- Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University) of Ministry of Education, Chengdu, Sichuan, China
Background and purpose: The relationship of the methylenetetrahydrofolate reductase (MTHFR) gene C677T polymorphism with the incidence of gestational diabetes mellitus (GDM) in the Chinese population remains controversial. This study aimed to further clarify the effect of the MTHFR gene C677T polymorphism on GDM risk among Chinese pregnant women based on current evidence.
Methods: Several databases were searched up to July 29, 2023 for relevant case-control studies. The numbers of patients with and without the T allele of the MTHFR gene C677T polymorphism in the GDM and control groups were determined, and all statistical analyses were performed by RevMan 5.3 software and STATA 15.0 software. Trial sequential analysis (TSA) was performed by TSA version 0.9 beta software to determine the required information size.
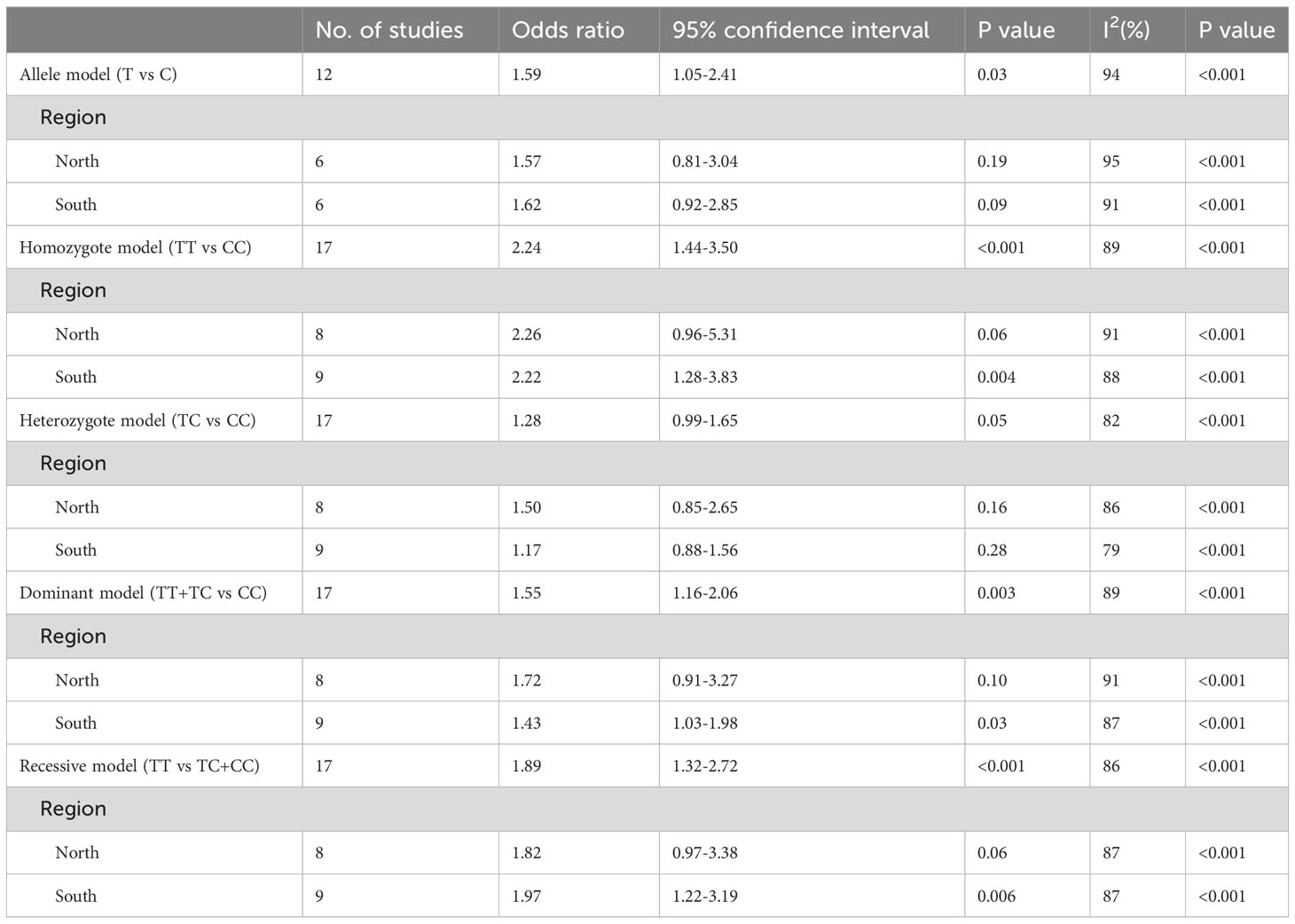
Results: A total of 17 case-control studies involving 12345 Chinese participants were included. The pooled results demonstrated that the T allele of the MTHFR gene C677T polymorphism was significantly associated with an increased risk of GDM, which was manifested by the five gene models of the MTHFR C677T polymorphism [T vs. C: odds ratio (OR)=1.59, P=0.03; TT vs. CC: OR=2.24, P<0.001; TC vs. CC: OR=1.28, P=0.05; (TT+TC) vs. CC: OR=1.55, P=0.003; TT vs. (TC+CC): OR=1.89, P<0.001]. Subgroup analysis based on the regions indicated that the significant relationship between the T allele of the MTHFR gene C677T polymorphism and an increased risk of GDM was detected only among the southern population [T vs. C: OR=1.62, P=0.09; TT vs. CC: OR=2.22, P=0.004; TC vs. CC: OR=1.17, P=0.28; (TT+TC) vs. CC: OR=1.43, P=0.03; TT vs. (TC+CC): OR=1.97, P=0.006]. TSA plots showed that the information sizes for the association between the MTHFR gene C677T polymorphism and GDM risk were sufficient in the homozygote (TT vs. CC) and recessive (TT vs. TC+CC) models.
Conclusion: The MTHFR gene C677T polymorphism is closely related to susceptibility to GDM in the southern Chinese population, and the C-T mutation serves as an important genetic risk factor for GDM. More well-designed large case-control studies are needed to further confirm the above findings.
Introduction
Gestational diabetes mellitus (GDM) is a common complication during pregnancy, posing significant health risks to both the fetus and the mother. The incidence of GDM has been rising, with reported rates ranging from 1% to 14% internationally and from 2% to 18% in China (1–4). Additionally, the onset of GDM is occurring at increasingly younger ages (2). GDM can have adverse effects on both the mother and the fetus, leading to complications such as miscarriage, preterm birth, diabetic ketoacidosis, infection, postpartum hemorrhage, fetal distress, macrosomia, neonatal respiratory distress syndrome, and neonatal hypoglycemia (5). Furthermore, both pregnant women with GSM and their offspring have an increased risk of developing type 2 diabetes in the long term (5). Therefore, identifying potential and modifiable risk factors for GDM is a valuable strategy to enhance the health status of pregnant women and their offspring.
Homocysteine (Hcy) is a reactive amino acid associated with vascular injury that impacts glucose and lipid metabolism. Previous studies have indicated that Hcy levels are higher in GDM patients than in women with normal pregnancies, suggesting a potential close relationship between Hcy levels and GDM and its complications (6). Relevant studies suggest that insulin resistance is a central factor in the development of GDM, and plasma Hcy levels are positively correlated with fasting insulin levels (7). The insulin resistance index directly influences plasma Hcy levels, although this remains a subject of significant debate (8, 9).
Methylenetetrahydrofolate reductase (MTHFR) can catalyze 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, participating in the remethylation of Hcy and keeping Hcy levels relatively low in the body. Variations in the MTHFR gene can lead to disruptions in multiple physiological and biochemical reactions in the body, such as modifications in the methylation of nucleic acids and proteins and the regulation of the cell cycle. The MTHFR gene is located on the short arm of human chromosome 1 (1p36.3), and polymorphisms in the MTHFR gene can result in reduced enzyme activity, causing folate metabolism disturbances, reduced 5-methyltetrahydrofolate production, impaired DNA methylation, metabolic disruptions of Hcy, and the accumulation of Hcy in the bloodstream. Currently, hundreds of single nucleotide polymorphisms (SNPs) have been identified in the MTHFR gene, with the C677T site being the most closely associated with diseases. When this gene polymorphism occurs, it may lead to reduced or impaired MTHFR enzyme activity, disrupted folate metabolism, and elevated Hcy levels and consequently result in related complications (10–13). Some studies have demonstrated that the MTHFR gene C677T polymorphism is closely related to the risk of diabetes mellitus (DM) (14, 15). Some investigators also revealed that the C-T mutation in the MTHFR C677T gene might lead to increased susceptibility to GDM (16, 17). However, whether the MTHFR gene C677T polymorphism leads to an increased risk of GDM in the Chinese population remains controversial.
Therefore, this meta-analysis aimed to further identify the effect of the MTHFR gene C677T polymorphism on GDM risk among Chinese pregnant women based on current evidence.
Materials and methods
The current meta-analysis was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analyses 2020 guidelines (18).
Literature search
In this meta-analysis, the CNKI, VIP, WanFang, Medline, Web of Science and EMBASE databases were searched from inception to July 29, 2023. The following terms were used during the search: single nucleotide polymorphism, SNP, polymorphism, gestational diabetes mellitus, pregnancy-induced diabetes, methylenetetrahydrofolate reductase and MTHFR. The detailed search strategy was as follows: (single nucleotide polymorphism OR SNP OR polymorphism) AND (gestational diabetes mellitus OR pregnancy-induced diabetes) AND (methylenetetrahydrofolate reductase OR MTHFR). Moreover, MeSH terms and free-text words were both applied, and references cited in the included studies were also reviewed.
Inclusion criteria
Studies that met the following criteria were included: 1) studies of the association between the MTHFR gene C677T polymorphism and the incidence of GDM among pregnant Chinese women; 2) studies in which the numbers of patients with and without the T allele of the MTHFR gene C677T polymorphism in the GDM and control groups were provided for the calculation of odds ratios (ORs) with 95% confidence intervals (CIs); and 3) high-quality studies with a Newcastle-Ottawa Scale (NOS) score of 6 or higher (19).
Exclusion criteria
Studies that met the following criteria were excluded: 1) letters, editorials, animal trials, reviews or case reports; and 2) studies with duplicated or overlapped data.
Data collection
The following information was extracted from each included study: the first author, publication year, sample size, number of patients with and without GDM, genotype detection method, number of patients with different genotypes and NOS score.
Quality assessment
The NOS was applied to assess the methodological quality of the included studies because all included studies were cohort studies. As mentioned above, only studies with an NOS score ≥ 6 were included.
The literature search, literature selection, data extraction and quality assessment were independently conducted by two authors, and all disagreements were resolved by team discussion.
Statistical analysis
The heterogeneity between studies was assessed by using I2 statistics and the Q test. If significant heterogeneity was detected, defined as I2 > 50% and/or P < 0.1, the random effects model was applied; otherwise, the fixed effects model was applied (20). ORs and 95% CIs were combined to evaluate the effect of this polymorphism on GDM risk under the allele model (T vs. C), homozygote model (TT vs. CC), heterozygote model (TC vs. CC), dominant model (TT+TC vs. CC) and recessive model (TT vs. TC+CC). The overall population was stratified according to region (northern vs. southern), and subgroup analysis was conducted by region. Sensitivity analysis was conducted to determine the sources of heterogeneity and assess the stability of the overall results. In addition, Begg’s funnel plot and Egger’s test were conducted to detect publication bias, and significant publication bias was defined as P < 0.05 (21, 22). If obvious publication bias was detected, then the fill-and-trim method was applied to identify potentially unpublished studies (23). The above analysis was performed by RevMan 5.3 software and STATA 15.0 software.
Trial sequential analysis (TSA) was conducted according to the guidelines of former publications (24, 25). A significance level of 5% for type I error and a significance level of 20% for type II error were set to evaluate the required sample size and TSA monitoring boundaries, which was performed by TSA version 0.9 beta software.
Results
Literature search and selection
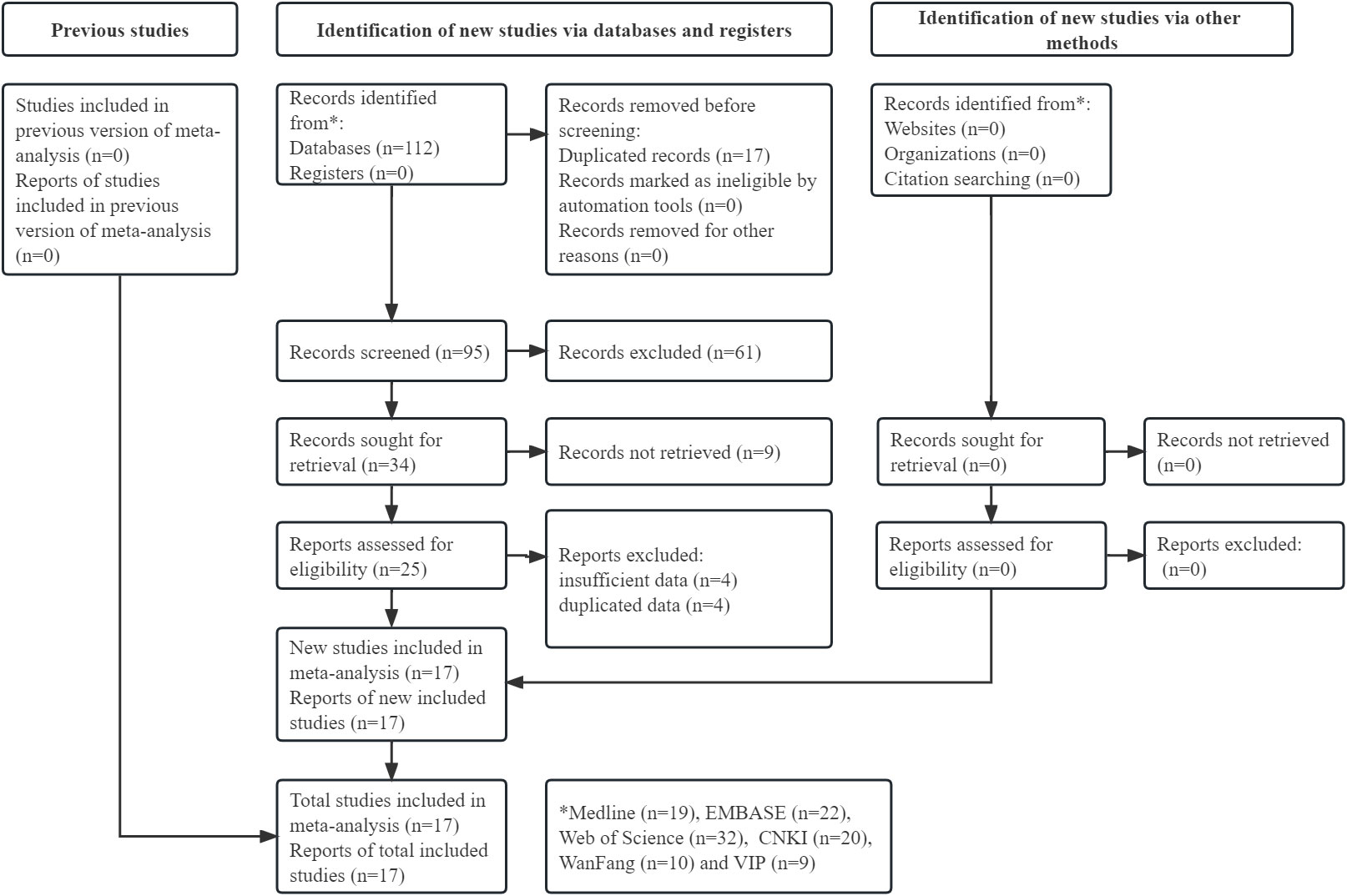
The specific literature search and selection process is shown in Figure 1. Initially, 112 records were identified from the six databases. Ultimately, a total of 18 available studies were included in this meta-analysis after reviewing the titles, abstracts and full texts (14, 26–41).
Basic characteristics of the included studies
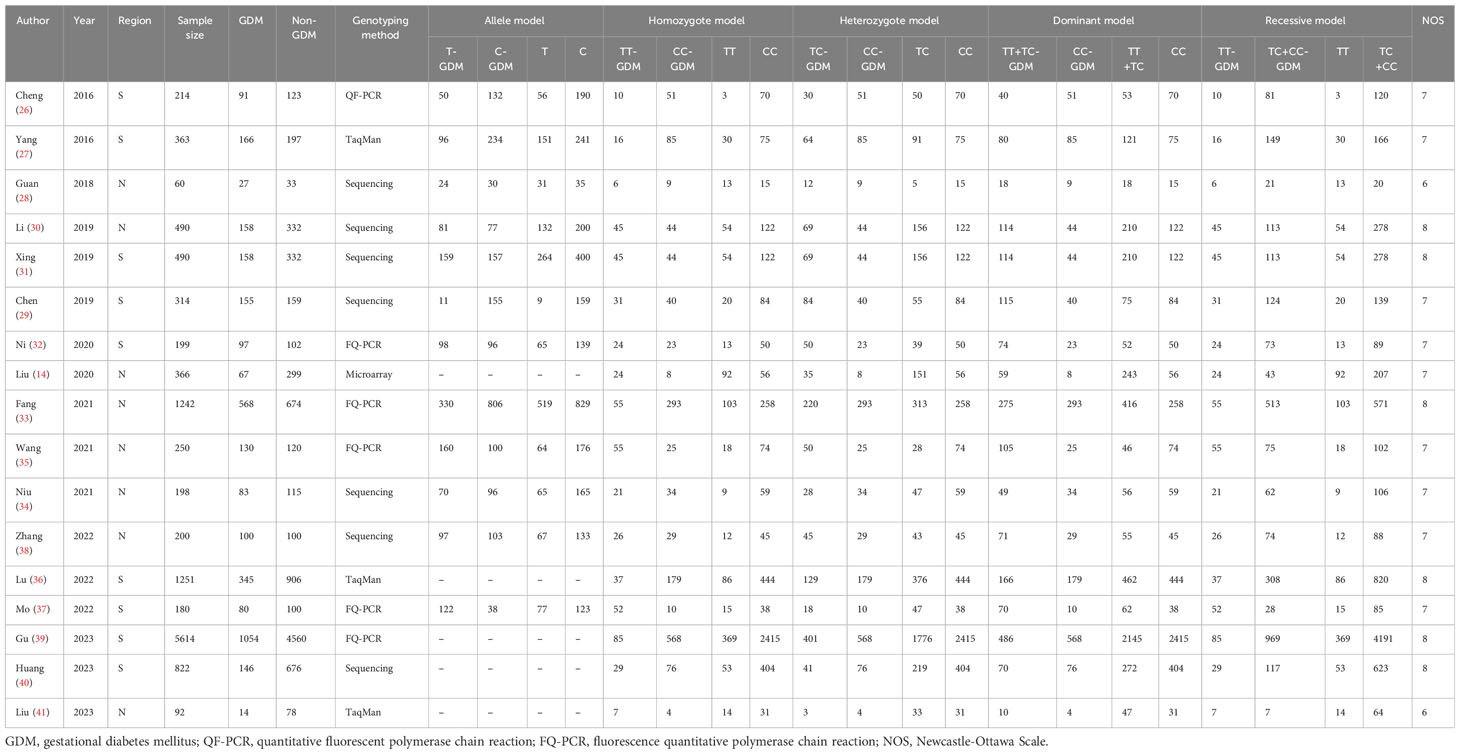
As shown in Table 1, 12345 Chinese participants were enrolled, with sample sizes ranging from 60 to 5614, and nine studies were from southern China. Among the enrolled patients, 3439 were diagnosed with GDM. Detailed data are presented in Table 1. Specific information about the NOS score is shown in Supplementary Table 1.
The association between the MTHFR gene C677T polymorphism and GDM risk in the Chinese population
The pooled results demonstrated that the MTHFR gene C677T polymorphism was significantly related to the risk of GDM in the allele model (OR=1.59, 95%:1.05-2.41, P=0.03; I2 = 94%, P<0.001) (Figure 2), homozygote model (OR=2.24, 95%:1.44-3.50, P<0.001; I2 = 89%, P<0.001) (Figure 3), heterozygote model (OR=1.28, 95%:0.99-1.65, P=0.05; I2 = 82%, P<0.001) (Figure 4), dominant model (OR=1.55, 95%:1.16-2.06, P=0.003; I2 = 89%, P<0.001) (Figure 5) and recessive model (OR=1.89, 95%:1.32-2.72, P<0.001; I2 = 86%, P<0.001) (Figure 6). Patients with the T variant allele were more likely to develop GDM (Table 2).
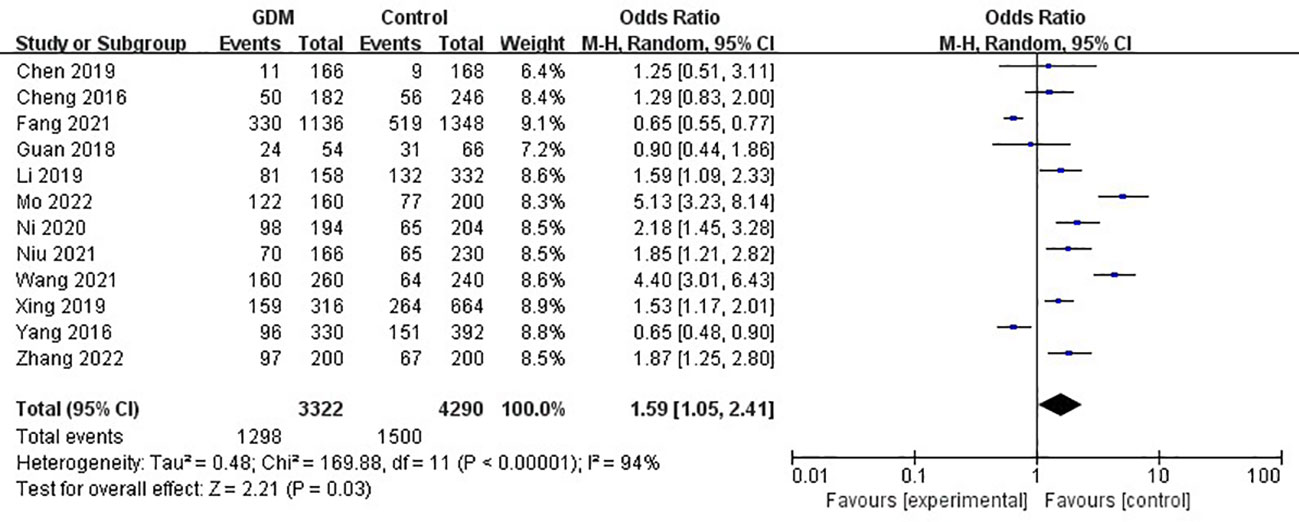
Figure 2 The association between MTHFR gene C677T polymorphism and risk of gestational diabetes mellitus in Chinese population under the allele model.
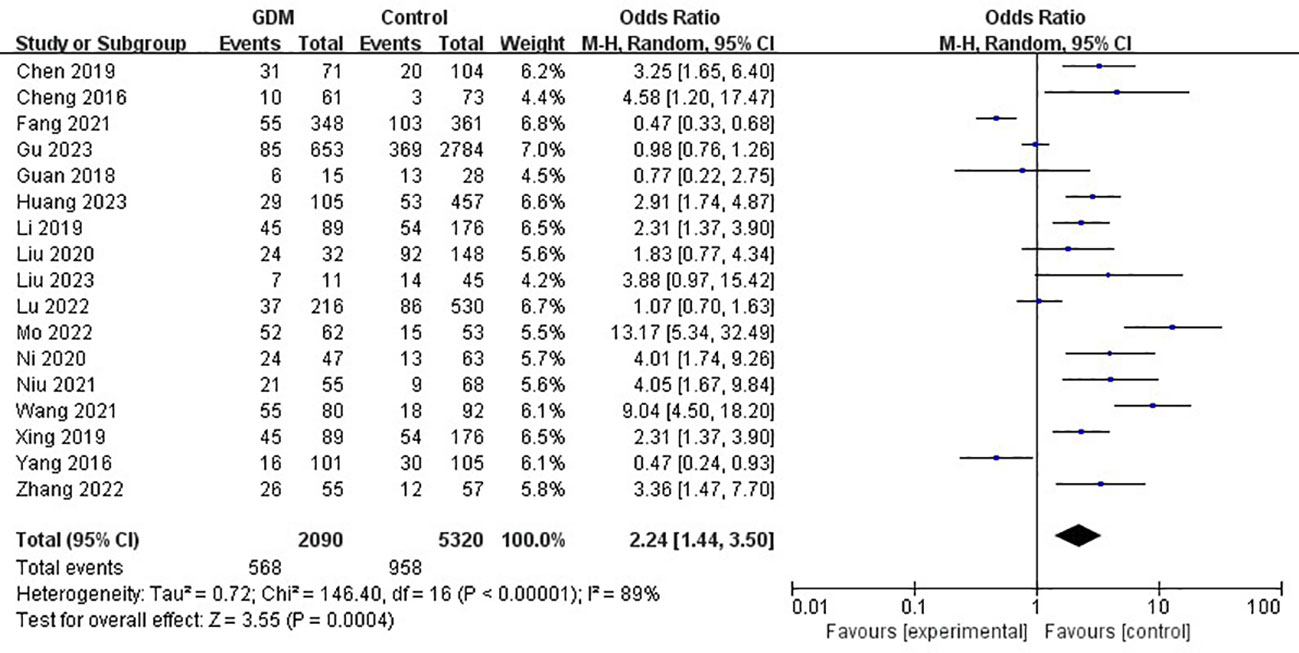
Figure 3 The association between MTHFR gene C677T polymorphism and risk of gestational diabetes mellitus in Chinese population under the homozygote model.
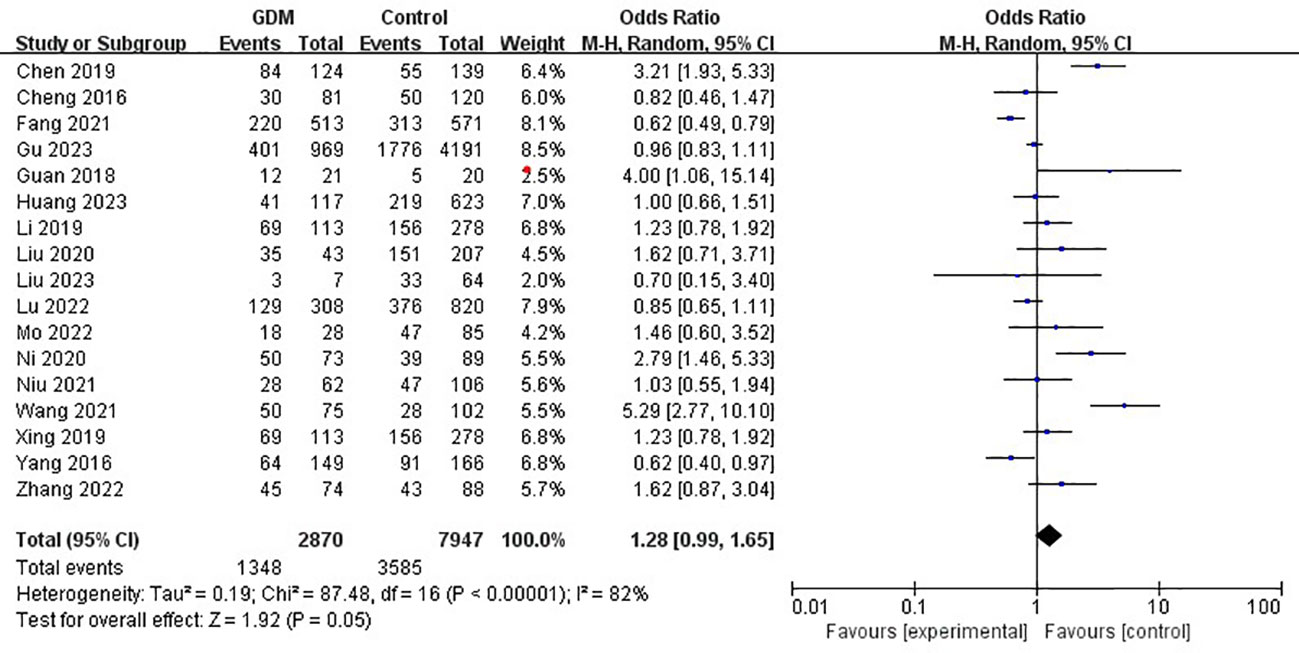
Figure 4 The association between MTHFR gene C677T polymorphism and risk of gestational diabetes mellitus in Chinese population under the heterozygote model.
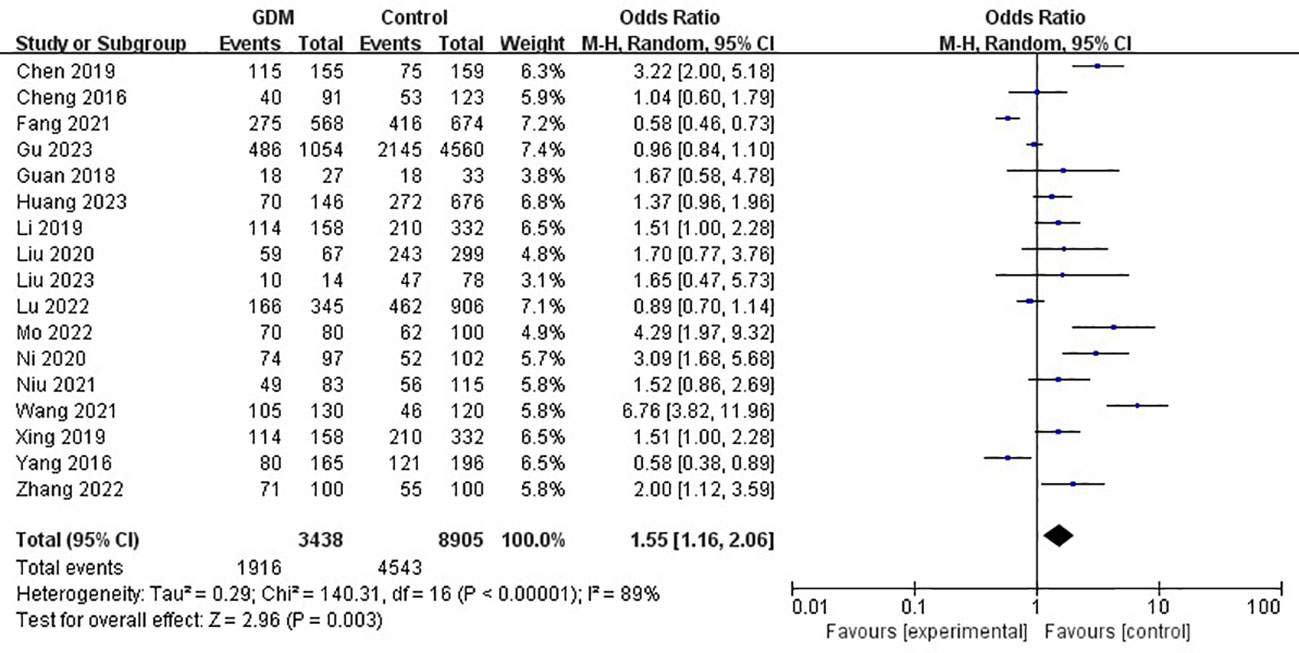
Figure 5 The association between MTHFR gene C677T polymorphism and risk of gestational diabetes mellitus in Chinese population under the dominant model.
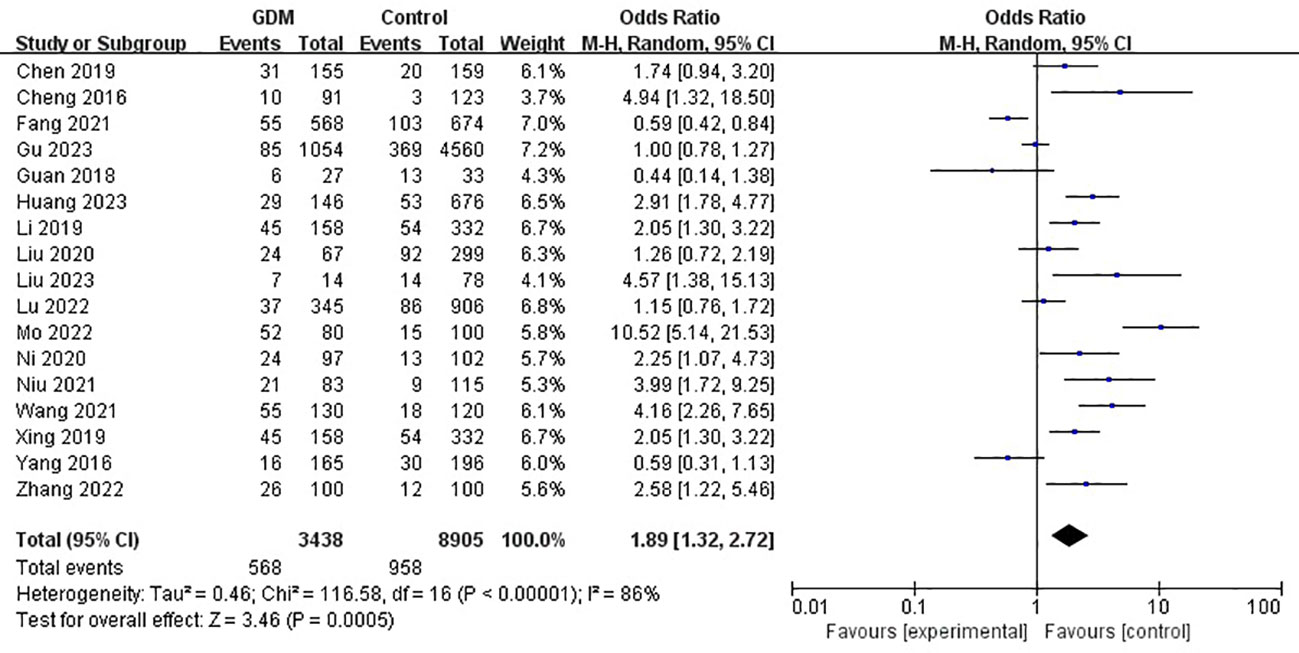
Figure 6 The association between MTHFR gene C677T polymorphism and risk of gestational diabetes mellitus in Chinese population under the recessive model.
However, subgroup analysis stratified by region revealed a significant association between the MTHFR gene C677T polymorphism and an increased risk of GDM only among the southern population in the allele model (OR=1.62, 95%:0.92-2.85, P=0.09; I2 = 91%, P<0.001), homozygote model (OR=2.22, 95%:1.28-3.83 P=0.004; I2 = 88%, P<0.001), heterozygote model (OR=1.17, 95%:0.88-1.56, P=0.28; I2 = 79%, P<0.001), dominant model (OR=1.43, 95%:1.03-1.98, P=0.03; I2 = 87%, P<0.001) and recessive model (OR=1.97, 95%:1.22-3.19, P=0.006; I2 = 87%, P<0.001). Although a significant difference in the allele model and heterozygote model was not shown, an obvious tendency was observed (Table 2). Thus, southern Chinese pregnant women with the T variant allele of the MTHFR gene C677T polymorphism showed significantly increased susceptibility to GDM overall.
Sensitivity analysis
Sensitivity analysis for the five gene models was performed. Overall, the results in the homozygote model (Supplementary Figure 1B), dominant model (Supplementary Figure 1D) and recessive model (Supplementary Figure 1E) were stable and reliable. However, studies by Mo et al. (37) and Wang et al. (35) showed an impact on the overall results in the allele model (Supplementary Figure 1A), and several studies (14, 28–30, 32, 37, 39, 4031, 35, 38) showed an impact on the overall results in the heterozygote model (Supplementary Figure 1C). Thus, more high-quality studies are still needed to further confirm our findings.
Publication bias
Similarly, publication bias for the five gene models was assessed. According to Begg’s funnel plots (Supplementary Figures 2A–E) and Egger’s test P values (allele model: P=0.045; homozygote model: P=0.011; heterozygote model: P=0.037; dominant model: P=0.010; recessive model: P=0.028), significant publication bias was observed. Thus, the fill-and-trim method was applied to identify potentially unpublished studies. According to filled funnel plots, no potentially unpublished publications for the allele model (Supplementary Figure 3A) or heterozygote model (Supplementary Figure 3C) were found. Three, six and two potentially unpublished studies were detected for the homozygote model (Supplementary Figure 3B), dominant model (Supplementary Figure 3D) and recessive model (Supplementary Figure 3E), respectively. The potentially unpublished studies had no significant effect on the overall results in the homozygote model (filled OR=1.74, 95% CI: 1.12-2.68, P=0.013) and recessive model (filled OR=1.63, 95% CI: 1.13-2.36, P=0.009). However, in the dominant model, a significant effect on the overall results caused by the six potentially unpublished studies was observed (filled OR=1.06, 95% CI: 0.80-1.42, P=0.678). Therefore, to further clarify the association between the MTHFR gene C677T polymorphism and the risk of GDM, more high-quality studies should be performed.
Trial sequential analysis
Supplementary Figure 4 shows the TSA results. In Supplementary Figures 4B and E, the cumulative Z-curve cut crossed TSA boundaries (red polylines), indicating that the information size was adequate and that the conclusions were robust. However, the Supplementary Figures 4A, C and D show negative results. Therefore, more case-control studies are still needed to further confirm our findings.
Discussion
The current meta-analysis included 17 relevant studies with 12345 participants and explored the relationship between the MTHFR gene C677T polymorphism and susceptibility to GDM among pregnant Chinese women. Based on currently available evidence, we demonstrated that the T variant allele could serve as a risk factor for GDM among the southern Chinese population. However, due to the limitations in the included studies and our analysis, more randomized controlled trials (RCTs) are needed to further confirm our findings.
It has been reported that the serum Hcy level in GDM patients is significantly higher than that in non-GDM patients (34). Serum Hcy levels have been identified as an independent risk factor for the occurrence of GDM, indicating a strong correlation between elevated Hcy levels and the development of GDM. Hcy can serve as a marker for monitoring the progression of GDM and as a potential predictive factor for evaluating microvascular changes, reflecting the status of blood sugar control (34). A possible reason is that during pregnancy, the maternal body and placenta secrete anti-insulin hormones, leading to increased insulin resistance, which in turn causes elevated Hcy levels. Elevated Hcy levels further inhibit pancreatic β-cell insulin secretion, resulting in an increase in blood glucose levels (42). These two factors have a reciprocal causal relationship and mutually influence each other. Therefore, elevated Hcy levels may serve as a pathological basis for promoting the occurrence and development of GDM (42).
The reduction in enzyme activity caused by the C677T mutation in the MTHFR gene is an important factor leading to elevated Hcy levels (43). MTHFR is a key enzyme involved in Hcy metabolism, catalyzing the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which promotes the conversion of Hcy to methionine (14). The C677T mutation site can lead to the substitution of methionine for alanine in MTHFR isoleucine, resulting in decreased MTHFR enzyme activity. This impedes the conversion of Hcy to methionine, leading to the accumulation of Hcy in the body and subsequently causing an increase in the serum Hcy concentration, which contributes to the development of GDM (44). The results from the study by Pawlik A et al. showed that MTHFR CT heterozygous mutations reduced enzyme activity to approximately 75% of the CC type, while TT genotype mutations led to even more significant decreases, reaching approximately 50% or lower (45). The aforementioned reports confirm that different genotypic mutations of MTHFR C677T can cause a reduction in MTHFR enzyme activity, resulting in elevated serum Hcy levels. Therefore, the T variant allele in the MTHFR gene C677T polymorphism leads to an increased risk of GDM.
Most included studies revealed a relationship between the MTHFR gene C677T polymorphism and an increased risk of GDM. However, studies by Fang et al. (33) and Yang et al. (27) reported opposite results, and they suggested several potential causes. Yang et al. did not find a significant correlation between mutations of MTHFR C677T and plasma Hcy levels, suggesting that the T variant allele in the MTHFR gene C677T polymorphism may have a relatively minor impact on Hcy metabolism in pregnant Han Chinese women. However, this mutation may reduce the risk of GDM through the alteration of other metabolic pathways within the body, thereby masking the potential adverse effects of Hcy metabolism changes caused by the C-T mutation in MTHFR C677T (27). In addition, the metabolism of Hcy is regulated by various factors, including genetics and nutrition. The potential influence of confounding factors such as plasma/red blood cell folate levels and the glomerular filtration rate in the study subjects might lead to biases in the research findings (27). Moreover, the sample size with 363 patients in the study by Yang et al. was relatively small, and the test performance of their results was only 0.48.
There are several limitations in our meta-analysis. First, the sample sizes in several included studies were relatively small, which might cause some bias. Second, the genotyping methods in the included studies differed, and the accuracy of different methods may vary. Third, a more detailed subgroup analysis based on other important parameters, such as race and age, could not be performed because the original data were unavailable. Fourth, significant heterogeneity among the included studies was detected.
Conclusion
The MTHFR gene C677T polymorphism is closely related to susceptibility to GDM in the southern Chinese population, and the C-T mutation serves as an important genetic risk factor for GDM.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XT: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft. HC: Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Sichuan Science and Technology Program (2023NSFSC1606) and the National Key Research and Development Program of China (2022YFC2704600, 2022YFC2704604).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1273218/full#supplementary-material
References
1. Meyer BN, Cash HL, Uso A, Eliapo-Unutoa I, Ropeti R, Muasau-Howard B. Gestational diabetes mellitus prevalence, screening, and treatment practices in American Samoa 2016. Hawaii J Health Soc Welf (2022) 81(7):185–92.
2. He LR, Yu L, Guo Y. Birth weight and large for gestational age trends in offspring of pregnant women with gestational diabetes mellitus in southern China 2012-2021. Front Endocrinol (Lausanne) (2023) 14:1166533. doi: 10.3389/fendo.2023.1166533
3. Lyu J, Sun Y, Ji Y, Liu N, Zhang S, Lin H, et al. Optimal gestational weight gain for women with gestational diabetes mellitus - China 2011-2021. China CDC Wkly (2023) 5(9):189–93. doi: 10.46234/ccdcw2023.034
4. Xia S, Du Y, Ren Z, Zhang J, Gao S, Wang J, et al. Periconceptional folic acid only versus multiple micronutrients containing folic acid and association with gestational diabetes mellitus - Beijing municipality, China 2017-2021. China CDC Wkly (2023) 5(23):505–10. doi: 10.46234/ccdcw2023.095
5. Luo R, Wen W, Corsi DJ, Fell DB, Taljaard M, Wen SW, et al. Comparison of adverse maternal and perinatal outcomes between induction and expectant management among women with gestational diabetes mellitus at term pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth (2023) 23(1):509. doi: 10.1186/s12884-023-05779-z
6. Mahesh M, Cheng G, Khalighi K. Association of methylenetetrahydrofolate reductase (MTHFR) A1298C polymorphism with lower high-density lipoprotein cholesterol level. Ann Clin Lab Sci (2019) 49(2):232–6.
7. Zhu C, Liu YW, Wang SZ, Li XL, Nie XL, Yu XT, et al. Associations between the C677T and A1298C polymorphisms of MTHFR and the toxicity of methotrexate in childhood Malignancies: a meta-analysis. Pharmacogenomics J (2018) 18(3):450–9. doi: 10.1038/tpj.2017.34
8. Xiang W, Yang Y, Weng L, Ye Z, Ding P, Li H, et al. Hyperhomocysteinemia activates NLRP3 inflammasome to cause hepatic steatosis and insulin resistance via MDM2-mediated ubiquitination of HSF1. Int Immunopharmacol (2023) 118:110085. doi: 10.1016/j.intimp.2023.110085
9. Yu X, Wen C, Xu R, Huang W. Dapagliflozin’s effect on serum homocysteine in patients with hypertension complicated with insulin resistance. J Clin Hypertens (Greenwich) (2023) 25(5):489–96. doi: 10.1111/jch.14662
10. Chen L, Chen H, Wang X, Wei B, Wu Z, Chen S, et al. Association of homocysteine with IVF/ICSI outcomes stratified by MTHFR C677T polymorphisms: a prospective cohort study. Reprod BioMed Online (2021) 43(1):52–61. doi: 10.1016/j.rbmo.2021.04.009
11. Liao S, Guo S, Ma R, He J, Yan Y, Zhang X, et al. Association between methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and H-type hypertension: A systematic review and meta-analysis. Ann Hum Genet (2022) 86(5):278–89. doi: 10.1111/ahg.12468
12. Wu X, Liu K, Zhao X, Zhang X, Guo H, Jiang H, et al. Correlation between the MTHFR C677T genotype and coronary heart disease in populations from Gansu, China. DNA Cell Biol (2022) 41(11):981–6. doi: 10.1089/dna.2022.0329
13. Li Z, Wu X, Huang H, Xu F, Liang G, Lin C, et al. MTHFR C677T polymorphism and cerebrovascular lesions in elderly patients with CSVD: A correlation analysis. Front Genet (2022) 13:987519. doi: 10.3389/fgene.2022.987519
14. Liu PJ, Liu Y, Ma L, Yao AM, Chen XY, Hou YX, et al. Associations between gestational diabetes mellitus risk and folate status in early pregnancy and MTHFR C677T polymorphisms in Chinese women. Diabetes Metab Syndr Obes (2020) 13:1499–507. doi: 10.2147/dmso.s250279
15. Ay A, Alkanli N, Kurt I, Ustundag S, Sipahi T, Sut N. The roles of MTHFR (C677T, A1298C) and MGP (G-7A, T-138C) gene variations in development of diabetic nephropathy in patients with type 2 diabetes mellitus. J Diabetes Metab Disord (2022) 21(2):1317–26. doi: 10.1007/s40200-022-01061-9
16. Li S, Tian X, Wang Y, Zhang X, Zhang L, Li C, et al. Associations of maternal rs1801131 genotype in MTHFR and serum folate and vitamin B-12 with gestational diabetes mellitus in Chinese pregnant women. Nutrients (2022) 14(6):1169. doi: 10.3390/nu14061169
17. Wang Y, Wang Y, Sun Y, Zhang N, Liang X, Luo S, et al. Serum folate mediates the associations of MTHFR rs1801133 polymorphism with blood glucose levels and gestational diabetes mellitus in Chinese Han pregnant women. Br J Nutr (2023) 130(8):1329–37. doi: 10.1017/s0007114523000314
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 372:n71. doi: 10.1136/bmj.n71
19. Wang Y, Li J, Chang S, Dong Y, Che G. Risk and influencing factors for subsequent primary lung cancer after treatment of breast cancer: A systematic review and two meta-analyses based on four million cases. J Thorac Oncol (2021) 16(11):1893–908. doi: 10.1016/j.jtho.2021.07.001
20. Li J, Wang Y, Li J, Cao S, Che G. Meta-analysis of lobectomy and sublobar resection for stage I non-small cell lung cancer with spread through air spaces. Clin Lung Cancer (2022) 23(3):208–13. doi: 10.1016/j.cllc.2021.10.004
21. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
23. Shi L, Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Med (Baltimore) (2019) 98(23):e15987. doi: 10.1097/md.0000000000015987
24. Mei TTY, Aung HH, Tung WS, Naing C. Association between IL-10 gene polymorphisms (- 1082 A/G, -819 T/C, -592 A/C) and hepatocellular carcinoma: a meta-analysis and trial sequential analysis. BMC Cancer (2023) 23(1):842. doi: 10.1186/s12885-023-11323-1
25. Zhou Y, Ma X, Sun J. Update on the relationship between the SLC4A7 variant rs4973768 and breast cancer risk: a systematic review and meta-analysis. J Int Med Res (2023) 51(4):3000605231166517. doi: 10.1177/03000605231166517
26. Cheng L, Wang D, Ye G, Yuan C, Peng J. Association between homocystine and C677T polymorphism with gestational diabetes mellitus. Int J Lab Med (2016) 37(06):736–7.
27. Yang M. Association of plasma homocysteine, leptin and related gene polymorphisms and gestational diabetes mellitus. Huazhong University of Science and Technology (2016).
28. Guan H, Yu T. The relationship between the polymorphism of MTHFR and β-CBS with GDM. China Health Standard Manage (2018) 9(03):140–1. doi: 10.3969/j.issn.1674-9316.2018.03.07
29. Chen Y, Yang F, Jia T, Liang B, Kong L, Shen J. Association of MTHFR and TCF7L2 gene polymorphisms with gestational diabetes susceptibility. Maternal Child Health Care China (2019) 34(20):4728–30. doi: 10.7620/zgfybj.j.issn.1001-4411.2019
30. Li S, Zhao R, Zheng J. Association of C677T polymorphism of MTHFR gene and overweight/obesity with the risk of gestational diabetes mellitus. Chin J Front Med Sci (2019) 11(10):114–7. doi: 10.12037/YXQY.2019.10-25
31. Xing W, Fei B, Ling L. Effect of MTHFR C677T polymorphism and overweight/obesity on the risk of GDM. Chin J Coal Industry Med (2019) 22(02):113–7.
32. Ni F, Zheng J, Xu X, Zheng X, Sun C, Huang Z, et al. Correlation between serum homocysteine, folic acid, vitamin B12 and MTHFR gene C677T polymorphism and gestational diabetes mellitus. China Modern Doctor (2020) 58(12):1–4.
33. Fang J, Zhang S, Xu M, Yang Y, Lu W. Effects of MTHFR gene polymorphism and serum folic acid level on pregnant women with gestational diabetes mellitus and their pregnancy outcomes. Chin J Obstet Gynecol Pediatr (Electron Ed) (2021) 17(03):291–7.
34. Niu X, Ding Y, Qiang D, Wang Y, Tao C, Tang M, et al. Association between Hcy and folate metabolism gene MTHFR C677T polymorphism in gestational diabetes mellitus. Chin J Hum Sexuality (2021) 30(09):93–6.
35. Wang L, Guo Z, Chen X. Association of MTHFR gene polymorphism with gestational diabetes. Int J Immunol (2021) 44(6):631–4.
36. Lu S, Liang X. Relationship between folate metabolism gene polymorphism and gestational diabetes mellitus. Prev Treat Cardiovasc Dis (2022) 12(35):23–6.
37. Mo W, Tai S, Wu H. Association between polymorphism of MTHFR C677T and risk of gestational diabetes mellitus in pregnant women of Han nationality in Xiaoshan District, Hangzhou City. Chin J Health Lab TeC (2022) 32(22):2777–83.
38. Zhang R. Analysis of the Relationship Between Preeclampsia Gestational Diabetes Mellitus and Blood Lipids Based on MTHFR C677T Gene Polymorphism. North China University of Science and Technology (2022).
39. Gu C, Wu W, Lai K, Li H, Wu L, Lu W, et al. Maternal pre-pregnancy BMI, MTHFR polymorphisms, and the risk of adverse pregnancy outcomes in pregnant women from South China: a retrospective cohort study. BMC Pregnancy Childbirth (2023) 23(1):295. doi: 10.1186/s12884-023-05605-6
40. Huang LL, Tong JR, Huang Y, Wei YN, Chen HF, Chen Y, et al. Association of MTHFR gene C677T polymorphism with pregnancy outcome. Eur Rev Med Pharmacol Sci (2023) 27(1):166–71. doi: 10.26355/eurrev_202301_30868
41. Liu H-Y, Qin S, Zhang Z, Qi J, Zhang W, Liu S-M, et al. Associations of MTHFR polymorphisms and cytosine modifications with early-gestational diabetes mellitus in Chinese pregnant women. Reprod Sci (2023) 30(10):2973–82. doi: 10.1007/s43032-023-01247-3
42. Chen Y, Lu M, Nie J, Liu J, Liu Y, Meng Y, et al. Increasing prevalence of gestational diabetes mellitus when carrying the T variant allele of the MTHFR gene C677T polymorphism: a systematic review and meta-analysis. Arch Gynecol Obstet (2022) 305(5):1193–202. doi: 10.1007/s00404-021-06303-4
43. Bahadır A, Eroz R, Türker Y. Does the MTHFR C677T gene polymorphism indicate cardiovascular disease risk in type 2 diabetes mellitus patients? Anatol J Cardiol (2015) 15(7):524–30. doi: 10.5152/akd.2014.5555
44. Pirozzi FF, Belini Junior E, Okumura JV, Salvarani M, Bonini-Domingos CR, Ruiz MA. The relationship between of ACE I/D and the MTHFR C677T polymorphisms in the pathophysiology of type 2 diabetes mellitus in a population of Brazilian obese patients. Arch Endocrinol Metab (2018) 62(1):21–6. doi: 10.20945/2359-3997000000005
Keywords: methylenetetrahydrofolate reductase, C677T polymorphism, gestational diabetes mellitus, China, meta-analysis, trial sequential analysis
Citation: Tan X and Chen H (2023) Association between MTHFR gene C677T polymorphism and gestational diabetes mellitus in Chinese population: a meta-analysis. Front. Endocrinol. 14:1273218. doi: 10.3389/fendo.2023.1273218
Received: 07 August 2023; Accepted: 13 October 2023;
Published: 30 October 2023.
Edited by:
Pradeep Kumar, Veer Bahadur Singh Purvanchal University, IndiaReviewed by:
Vandana Rai, Veer Bahadur Singh Purvanchal University, IndiaRami M. Elshazli, Horus University, Egypt
Umamaheswaran Gurusamy, Nationwide Children’s Hospital, United States
Copyright © 2023 Tan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongqin Chen, Y2hlbmhvbmdxaW4yMDIyQDE2My5jb20=
 Xi Tan
Xi Tan Hongqin Chen
Hongqin Chen