- 1Central Laboratory, Clinical Medical College & Affiliated Hospital of Chengdu University, Chengdu, Sichuan, China
- 2Department of Critical Care Medicine, Clinical Medical College & Affiliated Hospital of Chengdu University, Chengdu, Sichuan, China
- 3Clinical Medical College of Chengdu University, Chengdu, Sichuan, China
- 4Department of Cardiology, Clinical Medical College & Affiliated Hospital of Chengdu University, Chengdu, Sichuan, China
Background: Relationships of the polymorphisms in fat mass and obesity-associated gene (FTO) and peroxisome proliferator-activated receptor delta gene (PPARD) with metabolic-related diseases remain to be clarified.
Methods: One thousand three hundred and eighty-one subjects were enrolled. Metabolic-related diseases including obesity, dyslipidemia, hyperhomocysteinemia, hyperuricemia, hypertension, type 2 diabetes mellitus (T2DM) and coronary artery disease (CAD) were defined based on diagnostic criteria. FTO rs9939609 and rs17817449, and PPARD rs2016520 and rs2267668 polymorphisms were genotyped by using polymerase chain reaction-restricted fragment length polymorphism method.
Results: Patients with T2DM or dyslipidemia had a higher frequency of AA, AT or AA + AT genotypes as well as A allele of FTO rs9939609 polymorphism than those free of T2DM or dyslipidemia (P ≤ 0.04 for all). Patients with T2DM or dyslipidemia had a higher frequency of GG, GT or GG + GT genotypes as well as G allele of FTO rs17817449 polymorphism than those free of T2DM or dyslipidemia (P ≤ 0.03 for all). Multivariate logistic regression analyses showed that FTO rs9939609 and rs17817449 polymorphisms were independently associated with T2DM as well as dyslipidemia after adjustment for age, sex, smoking and other metabolic diseases. FTO rs9939609 and rs17817449 polymorphisms were not associated with obesity, hyperhomocysteinemia, hyperuricemia, hypertension and CAD. Obese or T2DM carriers of the AA or AT genotype of the FTO rs9939609 polymorphism had a higher prevalence of dyslipidemia compared to non-obese or non-T2DM carriers of the AA or AT genotype (P = 0.03 for both). Among the carriers of GG or GT genotype of the FTO rs17817449 polymorphism, the prevalence of dyslipidemia in obese patients was higher than that in non-obese subjects (P < 0.01). PPARD rs2016520 and rs2267668 polymorphisms were not correlated with any of the metabolic-related diseases in the study population.
Conclusion: Minor alleles of FTO rs9939609 and rs17817449 polymorphisms confer a higher risk of T2DM and dyslipidemia, and the risk is further increased among obese individuals. PPARD rs2016520 and rs2267668 polymorphisms are not associated with metabolic-related diseases.
Introduction
Metabolic-related diseases and chronic conditions, such as obesity, diabetes, dyslipidemia, hyperuricemia and hyperhomocysteinemia, can occur in people of all ages and confer a very high risk of developing life-threatening cardiovascular and cerebrovascular diseases (1). Metabolic-related diseases are a group of multifactorial diseases with a number of risk factors including genetic susceptibility, sedentary lifestyle, high-fat diet, old age, cigarette smoking, and alcohol drinking. Genetic susceptibility may be the most important risk factor for metabolic-related diseases (2, 3). Researchers around the world have paid a great effort to investigate the relations of genetic variants with metabolic-related diseases in the past few decades, but the findings were inconsistent and inconclusive due to various reasons such as small sample size and differences in race, dietary habit, health condition, and social and natural environments across the study populations (4–7).
Fat mass and obesity-associated gene (FTO) encodes a N6-methyladenosine (m6A) demethylase which belongs to the superfamily of 2-oxoglutarate- and Fe(II)-dependent dioxygenases. The main function of FTO is to remove m6A from specific RNA species, such as mRNAs, thereby affecting the stability of RNAs, translation efficiency of mRNAs or binding activity of m6A readers (8, 9). FTO mostly targets the mRNAs that are closely related to glucolipid metabolism and energy homoeostasis, such as sterol regulatory element binding protein 1c (SREBP1c) (10–12), carbohydrate responsive element binding protein (ChREBP) (12), peroxisome proliferator-activated receptor alpha (PPARα) (13), PPARγ (14), PPARγ coactivator 1α (PGC1α) (15), apolipoprotein E (APOE) (16), uncoupling protein 1 (UCP1) (17) and ghrelin (18). Based on the functions of the target mRNAs of FTO, it is logical to speculate that the genetic variations in FTO are involved in the occurrence and development of metabolic-related diseases. Human FTO is located on chromosome 16q12.2 and consists of 9 exons and 8 introns. The rs9939609 (g.53786615T>A) and rs17817449 (g.53779455T>G) variants are localized within intron 1 of FTO, and rs17817449 is positioned approximately 7.2 kb upstream of the rs9939609 polymorphism. The rs9939609 polymorphism is formed by a transversion from thymine (T) to adenine (A), while the rs17817449 polymorphism is formed from thymine (T) to guanine (G). These two loci have been reported to be associated to metabolic-related diseases such as obesity (19–23), dyslipidemia (22, 24), hypertension (22, 25–27), type 2 diabetes mellitus (T2DM) (28, 29) and cardiovascular disease (20, 27, 30). However, some other investigators have come to completely different and even conflicting conclusions, demonstrating that the minor alleles of these genetic variants were either not associated or negatively associated with metabolic-related diseases (31–33).
PPARδ (also known as PPARβ) is a transcription factor and an important member of the PPAR family of nuclear receptors, with the other two members PPARα and PPARγ. As a nuclear transcription factor, PPARδ mainly initiates transcription of the genes related to lipid metabolism (34–36), adaptive thermogenesis (37) and adipocyte formation (38). Human PPARδ gene (PPARD) is located on chromosome 6p21.3 and contains 8 exons and 7 introns. The rs2016520 and rs2267668 polymorphisms are positioned in the 5’ untranslated region (5’UTR) and intron 2 of PPARD, respectively. The rs2016520 variant is also known as +294T/C, -87T/C or +15T/C, with cytosine (C) as its minor allele. The rs2267668 polymorphism is localized 856 bp upstream of the rs2016520 variant. The two polymorphic loci have been proved to be related to metabolic-related diseases such as obesity (39) and dyslipidemia (40). However, several other studies did not support these findings (41, 42).
The relationships of FTO and PPARD variants with metabolic-related diseases deserve to be further explored since there were so many inconsistencies in the scientific publications. Moreover, the associations of the four polymorphisms with hyperuricemia and hyperhomocysteinemia have not yet been investigated to date. Here, a hospital-based study with the clinical diagnosis of metabolic-related diseases or chronic conditions including obesity, dyslipidemia, hypertension, hyperhomocysteinemia, hyperuricemia, T2DM and coronary artery disease (CAD) was conducted to systematically explore the relations of FTO rs9939609 and rs17817449, and PPARD rs2016520 and rs2267668 polymorphisms with metabolic-related diseases. The results from this study can provide an opportunity to unveil the interrelationships among variants in FTO and PPARD, metabolic disorders and CAD.
Subjects and methods
Subjects
A total of 1381 unrelated Chinese adult subjects who underwent coronary angiography and other examinations for suspected CAD at the Department of Cardiology, Affiliated Hospital of Chengdu University (Chengdu, China) were enrolled in the study between July 2021 and December 2022. The exclusion criteria were as follows: 1) subjects with renal, hepatic or valvular disease; 2) subjects who were taking antihypertensive drugs; 3) subjects taking the drugs that may influence plasma glucose, lipid, uric acid or homocysteine level. Subjects who were taking the drugs that were considered not to affect plasma glucose, lipid, uric acid or homocysteine level were still included in this study to increase sample size and statistical power. Written informed consent was provided by all participants or their legal guardians prior to participation in the study. The study protocol was reviewed and approved by the Ethics Committee of Chengdu University. The study adhered to the tenets of the Declaration of Helsinki.
Diagnosis of metabolic-related diseases
Smoking was defined as current smoking or having ever smoked cigarettes. T2DM was defined as fasting serum glucose level exceeding 7.0 mmol/L (126 mg/dL), or regular use of anti-diabetic medications. Dyslipidemia was defined as the presence of one or more of the following conditions: triglyceride (TG) ≥ 2.3 mmol/L, total cholesterol (TC) ≥ 6.2 mmol/L, low-density lipoprotein cholesterol (LDL-C) ≥ 4.1 mmol/L and high-density lipoprotein cholesterol (HDL-C) < 1.0 mmol/L (43). Serum glucose, TG, TC, LDL-C and HDL-C were measured by using enzymatic method, and all measurements were performed with an automatic clinical chemistry analyzer (Beckman Coulter AU5800, USA). CAD was diagnosed if one or more major coronary arteries (i.e., left main coronary artery, left anterior descending artery, left circumflex artery and right coronary arteries) had stenosis greater than 50%. Coronary angiography was conducted by using Allura Xper FD20 (Philips Medical Systems Nederland B.V. Netherlands) with at least two views of right coronary arteries and four views of the left coronary system. Obesity was defined as body mass index above 28 kg/m2 (44). Hypertension was diagnosed if systolic/diastolic blood pressure is greater than 140/90 mmHg or antihypertensive medications are regularly used (45). Hyperhomocysteinemia was defined as the fasting serum level of homocysteine exceeding 15 μmol/L. Hyperuricemia was defined as the fasting serum level of uric acid exceeding 7.3 mg/dL.
Genomic DNA extraction and genotyping
Genomic DNA was extracted from peripheral lymphocytes by using a whole blood DNA isolation kit according to the instruction manual (QIAGEN, Beijing, China). The genotyping of FTO rs9939609 and rs17817449, and PPARD rs2016520 and rs2267668 polymorphisms was performed by using polymerase chain reaction-restricted fragment length polymorphism method. PCR was carried out in a 25 μL reaction system consisting of 0.03 ug genomic DNA, 12.5 uL 2 × Taq PCR Mix (Tiangen, Beijing, China, cat # KT211), 0.01 uM forward primer, and 0.01 uM reverse primer. Next, PCR products were digested by restriction endonucleases and genotypes were determined by the size of the digested DNA fragments. Three uL PCR products were digested with 5 U restriction endonucleases (NEB, Beijing, China), and the DNA fragments were separated on a 2%-3% agarose gel containing nucleic acid dye (Goldview) and visualized under UV light. The PCR primer sequences, product size, amplification conditions, restriction endonucleases and the DNA fragment sizes of the FTO and PPARD polymorphisms are shown in Table S1. The gels displaying the different fragments of the FTO and PPARD polymorphisms are presented in Figures S1–S4.
Statistical analysis
Continuous data are expressed as mean and standard deviation. Differences between disease and disease-free groups, or among the subjects with different genotypes were analyzed by analysis of variance for continuous variables, and Chi-square test for categorical variables. Multivariate logistic regression analysis was carried out to analyze the associations between the FTO rs9939609 and rs17817449 polymorphisms and T2DM as well as dyslipidemia adjusting for possible confounding factors. The results of logistic regression analysis were presented as odds ratio (OR) and corresponding 95% confidence interval (CI). All P values were two-tailed and the differences were considered as significant if P ≤ 0.05. Statistical analysis was done by using 15.0 version SPSS (Chicago, IL, USA).
Results
Characteristics of the study population
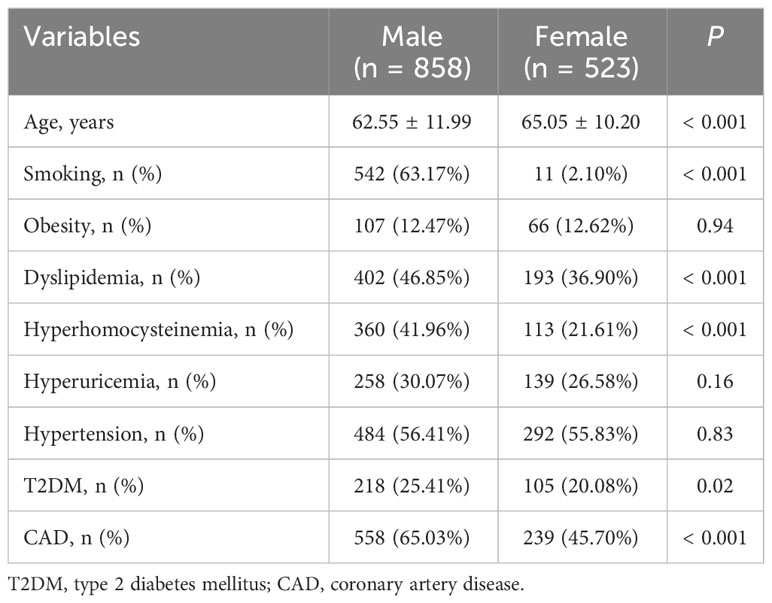
Characteristics of the study population are shown in Table 1. The study population consisted of 858 men and 523 women, and women had an older average age than men (P < 0.001). The prevalence of smoking (P < 0.001), dyslipidemia (P < 0.001), hyperhomocysteinemia (P < 0.001), T2DM (P = 0.02) and CAD (P < 0.001) in men were higher than in women. The prevalence of obesity, hypertension and hyperuricemia was comparable between men and women.
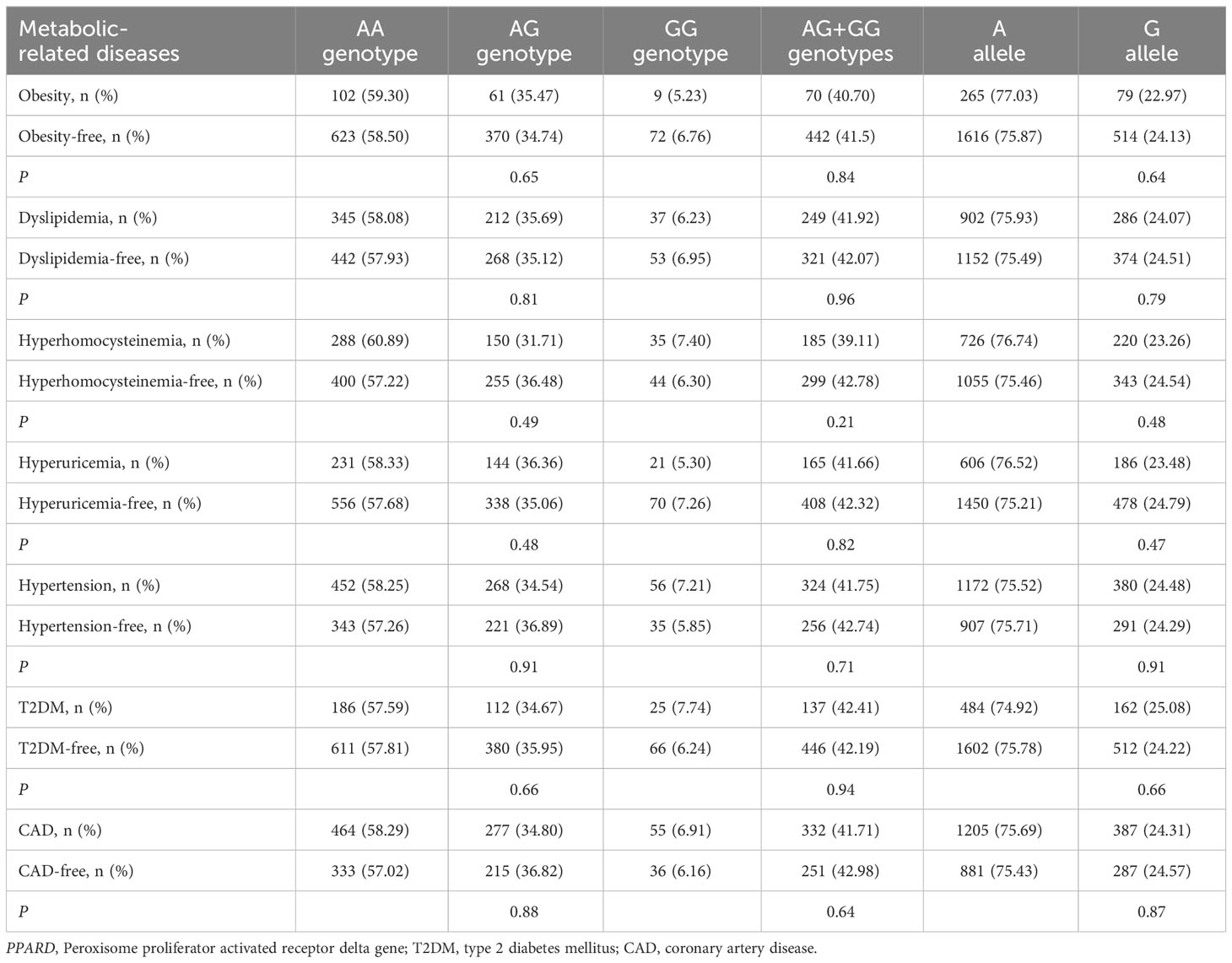
Associations of FTO rs9939609 and rs17817449 polymorphisms with metabolic-related diseases
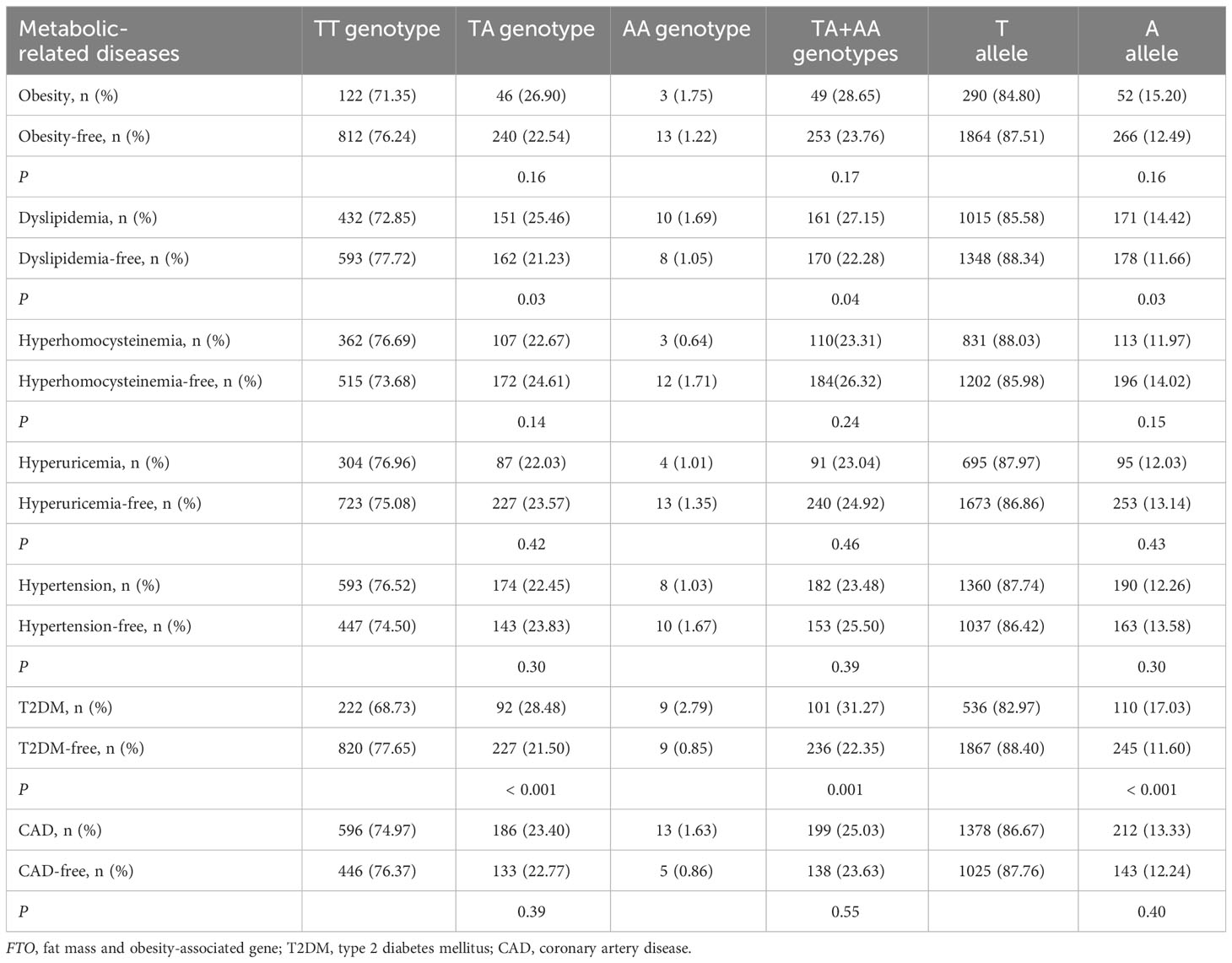
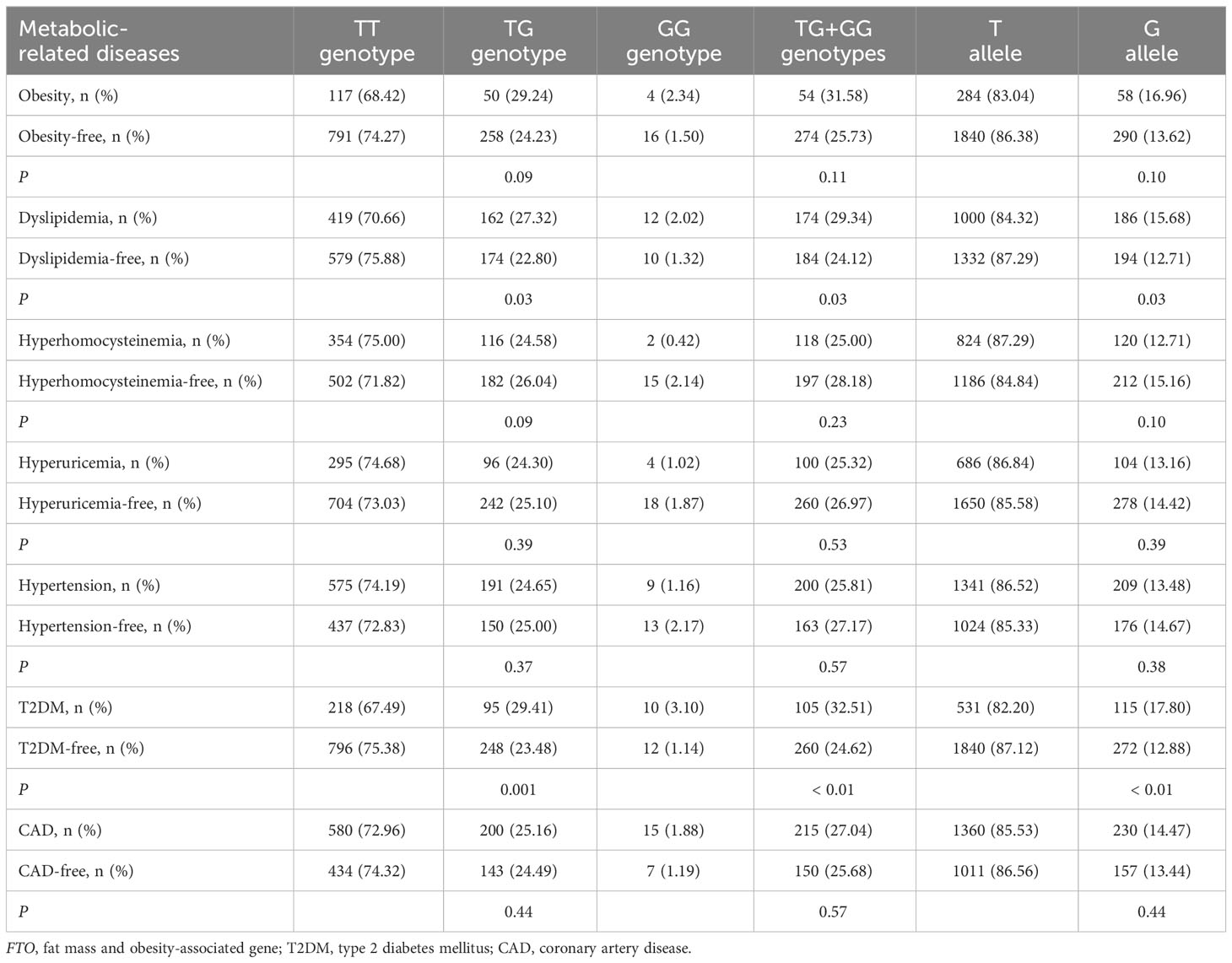
As shown in Table 2, there was a rising trend in the frequencies of AA genotype, AT genotype, AA + AT genotypes and A allele of the rs9939609 polymorphism in patients with dyslipidemia (P ≤ 0.04 for all) and T2DM (P ≤ 0.001 for all). The prevalence of AA genotype, AT genotype, AA + AT genotypes and A allele of the rs9939609 polymorphism was 1.69%, 25.46%, 27.15% and 14.42% in dyslipidemia patients, as compared to 1.05%, 21.23%, 22.28% and 11.66% in dyslipidemia-free subjects, respectively (P ≤ 0.04 for all). The prevalence of AA genotype, AT genotype, AA + AT genotypes and A allele of the rs9939609 polymorphism was 2.79%, 28.48%, 31.27% and 17.03% in T2DM patients, as compared to 0.85%, 21.50%, 22.35% and 11.60% in T2DM-free subjects, respectively (P ≤ 0.001 for all). Associations of FTO rs17817449 polymorphism with metabolic-related diseases are shown in Table 3. The prevalence of GG genotype, GT genotype, GG + GT genotypes and G allele of the rs17817449 polymorphism was 2.02%, 27.32%, 29.34% and 15.68% in dyslipidemia patients, as compared to 1.32%, 22.80%, 24.12% and 12.71% in dyslipidemia-free subjects, respectively (P = 0.03 for all). The prevalence of GG genotype, GT genotype, GG + GT genotypes and G allele of the rs17817449 polymorphism was 3.10%, 29.41%, 32.51% and 17.80% in T2DM patients, as compared to 1.14%, 23.48%, 24.62% and 12.88% in T2DM-free subjects, respectively (P < 0.01 for all). However, FTO rs9939609 and rs17817449 polymorphisms were not associated with obesity, hyperhomocysteinemia, hyperuricemia, hypertension and CAD in this study population.
Multivariate logistic regression analyses between the FTO rs9939609 and rs17817449 polymorphisms and T2DM as well as dyslipidemia
The results of multivariate logistic regression analyses between the FTO rs9939609 and rs17817449 polymorphisms and T2DM as well as dyslipidemia are shown in Table 4. The FTO rs9939609 polymorphism was independently associated with T2DM after controlling for the confounding variables including age, sex, smoking, obesity, dyslipidemia, hyperhomocysteinemia, hyperuricemia, hypertension and CAD (P < 0.01). The FTO rs9939609 polymorphism was independently associated with dyslipidemia after adusting for age, sex and smoking (P = 0.05), but the model lost its significance when the variables including obesity, dyslipidemia, hyperhomocysteinemia, hyperuricemia, hypertension and CAD were included (P = 0.12). The FTO rs17817449 polymorphism was independently associated with both T2DM (P = 0.04) and dyslipidemia (P = 0.05) after adjusting for the confounding variables including age, sex, smoking, obesity, dyslipidemia, hyperhomocysteinemia, hyperuricemia, hypertension and CAD.
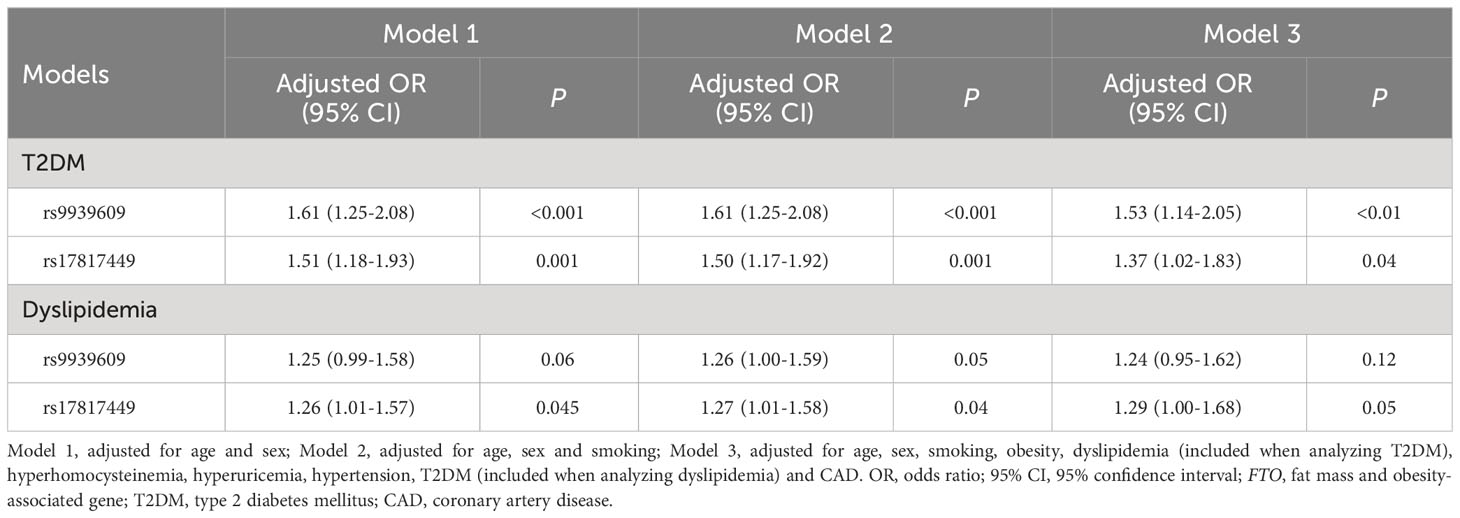
Table 4 Multivariate logistic regression analysis between the FTO rs9939609 and rs17817449 polymorphisms and T2DM as well as dyslipidemia.
Associations of PPARD rs2016520 and rs2267668 polymorphisms with metabolic-related diseases
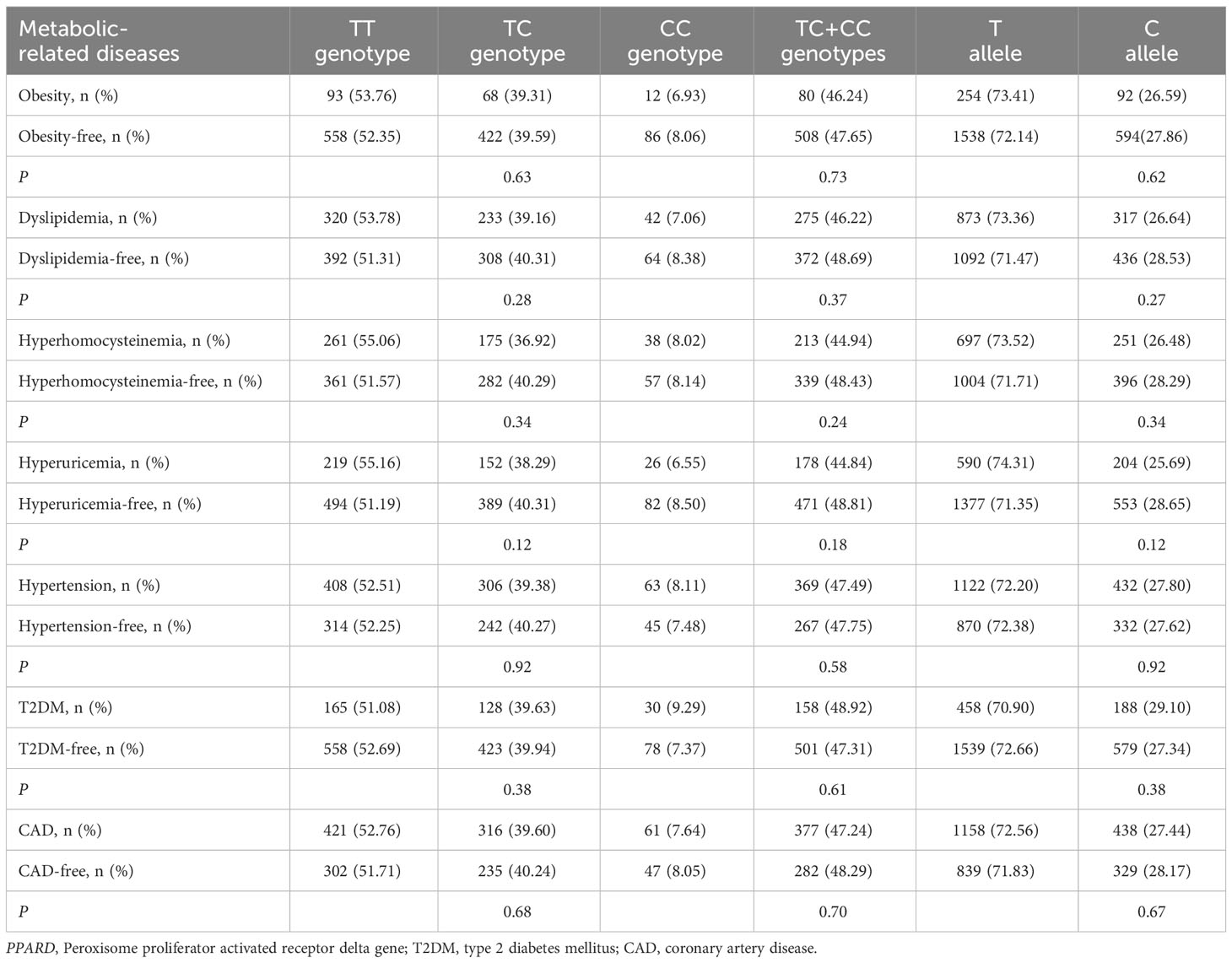
As shown in Tables 5, 6, no significant associations of PPARD rs2016520 and rs2267668 polymorphisms with obesity, dyslipidemia, hyperhomocysteinemia, hyperuricemia, hypertension, T2DM and CAD were detected in the present study (P > 0.05 for all).
Interactions of FTO rs9939609 and rs17817449 polymorphisms with obesity and other factors on the risk of dyslipidemia and T2DM
There was a significant interaction between FTO rs9939609 polymorphism and obesity or T2DM on dyslipidemia risk (Table 7). The prevalence of dyslipidemia was higher in obese or T2DM carriers of AA or AT genotype of the rs9939609 polymorphism than in non-obese or non-T2DM carriers of AA or AT genotype (P = 0.03 for both). Interestingly, carriers of AA or AT genotype of the rs9939609 polymorphism had a marginally nonsignificantly lower incidence of dyslipidemia in smokers than those in nonsmokers (P = 0.07). There may also be an interaction between the rs9939609 polymorphism and obesity on T2DM risk, since obese carriers of AA or AT genotype of the rs9939609 polymorphism had a marginally insignificantly higher incidence of T2DM compared to non-obese carriers of AA or AT genotype (P = 0.09). Significant interaction between FTO rs17817449 polymorphism and obesity on dyslipidemia risk has been observed (Table 8). Obese carriers of GG or GT genotype of the rs17817449 polymorphism had a higher incidence of dyslipidemia than non-obese carriers of GG or GT genotype (P < 0.01). Similar to the rs9939609 polymorphism, carriers of GG or GT genotype of the rs17817449 polymorphism had a marginally insignificantly lower incidence of dyslipidemia in smokers than those in nonsmokers (P = 0.08). No other interactions on dyslipidemia or T2DM risk were detected in the present study.
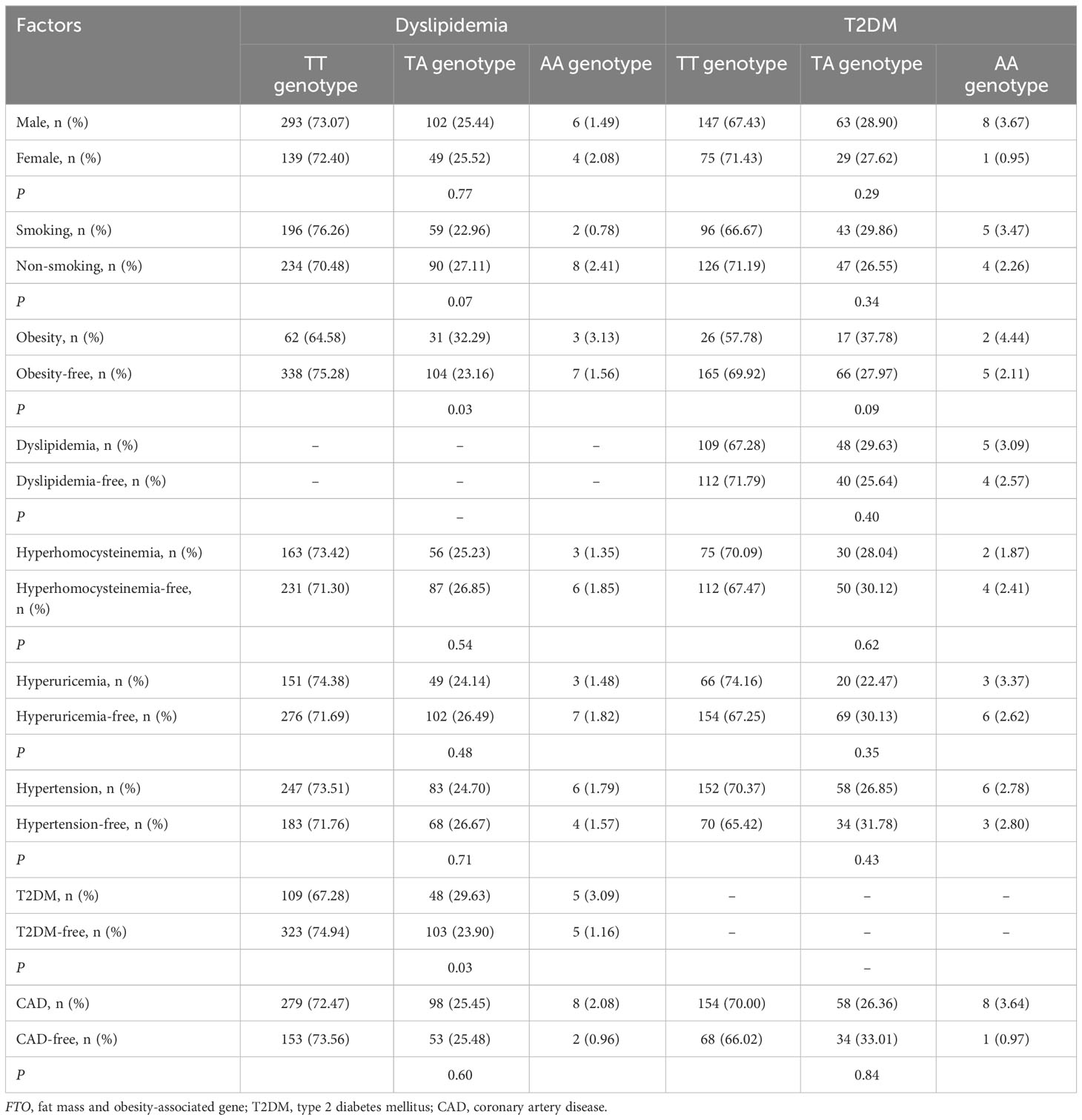
Table 7 Interactions of FTO rs9939609 polymorphism with obesity and other factors on the risk of T2DM or dyslipidemia.
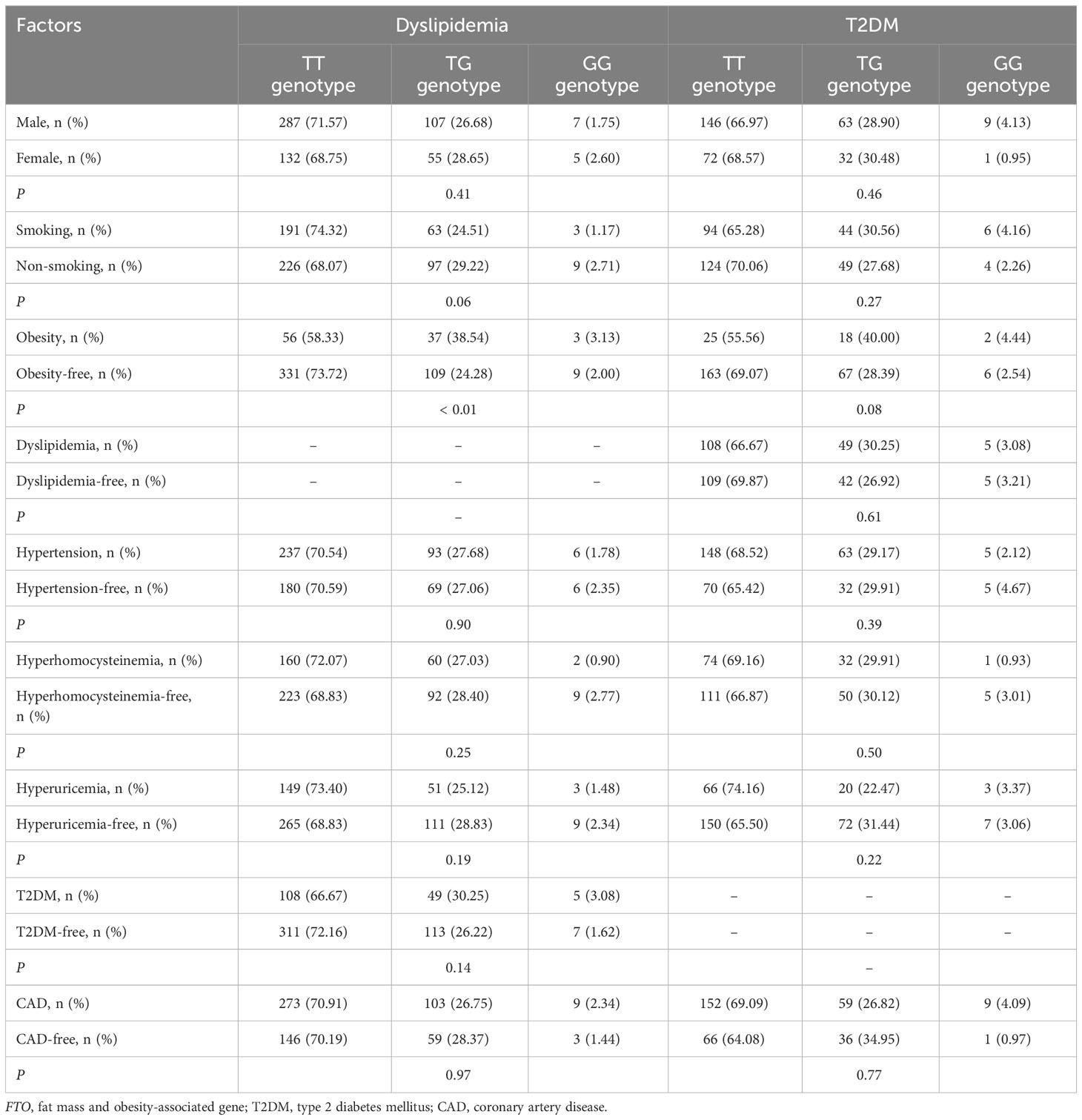
Table 8 Interactions of FTO rs17817449 polymorphism with obesity and other factors on the risk of T2DM and dyslipidemia.
Discussion
The present study demonstrates that FTO rs9939609 and rs17817449 polymorphisms are significantly and independently associated with the susceptibility to dyslipidemia and T2DM in a Chinese Han population. The A allele of rs9939609 and G allele of rs17817449 polymorphisms confer a higher risk of dyslipidemia and T2DM. In addition, carriers of the A allele of the rs9939609 polymorphism or G allele of the rs17817449 polymorphism have a higher risk of developing dyslipidemia in obese patients than those in non-obese individuals. To our knowledge, this is the first time to demonstrate that the relations between the rs9939609 and rs17817449 polymorphisms and dyslipidemia are modulated by obesity.
In line with our findings, a series of studies have reported significant correlations of FTO rs9939609 and rs17817449 polymorphisms with the risk of T2DM and dyslipidemia (24, 28, 29, 33, 46–51). Younus et al. conducted a case-control study to investigate the impact of the rs9939609 and rs17817449 polymorphisms on the development of T2DM in a sample of obese Iraqis, and found that the minor alleles of both variants are associated with an increased risk of T2DM (33). In addition, the significant relation between the rs9939609 and rs17817449 polymorphisms and T2DM risk was reported in Vietnamese (28), Indians (29), Egyptians (47) and Czechs (48) and Pakistanis (51). Sierra-Ruelas and teammates explored the association of the rs9939609 variant with serum lipids among Mexican adults, and found that the A allele carriers had significantly higher levels of TC and LDL-C (24). Jalili et al. observed that the A allele carriers of the rs9939609 polymorphism had a lower average level of HDL-C than noncarriers in a cohort of Iranian women (49). In a population of Romanian Children, researchers demonstrated that the G allele carriers of the rs17817449 variant had higher levels of TC and TG (50).
As the rs9939609 and rs17817449 polymorphic sites are localized within FTO, a gene with the strongest association with obesity discovered so far, a large number studies have reported a significant association between the rs9939609 and rs17817449 polymorphisms and obesity among various populations including East and South Asians (52–58), Latin Americans (23, 59–61), Europeans (62), Africans (63) and Middle Easterners (64). The A allele of the rs9939609 polymorphism was also found to be associated with an increased risk of obesity in several cohorts of children respectively from Romania (50), China (65), Portugal (66) and Chile (67). These relations were further strengthened with a genome-wide association study (68) and a couple of meta-analyses (69, 70) which consistently demonstrated that the A allele of rs9939609 and G allele of rs17817449 polymorphisms confer a higher risk of obesity in children and adults. Somehow, no significant associations between the rs9939609 and rs17817449 polymorphisms and obesity were found in our study population. One of the reasons could be that the present study population was not made up of general or healthy individuals, but those people who came to hospital to see doctors with chest pain symptoms and suspected CAD. In fact, more than half of these subjects had CAD or hypertension, and close to half of them had dyslipidemia. In addition, quite a few subjects in this study had T2DM, obesity, hyperhomocysteinemia, hyperuricemia, dyslipidemia and other diseases. Similarly, Sylwia et al. also reported that the rs17817449 polymorphism is not related to obesity in a population of patients with CAD, diabetes mellitus, hypertension and dyslipidemia (27).
The results of this study indicated that PPARD rs2016520 and rs2267668 polymorphisms are not associated with any of the metabolic-related diseases including obesity, dyslipidemia, hyperhomocysteinemia, hyperuricemia, hypertension, T2DM and CAD. In agreement with our findings, several previous studies also demonstrated no associations between the genotypes of PPARD rs2016520 or rs2267668 polymorphism and the risk of obesity (41), dyslipidemia (41, 71, 72), T2DM (41, 73) or CAD (42). However, Jguirim-Souissi et al. (72) concluded that the rs2016520 polymorphism is an independent risk factor of CAD, although it was not found to be associated with plasma lipid levels either in CAD patients or in control subjects. Chehaibi and colleagues (71) obtained a similar finding that the C allele frequency of PPARD rs2016520 polymorphism was higher in stroke patients than in controls, but plasma lipid levels did not differ significantly between the genotypes. Two studies demonstrated the rs2016520 polymorphism is associated with both CAD and blood lipids (74, 75). Maculewicz et al. (39) found that the rs2267668 polymorphism is significantly associated with BMI. As mentioned above, there were numerous inconsistent reports in the scientific community regarding these two PPARD polymorphic sites and metabolic-related diseases. The main reason for these inconsistencies may be that PPARδ as well as its gene polymorphisms just have a weak impact on the metabolic-related diseases, so their relations are easily affected by environmental and other factors. Among the three members of PPAR family, PPARγ is most closely related to metabolic-related diseases, PPARα being the next, and both of them have been served as drug targets for clinical use (3).
The mechanisms underlying the correlations between FTO rs9939609 and rs17817449 polymorphisms and T2DM as well as dyslipidemia have not been fully elucidated. The first potential explanation could be that the rs9939609 and rs17817449 polymorphisms have led to aberrant expression of FTO, which in turn disturbs the methylation status of FTO-targeted mRNAs, resulting in abnormalities in glucose and lipid metabolism. Indeed, Villalobos-Comparán et al. observed that the A allele carriers of the rs9939609 variant expressed significantly more FTO mRNA than those with the TT genotype in biopsies of subcutaneous fat tissue of Mexican women (61, 76). Karra et al. (18) and Berulava et al. (77) had similar finding that the A allele carriers of the rs9939609 polymorphism had significantly more abundant FTO transcripts in skin biopsies and/or blood cells than those with the TT genotype. The target mRNAs of FTO, such as SREBP1c (10–12), ChREBP (12), PPARα (13), PPARγ (14) and PGC1α (15), are all well-known to be extensively involved in glucose and lipid metabolism. The second explanation may involve energy intake. Ghrelin is a stomach hormone that has been proved to stimulate appetite and increase energy intake (78). Karra et al. found that subjects with the AA genotype of the rs9939609 polymorphism had elevated FTO transcripts, decreased ghrelin mRNA m6A methylation, and increased ghrelin levels as compared to the TT homozygotes (18). Several other studies have also demonstrated that FTO polymorphisms are highly associated with appetite, satiety, and energy intake in children and adolescents (79–81). Therefore, the association between the rs9939609 polymorphism and T2DM or dyslipidemia may be mediated by excessive energy intake. The third explanation may be that the rs9939609 and rs17817449 polymorphisms work in concert with other genetic variants to affect the susceptibility of obesity, diabetes and hypertension (82–84). For instance, Sirtuin 1 (SIRT1), a histone deacetylase and an anti-aging gene, is involved in the regulation of PPAR with relevance to lipid metabolism in the adipose tissue and liver, and is associated with metabolic-related diseases, such as metabolic syndrome and cardiovascular disease (85, 86). FTO polymorphisms may interact with the genetic variants in SIRT1 and other genes, and jointly regulate glucose and lipid metabolism.
There are several limitations to this study. First, the research population was not from general subjects, but a special group of people who had symptoms of chest pain came to the hospital for treatment and underwent coronary angiography examination. This may lead to sample-selection bias. Second, the research subjects are mainly made up of Chinese Han people, and hence the findings of this study may not apply to other ethnic origins. Third, the research adopted a cross-sectional design. It is necessary to carry out a follow-up investigation to determine the exact roles of the polymorphisms in FTO and PPARD in metabolic-related diseases.
Conclusions
Minor alleles of FTO rs9939609 and rs17817449 polymorphisms confer a higher risk of T2DM and dyslipidemia, and the risk is further increased among obese individuals. Relations between FTO polymorphisms and T2DM as well as dyslipidemia are possibly mediated by abnormal glucolipid metabolism and increased energy intake.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author/s.
Ethics statement
The study protocol was reviewed and approved by the Ethics Committee of Chengdu University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants or the participants’ legal guardians/next of kin.
Author contributions
YS, YZ and LC conceived of the study, participated in the design, analyzed the data, and drafted the manuscript. LC, JZ and CH made the diagnosis of metabolic-related diseases. YZ, HL, LX and YW carried out DNA extraction, genotyping and collection of clinical data. SY revised the manuscript critically for important intellectual content. All authors reviewed and approved the final version of the manuscript for submission.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Natural Science Foundation of Sichuan, China [2022NSFSC0740], the Chengdu Medical Research Project (2022554), the Research Project of Clinical Medical College & Affiliated Hospital of Chengdu University [Y202244], and the Innovation Team Project of Affiliated Hospital of Chengdu University [CDFYCX202202].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1249070/full#supplementary-material
References
1. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: A report from the american heart association. Circulation (2023) 147(8):e93–e621. doi: 10.1161/CIR.0000000000001123
2. Song Y, Wade H, Zhang B, Xu W, Wu R, Li S, et al. Polymorphisms of fat mass and obesity-associated gene in the pathogenesis of child and adolescent metabolic syndrome. Nutrients (2023) 15:2643. doi: 10.3390/nu15122643
3. Song Y, Li S, He C. PPARγ Gene polymorphisms, metabolic disorders, and coronary artery disease. Front Cardiovasc Med (2022) 9:808929. doi: 10.3389/fcvm.2022.808929
4. Vourdoumpa A, Paltoglou G, Charmandari E. The genetic basis of childhood obesity: A systematic review. Nutrients (2023) 15(6):1416. doi: 10.3390/nu15061416
5. Perumalsamy S, Huri HZ, Abdullah BM, Mazlan O, Wan Ahmad WA, Vethakkan SRDB. Genetic markers of insulin resistance and atherosclerosis in type 2 diabetes mellitus patients with coronary artery disease. Metabolites (2023) 13(3):427. doi: 10.3390/metabo13030427
6. Li S, Zhang Y, Xu W, Lv Z, Xu L, Zhao Z, et al. C Allele of the PPARδ+294T>C Polymorphism Confers a Higher Risk of Hypercholesterolemia, but not Obesity and Insulin Resistance: A Systematic Review and Meta-Analysis. Horm Metab Res (2023) 55(5):355–66. doi: 10.1055/a-2043-7707
7. Li S, He C, Nie H, Pang Q, Wang R, Zeng Z, et al. G Allele of the rs1801282 Polymorphism in PPARγ Gene Confers an Increased Risk of Obesity and Hypercholesterolemia, While T Allele of the rs3856806 Polymorphism Displays a Protective Role Against Dyslipidemia: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) (2022) 13:919087. doi: 10.3389/fendo.2022.919087
8. Xu ZY, Jing X, Xiong XD. Emerging role and mechanism of the FTO gene in cardiovascular diseases. Biomolecules (2023) 13(5):850. doi: 10.3390/biom13050850
9. Azzam SK, Alsafar H, Sajini AA. FTO m6A demethylase in obesity and cancer: implications and underlying molecular mechanisms. Int J Mol Sci (2022) 23(7):3800. doi: 10.3390/ijms23073800
10. Hu Y, Feng Y, Zhang L, Jia Y, Cai D, Qian SB, et al. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of m(6)A on lipogenic mRNAs. RNA Biol (2020) 17(7):930–42. doi: 10.1080/15476286.2020.1736868
11. Chen A, Chen X, Cheng S, Shu L, Yan M, Yao L, et al. FTO promotes SREBP1c maturation and enhances CIDEC transcription during lipid accumulation in HepG2 cells. Biochim Biophys Acta Mol Cell Biol Lipids (2018) 1863(5):538–48. doi: 10.1016/j.bbalip.2018.02.003
12. Tang Z, Sun C, Yan Y, Niu Z, Li Y, Xu X, et al. Aberrant elevation of FTO levels promotes liver steatosis by decreasing the m6A methylation and increasing the stability of SREBF1 and ChREBP mRNAs. J Mol Cell Biol (2023) 14(9):538–48. doi: 10.1093/jmcb/mjac061
13. Wei X, Zhang J, Tang M, Wang X, Fan N, Peng Y. Fat mass and obesity-associated protein promotes liver steatosis by targeting PPARα. Lipids Health Dis (2022) 21(1):29. doi: 10.1186/s12944-022-01640-y
14. Chen LS, Zhang M, Chen P, Xiong XF, Liu PQ, Wang HB, et al. The m(6)A demethylase FTO promotes the osteogenesis of mesenchymal stem cells by downregulating PPARG. Acta Pharmacol Sin (2022) 43(5):1311–23. doi: 10.1038/s41401-021-00756-8
15. Zhuang C, Zhuang C, Luo X, Huang X, Yao L, Li J, et al. N6-methyladenosine demethylase FTO suppresses clear cell renal cell carcinoma through a novel FTO-PGC-1α signalling axis. J Cell Mol Med (2019) 23(3):2163–73. doi: 10.1111/jcmm.14128
16. Huang J, Sun W, Wang Z, Lv C, Zhang T, Zhang D, et al. FTO suppresses glycolysis and growth of papillary thyroid cancer via decreasing stability of APOE mRNA in an N6-methyladenosine-dependent manner. J Exp Clin Cancer Res (2022) 41(1):42. doi: 10.1186/s13046-022-02254-z
17. Tews D, Fischer-Posovszky P, Fromme T, Klingenspor M, Fischer J, Rüther U, et al. FTO deficiency induces UCP-1 expression and mitochondrial uncoupling in adipocytes. Endocrinology (2013) 154(9):3141–51. doi: 10.1210/en.2012-1873
18. Karra E, O'Daly OG, Choudhury AI, Yousseif A, Millership S, Neary MT, et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest (2013) 123(8):3539–51. doi: 10.1172/JCI44403
19. Piwonska AM, Cicha-Mikolajczyk A, Sobczyk-Kopciol A, Piwonski J, Drygas W, Kwasniewska M, et al. Independent association of FTO rs9939609 polymorphism with overweight and obesity in Polish adults. Results from the representative population-based WOBASZ study. J Physiol Pharmacol (2022) 73(3):395–402. doi: 10.26402/jpp.2022.3.07
20. Shahid SU, Shabana Rehman A, Hasnain S. Role of a common variant of Fat Mass and Obesity associated (FTO) gene in obesity and coronary artery disease in subjects from Punjab, Pakistan: a case control study. Lipids Health Dis (2016) 15:29. doi: 10.1186/s12944-016-0200-0
21. Huđek A, Škara L, Smolkovič B, Kazazić S, Ravlić S, Nanić L, et al. Higher prevalence of FTO gene risk genotypes AA rs9939609, CC rs1421085, and GG rs17817449 and saliva containing Staphylococcus aureus in obese women in Croatia. Nutr Res (2017) 50:94–103. doi: 10.1016/j.nutres.2017.12.005
22. Ursu RI, Badiu C, Cucu N, Ursu GF, Craciunescu I, Severin E. The study of the rs9939609 FTO gene polymorphism in association with obesity and the management of obesity in a Romanian cohort. J Med Life (2015) 8(2):232–8.
23. Fonseca ACPD, Marchesini B, Zembrzuski VM, Voigt DD, Ramos VG, Carneiro JRI, et al. Genetic variants in the fat mass and obesity-associated (FTO) gene confer risk for extreme obesity and modulate adiposity in a Brazilian population. Genet Mol Biol (2020) 43(1):e20180264. doi: 10.1590/1678-4685-gmb-2018-0264
24. Sierra-Ruelas E, Campos-Pérez W, Torres-Castillo N, García-Solís P, Vizmanos B, Martínez-López E. The rs9939609 variant in FTO increases the risk of hypercholesterolemia in metabolically healthy subjects with excess weight. Lifestyle Genom (2022) 15(4):131–8. doi: 10.1159/000527097
25. Xi B, Zhao X, Shen Y, Wu L, Hotta K, Hou D, et al. Associations of obesity susceptibility loci with hypertension in Chinese children. Int J Obes (Lond) (2013) 37(7):926–30. doi: 10.1038/ijo.2013.37
26. Legry V, Cottel D, Ferrières J, Arveiler D, Andrieux N, Bingham A, et al. Effect of an FTO polymorphism on fat mass, obesity, and type 2 diabetes mellitus in the French MONICA Study. Metabolism (2009) 58(7):971–5. doi: 10.1016/j.metabol.2009.02.019
27. Mielcarska S, Stopińska K, Poręba M, Szywacz W, Macionga A, Szweda-Gandor N, et al. Influence of the FTO polymorphism rs17817449 on the risk of obesity, type 2 diabetes, and cardiovascular diseases in Upper Silesian population a preliminary, cross-sectional study. Med Res J (2022) 7(2):134–41. doi: 10.5603/MRJ.a2022.0019
28. Binh TQ, Linh DT, Chung LTK, Phuong PT, Nga BTT, Ngoc NA, et al. FTO-rs9939609 polymorphism is a predictor of future type 2 diabetes: A population-based prospective study. Biochem Genet (2022) 60(2):707–19. doi: 10.1007/s10528-021-10124-0
29. Irgam K, Reddy BS, Hari SG, Banapuram S, Reddy BM. The genetic susceptibility profile of type 2 diabetes and reflection of its possible role related to reproductive dysfunctions in the southern Indian population of Hyderabad. BMC Med Genomics (2021) 14(1):272. doi: 10.1186/s12920-021-01129-0
30. Zhong QQ, Zhu F. Genetic loci, rs17817449 and rs6567160, known for obesity and the risk of stroke events among middle-aged and older Chinese people. Front Neurol (2022) 13:1036750. doi: 10.3389/fneur.2022.1036750
31. Mofarrah M, Ziaee S, Pilehvar-Soltanahmadi Y, Zarghami F, Boroumand M, Zarghami N. Association of KALRN, ADIPOQ, and FTO gene polymorphism in type 2 diabetic patients with coronary artery disease: possible predisposing markers. Coron Artery Dis (2016) 27(6):490–6. doi: 10.1097/MCA.0000000000000386
32. Huang S, Qin P, Chen Q, Zhang D, Cheng C, Guo C, et al. Association of FTO gene methylation with incident type 2 diabetes mellitus: A nested case-control study. Gene (2021) 786:145585. doi: 10.1016/j.gene.2021.145585
33. Younus LA, Algenabi AHA, Abdul-Zhara MS, Hussein MK. FTO gene polymorphisms (rs9939609 and rs17817449) as predictors of Type 2 Diabetes Mellitus in obese Iraqi population. Gene (2017) 627:79–84. doi: 10.1016/j.gene.2017.06.005
34. Guisantes-Batan E, Mazuecos L, Rubio B, Pereira-Caro G, Moreno-Rojas JM, Andrés A, et al. Grape seed extract supplementation modulates hepatic lipid metabolism in rats. Implication of PPARβ/δ. Food Funct (2022) 13(21):11353–68. doi: 10.1039/D2FO02199D
35. Palomer X, Barroso E, Zarei M, Botteri G, Vázquez-Carrera M. PPARβ/δ and lipid metabolism in the heart. Biochim Biophys Acta (2016) 1861(10):1569–78. doi: 10.1016/j.bbalip.2016.01.019
36. Zarei M, Aguilar-Recarte D, Palomer X, Vázquez-Carrera M. Revealing the role of peroxisome proliferator-activated receptor β/δ in nonalcoholic fatty liver disease. Metabolism (2021) 114:154342. doi: 10.1016/j.metabol.2020.154342
37. Shin KC, Huh JY, Ji Y, Han JS, Han SM, Park J, et al. VLDL-VLDLR axis facilitates brown fat thermogenesis through replenishment of lipid fuels and PPARβ/δ activation. Cell Rep (2022) 41(11):111806. doi: 10.1016/j.celrep.2022.111806
38. Rachid TL, Silva-Veiga FM, Graus-Nunes F, Bringhenti I, Mandarim-de-Lacerda CA, Souza-Mello V. Differential actions of PPAR-α and PPAR-β/δ on beige adipocyte formation: A study in the subcutaneous white adipose tissue of obese male mice. PloS One (2018) 13(1):e0191365. doi: 10.1371/journal.pone.0191365
39. Maculewicz E, Mastalerz A, Maciejewska-Skrendo A, Cięszczyk P, Cywińska A, Borecka A, et al. Association between peroxisome proliferator-activated receptor-alpha, -delta and -gamma gene (PPARA, PPARD, PPARG) polymorphisms and overweight parameters in physically active men. Biol Sport (2021) 38(4):767–76. doi: 10.5114/biolsport.2022.109957
40. Ke T, Dorajoo R, Han Y, Khor CC, van Dam RM, Yuan JM, et al. Interaction between peroxisome proliferator activated receptor δ and epithelial membrane protein 2 polymorphisms influences HDL-C levels in the chinese population. Ann Hum Genet (2016) 80(5):282–93. doi: 10.1111/ahg.12164
41. Gouni-Berthold I, Giannakidou E, Faust M, Berthold HK, Krone W. The peroxisome proliferator-activated receptor delta +294T/C polymorphism in relation to lipoprotein metabolism in patients with diabetes mellitus type 2 and in non-diabetic controls. Atherosclerosis (2005) 183(2):336–41. doi: 10.1016/j.atherosclerosis.2005.03.016
42. Maciejewska-Skrendo A, Pawlik A, Sawczuk M, Rać M, Kusak A, Safranow K, et al. PPARA, PPARD and PPARG gene polymorphisms in patients with unstable angina. Gene (2019) 711:143947. doi: 10.1016/j.gene.2019.143947
43. Joint Committee on the Chinese Guidelines for Lipid Management. Chinese guidelines for lipid management (2023). Zhonghua Xin Xue Guan Bing Za Zhi (2023) 51(3):221–55. doi: 10.3760/cma.j.cn112148-20230119-00038
44. Chinese Nutrition Society Obesity Prevention and Control Section, Chinese Nutrition Society Clinical Nutrition Section, Chinese Preventive Medicine Association Behavioral Health Section, Chinese Preventive Medicine Association Sports and Health Section. Expert consensus on obesity prevention and treatment in China. Zhonghua Liu Xing Bing Xue Za Zhi (2022) 43(5):609–26. doi: 10.3760/cma.j.cn112338-20220402-00253
45. China Hypertension Prevention and Control Guidelines Revision Committee, Hypertension Alliance (China); Cardiovascular Branch of the Chinese Medical Association, Chinese Medical Doctor Association Hypertension Professional Committee, China Association for the Promotion of International Exchange in Healthcare, Hypertension Branch of the Chinese Geriatric Association Hypertension Branch. 2018 Chinese guidelines for the management of hypertension. Zhongguo Xin Xue Gua Za Zhi (2019) 24(1):24–56. doi: 10.3969/j.issn.1007-5410.2019.01.002
46. Velazquez-Roman J, Angulo-Zamudio UA, León-Sicairos N, Medina-Serrano J, DeLira-Bustillos N, Villamil-Ramírez H, et al. Association of FTO, ABCA1, ADRB3, and PPARG variants with obesity, type 2 diabetes, and metabolic syndrome in a Northwest Mexican adult population. J Diabetes Complications (2021) 35(11):108025. doi: 10.1016/j.jdiacomp.2021.108025
47. Barseem NF, El Ella SSA, Tawfik MA, El-Nehrawy RR. The potential implication of FTO rs17817449 gene polymorphism on BMI mediated risk for type2 diabetes among obese Egyptian children and adolescents. Endocr Metab Immune Disord Drug Targets (2019) 19(5):697–704. doi: 10.2174/1871530319666190101124751
48. Hubacek JA, Dlouha D, Klementova M, Lanska V, Neskudla T, Pelikanova T. The FTO variant is associated with chronic complications of diabetes mellitus in Czech population. Gene (2018) 642:220–4. doi: 10.1016/j.gene.2017.11.040
49. Jalili V, Mokhtari Z, Rastgoo S, Hajipour A, Bourbour F, Gholamalizadeh M, et al. The association between FTO rs9939609 polymorphism and serum lipid profile in adult women. Diabetol Metab Syndr (2021) 13(1):138. doi: 10.1186/s13098-021-00754-0
50. Duicu C, Mărginean CO, Voidăzan S, Tripon F, Bănescu C. FTO rs 9939609 SNP is associated with adiponectin and leptin levels and the risk of obesity in a cohort of Romanian children population. Med (Baltimore) (2016) 95(20):e3709. doi: 10.1097/MD.0000000000003709
51. Shaikh F, Shah T, Madkhali NAB, Gaber A, Alsanie WF, Ali S, et al. Frequency distribution and association of Fat-mass and obesity (FTO) gene SNP rs-9939609 variant with Diabetes Mellitus Type-II population of Hyderabad, Sindh, Pakistan. Saudi J Biol Sci (2021) 28(8):4183–90. doi: 10.1016/j.sjbs.2021.06.001
52. Li X, Song F, Jiang H, Zhang M, Lin J, Bao W, et al. A genetic variation in the fat mass- and obesity-associated gene is associated with obesity and newly diagnosed type 2 diabetes in a Chinese population. Diabetes Metab Res Rev (2010) 26(2):128–32. doi: 10.1002/dmrr.1066
53. Karasawa S, Daimon M, Sasaki S, Toriyama S, Oizumi T, Susa S, et al. Association of the common fat mass and obesity associated (FTO) gene polymorphism with obesity in a Japanese population. Endocr J (2010) 57(4):293–301. doi: 10.1507/endocrj.K09E-305
54. Chang YC, Liu PH, Lee WJ, Chang TJ, Jiang YD, Li HY, et al. Common variation in the fat mass and obesity-associated (FTO) gene confers risk of obesity and modulates BMI in the Chinese population. Diabetes (2008) 57(8):2245–52. doi: 10.2337/db08-0377
55. Hotta K, Nakata Y, Matsuo T, Kamohara S, Kotani K, Komatsu R, et al. Variations in the FTO gene are associated with severe obesity in the Japanese. J Hum Genet (2008) 53(6):546–53. doi: 10.1007/s10038-008-0283-1
56. Srivastava A, Mittal B, Prakash J, Srivastava P, Srivastava N, Srivastava N. Association of FTO and IRX3 genetic variants to obesity risk in north India. Ann Hum Biol (2016) 43(5):451–6. doi: 10.3109/03014460.2015.1103902
57. Prakash J, Srivastava N, Awasthi S, Agarwal CG, Natu SM, Rajpal N, et al. Association of FTO rs17817449 SNP with obesity and associated physiological parameters in a north Indian population. Ann Hum Biol (2011) 38(6):760–3. doi: 10.3109/03014460.2011.614278
58. Xi B, Mi J. FTO polymorphisms are associated with obesity but not with diabetes in East Asian populations: a meta-analysis. BioMed Environ Sci (2009) 22(6):449–57. doi: 10.1016/S0895-3988(10)60001-3
59. Castro GV, Latorre AFS, Korndorfer FP, de Carlos Back LK, Lofgren SE. The impact of variants in four genes: MC4R, FTO, PPARG and PPARGC1A in overweight and obesity in a large sample of the Brazilian population. Biochem Genet (2021) 59(6):1666–79. doi: 10.1007/s10528-021-10079-2
60. Saldaña-Alvarez Y, Salas-Martínez MG, García-Ortiz H, Luckie-Duque A, García-Cárdenas G, Vicenteño-Ayala H, et al. Gender-dependent association of FTO polymorphisms with body mass index in mexicans. PloS One (2016) 11(1):e0145984. doi: 10.1371/journal.pone.0145984
61. Villalobos-Comparán M, Teresa Flores-Dorantes M, Teresa Villarreal-Molina M, Rodríguez-Cruz M, García-Ulloa AC, Robles L, et al. The FTO gene is associated with adulthood obesity in the Mexican population. Obes (Silver Spring) (2008) 16(10):2296–301. doi: 10.1038/oby.2008.367
62. Carlos FF, Silva-Nunes J, Flores O, Brito M, Doria G, Veiga L, et al. Association of FTO and PPARG polymorphisms with obesity in Portuguese women. Diabetes Metab Syndr Obes (2013) 6:241–5. doi: 10.2147/DMSO.S45779
63. Ali EMM, Diab T, Elsaid A, Abd El Daim HA, Elshazli RM, Settin A. Fat mass and obesity-associated (FTO) and leptin receptor (LEPR) gene polymorphisms in Egyptian obese subjects. Arch Physiol Biochem (2021) 127(1):28–36. doi: 10.1080/13813455.2019.1573841
64. Saqlain M, Khalid M, Fiaz M, Saeed S, Mehmood Raja A, Mobeen Zafar M, et al. Risk variants of obesity associated genes demonstrate BMI raising effect in a large cohort. PloS One (2022) 17(9):e0274904. doi: 10.1371/journal.pone.0274904
65. Xu Y, Ling J, Yang M, Wang H, Zhang S, Zhang X, et al. Rs7206790 and rs11644943 in FTO gene are associated with risk of obesity in Chinese school-age population. PloS One (2014) 9(9):e108050. doi: 10.1371/journal.pone.0108050
66. Albuquerque D, Nóbrega C, Manco L. Association of FTO polymorphisms with obesity and obesity-related outcomes in Portuguese children. PloS One (2013) 8(1):e54370. doi: 10.1371/journal.pone.0054370
67. Riffo B, Asenjo S, Sáez K, Aguayo C, Muñoz I, Bustos P, et al. FTO gene is related to obesity in Chilean AmerIndian children and impairs HOMA-IR in prepubertal girls. Pediatr Diabetes (2012) 13(5):384–91. doi: 10.1111/j.1399-5448.2011.00834.x
68. Wang K, Li WD, Zhang CK, Wang Z, Glessner JT, Grant SF, et al. A genome-wide association study on obesity and obesity-related traits. PloS One (2011) 6(4):e18939. doi: 10.1371/journal.pone.0018939
69. Peng S, Zhu Y, Xu F, Ren X, Li X, Lai M. FTO gene polymorphisms and obesity risk: a meta-analysis. BMC Med (2011) 9:71. doi: 10.1186/1741-7015-9-71
70. da Silva TER, Andrade NL, Cunha DO, Leão-Cordeiro JAB, Vilanova-Costa CAST, Silva AMTC. The FTO rs9939609 polymorphism and obesity risk in teens: Evidence-based meta-analysis. Obes Res Clin Pract (2018) 12(5):432–7. doi: 10.1016/j.orcp.2018.08.001
71. Chehaibi K, Hrira MY, Rouis M, Najah M, Jguirim-Souissi I, Nouira S, et al. Effect of genetic polymorphism +294T/C in peroxisome proliferator-activated receptor delta on the risk of ischemic stroke in a Tunisian population. J Mol Neurosci (2013) 50(2):360–7. doi: 10.1007/s12031-013-9997-4
72. Jguirim-Souissi I, Jelassi A, Hrira Y, Najah M, Slimani A, Addad F, et al. +294T/C polymorphism in the PPAR-delta gene is associated with risk of coronary artery disease in normolipidemic Tunisians. Genet Mol Res (2010) 9(3):1326–33. doi: 10.4238/vol9-3gmr831
73. Lu L, Wu Y, Qi Q, Liu C, Gan W, Zhu J, et al. Associations of type 2 diabetes with common variants in PPARD and the modifying effect of vitamin D among middle-aged and elderly Chinese. PloS One (2012) 7(4):e34895. doi: 10.1371/journal.pone.0034895
74. Nikitin AG, Chistiakov DA, Minushkina LO, Zateyshchikov DA, Nosikov VV. Association of the CYBA, PPARGC1A, PPARG3, and PPARD gene variants with coronary artery disease and metabolic risk factors of coronary atherosclerosis in a Russian population. Heart Vessels (2010) 25(3):229–36. doi: 10.1007/s00380-009-1159-9
75. Skogsberg J, McMahon AD, Karpe F, Hamsten A, Packard CJ, Ehrenborg E, et al. Peroxisome proliferator activated receptor delta genotype in relation to cardiovascular risk factors and risk of coronary heart disease in hypercholesterolaemic men. J Intern Med (2003) 254(6):597–604. doi: 10.1111/j.1365-2796.2003.01236.x
76. Villalobos-Comparán M, Antuna-Puente B, Villarreal-Molina MT, Canizales-Quinteros S, Velázquez-Cruz R, León-Mimila P, et al. Interaction between FTO rs9939609 and the Native American-origin ABCA1 rs9282541 affects BMI in the admixed Mexican population. BMC Med Genet (2017) 18(1):46. doi: 10.1186/s12881-017-0410-y
77. Berulava T, Horsthemke B. The obesity-associated SNPs in intron 1 of the FTO gene affect primary transcript levels. Eur J Hum Genet (2010) 18(9):1054–6. doi: 10.1038/ejhg.2010.71
78. Smith A, Woodside B, Abizaid A. Ghrelin and the control of energy balance in females. Front Endocrinol (Lausanne) (2022) 13:904754. doi: 10.3389/fendo.2022.904754
79. Obregón Rivas AM, Santos JL, Valladares MA, Cameron J, Goldfield G. Association of the FTO fat mass and obesity-associated gene rs9939609 polymorphism with rewarding value of food and eating behavior in Chilean children. Nutrition (2018) 54:105–10. doi: 10.1016/j.nut.2018.03.001
80. Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obes (Lond) (2009) 33(1):42–5. doi: 10.1038/ijo.2008.174
81. Emond JA, Tovar A, Li Z, Lansigan RK, Gilbert-Diamond D. FTO genotype and weight status among preadolescents: Assessing the mediating effects of obesogenic appetitive traits. Appetite (2017) 117:321–9. doi: 10.1016/j.appet.2017.07.009
82. Ruiz-Díaz MS, Mena-Yi D, Gómez-Camargo D, Mora-García G. Interaction analysis of FTO and IRX3 genes with obesity and related metabolic disorders in an admixed Latin American population: a possible risk increases of body weight excess. Colomb Med (Cali) (2022) 53(2):e2044874. doi: 10.25100/cm.v53i2.4874
83. Xiao S, Zeng X, Fan Y, Su Y, Ma Q, Zhu J, et al. Gene polymorphism association with type 2 diabetes and related gene-gene and gene-environment interactions in a uyghur population. Med Sci Monit (2016) 22:474–87. doi: 10.12659/msm.895347
84. Kumar R, Kohli S, Alam P, Barkotoky R, Gupta M, Tyagi S, et al. Interactions between the FTO and GNB3 genes contribute to varied clinical phenotypes in hypertension. PloS One (2013) 8(5):e63934. doi: 10.1371/journal.pone.0063934
85. Andrikakou P, Reebye V, Vasconcelos D, Yoon S, Voutila J, George AJT, et al. Enhancing SIRT1 gene expression using small activating RNAs: A novel approach for reversing metabolic syndrome. Nucleic Acid Ther (2022) 32(6):486–96. doi: 10.1089/nat.2021.0115
Keywords: FTO, PPARD, polymorphism, T2DM, dyslipidemia
Citation: Zhang Y, Chen L, Zhu J, Liu H, Xu L, Wu Y, He C and Song Y (2023) Minor alleles of FTO rs9939609 and rs17817449 polymorphisms confer a higher risk of type 2 diabetes mellitus and dyslipidemia, but not coronary artery disease in a Chinese Han population. Front. Endocrinol. 14:1249070. doi: 10.3389/fendo.2023.1249070
Received: 02 August 2023; Accepted: 17 November 2023;
Published: 15 December 2023.
Edited by:
Shanel Raghubeer, Cape Peninsula University of Technology, South AfricaReviewed by:
Laith Younus, Jabir Ibn Hayyan Medical University, IraqIan James Martins, University of Western Australia, Australia
Copyright © 2023 Zhang, Chen, Zhu, Liu, Xu, Wu, He and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongyan Song, c29uZ3lvbmd5YW5AY2R1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Youjin Zhang1†
Youjin Zhang1† Yongyan Song
Yongyan Song