- 1Department of Surgical Oncology, Medical University of Gdansk, Gdansk, Poland
- 2Department of General, Endocrine, and Transplant Surgery, Medical University of Gdansk, Gdansk, Poland
- 3Regional Hepato-Pancreato-Biliary Surgical Unit, Manchester Royal Infirmary, Manchester, United Kingdom
- 4Department of Endocrinology and Internal Medicine, Medical University of Gdansk, Gdansk, Poland
Background: Evidence suggests that patients with Hashimoto thyroiditis (HT) are at significantly higher risk of developing papillary thyroid cancer (PTC). However, the course of PTC in patients with both diseases concomitantly has been found to be more indolent than conventional PTC. Additionally, it has been well proven that BRAF mutation results in an aggressive course of PTC. The aims of this meta-analysis were to identify prevalence of BRAF mutation and its impact on clinicopathological features in patients with concomitant PTC-HT.
Methods: Medline, Cochrane Library, Scopus, and Web of Science were searched until 16.09.2022, resulting in 227 articles, of which nine studies were included. Summary estimates, comparing patients with (A) BRAF (+) PTC-HT versus BRAF (+) PTC, and (B) BRAF (+) PTC-HT versus BRAF (-) PTC-HT, were generated with Review Manager 5.0.
Results: In total, 6395 patients were included in this review. PTC-HT patients had significantly less BRAF mutation than PTC patients (Odds Ratio (OR) (95% Confidence Interval (CI))=0.45 (0.35-0.58), P<0.001). BRAF (+) PTC-HT patients were significantly more likely to have multifocal lesions (OR (95% CI)=1.22 (1.04-1.44), P=0.01) but less likely to have lymph node metastasis (OR (95% CI)=0.65 (0.46-0.91), P=0.01) and extrathyroidal extension (OR (95% CI)=0.55 (0.32-0.96), P=0.03) compared to BRAF (+) PTC patients. BRAF (+) PTC-HT patients were more likely to have multifocal lesions (OR (95% CI)=0.71 (0.53-0.95), P=0.02), lymph node metastasis (OR (95% CI)=0.59 (0.44-0.78), P<0.001) and extrathyroidal extension (OR (95% CI)=0.72 (0.56-0.92), P=0.01) compared to BRAF (-) PTC-HT patients.
Conclusion: This meta-analysis highlights that the lower prevalence of BRAF mutation in patients with PTC-HT than conventional PTC may explain the indolent clinicopathological course in this cohort.
1 Introduction
Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer, with increasing incidence globally (1). Despite its malignant nature, patients with PTC tend to have excellent prognosis, with 10-year survival rate of approximately 90% (2–4). Hashimoto’s thyroiditis (HT) is an autoimmune inflammatory disease characterized by thyroid-specific autoantibodies [anti-thyroglobulin (Tg) and anti-thyroid peroxidase (TPO)] and T-cells infiltration of thyroid gland (5).
Decreased levels of T3 and T4 seen in hypothyroidism lead to an increase in thyroid-stimulating hormone (TSH) production, which in turn increases the risk of PTC development (6). Recent evidence suggests that, despite contributing to PTC development by causing hypothyroidism, HT may also ameliorate its malignant potential (1). It is suspected that the T-cells infiltrating thyroid tissues in HT secrete IL-1 responsible for inhibiting tumor cell growth and progression (7). Therefore, it is possible that patients with PTC and HT have favorable tumor characteristics (such as less lymph node metastasis (LNM), extrathyroidal extension (ETE), and decreased capsular and angio-invasion), and thereby better prognosis compared to those without HT (2, 8, 9). As HT has the potential to induce PTC, but also mitigate its progression, it can be referred to as a “double-edged sword” in relation to PTC (10).
The BRAF gene encodes a protein involved in the mitogen-activated protein kinases (MAPK) signaling pathway. BRAF mutation is a single nucleotide substitution, which results in continuous activation and signal transduction through the MAPK pathway, resulting in increased cell proliferation and differentiation (11). It is well known that mutation in the BRAF gene plays a crucial role in development of PTC (12). It is present in approximately 44% of PTC patients making it one of the most common somatic alterations in this type of cancer. An imperfect, but nevertheless a viable diagnostic marker (12). BRAF mutation in PTC has been associated with more aggressive clinicopathological features and outcomes such as LNM, ETE and overall mortality (13–15). Evidence also suggests that BRAF mutation in HT patients is associated with development of PTC and more aggressive clinical course (11, 16).
The aim of this meta-analysis is to identify the prevalence of BRAF mutation among patients with concomitant HT and PTC versus conventional PTC patients. The secondary aim is to evaluate the impact of BRAF mutation and HT on clinicopathological features in patients with PTC.
2 Methods
2.1 Literature search
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement using PICO (patients, interventions, comparisons, outcomes)-based questions. After formulating an appropriate search strategy, PubMed, Cochrane, Web of Science, and Scopus online databases were searched for suitable articles. No filters were applied during the search. Backward citation chaining of the chosen full-text studies was performed as well.
2.2 Evidence acquisition
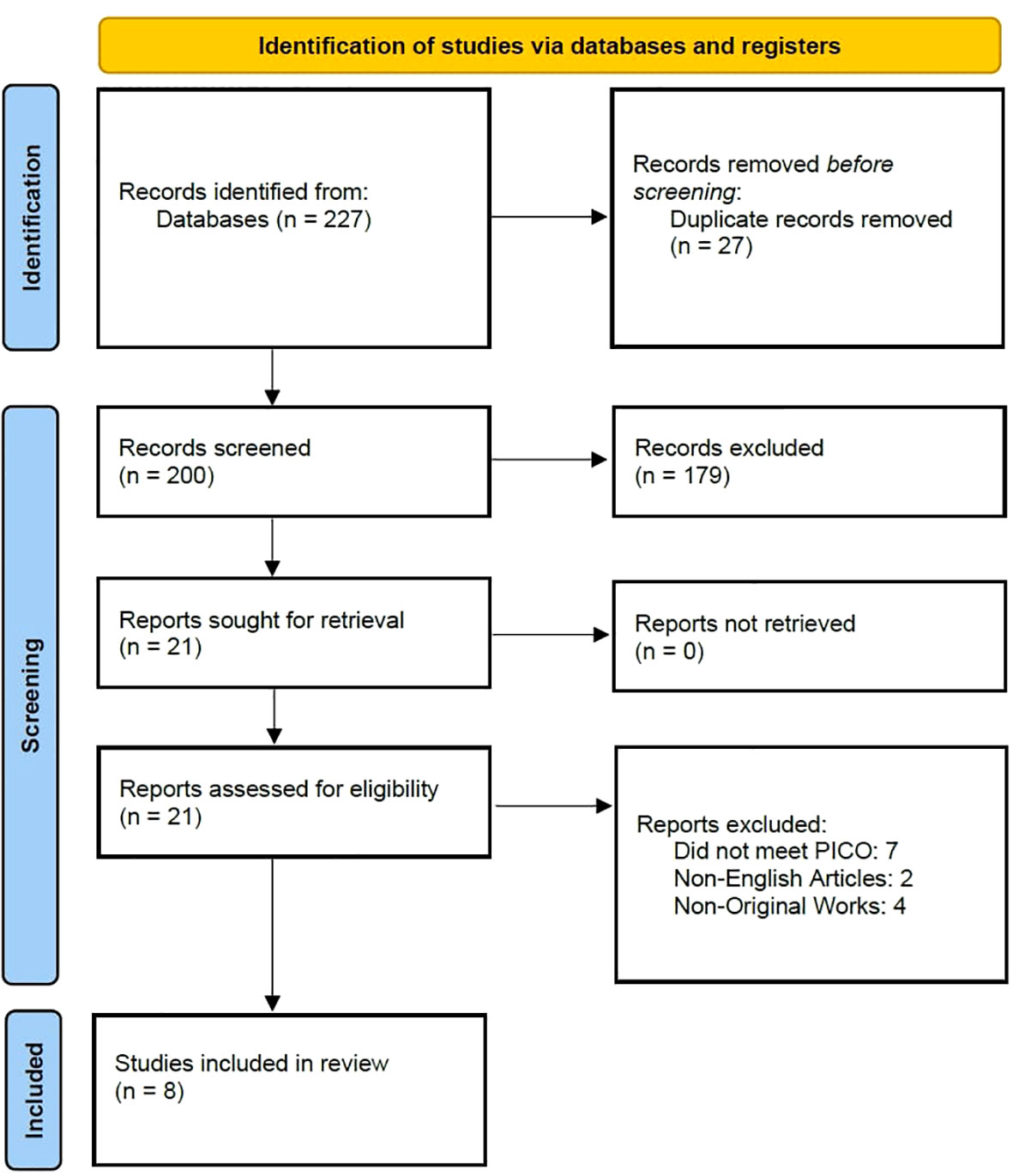
On September 16th, 2022, two independent researchers (LJ and AP) searched the previously mentioned databases for suitable studies. The search string used was (“thyroid cancer” OR “thyroid carcinoma” OR “thyroid microcarcinoma”) AND (“BRAF” OR “mutation”) AND (“Hashimoto thyroiditis” OR “chronic lymphocytic thyroiditis” OR “Hashimoto’s thyroiditis”). The preliminary search, after deleting duplicates, resulted in 227 articles which were later screened by two independent researchers (LJ and AP). The entire process is presented in a PRISMA flow chart (Figure 1).
2.3 Inclusion and exclusion criteria
PICO framework-based research questions were used for this review. Studies were included if they were full-text, original reports comparing BRAF mutation rates in patients with concomitant PTC-HT with conventional PTC. Additionally, studies were excluded if full text was not available, was not in English, were not original articles or did not conform with PICO.
2.4 Evidence synthesis and quality assessment
Information from the eligible studies was retrieved by two independent researchers (LJ and AP) and summarized in tables. Any conflicts relating to inclusion of studies and collected data were resolved via discussion between the two authors in consultation with a third author (AH). Data on the following parameter was collected: first author, year and country of publication, inclusion and exclusion criteria, method of BRAF mutation analysis, demographic information (age, gender), prevalence of BRAF mutation and clinicopathological features.
The quality of cohort studies was assessed by two independent researchers (AP and LJ) using the Newcastle-Ottawa scale (NOS). A maximum of nine points for each of the following items was awarded: four stars for selection, two for comparability and three for outcomes. High quality studies scored seven or more stars on NOS assessment.
2.5 Statistical analysis
Weighted arithmetic means for the prevalence of BRAF mutation in PTC-HT and PTC was calculated by multiplying the weight of each study with the percentage of BRAF (+) patients in each group. The pooled summary estimates were created to compare the prevalence of BRAF mutation between the PTC-HT and PTC patients. Additionally, to assess the impact of BRAF mutation on clinicopathological features (multifocality, extrathyroidal extension (ETE) and lymph node metastasis (LNM), subgroup analyses were performed comparing (1) BRAF (+) PTC-HT versus BRAF (+) PTC patients and (2) BRAF (-) PTC-HT versus BRAF (+) PTC-HT patients. The pooled estimates were generated as odds ratio (OR) with 95% confidence interval (CI) using the Mantel-Haenszel (M-H) test. Random effects model was used if there was significant statistical heterogeneity measured using the I2 coefficient, otherwise the fixed effect model was utilized. The statistical analysis was performed with the Review Manager 5.4.1 software.
3 Results
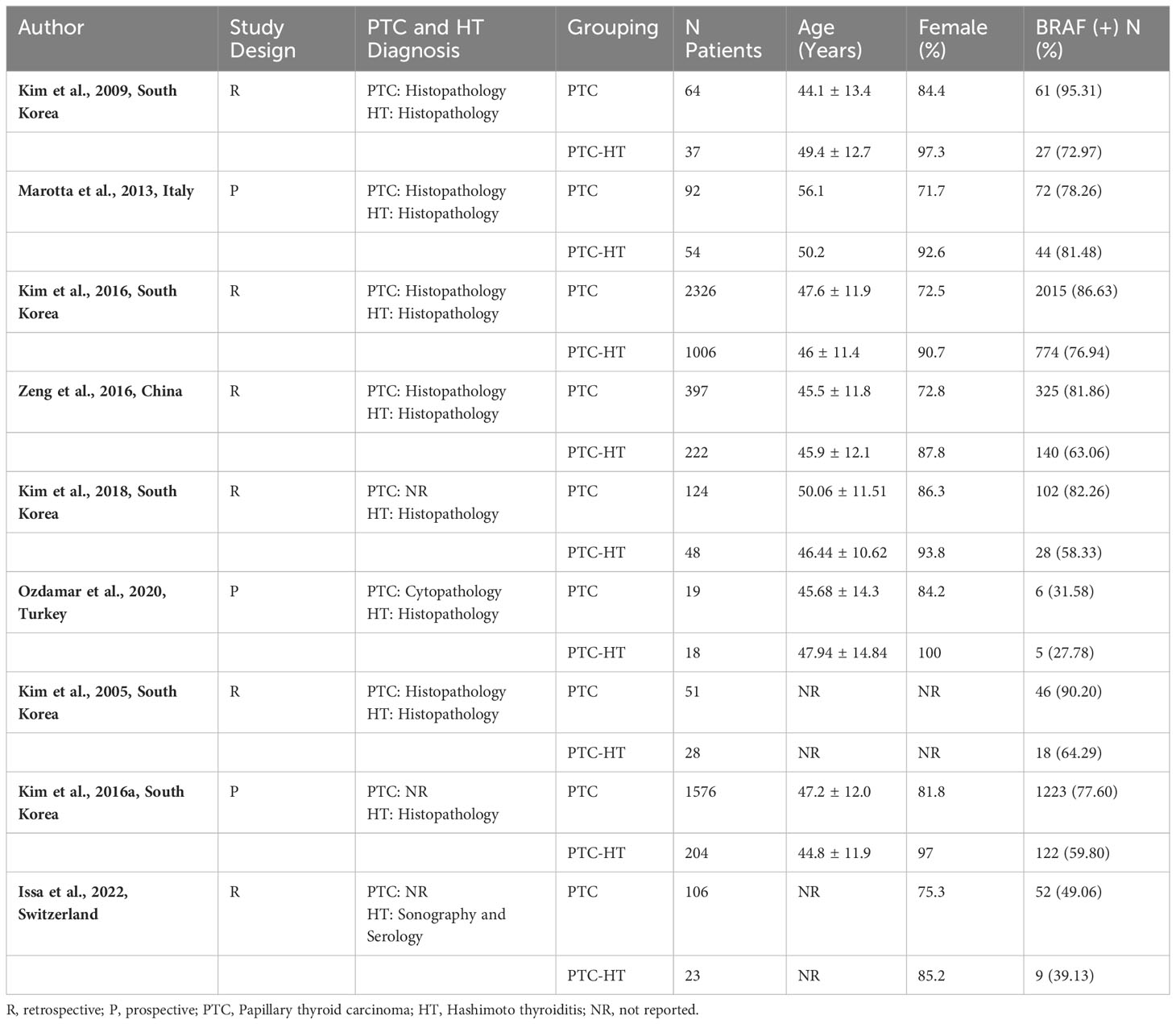
After screening and full text assessment, nine articles (six retrospective, three prospective cohort studies) were included in this review. The information retrieved from the eligible studies is shown in Table 1. Across all studies, concomitant HT was diagnosed based on histopathological evaluation (diffuse lymphocytic infiltration, reactive germinal centers, parenchymal atrophy, stromal fibrosis) while three studies also used anti-Tg and anti-TPO antibodies (3, 15, 17). PTC was diagnosed based on histopathological analysis in seven studies (3, 4, 11, 15, 16, 18, 19) while two studies did not provide information on diagnostic methods (17, 20).
A total of 6395 patients were examined for BRAF mutation, of which 1640 had PTC concomitant with HT (PTC-HT) and 4755 had conventional PTC. BRAF mutation analysis in the included studies was performed using PCR amplification and DNA sequencing. Across studies, percentage of females ranged from 71.7 to 100% while median age ranged from 44.1 to 56.1 years. On risk of bias assessment, all studies were found to be of high-quality scoring >7 points on NOS.
3.1 Prevalence of BRAF mutation
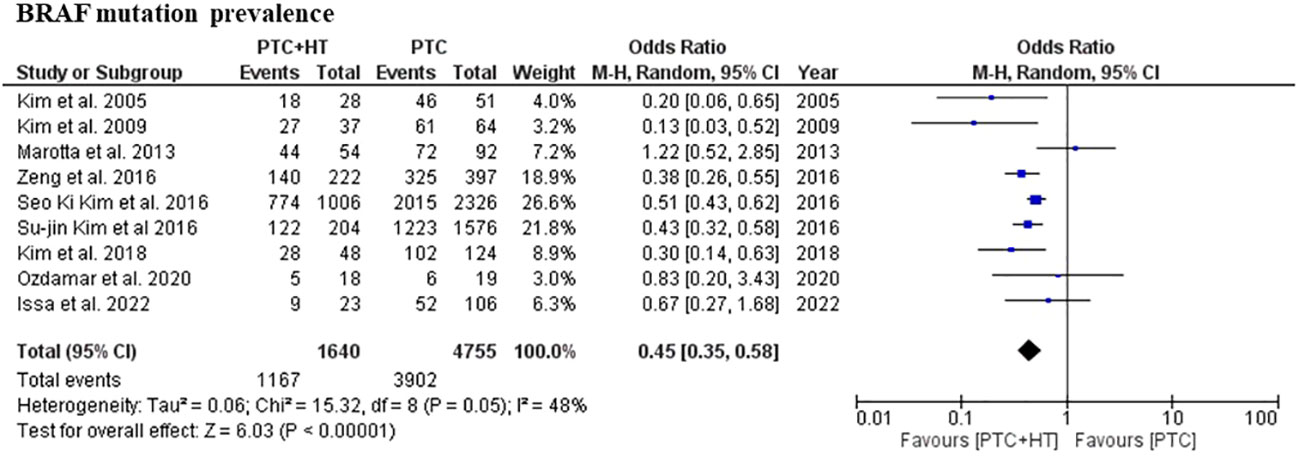
All included studies reported the prevalence of BRAF mutation in patients with concomitant PTC-HT and PTC. The weighted arithmetic mean for BRAF mutation was 71.6% and 82.8% in PTC-HT and PTC patients, respectively. Patients with concomitant PTC-HT were found to have significantly lower prevalence of BRAF mutation than patients with PTC (OR (95% CI) = 0.45 (0.35-0.58), I2 = 54%, P<0.001) (Figure 2).
3.2 BRAF (+) PTC-HT versus BRAF (+) PTC
Four studies presented information related to multifocality and ETE. In terms of multifocality and ETE, a total of 3381 patients with 963 patients in the BRAF (+) PTC-HT and 2418 patients in BRAF (+) PTC group were included (3, 15–17, 19). Patients with BRAF (+) PTC-HT were found to have significantly greater incidence of multifocal disease in comparison to BRAF (+) PTC (OR (95% CI) = 1.22 (1.04-1.44), I2 = 0%, P=0.01) (Figure 3A). On the other hand, the former group was found to have less ETE in comparison to BRAF (+) PTC (OR (95% CI) = 0.55 (0.32-0.96), I2 = 52%, P=0.03) (Figure 3B).
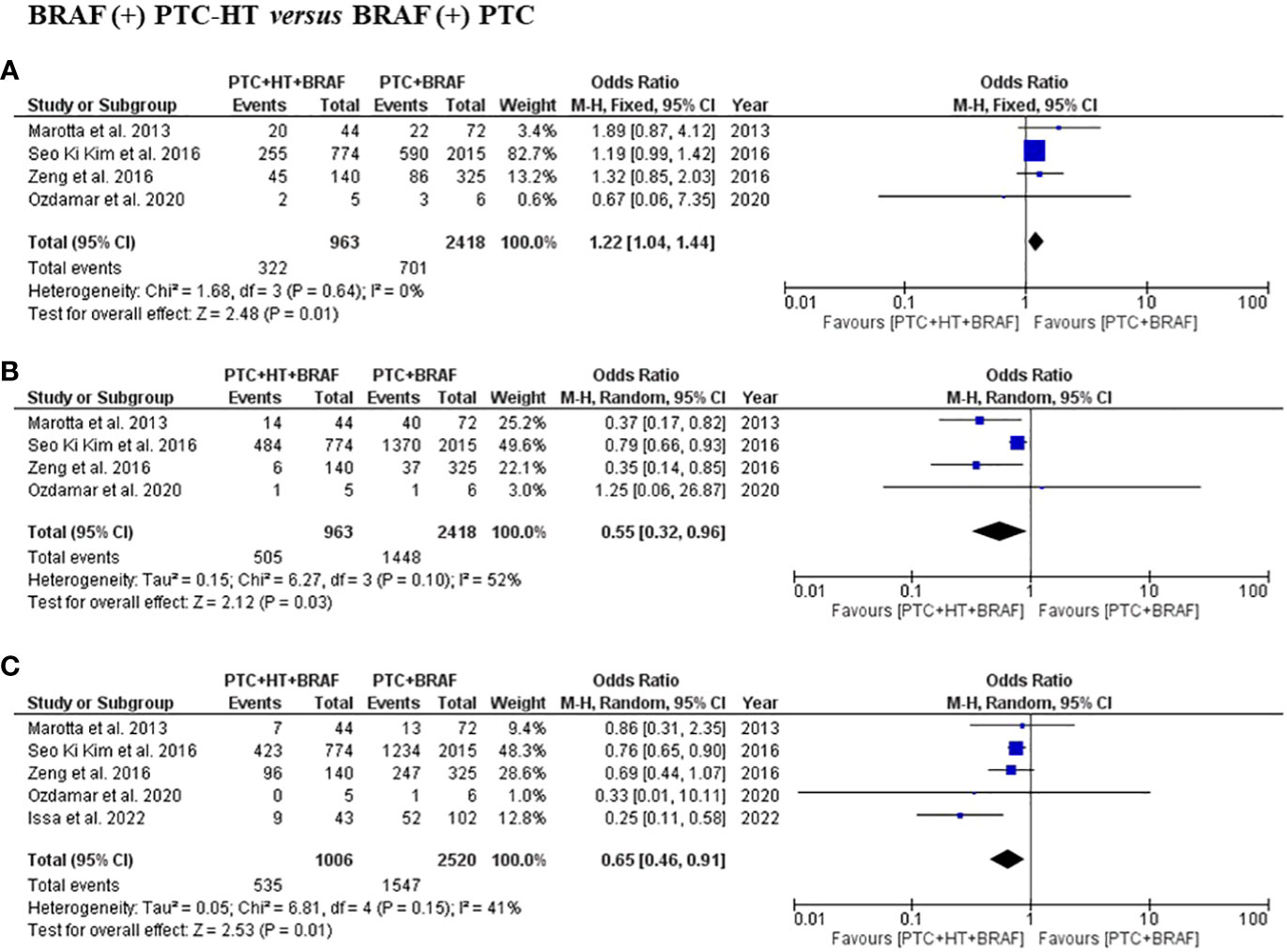
Figure 3 Summary estimates for (A) multifocality, (B) extrathyroidal extension and (C) lymph node metastasis comparing patients with BRAF (+) PTC-HT with BRAF (+) PTC.
Data on LNM was available in five studies, with a total of 3526 patients with 1006 in the BRAF (+) PTC-HT and 2520 in BRAF (+) PTC group were included (3, 15–17, 19). This subgroup analysis showed that patients with BRAF (+) PTC-HT had significantly lower risk of developing central and lateral LNM than patients with BRAF (+) PTC (OR (95% CI) = 0.65 (0.46-0.91), I2 = 41%, P=0.01) (Figure 3C).
3.3 BRAF (-) PTC-HT versus BRAF (+) PTC-HT
Three studies presented information related to multifocality and ETE. In terms of multifocality, a total of 1246 patients with 327 patients in the BRAF (-) PTC-HT and 919 patients in BRAF (+) PTC-HT group were included (3, 16, 19). BRAF (-) PTC-HT were found to have significantly less multifocal disease compared to BRAF (+) PTC-HT (OR (95% CI) = 0.71 (0.53-0.95), I2 = 0%, P=0.02) (Figure 4A). The same 3 studies provided data on 1246 patients in terms of ETE with 385 patients in the BRAF (-) PTC-HT and 861 in BRAF (+) PTC-HT group. The former group was found to have less occurrence of ETE (OR (95% CI) = 0.59 (0.44-0.78), I2 = 0%, P<0.001) (Figure 4B).
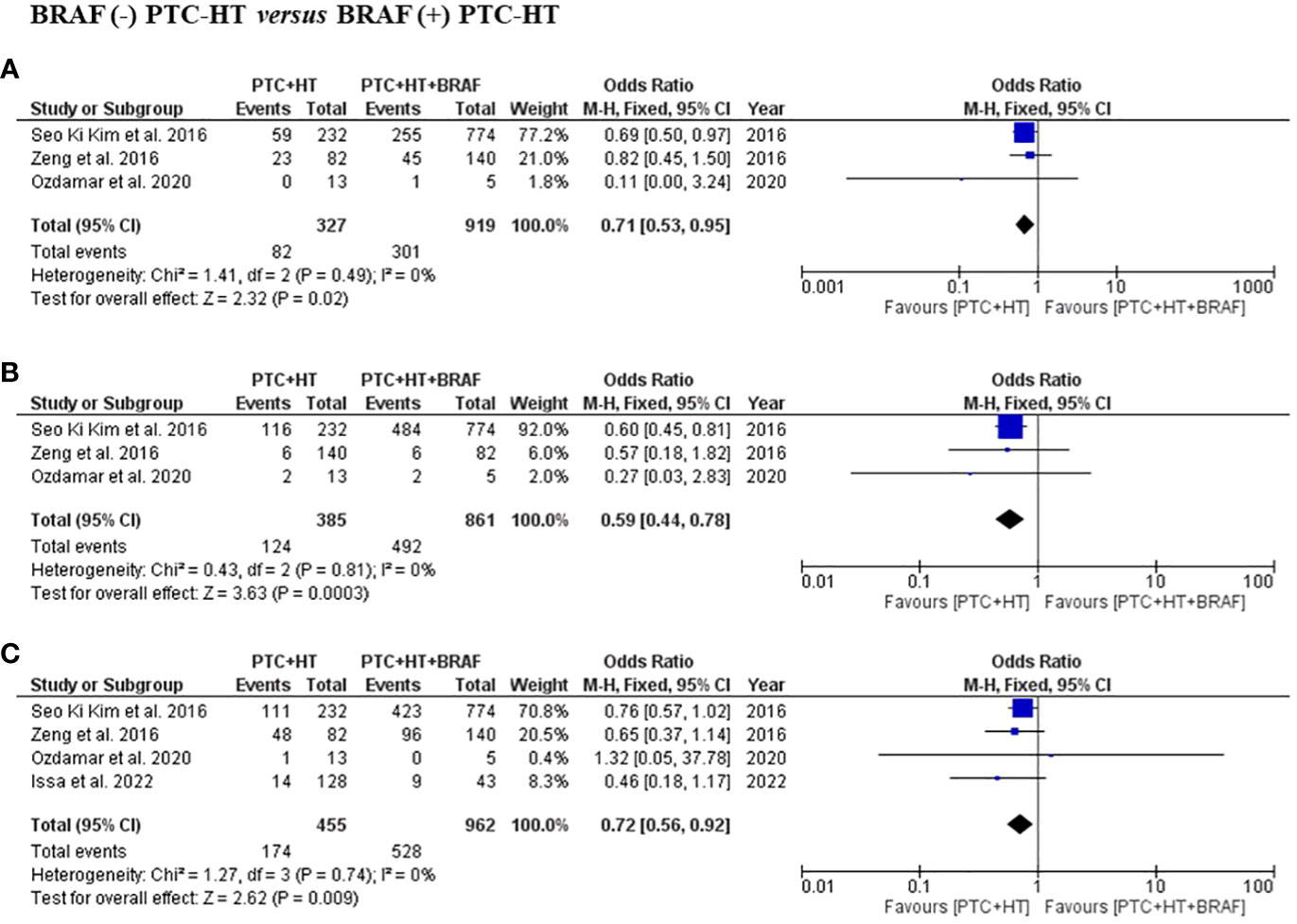
Figure 4 Summary estimates for (A) multifocality, (B) extrathyroidal extension and (C) lymph node metastasis comparing patients with BRAF (-) PTC-HT with BRAF (+) PTC-HT.
Information regarding LNM was available in four studies, with a total of 1417 patients with 455 in the BRAF (-) PTC-HT and 962 in BRAF (+) PTC-HT group (3, 16, 17, 19). This subgroup analysis showed that patients with BRAF (-) PTC-HT had significantly lower risk of developing central and lateral LNM compared to those with BRAF (+) PTC-HT (OR (95% CI) = 0.72 (0.56-0.92), I2 = 0%, P=0.01) (Figure 4C).
4 Discussion
To the best of our knowledge, this is the first meta-analysis reviewing the evidence on the relationship between BRAF mutation and concomitant PTC-HT. Most contemporary reviews have focused on the prevalence and clinicopathological features associated with concomitant PTC-HT. Previous systematic reviews have concluded that patients with HT are at increased risk of developing PTC, however, with a lower incidence of high-risk clinicopathological features (2, 10, 21). Over the recent years, increasing evidence suggested that BRAF mutation is associated with tumorigenesis and progression of PTC (22, 23). The BRAF mutation has been shown to be one of the strongest predictors for extrathyroidal invasion, lymph node metastasis and advanced tumor stages (24). Some have also proposed BRAF mutation scarcity among PTC-HT patients as an explanation for more indolent PTC course in this cohort (5). This review further investigates the above claims and demonstrates that patients with PTC and HT are significantly less likely to have BRAF mutation than those with conventional PTC.
The BRAF gene encodes a serine-threonine kinase, within the MAPK pathway, responsible for transferring signals from the extracellular matrix (ECM) to the nucleus. It regulates cell cycle, proliferation, growth, and survival. The BRAF mutation enables constitutive kinase activity, thus, elevating its activity almost 500-fold and making the MAPK continuously active (25). One of its actions in the nucleus is the methylation of tumor suppressing genes including tissue inhibitor of protein kinase 3, death-associated protein kinase, SLC5A8 and retinoic acid receptor β which are thought to be responsible for tumor inducing capabilities of BRAF (12). Moreover, PTCs harboring BRAF mutated alleles exhibit increased levels of MMP-2 and MMP-9 proteins, which break down ECM components as well as increase expression of vascular endothelial growth factor. These features play a role in BRAF-promoted PTC progression (12, 24, 26).
It is also postulated that the inherent characteristics of HT can facilitate development of PTC. One of the hypotheses points towards the increased production of thyroid stimulating hormone (TSH) as a consequence of HT-induced hypothyroidism. The elevated levels of TSH, a trophic hormone, may promote proliferation of thyroid follicular cells and increase the risk of tumorigenesis (6, 27, 28). Another theory suggests that diffuse lymphocytic infiltration associated with HT also leads to follicular cells dysregulation resulting in a tumor promoting effect (15). On the other hand, the presence of lymphocytic cells in the PTC-HT microenvironment increases the migration of myeloid-origin cells (Natural Killer-cells and macrophages), which are implicated in the late host-induced anti-tumor response (7, 15, 29). Ugolini et al. have demonstrated that anaplastic or poorly differentiated thyroid cancers, which have weaker lymphocytic infiltration, are associated with considerably poorer prognosis compared to PTC (30). Therefore, it appears that HT is a “promotor” of PTC, but may also simultaneously inhibit its progression. Conversely, some authors suggest the possibility that PTC arises first and triggers an immunological response against the thyroid gland resulting in HT (15, 17). Nonetheless, current evidence suggests a clear association between the two diseases, with HT having a protective effect towards PTC progression (1).
To investigate whether the protective effect of HT on PTC persists regardless of BRAF status, we analyzed the differences in clinicopathological features between BRAF (+) PTC, BRAF (+) PTC-HT and BRAF (-) PTC-HT. Firstly, our analysis demonstrated that patients with PTC-HT were 55% less likely to harbor BRAF mutation than PTC patients. Subsequently, BRAF (+) PTC-HT patients were found to have 45% and 35% less likelihood of ETE and LNM respectively compared to BRAF (+) PTC cohort. Similarly, BRAF (-) PTC-HT patients were 41% and 28% less likely to have ETE and LNM, respectively, than BRAF (+) PTC-HT patients. These findings confirm previous evidence suggesting a mitigating impact of HT on PTC, regardless of BRAF status (15). However, this effect may be slightly diminished in the presence of BRAF mutation. A potential explanation for lower LNM rates in PTC-HT patients may be due to the presence of HT-associated lymphadenopathy, resulting in more attentive monitoring and quicker PTC detection (15, 31). Furthermore, HT-induced inflammatory process involving IL-1, may be responsible for reduced ETE in PTC-HT patients (7, 31).
On the other hand, our analysis found an increased incidence of multifocal disease in patients with BRAF (+) PTC-HT compared to those with BRAF (+) PTC. These findings may be a result of varying cancerization processes. The increased levels of TSH implicated in HT-associated carcinogenesis influences follicular proliferation throughout the thyroid gland, resulting in greater number of lesions. Additionally, our analysis showed greater incidence of multifocal disease in patients with BRAF (+) PTC-HT than BRAF (-) PTC-HT. These findings are in line with the hypothesis presented by Lun et al. claiming that BRAF plays a key role in the TSH-involving cancerization process. As multifocality is not considered an aggravation feature, its increased incidence does not question the positive impact of HT on PTC (5, 21, 32).
Based on the results provided, we propose the foreknowledge of BRAF mutation status among PTC-HT patients can aid decisions on the extent of thyroid surgeries. It is known that inflammation associated with HT increases the risk of surgical complications (32). On the other hand, a recent randomized controlled trial has demonstrated that total thyroidectomy improves long-term health-related quality of life in patients with HT (33). In patients with BRAF (+) PTC-HT, total thyroidectomy should be favored, as per current guidelines, due to potentially more aggressive cancer course (34). However, in patients with BRAF (-) PTC-HT, a more conservative resection of the thyroid gland may be undertaken, in the absence of other high-risk features, to reduce the risk of complications. Therefore, it would be useful to assess BRAF mutation status as well as concomitant thyroid diseases while managing patients with PTC.
PTC microenvironment consists of a mixture of tumor cells with only a minority of them carrying BRAF. Therefore, it is plausible that BRAF mutation frequency in PTC-HT patients might be underestimated by the dilution of mutated cells caused by the wild type BRAF-containing lymphocytes. One of the limitations of our meta-analysis is that it is mainly based on retrospective studies. Some studies included a small number of patients, making it difficult to draw firm conclusions. Additionally, there was heterogeneity in reporting of PTC diagnostic criteria across studies. Moreover, majority of studies included in this meta-analysis originated from Asian countries, where the prevalence of BRAF mutation is known to be higher (22). Due to limited data on the BRAF-PTC-HT paradigm from Western countries, it is difficult to ascertain whether these findings translate into these patient cohorts. Therefore, there is a need for further prospective studies on the topic, including greater sample sizes, especially in the Western cohort.
5 Conclusion
Through our meta-analysis, we have shown that BRAF mutation occurs less frequently in patients with concomitant PTC-HT. BRAF (+) PTC-HT show greater chance of developing aggressive clinicopathological features compared to BRAF (-) PTC-HT, thus providing evidence that BRAF scarcity may be one of the factors responsible for the more indolent course of PTC in patients with concomitant HT. Based on the chosen clinicopathological features BRAF (+) PTC patients were more likely to develop poor prognosis than BRAF (+) PTC-HT confirming HT to be a protective factor against BRAF associated outcome.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
LJ: Writing – original draft, Data curation, Formal analysis, Investigation. AP: Writing – original draft, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Visualization. JJ: Supervision, Formal analysis, Investigation, Writing – review & editing. AH: Writing – review & editing, Conceptualization, Investigation, Methodology, Project administration, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J Cancer Epidemiol (2013) 2013, 1–10. doi: 10.1155/2013/965212
2. Osborne D, Choudhary R, Vyas A, Kampa P, Abbas LF, Chigurupati HD, et al. Hashimoto’s thyroiditis effects on papillary thyroid carcinoma outcomes: A systematic review. Cureus (2022) 14:e28054. doi: 10.7759/cureus.28054
3. Ozdamar OI, Acar GO, Ozen F, Zenginkinet T. Assessment of BRAF-V600E, KRAS, NRAS and EGFR mutations in papillary thyroid carcinoma and Hashimoto’s thyroiditis. ENT UPDATES (2020) 10:300–5. doi: 10.32448/entupdates.711666
4. Kim WW, Ha TK, Bae SK. Clinical implications of the BRAF mutation in papillary thyroid carcinoma and chronic lymphocytic thyroiditis. J Of Otolaryngology-Head \& Neck Surg (2018) 47:4. doi: 10.1186/s40463-017-0247-6
5. Moon S, Chung HS, Yu JM, Yoo HJ, Park JH, Kim DS, et al. Associations between Hashimoto thyroiditis and clinical outcomes of papillary thyroid cancer: A meta-analysis of observational studies. Endocrinol Metab (2018) 33:473–84. doi: 10.3803/EnM.2018.33.4.473
6. Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: Thyroid autonomy may play a protective role. Endocrine-Related Cancer (2009) 16:1251–60. doi: 10.1677/ERC-09-0036
7. Zhang Y, Dai J, Wu T, Yang N, Yin Z. The study of the coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma. J Of Cancer Res And Clin Oncol (2014) 140:1021–6. doi: 10.1007/s00432-014-1629-z
8. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: A meta-analysis. Eur J Endocrinol (2013) 168:343–9. doi: 10.1530/EJE-12-0903
9. Paulson LM, Shindo ML, Schuff KG. Role of chronic lymphocytic thyroiditis in central node metastasis of papillary thyroid carcinoma. OTOLARYNGOLOGY-HEAD AND Neck Surg (2012) 147:444–9. doi: 10.1177/0194599812445727
10. Xu J, Ding K, Mu L, Huang J, Ye F, Peng Y, et al. Hashimoto’s thyroiditis: A ``Double-edged sword{‘‘} in thyroid carcinoma. Front IN Endocrinol (2022) 13:801925. doi: 10.3389/fendo.2022.801925
11. Kim KH, Suh KS, Kang DW, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma and in Hashimoto’s thyroiditis. Pathol Int (2005) 55:540–5. doi: 10.1111/j.1440-1827.2005.01866.x
12. Xing M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocrine Rev (2007) 28:742–62. doi: 10.1210/er.2007-0007
13. Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab (2005) 90:6373–9. doi: 10.1210/JC.2005-0987
14. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA (2013) 309:1493–501. doi: 10.1001/jama.2013.3190
15. Marotta V, Guerra A, Zatelli MC, Uberti ED, Stasi V, Faggiano A, et al. BRAF mutation positive papillary thyroid carcinoma is less advanced when Hashimoto’s thyroiditis lymphocytic infiltration is present. Clin Endocrinol (2013) 79:733–8. doi: 10.1111/cen.12194
16. Kim SK, Woo JW, Lee JH, Park I, Choe JH, Kim JS, et al. Chronic lymphocytic thyroiditis and BRAF V600E in papillary thyroid carcinoma. ENDOCRINE-RELATED Cancer (2016) 23:27–34. doi: 10.1530/ERC-15-0408
17. Issa PP, Omar M, Buti Y, Issa CP, Chabot B, Carnabatu CJ, et al. Hashimoto’s thyroiditis minimizes lymph node metastasis in BRAF mutant papillary thyroid carcinomas. Biomedicines (2022) 10:2051. doi: 10.3390/biomedicines10082051
18. Kim SK, Song KH, Lim SD, Lim YC, Yoo YB, Kim JS, et al. Clinical and pathological features and the BRAFV600E mutation in patients with papillary thyroid carcinoma with and without concurrent Hashimoto thyroiditis. Thyroid (2009) 19:137–41. doi: 10.1089/thy.2008.0144
19. Zeng Rc, Jin Lp, Chen Ed, Dong Sy, Cai Yf, Huang G, et al. Potential relationship between Hashimoto’s thyroiditis and BRAFV600E mutation status in papillary thyroid cancer. Head Neck (2016) 36:1391. doi: 10.1002/hed.24149
20. Kim Sj, Myong JP, Jee HG, Chai YJ, Choi JY, Min HS, et al. Combined effect of Hashimoto’s thyroiditis and BRAFV600E mutation status on aggressiveness in papillary thyroid cancer Su-jin. Head Neck (2016) 38(1):95–101. doi: 10.1002/hed.23854
21. Tang Q, Pan W, Peng L. Association between Hashimoto thyroiditis and clinical outcomes of papillary thyroid carcinoma: A meta-analysis. PloS One (2022) 17:1–24. doi: 10.1371/journal.pone.0269995
22. Wei X, Wang X, Xiong J, Li C, Liao Y, Zhu Y, et al. Risk and prognostic factors for BRAFV600EMutations in papillary thyroid carcinoma. BioMed Res Int (2022) 2022:9959649. doi: 10.1155/2022/9959649
23. Fugazzola L, Puxeddu E, Avenia N, Romei C, Cirello V, Cavaliere A, et al. Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: Data from a multicentric Italian study and review of the literature. Endocrine-Related Cancer (2006) 13:455–64. doi: 10.1677/erc.1.01086
24. Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M, Stella M, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocrine-Related Cancer (2008) 15:191–205. doi: 10.1677/ERC-07-0212
25. Li DD, Zhang YF, Hui-Xiong X, Zhang XP. The role of BRAF in the pathogenesis of thyroid carcinoma. Front Bioscience - Landmark (2015) 20:1068–78. doi: 10.2741/4359
26. Mesa C, Mirza M, Mitsutake N, Sartor M, Medvedovic M, Tomlinson C, et al. Conditional Activation of RET/PTC3 and BRAFV600E in thyroid cells is associated with gene expression profiles that predict a preferential role of BRAF in extracellular matrix remodeling. Cancer Res (2006) 66:6521–9. doi: 10.1158/0008-5472.CAN-06-0739
27. Chui MH, Cassol CA, Asa SL, Mete O. Follicular epithelial dysplasia of the thyroid: morphological and immunohistochemical characterization of a putative preneoplastic lesion to papillary thyroid carcinoma in chronic lymphocytic thyroiditis. VIRCHOWS ARCHIV (2013) 462:557–63. doi: 10.1007/s00428-013-1397-1
28. Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z, et al. Hashimoto’s thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngol - Head Neck Surg (United States) (2013) 148:396–402. doi: 10.1177/0194599812472426
29. Ehlers M, Schott M. Hashimoto’s thyroiditis and papillary thyroid cancer: are they immunologically linked? Trends IN Endocrinol AND Metab (2014) 25:656–64. doi: 10.1016/j.tem.2014.09.001
30. Ugolini C, Basolo F, Proietti A, Vitti P, Elisei R, Miccoli P, et al. Lymphocyte and immature dendritic cell infiltrates in differentiated, poorly differentiated, and undifferentiated thyroid carcinoma. Thyroid (2007) 17:389–93. doi: 10.1089/thy.2006.0306
31. Jara SM, Carson KA, Pai SI, Agrawal N, Richmon JD, Prescott JD, et al. The relationship between chronic lymphocytic thyroiditis and central neck lymph node metastasis in North American patients with papillary thyroid carcinoma. SURGERY (2013) 154:1272–80. doi: 10.1016/j.surg.2013.07.021
32. McManus C, Luo J, Sippel R, Chen H. Is thyroidectomy in patients with Hashimoto thyroiditis more risky? J Surg Res (2012) 178:529–32. doi: 10.1016/j.jss.2012.09.017
33. Guldvog I, Reitsma LC, Johnsen L, Lauzike A, Gibbs C, Carlsen E, et al. Thyroidectomy versus medical management for euthyroid patients with hashimoto disease and persisting symptoms: A randomized trial. Ann Internal Med (2019) 170:453–64. doi: 10.7326/M18-0284
34. Krajewska J, Chmielik E, Dedecjus M, Jarząb B, Hubalewska-Dydejczyk A, Karbownik-Lewińska M, et al. Diagnosis and treatment of thyroid cancer in adult patients - Recommendations of Polish Scientific Societies and the National Oncological Strategy. Update of the 2022 Update [Diagnostyka i leczenie raka tarczycy u chorych dorosłych - Rekomendacje Polskich. Endokrynologia Polska (2022) 73:799–802. doi: 10.5603/EP.A2022.0087
Keywords: Hashimoto thyroiditis, papillary thyroid carcinoma, BRAF mutation, lymph node metastasis, extrathyroidal extension, multifocality
Citation: Janicki L, Patel A, Jendrzejewski J and Hellmann A (2023) Prevalence and Impact of BRAF mutation in patients with concomitant papillary thyroid carcinoma and Hashimoto’s thyroiditis: a systematic review with meta-analysis. Front. Endocrinol. 14:1273498. doi: 10.3389/fendo.2023.1273498
Received: 06 August 2023; Accepted: 25 October 2023;
Published: 17 November 2023.
Edited by:
Maria Laura Tanda, University of Insubria, ItalyReviewed by:
Armando Patrizio, University of Pisa, ItalyAndrii Dinets, Taras Shevchenko National University of Kyiv, Ukraine
Gloria Angélica González Villaseñor, Instituto Mexicano del Seguro Social, Mexico
Copyright © 2023 Janicki, Patel, Jendrzejewski and Hellmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrzej Hellmann, aGVsbG1hbm5hbmRyemVqQGd1bWVkLmVkdS5wbA==
†These authors have contributed equally to this work and share first authorship
 Lukasz Janicki
Lukasz Janicki Agastya Patel
Agastya Patel Jarosław Jendrzejewski4
Jarosław Jendrzejewski4 Andrzej Hellmann
Andrzej Hellmann