial or primary follicular state to mature follicles takes longer than non-pregnancy. In addition, CCIP patients can take delayed treatment when their condition is stable, and cervical cancer treatment, including surgery, radiotherapy, and chemotherapy, should be started as soon as possible when pregnancy is terminated or after delivery. These treatments, especially radiotherapy and chemotherapy, may lead to serious damage to ovarian function, premature ovarian failure, fertility loss, and various chronic diseases, seriously affecting patients’ quality of life and reproductive health (6, 7).
Because of the special physiological changes of CCIP, although preserving ovarian function and fertility is very important, the methods are very limited. There is no guideline or consensus on the preservation methods of ovarian function and fertility in this special period. However, due to the particularity of younger incidence of cervical cancer in China, some CCIP patients still face the special needs of ovarian function and fertility preservation. Therefore, the Committee of Fertility Protection and Preservation of China Association for the Promotion of Health Science and Technology, combined with the Chinese Society of Gynecological Endocrinology affiliated to the International Society of Gynecological Endocrinology, Society Endocrinology Branch of Beijing Institute of Obstetrics & Gynecology, combined with Society on Fertility Preservation affiliated with the Chinese Preventive Medicine Association, organized relevant experts from different disciplines to formulate this consensus, in order to guide ovarian function and fertility preservation of CCIP patients.
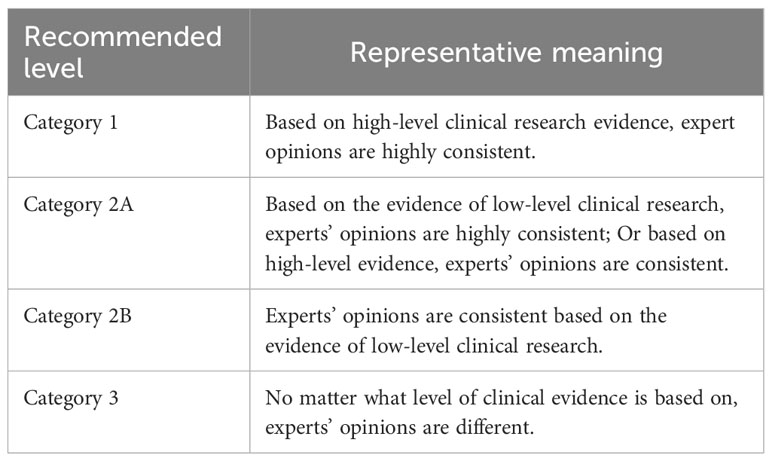
See Table 1 for the recommended level and representative significance of this consensus.
2 Multidisciplinary diagnosis and treatment in CCIP
Cervical cancer screening should be carried out according to the standard during pregnancy. The diagnosis and staging of CCIP are similar to those of non-pregnancy, and the clinical staging of CCIP is adopted by the International Federation of Gynecology and Obstetrics (FIGO), which should be differentiated from other diseases before diagnosis (8). Magnetic resonance imaging (MRI) with sufficient sensitivity and specificity is recommended for CCIP disease evaluation to provide lesion infiltration range, parametrial diffusion, and lymph node metastasis, which is helpful to make up for the dissatisfaction of gynecological examination during pregnancy and the lack of ultrasound examination (9).
The treatment of CCIP is more complex and challenging. Due to the lack of prospective studies and clinical trials and the lack of standardized procedures, it needs to be individualized, taking into account the patient’s age, tumor size, disease stage, histopathological type, lymph node involvement, pregnancy weeks, fetal intrauterine condition, the willingness of patients and their families to continue pregnancy, the desire to give birth in the future, and the advantages and disadvantages of various treatment methods. Obstetrics, neonatology, gynecological oncology, pathology, imaging, and other disciplines need to participate in formulating treatment plans. Strike a balance between effective treatment of tumors and protection of fetal health to avoid delayed treatment and premature delivery (10, 11). For details, please refer to the Chinese Expert Consensus on Diagnosis and Treatment of Pregnancy Complicated with Cervical Cancer, formulated by the Chinese Obstetricians and Gynecologists Association (COGA) (11).
Pregnancy itself may not have a negative impact on the prognosis of cervical cancer, but if the pregnancy continues and treatment is delayed, it will affect the maternal prognosis to a certain extent (5, 12, 13). In the first trimester of pregnancy, it takes a long time for the fetus to mature, which has a high risk of disease progression. Patients are generally not recommended to continue pregnancy, and they should receive routine cervical cancer treatment as soon as possible (5). For patients with early cervical cancer, delaying treatment to achieve fetal survival or improve fetal outcomes may be an option (14). For patients with 14 ~ 20 weeks of pregnancy, FIGO 2018 IB1 or IIA, neoadjuvant chemotherapy can be used until fetal maturity. The chemotherapy scheme is still managed regarding non-pregnancy chemotherapy guidelines. The platinum-based combined scheme is preferred, but chemotherapy is avoided 3 ~ 4 weeks before delivery to reduce bone marrow suppression of mother and child caused by chemotherapy (15, 16). The treatment can be postponed for 20 ~ 33 weeks of pregnancy until a cesarean section is performed to promote fetal lung maturation. For IB1 or above, neoadjuvant chemotherapy can be selected. The initial treatment time of delayed treatment should not exceed 32 ~ 34 weeks of pregnancy, and pregnancy can be terminated after fetal lung maturation (14). CCIP patients suggest a cesarean section to terminate the pregnancy, and cervical cancer should be treated routinely after delivery (13). The prognosis of CCIP patients is similar to that of non-pregnancy, related to FIGO staging and treatment methods (17).
Recommendation: CCIP’s ovarian function and fertility preservation need multidisciplinary cooperation, combined with factors such as tumor stage, gestational age, and patient’s pregnancy willingness, and choosing full communication between doctors and patients (recommended level 2A).
3 Ovarian function and fertility preservation strategy in CCIP
According to the National Cancer Institute of the National Institutes of Health data, the 5-year relative survival rate of patients with early cervical cancer is about 91%, and that of all patients with cervical cancer is 67%. However, surgery, radiotherapy, and chemotherapy will lead to ovarian insufficiency or failure, leading to early climacteric symptoms in young women, such as osteoporosis, cardiovascular and cerebrovascular diseases, and other chronic diseases, and the risk of death will increase significantly (8). The formulation of this consensus draws lessons from and strictly refers to the indications of ovarian preservation in cervical cancer (18, 19). On the premise of not affecting the survival outcome of patients with tumors, it is suggested that CCIP patients who meet the conditions of ovarian preservation should be protected in the diagnosis and treatment process to prevent the occurrence of iatrogenic premature ovarian insufficiency (POI). Because CCIP patients cannot perform ovarian stimulation and embryo and oocyte cryopreservation, this consensus only involves ovarian tissue cryopreservation and transplantation (OTCT) and ovarian transposition in CCIP patients.
3.1 Ovarian tissue cryopreservation and transplantation technology
3.1.1 Overview
OTCT technology includes removing part of ovarian tissue before gonadal toxicity treatment (including radiotherapy and chemotherapy), carrying out programmed freezing by cryobiology technology, and then cryopreservation at ultra-low temperatures. When patients need it and conditions permit, the cryopreserved ovarian tissue will be thawed and transplanted back into the body (20). In the 1860s, ovarian tissue was first cryopreserved and transplanted in rodents (21). In 1994, ovarian tissue was cryopreserved and transplanted in sheep (22). In 1996, Hovatta et al. successfully cryopreserved human ovarian tissue (23), and in 1998, the first xenogeneic model showed the feasibility of frozen-thawed human ovarian tissue transplantation (24). In 2000, the first human cryopreserved-thawed ovarian tissue was successfully transplanted in situ (25). The first human ovarian cortex was successfully heterotopic transplanted in 2001 (26), the first baby was born after human cryopreserved-thawed ovarian tissue transplantation in 2004, and the second baby was born in 2005 (27, 28). Our team successfully carried out the first Chinese orthotopic transplantation of human frozen-thawed ovarian tissue in 2016 (29), the first pregnancy after frozen-thawed ovarian tissue transplantation in 2020 (30), and the first healthy baby was born after frozen-thawed ovarian tissue transplantation in 2021 (31).
OTCT technology conforms to The European Society of Human Reproduction and Embryology (ESHRE), European Society for Medical Oncology (ESMO), American Society for Reproductive Medicine (ASRM), and French guidelines. It is a standard fertility and ovarian function preservation method and the only one for prepuberty girls and young women of reproductive age whose gonadal toxicity treatment cannot be delayed (20, 32–35). Over 200 healthy babies have been born by OTCT technology globally (36). However, only over 20 mature centers are globally developing OTCT technology (37). More than 500 cases of human ovarian tissue have been successfully cryopreserved in China’s first ovarian tissue cryobank, of which 31.6% are patients with cervical cancer, including three patients with CCIP, all of whom were cryopreserved at the same time when cesarean section terminated the pregnancy. Currently, 19 cases have been successfully transplanted in this center, and the success rate of transplantation is 100%, far exceeding the international average level (70%) (38–40).
3.1.2 Application of OTCT in CCIP patients
Because CCIP patients who receive neoadjuvant chemotherapy cannot effectively superovulation, OTCT can be selected at this time (41). Without additional anesthesia and surgery, ovarian tissue biopsy can be performed during lymph node resection or cesarean section (42). The screening criteria for OTCT in CCIP patients can refer to the Chinese Expert Consensus on Ovarian Tissue Cryopreservation and Transplantation and Group Standard on Technical Specifications for Ovarian Tissue Cryopreservation and Transplantation (20, 37).
3.1.2.1 Ovarian tissue biopsy
Ovarian tissue biopsy should avoid corpus luteum as much as possible, use a cold knife, and take more than 1/2 of the volume of one or both ovaries (the amount should be determined individually according to the patient’s condition). Energy instruments should not be used to contact the ovaries to avoid thermal damage, and the integrity of the taken ovarian tissue should be ensured as much as possible (37).
3.1.2.2 Ovarian tissue transport
The ovarian tissue removed by surgery should be immediately put into an aseptic transfer fluid, and a special transfer box should be used to maintain a low temperature (4°C ~ 8°C) and transfer to the ovarian tissue cryobank. The transport time should not exceed 24 hours. In order to achieve standard quality control, optimize patient management, and cost-effectiveness, organizational acquisition can be carried out locally. The cryopreservation and storage of ovarian tissue should be centralized (37).
3.1.2.3 Ovarian tissue cryopreservation
The ovarian tissue preparation, cryopreservation, and storage shall be performed in a laboratory meeting the requirements. When ovarian tissue is prepared, a sterile scalpel and forceps should be used to carefully remove the medulla and preserve the intact cortex. After preparation, the thickness of ovarian tissue should be about 1mm, and the size of each piece should be about 4mm × 8mm. After treatment, the ovarian tissue slices should be immediately put into cryopreservation solution for pre-cooling and balance for 20 min and then put into cryotubes containing cryopreservation solution to start freezing. Slow freezing is controlled by the computer program so that ovarian tissue is cooled to -140°C in stages according to the set rate, and then the cryopreservation tube is stored in liquid nitrogen at -196°C. Each cryopreservation tube should be marked with the patient’s name, date of birth, and code, record the storage location, and put in a liquid nitrogen tank (37).
3.1.2.4 Ovarian tissue transplantation
Ovarian tissue transplantation can be divided into orthotopic transplantation (pelvic cavity) and ectopic transplantation (extrapelvic cavity). Ovarian tissue transplantation should choose orthotopic transplantation, and ectopic transplantation can be considered if orthotopic transplantation cannot be carried out for various reasons. Orthotopic transplantation can occur in the original ovary, peritoneal bag, etc. It is advisable to make an incision where the peritoneal blood supply of the lateral wall of the ovary is good, make a peritoneal bag, and put and suture the resuscitated ovarian tissue piece (37).
Concerning the safety of ovarian tissue transplantation in patients with cervical cancer, the risk factors related to ovarian metastasis include age, FIGO stage, tumor size, pathological type (such as squamous cell carcinoma or adenocarcinoma), deep stroma, uterine cavity, endometrium, vagina, blood vessels and lymph nodes infiltration (43). Ovarian preservation is safe in young patients with early cervical cancer and has no significant effect on overall or progression-free survival (44, 45). The most common pathological type of CCIP is squamous cell carcinoma, and the ovarian metastasis rate is low (4). No tumor recurrence has been related to OTCT globally (36). For patients with severe climacteric symptoms before and after transplantation of cryopreserved ovarian tissue, traditional Chinese medicine is widely used as one of the drug therapies for climacteric syndrome. According to clinical syndromes, rational drug use (such as Kuntai Capsule) can improve menopause-related symptoms of POI (46).
3.1.2.5 Transplantation outcome
Most ovarian tissues recovered their ovarian function 3~4 months after transplantation, and the average maintenance time was 4~5 years (45). The ovarian function of the first cervical cancer patient with cryopreserved ovarian tissue transplantation in China has been maintained for more than seven years (29, 40), and it could be transplanted many times. The largest ovarian tissue cryobank in China reported that nine patients with cervical cancer had undergone cryopreserved ovarian tissue transplantation, and the ovarian function was restored after transplantation (20, 38). The age, ovarian reserve, and exposure to gonadal toxicity before cryopreservation of ovarian tissue are very important to the recovery of fertility, endocrine function, and ovarian functional life (47). Theoretically, when the function of the previous ovarian tissue begins to weaken, implanting another new cryopreserved ovarian tissue will avoid the simultaneous depletion of the reserves of multiple ovarian tissues and theoretically prolong the survival time of cryopreserved ovarian tissue and the maintenance time of estrogen and progesterone endocrine function (48).
3.2 Ovarian transposition
In recent years, the treatment of CCIP has gradually changed from active to more conservative treatment, especially for patients with early cervical cancer in the second and third trimesters of pregnancy (49). Radical resection of cervical cancer and postoperative selective pelvic radiotherapy with/without platinum drugs are the main methods to treat cervical cancer (50). In 2021, the Professional Committee of Gynecological Oncology (Study Group) of Micro-non-invasive Medicine Professional Committee of the Chinese Medical Doctor Association put forward the consensus of Chinese experts on fertility-preserving surgery for early cervical cancer (51). If combined with pelvic radiotherapy, the ovary can be moved to the planned irradiation field to protect the endocrine and reproductive function of the ovary. Ovarian transposition and shielding are recommended for cervical cancer patients under 40 (52), and the effective rate for women under 40 is about 20% ~ 90% (53). The success rate depends on the patient’s age, radiotherapy dose, transposition position, surgical skills, and whether chemotherapy is performed simultaneously (54). At present, some studies believe that the transposition of the ovary itself will also lead to the reduction of ovarian reserve (55), and it is necessary to conduct a prospective study on the long-term ovarian function of cervical cancer patients who receive ovarian transposition. Ovarian transposition and OTCT may be necessary for successful pregnancy (54, 56). Only ovarian transposition cannot protect against the adverse effects caused by chemotherapy (35).
Recommendation: Once CCIP patients are diagnosed, they need counseling on ovarian function and fertility preservation. OTCT technology does not delay the treatment of CCIP patients, which can preserve the fertility potential and the ovarian endocrine function of patients. Ovarian transposition alone is not recommended to protect patients’ ovarian function (recommended level: Class 2A).
4 Summary
With the development of cancer diagnosis and treatment technology, more and more attention has been paid to cancer survivors’ quality of life and fertility. Therefore, it is advocated to establish a multidisciplinary joint management mechanism for fertility preservation and protection of female cancer patients in all medical institutions that carry out cancer treatment and to implement a joint cancer fertility care plan in centers that treat young cancer patients so that once young women are diagnosed with cancer, they and their families can immediately get counseling on fertility preservation. Screening and evaluation of CCIP before and during pregnancy, through multidisciplinary team cooperation, will provide counseling for patients to maximize maternal and infant outcomes; those with strict indications can carry out OTCT and ovarian transposition.
First author and correspondence author
Xiangyan Ruan (E-mail:cnVhbnhpYW5neWFuQGNjbXUuZWR1LmNuLA== Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Beijing Maternal and Child Health Care Hospital).
Author contributions
RX: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Other experts involved in the consensus
Juan Du, Jiaojiao Cheng, Fengyu Jin, Muqing Gu, Yanglu Li, Yinmei Dai, Yumei Wu, Weimin Kong, Liying Zou, Li Zhou, Rui Ju, Chanwei Jia, Chenghong Yin, Alfred O. Mueck (Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Beijing Maternal and Child Health Care Hospital); Liangzhi Xu (West China Second University Hospital, Sichuan University); Mulan Ren (Zhongnan Hospital Southeast University); Ruifang Wu (Peking Univercity Shenzhen Hospital); Xin Yang (Peking University People’s Hospital); Rong Chen (Peking Union Medical College Hospital, Peking Union Medical College/Chinese Academy of Medical Sciences); Aizhen Zhu (Yuncheng Central Hospital); Wei Ma (Beijing Luhe Hospital, Capital Medical University); Aimei He (Fuqing City Hospital of Fujian); Fenge Huang (The Fifth Hospital of Cangzhou City, Hebei Province); Hongyan Sun (Shi jiazhuang TCM Hospital); Qianqing Wang (Xinxiang Central Hospital); Xin Du (Maternal and Child Health Hospital of Hubei Province); Lihua Lin (The Affiliated Hospital of Putian University); Ying Ye (Shangqiu First People’s Hospital); Songyun Han (Tongzhou Maternal and Child Health Hospital of Beijing); Chanyu Zhang (The Second Affiliated Hospital of Chongqing Medical University); Nuo Yi (Beijing Ditan Hospital, Capital Medical University); Lei Zhang (Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University); Lan Nie (Hunan Maternal and Child Health Hospital); Youguo Chen (The First Affiliated Hospital of Soochow University); Hua Zheng (Beijing Chaoyang District Maternal and Child Health Hospital); Chun Zhang (The Central Hospital of Wuhan); Junhua Bao (Changhai Hospital, Naval Medical University); Yanhong Shi (Beijing Chaoyang District Taiyanggong Community Health Service Center); Ruiying Li (Beijing Fengtai District Maternal and Child Health Hospital); Pengming Sun (Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University); Yamei Hou (The Fifth People’s Hospital in Hengshui); Yun Liu (Beijing Friendship Hospital Affiliated to Capital Medical University); Jian Gao (Hebei General Hospital); Yan Liu (Puren Hospital Affiliated to Wuhan University of Science and Technology); Liping Cai (First Affiliated Hospital of Nanchang University); Xiaodan Zhao (XingTai Infertility Specialized Hospital).
Funding
The article supported by China Association for Promotion of Health Science and Technology Special Fund project for Scientific research (JKHY2020003); Beijing Municipal Health Commission, demonstration construction project of Clinical Research ward (BCRW202109); Beijing Natural Science Foundation (No. 7202047); Capital’s Funds for Health Improvement and Research (2020-2-2112); Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20181401).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Amant F, Halaska MJ, Fumagalli M, Steffensen KD, Lok C, Calsteren KV, et al. Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting. Int J Gynecol Cancer (2014) 24(3):394–403. doi: 10.1097/IGC.0000000000000062
2. Beharee N, Shi Z, Wu D, Wang J. Diagnosis and treatment of cervical cancer in pregnant women. Cancer Med (2019) 8(12):5425–30. doi: 10.1002/cam4.2435
3. Hunter MI, Monk BJ, Tewari KS. Cervical neoplasia in pregnancy. Part 1: screening and management of preinvasive disease. Am J Obstet Gynecol (2008) 199(1):3–9. doi: 10.1016/j.ajog.2008.04.010
4. Li M, Zhao Y, Qie M, Zhang Y, Li Y, Lin B, et al. Management of cervical cancer in pregnant women: A multi-center retrospective study in China. Front Med (Lausanne) (2020) 7:538815. doi: 10.3389/fmed.2020.538815
5. Halaska MJ, Uzan C, Han SN, Fruscio R, Steffensen KD, Calster BV, et al. Characteristics of patients with cervical cancer during pregnancy: a multicenter matched cohort study. An initiative from the International Network on Cancer, Infertility and Pregnancy. Int J Gynecol Cancer (2019), ijgc–2018-000103. doi: 10.1136/ijgc-2018-000103
6. Okoth K, Chandan JS, Marshall T, Thangaratinam S, Thomas GN, Nirantharakumar K, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ (2020) 371:m3502. doi: 10.1136/bmj.m3502
7. Dolmans MM, Donnez J, Cacciottola L. Fertility preservation: the challenge of freezing and transplanting ovarian tissue. Trends Mol Med (2021) 27(8):777–91. doi: 10.1016/j.molmed.2020.11.003
8. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet (2019) 393(10167):169–82. doi: 10.1016/S0140-6736(18)32470-X
9. Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG Jr., Froelich JW, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol (2007) 188(6):1447–4. doi: 10.2214/AJR.06.1616
10. Ma J, Yu L, Xu F, Yi H, Wei W, Wu P, et al. Treatment and clinical outcomes of cervical cancer during pregnancy. Ann Transl Med (2019) 7(11):241. doi: 10.21037/atm.2019.04.76
11. Chinese obstetricians and gynecologists association (COGA), chinese expert consensus on diagnosis and treatment of pregnancy complicated with cervical cancer (2023). Chin J Pract Gynecology Obstetrics (2023) 39(3):310–7. doi: 10.19538/j.fk2023030113
12. Amant F, Berveiller P, Boere IA, Cardonick E, Fruscio R, Fumagalli M, et al. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Ann Oncol (2019) 30(10):1601–2. doi: 10.1093/annonc/mdz228
13. Gomez RS, Calderon J, Dionisi JN, Santi A, Mariconde JM, Rosato OD, et al. Cervical cancer in pregnancy at various gestational ages. Int J Gynecol Cancer (2021) 31(5):784–8. doi: 10.1136/ijgc-2020-002189
14. Wei L, Zhao J, Xing X, You Z, Bi H, Kong B, et al. Expert consensus on managing pregnancy complicated with cervical cancer. Chin J Clin Obstetrics Gynecology (2018) 19(2):190–2. doi: 10.13390/j.issn.1672-1861.2018.02.034
15. Ilancheran A. Neoadjuvant chemotherapy in cervical cancer in pregnancy. Best Pract Res Clin Obstet Gynaecol (2016) 33:102–7. doi: 10.1016/j.bpobgyn.2015.10.008
16. Halaska MJ, Drochytek V, Shmakov RG, Amant F. Fertility sparing treatment in cervical cancer management in pregnancy. Best Pract Res Clin Obstet Gynaecol (2021) 75:101–12. doi: 10.1016/j.bpobgyn.2021.03.014
17. Bigelow CA, Horowitz NS, Goodman A, Growdon WB, Carmen MD, Kaimal AJ. Management and outcome of cervical cancer diagnosed in pregnancy. Am J Obstet Gynecol (2017) 216(3):271–6. doi: 10.1016/j.ajog.2016.10.034
18. Zhou H, Liu Y, Luo M, Lin Z. Interpretation of clinical practice guide for 2023 NCCN cervical cancer (1st edition). Chin J Pract Gynecology Obstetrics (2023) 39(2):189–96. doi: 10.19538/j.fk2023020115
19. Zhang S, Ni X, Lin Z. Expert guidance on the indication of ovary preservation in the operation of cervical adenocarcinoma (2023). Chin J Pract Gynecology Obstetrics (2023) 39(2):185–8. doi: 10.19538/j.fk2023020114
20. Ruan X. Chinese society of gynecological endocrinology affiliated to the international society of gynecological endocrinology guideline for ovarian tissue cryopreservation and transplantation. Gynecol Endocrinol (2018) 34(12):1005–10. doi: 10.1080/09513590.2018.1488957
21. Parrott DMV. The fertility of mice with orthotopic ovarian grafts derived from frozen tissue. J Reprod Fertil (1960) 1:230–41.
22. Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196 degrees C. Hum Reprod (1994) 9(4):597–603. doi: 10.1093/oxfordjournals.humrep.a138556
23. Hovatta O, Silye R, Krausz T, Margara R, Trew G, Lass A, et al. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod (1996) 11(6):1268–72. doi: 10.1093/oxfordjournals.humrep.a019370
24. Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod (1998) 13(5):1133–8. doi: 10.1093/humrep/13.5.1133
25. Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. NJEM (2000) 342(25):1919. doi: 10.1056/NEJM200006223422516
26. Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA (2001) 286(12):1490–3. doi: 10.1001/jama.286.12.1490
27. Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet (2004) 364(9443):1405–10. doi: 10.1016/S0140-6736(04)17222-X
28. Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med (2005) 353(3):318–21. doi: 10.1056/NEJMc055237
29. Ruan X, Du J, Lu D, Kong W, Dai Y, Yin C, et al. Report on the first cryopreserved ovarian tissue transplantation case in China. J Capital Med Univ (2016) 06:840–2. doi: 10.3969/j.issn.1006-7795.2016.06.025
30. Ruan X, Du J, Lu D, Duan W, Jin F, Kong W, et al. First pregnancy in China after ovarian tissue transplantation to prevent premature ovarian insufficiency. Climacteric (2021) 24(6):624–8. doi: 10.1080/13697137.2022.2064215
31. Ruan X, Du J, Lu D, Duan W, Jin F, Kong W, et al. First live birth in China after cryopreserved ovarian tissue transplantation to prevent premature ovarian insufficiency. Climacteric (2022) 25(4):421–4. doi: 10.1080/13697137.2022.2064215
32. ASRM. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril (2019) 112(6):1022–33. doi: 10.1016/j.fertnstert.2019.09.013
33. Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol (2020) 31(12):1664–78. doi: 10.1016/j.annonc.2020.09.006
34. Nahata L, Woodruff TK, Quinn GP, Meacham LR, Chen D, Appiah LC, et al. Ovarian tissue cryopreservation as standard of care: what does this mean for pediatric populations? J Assist Reprod Genet (2020) 37(6):1323–6. doi: 10.1007/s10815-020-01794-7
35. Rives N, Courbiere B, Almont T, Kassab D, Berger C, Grynberg M, et al. What should be done in terms of fertility preservation for patients with cancer? The French 2021 guidelines. Eur J Cancer (2022) 173:146–66. doi: 10.1016/j.ejca.2022.05.013
36. Dolmans MM, von Wolff M, Poirot C, Diaz-Garcia C, Cacciottola L, Boissel N, et al. Transplantation of cryopreserved ovarian tissue in a series of 285 women: a review of five leading European centers. Fertil Steril (2021) 115(5):1102–15. doi: 10.1016/j.fertnstert.2021.03.008
37. Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Committee of Fertility Protection and Preservation of China Association for the Promotion of Health Science and Technology. Specification for ovarian tissue cryopreservation and transplantation. Chin Gen Pract (2023) 26(23):2836–41. doi: 10.12114/j.issn.1007-9572.2023.0237
38. Ruan X, Cheng J, Korell M, Du J, Kong W, Lu D, et al. Ovarian tissue cryopreservation and transplantation prevents iatrogenic premature ovarian insufficiency: first 10 cases in China. Climacteric (2020) 23(6):574–80. doi: 10.1080/13697137.2020.1767569
39. Khattak H, Malhas R, Craciunas L, Afifi Y, Amorim CA, Fishel S, et al. Fresh and cryopreserved ovarian tissue transplantation for preserving reproductive and endocrine function: a systematic review and individual patient data meta-analysis. Hum Reprod Update (2022) 28(3):400–16. doi: 10.1093/humupd/dmac003
40. Ruan X, Du J, Korell M, Kong W, Lu D, Jin F, et al. Case report of the first successful cryopreserved ovarian tissue retransplantation in China. Climacteric (2018) 21(6):613–6. doi: 10.1080/13697137.2018.1514005
41. Spears N, Lopes F, Stefansdottir A, Rossi V, Felici MD, Anderson RA, et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update (2019) 25(6):673–93. doi: 10.1093/humupd/dmz027
42. Ruan X. Expert consensus on fertility preservation in patients with breast cancer occurring during pregnancy. Gynecol Endocrinol (2022) 38(10):797–802. doi: 10.1080/09513590.2022.2122432
43. Matsuo K, Shimada M, Yamaguchi S, Kanao H, Nakanishi T, Saito T, et al. Identifying a candidate population for ovarian conservation in young women with clinical stage IB-IIB cervical cancer. Int J Cancer (2018) 142(5):1022–32. doi: 10.1002/ijc.31084
44. Obermair A, Asher R, Pareja R, Frumovitz M, Lopez A, Moretti-Marques R, et al. Incidence of adverse events in minimally invasive vs open radical hysterectomy in early cervical cancer: results of a randomized controlled trial. Am J Obstet Gynecol (2020) 222(3):241–9. doi: 10.1016/j.ajog.2019.09.036
45. Chen J, Wang R, Zhang B, Lin X, Wei J, Jia Y, et al. Safety of ovarian preservation in women with stage I and II cervical adenocarcinoma: a retrospective study and meta-analysis. Am J Obstet Gynecol (2016) 215(4):460–1. doi: 10.1016/j.ajog.2016.04.023
46. Ruan X, Committee of Fertility Preserveion and Preservation of China Association for the Promotion of Health Science and Technology, Chinese Society of Gynecological Endocrinology affiliated to the International Society of Gynecological Endocrinology, Society Endocrinology Branch of Beijing Institute of Obstetrics & Gynecology, Society on Fertility Preservation affiliated to Chinese Preventive Medicine Association, Reproductive Endocrine Professional Committee of China Maternal and Child Health Research Association. Practice Guideline of ovarian tissue cryopreservation and transplantation in the prevention and treatment of iatrogenic premature ovarian insufficiency. J Capital Med Univ (2023) 44(5):695–703. doi: 10.3969/j.issn.1006-7795.2023.05.001
47. Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Serrano MS, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril (2013) 99(6):1503–13. doi: 10.1016/j.fertnstert.2013.03.030
48. Lotz L, Bender-Liebenthron J, Dittrich R, Häberle L, Beckmann MW, Germeyer A, et al. Determinants of transplantation success with cryopreserved ovarian tissue: data from 196 women of the FertiPROTEKT network. Hum Reprod (2022) 37(12):2787–96. doi: 10.1093/humrep/deac225
49. Perrone AM, Bovicelli A, D'Andrilli G, Borghese G, Giordano A, De Iaco P. Cervical cancer in pregnancy: analysis of the literature and innovative approaches. J Cell Physiol (2019) 234(9):14975–90. doi: 10.1002/jcp.28340
50. Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health (2022) 11(2):e197–206. doi: 10.1016/S2214-109X(22)00501-0
51. Professional Committee of Gynecological Oncology (Study Group) of Micro-non-invasive Medicine Professional Committee of the Chinese Medical Doctor Association. The consensus of Chinese experts on fertility-preserving surgery for early cervical cancer. Chin J Minimally Invasive Surg (2021) 21(8):673–9. doi: 10.3969/j.issn.1009-6604.2021.08.001
52. Hwang JH, Yoo HJ, Park SH, Lim MC, Seo S, Kang S, et al. Association between the location of transposed ovary and ovarian function in patients with uterine cervical cancer treated with (postoperative or primary) pelvic radiotherapy. Fertil Steril (2012) 97(6):1387–93. doi: 10.1016/j.fertnstert.2012.02.052
53. Hoekman EJ, Broeders E, Louwe LA, Nout RA, Jansen FW, de Kroon CD. Ovarian function after ovarian transposition and additional pelvic radiotherapy: a systematic review. Eur J Surg Oncol (2019) 45(8):1328–40. doi: 10.1016/j.ejso.2019.02.017
54. Laios A, Duarte PS, Papadopoulou A, Gallos ID, Otify M, Ind T. Ovarian transposition and cervical cancer. Best Pract Res Clin Obstet Gynaecol (2021) 75:37–53. doi: 10.1016/j.bpobgyn.2021.01.013
55. Buonomo B, Multinu F, Casarin J, Betella I, Zanagnolo V, Aletti G, et al. Ovarian transposition in patients with cervical cancer prior to pelvic radiotherapy: a systematic review. Int J Gynecol Cancer (2021) 31(3):360–70. doi: 10.1136/ijgc-2020-001774
56. Metzger ML, Meacham LR, Patterson B, Casillas JS, Constine LS, Hijiya N, et al. Female reproductive health after childhood, adolescent, and young adult cancers: guidelines for the assessment and management of female reproductive complications. J Clin Oncol (2013) 31(9):1239–47. doi: 10.1200/JCO.2012.43.5511
Keywords: cervical cancer in pregnancy, ovarian function, fertility preservation, expert consensus, premature ovarian insufficiency
Citation: Ruan X (2023) Chinese Expert Consensus on ovarian function and fertility preservation of cervical cancer in pregnancy (2023). Front. Endocrinol. 14:1280631. doi: 10.3389/fendo.2023.1280631
Received: 21 August 2023; Accepted: 08 November 2023;
Published: 13 December 2023.
Edited by:
Scott Christley, University of Texas Southwestern Medical Center, United StatesReviewed by:
Thomas Rabe, Heidelberg University Hospital, GermanyMark P. Brincat, Queen Mary University of London, United Kingdom
Copyright © 2023 Ruan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangyan Ruan, cnVhbnhpYW5neWFuQGNjbXUuZWR1LmNu
†Committee of Fertility Protection and Preservation of China Association for the Promotion of Health Science and Technology, Chinese Society of Gynecological Endocrinology affiliated to the International Society of Gynecological Endocrinology, Society Endocrinology Branch of Beijing Institute of Obstetrics & Gynecology, and The Society on Fertility Preservation affiliated with the Chinese Preventive Medicine Association
 Xiangyan Ruan
Xiangyan Ruan