- 1State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao, Macao SAR, China
- 2School of Public Health and Management, Guangzhou University of Chinese Medicine, Guangzhou, China
- 3School of Pharmacy, Nanjing Medical University, Nanjing, Jiangsu, China
- 4Department of Public Health and Medicinal Administration, Faculty of Health Sciences, University of Macau, Macao, Macao SAR, China
Introduction: Pharmacological therapy is recommended as a second-line alternative to reverse obesity. Currently, five anti-obesity drugs (AODs) have been approved by the U.S. Food and Drug Administration (FDA) for chronic weight management. The aim of this paper is to investigate the pharmacoeconomic evaluation of AODs through a systematic review with a special focus on methodological considerations.
Methods: We searched the general and specific databases to identify the primary pharmacoeconomic evaluation of AODs.
Results: A total of 18 full-text articles and three conference abstracts were included in this review. Most of the economic assessments were still about Orlistat. And the observations we could make were consistent with the previous systematic review. A few studies were on the combined therapies (i.e. PHEN/TPM ER and NB ER) compared to different comparators, which could hardly lead to a generalized summary of the cost-effectiveness. Most recently, pharmacoeconomic evidence on the newest GLP 1 RA approved for the indication of obesity or obesity with at least one comorbidity emerged gradually. Modelling-based cost-utility analysis is the major type of assessment method. In the modelling studies, a manageable number of the key health states and the state transitions were structured to capture the disease progression. In particular, the principal structure of the decision model adopted in the three studies on the newly approved drug was nearly the same, which enables more in-depth comparisons and generalizations of the findings.
Conclusion: This study provided an up-to-date overview of the strengths and areas for improvement in the methodological design of the pharmacoeconomic evaluation of the licensed drugs for chronic weight management. Future modelling evaluations would benefit from a better understanding of the long-term weight loss effects of the current therapeutic options and the weight rebound process after the discontinuation of treatment.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022302648, identifier CRD42022302648.
1 Introduction
The world has been experiencing an obesity crisis (1–4). According to the latest statistics of the World Health Organization, more than 1.9 billion adults (aged or older than 18 years) were overweight and around 650 million were obese. Between 1980 and 2015, a mounting prevalence of obesity was recorded at the global level (5). In the United States, more than 42% of adults were estimated to have obesity in 2018 (6). In China, the prevalence of obesity in adults was 16.4% from 2015 to 2019 according to recent national-wide nutrition surveys (7–9). The worldwide childhood and adolescent obesity issue is also worrying with consideration of its strong connection with adulthood obesity and other conditions in the long run (4, 10).
The elevated prevalence and incidence of obesity and overweight have been pressurizing the healthcare systems worldwide with complicated and serious health outcomes as well as multiplicatively unfavorable economic consequences. The linkage between obesity and overweight with increased occurrence of premature deaths, cardiovascular diseases, hypertension, type 2 diabetes, several types of cancers, as well as mental illnesses has been substantiated in various studies (5, 11–15). Besides the cosmetic concerns, undesirable health-related quality of life (HRQOL) has been consistently observed in the population with obesity (16–18). More recently, high-quality evidence was pooled to prove that the population group with obesity is vulnerable to COVID-19 in terms of incidence, morbidity and mortality, and is subject to compromised effectiveness of COVID-19 vaccines (19, 20). Financially, obesity and its related conditions lead to not only reduction and even loss of personal or family incomes, but also an increase in healthcare expenditure and other social costs (11, 21–23). Within the OECD countries, overweight and obesity were estimated to be responsible for 8% of their overall health budgets impacting 0.5%-1.6% of GDP (24).
Despite the profound implications of excessive weight, obesity remains an undertreated chronic disease and is often treated merely as a risk factor for other conditions (25–28). To reverse the trend of the obesity epidemic, both preventative and treatment interventions for weight normalization are needed (28–31). Life-style management has been prioritized for weight loss mainly by controlling energy intake from diets or boosting energy consumption with physical activities (32, 33). Bariatric surgeries are the recommended procedures for severe obesity with comorbidities owing to their proven effectiveness in sizeable weight reduction (34, 35). Pharmacological therapies are still categorized as a second-line auxiliary approach to treat obesity at designated obese stages or body mass index (BMI) levels with consideration of the occurrence of comorbidities (32, 33, 35–37).
The Food and Drug Administration (FDA) in the U.S. currently approves a handful of general anti-obesity drugs for long-term use, namely, orlistat, phentermine/topiramate extended-release (PHN/TPM ER), naltrexone/bupropion extended-release (NB ER), liraglutide (LIRA) 3.0 mg, and semaglutide (SEMA) 2.4 mg (38). In the latest network meta-analysis of the relevant randomized controlled trials, these pharmaceutical options could reduce 2.78 to 12.54% of the original weight (39) (please see details in Supplementary Table S1). Safety concerns pertaining to anti-obesity drugs (AODs), which are typified by high-profile market withdrawals due to severe adverse events of sibutramine, rimonabant, and the more recent lorcaserin, have led to more discretion in the approval of new drugs for weight loss purposes (40, 41). Orlistat (Xenical®) has been available on the market for more than 20 years and is the only one among the five long-term AODs approved by different major drug regulatory authorities including the U.S. FDA, the European Medicines Agency (EMEA), and the National Medical Products Administration (NMPA) in China. Notably, the recent discovery of novel treatment targets opened up new anticipated possibilities in pharmaceutical therapies for obesity with improved effectiveness and safety (42–44). In 2021, semaglutide 2.4 mg (Wegovy®) was approved to be on the American and European markets, which is the first drug authorized for chronic weight normalization since 2014 (38, 42, 45).
Cost-effectiveness evaluation is not only essential for pharmaceutical companies to prove the value for money of their innovative products to the regulatory authorities but also enables the manufacturers to predict the returns of their investment in a specific product (46). The pharmacoeconomic evidence on anti-obesity drugs has been emerging in several reviews which primarily focused on either pharmacologic treatment or various interventions (47, 48). Some of the drugs covered in those reviews have been de-licensed due to severe adverse events, e.g. sibutramine, rimonabant, lorcaserin while emerging studies on the cost-effectiveness of the two Glucagon-like peptide-1 Receptor Agonists (GLP-1 RAs) approved in 2014 and 2021 respectively have yet been included in any of the previous reviews. Therefore, it would be meaningful to pool the up-to-date relevant pharmacoeconomic studies together to obtain a more comprehensive overview of the currently available anti-obesity drugs for long-term use with a primary focus on the understanding of the pharmacoeconomic evaluation methods.
The aim of this paper is to investigate the published pharmacoeconomic evaluation of AODs through a systematic review with a special focus on methodological considerations. In particular, we aim to evaluate the model-based cost-effectiveness studies on their potential impact on the estimation of economic outcomes and discuss the possible structural uncertainty in the modelling approaches in the pharmacoeconomic evaluations of the drugs for chronic weight management.
2 Methods
The whole process of screening and selection of studies for inclusion according to the predefined eligibility criteria followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (49) (see Supplementary Table S2). The study protocol outlining the study design has been previously registered on the international prospective register of systematic reviews PROSPERO (reg. no. CRD42022302648).
2.1 Data sources and search strategy
The search for relevant studies was conducted in the mainstream electronic databases PubMed and EMBASE, as well as on the specific databases including ISPOR, Centre for Reviews and Dissemination (CRD) Databases (Database of Abstracts of Reviews of Effects (DARE), the National Health Service Economic Evaluation Databases (NHS EED), Health Technology Assessment Database (HTA). In addition, a snowball manual search was also performed by scanning the citation of eligible studies or relevant reviews. Both free texts and subject headings were adopted for searching the key concepts about obesity, anti-obesity drugs approved by the FDA for long-term use, as well as pharmacoeconomic evaluation. Zotero (5.0) and EndNote 9 (20.0 version) were employed for recording and managing the de-duplication and screening of articles retrieved from various sources, as well as reference management in writing the manuscript. We conducted the search on 23 January 2023 and no time limitation was set in the search. The language of studies was limited to English. See the Supplementary Table S3 for the detailed search strategies used on different databases.
2.2 Eligibility criteria
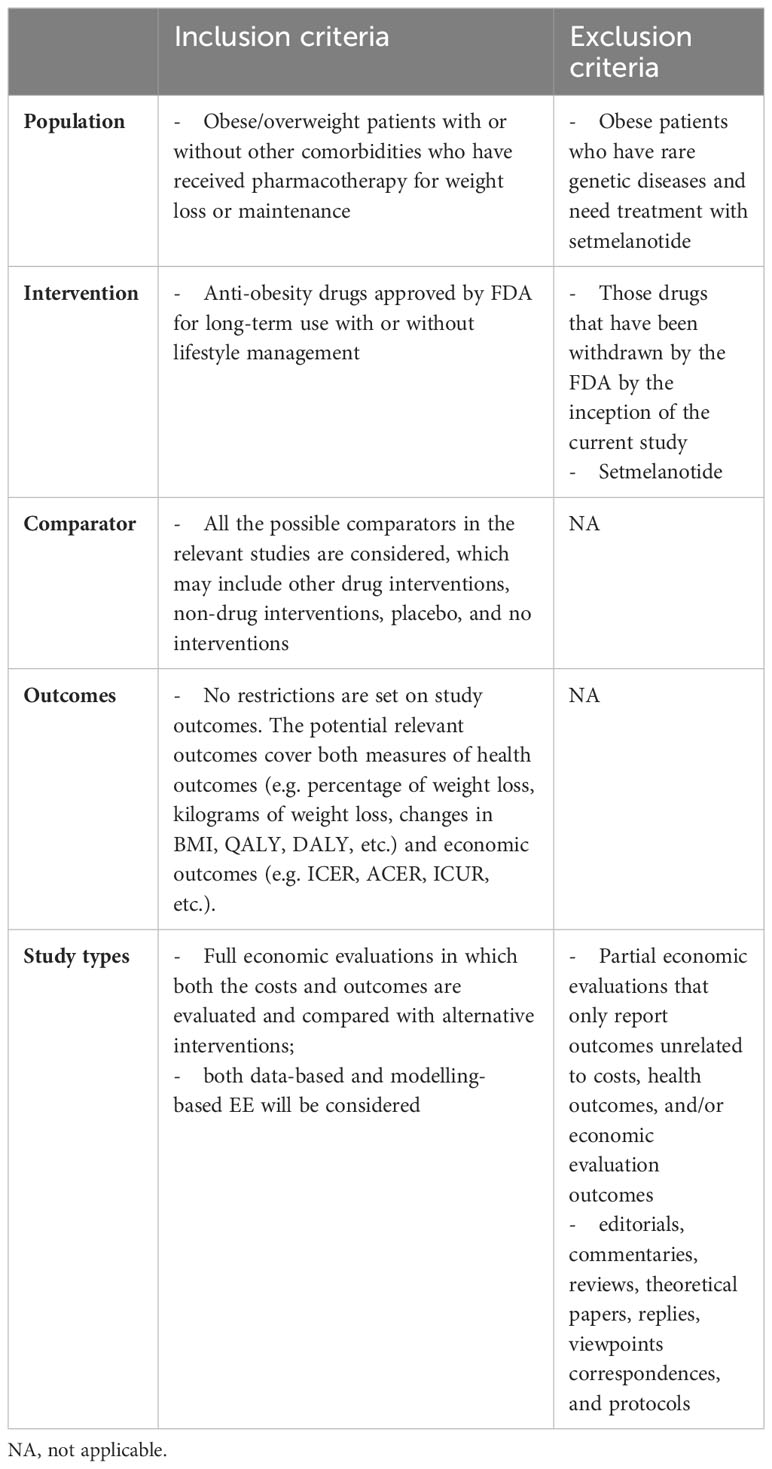
Based on our study scope and aims, the eligibility criteria were predefined as outlined in Table 1. We primarily considered the original full pharmacoeconomic evaluations on any pharmacotherapy for chronic weight management currently approved by the FDA.
2.3 Study selection
Based on the eligibility criteria, two reviewers first screened the titles and abstracts independently for initial inclusion. Then, full texts of articles considered eligible were reviewed by the two reviewers for the final inclusion. In both steps, reasons for exclusion were noted. And consensus between the two reviewers was reached over the final inclusion of studies by discussion.
2.4 Data extraction
An Excel form for data was designed and piloted by the main reviewer. The information to be extracted from the selected articles included the basic information of the study, the economic outcomes and conclusions on the cost-effectiveness, and the design of the pharmacoeconomic evaluations. The extraction of data was first performed by one reviewer, while the extracted data was later confirmed by another reviewer to ensure no omission or mistakes.
2.5 Quality assessment
The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Checklist (50) was employed for assessing the quality of the included studies on the 28 items. The full-text articles were evaluated against the 28 items with “yes” if they reported the relevant information and “no”, if not. The percentages of the studies reporting the items were calculated to obtain a general view of the completeness and quality of the studies.
3 Results
3.1 Selection of the included studies
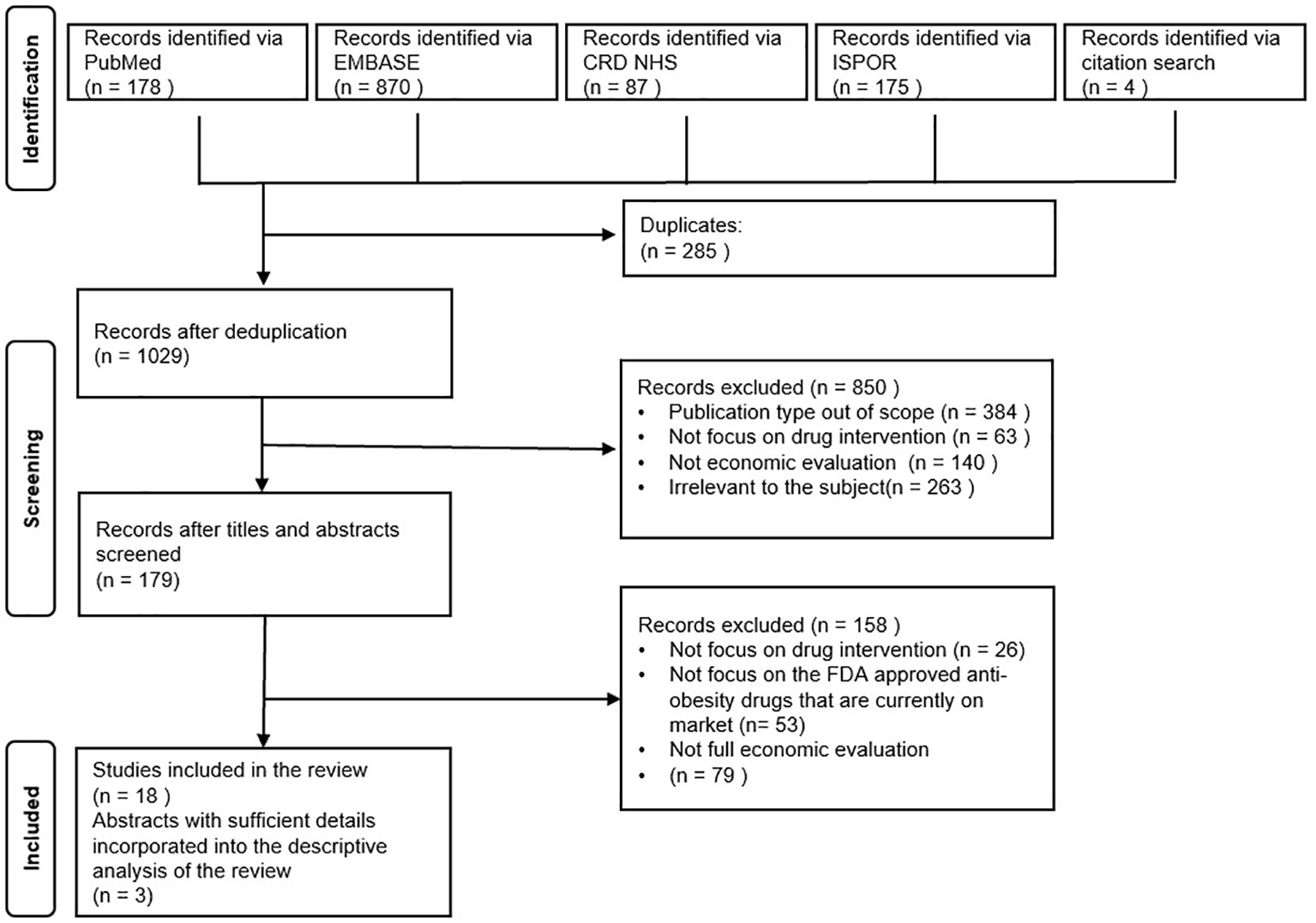
A total of 1314 titles and abstracts were obtained initially for examination of their potential relevance to the current research focus based on the preset search strategy as illustrated in the previous section. After the removal of duplications, 1029 records were found valid for further screening. With a closer study of the titles and abstracts, a total of 179 records were identified as relevant to our research questions as outlined in the PRISMA Flowchart Figure 1. Thereafter, the full-text articles were retrieved and examined, and four additional research articles were found from the core references that fit with the eligibility criteria. Finally, 18 full-text published articles were included for the systematic review. Considering that only a limited number of primary pharmacoeconomic studies on some drugs could be searched, three conference abstracts with relatively sufficient information on their methodological design were also incorporated into the synthesis of information in the current study.
3.2 Quality assessment of the included studies
The quality of the 18 full-text articles included in the review was evaluated according to the CHEERS Checklist. The percentages of the studies reporting the 28 items were calculated and presented in Supplementary Table S4. All the included studies depicted their study context and settings, the objectives of conducting the economic evaluation, interventions or strategies for investigation, the baseline characteristics, and time horizon. Moreover, the measurement and estimation of health outcomes, resources, and costs were specified in all the full-text articles. However, the explanation of the reason for selecting a particular model structure and a very detailed description of the model were only seen in 2/3 of the studies. In the report of the results, the major study parameters and the main review findings were summarized. The effect of uncertainty was also included and discussed in all the studies. The limitations and generalizability of all the full-text studies were clarified. Notably, none of the studies have included any explicit efforts to engage patients or other stakeholders who are affected by the study, which is a new focus reflected in the latest version of the CHEERS checklist. All the studies in full text either reported their funding sources or disclosed conflicts of interest. Details of the quality assessment are presented in the Supplementary Table S5.
3.3 Descriptive characteristics of the included studies
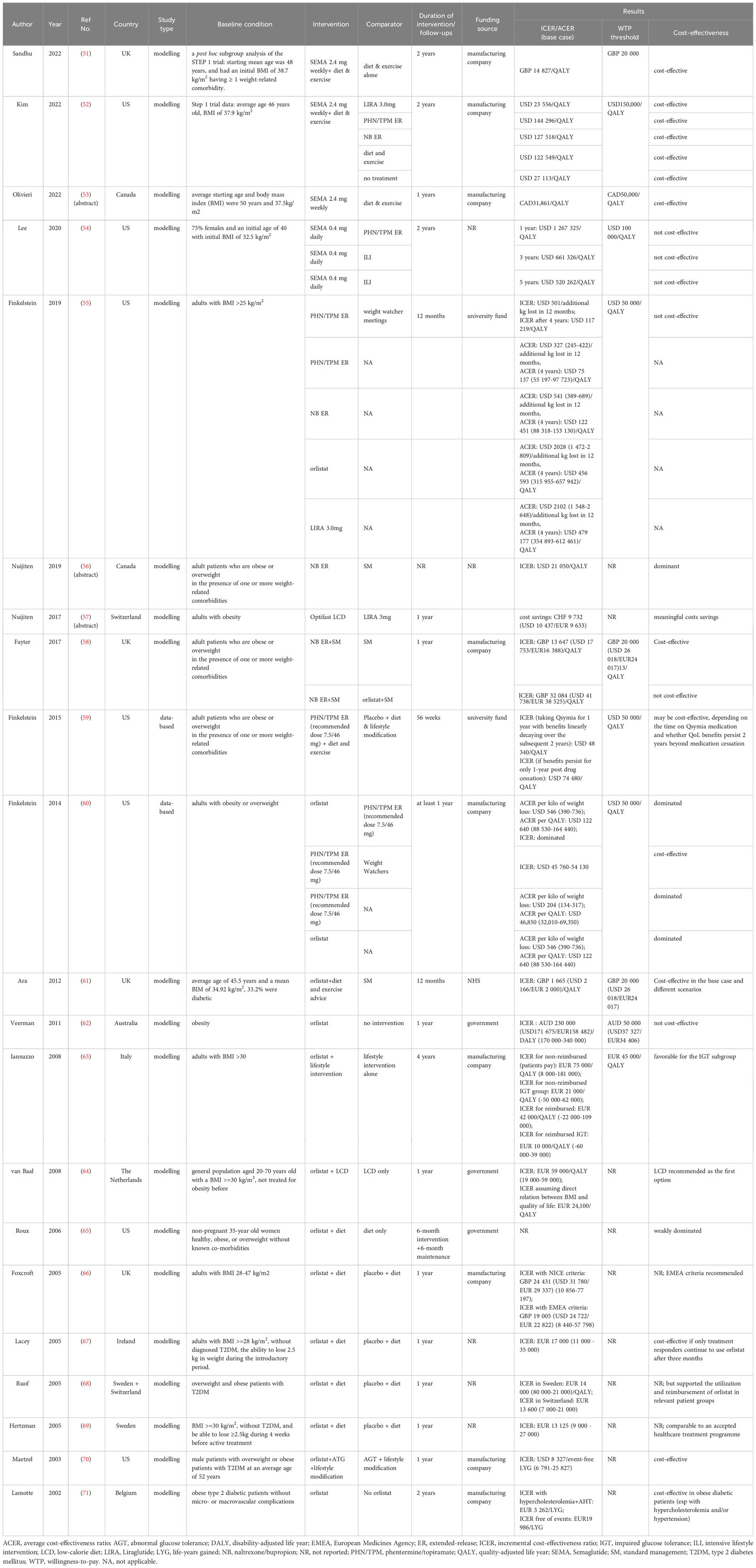
The general characteristics of the included studies are presented in Table 2. Most of the studies were conducted in the UK and the European settings (51, 57, 58, 61, 63, 64, 66–69, 71), while 10 other studies were conducted in the US, Canada, or Australia (52–56, 59, 60, 62, 65, 70). One study analyzed the cost-effectiveness of AODs in more than one country (68). 14 studies examined the costs and benefits of orlistat as an adjunct intervention to either lifestyle interventions or dietary programs relative to other interventions, placebo or no treatment (55, 58, 60–71). The cost-effectiveness of phentermine/topiramate ER (PHN/TPM ER) was evaluated in five studies (52, 54, 55, 59, 60). Three studies examined naltrexone/bupropion ER (NB ER) (55, 56, 58), three examined liraglutide (LIRA) 3.0 mg (52, 55, 57). Regarding the latest approved semaglutide (SEMA), one study in 2020 examined semaglutide (SEMA) 0.4 mg (54), while the three most recent studies investigated the cost-effectiveness of the regimen in the approved dosage 2.4mg (51–53). Moreover, five studies performed comparisons between various approved AODs (52, 54, 55, 58, 60). The treatment duration either modelled or implemented in most studies normally lasted for around one year.
Most of the included studies are modelling-based (51–58, 61–71), which aimed to estimate the outcomes of weight reduction beyond the treatment duration by building mathematical models. And the baseline characteristics of the target population in these studies included the following categories: 1) obesity population with/without comorbidities, 2) overweight population with at least one obesity-related comorbidities, and 3) both conditions. Two studies exclusively focused on a gender-specific obesity group (65, 70).
All the studies with full-text research articles either disclosed the funding sources or the conflicts of interest, or both. Except for four funded by the government (61, 62, 64, 65), all the other studies involved the relevant pharmaceutical companies (e.g. Roche, Novo Nordisk, Vivus, etc.) in various forms.
Studies revealed that the general cost-effectiveness picture of the four anti-obesity drugs approved earlier for long-term use (i.e. orlistat, PHN/TPM ER, NB ER, LIRA 3.0mg) was not desirable. The cost-effectiveness of Orlistat varies largely in countries. For example, the model-based estimation of cost-effectiveness in the UK indicated that orlistat was cost-effective in the base case with an ICER of GBP 1 665 (USD 2 166/EUR 2 000) relative to placebo (61). However, in the Australian health care setting in 2003, orlistat was found to be not cost-effective with the ICER of AUD 230 000 (171 675 USD/158 482 EUR) per DALY (95% CI: 170 000 – 340 0000) in the base case in any of the costing scenarios (62). In addition, in the studies on the cost-effectiveness of orlistat, a range of cost-effectiveness thresholds was employed in the probabilistic sensitivity analysis to evaluate the impact of this threshold on the probability of cost-effectiveness of this intervention investigated. In terms of PHN/TPM ER, the ICER in a data-based CEA study turned out to be slightly below the WTP threshold of USD 50 000 per QALY, only if the benefit of the one-year treatment could be sustained for the following two years after drug cessation (59). And in another data-based study on PHN/TPM ER, the ICER was found to be at USD 54 130 per QALY and the average cost-effectiveness ratio (ACER) at USD 46 850 (32 010–69 350) per QALY with an assumed WTP threshold of USD 50 000 (60). In a more recent study, the ICER of PHN/TPM ER relative to a lifestyle management program called Weight Watcher was found to be as high as USD 117 219 per QALY (55). In the last two studies mentioned above, the ACERs of other pharmaceutical treatments including orlistat, NB ER, and LIRA 3.0mg were considerably higher than the commonly accepted WTP threshold of USD 50 000 (55, 60). Furthermore, the selection of different comparators led to different conclusions on the cost-effectiveness of NB ER. For instance, the two CEA studies of NB ER conducted in the health care setting of Canada and the UK respectively reported NB ER to be a cost-effective weight loss option relative to standard weight management for long-term use (56) and even in a lifetime horizon (58). However, in the later study, this combination therapy was found to be not cost-effective relative to orlistat (58).
Notably, the latest three studies on SEMA with the approved dosage at 2.4mg conducted in different settings converged on the conclusions about the cost-effectiveness of this newest anti-obesity drug approved by the major drug authorities. From the UK National Health Service (NHS) and Personal Social Services perspectives, the SEMA 2.4mg injection could benefit the population with obesity and relevant comorbidities with an ICER of GBP 14 827 per QALY relative to the treatment of diet and exercise alone (51). And a series of sensitivity analyses proved the robustness of its cost-effectiveness in different scenarios under the prespecified willingness-to-pay (WTP) threshold as GBP 20 000 per QALY. In a setting of US third-party payer, this newly approved therapy also showed its cost-effectiveness against all the selected comparators including three branded AOMs under the WTP threshold of USD 150 000 per QALY (52). In another assessment of the cost-effectiveness of SEMA 2.4mg injection in a Canadian setting, the therapy showed a favorable ICER at CAD31 861 per QALY when compared with diet and exercise under the WTP threshold suggested in the relevant Canadian Guidelines (CADTH) (53). However, in an earlier CEA study on SEMA 0.4mg administered per day from the US healthcare perspective, the ICER of the same therapy option given in a daily pattern with favorable weight loss effects was found to be not cost-effective in all the projected time horizons (54).
3.4 Analysis of the pharmacoeconomic evaluation methods
3.4.1 Types of cost-effectiveness analysis
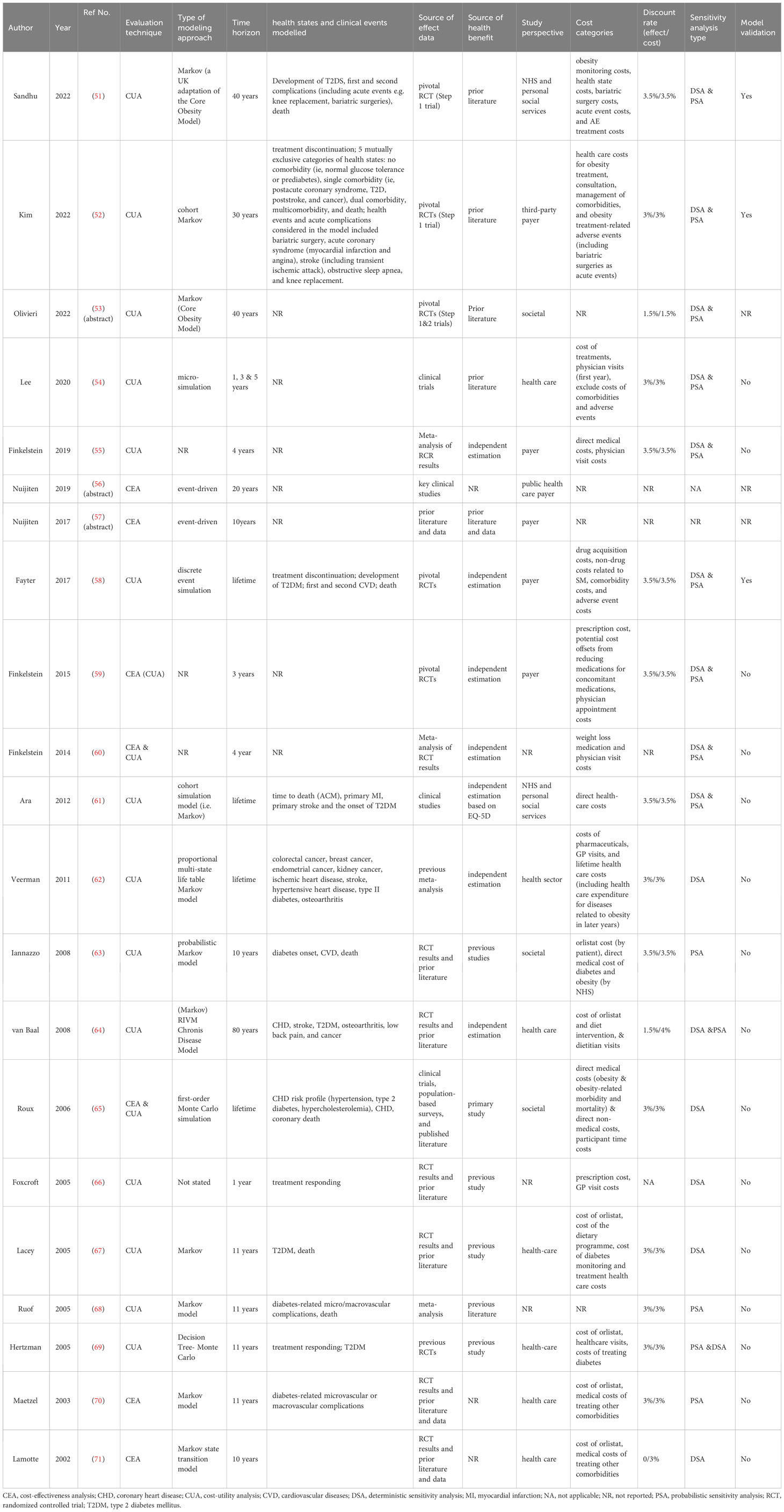
As summarized in Table 3, cost-utility analysis (CUA) is the major type of assessment method among all the included studies, with quality-adjusted life year (QALY) as a proxy of the health outcome (51–58, 61–64, 66–69). There was one study conducted in the Australian setting that used disability-adjusted life year (DALY) as a measure of health loss (62). A few studies undertook both CUA and cost-effectiveness analysis (CEA) with QALYs and kilograms of weight reduction as the measure of health outcome, respectively (59, 60, 65). In addition, two early studies only adopted event-free life years gained (LYG) as the measure of health benefit (70, 71). No cost-benefit analysis or cost-minimization analysis was observed in the economic evaluations of pharmacologic treatment for obesity.
3.4.2 Decision analytic approaches
Various decision- analytic approaches were observed in the modelling-based studies. Cohort-based Markov model was commonly applied to conceptualize a series of health states in relation to obesity and transitions between the states in most of the studies (51–53, 61–64, 67, 68, 70, 71). In particular, the latest publications on the SEMA 2.4mg adopted the Core Obesity Model with adaptations to various extents, which is indeed a typical Markov structure (51–53). The individual-based state-transition Monte Carlo simulation was also employed by modelling different patient characteristics with multiple runs in the model cycle representing the state changes in a few of the studies (54, 65, 69). In addition, the event-driven simulation was used in three studies to capture the complex disease course of obesity (56–58). The modelled health states or events include discontinuation of treatment, and occurrence of obesity-related events (e.g. type 2 diabetes, primary and secondary cardiovascular events, death). 10 of the studies provided a justification for selecting a particular model and a relatively detailed account of the decision model structure (51, 52, 58, 61–63, 65, 68, 70, 71). Moreover, the explicit model validation procedure was only mentioned briefly in two of the latest investigations on SEMA 2.4mg (51, 52).
3.4.3 Perspective of the evaluation and cost categories
Most of the included studies specified their evaluation perspectives. The selection of cost categories also differs according to the study perspectives. Eight of the studies adopted a health-care perspective, involving the costs of the anti-obesity drugs, direct medical costs of treating obesity-related conditions, health care costs, and even the costs of the dietary programs (54, 56, 62, 64, 67, 69–71). The payer perspective was undertaken in eight of the studies, which mainly considered the costs of interventions and physician visit costs, and other medication costs for reducing obesity-related conditions (51, 52, 55–59, 61). Three studies performed their evaluation from a societal perspective (53, 63, 65).
3.4.4 Time horizon projected and discounting
The two data-based studies focus on the outcomes within the one-year treatment period, no discounting was performed as unnecessary (59, 60). The modelling-based studies adopted various time horizons, among which five stretched the evaluation to a lifetime or around (58, 61, 62, 64, 65), three projected the outcomes in a period of 30 or 40 years (51–53), eight selected a time horizon between 10-20 years (56, 57, 63, 67–71), while the rest used a short-term time horizon no more than five years (54, 55, 66). Correspondingly, for the modelling-based evaluation with more than a one-year time horizon, discount rates that followed the guideline or consensus in a specific country or setting were applied to future effects and costs in most of the studies (51–55, 58, 59, 61–65, 67–71). Moreover, time horizon and discount rates were estimated at values different from the base case in the sensitivity analysis to investigate the parameter uncertainty in some of the studies (51, 52, 54, 58, 59, 61, 65, 67, 68, 70, 71).
3.4.5 Sources of evidence and estimation of outcomes
Most of the studies reported the sources of data about effectiveness and health utility. The extrapolation of effectiveness (e.g. discontinuation data, rate of responders, mean change in body weight, risk of obesity-related sequelae, and adverse events) was mainly derived from the pivotal large-scale randomized control trials or meta-analysis (51–56, 58–71).
The valuing of health-related utility (i.e. QALY or DALY) in some of the studies was directly informed by published literature (51–54, 56, 57, 63, 65–69). Independent computation of health utilities was also found in several studies by transforming the effectiveness data into QALY or DALY with the aid of established algorithms (55, 58, 62, 64).
3.4.6 Sensitivity and uncertainty analysis
Sensitivity analysis was carried out in all the studies in full text to check the robustness of the base case estimates. More than half of these studies performed both deterministic and probabilistic sensitivity analyses (51–55, 58–61, 64, 69). The covariates in the sensitivity analysis of these 18 studies fell into the following categories, namely, baseline characteristics, efficacy of interventions in comparison, natural weight increase rate, duration of weight loss benefit decay, occurrence of obesity-related conditions, costs and discount rates, valuation of health utility, and so on. However, there was no consistent inclusion of covariates among these studies. In addition, in many studies, authors solely listed specific variables or scenarios for analysis without giving detailed justification for selecting a specific parameter for the sensitivity analysis in advance. Among the evaluations on orlistat that were performed in the early 2000s, five of the studies only conducted a series of univariate sensitivity analyses by variating one of the input parameters each time (62, 65–67, 71), while the other three studies only performed probabilistic sensitivity analysis (PSA) with results displayed in scatter plots, cost-effectiveness/utility curves as well as planes as a measure of uncertainty (63, 68, 70).
4 Discussion
The current review comprehensively consolidated the pharmacoeconomic evidence relevant to drug options for long-term weight control. Different from the previous reviews on similar topics, the primary focus of this study rests on the methodological design of the pharmacoeconomic evaluations in the synthesis and analysis of the included studies.
Our predefined search strategy and selection process enabled us to access the relevant and up-to-date studies, of which the interventions cover all the five currently available anti-obesity drugs approved by the FDA. In general, these five AODs work on various peripheral and central pathways to regulate energy intake, suppress appetite, or increase fullness (72). Orlistat is an agent acting via peripheral pathway. It acts as an inhibitor of gastrointestinal and pancreatic lipase by preventing the catalysis of hydrolyzing triglycerides. Therefore, free fatty acids are not absorbed by the intestinal endothelium (45). Phentermine is a sympathomimetic amine anorectic acting as a norepinephrine agonist in the central nervous system, thus, decreasing the appetite. Its common anticonvulsant, topiramate, which is a gamma-aminobutyric acid agonist, glutamate antagonist and carbonic anhydrase inhibitor, shows several potential mechanisms of topiramate on weight loss (73). However, the clear mechanism of action of the combination therapy of PHN/TPM ER still awaits confirmation in animal and human studies (45, 74). NB ER is another combination therapy for long-term weight management that makes use of the synergistic effect of two distinct agents. Naltrexone originally is an opioid receptor antagonist, while bupropion a dopamine and norepinephrine reuptake inhibitor. In the hypothalamus, bupropion enhances the effects of pro-opiomelanocortin (POMC) cells in producing melanocyte-stimulating hormone (alpha-MSH) and beta-endorphin. The alpha-MSH activates melanocortin-4 receptor; which can decrease suppress appetite, and increase energy expenditure and weight loss. Naltrexone blocks mu-opioid receptor, so preventing the inhibitory feedback from beta-endorphin on POMC cells. Therefore, bupropion and naltrexone work complementarily to reduce bodyweight (45, 74). Lastly, both LIRA and SEMA are analogs of human glucagon like peptide (GLP-1) and act as GLP-1 receptor agonist. They stimulate pancreas to release insulin, which can regulate glucose concentration to reach euglycemia. They also inhibit the secretion of glucagon which triggers glycogenolysis and gluconeogenesis. In this approach, appetite and digestion are suppressed, thus calorie intake is reduced (45, 74, 75). Interestingly, the dose-dependent weight reduction effect of four among these five approved AODs (except Orlistat) was observed in the exploration of multiple sites of action and mechanisms of the therapeutic agent(s) involved, which were originally for other pathophysiological conditions (45, 75). This development process of these critical weight loss therapies benefits from the recent advances in the understanding of the pathophysiology of obesity as a complex disease and the metabolic processes (74).
The cost-effectiveness of the four AOMs before the approval of SEMA 2.4mg was not favorable for market access in general. As orlistat has been the only pharmacotherapy option on the market for around two decades, more than half of the studies included in our review evaluated the cost-effectiveness of this AOD either as the primary intervention or as a comparator. Patients in overweight or obesity who are with or without diabetes were observed in these studies. And the evaluations were conducted in various countries from different perspectives. Both cohort-based Markov model and patient-based Monte Carlo simulations were adopted in the modelling construction. Although the generalizability of these evaluations was undesirable, it is observed that the models have evolved to capture a relatively more complex disease progression course. Specifically, the early studies adopted a shorter time horizon, while the more recent studies made efforts to extrapolate the weight loss effects to the long term by incorporating the occurrence risks of complications such as type 2 diabetes and cardiovascular events. The economic evaluations focusing on LIRA 3.0mg, NB ER and PHN/TPM ER were relatively insufficient, which makes comparisons across challenging. By contrast, the latest studies on the SEMA 2.4 mg sponsored by the manufacturer seem to alter this situation. Although the three evaluations included were conducted in different settings, the cost-effectiveness of this newly approved GLP-1 drug for long-term weight management based on the Core Obesity Model was consistently promising.
The five licensed AODs for long-term weight reduction identified in this study have been approved in North America, and four of them except NB ER are available in the European markets. This scenario is probably the main reason that nearly all the included economic evaluations were conducted in countries from these regions. There was one study that was carried out in Australia, where orlistat, phentermine, and liraglutide are officially available for weight reduction. No pharmacoeconomic evidence was generated from a Chinese setting, as orlistat has been the only approved pharmaceutical option for weight reduction in China for a long time. The emerging novel drug targets for weight loss have attracted domestic pharmaceuticals and research teams. To facilitate the research and development of AODs, the Technical Guiding Principles on the Clinical Trials of Weight Management Drugs was enacted in 2021 by the Center for Drug Evaluation (CDE) of the NMPA as a move at the institutional level to combat obesity.
As modelling-based economic evaluations are relatively less time- and money-consuming, the majority of the included studies constructed a mathematical model to calculate the possible costs and health outcomes of the intervention of interest. Two evaluations were data-based (59, 60), and one of them was a typical piggyback study alongside the phase III clinical trial on Qsymia (59). State-transition decision analytical approaches including the Markov model and microsimulation were predominantly adopted in most of the studies on the AOMs approved earlier. In these models, a manageable number of the key health states and the state transitions were structured to capture the disease progression. One of the key model assumptions found in these studies was primarily about the length of weight loss decay. Studies proved the sensitivity of effect persistence in the model by assuming either a longer or shorter course of weight regain in the sensitivity analysis than in the base case scenario (51, 52, 54, 55, 58–62, 67, 69, 70). Naturally, the longer the weight loss sustained after the treatment cessation, the better benefit was observed. And in the modelling of these studies, the decay process of the weight loss effect was normally assumed linearly after the treatment cessation. Moreover, adverse events associated with the pharmacologic treatments were seldom explicitly incorporated into the models in the included studies. The Core Obesity Model was the only one that was applied in different studies on the same AOM SEMA 2.4 mg (51–53). It also follows a Markov model structure, which aims to reflect the natural disease course in a real-world setting by incorporating a series of obesity-related comorbidities including the occurrence of pre-diabetes evidenced in literature or pivotal clinical studies (76). The uncertainty of the modelling evaluations on AODs would be mitigated to a greater extent if more solid evidence could be achieved in the understanding of the weight rebound process after the discontinuation of treatment.
The major evaluation technique adopted in the included studies was cost-utility evaluation. Quality of life has been proved to be negatively associated with the BMI value, so in these CUA studies, quality-adjusted-life-years (QALY) gained per weight loss effect and disability-adjusted-life-years (DALY) were used as the surrogates of health utility. The methods employed in the estimation of health utilities include direct elicitation (55, 62, 64), indirect measurement with self-reported questionnaires such as EQ-5D and SF-12 v2 (58–61), as well as extraction of reference value from previous literature (51–54, 56, 57, 63, 65–69). Although using QALYs aims to facilitate the comparison across studies, the health utility values associated with one unit reduction in BMI were found to differ considerably in these studies. The comparability between studies on the cost-effectiveness of AODs would be improved if a more in-depth understanding of the linkage between quality of life, weight reduction effect, adverse events, and side effects could be obtained through clinical and real-world studies, and correspondingly better measurement of utility value could be performed.
All the included studies made efforts to examine the uncertainty through various sensitivity analyses, which constituted the good practice of reporting (77). Future studies could provide proper justifications on the selection of parameters or inputs as the covariates in the sensitivity analysis with evidence-based consideration of the nature of the disease and statistical significance. The transparency of the model structure, parameter values, and key assumptions in the included studies were found to be improved in more recent studies to facilitate stakeholders or decision-makers to obtain a fuller understanding of the generation of evaluation results from the models (78). On the other hand, the included studies except the recent two (51, 52) commonly were lack of explicit validation procedures to check the accuracy of the model.
Despite the effort, we managed to make, the current review still has some limitations. Firstly, as we only focused on the published studies, it is very likely that pharmacoeconomic evaluations not yet accessible to the public in any form were missed. Secondly, the heterogeneity in the methodological design of the included studies made the synthesis of information challenging. Thirdly, as inherent in the currently available evaluations, it would be difficult to make a judgment about the prediction of long-term weight loss effects and their impact on morbidity and mortality without the presence of long-term large-scale clinical trials and real-world observational studies.
5 Conclusions
This systematic review rendered a comprehensive and updated analysis of strengths and areas for improvement in the methodological design and quality of the pharmacoeconomic evaluations on the currently licensed drugs for chronic weight management. Recent CEA studies on the new-generation AOD licensed for long-term weight management indicated its great potential to better meet the clinical and market needs. More in-depth understanding of obesity and its natural trajectory as well as solid data on the long-term effectiveness and safety of AODs from future studies would facilitate the generation of pharmacoeconomic evidence with enhanced quality.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YX: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. HZ: Data curation, Formal analysis, Writing – review & editing. ZR: Investigation, Writing – review & editing. XC: Writing – review & editing. YL: Writing – review & editing. DY: Writing – review & editing. CU: Conceptualization, Writing – review & editing. HH: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
This research is supported by the fundings of the University of Macau (MYRG2020-00230-ICMS) and The Science and Technology Development Fund, Macao SAR (001/2023/ALC).
Acknowledgments
We would like to express our gratitude to Dr Menghuan Song for her technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1254398/full#supplementary-material
References
1. Malik VS, Willet WC, Hu FB. Nearly a decade on — trends, risk factors and policy implications in global obesity. Nat Rev Endocrinol (2020) 16(11):615–6. doi: 10.1038/s41574-020-00411-y
2. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol (2013) 9(1):13–27. doi: 10.1038/nrendo.2012.199
3. McPherson K. Reducing the global prevalence of overweight and obesity. Lancet (2014) 384(9945):728–30. doi: 10.1016/S0140-6736(14)60767-4
4. The GBD 2013 Obesity Collaboration, Ng M, Fleming T, Robinson M, Blake Thomson B, Graetz N, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (2014) 384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8
5. The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med (2017) 377(1):13–27. doi: 10.1056/NEJMoa1614362
6. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief No 360 (2020) 360):8. Available at: https://www.cdc.gov/nchs/products/databriefs/db360.htm#fig1.
7. Nie P, Ding L, Sousa-Poza A. Decomposing adult obesity trends in China (1991–2011). Econ& Hum Biol (2019) 34:5–15. doi: 10.1016/j.ehb.2019.02.001
8. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol (2021) 9(6):373–92. doi: 10.1016/S2213-8587(21)00045-0
9. Zhang J, Wang H, Wang Z, Du W, Su C, Zhang J, et al. Prevalence and stabilizing trends in overweight and obesity among children and adolescents in China, 2011-2015. BMC Public Health (2018) 18(1):571. doi: 10.1186/s12889-018-5483-9
10. Zhang L, Wang Z, Wang X, Chen Z, Shao L, Tian Y, et al. Prevalence of overweight and obesity in China: Results from a cross-sectional study of 441 thousand adults, 2012–2015. Obes Res Clin Pract (2020) 14(2):119–26. doi: 10.1016/j.orcp.2020.02.005
11. Chu DT, Minh Nguyet NT, Dinh TC, Thai Lien NV, Nguyene KH, Nhu Ngoc VT, et al. An update on physical health and economic consequences of overweight and obesity. Diabetes Metab Syndr: Clin Res Rev (2018) 12(6):1095–100. doi: 10.1016/j.dsx.2018.05.004
12. Yu S, Xing L, Du Z, Tian Y, Jing L, Yan H, et al. Prevalence of obesity and associated risk factors and cardiometabolic comorbidities in rural Northeast China. BioMed Res Int (2019) 2019:1–9. doi: 10.1155/2019/6509083
13. Song N, Liu F, Han M, Zhao Q, Zhao Q, Zhai H, et al. Prevalence of overweight and obesity and associated risk factors among adult residents of northwest China: a cross-sectional study. BMJ Open (2019) 9(9):e028131. doi: 10.1136/bmjopen-2018-028131
14. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med (2017) 376(3):254–66. doi: 10.1056/NEJMra1514009
15. Su W, Huang J, Chen F, Iacobucci W, Mocarski M, Dall TM, et al. Modeling the clinical and economic implications of obesity using microsimulation. J Med Econ (2015) 18(11):886–97. doi: 10.3111/13696998.2015.1058805
16. Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev (2001) 2(3):173–82. doi: 10.1046/j.1467-789x.2001.00032.x
17. Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life: Quality of life systematic review of reviews. Clin Obes (2017) 7(5):273–89. doi: 10.1111/cob.12203
18. Park S. Pathways linking obesity to health-related quality of life. Qual Life Res (2017) 26(8):2209–18. doi: 10.1007/s11136-017-1565-x
19. Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: A systematic review. Diabetes Metab Syndr: Clin Res Rev (2020) 14(4):655–9. doi: 10.1016/j.dsx.2020.05.020
20. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev (2020) 21(11):e13128. doi: 10.1111/obr.13128
21. Tremmel M, Gerdtham UG, Nilsson P, Saha S. Economic burden of obesity: A systematic literature review. Int J Environ Res Public Health (2017) 14(4):435. doi: 10.3390/ijerph14040435
22. Hamilton D, Dee A, Perry IJ. The lifetime costs of overweight and obesity in childhood and adolescence: a systematic review: Lifetime costs of childhood obesity. Obes Rev (2018) 19(4):452–63. doi: 10.1111/obr.12649
23. Grieve E, Fenwick E, Yang HC, Lean M. The disproportionate economic burden associated with severe and complicated obesity: a systematic review: Economic burden of severe obesity. Obes Rev (2013) 14(11):883–94. doi: 10.1111/obr.12059
24. Vuik S, Lerouge A, Guillemette Y, Feigl A, Aldea A. The economic burden of obesity. In: The Heavy Burden of Obesity. OECD Health Policy Studies. Paris(FR):OECD Publishing (2019). p. 74–100. doi: 10.1787/6cc2aacc-en
25. Tucker S, Bramante C, Conroy M, Fitch A, Gilden A, Wittleder S, et al. The most undertreated chronic disease: addressing obesity in primary care settings. Curr Obes Rep (2021) 10(3):396–408. doi: 10.1007/s13679-021-00444-y
26. Zeng Q, Li N, Pan XF, Chen L, Pan A. Clinical management and treatment of obesity in China. Lancet Diabetes Endocrinol (2021) 9(6):393–405. doi: 10.1016/S2213-8587(21)00047-4
27. Burki T. European Commission classifies obesity as a chronic disease. Lancet Diabetes Endocrinoly (2021) 9(7):418. doi: 10.1016/S2213-8587(21)00145-5
28. Xue Y, Ruan Z, Ung CO, Lai Y, Hu H. Policy analysis of system responses to addressing and reversing the obesity trend in China: a documentary research. BMC Public Health (2023) 23(1):1198. doi: 10.1186/s12889-023-15890-7
29. Twells LK, Harris Walsh K, Blackmore A, Adey T, Donnan J, Peddle J, et al. Nonsurgical weight loss interventions: A systematic review of systematic reviews and meta-analyses. Obes Rev (2021) 22(11):e13320. doi: 10.1111/obr.13320
30. Bray GA, Frühbeck G, Ryan DH, Wilding JPH. Management of obesity. Lancet (2016) 387(10031):1947–56. doi: 10.1016/S0140-6736(16)00271-3
31. Semlitsch T, Stigler FL, Jeitler K, Horvath K, Siebenhofer A. Management of overweight and obesity in primary care—A systematic overview of international evidence-based guidelines. Obes Rev (2019) 20(9):1218–30. doi: 10.1111/obr.12889
32. Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, et al. Obesity in adults: a clinical practice guideline. CMAJ (2020) 192(31):E875–91. doi: 10.1503/cmaj.191707
33. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European guidelines for obesity management in adults. Obes Facts (2015) 8(6):402–24. doi: 10.1159/000442721
34. Avenell A, Robertson C, Skea Z, Jacobsen E, Boyers D, Cooper D, et al. Bariatric surgery, lifestyle interventions and orlistat for severe obesity: the REBALANCE mixed-methods systematic review and economic evaluation. Health Technol Assess (2018) 22(68):1–246. doi: 10.3310/hta22680
35. Schneider R, Kraljević M, Peterli R, Rohm TV, Klasen JM, Cavelti-Weder C, et al. GLP-1 analogues as a complementary therapy in patients after metabolic surgery: a systematic review and qualitative synthesis. Obes Surg (2020) 30(9):3561–9. doi: 10.1007/s11695-020-04750-7
36. Anekwe CV, Knight MG, Seetharaman S, Dutton WP, Chhabria SM, Stanford FC. Pharmacotherapeutic options for weight regain after bariatric surgery. Curr Treat Options Gastro (2021) 19(3):524–41. doi: 10.1007/s11938-021-00358-7
37. Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2015) 100(2):342–62. doi: 10.1210/jc.2014-3415
38. Singh G, Krauthamer M, Bjalme-Evans M. Wegovy (semaglutide): a new weight loss drug for chronic weight management. J Investig Med (2022) 70(1):5–13. doi: 10.1136/jim-2021-001952
39. Shi Q, Wang Y, Hao Q, Vandvik PO, Guyatt G, Li J, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Lancet (2021) 399(10321):259–69. doi: 10.1016/S0140-6736(21)01640-8. S0140673621016408.
40. Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: A systematic review and meta-analysis. JAMA (2016) 315(22):2424. doi: 10.1001/jama.2016.7602
41. Zhang L, Liu Z, Liao S, He H, Zhang M. Cardiovascular safety of long-term anti-obesity drugs in subjects with overweight or obesity: a systematic review and meta-analysis. Eur J Clin Pharmacol (2021) 77(11):1611–21. doi: 10.1007/s00228-021-03160-7
42. Ryan DH. Semaglutide for obesity: four STEPs forward, but more to come. Lancet Diabetes Endocrinol (2021) 9(5):252–4. doi: 10.1016/S2213-8587(21)00081-4
43. Rosenstock J, Wysham C, Kaneko S, Lee CJ, Fernández Landó L, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet (2021) 398(10295):143–55. doi: 10.1016/S0140-6736(21)01324-6
44. Angelidi AM, Belanger MJ, Kokkinos A, Koliaki CC, Mantzoros CS. Novel noninvasive approaches to the treatment of obesity: from pharmacotherapy to gene therapy. Endocr Rev (2021) 43(3):507–57. doi: 10.1210/endrev/bnab034
45. Tak YJ, Lee SY. Long-term efficacy and safety of anti-obesity treatment: where do we stand? Curr Obes Rep (2021) 10(1):14–30. doi: 10.1007/s13679-020-00422-w
46. Dimasi JA, Caglarcan E, Wood-Armany M. Emerging role of pharmacoeconomics in the research and development decision-making process. Pharmacoeconomics (2001) 19(7):753–66. doi: 10.2165/00019053-200119070-00004
47. Boyers D, Avenell A, Stewart F, Robertson C, Archibald D, Douglas F, et al. A systematic review of the cost-effectiveness of non-surgical obesity interventions in men. Obes Res Clin Pract (2015) 9(4):310–27. doi: 10.1016/j.orcp.2015.03.001
48. Neovius M, Narbro K. Cost-effectiveness of pharmacological anti-obesity treatments: a systematic review. Int J Obes (2008) 32(12):1752–63. doi: 10.1038/ijo.2008.189
49. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021), n71. doi: 10.1136/bmj.n71
50. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated health economic evaluation reporting standards (CHEERS) 2022 explanation and elaboration: A report of the ISPOR CHEERS II good practices task force. Value Health (2022) 25(1):10–31. doi: 10.1016/j.jval.2021.10.008
51. Sandhu H, Xu W, Olivieri AV, Lübker C, Smith I, Antavalis V. Once-weekly subcutaneous semaglutide 2.4 mg injection is cost-effective for weight management in the United Kingdom. Adv Ther (2023). 40(3):1282–91. doi: 10.1007/s12325-022-02423-8
52. Kim N, Wang J, Burudpakdee C, Song Y, Ramasamy A, Xie Y, et al. Cost-effectiveness analysis of semaglutide 2.4 mg for the treatment of adult patients with overweight and obesity in the United States. J Manag Care Spec Pharm (2022) 28(7):740–52. doi: 10.18553/jmcp.2022.28.7.740
53. Olivieri A, Larsen S, Luckevich M, Chan K, Lamotte M. EE464 the cost-effectiveness of subcutaneous semaglutide 2.4MG injection in the management of obesity in Canada using the core obesity model. Value Health (2022) 25(7):S426. doi: 10.1016/j.jval.2022.04.712
54. Lee M, Lauren BN, Zhan T, Choi J, Klebanoff M, Abu Dayyeh B, et al. The cost-effectiveness of pharmacotherapy and lifestyle intervention in the treatment of obesity. Obes Sci Pract (2020) 6(2):162–70. doi: 10.1002/osp4.390
55. Finkelstein EA, Verghese NR. Incremental cost-effectiveness of evidence-based non-surgical weight loss strategies. Clin Obes (2019) 9(2):e12294. doi: 10.1111/cob.12294
56. Nuijten M, Haig J, Barbeau M. PDB14 cost-effectiveness of Contrave® in obesity management in Canada. Value Health (2019) 22:S574. doi: 10.1016/j.jval.2019.09.896
57. Nuijten M, Marczewska A, Araujo Torres K, Morton M, Perugini M. Cost effectiveness of optifast® LCD as compared with liraglutide 3 mg and “No intervention” In Switzerland. Value Health (2017) 20(9):A554–5. doi: 10.1016/j.jval.2017.08.885
58. National Institute for Health and Care Excellence. Naltrexone-bupropion for managing overweight and obesity: technology appraisal guidance [TA494]. London(GB):National Institute of For Health and Care Excellence: Technology appraisal guidance [TA494] (2017). Available at: https://www.nice.org.uk/guidance/ta494.
59. Finkelstein EA, Kruger E, Karnawat S. Cost-effectiveness analysis of qsymia for weight loss. Pharmacoeconomics (2015) 33(7):699–706. doi: 10.1007/s40273-014-0182-6
60. Finkelstein EA, Kruger E. Meta- and cost-effectiveness analysis of commercial weight loss strategies: CEA of Commercial Weight Loss Strategies. Obesity (2014) 22(9):1942–51. doi: 10.1002/oby.20824
61. Ara R, Blake L, Gray L, Hernández M, Crowther M, Dunkley A, et al. What is the clinical effectiveness and cost-effectiveness of using drugs in treating obese patients in primary care? A systematic review. Health Technol Assess (2012). 16(5):iii–195. doi: 10.3310/hta16050
62. Veerman JL, Barendregt JJ, Forster M, Vos T. Cost-effectiveness of pharmacotherapy to reduce obesity. PloS One (2011) 6(10):e26051. doi: 10.1371/journal.pone.0026051
63. Iannazzo S, Zaniolo O, Pradelli L. Economic evaluation of treatment with orlistat in Italian obese patients. Curr Med Res Opin (2008) 24(1):63–74. doi: 10.1185/030079908X253591
64. van Baal PHM, van den Berg M, Hoogenveen RT, Vijgen SMC, Engelfriet PM. Cost-effectiveness of a low-calorie diet and orlistat for obese persons: modeling long-term health gains through prevention of obesity-related chronic diseases. Value Health (2008) 11(7):1033–40. doi: 10.1111/j.1524-4733.2008.00328.x
65. Roux L, Kuntz KM, Donaldson C, Goldie SJ. Economic evaluation of weight loss interventions in overweight and obese women*. Obesity (2006) 14(6):1093–106. doi: 10.1038/oby.2006.125
66. Foxcroft DR. Orlistat for the treatment of obesity: cost utility model. Obes Rev (2005) 6(4):323–8. doi: 10.1111/j.1467-789X.2005.00211.x
67. Lacey LA, Wolf A, O’Shea D, Erny S, Ruof J. Cost-effectiveness of orlistat for the treatment of overweight and obese patients in Ireland. Int J Obes (2005) 29(8):975–82. doi: 10.1038/sj.ijo.0802947
68. Ruof J, Golay A, Berne C, Collin C, Lentz J, Maetzel A. Orlistat in responding obese type 2 diabetic patients: meta-analysis findings and cost-effectiveness as rationales for reimbursement in Sweden and Switzerland. Int J Obes (2005) 29(5):517–23. doi: 10.1038/sj.ijo.0802925
69. Hertzman P. The cost effectiveness of orlistat in a 1-year weight-management program for treating overweight and obese patients in Sweden: A treatment responder approach. Pharmacoeconomics (2005) 23(10):1007–20. doi: 10.2165/00019053-200523100-00004
70. Maetzel A, Ruof J, Covington M, Wolf A. Economic evaluation of orlistat in overweight and obese patients with type 2 diabetes mellitus. Pharmacoeconomics (2003) 21(7):501–12. doi: 10.2165/00019053-200321070-00005
71. Lamotte M, Annemans L, Lefever A, Nechelput M, Masure J. A health economic model to assess the long-term effects and cost-effectiveness of orlistat in obese type 2 diabetic patients. Diabetes Care (2002) 25(2):303–8. doi: 10.2337/diacare.25.2.303
72. Chakhtoura M, Haber R, Ghezzawi M, Rhayem C, Tcheroyan R, Mantzoros CS. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. eClinicalMedicine (2023) 58:101882. doi: 10.1016/j.eclinm.2023.101882
73. Wajid I, Vega A, Thornhill K, Jenkins J, Merriman C, Chandler D, et al. Topiramate (Topamax): Evolving role in weight reduction management: a narrative review. Life (2023) 13(9):1845. doi: 10.3390/life13091845
74. Kokkorakis M, Katsarou A, Katsiki N, Mantzoros CS. Milestones in the journey towards addressing obesity; Past trials and triumphs, recent breakthroughs, and an exciting future in the era of emerging effective medical therapies and integration of effective medical therapies with metabolic surgery. Metabolism (2023) 148:155689. doi: 10.1016/j.metabol.2023.155689
75. Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: Advances and challenges. Nat Rev Drug Discovery (2022) 21(3):201–23. doi: 10.1038/s41573-021-00337-8
76. Lopes S, Meincke HH, Lamotte M, Olivieri A, Lean MEJ. A novel decision model to predict the impact of weight management interventions: The Core Obesity Model. Obes Sci Pract (2021) 7(3):269–80. doi: 10.1002/osp4.495
77. Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: A report of the ISPOR-SMDM modeling good research practices task force-6. Value Health (2012) 15(6):835–42. doi: 10.1016/j.jval.2012.04.014
Keywords: obesity, anti-obesity drugs, cost-effectiveness, modeling, methodology
Citation: Xue Y, Zou H, Ruan Z, Chen X, Lai Y, Yao D, Ung COL and Hu H (2023) Pharmacoeconomic evaluation of anti-obesity drugs for chronic weight management: a systematic review of literature. Front. Endocrinol. 14:1254398. doi: 10.3389/fendo.2023.1254398
Received: 07 July 2023; Accepted: 17 October 2023;
Published: 06 November 2023.
Edited by:
Katsunori Nonogaki, Tohoku University, JapanReviewed by:
M. Ishaq Geer, University of Kashmir, IndiaLihua Jin, City of Hope, United States
Tessa Weir, Nepean and Blue Mountains Local Health District, Australia
Copyright © 2023 Xue, Zou, Ruan, Chen, Lai, Yao, Ung and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolina Oi Lam Ung, Y2Fyb2xpbmF1bmdAdW0uZWR1Lm1v; Hao Hu, aGFvaHVAdW0uZWR1Lm1v
 Yan Xue
Yan Xue Huimin Zou
Huimin Zou Zhen Ruan
Zhen Ruan Xianwen Chen
Xianwen Chen Yunfeng Lai
Yunfeng Lai Dongning Yao
Dongning Yao Carolina Oi Lam Ung
Carolina Oi Lam Ung Hao Hu
Hao Hu