
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 25 September 2023
Sec. Adrenal Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1254221
 Sandy Maumus-Robert1
Sandy Maumus-Robert1 Ana Jarne-Munoz1
Ana Jarne-Munoz1 Antoine Pariente1,2
Antoine Pariente1,2 Thomas Duroux3
Thomas Duroux3 Lise Duranteau4
Lise Duranteau4 Julien Bezin1,2*
Julien Bezin1,2*Introduction: Statins could reduce the synthesis of steroid hormones, thereby could cause adrenal insufficiency. We investigated this risk in a large nationwide database.
Methods: We conducted a nested case-control study using a cohort of individuals affiliated to the French health insurance system in 2010, ≥18y and without adrenal insufficiency history. Each case had a first event of adrenal insufficiency between 2015 and 2017 and was matched to up to ten controls on age, sex, and prior treatment with corticosteroids. Statin exposure was measured over the five years preceding the index date, considering a six-month censoring lag-time. Association was estimated using a conditional logistic regression adjusted for confounders included in a disease risk score. Analyses were stratified on age, sex and corticosteroid history of use.
Results: 4 492 cases of adrenal insufficiency were compared with 44 798 controls (median age 66y, 58% women), of which 39% vs. 33% were exposed to statins, respectively. No association between statin use and adrenal insufficiency was found when adjusting the model for confounders (adjusted odds ratio 0.98; 95% confidence interval 0.90-1.05). These results were consistent regardless of the exposure definition and stratifications considered.
Conclusion: Statin-related adrenal insufficiency risk, if any, seems to be very limited and does not compromise the benefit of statin treatment.
Statins are lipid-lowering drugs used to prevent cardiovascular diseases. They act by inhibiting the catalysis of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) into mevalonate by HMG CoA reductase, thus reducing cholesterol synthesis by the liver. Beside this effect on cholesterol reduction, statins reduce inflammation and oxidative stress and up-regulate endothelial NO-synthase activity, thereby improving endothelial functions and stabilizing the atherosclerotic plaque (1). Benefit of statins have been shown for both secondary and primary cardiovascular prevention (2–4). Statins are among the most commonly prescribed drugs worldwide (5). Around 200 million people take a daily statin worldwide, including over 30 million people in the United States alone (6). In France, about 5 million people were using such drugs in 2014 (7).
Statins have already been shown to increase the risk of diabetes through endocrine mechanisms related to HMG-CoA reductase activity (2, 8, 9). Steroid hormones are produced from cholesterol of low-density lipoproteins, which is used as a substrate of steroidogenesis in adrenal cortex and gonads (10, 11). By inhibiting cholesterol synthesis, statins could be responsible of a reduction of steroid hormones synthesis and thereby cause adrenal insufficiency (AI). Today, only a few studies concerning a limited number of patients showed contradictory results on steroid hormone synthesis (12–14).
In this context, we conducted a large, nationwide study based on French claims data to investigate if the use of statin is associated to occurrence of AI.
This study used the nationwide database of the French health care insurance system, SNDS (Système National des Données de Santé). SNDS provides individual and anonymous information for almost all French population (99%). It contains all outpatient healthcare reimbursements, including drugs, sociodemographic data (age, sex, social deprivation index based on geographic area, date of death), and all hospitalization discharge reports (dates, diagnoses, and performed procedures). For each reimbursed drug, data collected in the database include date of dispensing, active ingredients, route of administration, dosage, packaging, but not the prescribed daily dosage. Details on the French medico-administrative databases have been described elsewhere (15).
This study focused on the beneficiaries of the major health insurance scheme for employees (salaried workers and their relatives, retired salaried workers and their relatives); that is, 77% of the French population for whom the SNDS database has comprehensively recorded data since 2006.
We conducted a nested case-control study. The source cohort was constituted by all individuals affiliated on January 1st 2010 to the major health insurance scheme for at least three years before this date, aged 18 years or older in 2010, with no history of primary or secondary AI in the three years preceding baseline (without any hospitalization presenting ICD-10 diagnosis codes E271-E274 and without any use of hydrocortisone or desoxycortone identified by their respective ATC codes H02AB09 and H02AA03). Participants with AI diagnosis between January 1st 2010 and December 31st 2014 or with less than 5 years of follow-up were excluded from source cohort. Participants were followed until study outcome occurrence, December 31st 2017, lost to follow-up or death, whichever came first.
Incident cases of AI were identified from January 1st 2015 to December 31st 2017. It was defined as the presence of both a first hospital stay with a diagnosis of AI (ICD-10 code E271 to E274), and a first dispensing of hydrocortisone up to 30 days before or after hospital admission date. The index date was hospital admission date or hydrocortisone dispensing date, whichever came first.
For each case was established a risk set of potential controls. Any subject included in the source cohort could be a potential control until the index date of the corresponding case. Each case was matched to up to ten controls using the Risk Set Sampling method. Matching criteria were age at index date (± two years), sex, and prior treatment with corticosteroids in the three years before the index date. Corticosteroid treatment history was assessed separately for each administration route (systemic, inhaled and topic), using two variables: (i) quartiles of the number of dispensings, and (ii) time of use (recent: at least one dispensing in the year before the index date; past: last dispensing occurring more than one year before the index date; non-use: no use within the preceding three years). Corticosteroids dispensing was identified using ATC codes (Supplementary Data, Table S1).
Exposure to statins was measured through dispensings in the five years prior to the index using ATC codes (Supplementary Data, Table S2). A six-month lag-time before the index date was used to censor exposure measurement, in order to minimize protopathic bias (related to the possible time between the occurrence of the first AI symptoms and the diagnosis date or drug initiation date) or detection bias (related to an increase of diagnostic procedures in the months before the AI diagnosis date and that could have potentially led to prescribe statins).
The exposure to statins was measured in four different ways: (i) statin use (≥ one dispensing), (ii) statin history of use (new users: first dispensing within the 6 months before lag-time period; current users: first dispensing more than six months before lag-time period and last dispensing within the six months before lag-time period; past users: last dispensing more than six months before lag-time period), (iii) statin cumulative use (total dose dispensed before lag-time period converted and expressed in cumulative years of use by dividing it by the Defined Daily Dose (DDD) of the drug), and (iv) intensity of the last dispensed statin; the reference being always no statin dispensing before lag-time period.
We used conditional logistic regression models to compute the odds of AI associated with statin exposure (odds ratios OR and corresponding 95% confidence intervals, 95% CI). Conditional logistic regression models were adjusted for fibrate and ezetimibe use. In addition, statistical models were adjusted for deciles of a disease risk score (DRS) to balance the treatment groups for all potential confounding factors that could alter risk estimation (16). The DRS was estimated by a logistic regression model in all subjects who were never exposed to statins during their follow-up. Values of covariates included in the DRS were measured in the two years before the index date. Potential confounding factors are presented in details in Supplementary Data, Table S3. The standardized difference between cases and controls was computed for each covariate used as an independent variable in the DRS estimation. A maximal value of 0.1 for standardized difference was considered as satisfying, covariates with a higher value were excluded from the DRS and included into the final model for adjustment.
Secondary statistical analyses consisted in stratifications according to sex, age classes, qualitative use of corticosteroids (non-use vs. at least one dispensing within the three years before index date). Sensitivity analyses were performed to study lag-time variation (assessment with a three-month period and without lag-time), and impact of hospital stay length, by adjusting models for the existence of hospital stays of at least three months within the three years preceding the index date (to take into account periods of hospitalizations when drug exposure could not be identified). Finally, low-dose aspirin (ATC code B01AC06) was used as a negative exposure, this drug having similar indications to statins in cardiovascular prevention.
All analyses were performed using SAS Enterprise Guide 9.4 (SAS Institute, Inc., Cary, NC).
By agreement of the French Data Protection Supervisory Authority (Commission Nationale de l’Informatique et des Libertés), neither ethics committee approval nor informed consent were required for this observational study based on anonymized French medico-administrative database.
The source cohort included 27 028 250 participants eligible for case and control selection. A total of 4 496 AI cases were identified, of which 4 492 cases were finally matched to 44 798 controls (Figure 1). Participants included in the analyses had a median age of 66 years (IQR 52-78), and were mostly women (58.2%). Around 75% had at least one dispensing of systemic corticosteroids or of topical corticosteroids within the three years prior to index date and 40% of inhaled corticosteroids.
Cases appeared to present with a higher burden of comorbidities and a higher use of drugs, potentially confounders in the association between statins and adrenal insufficiency (Table 1).
Given the very low frequency of tyrosine kinase inhibitors, interferon or ribavirin and immunotherapy (less than 0.1% of controls), these covariates were not included in the DRS. Among the remaining covariates, all showed standardized differences lower than 0.1, except the covariate “radiotherapy, local tumors and related procedures that can lead to a secondary adrenal insufficiency”. This covariate was, as a result, excluded from the DRS and included into the final model for adjustment. Validation information about DRS is detailed in Supplementary Data, Figure S1 and Table S4.
Analyses showed no association between statin use and the risk of adrenal insufficiency after adjustment for potential confounders. This absence of association was found regardless of the definition of statin exposure used (statin use, statin history of use, statin cumulative use, or intensity of last statin dispensed). A non-significant increase in AI risk was observed with a cumulative use of]0-1] year (OR 1.09, 95%CI 0.97; 1.27) and for new users (OR 1.12, 95%CI 0.81; 1.55) compared with non-users of statin (Table 2). Stratified analyses on sex, age or corticosteroid use did not show any association in some subgroups of patients (Table 3).
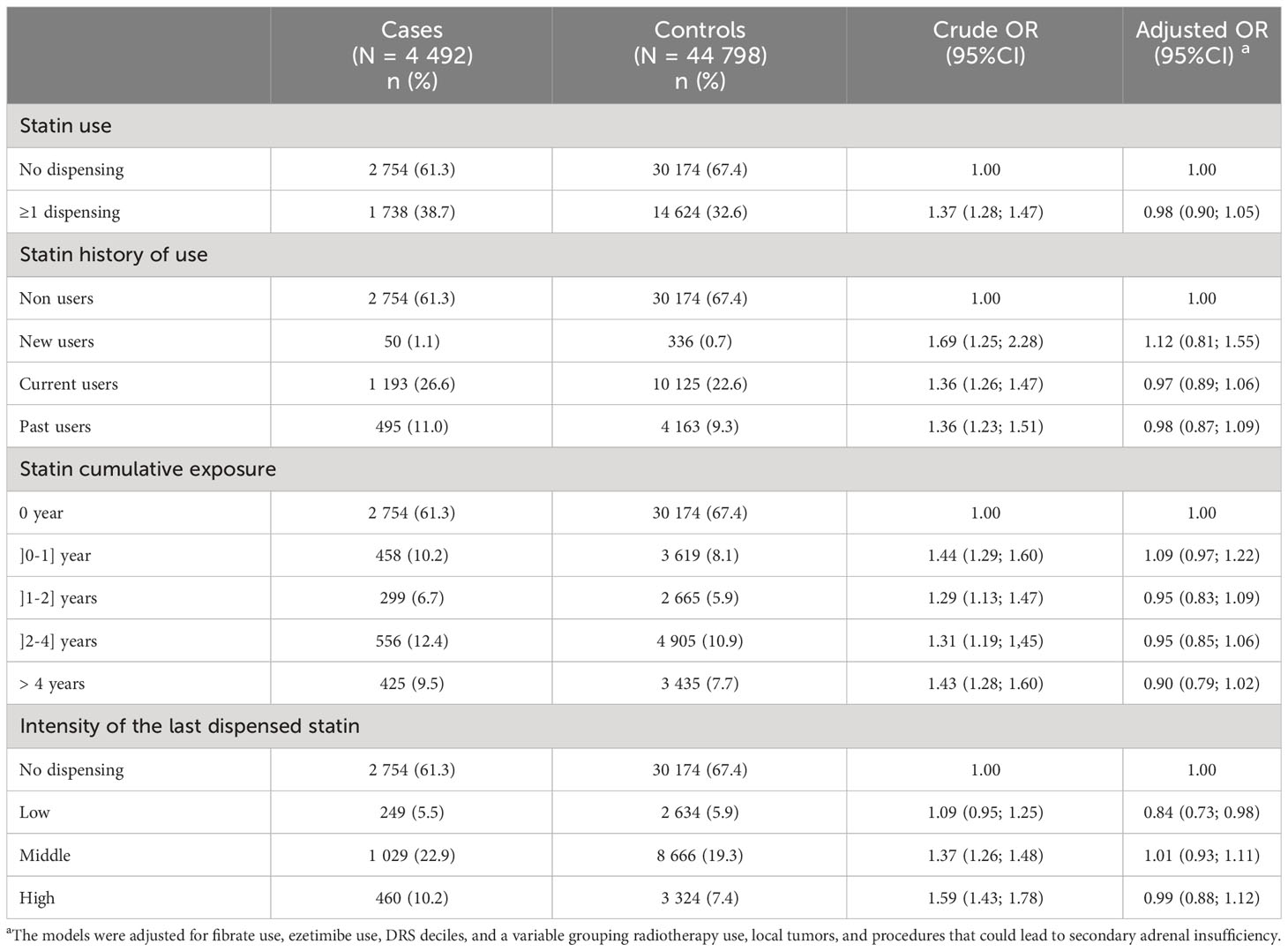
Table 2 Results of the analyses assessing the association between statin exposure and risk of adrenal insufficiency.
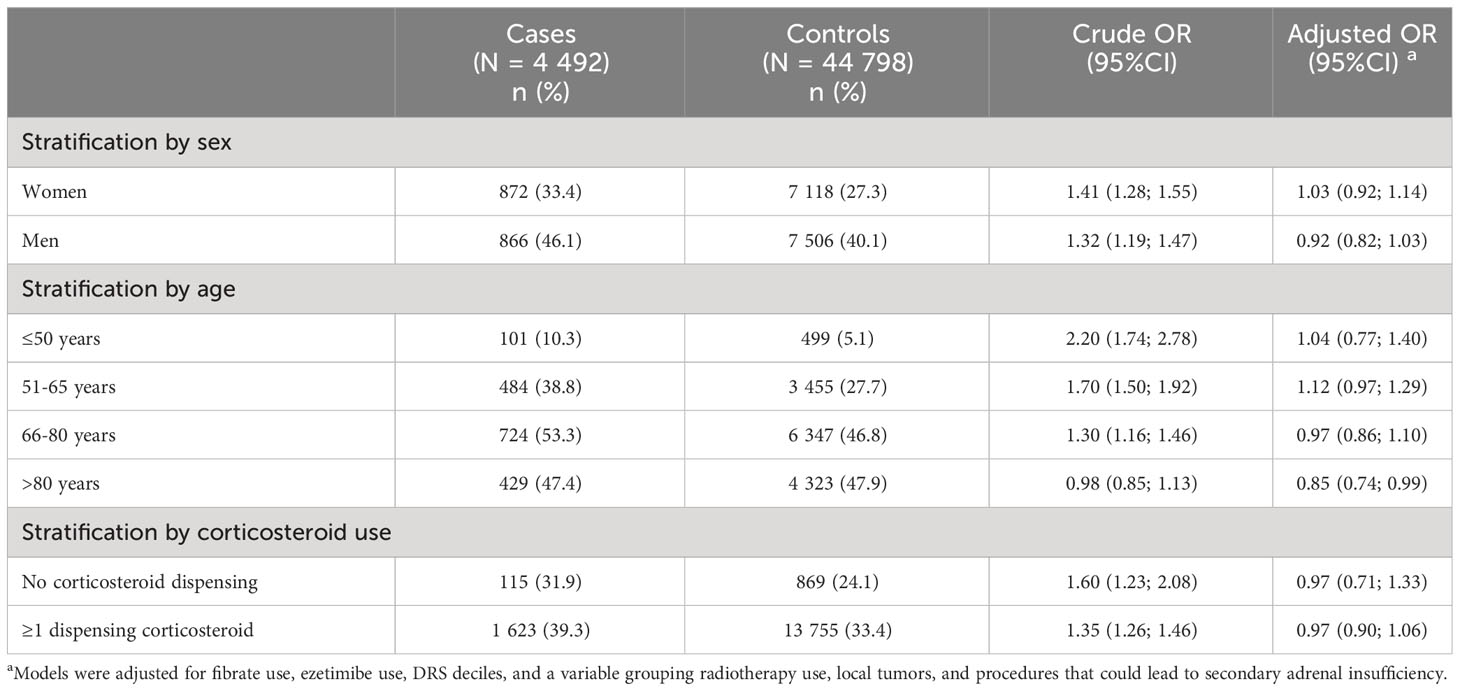
Table 3 Results of secondary analyses assessing the association between statin use (at least one statin dispensing vs. no dispensing) and risk of adrenal insufficiency stratified by sex, age and corticosteroid use.
The results of sensitivity analyses assessing the impact of lag-time length variation and hospital stays longer than three weeks were consistent with those of the main analyses (Supplementary Data, Table S5). The negative exposure analysis using low-dose aspirin as exposure confirmed also our negative results (Supplementary Data, Table S6).
We found no relevant association between statin exposure and AI risk after multivariate adjustment for potential confounders and regardless of statin definitions of exposure we used. This result is consistent with those of a recent study describing drug-induced AI using pharmacovigilance reports (17). Our results showed a non-significant increase in AI occurrence for new users of statins and for cumulative use of statins lower than one year. This risk, if any, seems to be very limited given the risk estimates not exceeding 1.15. This non-significant association observed when treatment with statin was initiated may be due to a residual detection bias rather than to an effect of statin on AI. From a biological and physiological viewpoint, a cumulative dose-effect would rather have been expected.
London et al. had shown in their study of 14 patients that a single treatment with a lipophilic statin reduced the production of some glucocorticoid precursors (12). This effect could have a clinical impact but it is possible that a compensatory phenomena allow glucocorticoid synthesis to be maintained or regulated.
Our study presents with strengths and limitations common to all studied performed from medico-administrative databases. As for their strengths, the nationwide healthcare database of the French population is frequently used for safety studies owing to their interest in terms of data exhaustiveness, and representativeness of the population (15, 18–20). Our study hence focused on data relating to beneficiaries of the major health insurance scheme, which concern about 80% of the French population. As for their limitations, this database only informs on reimbursed dispensings for prescribed drugs, which is only a proxy of patients’ exact use. As for the strengths specific to our study, the multivariate adjustment including a DRS succeeded in considering for a big part of confounding as it removed the association initially found with the crude analysis. In addition, all performed sensitivity analyses were consistent, including the analyses using low-dose aspirin as negative tracer exposure which eliminates a possible indication bias. As for the limitations of our study, the way we identified AI has not been validated elsewhere, and did not used steroid levels. However, it has been internally discussed with clinical experts in endocrinology and hospital coding. In addition, other studies on the same topic have used similar identification algorithm (AI ICD codes associated with hydrocortisone use) to define AI in claim databases (21, 22). Finally, our study only involved adult subjects, whereas in heterozygous familial hypercholesterolemia statin treatment can be initiated at a very young age. The impact of such treatment at this young age on steroid hormone synthesis could not therefore be studied here and would require further investigations, even if clinical trials conducted in this population have been reassuring (23, 24).
To the best of our knowledge, our study is the first to investigate the risk of AI associated with statin use in nationwide data. Our results do not indicate an increase in the occurrence of AI associated with statin use, either at initiation, with high cumulative use or high intensity treatment. The risk of AI related to statin exposure, if any, seems to be very limited and does not compromise statin treatment. Further studies could be carried out to assess the impact of these drugs on steroid hormone synthesis in more at-risk population or young patients.
The data analyzed in this study is subject to the following licenses/restrictions: French law to access SNDS. Requests to access these datasets should be directed to https://www.health-data-hub.fr.
SM: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. AJ: Conceptualization, Formal Analysis, Methodology, Writing – review & editing. AP: Conceptualization, Methodology, Supervision, Writing – review & editing. TD: Conceptualization, Methodology, Writing – review & editing. LD: Conceptualization, Methodology, Writing – review & editing. JB: Conceptualization, Methodology, Supervision, Writing – review & editing.
This work was supported by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, ANSM) (grant number 2019S015), in the context of a partnership with the Health Product Epidemiology Scientific Interest Group (EPI-PHARE). The ANSM played no role in the study design, conduct, and results interpretation or discussion. This publication represents the views of the authors and does not necessarily represent the opinion of the ANSM.
We thank Professor Bernard Bégaud for his advices at the beginning of the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1254221/full#supplementary-material
1. Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol (2005) 96(5):24–33. doi: 10.1016/j.amjcard.2005.06.009
2. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet (2016) 388(10059):2532–61. doi: 10.1016/S0140-6736(16)31357-5
3. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet (2010) 376(9753):1670–81. doi: 10.1016/S0140-6736(10)61350-5
4. Andersson T, Nåtman J, Mourtzinis G, Bager JE, Bengtsson Boström K, Franzén S, et al. The effect of statins on mortality and cardiovascular disease in primary care hypertensive patients without other cardiovascular disease or diabetes. Eur J Prev Cardiol (2023) 27:zwad212. doi: 10.1093/eurjpc/zwad212
5. Blais JE, Wei Y, Yap KKW, Alwafi H, Ma TT, Brauer R, et al. Trends in lipid-modifying agent use in 83 countries. Atherosclerosis (2021) 328:44–51. doi: 10.1016/j.atherosclerosis.2021.05.016
6. Blaha MJ, Martin SS. How do statins work? J Am Coll Cardiol (2013) 62(25):2392–4. doi: 10.1016/j.jacc.2013.08.1626
7. Bonnet F, Bénard A, Poulizac P, Afonso M, Maillard A, Salvo F, et al. Discontinuing statins or not in the elderly? Study protocol for a randomized controlled trial. Trials (2020) 21(1):342. doi: 10.1186/s13063-020-04259-5
8. Ruscica M, Ferri N, Banach M, Sirtori CR, Corsini A. Side effects of statins: from pathophysiology and epidemiology to diagnostic and therapeutic implications. Cardiovasc Res (2022) 118(17):3288–3304. doi: 10.1093/cvr/cvac020
9. Betteridge DJ, Carmena R. The diabetogenic action of statins — mechanisms and clinical implications. Nat Rev Endocrinol (2016) 12(2):99–110. doi: 10.1038/nrendo.2015.194
10. Miller WL. Steroidogenic enzymes. In: Flück CE, Miller WL, editors. Endocrine Development, vol. 13 . KARGER (2008). p. 1–18. doi: 10.1159/000134751
11. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev (2011) 32(1):81–151. doi: 10.1210/er.2010-0013
12. London E, Tatsi C, Soldin SJ, Wassif CA, Backlund P, Ng D, et al. Acute statin administration reduces levels of steroid hormone precursors. Horm Metab Res (2020) 52(10):742–6. doi: 10.1055/a-1099-9556
13. Baudrand R, Pojoga LH, Vaidya A, Garza AE, Vöhringer PA, Jeunemaitre X, et al. Statin use and adrenal aldosterone production in hypertensive and diabetic subjects. Circulation (2015) 132(19):1825–33. doi: 10.1161/CIRCULATIONAHA.115.016759
14. Santini SA, Carrozza C, Lulli P, Zuppi C, Tonolo GC, Musumeci S. Atorvastatin treatment does not affect gonadal and adrenal hormones in type 2 diabetes patients with mild to moderate hypercholesterolemia. JAT (2003) 10(3):160–4. doi: 10.5551/jat.10.160
15. Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: Powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf (2017) 26(8):954–62. doi: 10.1002/pds.4233
16. Arbogast PG, Ray WA. Use of disease risk scores in pharmacoepidemiologic studies. Stat Methods Med Res (2009) 18(1):67–80. doi: 10.1177/0962280208092347
17. Raschi E, Fusaroli M, Massari F, Mollica V, Repaci A, Ardizzoni A, et al. The changing face of drug-induced adrenal insufficiency in the food and drug administration adverse event reporting system. J Clin Endocrinol Metab (2022) 107(8):e3107–14. doi: 10.1210/clinem/dgac359
18. Bezin J, Mansiaux Y, Noize P, Salvo F, Bégaud B, Pariente A. Use of lipid-lowering drugs and the risk of cataract: A population-based nested case-control study. Clin Pharmacol Ther (2019) 105(2):458–65. doi: 10.1002/cpt.1176
19. Bénard-Laribière A, Hucteau E, Debette S, Kirchgesner J, Bezin J, Pariente A. Risk of first ischaemic stroke and use of antidopaminergic antiemetics: nationwide case-time-control study. BMJ (2022) 376:e066192. doi: 10.1136/bmj-2021-066192
20. Weill A, Nguyen P, Labidi M, Cadier B, Passeri T, Duranteau L, et al. Use of high dose cyproterone acetate and risk of intracranial meningioma in women: cohort study. BMJ (2021) 372:n37. doi: 10.1136/bmj.n37
21. Iwasaku M, Shinzawa M, Tanaka S, Kimachi K, Kawakami K. Clinical characteristics of adrenal crisis in adult population with and without predisposing chronic adrenal insufficiency: a retrospective cohort study. BMC Endocr Disord (2017) 17(1):58. doi: 10.1186/s12902-017-0208-0
22. Iwasaku M, Tanaka S, Shinzawa M, Kawakami K. Impact of underlying chronic adrenal insufficiency on clinical course of hospitalized patients with adrenal crisis: A nationwide cohort study. Eur J Internal Med (2019) 64:24–8. doi: 10.1016/j.ejim.2019.04.001
23. Stein EA, Illingworth DR, Kwiterovich PO Jr., Liacouras CA, Siimes MA, Jacobson MS, et al. Efficacy and safety of lovastatin in adolescent males with heterozygous familial hypercholesterolemia: A randomized controlled trial. JAMA (1999) 281(2):137. doi: 10.1001/jama.281.2.137
Keywords: statins, adrenal insufficiency, risk, pharmacoepidemiology, database
Citation: Maumus-Robert S, Jarne-Munoz A, Pariente A, Duroux T, Duranteau L and Bezin J (2023) Statin treatment is not associated with an increased risk of adrenal insufficiency in real-world setting. Front. Endocrinol. 14:1254221. doi: 10.3389/fendo.2023.1254221
Received: 06 July 2023; Accepted: 08 September 2023;
Published: 25 September 2023.
Edited by:
Giuseppe Reimondo, University of Turin, ItalyReviewed by:
Massimiliano Ruscica, Faculty of Pharmacy, University of Milan, ItalyCopyright © 2023 Maumus-Robert, Jarne-Munoz, Pariente, Duroux, Duranteau and Bezin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julien Bezin, anVsaWVuLmJlemluQHUtYm9yZGVhdXguZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.