
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 12 December 2023
Sec. Cancer Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1280060
This article is part of the Research Topic Metabolic Abnormalities on the Incidence and Prognosis of Gynecological Malignant Tumors View all 5 articles
Background: Metabolic disorders are involved in the development of numerous cancers, but their association with the progression of cervical cancer is unclear. This study aims to investigate the association between metabolic disorders and the pathological risk factors and survival in patients with early cervical cancer.
Methods: Patients with FIGO IB1 (2009) primary cervical cancer who underwent radical hysterectomy and systematic pelvic lymph node dissection at our institution from October 2014 to December 2017 were included retrospectively. Clinical data regarding the metabolic syndrome and surgical pathology of the patient were collected. The correlations between metabolic disorders (hypertension, hyperglycemia, and obesity) and clinicopathological characteristics as well as survival after surgery were analyzed.
Results: The study included 246 patients with clinical IB1 cervical cancer, 111 (45.1%) of whom had at least one of the comorbidities of hypertension, obesity, or hyperglycemia. Hypertension was positively correlated with parametrial invasion and poorly differentiated histology; hyperglycemia was positively correlated with stromal invasion; obesity was negatively associated with lymph node metastasis; but arbitrary disorder did not show any correlation with pathologic features. Hypertension was an independent risk factor for parametrial invasion (OR=6.54, 95% CI: 1.60-26.69); hyperglycemia was an independent risk factor for stromal invasion (OR=2.05, 95% CI: 1.07-3.95); and obesity was an independent protective factor for lymph node metastasis (OR=0.07, 95% CI: 0.01-0.60). Moreover, the patients with hypertension had a significantly lower 5-year OS rate (70.0% vs. 95.3%, P<0.0001) and a significantly lower 5-year PFS rate than those without hypertension (70.0% vs. 91.2%, P=0.010).
Conclusion: Hypertension and hyperglycemia are positively associated with local invasion of early cervical cancer, which need to be verified in multi-center, large scale studies.
Noncommunicable diseases are the leading cause of mortality on the global scale (1). Metabolic syndrome is the most prevalent condition among these noncommunicable disorders. According to global statistics, more than one-third of adults in the United States suffer from metabolic syndrome, of which more than 50% are older than 60, and in some Asian countries, the prevalence is as high as 10% (2), making it a pervasive and potentially fatal condition worldwide. Metabolic syndrome is characterized by obesity, insulin resistance, hypertension, and hyperlipidemia (1) and is associated with the progression of diseases such as type 2 diabetes mellitus, cardiovascular disease, and nonalcoholic steatohepatitis (3, 4). Recent epidemiological studies have indicated that metabolic syndrome is also closely associated with the development of malignant tumors such as liver, bladder, renal, endometrial, pancreatic, and breast cancers (5–11). Moreover, recovery from metabolic syndrome by making lifestyle adjustments is associated with a reduced risk of pancreatic cancer compared to persistent metabolic syndrome, implying that alterations in metabolic syndrome can affect pancreatic cancer risk (12). Experiments have also shown that organismal metabolism can influence the development and progression of cancers, for instance, by modulating diet to control tumor progression (13–15). The introduction of a high-fat diet in a mouse model has been observed to increase the growth and metastasis of a primary tumor (16). Hyperglycemia promotes upregulation of mitochondrial protein GRP75 in megakaryocytes, which increases platelet activation to promote cancer metastasis in the mouse model of melanoma (17). However, how alterations in metabolic levels affect cancer risk and progression is not yet fully understood (18).
Cervical cancer ranks as the fourth most prevalent malignant tumor in females worldwide in terms of incidence and mortality (19), representing a substantial global health challenge. A retrospective study suggested that metabolic syndrome increases the risk of cervical epithelial cell abnormalities and persistent HPV infection (20–22). Large prospective epidemiologic studies and retrospective analyses have found that metabolic abnormalities may increase the risk of cervical cancer (23, 24). Nevertheless, the correlation between metabolic syndrome and cervical cancer progression is currently uncertain. In terms of mechanisms, there is limited relevant research. Hyperglycemia might accelerate the growth of cervical cancer by increasing the expression of the proteasome alpha 2 subunit (25). In obese women, the communication between tumor cells and adipocyte-derived stem cells may be enhanced, which can regulate the expression of diverse chemokines that favor malignancy-associated capacities such as migration (26). So, we were prompted to explore the clinical evidence for the role of metabolic abnormalities in cervical cancer progression, which can fuel studies on underlying mechanisms that may help to develop novel strategies for treatment of cervical cancer. Moreover, clarifying the risk factors of tumor progression can be important to guide clinicians towards better therapeutic choices, such as radiochemotherapy for more advanced disease (27).
In early-stage cervical cancers, local invasion is the principal way of tumor to spread, and lymphatic metastatic can be found postoperatively in about 15% of clinically early tumors (28, 29). Therefore, we designed a retrospective study to preliminarily investigate the association between metabolic disorders and pathological features of cervical cancer, including tumor size, parametrial invasion, stromal invasion, and lymph node metastasis. To minimize the effect of confounding factors, only patients undergoing radical surgery at clinical stage IB1 (FIGO, 2009) were included in this study. This is the first study to examine the relationship between metabolic disturbance and the pathological characteristics of cervical cancer, and it is anticipated that it will provide clinical evidence for the body’s metabolic role in the progression of cervical cancer.
This study involved a cohort of patients diagnosed with clinical stage IB1 cervical cancer who underwent surgery at our institution between October 2014 and December 2017. The studies were conducted in accordance with the local legislation and institutional requirements. The study protocol was approved by the ethics committee of Tongji Medical College of Huazhong University of Science and Technology. Patients met the following inclusion criteria: (1) surgical pathology diagnosis of primary cervical cancer with any type of pathology; (2) preoperative stage IB1 diagnosis according to 2009 FIGO; and (3) radical C-type surgery and postoperative pathology testing at our institution. Patients who met the following criteria were excluded: (1) received preoperative neoadjuvant chemotherapy or radiotherapy; (2) had incomplete data regarding blood glucose, blood pressure, body mass index (BMI), tumor size, or important pathological details (such as pathological type, histologic grading, parametrial invasion, stromal invasion, lymphovascular space involvement (LVSI), removed lymph nodes, and lymph node metastasis); and (3) had a combination of other malignant tumors.
Data regarding the patient’s age, preoperative BMI, blood pressure, glucose levels, and medical history related to metabolism (such as hypertension, abnormal glucose tolerance, and obesity) were obtained from the patient’s medical records. The tumor size was determined based on preoperative imaging and postoperative pathology data, with the largest lesion being selected for analysis. Additional information collected from medical records included clinical FIGO staging, pathological type, histology grading, parametrial invasion, stromal invasion, LVSI, removed lymph nodes, and lymph node metastasis. At our hospital, during the period of October 2014 and December 2017, clinical staging of cervical cancer was mainly based on physical examination and imaging of pelvic organs. The most frequently used imaging examination was magnetic resonance imaging, while computed tomography and ultrasound were used as alternatives occasionally. In this investigation, we applied the diagnostic criteria for obesity, hypertension, and hyperglycemia in metabolic syndrome established by the Chinese Diabetes Association in 2004 (30). (1) obesity: ≥25.0 kg/m2; (2) hyperglycemia: fasting blood glucose ≥6.1 mmol/L; postprandial blood glucose ≥7.8 mmol/L; or diabetes mellitus diagnosed and treated; (3) blood pressure ≥140/90 mmHg (1 mmHg = 0.133333 kPa) or hypertension diagnosed and treated. The exclusion of lipids from the study was due to their not being routinely tested preoperatively in our hospital, hence posing challenges in the collection of data pertaining to lipid metabolism. The survival outcomes were collected from the medical records in combination with telephone follow-up data. Overall survival (OS) was defined as the interval between the date of surgery and the date of death or to the end of follow-up. Progression-free survival (PFS) was defined as the interval between the date of surgery and the diagnosis of recurrence or to the end of follow-up.
SPSS 27.0 software was applied to process the data. Survival curves were generated using GraphPad Prism 10.0. Continuous variables that followed a normal distribution are presented as the mean ± standard deviation, while nonnormally distributed continuous variables are presented as the median (range). The t test and the Mann−Whitney U test were used to compare normally distributed and nonnormally distributed variables, respectively. Categorical variables were compared using the chi-square (χ2) test—the ordinary χ2 test for n ≥ 40 and T ≥ 5 in chi-square tests. When n ≥40 and 1≤T<5, continuity correction was implemented. Fisher’s test was applied when n<40 or there was T<1. Univariate and multivariate logistic regression analyses were used to assess the risk of tumor parametrial invasion, stromal invasion, and lymph node metastasis. Additionally, those variables with P values <0.05 in univariate logistic regression analyses were included in multivariate logistic regression analysis. The results were expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs). Survival curves were plotted by the Kaplan-Meier method and compared using the log-rank test. Two-sided P values were considered statistically significant at P < 0.05.
This investigation enrolled a total of 246 patients with clinical stage IB1 cervical cancer, and the median age of the patients was 47 (range 25-75) years. A total of 111 patients (45.1%) had at least one of the metabolic disorders of hypertension, hyperglycemia or obesity, with hypertension accounting for 28 patients (11.4%), hyperglycemia accounting for 83 patients (33.7%) and obesity accounting for 47 patients (19.1%). There were 170 cases (69.1%) with a tumor size of 2-4 cm, with squamous carcinoma accounting for 76.8% (189/246). Patients with parametrial invasion, stromal invasion (outer 1/3), LVSI, and lymph node metastases were found in 21 (8.5%), 68 (27.6%), 80 (32.5%), and 32 (13.0%) patients, respectively (Table 1).
Patients older than 50 were more likely to have hypertension and obesity, whereas hyperglycemia was not significantly associated with patient age. Moreover, the proportion of positive parametrial invasion patients with comorbid hypertension (28.6%) was significantly higher than the proportion of negative parametrial invasion patients with comorbid hypertension (9.8%, P=0.025); the proportion of positive stromal invasion patients with comorbid hyperglycemia (44.1%) was significantly higher than the proportion of negative stromal invasion patients with comorbid hyperglycemia (29.8%, P=0.033). In addition, the proportion of obese patients with lymph node metastasis was significantly lower than the proportion of obese patients without lymph node metastasis (3.1% vs. 21.5%, P=0.014). There was no statistical correlation between arbitrary metabolic abnormalities and pathologic risk factors in patients (Table 2).
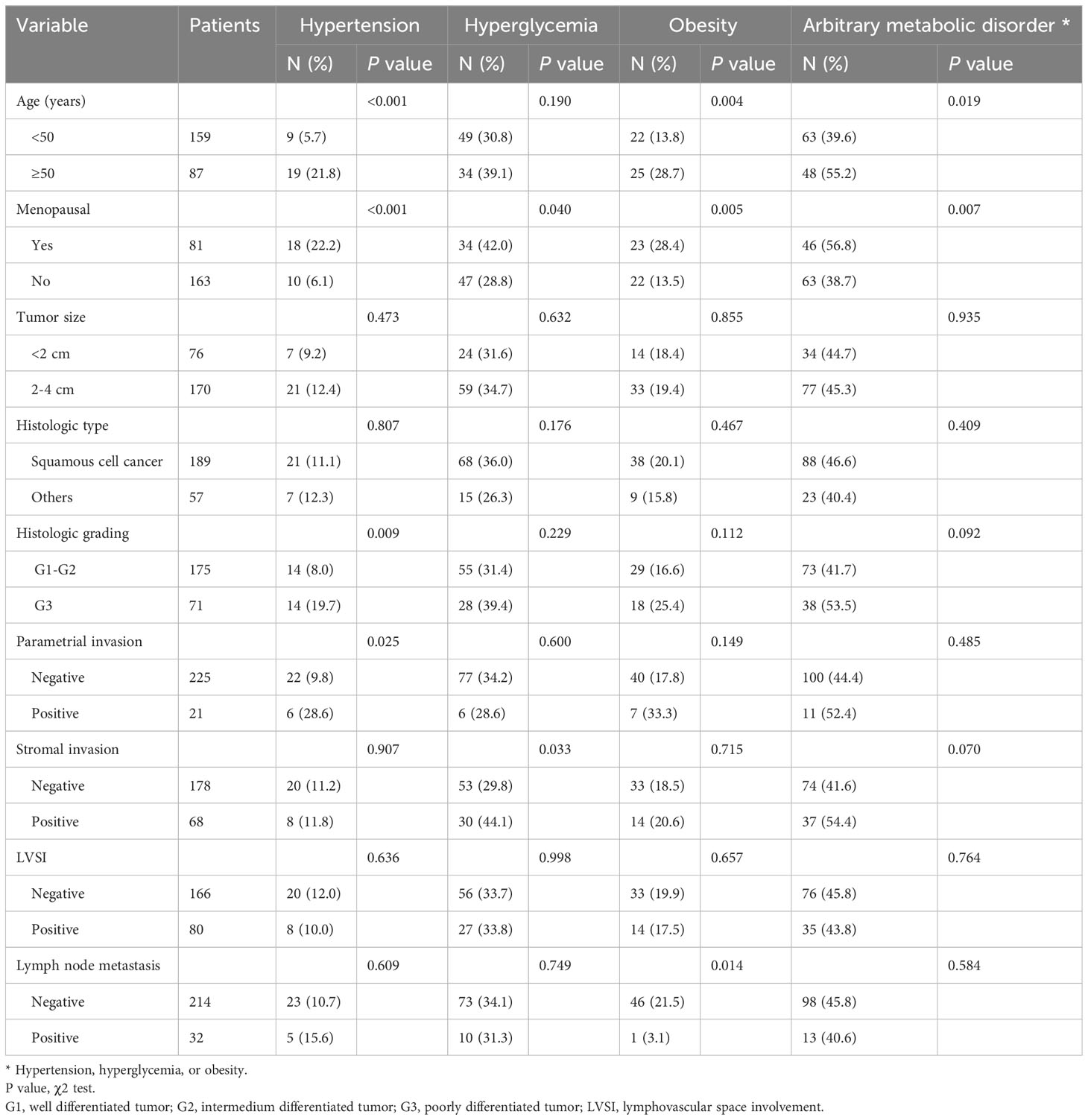
Table 2 Correlation of metabolic disorders with clinicopathologic characteristics in patients with IB1 cervical cancers (N=246).
Based on the results of the above correlation analysis, we further investigated the risk factors for parametrial invasion, stromal invasion, and lymph node metastasis (see Table 3). Univariate logistic regression results revealed that hypertension (OR=3.69, 95% CI: 1.30-10.48), tumor size, LVSI, stromal invasion and lymph node metastasis were all risk factors for parametrial invasion. Hyperglycemia (OR=1.86, 95% CI: 1.05-3.31), menopause, tumor size, LVSI, parametrial invasion and lymph node metastasis were associated with an elevated risk of stromal invasion. Tumor size, LVSI, parametrial invasion and stromal invasion increased the risk of lymph node metastasis, while obese patients had a lower risk of lymph node metastasis (OR=0.12, 95% CI: 0.02-0.89).

Table 3 Univariate regression analysis of risk factors for parametrial invasion, stromal invasion, and lymph node metastasis.
To clarify whether hypertension, hyperglycemia, and obesity were independent risk factors for parametrial invasion, stromal invasion, and lymph node metastasis, we performed a multivariate logistic regression analysis (see Table 4). The results indicated that hypertension was an independent risk factor for parametrial invasion (OR=6.54, 95% CI: 1.60-26.69), whereas hyperglycemia was an independent risk factor for stromal invasion (OR=2.05, 95% CI: 1.07-3.95). Obesity was associated with a low risk of lymph node metastasis (OR=0.07, 95% CI: 0.01-0.60). The presence of obesity in surgical patients can pose difficulties, potentially resulting in inadequate lymph node clearance and failure to detect lymph node metastases (31, 32). To exclude the influence of this variable, we compared the number of lymph node dissections performed on obese and nonobese patients and found no significant difference (median 32 (range 8-89) vs. median 31 (range 14-75); Mann−Whitney U test, P=0.654).
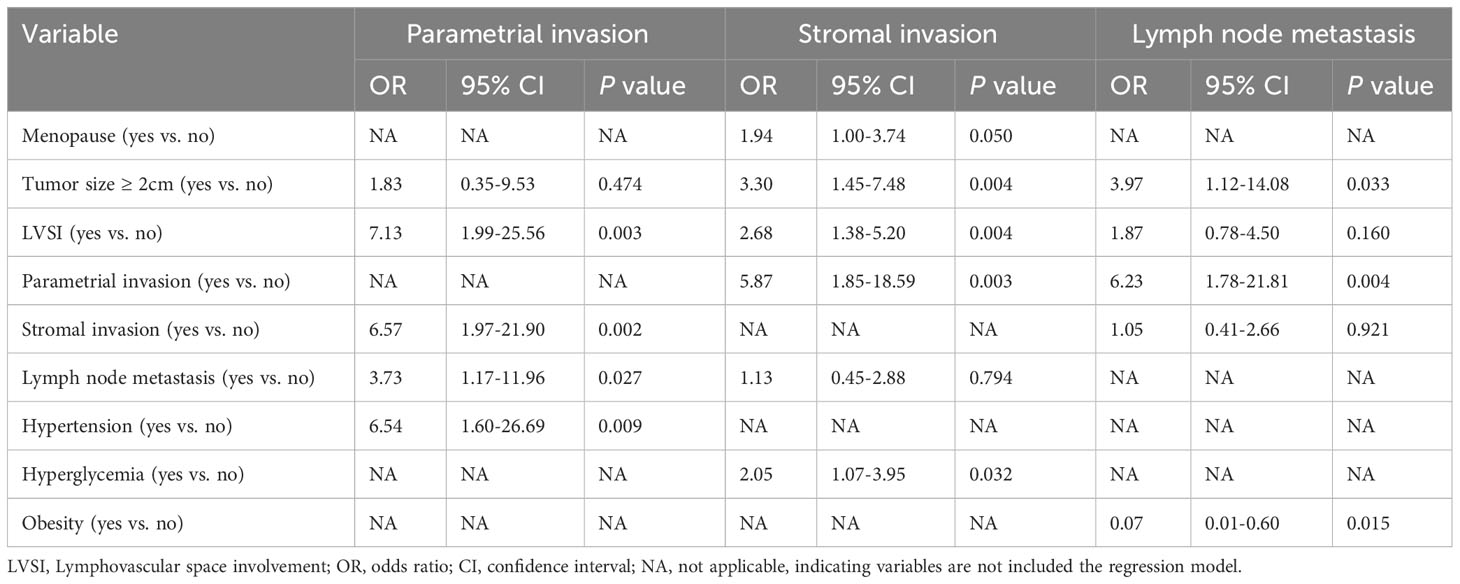
Table 4 Multivariate regression analysis of risk factors for parametrial invasion, stromal invasion, and lymph node metastasis.
To assess the association of hypertension, hyperglycemia, and obesity with the prognosis of early-stage cervical cancer, we performed a survival analysis. With a median follow-up time of 83 months (range, 14-107), we found a 5-year OS of 92.6% and a 5-year PFS of 88.9% in 190 patients with available follow-up data. The patients with hypertension showed had a significantly lower 5-year OS rate (70.0% vs. 95.3%, P<0.0001) and a significantly lower 5-year PFS rate than those without hypertension (70.0% vs. 91.2%, P= 0.010). Neither hyperglycemia nor obesity was significantly correlated with OS or PFS in the patients with IB1 cervical cancer (Figure 1).
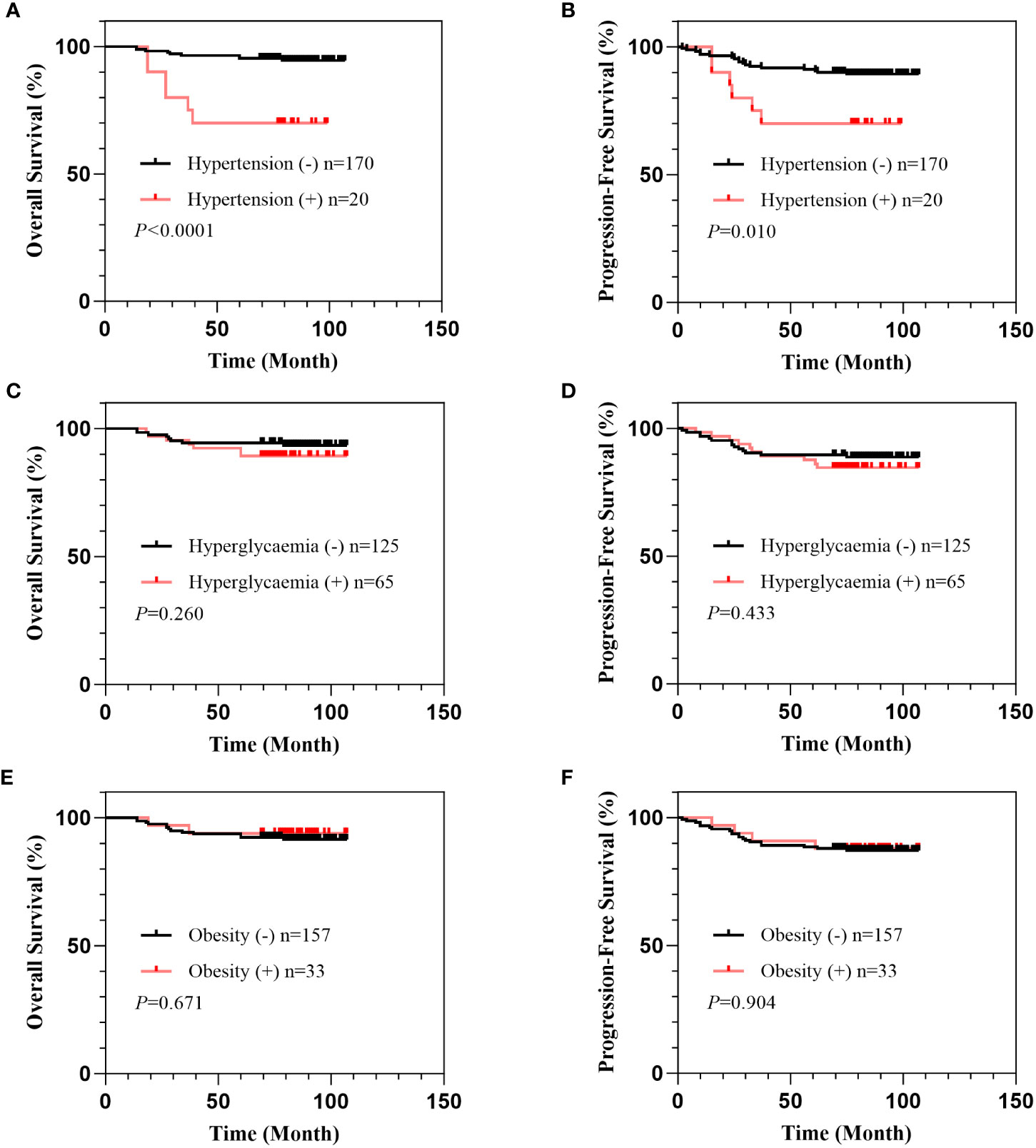
Figure 1 Correlation of hypertension, hyperglycemia, and obesity with survival in patients with IB1 cervical cancer. Overall survival curves of patients stratified by comorbidity of hypertension (A), hyperglycemia (B), and obesity (C). Progression-free survival curves of patients stratified by comorbidity of hypertension (D), hyperglycemia (E), and obesity (F).
In various tumors, a correlation between metabolic syndrome and pathological risk factors was discovered. According to retrospective studies, endometrial cancer patients with metabolic syndrome have higher lymph node metastasis rates, more LVSI, and greater parametrial invasion (33). Another small retrospective study revealed that thyroid cancer patients with metabolic syndrome had larger tumors, a greater proportion of lymph node metastases, and more advanced tumor staging (34). Additionally, for colorectal cancer, the proportion of lymph node metastasis and LVSI were higher in male patients with metabolic syndrome, whereas there was little association in female patients (35). To investigate the relationship between metabolic syndrome and the pathological characteristics of cervical cancer, we retrospectively analyzed the clinicopathological data of clinical early-stage cervical cancer patients. Hypertension was identified as a distinct risk factor for parametrial invasion, while hyperglycemia was determined to be an independent risk factor for stromal invasion. This suggests that abnormalities in body metabolism may play an important role in cervical cancer progression; however, further research is necessary.
We found that patients with hypertension had an increased risk of parametrial invasion and poorer overall survival. According to epidemiological studies, hypertension is associated with the occurrence of malignant tumors at various sites and can affect the prognosis of these cancers, particularly kidney, colon, esophageal, breast, skin, prostate, liver, uterine, and pancreatic cancers (36, 37). Furthermore, regarding the connection between hypertension and pathological characteristics, a retrospective study revealed that hypertension was associated with advanced TNM staging in gastric cancer (38). Likewise, large retrospective studies have discovered that hypertension is linked to a higher risk of larger tumor size, positive lymph node metastasis, and later tumor stage in thyroid cancer (39). The potential mechanistic correlation between hypertension and tumor progression may involve the influence of hormones, such as angiotensin II, which can enhance tumor angiogenesis by stimulating the production of vascular endothelial growth factor (40, 41). Additionally, estrogen, known for its protective effects against hypertension, may play a role in regulating the proliferation and apoptosis of tumor cells through receptor binding (42, 43). Additionally, hypertension-induced chronic inflammation, the upregulation of hypoxia-inducible factors, and reactive oxygen species promote tumor progression (44). In addition, cancer patients are more likely to develop hypertension because anti-VEGF drugs and targeted medications can dramatically increase blood pressure (45, 46). Meanwhile, the drugs used in hypertensive patients may affect tumor progression (47). As a result, hypertension may contribute to cancer progression through multiple mechanisms.
Not only is hypertension linked to cervical cancer invasion, but we discovered that hyperglycemia increased the risk of stromal invasion. Epidemiological studies revealed a correlation between diabetes and decreased overall survival as well as an increased susceptibility to cancer-related mortality, including pancreatic, ovarian, breast, and uterine cancers (48–50). Moreover, the link between hyperglycemia and tumor pathology risk factors has been assessed in several tumors. In a large retrospective study on breast cancer, Hou et al. found that diabetic patients had an advanced tumor stage, a higher incidence of lymph node metastases, and a poor prognosis overall (51, 52). Furthermore, a retrospective study found a significant association between diabetes mellitus and an increased risk of stromal invasion in colorectal cancer (53), which is consistent with our findings. The mechanism by which hyperglycemia promotes tumor progression may be related to insulin resistance. Insulin and IGF-1 receptors can be observed in most types of cancer tissue. Insulin receptors in cancer cells are engaged to activate proliferative signaling pathways, protect against apoptotic stimuli, and promote cancer invasion and metastasis (54). Moreover, hyperglycemia induces the production of numerous inflammatory factors, such as IL-6 and TNF-α, that can induce epithelial mesenchymal transition, thereby promoting tumor cell invasion and inhibiting apoptosis (55). Additionally, hyperglycemia induces cellular hypoxia, and the exacerbation of microenvironmental hypoxia leads to an increase in HIF-1 expression, which promotes pancreatic cancer metastasis (56). In addition, high blood glucose can provide nutrients for the rapid proliferation of malignant tumor cells, thereby accelerating the proliferation and invasion of tumor cells (57).
Obesity is an epidemiologic risk factor for the development of many cancers, and it has a negative impact on the prognosis of many, but not all, cancers (58–60). We noted that obesity correlated with a low risk of lymph node metastasis in obese and nonobese patients, with no significant difference in the number of lymph nodes removed. This finding is consistent with the results of a previous retrospective study on gastric cancer, which also found obesity to be a factor protecting against lymph node metastasis (61). However, this is contrary to the findings of Lucas et al., who discovered that higher BMI was an independent risk factor for lymph node metastasis in stage IA2 or IB1 cervical cancer (62). Mechanistically, the correlation between organismal metabolism and lymph node metastasis is unclear. Experimental studies have revealed that the monounsaturated fatty acid (MUFA) oleic acid in lymphatic vessels mitigates oxidative stress and promotes cancer cell circulation in lymphatic systems (63). Inmaculada et al. hypothesized that a high-fat diet rich in MUFAs may prevent ferroptosis to potentially promote tumor growth and metastasis, but this has not been demonstrated (64). Currently, the relationship between obesity and lymph node metastasis remains unclear. Therefore, large prospective studies and fundamental research are required for further investigation.
Additionally, there are limitations to this study. First, this is a single-center study with a sample size of 246 patients, which, by its nature, cannot avoid selective bias or confirm causality; therefore, a multi-center investigation employing a more substantial sample size is required to validate the results. Second, the association between metabolic syndrome and pathological characteristics was not investigated due to a lack of information on lipid metabolism and waist circumference in medical records. Although the overall analysis of metabolic disorders did not reveal a significant correlation with each pathological feature, we discovered that hypertension, hyperglycemia, and obesity were all associated with cancer invasion. This suggests that any metabolic components of the metabolic syndrome should be analyzed apart from the syndrome itself.
Hypertension and hyperglycemia may increase the risk of parametrial invasion or stromal invasion in patients with early clinical stage cervical cancer, indicating that metabolic abnormalities may play an important role in the local progression of early cervical cancer; however, multi-center, large scale studies are needed to verify the observation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
TS: Writing – original draft, Writing – review & editing, Data curation, Formal Analysis, Investigation, Methodology. JZ: Supervision, Writing – review & editing, Investigation, Methodology. WL: Investigation, Writing – review & editing, Validation. XW: Validation, Writing – review & editing. YG: Validation, Writing – review & editing. ZW: Conceptualization, Project administration, Writing – review & editing. SH: Project administration, Supervision, Writing – review & editing. JC: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. National Natural Science Foundation of China (82072891).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SL declared a shared parent affiliation with the author(s) to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Boerma T, Mathers CD. The World Health Organization and global health estimates: improving collaboration and capacity. BMC Med (2015) 13:50. doi: 10.1186/s12916-015-0286-7
2. O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev (2015) 16(1):1–12. doi: 10.1111/obr.12229
3. Scholze J, Alegria E, Ferri C, Langham S, Stevens W, Jeffries D, et al. Epidemiological and economic burden of metabolic syndrome and its consequences in patients with hypertension in Germany, Spain and Italy; a prevalence-based model. BMC Public Health (2010) 10:529. doi: 10.1186/1471-2458-10-529
4. Pothiwala P, Jain SK, Yaturu S. Metabolic syndrome and cancer. Metab Syndr Relat Disord (2009) 7(4):279–88. doi: 10.1089/met.2008.0065
5. Martin RM, Vatten L, Gunnell D, Romundstad P, Nilsen TI. Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes Control (2009) 20(7):1181–92. doi: 10.1007/s10552-009-9319-x
6. Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer (2009) 115(24):5651–61. doi: 10.1002/cncr.24687
7. Montella M, Di Maso M, Crispo A, Grimaldi M, Bosetti C, Turati F, et al. Metabolic syndrome and the risk of urothelial carcinoma of the bladder: a case-control study. BMC Cancer (2015) 15:720. doi: 10.1186/s12885-015-1769-9
8. Du W, Guo K, Jin H, Sun L, Ruan S, Song Q. Association between metabolic syndrome and risk of renal cell cancer: A meta-analysis. Front Oncol (2022) 12:928619. doi: 10.3389/fonc.2022.928619
9. Rosato V, Zucchetto A, Bosetti C, Dal Maso L, Montella M, Pelucchi C, et al. Metabolic syndrome and endometrial cancer risk. Ann Oncol (2011) 22(4):884–9. doi: 10.1093/annonc/mdq464
10. Zhong L, Liu J, Liu S, Tan G. Correlation between pancreatic cancer and metabolic syndrome: A systematic review and meta-analysis. Front Endocrinol (Lausanne) (2023) 14:1116582. doi: 10.3389/fendo.2023.1116582
11. Dong S, Wang Z, Shen K, Chen X. Metabolic syndrome and breast cancer: prevalence, treatment response, and prognosis. Front Oncol (2021) 11:629666. doi: 10.3389/fonc.2021.629666
12. Park JH, Han K, Hong JY, Park YS, Hur KY, Kang G, et al. Changes in metabolic syndrome status are associated with altered risk of pancreatic cancer: A nationwide cohort study. Gastroenterology (2022) 162(2):509–520.e7. doi: 10.1053/j.gastro.2021.09.070
13. Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol (2008) 180(7):4476–86. doi: 10.4049/jimmunol.180.7.4476
14. Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature (2019) 572(7769):397–401. doi: 10.1038/s41586-019-1437-3
15. Maddocks ODK, Athineos D, Cheung EC, Lee P, Zhang T, van den Broek NJF, et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature (2017) 544(7650):372–6. doi: 10.1038/nature22056
16. Peck B, Schulze A. Lipid metabolism at the nexus of diet and tumor microenvironment. Trends Cancer (2019) 5(11):693–703. doi: 10.1016/j.trecan.2019.09.007
17. Wu B, Ye Y, Xie S, Li Y, Sun X, Lv M, et al. Megakaryocytes mediate hyperglycemia-induced tumor metastasis. Cancer Res (2021) 81(21):5506–22. doi: 10.1158/0008-5472.CAN-21-1180
18. Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer (2021) 21(10):669–80. doi: 10.1038/s41568-021-00378-6
19. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
20. Huang X, Zhao Q, Yang P, Li Y, Yuan H, Wu L, et al. Metabolic syndrome and risk of cervical human papillomavirus incident and persistent infection. Med (Baltimore) (2016) 95(9):e2905. doi: 10.1097/MD.0000000000002905
21. Molokwu JC, Penaranda E, Lopez DS, Dwivedi A, Dodoo C, Shokar N. Association of metabolic syndrome and human papillomavirus infection in men and women residing in the United States. Cancer Epidemiol Biomarkers Prev (2017) 26(8):1321–7. doi: 10.1158/1055-9965.EPI-17-0129
22. Lee D, Lee TS. The association between metabolic syndrome and epithelial cell abnormalities detected on pap smear: A nationwide population-based study. J Clin Med (2023) 12(8). doi: 10.3390/jcm12082954
23. Stocks T, Bjørge T, Ulmer H, Manjer J, Häggström C, Nagel G, et al. Metabolic risk score and cancer risk: pooled analysis of seven cohorts. Int J Epidemiol (2015) 44(4):1353–63. doi: 10.1093/ije/dyv001
24. Ulmer H, Bjørge T, Concin H, Lukanova A, Manjer J, Hallmans G, et al. Metabolic risk factors and cervical cancer in the metabolic syndrome and cancer project (Me-Can). Gynecol Oncol (2012) 125(2):330–5. doi: 10.1016/j.ygyno.2012.01.052
25. Mao D, Cao H, Shi M, Wang CC, Kwong J, Li JJX, et al. Increased co-expression of PSMA2 and GLP-1 receptor in cervical cancer models in type 2 diabetes attenuated by Exendin-4: A translational case-control study. EBioMedicine (2021) 65:103242. doi: 10.1016/j.ebiom.2021.103242
26. De la Fuente-Hernandez MA, Alanis-Manriquez EC, Ferat-Osorio E, Rodriguez-Gonzalez A, Arriaga-Pizano L, Vazquez-Santillan K, et al. Molecular changes in adipocyte-derived stem cells during their interplay with cervical cancer cells. Cell Oncol (Dordr) (2022) 45(1):85–101. doi: 10.1007/s13402-021-00653-6
27. Mazzola R, Ricchetti F, Fiorentino A, Levra NG, Fersino S, Di Paola G, et al. Weekly cisplatin and volumetric-modulated arc therapy with simultaneous integrated boost for radical treatment of advanced cervical cancer in elderly patients: feasibility and clinical preliminary results. Technol Cancer Res Treat (2017) 16(3):310–5. doi: 10.1177/1533034616655055
28. Matsuo K, Machida H, Blake EA, Takiuchi T, Mikami M, Roman LD, et al. Significance of uterine corpus tumor invasion in early-stage cervical cancer. Eur J Surg Oncol (2017) 43(4):725–34. doi: 10.1016/j.ejso.2017.01.017
29. Ferrandina G, Pedone Anchora L, Gallotta V, Fagotti A, Vizza E, Chiantera V, et al. Can we define the risk of lymph node metastasis in early-stage cervical cancer patients? A large-scale, retrospective study. Ann Surg Oncol (2017) 24(8):2311–8. doi: 10.1245/s10434-017-5917-0
30. Zhou H, Guo ZR, Yu LG, Hu XS, Xu BH, Liu HB, et al. Evidence on the applicability of the ATPIII, IDF and CDS metabolic syndrome diagnostic criteria to identify CVD and T2DM in the Chinese population from a 6.3-year cohort study in mid-eastern China. Diabetes Res Clin Pract (2010) 90(3):319–25. doi: 10.1016/j.diabres.2010.09.001
31. Papadia A, Ragni N, Salom EM. The impact of obesity on surgery in gynecological oncology: a review. Int J Gynecol Cancer (2006) 16(2):944–52. doi: 10.1111/j.1525-1438.2006.00577.x
32. Li C, Dionigi G, Liang N, Guan H, Sun H. The relationship between body mass index and different regional patterns of lymph node involvement in papillary thyroid cancers. Front Oncol (2021) 11:767245. doi: 10.3389/fonc.2021.767245
33. Yang X, Li X, Dong Y, Fan Y, Cheng Y, Zhai L, et al. Effects of metabolic syndrome and its components on the prognosis of endometrial cancer. Front Endocrinol (Lausanne) (2021) 12:780769. doi: 10.3389/fendo.2021.780769
34. Song JL, Li LR, Yu XZ, Zhan L, Xu ZL, Li JJ, et al. Association between metabolic syndrome and clinicopathological features of papillary thyroid cancer. Endocrine (2022) 75(3):865–71. doi: 10.1007/s12020-021-02940-6
35. Healy LA, Howard JM, Ryan AM, Beddy P, Mehigan B, Stephens R, et al. Metabolic syndrome and leptin are associated with adverse pathological features in male colorectal cancer patients. Colorectal Dis (2012) 14(2):157–65. doi: 10.1111/j.1463-1318.2011.02562.x
36. Stocks T, Van Hemelrijck M, Manjer J, Bjørge T, Ulmer H, Hallmans G, et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension (2012) 59(4):802–10. doi: 10.1161/HYPERTENSIONAHA.111.189258
37. Christakoudi S, Kakourou A, Markozannes G, Tzoulaki I, Weiderpass E, Brennan P, et al. Blood pressure and risk of cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer (2020) 146(10):2680–93. doi: 10.1002/ijc.32576
38. Li F, Du H, Li S, Liu J. The association between metabolic syndrome and gastric cancer in Chinese. Front Oncol (2018) 8:326. doi: 10.3389/fonc.2018.00326
39. Li LR, Song JL, Liu HQ, Chen C. Hypertension was associated with higher tumor stages in papillary thyroid cancer: A large sample single-center study. Metab Syndr Relat Disord (2022) 20(8):466–72. doi: 10.1089/met.2022.0033
40. Egami K, Murohara T, Shimada T, Sasaki K, Shintani S, Sugaya T, et al. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest (2003) 112(1):67–75. doi: 10.1172/JCI16645
41. Hashemzehi M, Beheshti F, Hassanian SM, Ferns GA, Khazaei M, Avan A. Therapeutic potential of renin angiotensin system inhibitors in cancer cells metastasis. Pathol Res Pract (2020) 216(7):153010. doi: 10.1016/j.prp.2020.153010
42. Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Curr Hypertens Rep (2006) 8(5):368–76. doi: 10.1007/s11906-006-0080-1
43. Pagano MT, Ortona E, Dupuis ML. A role for estrogen receptor alpha36 in cancer progression. Front Endocrinol (Lausanne) (2020) 11:506. doi: 10.3389/fendo.2020.00506
44. Kim CS, Han KD, Choi HS, Bae EH, Ma SK, Kim SW. Association of hypertension and blood pressure with kidney cancer risk: A nationwide population-based cohort study. Hypertension (2020) 75(6):1439–46. doi: 10.1161/HYPERTENSIONAHA.120.14820
45. Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol (2008) 9(2):117–23. doi: 10.1016/S1470-2045(08)70003-2
46. Sahni G. Onco-hypertension: changing paradigm of treating hypertension in patients with cancer. J Clin Oncol (2023) 41(5):958–63. doi: 10.1200/JCO.22.01875
47. Tadic M, Cuspidi C, Belyavskiy E, Grassi G. Intriguing relationship between antihypertensive therapy and cancer. Pharmacol Res (2019) 141:501–11. doi: 10.1016/j.phrs.2019.01.037
48. van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer (2007) 120(9):1986–92. doi: 10.1002/ijc.22532
49. Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care (2012) 35(9):1835–44. doi: 10.2337/dc12-0002
50. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev (2015) 95(3):727–48. doi: 10.1152/physrev.00030.2014
51. Hou G, Zhang S, Zhang X, Wang P, Hao X, Zhang J. Clinical pathological characteristics and prognostic analysis of 1,013 breast cancer patients with diabetes. Breast Cancer Res Treat (2013) 137(3):807–16. doi: 10.1007/s10549-012-2404-y
52. Juanjuan L, Wen W, Zhongfen L, Chuang C, Jing C, Yiping G, et al. Clinical pathological characteristics of breast cancer patients with secondary diabetes after systemic therapy: a retrospective multicenter study. Tumour Biol (2015) 36(9):6939–47. doi: 10.1007/s13277-015-3380-8
53. He Q, Zhang H, Yao S, Zhu D, Lv D, Cui P, et al. A study on relationship between metabolic syndrome and colorectal cancer. J buon (2018) 23(5):1362–8.
54. Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol (2016) 34(35):4270–6. doi: 10.1200/JCO.2016.67.4283
55. Li W, Zhang X, Sang H, Zhou Y, Shang C, Wang Y, et al. Effects of hyperglycemia on the progression of tumor diseases. J Exp Clin Cancer Res (2019) 38(1):327. doi: 10.1186/s13046-019-1309-6
56. Li W, Liu H, Qian W, Cheng L, Yan B, Han L, et al. Hyperglycemia aggravates microenvironment hypoxia and promotes the metastatic ability of pancreatic cancer. Comput Struct Biotechnol J (2018) 16:479–87. doi: 10.1016/j.csbj.2018.10.006
57. Joshi S, Liu M, Turner N. Diabetes and its link with cancer: providing the fuel and spark to launch an aggressive growth regime. BioMed Res Int (2015) 2015:390863. doi: 10.1155/2015/390863
58. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med (2003) 348(17):1625–38. doi: 10.1056/NEJMoa021423
59. Parekh N, Okada T, Lu-Yao GL. Obesity, insulin resistance, and cancer prognosis: implications for practice for providing care among cancer survivors. J Am Diet Assoc (2009) 109(8):1346–53. doi: 10.1016/j.jada.2009.05.001
60. LeRoith D, Novosyadlyy R, Gallagher EJ, Lann D, Vijayakumar A, Yakar S. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes (2008) 116 Suppl 1:S4–6. doi: 10.1055/s-2008-1081488
61. Minig L, Fagotti A, Scambia G, Salvo G, Patrono MG, Haidopoulos D, et al. Incidence of lymph node metastases in women with low-risk early cervical cancer (<2 cm) without lymph-vascular invasion. Int J Gynecol Cancer (2018) 28(4):788–93. doi: 10.1097/IGC.0000000000001236
62. Zou Y, Wu L, Yang Y, Shen X, Zhu C. Risk factors of tumor invasion and node metastasis in early gastric cancer with undifferentiated component: a multicenter retrospective study on biopsy specimens and clinical data. Ann Transl Med (2020) 8(6):360. doi: 10.21037/atm.2020.02.42
63. Ubellacker JM, Tasdogan A, Ramesh V, Shen B, Mitchell EC, Martin-Sandoval MS, et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature (2020) 585(7823):113–8. doi: 10.1038/s41586-020-2623-z
Keywords: metabolic disorders, pathological characteristics, cervical cancer, hypertension, obesity, hyperglycemia
Citation: Shen T, Zhao J, Li W, Wang X, Gao Y, Wang Z, Hu S and Cai J (2023) Hypertension and hyperglycaemia are positively correlated with local invasion of early cervical cancer. Front. Endocrinol. 14:1280060. doi: 10.3389/fendo.2023.1280060
Received: 19 August 2023; Accepted: 28 November 2023;
Published: 12 December 2023.
Edited by:
Zhongqiu Xie, University of Virginia, United StatesReviewed by:
Shitong Lin, Huazhong University of Science and Technology, ChinaCopyright © 2023 Shen, Zhao, Li, Wang, Gao, Wang, Hu and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sha Hu, YWJjZGU4MzEyM0AxNjMuY29t; Jing Cai, amluZ2NhaUBodXN0LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.