A Commentary on
Association between Helicobacter pylori infection and metabolic syndrome and its components
by Liu Y, Shuai P, Chen W, Liu Y and Li D (2023) Front. Endocrinol. 14:1188487. doi: 10.3389/fendo.2023.1188487
1 Introduction
Liu et al. (1) published “Association between Helicobacter pylori (H. pylori) infection and metabolic syndrome (MetS) and its components,” suggesting that H. pylori infection is associated with MetS in males. Males and females in the H. pylori-infected group had a significantly higher prevalence of obesity, one of the components of MetS, than those in the uninfected group. Most studies of adults from different countries and regions have demonstrated a positive correlation between H. pylori infection and MetS (2). However, although studies have evaluated the relationship between H. pylori infection and body weight in children (3), no studies have directly investigated the relationship between H. pylori infection and MetS.
H. pylori infection, an established cause of gastritis, peptic ulcer, and gastric cancer, has an estimated worldwide prevalence of 50% (4) and 80%–90% among adults and adolescents in developing countries, respectively (5, 6). Although pediatric obesity has become an international problem (7) and the acquisition of H. pylori infection frequently occurs in childhood (8), a major problem is the lack of research on the association between H. pylori and MetS.
2 Association between MetS and H. pylori in children
2.1 Body height and weight of children undergoing H. pylori eradication treatment
An H. pylori testing and treatment strategy for children and adolescents was reported in Japan (9). Since 2016, we have been implementing a testing and treatment program for H. pylori among third-year junior high school students in Saga Prefecture (10, 11). The pediatric guidelines from several learned societies on the management of H. pylori in children have recommended against a “testing and treatment” strategy for H. pylori (12). Conversely, the guidelines have also added that in regions with high rates of gastric cancer including China and Japan, the benefits of treatments that reduce the risk of gastric cancer development may outweigh the risks of treatment. In Japan, where gastric cancer incidence is high, the H. pylori testing and treatment strategy for children and adolescents is implemented in several local governments. Between 2016 and 2022, 126 students underwent eradication treatment at the age of 15 at our hospital, of whom 91 (males, 50; females, 41) had their body height and weight measured. The average height and weight of male students were 166.92 ± 6.09 cm and 58.83 ± 11.51 kg, respectively. The average height and weight of female students were 156.23 ± 7.34 cm and 52.05 ± 9.40 kg, respectively. These results were compared with those of the annual report of school health statistics research compiled by the Ministry of Education, Culture, Sports, Science and Technology of Japan for male and female students, and no differences were observed. The results of this report were the average data of 57,966 individuals, accounting for 5.3% of 15-year-old individuals in Japan in 2021.
2.2 Analysis of the intestinal microbiota of children with H. pylori infection
We analyzed the effects of H. pylori infection on the intestinal microbiota of children (13). This study included 80 H. pylori-infected and 79 noninfected 15-year-old adolescents. No significant differences could be observed in sex, age, and body mass index (BMI) between the groups. H. pylori infection-associated symptoms including abdominal symptoms were not evaluated in both groups. Intestinal microbiota samples were collected from feces. The H. pylori-infected group had a significantly higher relative prevalence of the Prevotella genus than the uninfected group (p < 0.01) (Figure 1A). Furthermore, the relative occupancy of the Prevotella genus in the H. pylori-infected group increased in proportion to the BMI (Figure 1B). Thus, H. pylori infection had a significant impact on the intestinal microbiota of Japanese adolescents.
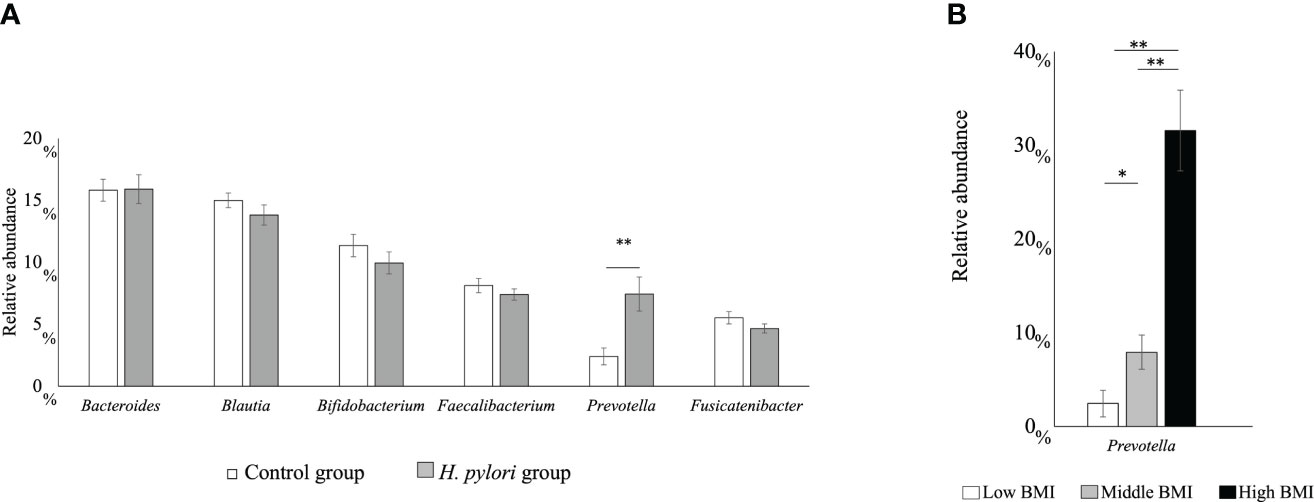
Figure 1 (A) The six main bacterial types present in the intestinal microbiota at the genus level. Comparison between the H pylori-infected and control groups (**p < 0.01). (B) Relative abundance of the Prevotella genus with regard to the body mass index (BMI) category in the H pylori-infected and control groups (*p < 0.05; **p < 0.01; ***p < 0.001). Low BMI, <15 kg/m2; mid BMI, 15–25 kg/m2; high BMI, >25 kg/m2.
3 Discussion
Currently, consistent diagnostic criteria for defining MetS in childhood and adolescence are not available. Since 1980, the prevalence of childhood obesity has more than doubled, and 6%–39% of obese children and adolescents already present with MetS, depending on the definition applied (14). The lack of diagnostic criteria for MetS in children is a major impediment to progress in research on the relationship between H. pylori and MetS.
Previous studies have evaluated the relationship between H. pylori infection and body weight in adults. Furthermore, this relationship has been evaluated in children, with various results reported including positive (15), negative (16–19), and no correlations (20). Our data did not differ when compared with randomized data from Japan (the annual report of school health statistics research). Previously, the H. pylori infection rate among 15-year-old individuals in Japan was approximately 2% (11). Although there may be various biases, in general, H. pylori infection seems to lead to poor weight gain in children. This is contrary to the findings of Liu et al., where H. pylori was associated with obesity, one of the components of MetS in adults. However, H. pylori eradication was reported to improve the nutritional status and weight gain in children (18, 20). The reasons for these differences in H. pylori and body weight between children and adults are unclear. As one of the reasons, pediatric studies on changes in weight come from studies on symptomatic pediatric patients, wherein ruling out the restriction of intakes owing to pain, dyspeptic symptoms, or adverse effects of any H. pylori eradication treatment is not possible. Regarding the relationship between H. pylori and MetS in children, whether the results are similar to those in adults is an issue that requires further investigation.
We compared the fecal intestinal microbiota of H. pylori-infected and noninfected children (13). Most studies on the effects of H. pylori on the intestinal microbiota based on the analysis of fecal samples were conducted in adults, and data from children are lacking (21). H. pylori infection in children with an increased BMI without diabetes mellitus, was associated with an increase in the prevalence of the Prevotella genus (22), which was concomitant with an increase in the BMI in our previous study (13) (Figure 1B). The prevalence of the Prevotella genus was increased in patients with obesity (23), nonalcoholic steatohepatitis (24), hyperlipidemia (25), and even in gestational diabetes, which is considered a diabetes mellitus preliminary group disease. It is believed that an increase in the Prevotella genus may be involved in abnormal glucose metabolism development as a result of obesity (26). Although there are no studies regarding an increase in the Prevotella genus in adults infected with H. pylori, the Prevotella genus might be increased owing to H. pylori infection, thereby resulting in MetS. This suggests that children infected with H. pylori are at risk of MetS.
4 Conclusion
To elucidate the relationship between H. pylori and MetS in children, developing international standards for MetS in children and clarifying the pathogenesis of H. pylori-induced MetS in adults are necessary.
Author contributions
TK: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author declares financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The author thanks J. Ludovic Croxford, PhD, from Edanz (https://jp.edanz.com/ac) for editing the draft of this manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu Y, Shuai P, Chen W, Liu Y, Li D. Association between Helicobacter pylori infection and metabolic syndrome and its components. Front Endocrinol (Lausanne) (2023) 14:1188487. doi: 10.3389/fendo.2023.1188487
2. Azami M, Baradaran HR, Dehghanbanadaki H, Kohnepoushi P, Saed L, Moradkhani A, et al. Association of Helicobacter pylori infection with the risk of metabolic syndrome and insulin resistance: an updated systematic review and meta-analysis. Diabetol Metab Syndr (2021) 13:145. doi: 10.1186/s13098-021-00765-x
3. Pundak OY, Topf Olivestone C, Hofi L, Kori M. Lack of association between Helicobacter pylori infection and childhood overweight/obesity. Helicobacter (2020) 25:e12728. doi: 10.1111/hel.12728
4. Jeffery PL, McGuckin MA, Linden SK. Endocrine impact of Helicobacter pylori: focus on ghrelin and ghrelin o-acyltransferase. World J Gastroenterol (2011) 17:1249–60. doi: 10.3748/wjg.v17.i10.1249
5. Goh KL, Chan WK, Shiota S, Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter (2011) 16:1–9. doi: 10.1111/j.1523-5378.2011.00874.x
6. Mišak Z, Hojsak I, HOman M. Review: Helicobacter pylori in pediatrics. Helicobacter (2019) 24:e12639. doi: 10.1111/hel.12639
7. York DA, Rössner S, Caterson I, Chen CM, James WP, Kumanyika S, et al. Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: Group I: worldwide demographics of obesity. Circulation (2004) 110:e463–70. doi: 10.1161/01.Cir.0000140125.26161.49
8. Chong SK, Lou Q, Zollinger TW, Rabinowitz S, Jibaly R, Tolia V, et al. The seroprevalence of Helicobacter pylori in a referral population of children in the United States. Am J Gastroenterol (2003) 98:2162–8. doi: 10.1111/j.1572-0241.2003.07683.x
9. Saito H, Nishikawa Y, Masuzawa Y, Tsubokura M, Mizuno Y. Helicobacter pylori infection mass screening for children and adolescents: a systematic review of observational studies. J Gastrointest Cancer (2021) 52:489–97. doi: 10.1007/s12029-021-00630-0
10. Kakiuchi T, Matsuo M, Endo H, Nakayama A, Sato K, Takamori A, et al. A Helicobacter pylori screening and treatment program to eliminate gastric cancer among junior high school students in Saga Prefecture: a preliminary report. J Gastroenterol (2019) 54:699–707. doi: 10.1007/s00535-019-01559-9
11. Kakiuchi T, Matsuo M, Endo H, Sakata Y, Esaki M, Noda T, et al. Efficacy and safety of vonoprazan-based regimen for Helicobacter pylori eradication therapy in Japanese adolescents: a prospective multicenter study. J Gastroenterol (2023) 58:196–204. doi: 10.1007/s00535-022-01942-z
12. Jones NL, Koletzko S, Goodman K, Bontems P, Cadranel S, Casswall T, et al. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (update 2016). J Pediatr Gastroenterol Nutr (2017) 64:991–1003. doi: 10.1097/mpg.0000000000001594
13. Kakiuchi T, Tanaka Y, Ohno H, Matsuo M, Fujimoto K. Helicobacter pylori infection-induced changes in the intestinal microbiota of 14-year-old or 15-year-old Japanese adolescents: a cross-sectional study. BMJ Open (2021) 11:e047941. doi: 10.1136/bmjopen-2020-047941
14. Weihe P, Weihrauch-Blüher S. Metabolic syndrome in children and adolescents: diagnostic criteria, therapeutic options and perspectives. Curr Obes Rep (2019) 8:472–9. doi: 10.1007/s13679-019-00357-x
15. Bruera MJ, Amezquita MV, Riquelme AJ, Serrano CA, Harris PR. Helicobacter pylori infection and UBT-13C values are associated with changes in body mass index in children and adults. Rev Med Chil (2022) 150:1467–76. doi: 10.4067/s0034-98872022001101467
16. Mera RM, Correa P, Fontham EE, Reina JC, Pradilla A, Alzate A, et al. Effects of a new Helicobacter pylori infection on height and weight in Colombian children. Ann Epidemiol (2006) 16:347–51. doi: 10.1016/j.annepidem.2005.08.002
17. Boyanova L, Hadzhiyski P. Helicobacter pylori infection is associated with anemia, weight loss or both conditions among Bulgarian children. Acta Microbiol Immunol Hung (2020) 67:239–42. doi: 10.1556/030.2020.01158
18. Dror G, Muhsen K. Helicobacter pylori infection and children’s growth: an overview. J Pediatr Gastroenterol Nutr (2016) 62:e48–59. doi: 10.1097/mpg.0000000000001045
19. Erdemir G, Ozkan TB, Ozgur T, Altay D, Cavun S, Goral G. Helicobacter pylori infection in children: nutritional status and associations with serum leptin, ghrelin, and IGF-1 levels. Helicobacter (2016) 21:317–24. doi: 10.1111/hel.12288
20. Choi JS, Ko KO, Lim JW, Cheon EJ, Lee GM, Yoon JM. The association between Helicobacter pylori infection and body weight among children. Pediatr Gastroenterol Hepatol Nutr (2016) 19:110–5. doi: 10.5223/pghn.2016.19.2.110
21. Dash NR, Khoder G, Nada AM, Al Bataineh MT. Exploring the impact of Helicobacter pylori on gut microbiome composition. PloS One (2019) 14:e0218274. doi: 10.1371/journal.pone.0218274
22. Benavides-Ward A, Vasquez-Achaya F, Silva-Caso W, Aguilar-Luis MA, Mazulis F, Urteaga N, et al. Helicobacter pylori and its relationship with variations of gut microbiota in asymptomatic children between 6 and 12 years. BMC Res Notes (2018) 11:468. doi: 10.1186/s13104-018-3565-5
23. Marietti M, Gasbarrini A, Saracco G, Pellicano R. Helicobacter pylori infection and diabetes mellitus: the 2013 state of art. Panminerva Med (2013) 55:277–81.
24. Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology (2013) 58:120–7. doi: 10.1002/hep.26319
25. Roager HM, Licht TR, Poulsen SK, Larsen TM, Bahl MI. Microbial enterotypes, inferred by the Prevotella-to-Bacteroides ratio, remained stable during a 6-month randomized controlled diet intervention with the new Nordic diet. Appl Environ Microbiol (2014) 80:1142–9. doi: 10.1128/aem.03549-13
26. Leiva-Gea I, Sánchez-Alcoholado L, Martín-Tejedor B, Castellano-Castillo D, Moreno-Indias I, Urda-Cardona A, et al. Gut microbiota differs in composition and functionality between children with type 1 diabetes and MODY2 and healthy control subjects: a case-control study. Diabetes Care (2018) 41:2385–95. doi: 10.2337/dc18-0253
Keywords: Helicobacter pylori, metabolic syndrome, child, obesity, Prevotella genus
Citation: Kakiuchi T (2023) Commentary: Association between Helicobacter pylori infection and metabolic syndrome and its components. Front. Endocrinol. 14:1270855. doi: 10.3389/fendo.2023.1270855
Received: 01 August 2023; Accepted: 10 August 2023;
Published: 21 August 2023.
Edited by:
Mei-zhou Huang, The Affiliated Hospital of Southwest Medical University, ChinaReviewed by:
Paul R Harris, Pontificia Universidad Católica de Chile, ChileCopyright © 2023 Kakiuchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshihiko Kakiuchi, a2FraXVjaHRAY2Muc2FnYS11LmFjLmpw
 Toshihiko Kakiuchi
Toshihiko Kakiuchi