- 1Department of Cardiology, The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University, Changzhou, Jiangsu, China
- 2Department of Geriatrics, Nanjing Tongren Hospital, School of Medicine, Southeast University, Nanjing, China
- 3Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 4Medical Research Center, The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University, Changzhou, Jiangsu, China
Background and aims: Increasing evidence supports a causal relationship between lipoprotein(a) [Lp(a)] and atherosclerotic cardiovascular disease, yet its association with left ventricular hypertrophy (LVH) assessed by electrocardiogram (ECG) remains unknown. The aim of this study was to explore the relationship between Lp(a) and LVH assessed by ECG in general population.
Methods and results: In this cross-sectional study, we screened 4,052 adults from the participants of the third National Health and Nutrition Examination Survey for analysis. Lp(a) was regarded as an exposure variable. LVH defined by the left ventricular mass index estimated from ECG was considered as an outcome variable. Multivariate logistic regression and restricted cubic spline (RCS) were used to assess the relationship between Lp(a) and LVH. Individuals with LVH had higher Lp(a) compared to individuals without LVH (P< 0.001). In the fully adjusted model, Lp(a) was strongly associated with LVH when as a continuous variable (per 1-unit increment, OR: 1.366, 95% CI: 1.043-1.789, P = 0.024), and higher Lp(a) remained independently associated with a higher risk of LVH when participants were divided into four groups according to quartiles of Lp(a) (Q4 vs Q1, OR: 1.508, 95% CI: 1.185-1.918, P = 0.001). And in subgroup analysis, this association remained significant among participants< 60 years, ≥ 60 years, male, with body mass index< 30 kg/m2, with hypertension and without diabetes (P< 0.05). In addition, we did not observe a nonlinear and threshold effect of Lp(a) with LVH in the RCS analysis (P for nonlinearity = 0.113).
Conclusion: Lp(a) was closely associated with LVH assessed by ECG in general population.
Introduction
Left ventricular hypertrophy (LVH) is defined as an increase in left ventricular mass (LVM) that can be secondary to an increase in ventricular wall thickness or chamber size (1). Electrocardiography (ECG), echocardiography and magnetic resonance imaging are currently the main diagnostic tools for the evaluation of LVH. Although ECG is less accurate than the other two diagnostic methods in diagnosing LVH, it is widely used in epidemiological studies because of its low cost, convenience and easy availability. However, LVH detected by either method is strongly associated with a higher risk of cardiovascular disease (CVD) and CVD-related mortality (2–6). Therefore, the 2018 European Society of Hypertension (ESH)/European Society of Cardiology (ESC) clinical practice guideline added LVH as a high-risk factor to the CVD risk assessment system and recommended screening for LVH in high-risk populations as well as early identification and intervention of controllable risk factors for LVH to prevent premature CVD or CVD-related death (7). Although current evidence suggests that the risk factors for LVH are composed of some non-modifiable and modifiable factors, including uncontrollable factors such as age, gender and genetic susceptibility and controllable risk factors such as hypertension, diabetes, chronic kidney disease, metabolic disorders, obesity, lack of exercise or unhealthy diet, other potential risk factors may still exist (1, 8–10).
Lipoprotein(a) [Lp(a)] is a low-density lipoprotein cholesterol-like particle bound to apolipoprotein(a), which can participate in the occurrence and development of CVD by promoting oxidation, inflammation, calcification, thrombosis and atherosclerosis (11). Because circulating Lp(a) levels are largely regulated by genes, its absolute risk threshold has not yet reached unity, but current evidence suggests that higher Lp(a) is associated with a higher risk of CVD (12). In recent years, studies of Lp(a) and CVD have been widely conducted as Lp(a) has gradually gained attention and improved measurement methods have been developed. As a result, a growing number of observational studies have shown that Lp(a) is closely related to coronary heart disease (CHD) (13), stroke (14), calcific aortic valve disease (CAVD) (15), hypertension (16), atrial fibrillation (17) and venous thromboembolism (18), and evidence from genetic studies has also confirmed the causal association of Lp(a) with CVD and CVAD (19, 20). A recent study revealed the relationship between Lp(a) and LVH assessed by echocardiography only in patients with acute myocardial infarction (21), whereas the correlation between Lp(a) and LVH assessed by ECG in the general population remains unknown.
Therefore, in order to fill the gap in this research field and provide reference for formulating early prevention and treatment strategies of LVH under the background of higher Lp(a), the aim of this study was to explore the relationship between Lp(a) and LVH assessed by ECG in the general population.
Methods
Study population
After excluding individuals younger than 17 years old, without Lp(a) and left ventricular mass, left ventricular mass index (LVMI) or LVH data, we screened 4,052 participants from the third National Health and Nutrition Examination Survey (NHANES III). The flow chart of the study population was shown in Figure 1. The NHANES study protocol was approved by the National Center for Health Statistics of the Center for Disease Control and Prevention Institutional Review Board, and all participants signed a written informed consent form when participating in the NHANES. And this study was in line with the Declaration of Helsinki.
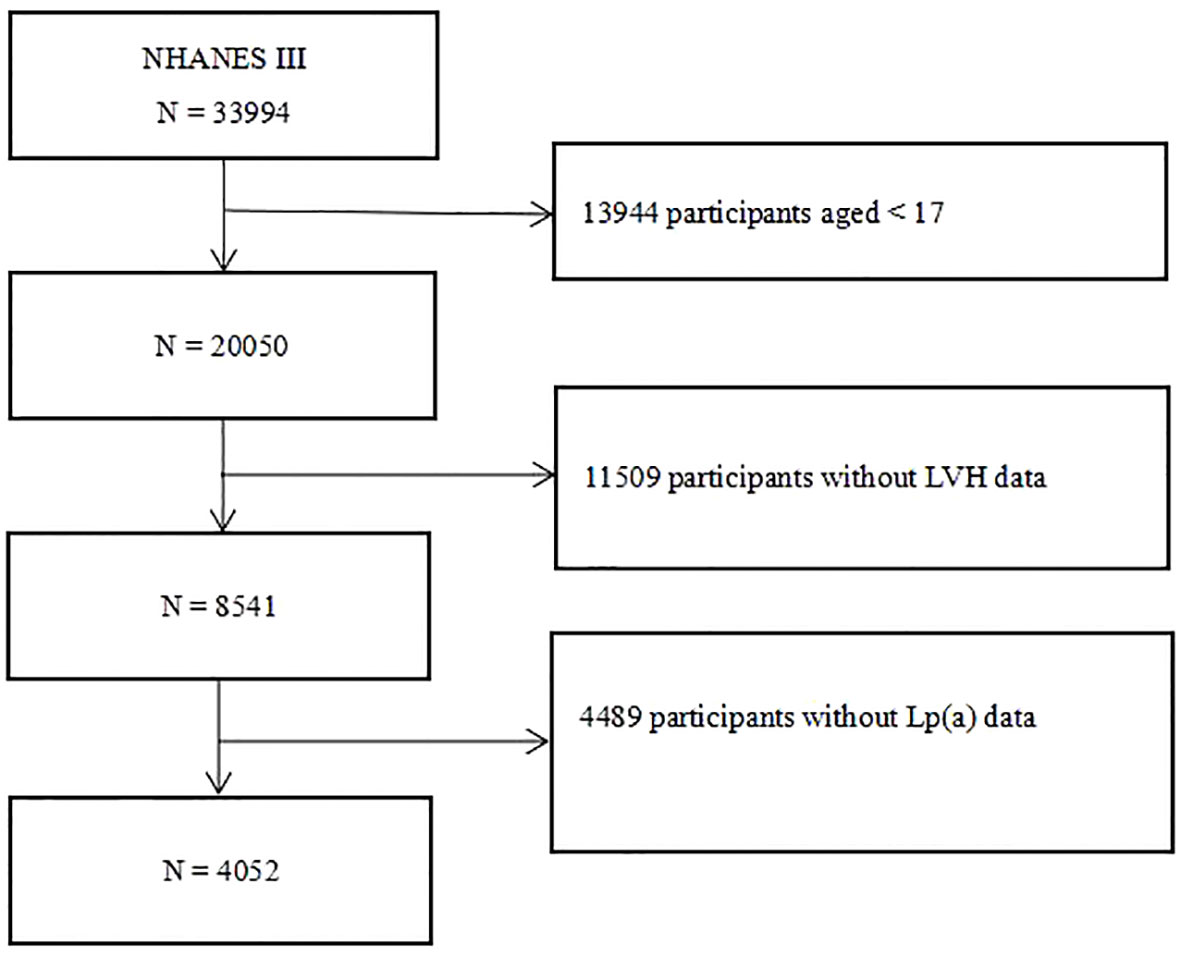
Figure 1 Flow chart of the study participants. NHANES III, the third National Health and Nutrition Examination Survey; Lp(a), lipoprotein(a); LVH, left ventricular hypertrophy.
Data collection and definitions
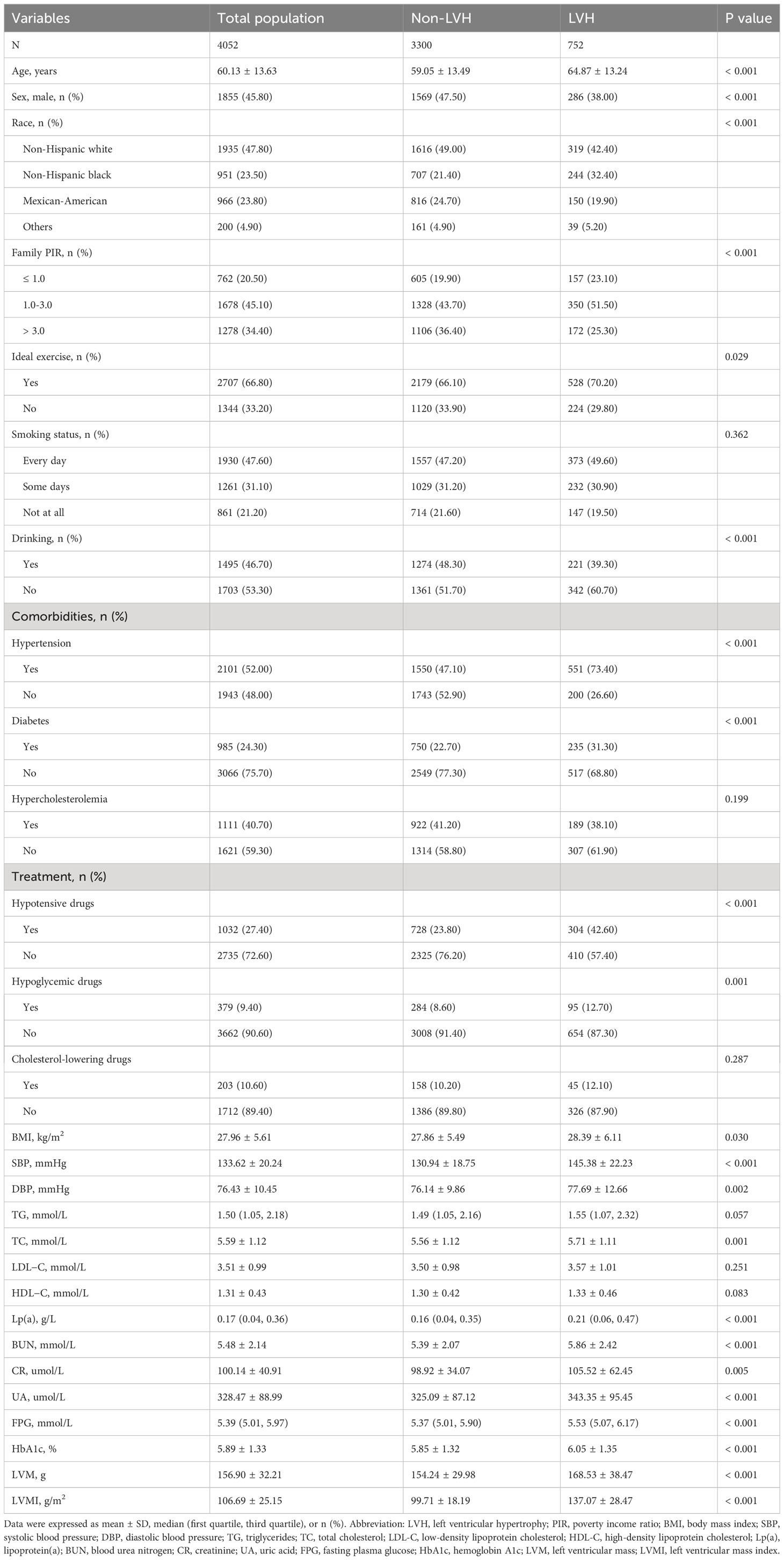
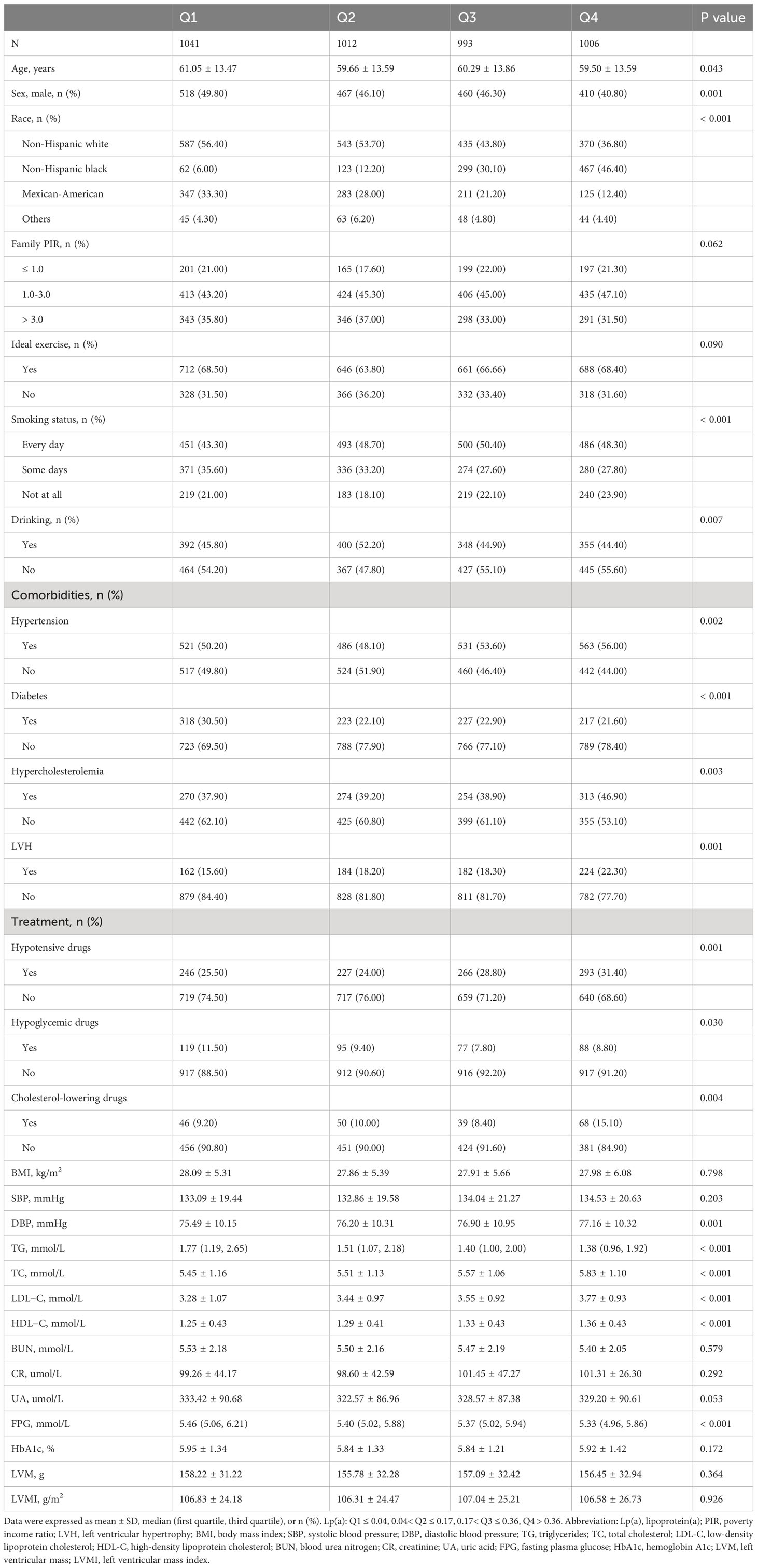
Variables used for analysis in this study included age, sex, race, family poverty income ratio (PIR), ideal exercise, smoking status, drinking, hypertension, diabetes, hypercholesterolemia, hypotensive drugs, hypoglycemic drugs, cholesterol-lowering drugs, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), triglycerides, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), Lp(a), blood urea nitrogen (BUN), creatinine, uric acid (UA), fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), LVM, LVMI, and LVH. All of the above demographic data, comorbidity data, medication data, and biomarker data were obtained using standardized household questionnaires and standard biochemical measurement procedures, the specific methods and contents of which are available on the publicly available NHANES website. In this study, race was divided into four groups: non-Hispanic White, non-Hispanic Black, Mexican-American and Others. Family PIR was divided into three groups: ≤ 1.0, 1.0-3.0, > 3.0. Ideal exercise was defined as ≥ 150 minutes of moderate intensity activity or ≥ 75 minutes of high intensity activity per week. Smoking status was divided into three groups: every day, some days, and not at all. Drinking was defined as drinking at least 12 drinks in a year. Hypertension was defined as pre-existing hypertension, or a mean SBP ≥ 140 mmHg or mean DBP ≥ 90 mmHg when participating in NHANES or taking oral antihypertensive medication. Diabetes was defined as pre-existing diabetes, or FPG ≥ 7.0 mmol/L or HbA1c ≥ 6.5% when participating in NHANES or using hypoglycemic medication. Hypercholesterolemia was defined as having previous hypercholesterolemia.
All participants in this study underwent a 12-lead resting ECG. Trained professionals collected ECG signal data of participants through a Marquette MAC 12 system (Marquette Medical Systems, Milwaukee, Wisconsin), which was then transmitted to an ECG reading centre where the acquired ECG data were coded by Minnesota codes and analyzed by a Novacode ECG measurement, A classification program with an algorithm for classifying ECGs according to Minnesota codes, an algorithm for classifying LVH according to various ECG criteria, and a multivariate statistical model for estimating LVM and LVMI by the routine 12-lead ECG were used (22–28). Specific multivariate linear regression equations for the estimated LVM were shown below (24):
White and black males: .
White female: .
Black females: .
LVH is defined as LVMI > 150 g/m2 for men or LVMI > 120 g/m2 for women. The above reference values of LVMI for LVH defined by sex correspond to the normal upper limits for LVMI based on echocardiographic detection established by the American Society of Echocardiography (23), and these ECG-LVH standards have been proved to have good diagnostic efficacy in a large population-based epidemiological study (24). In addition, we evaluated LVH according to other ECG criteria such as Sokolow-Lyon criterion, Cornell criterion and Cornell product (29, 30).
Statistical analysis
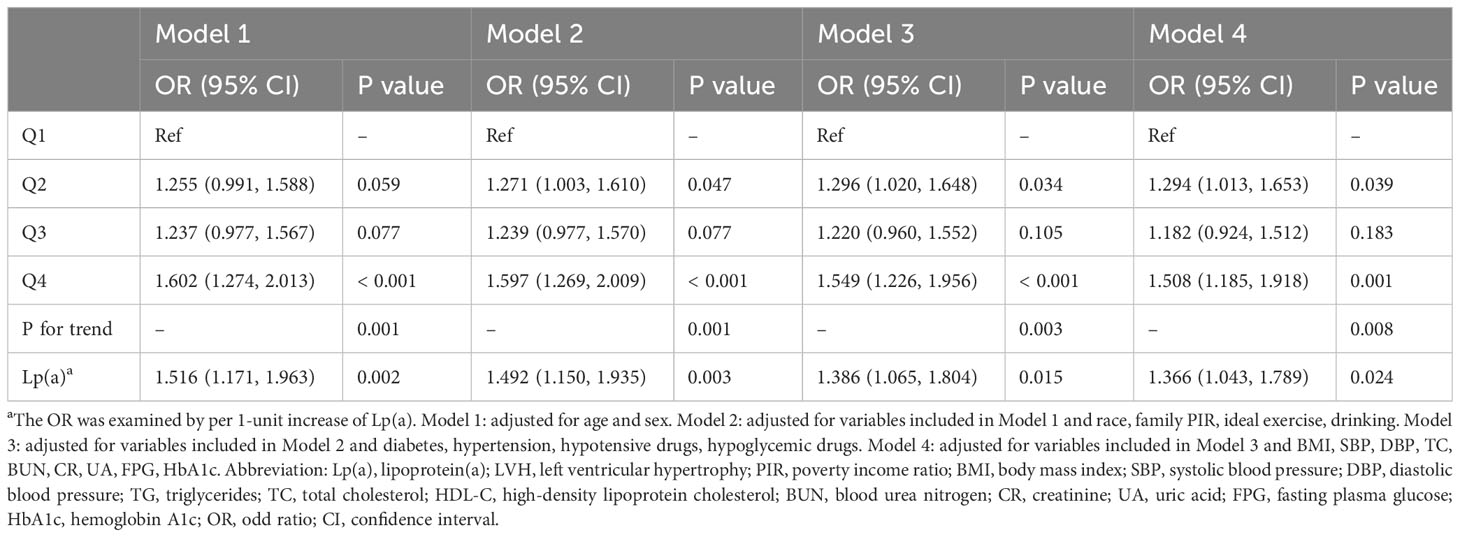
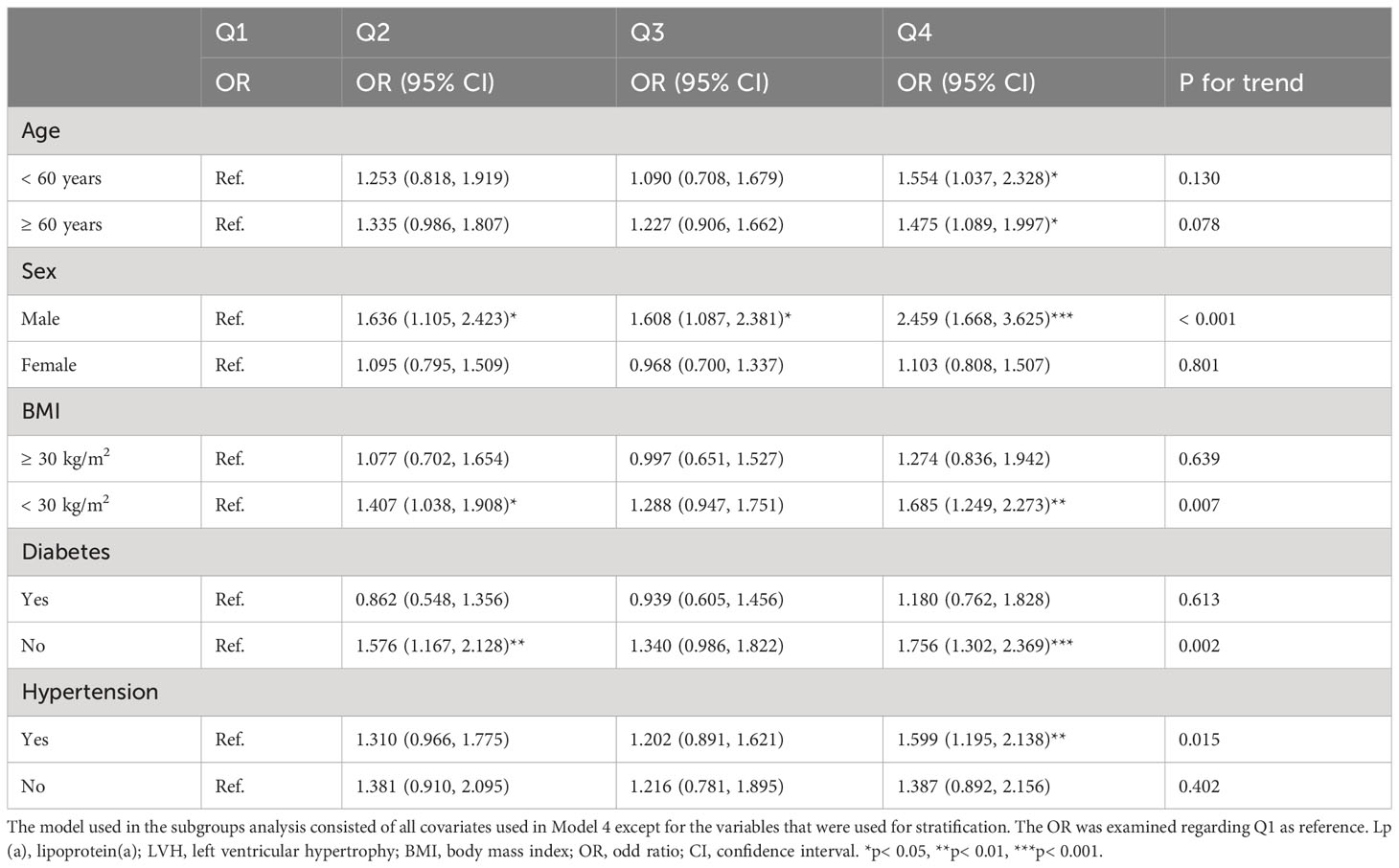
First, continuous variables that were normally distributed were expressed as mean ± standard deviation and compared between two or four groups using independent samples t-test or one-way ANOVA, respectively, when the variables met both the homogeneity of variance. Continuous variables as non-normally distributed were expressed as median (first quartile, third quartile) and compared between two or four groups using the Mann-Whitney U or Kruskal-Wallis H test, respectively. Categorical variables were expressed as frequencies and percentages, and differences in percentages of categorical variables between groups were assessed using the chi-square or Fisher’s exact test. Second, univariate logistic regression model was used to examine variables associated with LVH (P< 0.05), and these variables were then constructed into four models for multivariate logistic regression analysis of the relationship between Lp(a) and LVH. Model 1 adjusted for age and sex; model 2 adjusted for age, sex, race, family PIR, ideal exercise, and drinking; model 3 adjusted for the variables in model 2 plus diabetes, hypertension, hypoensive drugs, and hypoglycemic drugs; model 4 adjusted the variables in model 3 plus BMI, SBP, DBP, TC, BUN, creatinine, UA, FPG and HbA1c. Subsequently, we assessed the robustness of the relationship between Lp(a) and LVH in five subgroups, including age (< 60 or ≥ 60 years), sex (male or female), BMI (≥ 30 or< 30 kg/m2), hypertension (yes or no), and diabetes (yes or no). Third, we explored possible nonlinear relationships and threshold effects of Lp(a) with LVM, LVMI, and LVH using restricted cubic splines (RCS) with three nodes. All statistical methods in this study were performed by SPSS 26.0 (SPSSInc., Chicago, Illinois, USA) and the R programming language (version 4.1.3). A two-tailed P< 0.05 was viewed as statistically significant.
Results
Baseline characteristics
First, 4,052 participants (mean age: 60.13 years; 45.80% men) were divided into two groups: non-LVH and LVH groups (Table 1). Compared with the non-LVH group, the LVH group had more older people, more women, more non-Hispanic Black, more family PIR of 1.0-3.0, more ideal exercise, more prevalence of hypertension, more prevalence of diabetes, more use of hypotensive drugs, more use of hypoglycemic drugs, fewer drinkers and higher levels of BMI, SBP, DBP, TC, Lp(a), BUN, creatinine, UA, FPG, HbA1c, LVM, and LVMI (P< 0.05). Then, all participants were divided into four groups according to the quartiles of Lp(a): Q1 ≤ 0.04, 0.04< Q2 ≤ 0.17, 0.17< Q3 ≤ 0.36, Q4 > 0.36 (Table 2). Age, sex, race, smoking status, drinking, hypertension, diabetes, hypercholesterolemia, hypotensive drugs, hypoglycemic drugs, cholesterol-lowering drugs, DBP, triglycerides, TC, LDL-C, HDL-C, and FPG were statistically significant between these four groups, and the group with higher Lp(a) had higher prevalence of LVH than the group with lower Lp(a) (P< 0.05).
Association between Lp(a) and LVH
In multivariate logistic regression analyses (Table 3), Lp(a) was strongly associated with LVH whether it was used as a continuous variable or a categorical variable when adjusting for age and sex only (per 1-unit increment, OR: 1.516, 95% CI: 1.171-1.963, P = 0.002; Q4 vs Q1, OR: 1.602, 95% CI: 1.274-2.013, P< 0.001; respectively). And in the fully adjusted model, higher Lp(a) was still associated with a higher risk of LVH (per 1-unit increment, OR: 1.366, 95% CI: 1.043-1.789, P = 0.024; Q4 vs Q1, OR: 1.508, 95% CI: 1.185-1.918, P = 0.001; respectively).
And in the subgroup analysis (Table 4), the risk of developing LVH in participants with higher Lp(a) was 1.6, 1.5, 2.5, 1.7, 1.6, and 1.8 times higher than in participants with lower Lp(a) in the subgroups aged< 60 or ≥ 60 years, male, with BMI< 30 kg/m2, and with hypertension or without diabetes, respectively (P< 0.05). Additionally, we evaluated LVH according to the Sokolow-Lyon criterion, Cornell criterion and Cornell product methods, respectively, and found that after adjusting for confounding variables, only Lp(a) as a four-categorical variable was strongly associated with the LVH assessed by the Sokolow-Lyon criterion (P< 0.05). However, the correlation of Lp(a) with LVH assessed by the Cornell criterion and Cornell product methods could not be further determined (P > 0.05) (Table 5). In addition, we did not find a nonlinear relationship between Lp(a) and LVH, LVM, and LVMI in the RCS analysis (P for nonlinearity > 0.05) (Figure 2).
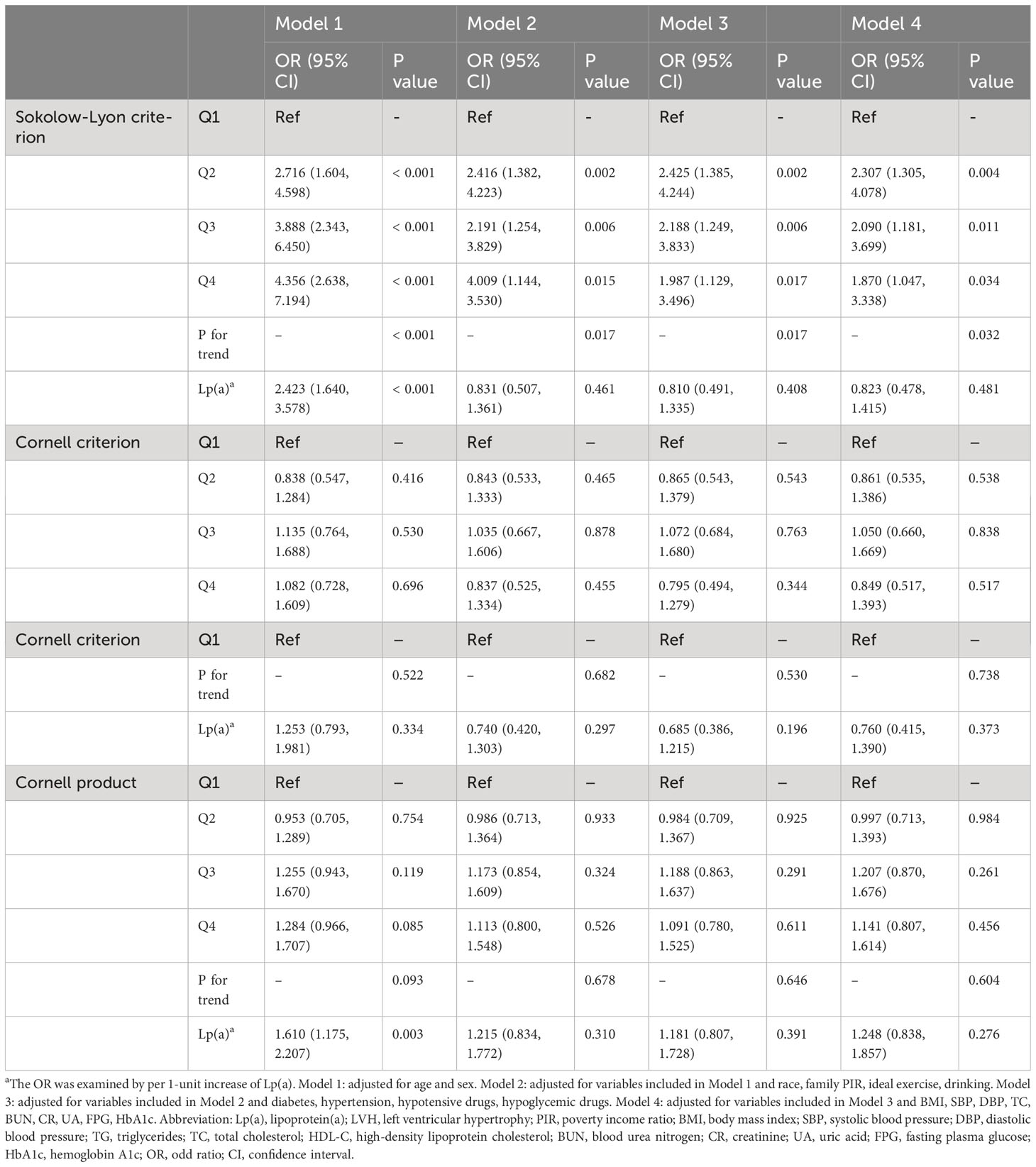
Table 5 Association between Lp(a) and LVH assessed by the Sokolow-Lyon criterion, Cornell criterion, and Cornell product, respectively.
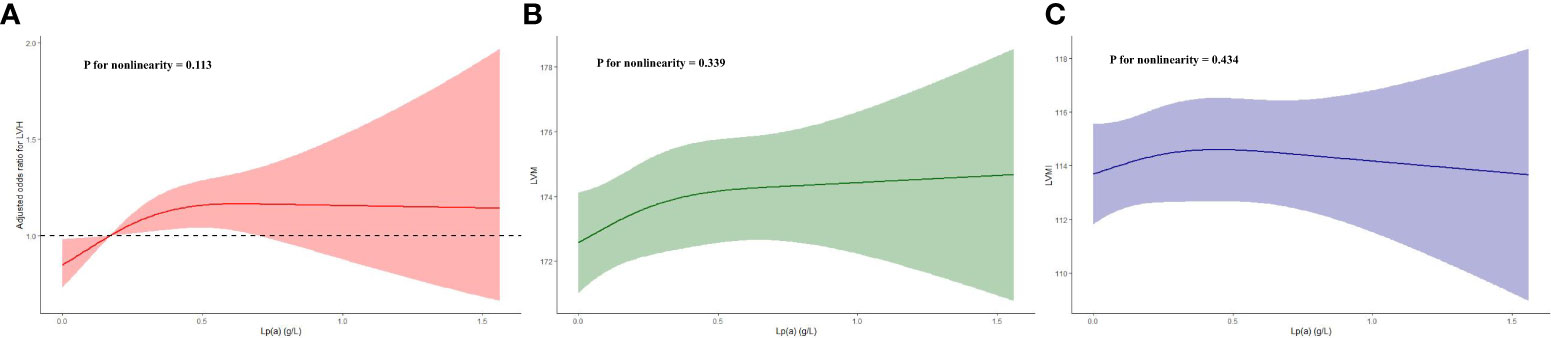
Figure 2 Restricted cubic spline plots of the association between Lp(a) and LVH (A), LVM (B) and LVMI (C). Lp(a), lipoprotein(a); LVH, left ventricular hypertrophy; LVM, left ventricular mass; LVMI, left ventricular mass index.
Discussion
In this large population-based cross-sectional observational study, we found that higher Lp(a) was strongly associated with a higher prevalence of LVH assessed by ECG in the general population, and this association was further confirmed to be linear. Additionally, we also demonstrated robustness of the association of Lp(a) with LVH in people aged< 60 or ≥ 60 years, male, with BMI< 30 kg/m2, with hypertension or without diabetes.
Current evidence from epidemiological studies suggests that LVH is strongly associated with CVD as well as cardiovascular and all-cause mortality (4, 31–33). Therefore, it is very important to identify the risk factors of LVH and carry out early intervention to prevent the premature occurrence of LVH. Currently, an increasing number of studies have identified independent risk factors for LVH, such as age, hypertension, diabetes and obesity (1, 34, 35). However, to our knowledge, a previous study only showed an association between Lp(a) and LVH assessed by echocardiography in patients with acute myocardial infarction (21), whereas the correlation between Lp(a) and LVH in the general population remains unclear, especially for LVH diagnosed by ECG. Recently, a large observational epidemiological study involving 309,400 participants showed that Lp(a) in the highest tertile was significantly associated with a higher prevalence of LVH diagnosed by echocardiography compared with Lp(a) in the lowest tertile, but not with a higher incidence of LVH during follow-up (36). Although this study demonstrated in the cross-sectional section that participants in the highest tertile of Lp(a) had a 1.3 times higher risk of developing LVH compared with those in the lowest tertile, because LVH in this study was defined by expensive and highly subjective echocardiography, the results may not be applicable to large epidemiological studies based on the general population. Nevertheless, fortunately, our findings were consistent with the conclusion of the above study on the correlation between Lp(a) and LVH, that is, participants in the highest quartile of Lp(a) had a 1.5 times higher risk of suffering from ECG-defined LVH than those in the lowest quartile of Lp(a). Furthermore, in addition to the current evidence confirming the causal association of Lp(a) with atherogenic CVD as well as CAVD (15, 19, 20), several studies have identified other pathogenic phenotypes of Lp(a). For example, Dentali et al. demonstrated in a systematic review and meta-analysis including 14,011 participants and 14 observational studies that higher Lp(a) was significantly associated with higher venous thromboembolism (OR:1.56,95% CI:1.36-1.79) using a random effects model (18). Another observational study showed that although familial hypercholesterolemia did not cause elevated Lp(a), elevated Lp(a) predicted the occurrence of familial hypercholesterolemia (37). However, higher Lp(a) is not always detrimental. For example, Garg et al. found that higher levels of Lp(a) were independently associated with a lower incidence of atrial fibrillation during follow-up in a community-based prospective cohort of 6,593 adults without CVD (17). And Lamina et al. also revealed a negative association of Lp(a) with diabetes (38). However, the above studies only measured the concentration of circulating Lp(a) at baseline, but did not observe the effect of dynamic changes of Lp (a) on CVD. Trinder et al. refined the study design based on the above studies and showed that there was no significant change in the circulating molar concentration of Lp(a) during the median follow-up period of 4.42 years [baseline vs follow-up: 19.50 (7.56-72.50) vs 20.40 (7.70-77.50)], and that Lp(a) at baseline and follow-up were both significantly associated with the occurrence of CVD during follow-up, while changes in Lp(a) had no significant effect on the incidence of CVD (39), indicating that a single measurement of Lp(a) is essential for the primary prevention of CVD in the general population without treatment that significantly changes the level of circulating Lp(a).
Although our study demonstrated the association of Lp(a) with LVH, the mechanisms involved are still unknown. Based on published studies, we proposed the following hypotheses. First, Bergmark et al. used immunoprecipitation and ultracentrifugation experiments, in vitro transfer studies and chemiluminescence ELISAs experiments to evaluate the priority of Lp(a) as a carrier of oxidized phospholipids in human plasma. The results showed that most of oxidized phospholipids and Lp(a) co-precipitated in immunoprecipitation experiments, and most of oxidized phospholipids existed in components containing apolipoprotein(a) after subsequent ultracentrifugation experiments, and apolipoprotein(a) was the most important part of Lp(a) structure. Further in vitro transfer studies showed that oxidized phospholipids could be preferentially transferred to Lp(a) by oxidized LDL in a time-and temperature-dependent manner regardless of the nature of the buffer. Based on these data, we could draw a conclusion that apolipoprotein(a) or Lp(a) could indirectly participate in oxidative stress as the priority carrier of human plasma oxidized phospholipids, and then activate the markers related to oxidative stress, which might eventually promote the occurrence and development of LVH (40). Second, Aung et al. first conducted a single-sample Mendelian randomized study on 17,311 European individuals from British Biobank, which showed that there was an exact causal relationship between higher LDL and higher LVM, and then conducted a two-sample Mendelian randomized study on another genetic data, which also confirmed this conclusion (41), and since Lp(a) as an LDL-like particle has a cholesterol component that acts similarly to LDL-C (42), we hypothesized that the cholesterol component in Lp(a) plays a role in promoting LVH. Third, a previous study has shown that Lp(a) has a ring structure and inactive protease region similar to plasmin precursor protein structure, which can inhibit fibrinolysis and promote thrombosis through competitive binding of fibrinolytic proteins (43), and Lip et al. conducted a cross-sectional study of 178 patients from hypertension clinic, they estimated LVM, LVMI and LVH by echocardiography, the results showed that hypertensive patients had higher Lp(a) levels and left ventricular septum and posterior wall thickness. Further analysis revealed the correlation between plasma fibrinogen level with homology to Lp(a) and LVM, LVMI and LVH. Therefore, based on these complicated relationships, the hypothesis that Lp(a) indirectly affects left ventricular structure and LVH through fibrinogen or thrombogenic state may also be widely recognized (44). Additionally, current evidence confirms the association of Lp(a) with aortic stenosis and hypertension, and aortic stenosis or hypertension has been proved to be closely related to the left ventricular afterload and occurrence and development of LVH (15, 16).Consequently, we assumed that Lp(a) could promote LVH by causing aortic stenosis and hypertension in the early stages of the disease. In addition to the above findings, we believe that there are still other potential mechanisms that need to be further explored.
Despite the valuable findings, this study still had several limitations. First, we failed to confirm the causal association of Lp(a) with LVH due to the limitations of cross-sectional observational studies. Second, we only studied LVH from ECG sources and did not compare it with LVH detected by echocardiography or cardiac magnetic resonance, and the study population was limited to adults in the United States, so the robustness of the association between Lp(a) and LVH still needs to be further explored. Third, although we controlled for some confounding factors in our study, there may still be other potential risk factors for LVH, such as unhealthy diet and genetic susceptibility. Finally, there are up-to-date criteria for assessing LVH by ECG or echocardiography (45), whereas in this study we used previous criteria for assessing LVH by ECG, so the results might not be representative and more studies are needed to further validate the stability and outreach of the results.
Conclusions
In this large adult-based observational study, we found that higher Lp(a) levels were significantly associated with a higher risk of developing LVH assessed by ECG in the general population, and we further confirmed the consistency of this association in some specific populations.These findings suggest that early intervention for excessive Lp(a) levels in adults and the development of prevention and treatment measures matched to high-risk populations may help prevent premature onset and excessive prevalence of LVH. Nevertheless, due to several hypothesized causative mechanisms the intervention for excessive Lp(a) levels is due to be further examined in experimental and clinical studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics of the Center for Disease Control and Prevention Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XJY, JG, ZWW and CJQ: Writing – review & editing. JG: Data curation. XJY: Data curation, Writing – original draft. XJY and ZWW: Conceptualization, Methodology, Software. FFW and CJQ: Conceptualization, Funding acquisition, Project administration, Supervision. All authors read and approved the final manuscript.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 81900453), 2020 Postdoctoral Research Funding Program of Jiangsu Province (No. 2020Z089), 2021 China Postdoctoral Science Funding Program (No. 2021M690480).
Acknowledgments
This work thank the other investigators, the staff, and the participants of the NHANES III for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yildiz M, Oktay AA, Stewart MH, Milani RV, Ventura HO, Lavie CJ. Left ventricular hypertrophy and hypertension. Prog Cardiovasc Dis (2020) 63(1):10–21. doi: 10.1016/j.pcad.2019.11.009
2. Ferdinand KC, Maraboto C. Is electrocardiography-left ventricular hypertrophy an obsolete marker for determining heart failure risk with hypertension? J Am Heart Assoc (2019) 8(8):e012457. doi: 10.1161/JAHA.119.012457
3. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Hypertension. 2018 Jun;71(6):e140-e144]. Hypertension (2018) 71(6):e13–e115. doi: 10.1161/HYP.0000000000000065
4. Bang CN, Soliman EZ, Simpson LM, Davis BR, Devereux RB, Okin PM, et al. Electrocardiographic left ventricular hypertrophy predicts cardiovascular morbidity and mortality in hypertensive patients: the ALLHAT study. Am J Hypertens (2017) 30(9):914–22. doi: 10.1093/ajh/hpx067
5. Okin PM, Hille DA, Kjeldsen SE, Devereux RB. Combining ECG criteria for left ventricular hypertrophy improves risk prediction in patients with hypertension. J Am Heart Assoc (2017) 6(11):e007564. doi: 10.1161/JAHA.117.007564
6. Lavie CJ, Patel DA, Milani RV, Ventura HO, Shah S, Gilliland Y. Impact of echocardiographic left ventricular geometry on clinical prognosis. Prog Cardiovasc Dis (2014) 57(1):3–9. doi: 10.1016/j.pcad.2014.05.003
7. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension [published correction appears in Eur Heart J. 2019 Feb 1;40(5):475]. Eur Heart J (2018) 39(33):3021–104. doi: 10.1093/eurheartj/ehy339
8. Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation (2000) 102(4):470–9. doi: 10.1161/01.cir.102.4.470
9. Gardin JM, Lauer MS. Left ventricular hypertrophy: the next treatable, silent killer? JAMA (2004) 292(19):2396–8. doi: 10.1001/jama.292.19.2396
10. Liu CW, Chen KH, Tseng CK, Chang WC, Wu YW, Hwang JJ. The dose-response effects of uric acid on the prevalence of metabolic syndrome and electrocardiographic left ventricular hypertrophy in healthy individuals. Nutr Metab Cardiovasc Dis (2019) 29(1):30–8. doi: 10.1016/j.numecd.2018.10.001
11. Duarte Lau F, Giugliano RP. Lipoprotein(a) and its significance in cardiovascular disease: A review. JAMA Cardiol (2022) 7(7):760–9. doi: 10.1001/jamacardio.2022.0987
12. Kamstrup PR. Lipoprotein(a) and cardiovascular disease. Clin Chem (2021) 67(1):154–66. doi: 10.1093/clinchem/hvaa247
13. Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA (2009) 302(4):412–23. doi: 10.1001/jama.2009.1063
14. Brandt EJ, Mani A, Spatz ES, Desai NR, Nasir K. Lipoprotein(a) levels and association with myocardial infarction and stroke in a nationally representative cross-sectional US cohort. J Clin Lipidol (2020) 14(5):695–706.e4. doi: 10.1016/j.jacl.2020.06.010
15. Santangelo G, Faggiano A, Bernardi N, Carugo S, Giammanco A, Faggiano P. Lipoprotein(a) and aortic valve stenosis: A casual or causal association? Nutr Metab Cardiovasc Dis (2022) 32(2):309–17. doi: 10.1016/j.numecd.2021.10.015
16. Ward NC, Nolde JM, Chan J, Carnagarin R, Watts GF, Schlaich MP. Lipoprotein (a) and hypertension. Curr Hypertens Rep (2021) 23(12):44. doi: 10.1007/s11906-021-01161-6
17. Garg PK, Guan W, Karger AB, Steffen BT, O'Neal W, Heckbert SR, et al. Lp(a) (Lipoprotein [a]) and risk for incident atrial fibrillation: multi-ethnic study of atherosclerosis. Circ Arrhythm Electrophysiol (2020) 13(5):e008401. doi: 10.1161/CIRCEP.120.008401
18. Dentali F, Gessi V, Marcucci R, Gianni M, Grandi AM, Franchini M. Lipoprotein(a) as a risk factor for venous thromboembolism: A systematic review and meta-analysis of the literature. Semin Thromb Hemost (2017) 43(6):614–20. doi: 10.1055/s-0036-1598002
19. Reyes-Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, et al. Lipoprotein(a): A genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: A scientific statement from the American heart association. Arterioscler Thromb Vasc Biol (2022) 42(1):e48–60. doi: 10.1161/ATV.0000000000000147
20. Liu Q, Yu Y, Xi R, Li J, Lai R, Wang T, et al. Association between lipoprotein(a) and calcific aortic valve disease: A systematic review and meta-analysis. Front Cardiovasc Med (2022) 9:877140. doi: 10.3389/fcvm.2022.877140
21. Wang ZW, Xiao SJ, Liu NF. Association of lipoprotein(a) with left ventricular hypertrophy in patients with new-onset acute myocardial infarction: A large cross-sectional study. Clin Chim Acta (2023) 540:117226. doi: 10.1016/j.cca.2023.117226
22. Kornreich F, Montague TJ, van Herpen G, Rautaharju PM, Smets P, Dramaix M. Improved prediction of left ventricular mass by regression analysis of body surface potential maps. Am J Cardiol (1990) 66(4):485–92. doi: 10.1016/0002-9149(90)90710-i
23. Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol (1987) 59(9):956–60. doi: 10.1016/0002-9149(87)91133-7
24. Rautaharju PM, Manolio TA, Siscovick D, Zhou SH, Gardin JM, Kronmal R, et al. Utility of new electrocardiographic models for left ventricular mass in older adults. The Cardiovascular Health Study Collaborative Research Group. Hypertension (1996) 28(1):8–15. doi: 10.1161/01.hyp.28.1.8
25. Rautaharju PM, LaCroix AZ, Savage DD, Haynes SG, Madans JH, Wolf HK, et al. Electrocardiographic estimate of left ventricular mass versus radiographic cardiac size and the risk of cardiovascular disease mortality in the epidemiologic follow-up study of the First National Health and Nutrition Examination Survey. Am J Cardiol (1988) 62(1):59–66. doi: 10.1016/0002-9149(88)91365-3
26. Wolf HK, Burggraf GW, Cuddy E, Milliken JA, Rautaharju PM, Smith ER, et al. Prediction of left ventricular mass from the electrocardiogram. J Electrocardiol (1991) 24(2):121–7. doi: 10.1016/0022-0736(91)90003-5
27. Rautaharju PM, MacInnis PJ, Warren JW, Wolf HK, Rykers PM, Calhoun HP. Methodology of ECG interpretation in the Dalhousie program; NOVACODE ECG classification procedures for clinical trials and population health surveys. Methods Inf Med (1990) 29(4):362–74. doi: 10.1055/s-0038-1634798
28. Rautaharju PM, Calhoun HP, Chaitman BR. NOVACODE serial ECG classification system for clinical trials and epidemiologic studies. J Electrocardiol (1992) 24 Suppl:179–87. doi: 10.1016/s0022-0736(10)80041-x
29. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens (2013) 31(7):1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc
30. Okin PM, Devereux RB, Gerdts E, Snapinn SM, Harris KE, Jern S, et al. Impact of diabetes mellitus on regression of electrocardiographic left ventricular hypertrophy and the prediction of outcome during antihypertensive therapy: the Losartan Intervention For Endpoint (LIFE) Reduction in Hypertension Study. Circulation (2006) 113(12):1588–96. doi: 10.1161/CIRCULATIONAHA.105.574822
31. Havranek EP, Froshaug DB, Emserman CD, Hanratty R, Krantz MJ, Masoudi FA, et al. Left ventricular hypertrophy and cardiovascular mortality by race and ethnicity. Am J Med (2008) 121(10):870–5. doi: 10.1016/j.amjmed.2008.05.034
32. Afify HMA, Waits GS, Ghoneum AD, Cao X, Li Y, Soliman EZ. Peguero electrocardiographic left ventricular hypertrophy criteria and risk of mortality. Front Cardiovasc Med (2018) 5:75. doi: 10.3389/fcvm.2018.00075
33. Giamouzis G, Dimos A, Xanthopoulos A, Skoularigis J, Triposkiadis F. Left ventricular hypertrophy and sudden cardiac death. Heart Fail Rev (2022) 27(2):711–24. doi: 10.1007/s10741-021-10134-5
34. Zhang Y, Zhao M, Bovet P, Xi B. Association of abdominal obesity and high blood pressure with left ventricular hypertrophy and geometric remodeling in Chinese children. Nutr Metab Cardiovasc Dis (2021) 31(1):306–13. doi: 10.1016/j.numecd.2020.09.007
35. Wang H, Zhao M, Magnussen CG, Xi B. Change in waist circumference over 2 years and the odds of left ventricular hypertrophy among Chinese children. Nutr Metab Cardiovasc Dis (2021) 31(8):2484–9. doi: 10.1016/j.numecd.2021.04.027
36. Huang XW, Deng KQ, Qin JJ, Lei F, Zhang XY, Wang WX, et al. Association between lipid profiles and left ventricular hypertrophy: new evidence from a retrospective study. Chin Med Sci J (2022) 37(2):103–17. doi: 10.24920/004066
37. Trinder M, DeCastro ML, Azizi H, Cermakova L, Jackson LM, Frohlich J, et al. Ascertainment bias in the association between elevated lipoprotein(a) and familial hypercholesterolemia. J Am Coll Cardiol (2020) 75(21):2682–93. doi: 10.1016/j.jacc.2020.03.065
38. Lamina C, Ward NC. Lipoprotein (a) and diabetes mellitus. Atherosclerosis (2022) 349:63–71. doi: 10.1016/j.atherosclerosis.2022.04.016
39. Trinder M, Paruchuri K, Haidermota S, Bernardo R, Zekavat SM, Gilliland T, et al. Repeat Measures of Lipoprotein(a) Molar Concentration and Cardiovascular Risk [published correction appears in J Am Coll Cardiol. 2022 Aug 9;80(6):651]. J Am Coll Cardiol (2022) 79(7):617–28. doi: 10.1016/j.jacc.2021.11.055
40. Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res (2008) 49(10):2230–9. doi: 10.1194/jlr.M800174-JLR200
41. Aung N, Sanghvi MM, Piechnik SK, Neubauer S, Munroe PB, Petersen SE. The effect of blood lipids on the left ventricle: A mendelian randomization study. J Am Coll Cardiol (2020) 76(21):2477–88. doi: 10.1016/j.jacc.2020.09.583
42. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol (2017) 69(6):692–711. doi: 10.1016/j.jacc.2016.11.042
43. Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res (2016) 57(8):1339–59. doi: 10.1194/jlr.R067314
44. Lip GY, Blann AD, Jones AF, Lip PL, Beevers DG. Relation of endothelium, thrombogenesis, and hemorheology in systemic hypertension to ethnicity and left ventricular hypertrophy. Am J Cardiol (1997) 80(12):1566–71. doi: 10.1016/s0002-9149(97)00749-2
45. Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE)†. Eur Heart J Cardiovasc Imaging (2015) 16(6):577–605. doi: 10.1093/ehjci/jev076
Keywords: lipoprotein(a), left ventricular hypertrophy, left ventricular mass index, cardiovascular disease, general population
Citation: Yan X, Gong J, Wang Z, Wang F and Qi C (2023) Association of lipoprotein(a) with left ventricular hypertrophy assessed by electrocardiogram in adults: a large cross-sectional study. Front. Endocrinol. 14:1260050. doi: 10.3389/fendo.2023.1260050
Received: 17 July 2023; Accepted: 27 October 2023;
Published: 30 November 2023.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Indre Ceponiene, Lithuanian University of Health Sciences, LithuaniaPanagiotis Vasileiou, National and Kapodistrian University of Athens, Greece
Alena Hrubeš Krajčoviechová, Thomayer University Hospital, Czechia
Copyright © 2023 Yan, Gong, Wang, Wang and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunjian Qi, cWljaHVuamlhbkBuam11LmVkdS5jbg==; Fangfang Wang, bGlnaHR5ZWFyd2ZmQDE2My5jb20=
†These authors have contributed equally to this work
 Xuejiao Yan1†
Xuejiao Yan1† Zhenwei Wang
Zhenwei Wang