- 1Department of Endocrinology, Xinhua Hospital Affiliated with Shanghai Jiaotong University School of Medicine, Shanghai, China
- 2Health Management Center, Xinhua Hospital Affiliated with Shanghai Jiaotong University School of Medicine, Shanghai, China
Objectives: The objective of this study was to investigate changes in serum tumor markers in type 2 diabetes mellitus (T2DM) with microalbuminuria and analyze the relationship between tumor markers and microalbuminuria.
Methods: A total of 956 T2DM patients aged 40–70 years hospitalized in the Department of Endocrinology, Xinhua Hospital, China, affiliated with Shanghai Jiaotong University School of Medicine, were enrolled from January 2018 to December 2020. The sample comprised 313 T2DM patients with microalbuminuria and 643 T2DM patients with normal urinary microalbumin levels. After assessing the changes in serum tumor markers in T2DM with microalbuminuria, we analyzed the risk of microalbuminuria by the serum tumor marker category using multiple logistic regression analysis.
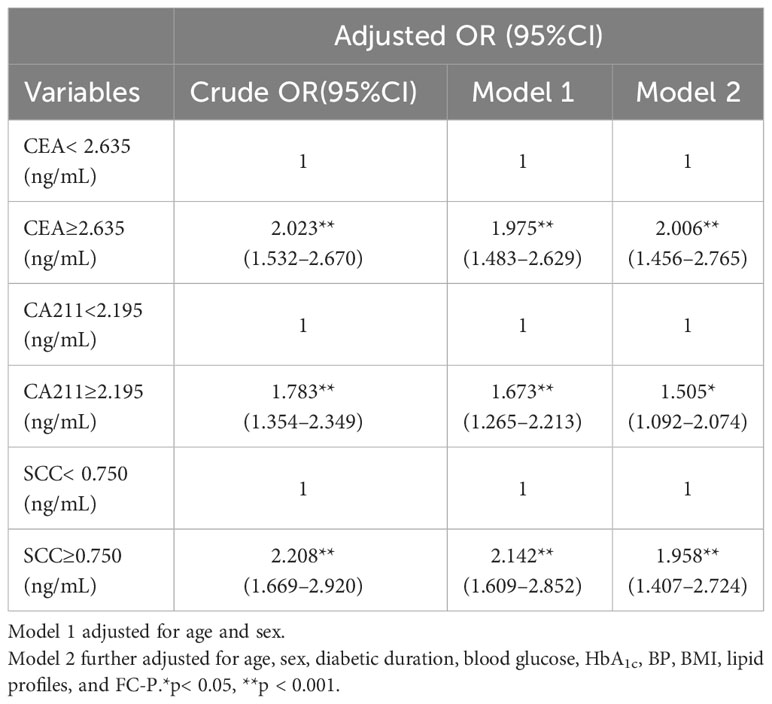
Results: Serum CEA, CA199, CA125, CA153, CA211, SCC, CA242, and CA50 levels were significantly higher in T2DM patients with microalbuminuria than in those without microalbuminuria, while serum AFP levels were lower in the microalbuminuria group (P < 0.05). Following adjustment of confounders, serum CEA, CA211, and SCC were independently associated with microalbuminuria in T2DM. An ROC curve was used to estimate the cutoff point of tumor markers for microalbuminuria. Taking the values under the cutoff points as a reference, values for CEA, CA211, and SCC above the cutoff points indicated a significantly high risk of microalbuminuria. The OR of increased CEA for microalbuminuria was 2.006 (95%CI 1.456–2.765), the OR of increased CA211 for microalbuminuria was 1.505 (95%CI 1.092–2.074), and the OR of increased SCC for microalbuminuria was 1.958 (95%CI 1.407–2.724).
Conclusion: Several serum tumor markers were related to microalbuminuria in T2DM. Serum tumor markers such as CEA, SCC, and CA211 may indicate early diabetic nephropathy, particularly when elevated in combination.
Introduction
Diabetes mellitus is a heterogeneous disease characterized by elevated blood glucose. Various genetic factors and environmental factors can lead to the dysfunction of pancreatic islet beta cells, resulting in a relative or absolute lack of insulin in the body, presenting as a hyperglycemic state (1). The International Diabetes Federation reported that global diabetes prevalence in adults aged 20–79 years reached 10.2% in 2021 and is projected to increase to 12.2% by 2045 (2). In 2021, global diabetes-related health costs were estimated to be 966 billion USD and are expected to reach 1054 billion USD by 2045 (2). China has witnessed one of the most dramatic rises in diabetes prevalence of anywhere in the world (3). It is well established that the prevalence of various cancers is higher in T2DM patients than in the general population (4). Serum tumor markers are widely used for cancer screening in clinical practice. Previous studies have reported connections between T2DM and several tumor markers are elevated in diabetic patients (5). Diabetic kidney disease (DKD) characterized by albuminuria is one of the most common vascular complications of diabetes. The urinary microalbumin-to-creatinine ratio (UACR) is usually recommended to screen and diagnose DKD. Whether a relationship exists between serum tumor markers and diabetic complications, particularly DKD, has rarely been reported. Hence, we conducted a cross-sectional study to explore the relationship between serum tumor markers and UACR and verify whether tumor markers are associated with microalbuminuria in T2DM. We also aimed to identify a new marker of early diabetic nephropathy and explain why tumor markers are increased in T2DM patients in the absence of malignant tumors.
Methods
Subjects
A total of 956 adult T2DM patients without a history of malignant tumor hospitalized in the Department of Endocrinology, Xinhua Hospital, China, affiliated with Shanghai Jiaotong University School of Medicine, were enrolled from January 2018 to December 2020. The sample comprised 313 T2DM patients with microalbuminuria and 643 T2DM patients with normal urinary microalbumin levels. The enrolled subjects were all hospitalized patients who signed the informed consent form for hospitalization when they were admitted and agreed that all their data during hospitalization could be used for future scientific research by our hospital. Extra informed consent was waived by the hospital ethics committee as the research was about hospitalized patients. Patients with a history of kidney disease, including chronic glomerulonephritis, acute nephritis, or urinary tract infection, were excluded, as were those with acute infection and autoimmune disease or those who received a malignant tumor diagnosis while hospitalized.
Anthropometric and biochemical measurements
Anthropometric measurements, including height, weight, and blood pressure, were collected by medical staff. Other data collected included fasting blood glucose (FPG), 2-hour postprandial blood glucose (2hPG), glycosylated hemoglobin (HbA1c), fasting C-peptide (FC-P), 2-hour postprandial C-peptide (2hC-P), fasting insulin (FINS), 2-hour postprandial insulin (2hINS), liver function indexes, kidney function indexes, lipids profiles, tumor markers, and urinary microalbumin-to-creatinine ratio. We used the formulas to calculate the index as follows: BMI was calculated with the formula: weight (kg)/square of height (m²). HOMA-IR was calculated with the formula: FPG (mmol/L) * FINS (μU/mL)/22.5. Serum tumor markers were determined by the immunoassay method (cobas e 801 analyzer, Roche), while blood glucose, blood lipids, hepatic function, and renal function were determined by an automatic biochemical analyzer (Hitachi LABOSPECT 008 AS, Japan). Blood C-peptide and insulin levels were measured with the chemiluminescence method (BECKMAN COULTER UniCeL Dxl 800 Access immunoassay system, USA). Glycated hemoglobin was determined using high-performance liquid chromatography (Bio-Rad Variant II Turbo, USA). Urine microalbumin was measured by immunoturbidimetry (Siemens automatic protein analyzer, Germany).
Statistical analysis
SPSS 22.0 software (SPSS Inc., Chicago, IL) was used to analyze the data. The data were expressed as mean± standard deviation or median with interquartile range. The comparison between the two groups was performed using the Mann–Whitney U test or independent samples t-test. Spearman correlation analysis and multiple stepwise regression analysis were used to estimate the associations of tumor markers with other variables. Binary logistic regression models were adopted to evaluate the odds ratios (ORs) for microalbuminuria. P values < 0.05 were considered statistically significant. An ROC curve was used to estimate the cutoff point of tumor markers for microalbuminuria.
Results
Clinical characteristics of the two groups
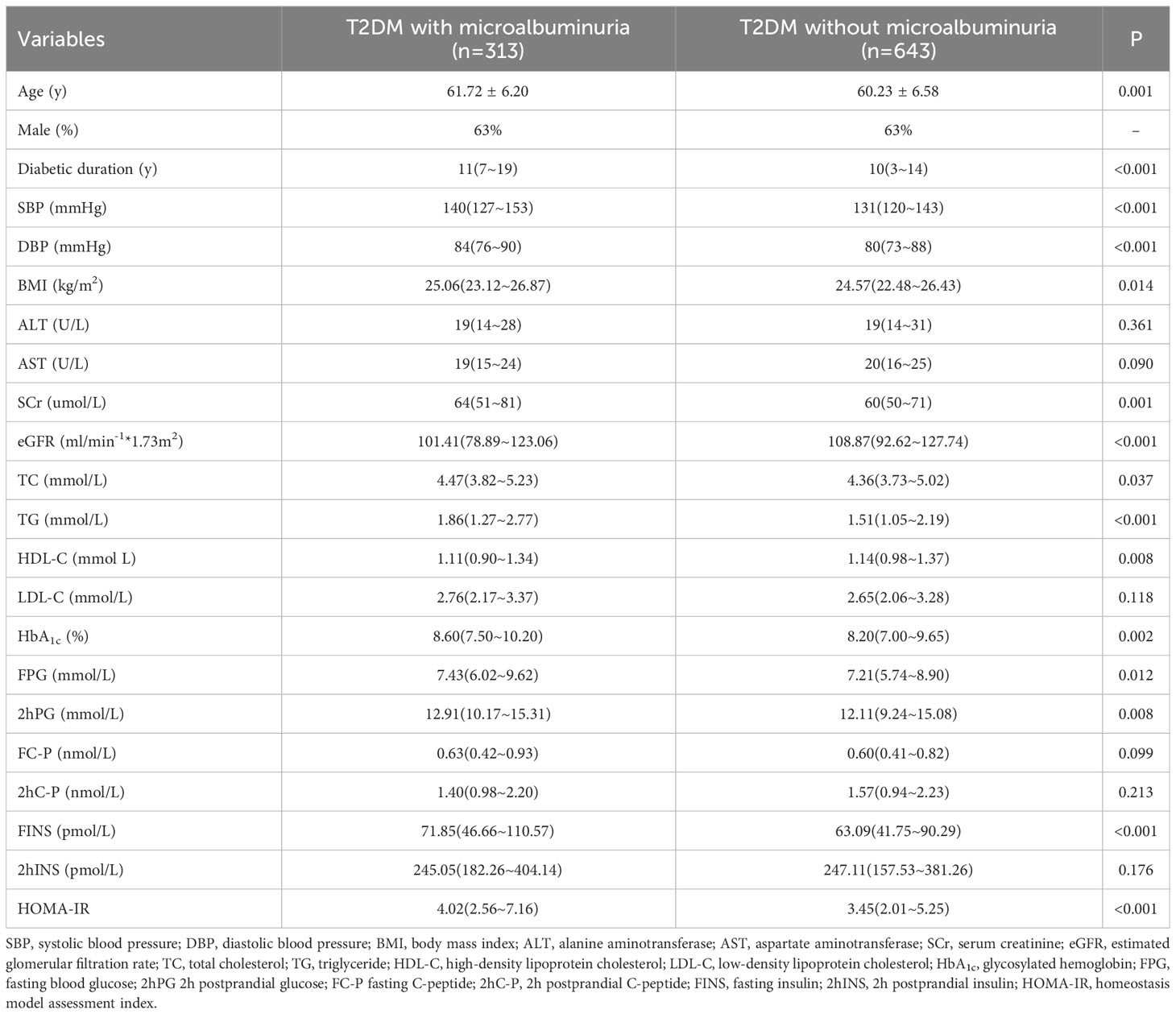
Table 1 shows the basic clinical characteristics of the two groups. There was no difference in sex proportion, ALT, AST, LDL-C, FC-P, 2hC-P,and 2hFINS between the two groups. Compared to the group without microalbuminuria, age, diabetes duration, blood pressure, and BMI, SCr, TC, TG, HbA1c, FPG, 2hPG, FINS, and HOMA-IR were all higher in patients with microalbuminuria. Estimated glomerular filtration rate (eGFR) and HDL-C were lower in patients with microalbuminuria (detailed in Table 1).
Comparison of tumor markers between the two groups
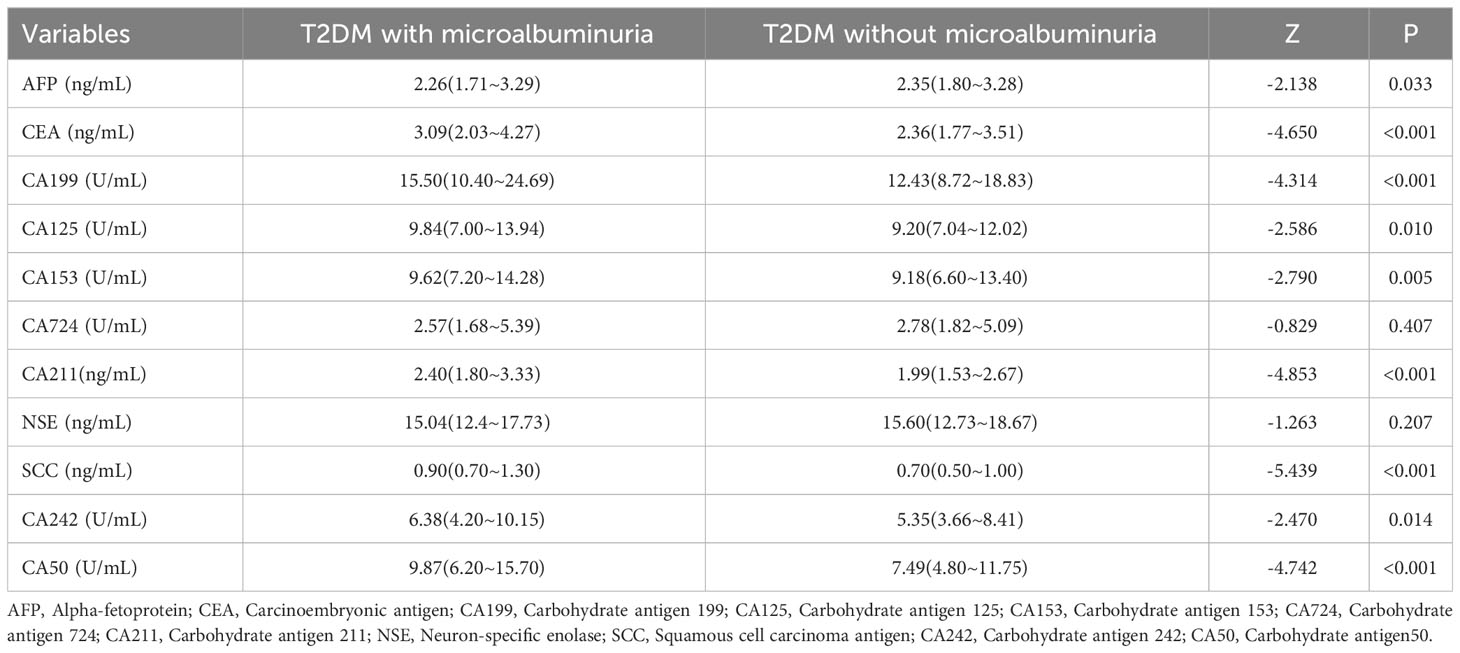
Data were analyzed using the Mann–Whitney U test (Table 2). There was no statistically significant difference in CA724 and NSE between the two groups. AFP was lower in patients with microalbuminuria (P = 0.033), while serum CEA, CA199, CA125, CA153, CA211, SCC, CA242, and CA50 were all higher (detailed in Table 2).
Variables independently associated with microalbuminuria
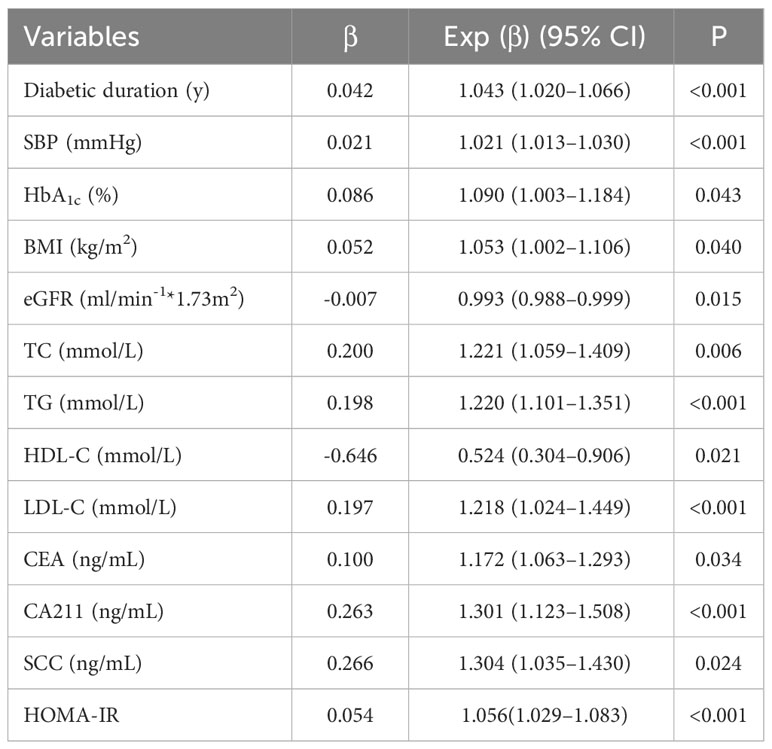
As shown in Table 3, following adjustment for all confounders, variables independently associated with microalbuminuria in T2DM patients were duration of T2DM (OR 1.043, 95%CI 1.020–1.066), SBP (OR 1.021, 95%CI 1.013–1.030), HbA1c (OR 1.090, 95%CI 1.003–1.184), BMI (OR 1.053, 95%CI 1.002–1.106), TC (OR 1.221, 95%CI 1.059–1.409), TG (OR 1.220, 95%CI 1.101–1.351), HDL-C (OR 0.524, 95%CI 0.304–0.906), LDL-C (OR 1.218, 95%CI, 1.024–1.449), eGFR (OR 0.993, 95%CI 0.988–0.999), and HOMA-IR (OR 1.056, 95%CI 1.029–1.083). An ROC curve was used to estimate the cutoff point of tumor markers for microalbuminuria. Taking the values under the cutoff points as a reference, values of CEA, CA211, and SCC above the cutoff points indicated a significantly high risk of microalbuminuria. The OR of increased CEA for microalbuminuria was 2.006 (95%CI 1.456–2.765), the OR of increased CA211 for microalbuminuria was 1.505 (95%CI 1.092–2.074), and the OR of increased SCC for microalbuminuria was 1.958 (95%CI 1.407–2.724) (see Table 4).
Variables independently related to CEA, CA211, and SCC
As detailed in Table 5, variables independently associated with CEA were HbA1c, LDL-C, age, and sex. Variables independently associated with CA211 were HbA1c, LDL-C, age, diabetes duration, SCr, DBP, and FC-P. Variables independently associated with SCC were SCr, age, and FC-P.
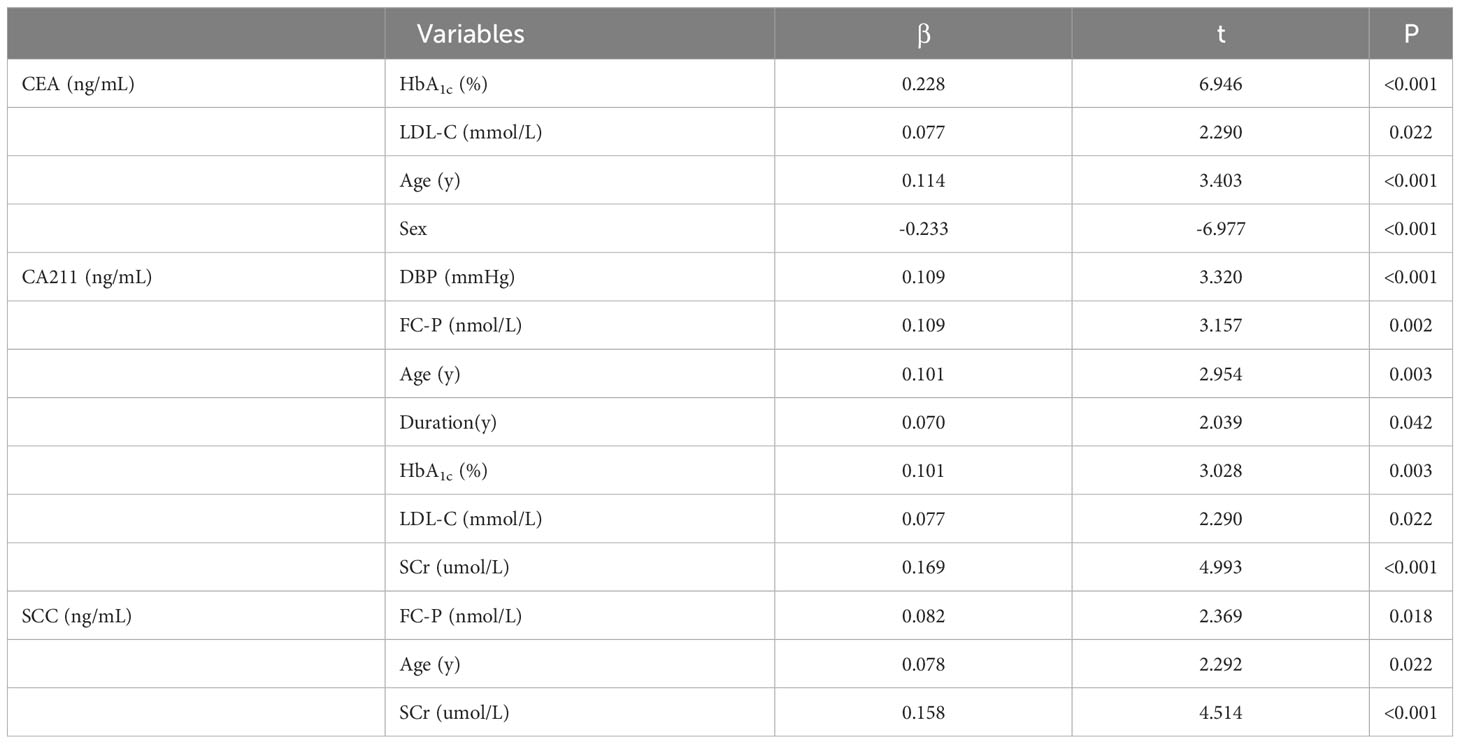
Table 5 Variables independently related to CEA, CA211, and SCC by multiple linear regression analysis.
Discussion
China is among the countries with the highest prevalence of diabetes worldwide, with the number of patients estimated to exceed 140 million in 2021 and projected to reach over 174 million by 2045 (2). As reported by Zheng et al., diabetes mellitus is the ninth leading cause of mortality globally (6). A previous study that investigated the relationship between T2DM and malignant tumors observed a significant correlation (7). Chronic complications of T2DM lead to increased mortality and morbidity and severely affect the life expectancy and quality of life of patients. DKD is one of the most common vascular complications of diabetes. Clinically, the stage of diabetic nephropathy is primarily determined based on the urinary albumin excretion rate, glomerular filtration rate, creatinine, and total urinary protein. The American Diabetes Association screens and diagnoses DKD according to UACR, defining UACR < 30μg/mg, 30-299μg/mg, and > 300μg/mg as normal, microalbuminuria, and macroalbuminuria, respectively. Gerstein et al. showed that any degree of albuminuria is a risk factor for cardiovascular events in individuals with T2DM and that risk increases progressively with UACR elevation (8). A previous study found that CA153 was negatively related to eGFR and positively related to HbA1c and FPG in patients with T2DM (9). Turgutalp et al. found that the urinary protein excretion rate was correlated with CA125, CA153, and CA199 (10). So far, numerous studies have shown that some serum tumor markers are higher in patients with T2DM than in healthy individuals, but few studies have investigated the relationship between serum tumor markers and microalbuminuria in T2DM patients.
Our present study revealed that tumor markers were related to microalbuminuria in T2DM. Most of the serum tumor markers, namely, CEA, CA199, CA125, CA153, CA211, SCC, CA242, and CA50, were increased in the T2DM with microalbuminuria group, while serum AFP was decreased. CA199 has high sensitivity in the diagnosis of pancreatic cancer and, as such, is regarded as the best validated biomarker of pancreatic cancer (11). T2DM is associated with a status of chronic inflammation; pancreatic inflammation leads to impairment of pancreatic exocrine gland function, resulting in increased CA199 levels (12). A previous study indicated that CA199 levels in T2DM patients with microvascular complications including neuropathy, diabetic nephropathy, and retinopathy were significantly increased in comparison with those without microvascular complications (13). It has also been reported that renal impairment could lead to increased serum CA125 levels, which have been independently correlated with urinary microalbumin (14). Our results are in agreement with this finding. Furthermore, as our studied subjects were T2DM patients, the levels of CA125 were significantly higher in T2DM patients with microalbuminuria than in those without microalbuminuria. In recent years it has been well established that inflammation promotes the occurrence and progression of T2DM, and the activated inflammatory status may play an important role in the occurrence and progression of DKD (15, 16). DKD is an inflammatory disease with increased serum high-sensitivity C-reactive protein levels (17).
CA153 is a glycoprotein that has a proven association with a wide range of cancers (18). CA153 is a product of the Mucin 1 (MUC1) gene, a transmembrane protein expressed on the surface of most epithelial cells (19, 20). Some inflammatory factors such as tumor necrosis factor (TNF), interleukin-1 (IL-1), and interleukin-6 (IL-6) can promote the expression of MUC1 (21), so in an inflammatory state, serum CA153 may be increased. Accordingly, our results showed serum CA153 levels were higher in T2DM patients with microalbuminuria than in those without microalbuminuria. Other researchers have found that serum CA153 levels were negatively correlated with eGFR in diabetic patients (9). CA50, initially reported as a specific antigen expressed on the surface of colorectal cancer cells (22), has also been discovered in other malignant tumors, including lung cancer, pancreatic cancer, liver cancer, gastric cancer, uterine cancer, and bladder cancer (22). Nevertheless, it has been observed to be increased in some benign diseases and notably in patients who suffer from T2DM and pancreatitis. In such cases, the level of serum CA50 decreases after remission of inflammation (22). We suppose that inflammation may be the underlying reason for increased serum CA50 among T2DM patients with microalbuminuria. Our study found that the level of AFP was markedly lower in T2DM patients with microalbuminuria than in patients without microalbuminuria, which is consistent with Turgutalp’s conclusions. Turgutalp et al. (10) found that urinary protein excretion was correlated with tumor markers. The possible reasons for the decrease in AFP are loss from urine, increased catabolic rate, decreased synthesis rate, changes in molecular structure, and usage of drugs.
Following adjustment for all confounders, the tumor markers independently associated with microalbuminuria in T2DM patients were CEA, CA211, and SCC. The increased serum CEA levels in T2DM patients with microalbuminuria in our study are consistent with the conclusion of previous research (23). As DKD is more common in patients with poor glycemic control, in the long-term, serum CEA level would be expected to significantly increase due to glucotoxicity damage to the digestive tract. Long-term hyperglycemia could lead to raised levels of advanced glycation end products, resulting in vascular endothelial dysfunction and oxidative stress (23). Another study demonstrated that there exists a positive relationship between serum CEA levels and leukocyte counts in adults; as such, elevated serum CEA levels may reflect a chronic state of inflammation (24). Diabetes mellitus and DKD are chronic inflammatory diseases.
Another reason for increased CEA levels in microalbuminuria in T2DM may be because of the glomerular ultrafiltrate function. According to a previous study, plasma proteins with molecular masses higher than albumin are mostly restricted from passing into the glomerular ultrafiltrate, and thus, only a small proportion can be detected in urine (25). CEA is one of several proteins of this type. In our study, serum levels of SCC and CA211 were also independently associated with microalbuminuria in T2DM. The specific mechanism remains unclear, and we did not find any relevant studies reporting on the relationship between SCC, CA211, and microalbuminuria. The possible reasons may include inflammation, oxidative stress, imbalance of synthesis, catabolism and excretion, and other related factors.
In conclusion, compared to T2DM patients without microalbuminuria, T2DM patients with microalbuminuria were found to have higher levels of CEA, CA199, CA125, CA153, CA211, SCC, CA242, and CA50. Serum CEA, CA211, and SCC were independently correlated with microalbuminuria in T2DM. When serum tumor markers are elevated in diabetic patients, especially when they are increased in combination, it is necessary to screen for diabetic nephropathy in addition to malignant tumors.
Our research has some advantages and limitations. Firstly, the studied population was relatively large. Second, the items of tumor indicators were completely detected, and almost all of the tumor markers were analyzed. Third, our study was the first study to report the relationship between all of the tumor markers and DKD. The limitation of the current study is that it was cross-sectional, and so we cannot ascertain the causality of serum tumor markers for the risk of DKD. As such, further studies are warranted.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The enrolled subjects are all hospitalized patients who have signed the informed consent form for hospitalization when they were admitted, and have agreed that all their data during hospitalization could be used for future scientific research by our hospital. Extra informed consent form was waived by the hospital ethics committee if the research is about hospitalized patients.
Author contributions
HZ designed the study. LC and SD performed the statistical analysis and drafted the manuscript with assistance from HZ. JZ and LC collected the data. QS and JZ contributed to the specification of the analyses and critically reviewed and edited the manuscript. HZ and JZ are the guarantors of this work, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Shanghai Pujiang Program (2019PJD033).
Acknowledgments
We would like to thank all the members who contributed to this study and all the participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Diabetic Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care (2017) 41(Supplement_1):S13–27. doi: 10.2337/dc18-S002
2. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
3. Ma RCW. Epidemiology of diabetes and diabetic complications in China. Diabetologia (2018) 61(6):1249–60. doi: 10.1007/s00125-018-4557-7
4. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev (2015) 95(3):727–48. doi: 10.1152/physrev.00030.2014
5. Shang X, Song C, Du X, Shao H, Xu D, Wang X. The serum levels of tumor marker CA19-9, CEA, CA72-4, and NSE in type 2 diabetes without Malignancy and the relations to the metabolic control. Saudi Med J (2017) 38(2):204–8. doi: 10.15537/smj.2017.2.15649
6. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol (2018) 14(2):88–98. doi: 10.1038/nrendo.2017.151
7. Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, et al. Type 2 diabetes and cancer: an umbrella review of observational and mendelian randomization studies. Cancer Epidemiol Biomarkers Prev (2021) 30(6):1218–28. doi: 10.1158/1055-9965
8. Gerstein HC, Mann JFE, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA (2001) 286(4):421–6. doi: 10.1001/jama.286.4.421
9. Peng YF, Lin H, Han MM, Li L. Serum carbohydrate antigen 153 and renal function in patients with type 2 diabetes mellitus. J Clin Lab Anal (2018) 32(7):e22461. doi: 10.1002/jcla.22461
10. Turgutalp K, Ozhan O, Helvacı İ, Ata A, Arican A, Boztepe B, et al. Serum levels of cancer biomarkers in diabetic and non-diabetic proteinuric patients: a preliminary study. Clin Chem Lab Med (CCLM) (2013) 51(4):889–95. doi: 10.1515/cclm-2012-0657
11. Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong Y, et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer (2021) 1875(2):188409. doi: 10.1016/j.bbcan.2020.188409
12. Zelenko Z, Gallagher EJ, Tobin-Hess A, Belardi V, Rostoker R, Blank J, et al. Silencing vimentin expression decreases pulmonary metastases in a pre-diabetic mouse model of mammary tumor progression. Oncogene (2017) 36(10):1394–403. doi: 10.1038/onc.2016.305
13. Tong W, Gao H, Wei X, Mao D, Zhang L, Chen Q, et al. Correlation of serum CA199 levels with glycemic control and microvascular complications in patients with type 2 diabetes mellitus. Am J Transl Res (2021) 13(4):3302–8.
14. Peng Y, Sun J, Li L. Renal impairment increases serum cancer antigen 125 levels in patients with type 2 diabetes mellitus. Ann Clin Lab Sci (2020) 50(3):361–3.
15. Moreno JA, Gomez-Guerrero C, Mas S, Sanz AB, Lorenzo O, Ruiz-Ortega M, et al. Targeting inflammation in diabetic nephropathy: a tale of hope. Expert Opin Investig Drugs (2018) 27(11):917–30. doi: 10.1080/13543784.2018.1538352
16. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) (2013) 124(3):139–52. doi: 10.1042/CS20120198
17. Hayashino Y, Mashitani T, Tsujii S, Ishii H, Diabetes D, Care Registry at Tenri Study G. Serum high-sensitivity C-reactive protein levels are associated with high risk of development, not progression, of diabetic nephropathy among Japanese type 2 diabetic patients: a prospective cohort study (Diabetes Distress and Care Registry at Tenri [DDCRT7]). Diabetes Care (2014) 37(11):2947–52. doi: 10.2337/dc14-1357
18. Li X, Xu Y, Zhang L. Serum CA153 as biomarker for cancer and noncancer diseases. Prog Mol Biol Transl Sci (2019) 162:265–76. doi: 10.1016/bs.pmbts.2019.01.005
19. Cascio S, Finn OJ. Intra- and extra-cellular events related to altered glycosylation of MUC1 promote chronic inflammation, tumor progression, invasion, and metastasis. Biomolecules (2016) 6(4):39. doi: 10.3390/biom6040039
20. Adachi Y, Kikumori T, Miyajima N, Inaishi T, Onishi E, Shibata M, et al. Postoperative elevation of CA15-3 due to pernicious anemia in a patient without evidence of breast cancer recurrence. Surg Case Rep (2015) 1(1):126. doi: 10.1186/s40792-015-0128-z
21. Li X, Wang L, Nunes DP, Troxler RF, Offner GD. Pro-inflammatory cytokines up-regulate MUC1 gene expression in oral epithelial cells. J Dent Res (2003) 82(11):883–7. doi: 10.1177/154405910308201107
22. Shan M, Tian Q, Zhang L. Serum CA50 levels in patients with cancers and other diseases. Prog Mol Biol Transl Sci (2019) 162:187–98. doi: 10.1016/bs.pmbts.2018.12.006
23. Cai R, Kong Q, Wang Z, Gao Z, Huo Y. Correlation between tumor markers and type 2 diabetes mellitus complications and their related influencing factors. Ann Palliative Med (2022) 11(1):58–67. doi: 10.21037/apm-21-3429
24. Kwon YJ, Lee HS, Shim JY, Lee YJ. Serum carcinoembryonic antigen is positively associated with leukocyte count in Korean adults. J Clin Lab Anal (2018) 32(3):e22291. doi: 10.1002/jcla.22291
Keywords: tumor markers, microalbuminuria, type 2, diabetes mellitus, UACR
Citation: Chen L, Du S, Li YB, Su Q, Zhang J and Zhang H (2023) Changes in serum tumor markers in type 2 diabetes mellitus with microalbuminuria. Front. Endocrinol. 14:1247099. doi: 10.3389/fendo.2023.1247099
Received: 25 June 2023; Accepted: 20 November 2023;
Published: 07 December 2023.
Edited by:
Maria Margherita Rando, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Laura Mitrea, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaXiang Dong Jian, Shandong University, China
Copyright © 2023 Chen, Du, Li, Su, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmei Zhang, emhhbmdob25nbWVpMDJAeGluaHVhbWVkLmNvbS5jbg==; Jiangrong Zhang, emhhbmdqaWFuZ3JvbmdAeGluaHVhbWVkLmNvbS5jbg==
†These authors have contributed equally to this work
 Lina Chen1†
Lina Chen1† Qing Su
Qing Su Hongmei Zhang
Hongmei Zhang