- 1Department of Urology, Affiliated Hospital of North Sichuan Medical College, Nan chong, China
- 2Physical Examination Center, Affiliated Hospital of North Sichuan Medical College, Nan chong, China
Background: The comparative advantages of robotic posterior retroperitoneal adrenalectomy (RPRA) over laparoscopic posterior retroperitoneal adrenalectomy (LPRA) remain a topic of ongoing debate within the medical community. This systematic literature review and meta-analysis aim to assess the safety and efficacy of RPRA compared to LPRA, with the ultimate goal of determining which procedure yields superior clinical outcomes.
Methods: A systematic search was conducted on databases including PubMed, Embase, Web of Science, and the Cochrane Library database to identify relevant studies, encompassing both randomized controlled trials (RCTs) and non-RCTs, that compare the outcomes of RPRA and LPRA. The primary focus of this study was to evaluate perioperative surgical outcomes and complications. Review Manager 5.4 was used for this analysis. The study was registered with PROSPERO (ID: CRD42023453816).
Results: A total of seven non-RCTs were identified and included in this study, encompassing a cohort of 675 patients. The findings indicate that RPRA exhibited superior performance compared to LPRA in terms of hospital stay (weighted mean difference [WMD] -0.78 days, 95% confidence interval [CI] -1.46 to -0.10; p = 0.02). However, there were no statistically significant differences observed between the two techniques in terms of operative time, blood loss, transfusion rates, conversion rates, major complications, and overall complications.
Conclusion: RPRA is associated with a significantly shorter hospital stay compared to LPRA, while demonstrating comparable operative time, blood loss, conversion rate, and complication rate. However, it is important to note that further research of a more comprehensive and rigorous nature is necessary to validate these findings.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=453816, identifier CRD42023453816.
1 Introduction
Laparoscopic transperitoneal adrenalectomy (LTA) was first elucidated by Gagner et al. in 1992 (1). Subsequent empirical evidence has unequivocally unveiled a spectrum of advantages inherently associated with LTA, transcending those of conventional open adrenalectomy. These encompass a notable reduction in estimated blood loss, abbreviated hospitalization periods, alleviated postoperative discomfort, and a diminished incidence of complications (2, 3). In the year 1995, Mercan et al. introduced the laparoscopic posterior retroperitoneal adrenalectomy (LPRA), an innovative surgical paradigm that has since been methodically established as both a practicable and secure operative approach (4–6). From an anatomical vantage point, LPRA presents a more direct conduit to reach the adrenal gland, obviating the necessity for the mobilization of contiguous structures. This tactical approach concomitantly mitigates the potential for complications entailed in peritoneal cavity ingress. Noteworthy is LPRA’s specific commendation for patients harboring bilateral tumors and grappling with abdominal adhesions (7). However, juxtaposed against LTA, LPRA does encounter certain limitations stemming from its confined surgical arena, rigid instrumentation, and plausible interactions with neighboring anatomical architecture (8).
In the realm of surgical innovation, propelled by advancements in technology, robotic posterior retroperitoneal adrenalectomy (RPRA) has ascended as a preeminent surgical modality. RPRA affords an array of advantages, including heightened visual acuity through three-dimensional optics and an expanded panoramic canvas of the operative field. This is coupled with a broader range of maneuverability, encompassing seven degrees of freedom compared to the conventional four, thereby enhancing the ergonomic milieu for surgical practitioners (9, 10). However, it is imperative to acknowledge the attendant limitations inherent in robotic surgical systems, encompassing the requisites of setup, the intricacies of instrumentation, augmented expenses, and an extended surgical duration. As a result, the quest for the optimal surgical paradigm within the constricted confines of the retroperitoneal space remains a matter of ongoing scholarly discourse.
Therefore, the aim of this study is to amalgamate data derived from comparative studies and evaluate the efficacy and safety of RPRA and LPRA. The results of this study are intended to function as an all-encompassing guide for clinical decision-making, thereby assisting physicians in the discernment of the most fitting surgical approach for their patients.
2 Methods
This study was executed in strict adherence to the protocols delineated within the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [13]. Furthermore, it underwent registration within the PROSPERO database (ID: CRD42023453816) in accordance with established practices.
2.1 Literature search strategy, study selection and data collection
A thorough and exhaustive electronic survey of the academic databases, encompassing PubMed, Embase, Web of Science, and the Cochrane Library, was meticulously conducted. The data collection process was finalized in July 2023. The search strategy seamlessly integrated pertinent terms concerning the intervention and patient demographics, culminating in the formulation of the subsequent search query: ((Laparoscopic OR Robot-assisted OR Minimally invasive) AND (Retroperitoneoscopic OR Retroperitoneal OR Direct posterior OR Posterior) AND (Adrenalectomy)). Additionally, a comprehensive manual inquiry and scrupulous assessment of pertinent studies were undertaken to ensure the preemptive mitigation of potential oversights. It is noteworthy that the search was specifically delimited to publications presented in the English language. In instances of discordance, a consensus was judiciously attained through deliberation or, when deemed necessary, through consultation with a third reviewer.
The criteria for inclusion were delineated utilizing the PICOS methodology. P (patients): Patients aged 18 years or older who were due to undergo adrenalectomy for any indication. I (intervention): Encompassing patients subjected to RPRA. C (comparator): LPRA was employed as the comparative modality. O (outcome): The primary objective of this inquiry was to evaluate one or more of the ensuing outcomes: perioperative ramifications, surgical outcomes, and associated complications. S (study type): This investigation encompassed randomized controlled trials (RCTs), alongside both retrospective and prospective comparative analyses. Exclusionary criteria were employed as follows: (1) Studies bereft of comparative designs were systematically excluded. (2) Editorial commentaries, epistolary exchanges with the editor, abstracts from meetings, and singular case reports were not integrated into the analytical framework. (3) Studies that did not undertake an evaluation of the stipulated outcome metrics were purposefully excluded from consideration.
Following that, two independent reviewers meticulously extracted the subsequent dataset from the incorporated studies: (1) General manuscript details encompassing the year of publication, lead author, and country of origin. (2) Characteristics of the study population including sample size, age distribution, and body mass index (BMI). (3) Attributes specific to the tumors under investigation: tumor diameter, tumor site, and oncologic outcomes. (4) Perioperative effectiveness metrics: procedural duration, volume of blood loss, duration of hospitalization, rate of conversions, and frequency of transfusions. (5) Considerations regarding perioperative safety: overall complications (as defined by Clavien grade ≥ 1) and major complications (as defined by Clavien grade ≥ 3) (11). The process of data extraction was autonomously executed by the two reviewers to ensure meticulousness and uniformity.
In order to assess the quality of the literature, a comprehensive evaluation was conducted on the studies incorporated in the analysis, employing the “risk of bias in non-randomized studies of interventions” (ROBINS-I) framework (12). This assessment was executed independently by two evaluators (Y.L. and X.C.), who conducted a meticulous scrutiny of the studies for potential biases, encompassing confounding factors or other potential sources of systematic deviation. Any inconsistencies or differences that emerged during the assessment process were resolved through in-depth discourse.
2.2 Statistical analysis
For the purpose of data analysis within this study, we used the Cochrane Collaborative RevMan 5.4 software. Odds ratios (OR) were applied to assess dichotomous outcomes, while weighted mean differences (WMD) were employed to quantify continuous outcomes, accompanied by 95% confidence intervals (CI) for all evaluated measures. The evaluation of inter-study heterogeneity was accomplished using the I2 test (13). Given the anticipated existence of inter-trial heterogeneity, we adopted the random-effects model for all analyses, and statistical significance was determined at a significance threshold of p < 0.05. In instances where substantial heterogeneity was observed among outcomes (I2 > 75%), sensitivity analyses were undertaken to ascertain the origin of inter-study variability and to verify the robustness of our findings. However, sensitivity analyses could not be conducted for outcomes predicated on three or fewer studies.
2.3 Subgroup analysis
We performed a subgroup analysis considering several factors, such as age, BMI, sample size, and tumor diameter.
2.4 Publication bias
To evaluate the possibility of publication bias, we employed Begg’s method funnel plot.
3 Results
3.1 Baseline characteristics
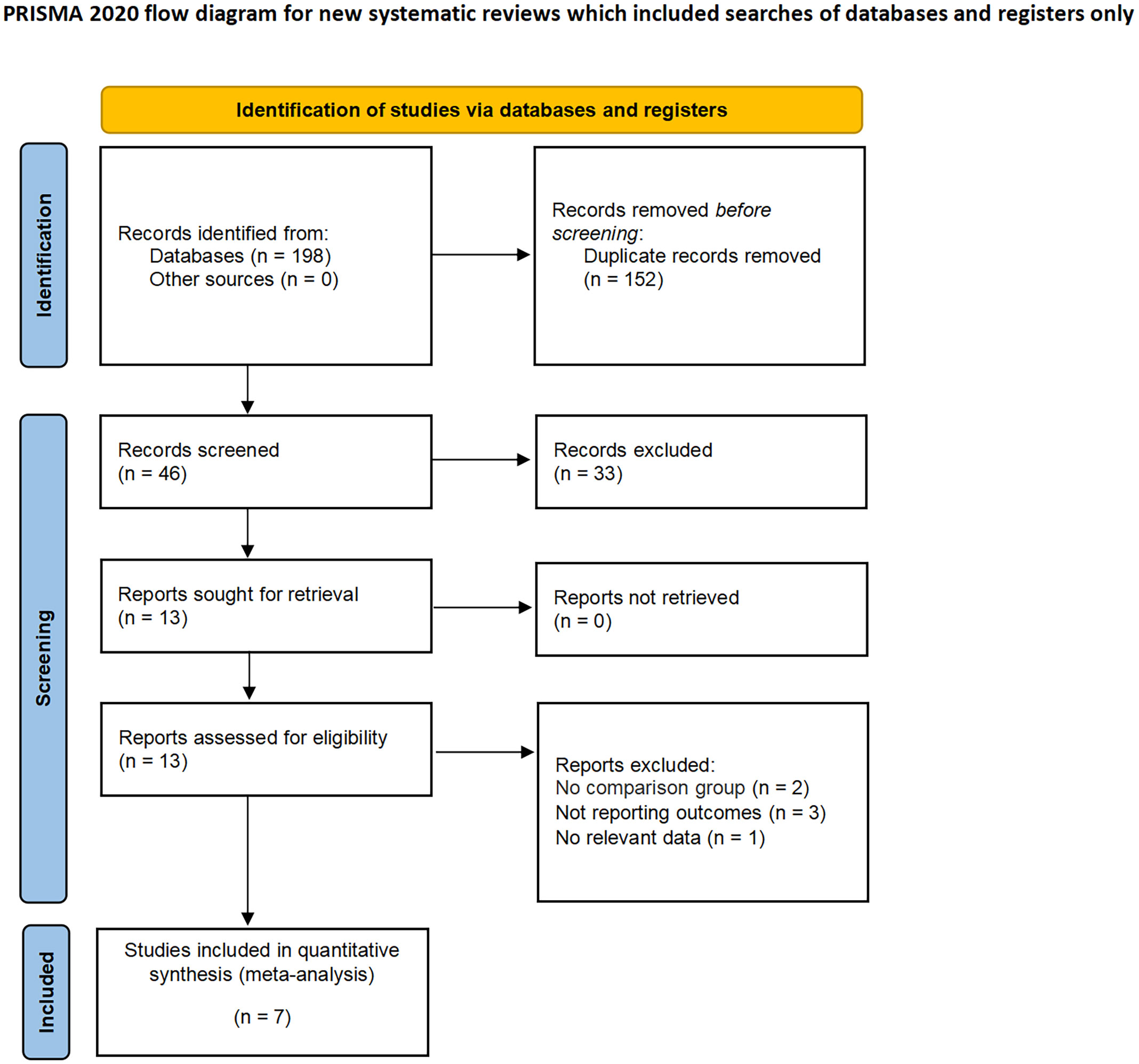
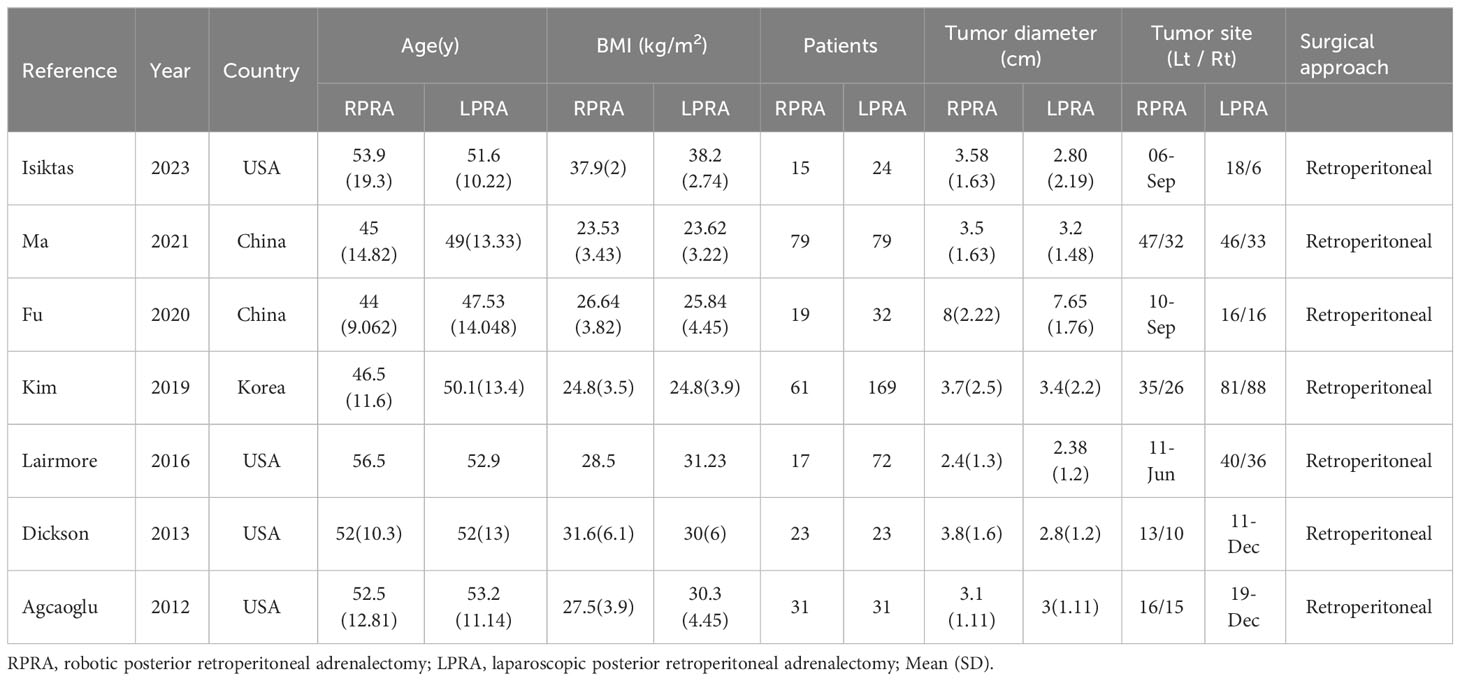
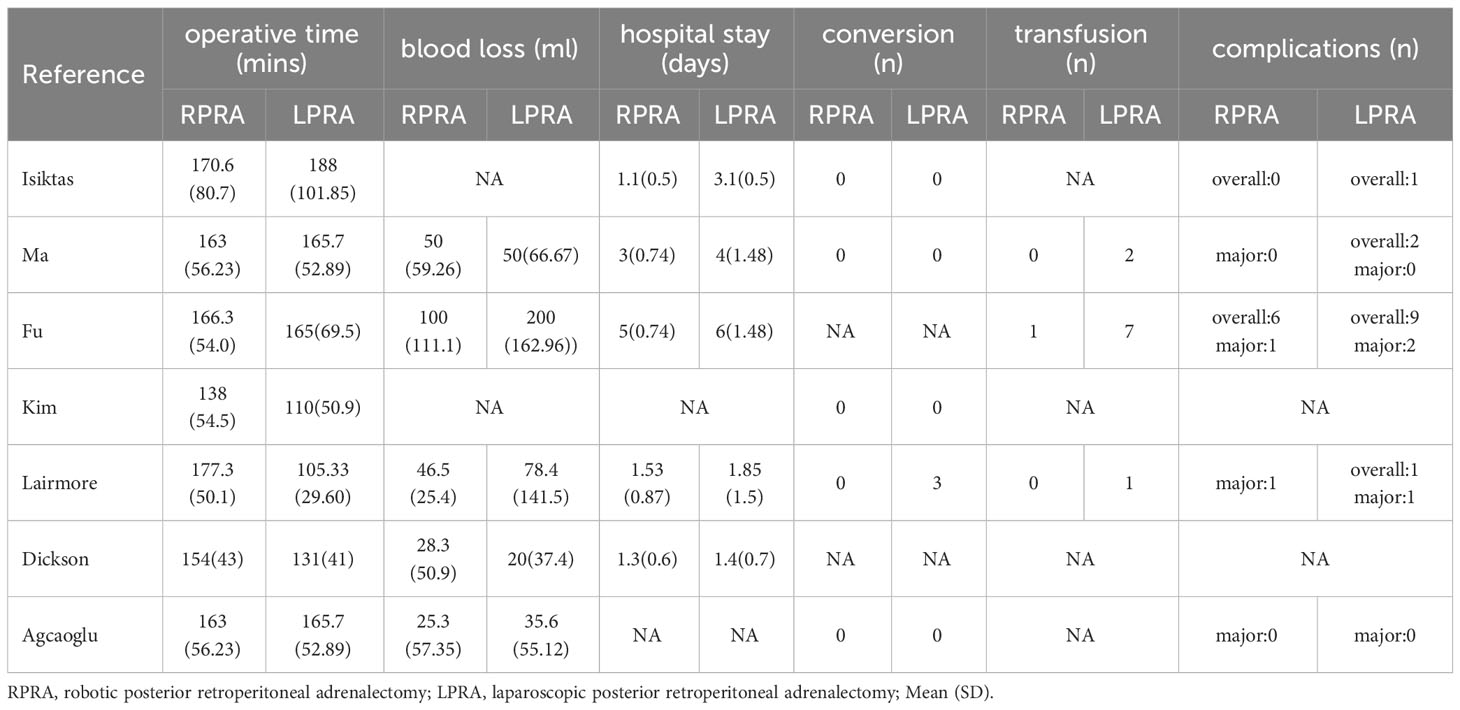
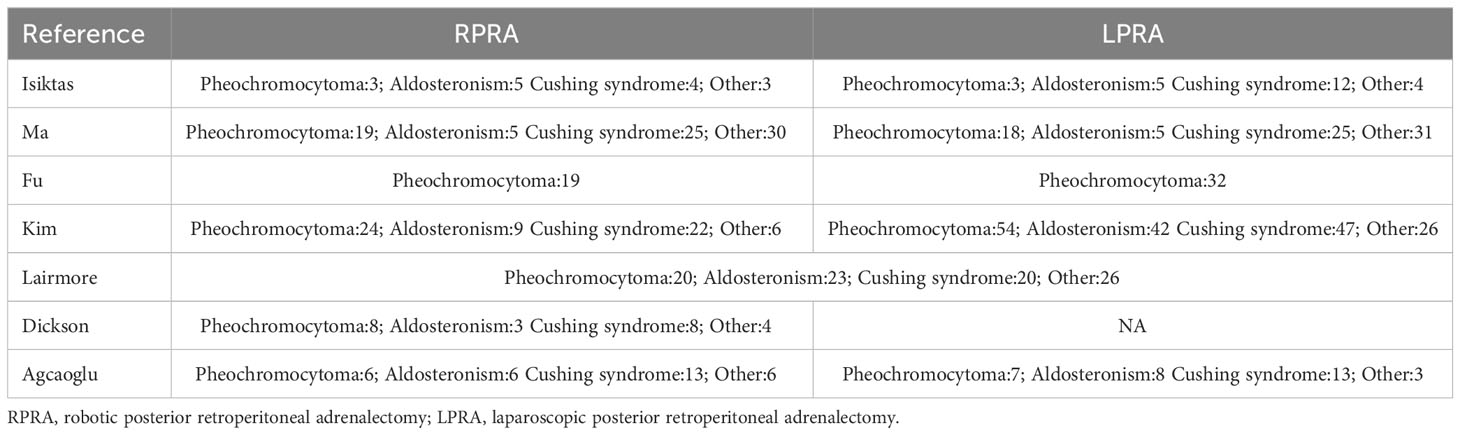
The applied search algorithm initially identified a total of 139 studies within the databases. Following an extensive review of full-text materials and a meticulous screening process, seven studies, comprising 675 patients in total, were deemed suitable for inclusion in the comprehensive meta-analysis (Figure 1) (14–20). All seven investigations adopted the posterior retroperitoneal adrenalectomy approach. The succinct overview in Table 1 offers a synopsis of fundamental patient characteristics, accompanied by the corresponding interventions and associated preoperative variables (including sample size, age, BMI, surgical approach, tumor diameter, and location). These studies were conducted across diverse countries, including China, the United States, and Korea, and were published between 2012 and 2023. Table 2 delineates perioperative and surgical outcomes, encompassing pivotal parameters such as operative duration, blood loss, hospitalization duration, conversion rate, transfusion frequency, and occurrences of complications. The compendious summation of oncologic outcomes is presented in Table 3.
3.2 Assessment of quality
No randomized controlled trials (RCTs) comparing RPRA to LPRA were identified. The current meta-analysis meticulously scrutinized a cumulative of seven meticulously chosen investigations, with six of these displaying a discernible, moderate proclivity for bias, while only a study exhibited a notably diminished susceptibility to bias (14). Notably, each of the incorporated studies executed a meticulous comparative scrutiny, as elucidated in Table S1.
3.3 Outcome analysis
3.3.1 Perioperative effectiveness
Following the amalgamation of findings from seven studies, no statistically significant distinction emerged in operative time between the RPRA and LPRA approaches (p = 0.22) (14–20). Upon pooling data from six distinct studies, the RPRA cohort exhibited a diminished duration of hospitalization compared to their LPRA counterparts (WMD -0.78 days, 95% CI -1.46 to -0.10; p = 0.02) (14–16, 18–20) (Figure 2).
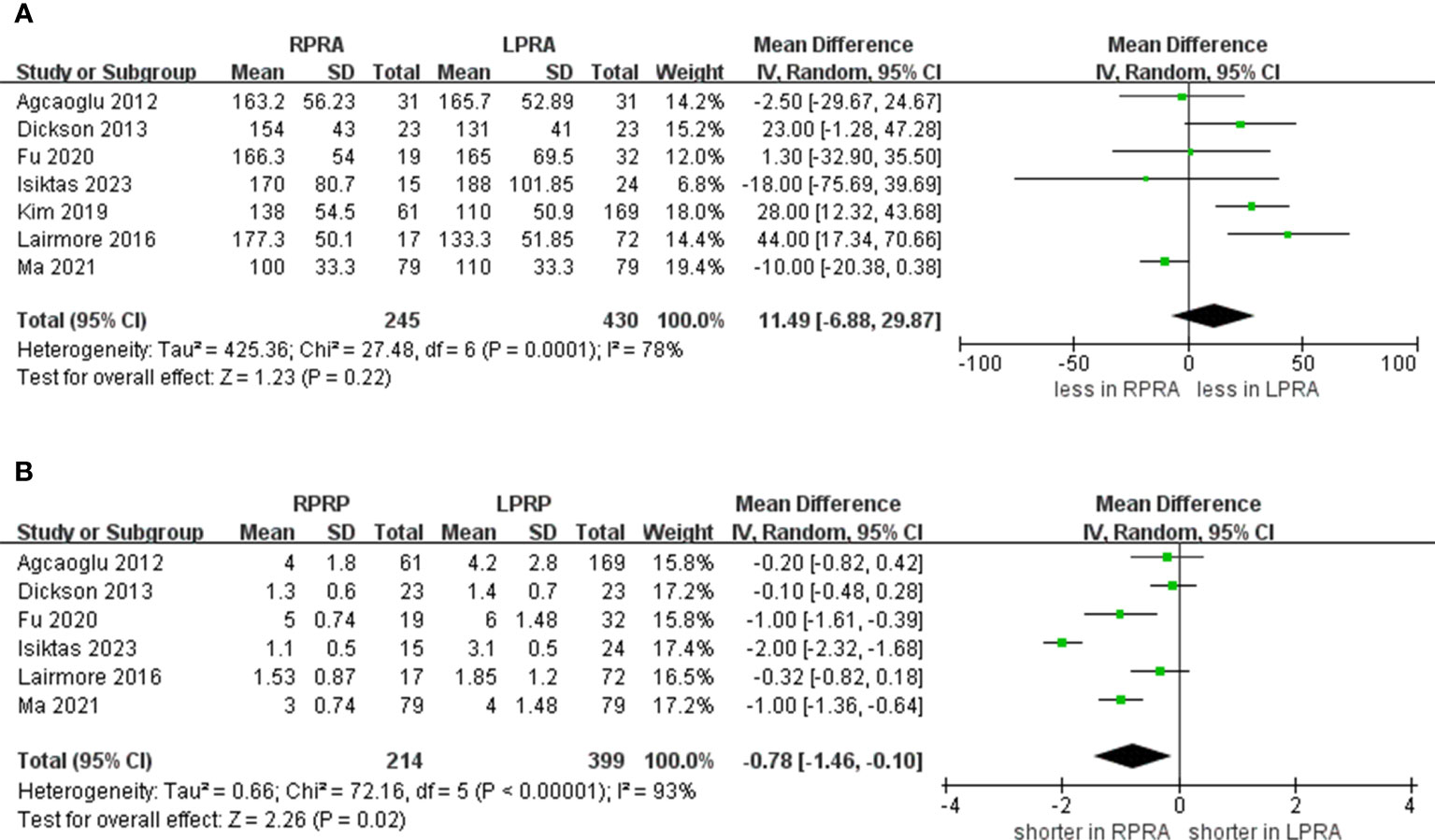
Figure 2 Forest plots of perioperative outcomes for RPRA versus LPRA. (A) operative time, (B) length of hospital stay.
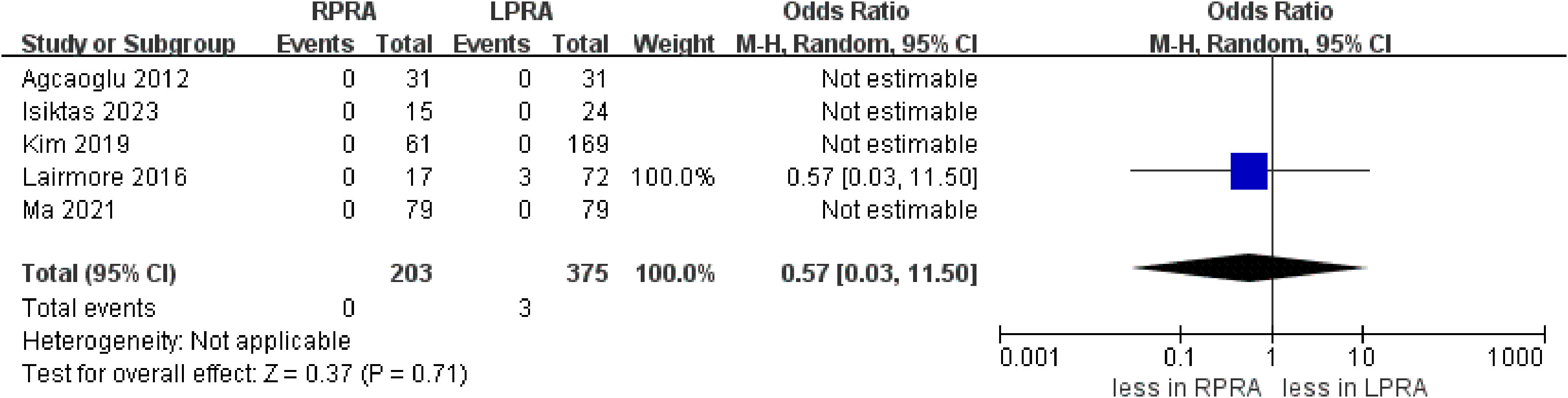
The cumulative revealed no statistically significant disparity in the occurrence of blood loss (five studies; p = 0.25) (15, 16, 18–20). Likewise, no substantial difference emerged in the prevalence of transfusion rate between RPRA and LPRA (p = 0.14, three studies) (Figure 3) (15, 16, 18). The frequency of conversion to open surgery was documented in five studies. However, the aggregated analysis did not reveal any statistically significant disparities in the reduction of conversion to open surgery between RPRA and LPRA (p = 0.71) (Figure 4) (14, 15, 17, 18, 20).
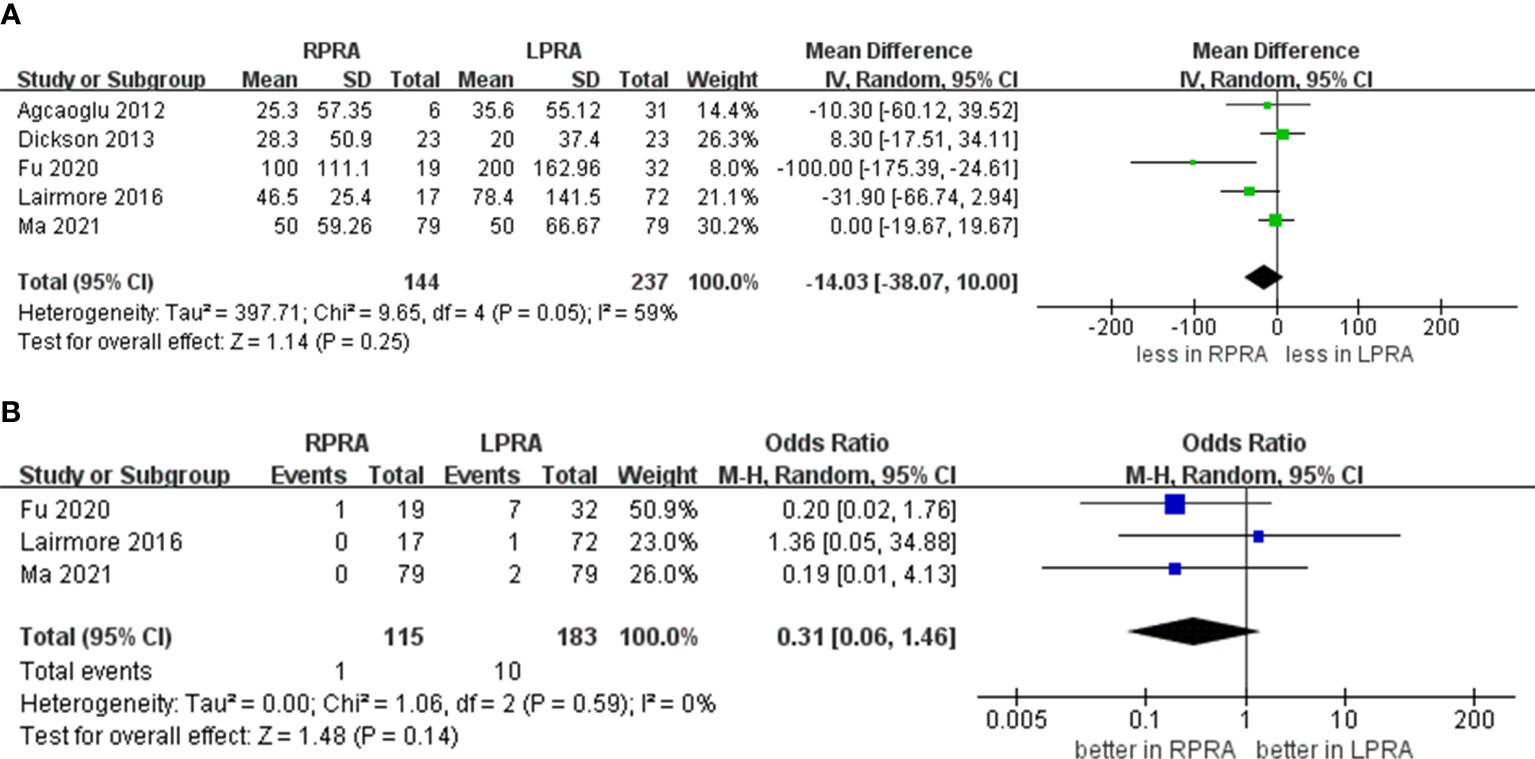
Figure 3 Forest plots of perioperative outcomes for RPRA versus LPRA. (A) blood loss, (B) transfusion rates.
3.3.2 Complications
The collective incidence of overall complications was 9.2% (12 out of 130 cases) for RPRA and 10.6% (22 of 207 cases) for LPRA (14–16, 18). Notably, no substantial disparities emerged in the prevalence of postoperative overall complications (graded as Clavien ≥1) (p = 0.99). Moreover, the rates of major complications were 1.3% (2 out of 146 cases) for RPRA and 1.4% (3 of 214 cases) for LPRA. Similarly, no statistically significant differences were identified in the occurrence of major complications between RPRA and LPRA (four studies; p = 0.57) (Figure 5) (15–18).
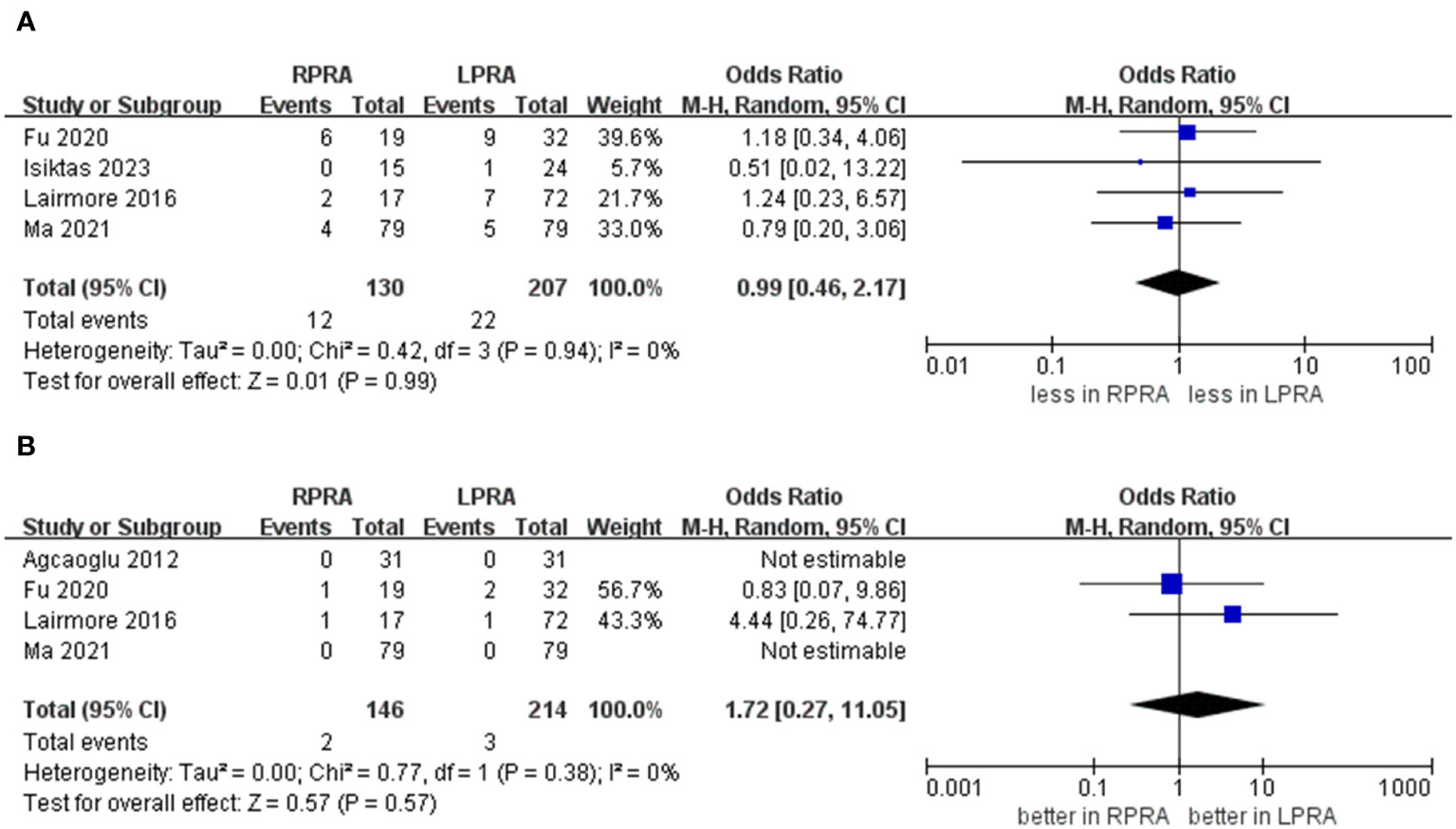
Figure 5 Forest plots of complication for MIPN versus OPN. (A) overall complication, (B) major complications.
3.3.3 Subgroup
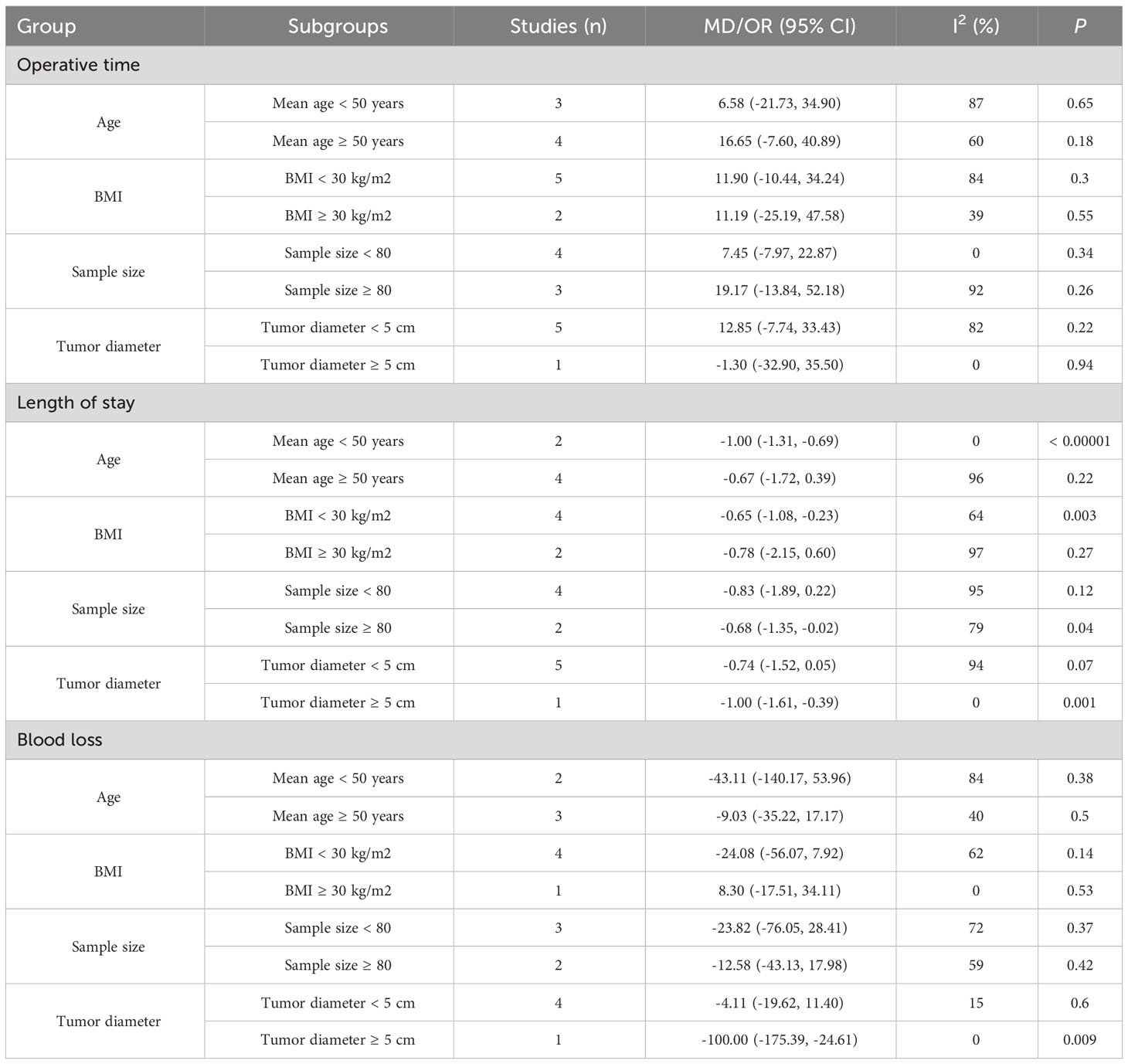
We undertook a subgroup analysis through the stratification of data according to age, BMI, sample size, and tumor diameter. This rigorous analysis encompassed pivotal outcomes, including operative time, length of hospitalization, and blood loss, all of which are presented in Table 4.
All subgroup analyses consistently indicated no significant disparity in operative time between the two groups. The heterogeneity across studies concerning length of hospital stay was found to be influenced by both age and tumor diameter. Specifically, within the subset of studies involving individuals aged < 50 years, RPRA exhibited a markedly reduced length of stay compared to LPRA (p < 0.00001). In contrast, within the subgroup of studies encompassing individuals aged ≥ 50 years, no significant variance in length of stay was observed between the two groups (p = 0.22). Furthermore, within the subgroup characterized by a tumor diameter ≥ 5 cm, patients who underwent RPRA displayed a significantly shorter length of hospital stay compared to those who underwent LPRA (p = 0.001). Conversely, no noteworthy distinction was noted within the subgroup of cases with a tumor diameter < 5 cm (p = 0.07).
Our analysis revealed tumor diameter as a substantive source of heterogeneity concerning blood loss. In particular, within the subset characterized by a tumor diameter ≥ 5 cm, RPRA exhibited an association with diminished blood loss in contrast to LPRA (p = 0.009), whereas in the subgroup marked by a mean tumor diameter < 5 cm, no noteworthy distinction was evident (p = 0.6).
3.4 Heterogeneity
A prevailing trend toward low to moderate heterogeneity was evident in most of the findings. Even with the incorporation of studies of intermediate and high quality, substantial variability was discerned in two of the outcomes (operative time, I2 = 78%; length of hospital stay, I2 = 93%).
3.5 Sensitivity analysis
Within the context of this study, the evident heterogeneity present in factors such as operative time and hospital stay prompted the implementation of a sensitivity analysis. This analytical endeavor aimed to unveil the fundamental origins of the heterogeneity while also evaluating the robustness and steadfastness of the study’s outcomes. The findings of this comprehensive analysis unveiled a lack of substantial shifts in the extent of heterogeneity, signifying the enduring consistency of the underlying heterogeneity sources in both operative time and hospital stay over the course of the study.
3.6 Publication bias
To ascertain the potential for publication bias within the examined studies, an analysis was conducted involving operative time, and length of stay as variables. Our findings revealed that the distribution among the studies exhibited an almost symmetrical pattern, suggesting a minimal probability of publication bias (Figure 6).
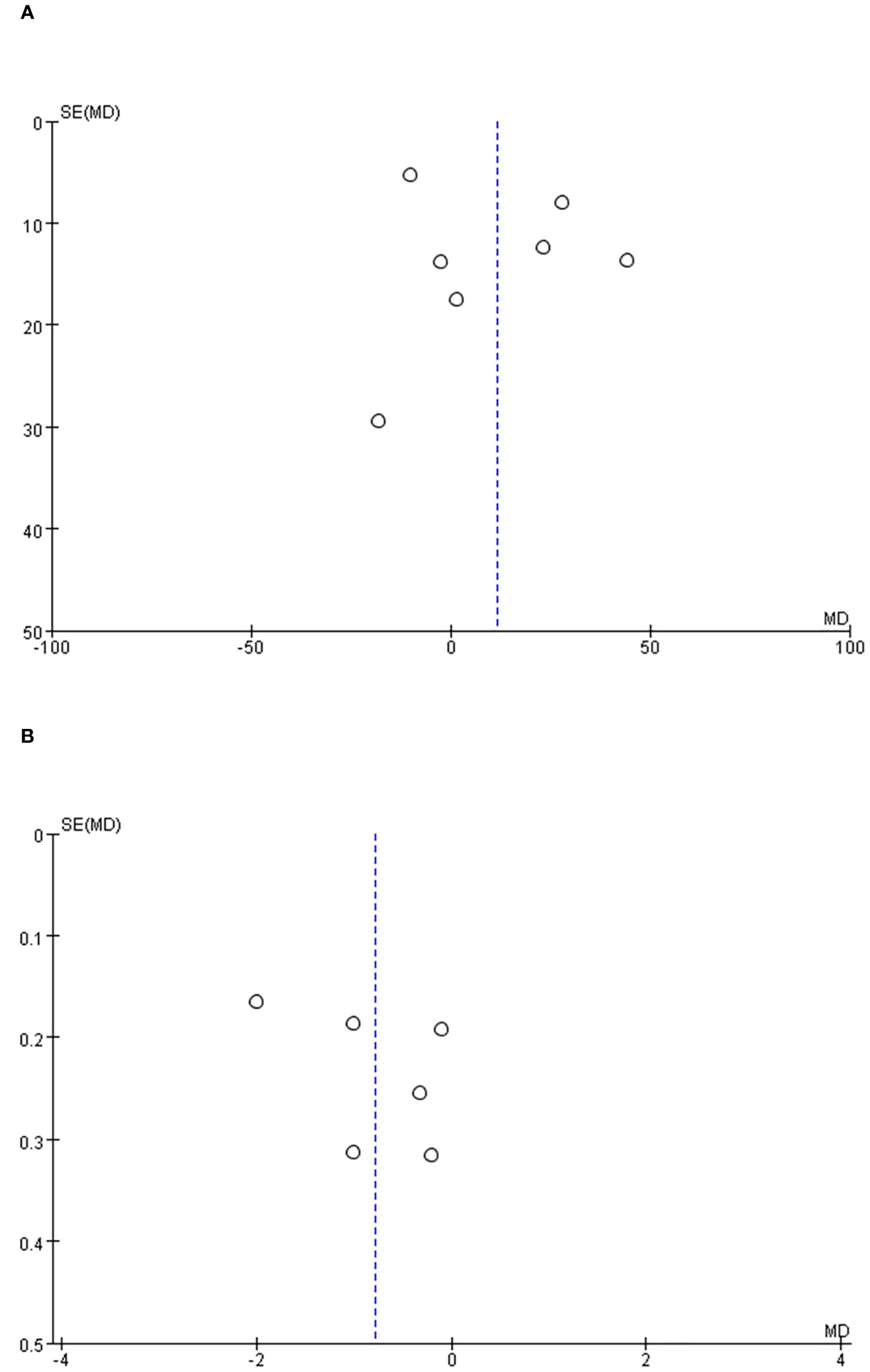
Figure 6 Funnel plot of the studies represented in the meta-analysis. (A) operative time, (B) length of hospital stay.
4 Discussion
This represents the first systematic review and meta-analysis examining the comparative outcomes between RPRA and LPRA. Several pivotal discoveries within this study merit comprehensive elucidation and discourse.
Seven studies were encompassed in the analysis of operative duration. No statistically significant difference was observed in operative time between RPRA and LPRA. Nevertheless, earlier investigations revealed a substantial elongation in the procedural duration for robotic-assisted posterior retroperitoneoscopic adrenalectomy in contrast to its posterior retroperitoneoscopic counterpart (21, 22). After establishing pneumoperitoneum, three to four robotic ports are typically positioned two finger-widths below the rib edge. Additionally, there are instances where it becomes necessary to create an initial auxiliary opening near the border of the rectus muscle to facilitate retraction or suction (23, 24). The surgeon’s preparatory actions, encompassing the orchestration of the operative field, calibration of camera perspectives, and manipulative proficiency, may have exerted influence on the temporal course of RPRA procedures. Furthermore, the variable levels of surgical expertise possessed by individual practitioners bore impact on the operative time within the aggregate studies. In light of recent investigations, as surgeons traverse the learning curve, the temporal demands associated with the robotic approach are anticipated to diminish. In addition, the favorable outcomes observed in robotic urology surgeries conducted by RPRA surgeons may be ascribed to their expertise gained through previous experience in other robotic procedures, such as robotic prostatectomy and nephrectomy (1). An explicable conjecture could attribute this phenomenon to the prevalence of more contemporary RPRA interventions, a manifestation likely stemming from the evolutionary aspects of the surgical technique. Hence, it is conceivable that the surgeon’s navigation through the learning curve could inadvertently protract the operative duration (8, 25). Indeed, within the recent inclusions, RPRA has demonstrated a notably reduced operative time in comparison to LPRA. The integration of robotic articulatory instruments with a more robust camera platform and the provision of high-definition 3D visualizations have the potential to expedite the dissection process. Therefore, it remains conceivable that RPRA may have the potential to necessitate a reduced operative duration compared to LARP in subsequent periods (26, 27). Accordingly, a more substantial body of high-caliber evidence is imperative to substantiate our findings. No statistically significant disparity surfaced in the conversion rate between RPRA and LARP. A prior investigation documented a RPRA conversion rate reaching a magnitude of 40%, thereby unveiling an elevated conversion propensity within the RPRA domain as juxtaposed with LARP (28). Concomitant with the accumulated proficiency of individual surgeons utilizing the robotic platform, the conversion rates exhibited a parallel reduction akin to the corresponding levels observed within the LARP frame (29).
Notwithstanding the divergent findings reported in antecedent studies regarding hospital stay (20, 30), our conducted meta-analysis tends to corroborate that RPRA was linked to a briefer hospitalization interval in comparison to LPRA. The variance in hospital stay can be elucidated through the subsequent rationales. Primarily, this variance could potentially stem from the advantages intrinsic to the robotic platform. The benefits conferred by robotic technology encompass high-resolution three-dimensional optics, augmented dexterity, and improved ergonomics, enabling quiver-free and meticulous movements (31). Additionally, the sensitivity analysis indicates the robustness of the estimations. Secondly, bearing in mind the consistent demonstration in prior research of the pivotal role played by institutional caseload and surgeon expertise as crucial determinants influencing the outcomes of minimally invasive procedures, RPRA and LARP are not exempt from this paradigm (32). Hence, exercising prudence is imperative while appraising the hospitalization period following RPRA and LPRA.
Blood loss stands as a pivotal metric for assessing surgical quality. While a statistically significant discrepancy in blood loss between the two groups was not observed, the majority of the encompassed studies exhibited that the RPRA cohort manifested a diminished transfusion incidence and reduced blood loss in contrast to the LARP cohort. The variance in estimated blood loss can be explicated through the ensuing rationales. The adaptability of the robotic flexible arm coupled with the enhanced precision afforded by magnified high-definition stereoscopic vision facilitates the identification and management of intraoperative bleeding and the precise delineation of intricate anatomical structures and separations (33). However, what is worth our attention is that estimate blood loss is not a relevant parameter to assess the surgical efficacy, because of a difference of few ml may not be clinically significant. Furthermore, it is essential to consider that the postoperative blood transfusion rate among patients may be contingent on the surgical expertise of the healthcare professionals involved and the specific blood transfusion protocols adhered to by the hospitals (34). In forthcoming times, a deeper reservoir of research focusing on blood loss is requisite to further corroborate this assertion.
Regarding morbidity, no noteworthy distinction emerged between RPRA and LARP in relation to both overall and major complications. A precedent meta-analysis substantiated that RPRA exhibited a higher incidence of complications in contrast to LPRA (35). Nonetheless, contemporary investigations have demonstrated a lack of significant divergence between the two groups concerning complication rates (36). The variance in complication rates can be elucidated through the subsequent rationales. Primarily, the accumulated surgeon expertise in robotic utilization has contributed to the reduction in RPRA-associated complications. Secondly, the abbreviated hospital stay and mitigated blood loss appear to equilibrate the physiological strain endured by the surgical patient, thereby culminating in commensurate complication rates.
Given that the elevated cost associated with robotic surgery constitutes a drawback of the procedure, cost assumes a pivotal role in the contemplation of robotic utilization. Several studies have indicated that robotic surgery has been documented to be 1.3 -3.2 times pricier compared to laparoscopy (28, 37). On one hand, the depreciation of the robotic system and augmenting the annual volume of robotic cases employed proved more efficacious in cost mitigation. On the other hand, patients’ selection of an approach was influenced by their individual financial capacity. Hence, cost may not be deemed a salient determinant impacting outcomes. Nevertheless, it exerted influence on infrastructure and medical insurance provisions. It is incumbent upon us to deliberate upon which patients are suitable candidates for the robotic approach, taking into account the social and economic costs.
A subgroup analysis was conducted to ascertain the patient cohort that could potentially derive advantages from RPRA as opposed to LPRA. Certain studies have posited that LTA for tumors surpassing the 5 cm threshold is both secure and feasible under the supervision of a seasoned practitioner. Despite the anticipation of lengthier operative durations associated with LPRA in comparison to LTA, select research endeavors have indicated that the employment of a robotic platform could potentially truncate the procedural chronometry for adrenal tumors > 5 cm (9, 38). Nevertheless, our study did not disclose statistically significant disparities in operative time. Despite the subgroup analysis unveiling a shorter postoperative hospitalization duration within the RPRA cohort, no marked distinctions between the two surgical methodologies were discerned in relation to other outcomes. This could potentially be attributed to the diminutive sample size. Given the inherent advantages of robotic platforms, some surgeons may opt for robotic methods to manage more challenging cases, such as larger tumors or patients with higher BMI (39). Consequently, additional investigations are imperative to facilitate a comprehensive comparison of the advantages intrinsic to both approaches within these cases. Upon stimulation, pheochromocytomas can exude catecholamines, precipitating hemodynamic fluctuations that may culminate in severe complications or morbidity. Consequently, adrenalectomy for pheochromocytoma presents a substantial challenge for operators. In recent years, several studies have postulated that the robotic platform confers superior therapeutic efficacy in the context of adrenal tumors with pheochromocytoma (40, 41). Particularly for pheochromocytomas, neoplasms characterized by a profuse vascular supply, precision in surgical execution holds paramount significance for efficacious hemostatic management. With the exception of operative time, no significant distinctions were observed between the two groups. In light of the paucity of data within our study, circumspection is warranted while appraising the outcomes differential between RPRA and LPRA in the context of pheochromocytoma. Furthermore, Li et al. (42) conducted a meticulous meta-analysis to comprehensively assess the safety and efficacy of partial adrenalectomy (PA) in comparison to total adrenalectomy (TA), with a focus on perioperative and functional outcomes. Their analysis reveals that surgical outcomes in both TA and PA procedures are indeed comparable. The robotic system appears exceptionally well-suited for this technology, owing to its remarkable capacity for achieving precise operations. This is primarily attributed to its multi-degree-of-freedom articulated wrist and the advantage of 3D magnified vision (24). However, further research is imperative to corroborate this conclusion. We have added these relevant findings in the current manuscript.
The current study bears certain limitations that necessitate acknowledgment prior to the interpretation of our findings. First and foremost, the composition of non-randomized controlled trials (non-RCTs) with intermediate methodological quality engenders susceptibility to potential misclassification bias and latent confounding variables. Moreover, a subset of the incorporated studies exhibited diminutive sample sizes. Secondly, albeit the utilization of subgroup analysis, it is noteworthy that the amalgamated studies encompass diverse clinical diagnoses for tumors, a factor that potentially introduces confounding elements into the results. Thirdly, the preponderance of outcomes is contingent upon a selection of the seven studies, precipitated by a dearth of data within the remaining studies. This aspect conspicuously underscores the limitations inherent in the comparison. A substantial portion of the studies are characterized by limited sample sizes and inadequate statistical power.
5 Conclusions
The outcomes of this meta-analysis provide encouragement for the adoption of RPRA within the retroperitoneal space. Specifically, RPRA exhibited a reduced hospitalization duration and diminished invasiveness. Furthermore, the study indicates comparable perioperative outcomes and complication rates when juxtaposed with LPRA. Given that the encompassed studies were characterized by non-randomized controlled trials (non-RCTs) with intermediate methodological quality, the substantiation of RPRA’s superiority and identification of the patients most predisposed to gain from RPRA mandate the execution of prospective randomized controlled trials (RCTs) with extended follow-up periods and elevated-level evidence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Y-GL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. X-BC: Conceptualization, Formal analysis, Resources, Visualization, Writing – original draft, Writing – review & editing. C-MW: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. X-DY: Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. X-ZD: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. BL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the City and College Technology Strategic Cooperation Project of Nanchong (18SXHZ0576); City and College Technology Strategic Cooperation Project of Nanchong (22SXQT0215); Project of North Sichuan Medical College (CBY21-QA38); Five-Year Plan” for Social Science Research in Nanchong City (NC2018B035).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1278007/full#supplementary-material
References
1. Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. N Engl J Med (1992) 327(14):1033. doi: 10.1056/nejm199210013271417
2. Gonzalez R, Smith CD, McClusky DA 3rd, Ramaswamy A, Branum GD, Hunter JG, et al. Laparoscopic approach reduces likelihood of perioperative complications in patients undergoing adrenalectomy. Am Surg (2004) 70(8):668–74. doi: 10.1177/000313480407000803
3. Hazzan D, Shiloni E, Golijanin D, Jurim O, Gross D, Reissman P. Laparoscopic vs open adrenalectomy for benign adrenal neoplasm. Surg Endosc (2001) 15(11):1356–8. doi: 10.1007/s004640080052
4. Mercan S, Seven R, Ozarmagan S, Tezelman S. Endoscopic retroperitoneal adrenalectomy. Surgery (1995) 118(6):1071–5. doi: 10.1016/s0039-6060(05)80116-3
5. Barczyński M, Konturek A, Nowak W. Randomized clinical trial of posterior retroperitoneoscopic adrenalectomy versus lateral transperitoneal laparoscopic adrenalectomy with a 5-year follow-up. Ann Surg (2014) 260(5):740–7. doi: 10.1097/sla.0000000000000982
6. Arezzo A, Bullano A, Cochetti G, Cirocchi R, Randolph J, Mearini E, et al. Transperitoneal versus retroperitoneal laparoscopic adrenalectomy for adrenal tumours in adults. Cochrane Database Syst Rev (2018) 12(12):Cd011668. doi: 10.1002/14651858.CD011668.pub2
7. Koehne EL, Bajic P, Gupta GN. Robotic-assisted laparoscopic retroperitoneal adrenalectomy. Surg Oncol (2019) 31:7. doi: 10.1016/j.suronc.2019.06.005
8. Berber E, Mitchell J, Milas M, Siperstein A. Robotic posterior retroperitoneal adrenalectomy: operative technique. Arch Surg (2010) 145(8):781–4. doi: 10.1001/archsurg.2010.148
9. Agcaoglu O, Aliyev S, Karabulut K, Mitchell J, Siperstein A, Berber E. Robotic versus laparoscopic resection of large adrenal tumors. Ann Surg Oncol (2012) 19(7):2288–94. doi: 10.1245/s10434-012-2296-4
10. Karabulut K, Agcaoglu O, Aliyev S, Siperstein A, Berber E. Comparison of intraoperative time use and perioperative outcomes for robotic versus laparoscopic adrenalectomy. Surgery (2012) 151(4):537–42. doi: 10.1016/j.surg.2011.09.047
11. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
12. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj (2016) 355:i4919. doi: 10.1136/bmj.i4919
13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
14. Isiktas G, Avci SN, Erten O, Ergun O, Krishnamurthy V, Shin J, et al. Laparoscopic versus robotic adrenalectomy in severely obese patients. Surg Endosc (2023) 37(2):1107–13. doi: 10.1007/s00464-022-09594-z
15. Ma W, Mao Y, Dai J, Alimu P, Zhuo R, He W, et al. Propensity score matched analysis comparing robotic-assisted with laparoscopic posterior retroperitoneal adrenalectomy. J Invest Surg (2021) 34(11):1248–53. doi: 10.1080/08941939.2020.1770377
16. Fu SQ, Zhuang CS, Yang XR, Xie WJ, Gong BB, Liu YF, et al. Comparison of robot-assisted retroperitoneal laparoscopic adrenalectomy versus retroperitoneal laparoscopic adrenalectomy for large pheochromocytoma: a single-centre retrospective study. BMC Surg (2020) 20(1):227. doi: 10.1186/s12893-020-00895-5
17. Kim WW, Lee YM, Chung KW, Hong SJ, Sung TY. Comparison of robotic posterior retroperitoneal adrenalectomy over laparoscopic posterior retroperitoneal adrenalectomy: A single tertiary center experience. Int J Endocrinol (2019), 9012910. doi: 10.1155/2019/9012910
18. Lairmore TC, Folek J, Govednik CM, Snyder SK. Improving minimally invasive adrenalectomy: selection of optimal approach and comparison of outcomes. World J Surg (2016) 40(7):1625–31. doi: 10.1007/s00268-016-3471-8
19. Dickson PV, Alex GC, Grubbs EG, Jimenez C, Lee JE, Perrier ND. Robotic-assisted retroperitoneoscopic adrenalectomy: making a good procedure even better. Am Surg (2013) 79(1):84–9.
20. Agcaoglu O, Aliyev S, Karabulut K, Siperstein A, Berber E. Robotic vs laparoscopic posterior retroperitoneal adrenalectomy. Arch Surg (2012) 147(3):272–5. doi: 10.1001/archsurg.2011.2040
21. Tang K, Li H, Xia D, Yu G, Guo X, Guan W, et al. Robot-assisted versus laparoscopic adrenalectomy: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A (2015) 25(3):187–95. doi: 10.1089/lap.2014.0431
22. Pineda-Solís K, Medina-Franco H, Heslin MJ. Robotic versus laparoscopic adrenalectomy: a comparative study in a high-volume center. Surg Endosc (2013) 27(2):599–602. doi: 10.1007/s00464-012-2496-9
23. Nomine-Criqui C, Germain A, Ayav A, Bresler L, Brunaud L. Robot-assisted adrenalectomy: indications and drawbacks. Updates Surg (2017) 69(2):127–33. doi: 10.1007/s13304-017-0448-6
24. Materazzi G, Rossi L. Robot-assisted adrenalectomy: state of the art. Updates Surg (2021) 73(3):1131–46. doi: 10.1007/s13304-020-00915-2
25. Gu L, Ma X, Wang B, Xie Y, Li X, Gao Y, et al. Laparoscopic vs robot-assisted partial nephrectomy for renal tumours of >4 cm: a propensity score-based analysis. BJU Int (2018) 122(3):449–55. doi: 10.1111/bju.14386
26. Asher KP, Gupta GN, Boris RS, Pinto PA, Linehan WM, Bratslavsky G. Robot-assisted laparoscopic partial adrenalectomy for pheochromocytoma: the National Cancer Institute technique. Eur Urol (2011) 60(1):118–24. doi: 10.1016/j.eururo.2011.03.046
27. Boris RS, Gupta G, Linehan WM, Pinto PA, Bratslavsky G. Robot-assisted laparoscopic partial adrenalectomy: initial experience. Urology (2011) 77(4):775–80. doi: 10.1016/j.urology.2010.07.501
28. Morino M, Benincà G, Giraudo G, Genio GMD, Rebecchi F, Garrone C. Robot-assisted vs laparoscopic adrenalectomy: a prospective randomized controlled trial. Surg Endosc (2004) 18(12):1742–6. doi: 10.1007/s00464-004-9046-z
29. Mishra K, Maurice MJ, Bukavina L, Abouassaly R. Comparative efficacy of laparoscopic versus robotic adrenalectomy for adrenal Malignancy. Urology (2019) 123:146–50. doi: 10.1016/j.urology.2018.08.037
30. Melquist JJ, Redrow G, Delacroix S, Park A, Faria EE, Karam JA, et al. Comparison of single-docking robotic-assisted and traditional laparoscopy for retroperitoneal lymph node dissection during nephroureterectomy with bladder cuff excision for upper-tract urothelial carcinoma. Urology (2016) 87:216–23. doi: 10.1016/j.urology.2015.07.070
31. Mack MJ. Minimally invasive and robotic surgery. Jama (2001) 285(5):568–72. doi: 10.1001/jama.285.5.568
32. Fleming ND, Axtell AE, Lentz SE. Operative and anesthetic outcomes in endometrial cancer staging via three minimally invasive methods. J Robot Surg (2012) 6(4):337–44. doi: 10.1007/s11701-011-0319-y
33. Takagi T, Kondo T, Tachibana H, Iizuka J, Omae K, Kobayashi H, et al. Robot-assisted laparoscopic versus open partial nephrectomy in patients with chronic kidney disease: A propensity score-matched comparative analysis of surgical outcomes. Int J Urol (2017) 24(7):505–10. doi: 10.1111/iju.13363
34. Li KP, Chen SY, Wang CY, Yang L. Comparison between minimally invasive partial nephrectomy and open partial nephrectomy for complex renal tumors: a systematic review and meta-analysis. Int J Surg (2023) 109(6):1769–82. doi: 10.1097/js9.0000000000000397
35. Brandao L, Zargar H, Autorino R, Economopoulos KP, Stamou A, Sergentanis TN, et al. Robotic versus laparoscopic adrenalectomy: a systematic review and meta-analysis. Eur Urol (2014)67(2):1154–61e33–4. doi: 10.1016/j.eururo.2014.09.053
36. Perivoliotis K, Baloyiannis I, Sarakatsianou C, Tzovaras G. Comparing the efficacy and safety of laparoscopic and robotic adrenalectomy: a meta-analysis and trial sequential analysis. Langenbecks Arch Surg (2020) 405(2):125–35. doi: 10.1007/s00423-020-01860-9
37. Winter JM, Talamini MA, Stanfield CL, Chang DC, Hundt JD, Dackiw AP, et al. Thirty robotic adrenalectomies: a single institution’s experience. Surg Endosc (2006) 20(1):119–24. doi: 10.1007/s00464-005-0082-0
38. Brunaud L, Bresler L, Ayav A, Zarnegar R, Raphoz AL, Levan T, et al. Robotic-assisted adrenalectomy: what advantages compared to lateral transperitoneal laparoscopic adrenalectomy? Am J Surg (2008) 195(4):433–8. doi: 10.1016/j.amjsurg.2007.04.016
39. Sforza S, Minervini A, Tellini R, Ji C, Bergamini C, Giordano A, et al. Perioperative outcomes of robotic and laparoscopic adrenalectomy: a large international multicenter experience. Surg Endosc (2021) 35(4):1801–7. doi: 10.1007/s00464-020-07578-5
40. Manny TB, Pompeo AS, Hemal AK. Robotic partial adrenalectomy using indocyanine green dye with near-infrared imaging: the initial clinical experience. Urology (2013) 82(3):738–42. doi: 10.1016/j.urology.2013.03.074
41. Pahwa M, Pahwa AR, Batra R, Abraham RR, Chawla A, Kathuria S, et al. Robotic assisted laparoscopic adrenalectomy: Initial experience from a tertiary care centre in India. J Minim Access Surg (2015) 11(1):83–6. doi: 10.4103/0972-9941.147704
Keywords: robotic, laparoscopic, posterior, adrenalectomy, meta-analysis
Citation: Li Y-g, Chen X-b, Wang C-m, Yu X-d, Deng X-z and Liao B (2023) Robotic posterior retroperitoneal adrenalectomy versus laparoscopic posterior retroperitoneal adrenalectomy: outcomes from a pooled analysis. Front. Endocrinol. 14:1278007. doi: 10.3389/fendo.2023.1278007
Received: 15 August 2023; Accepted: 06 November 2023;
Published: 28 November 2023.
Edited by:
Marta Araujo-Castro, Ramón y Cajal University Hospital, SpainReviewed by:
Leonardo Rossi, University of Pisa, ItalyIoannis Koutelidakis, Aristotle University of Thessaloniki, Greece
Simone Sforza, University of Florence, Italy
Copyright © 2023 Li, Chen, Wang, Yu, Deng and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Liao, Ym95ZWRsb3ZlckBzaW5hLmNvbQ==
†These have authors contributed equally to this work
 Yu-gen Li1†
Yu-gen Li1† Xiao-dong Yu
Xiao-dong Yu Bo Liao
Bo Liao