- Key Specialty of Clinical Pharmacy, The First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, China
Purpose: This study aimed to perform a network meta-analysis to objectively evaluate the efficacy and safety of 10 Glucagon-like peptide-1 receptor agonists (GLP-1RAs) in combination with metformin that is approved for use worldwide in patients with type 2 diabetes and to provide evidence-based support and reference for the selection of clinical treatment.
Methods: Three databases (PubMed, Embase, and Cochrane Library) were searched from their respective inception until September 30, 2022. Only randomized controlled trials comparing the efficacy and safety of GLP-1RAs for treating type 2 diabetes (T2D) were included. The 10 GLP-1RAs are exenatide (including exenatide twice daily and once weekly), liraglutide, lixisenatide, dulaglutide, PEX168, semaglutide (subcutaneous and oral semaglutide), tirzepatide and albiglutide.
Results: 34 RCTs with 10 GLP-1RAs and 12993 patients were included in the Network Meta-Analysis (NMA). According to the NMA, tirzepatide 15 mg, semaglutide 1.0 mg, PEX168-200μg, oral semaglutide 14 and dulaglutide 1.5 mg reduced HbA1c by -2.23%, -1.57%, -1.12%, -1.10%, -1.09% and body weight by -11.33 kg, -5.99 kg, +0.40 kg, -3.95 kg, -1.87 kg, respectively. There was no significant difference in the rate of adverse events for tirzepatide 15 mg, oral-semaglutide 14 mg, and semaglutide 1.0 mg. PEX168-200μg, tirzepatide 15mg, and oral semaglutide 14mg had Surface Under the Cumulative Ranking (SUCRA) values greater than placebo, and only tirzepatide 15mg and oral semaglutide 14mg were significantly different from placebo in the rate of serious adverse events. All GLP-1RA did not lead to increased incidence of hypoglycemia. Albiglutide 30mg and semaglutide 1.0mg significantly differed from placebo in Adverse Event (AE) withdrawal. Finally, the sensitivity analysis and publication bias analysis results indicate that the study results are reliable.
Conclusion: This study’s results showed that GLP-1RAs were effective in lowering HbA1c and reducing body weight without increased incidence of hypoglycemic reactions. In addition, this study may provide reference and evidence-based medical evidence for clinicians to select GLP-1RAs in patients with T2D and high body mass index (BMI). Based on the NMA results, tirzepatide 15mg and semaglutide 1.0mg may be preferred.
1 Introduction
As people’s diets and lifestyles change, diabetes prevalence is increasing, with type 2 diabetes accounting for 90-95% of all cases of diabetes. Diabetes imposes a heavy economic burden on individuals and society, with the annual cost of diagnosing diabetes estimated at $327 billion in 2017 in the US (1). Diabetes prevalence is significantly higher in people who are overweight or obese. Studies have shown that the higher the BMI, the higher the risk of type 2 diabetes (2–4), and patients with pre-diabetes often have associated cardiovascular risk factors, including hypertension and dyslipidemia, and may be at increased risk of cardiovascular disease (5–7).
The American Diabetes Association (ADA) Medical Standards of Care for Diabetes 2022 Edition recommends metformin as the first-line treatment and GLP-1RAs as part of the treatment regimen for patients with T2D in combination with atherosclerotic cardiovascular disease or high-risk factors, renal disease, or heart failure (8). GLP-1RA has been shown to improve hemoglobin A1c (HbA1c), reduce body weight, and have cardiovascular benefits, and should be more widely used in clinical practice (9–15).
In 2019, 48% of diabetes deaths occurred before age 70. Elevated blood glucose contributes to about 20% of deaths from cardiovascular disease (16). In 2021, approximately 537 million adults (aged 20-79) worldwide had diabetes, and an estimated 6.7 million adults will have died from diabetes or its complications (17). However, in the CAPTURE study (18) (a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries), results showed that the prevalence of cardiovascular disease in Chinese patients with T2DM was 33.9%, of which 94.9% was atherosclerotic cardiovascular disease (ASCVD). Only 1.5% of Chinese patients with T2DM and combined ASCVD were treated with GLP-1RAs with cardiovascular benefit, a large gap between clinical practice and guideline recommendations (19, 20).
An NMA of metformin in combination of GLP-1RAs has not been previously performed. Therefore, we sought to objectively evaluate the efficacy and safety of 10 globally approved and marketed GLP-1RAs in combination with metformin for treating patients with T2DM through NMA and to provide evidence-based support and reference for the clinical selection of GLP-1RAs.
2 Methods
2.1 Registration
The Preferred Items report for this systematic review, the NMA for Systematic Reviews and Meta-Analyses (PRISMA) statement, and the PRISMA checklist is provided in Supplementary File 1. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42023390347).
2.2 Data source
The Cochrane Library, PubMed, and Embase databases were searched from the creation of the databases to September 30, 2022, with no language restrictions. We used search terms including “diabetes mellitus, type 2”, “type 2 diabetes”, “glucagon-like peptide-1 receptor agonist”, “GLP-1 receptor agonist”, and “randomized controlled trial,” including any of these terms in any field. The search strategy, including all search terms, is shown in Supplementary File 2.
2.3 Inclusion criteria
Inclusion and exclusion criteria were developed according to the principles of Participants, Intervention, Comparison, Outcomes, and Study (PICOS). Inclusion criteria: (1) Participants: Patients diagnosed with type 2 diabetes, HbA1c ≥7.0%, age ≥18 years, no diabetes-related complications, no gender or race restrictions, and drug treatment cycle ≥12 weeks (initial dose + maintenance dose). (2) Intervention: 10 GLP-1RAs (“exenatide (including exenatide twice daily and once weekly)” or “liraglutide” or “lixisenatide” or “dulaglutide” or “loxenatide” or “PEX168” or “semaglutide (including oral semaglutide)” or “tirzepatide” or “albiglutide”) +/- other oral antidiabetic drugs (OADs) were taken in the treatment group. (3) Comparison: Placebo or another GLP-1RA in the control group. (4) Outcomes: Primary outcomes were HbA1c and rate of all adverse events. Secondary outcomes were weight loss, serious adverse events, hypoglycemic events, and withdrawal due to adverse events. (5) Study: The study type was RCT.
2.4 Exclusion criteria
Exclusion criteria: (1) The article was republished, case report, review, conference, monotherapy, animal studies, retrospective studies, and data could not be extracted. (2) The patient’s age <18, HbA1c<7.0%, and treatment cycle <12 weeks, presence of associated diabetic complications. (3) High-risk trials and type of non-randomized controlled trials.
2.5 Literature screening and data extraction
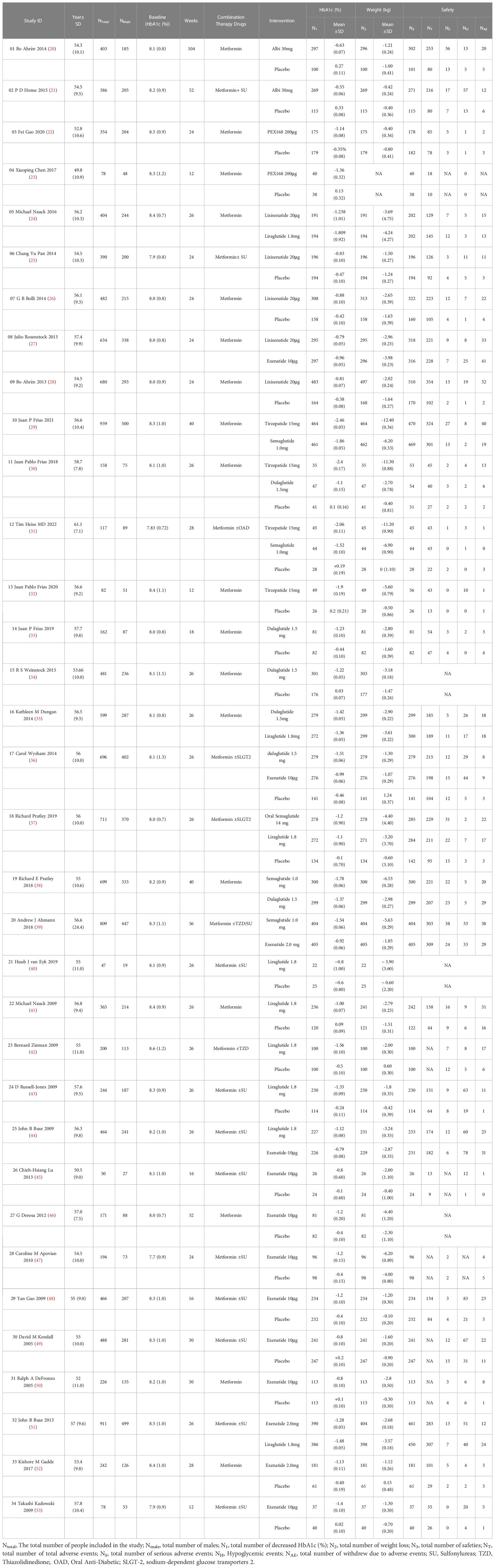
The Note Express software has been used to eliminate duplicates of articles, then read the titles and abstracts to exclude articles that did not meet the inclusion criteria, and finally read the full text according to the predefined inclusion and exclusion criteria to determine the final included studies, and extracted data from the included studies. Two investigators extracted data independently (Gu and Hu), and a third investigator resolved conflicting data (Chen). The extracted data include the following information: (1) study characteristics (e.g., year of publication, first author, mean age, sex, mean HbA1c (%), and total number of people included in the study). (2) Therapeutic interventions (e.g., drugs, dose, and cycle). (3) Clinical data (e.g., HbA1c reduction (%), weight loss (kg), number of adverse events, serious adverse events, withdrawals due to adverse events, and hypoglycemia events).
2.6 Risk of bias assessment
The risk of bias in the included studies was assessed independently by two investigators (Gu and Hu) using the software Review Manager 5.4.1 according to the Cochrane Risk of Bias Assessment Tool criteria, and a third investigator (Chen) adjudicated conflicting studies.
2.7 Analysis of data
Frequentist NMA was performed using software (Stata 16.1). The mean difference (MD) value was used to calculate continuous variables. Each effect size was expressed as a 95% confidence interval (95%_CI). The odds ratio (OR) value is used to calculate dichotomous variables (the number of adverse events), and a higher value means more adverse events, which means worse outcomes. We used the surface under the cumulative ranking (SUCRA) to rank the outcome of each treatment and finally expressed it as a percentage. For heterogeneity results of each outcome, we used software (Review Manager 5.4.1) to calculate, and the results are expressed in I2 and p-value (if p-value for Q test < 0.10 or I2 > 50% was defined as significant heterogeneity).
For the inconsistency test of the whole network meta, we used software (Stata 16.1) to test global inconsistency, local inconsistency (node splitting method), and closed-loop inconsistency (If the p-value is greater than 0.05, it means that the inconsistency is not significantly different). If the results are consistent (p>0.05), the results of the network meta are reliable. Meanwhile, we evaluated the included studies by drawing the network plots, funnel plots of outcome indicators, and risk of publication bias plots. In addition, a cluster analysis was performed to compare the effect of GLP-1RAs on efficacy (reduction in HbA1c) and safety (rate of adverse events). Finally, a sensitivity analysis is required if the included studies are high-risk.
3 Result
3.1 Inclusion process and study characteristics
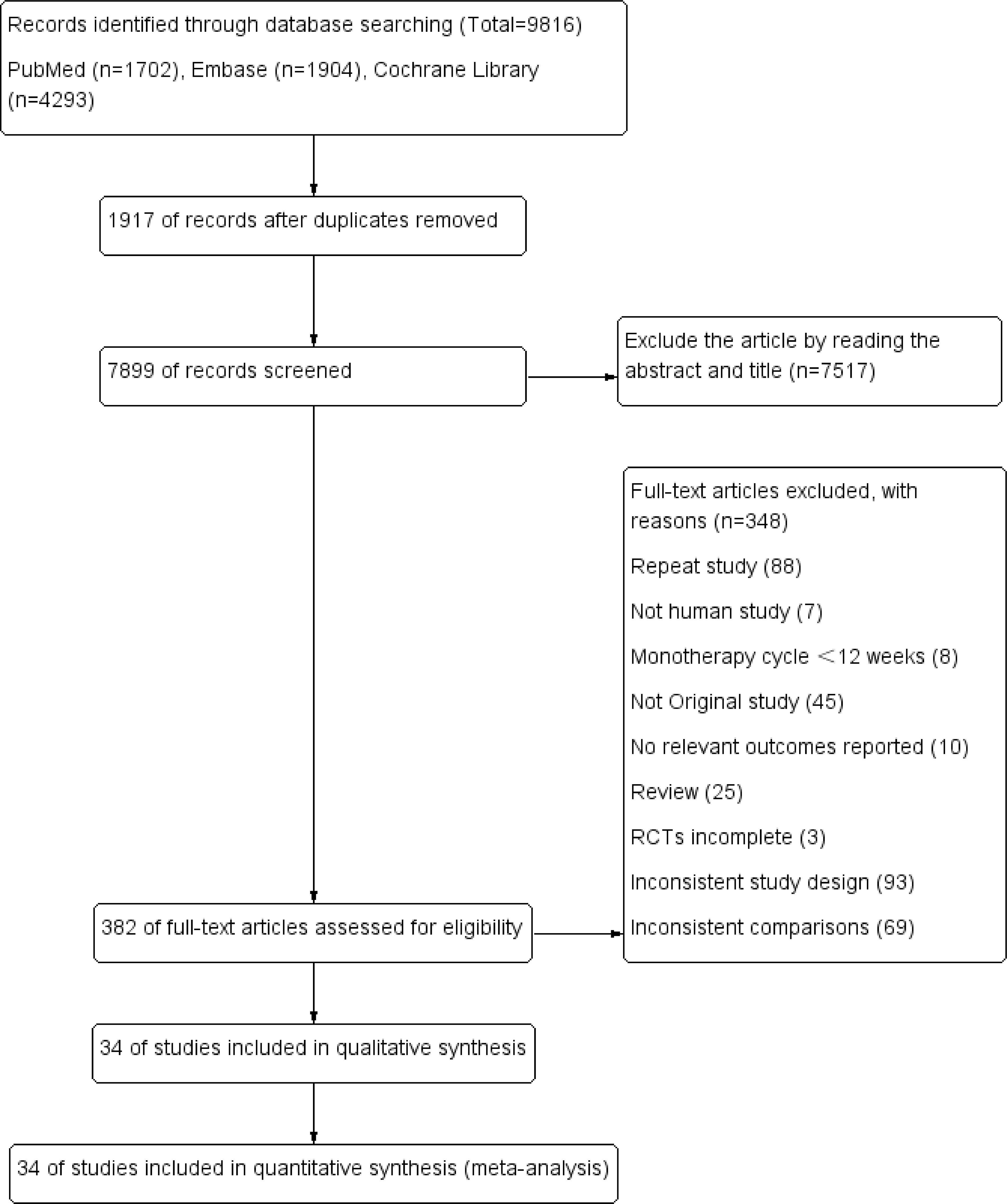
The study’s screening process and the patients’ characteristics included are shown in Figure 1; Table 1. A total of 9816 articles were initially included by searching the databases according to the predefined search criteria, including PubMed (1702), Embase (1904), and the Cochrane Library (4293), further removing duplicates (7517) and reading the full text and abstracts (348). Finally, 34 studies with 11 treatments (involving 12993 patients) were included in the NMA. In the 34 included studies, 18 used metformin + GLP-1RA as the therapeutic agent, and 16 used metformin + GLP-1RA ± OAD (e.g., sulfonylurea, thiazolidinedione, and sodium-glucose cotransporter-2 inhibitors).
3.2 Quality assessment of included study
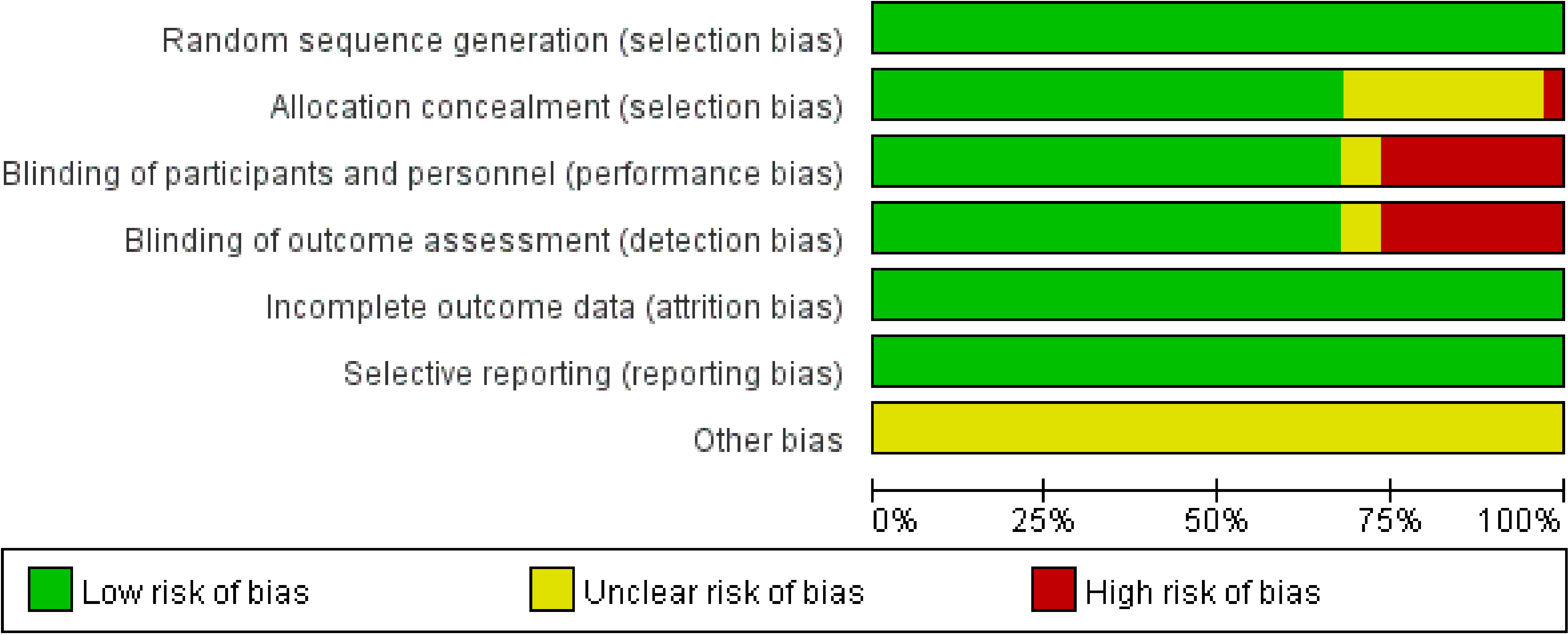
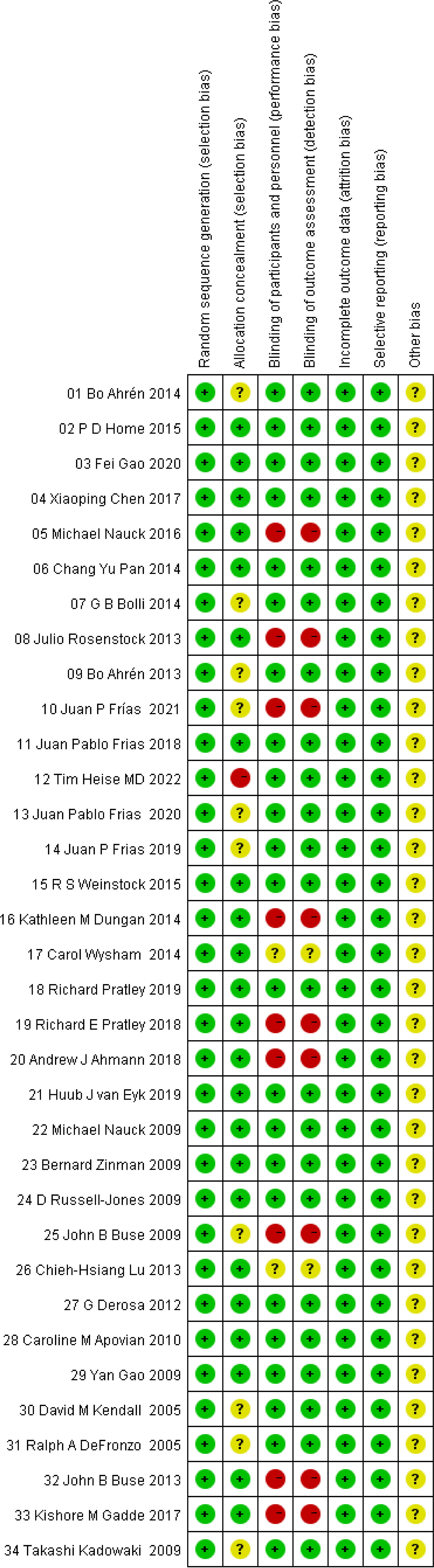
The risk of bias assessment plots and risk summary plots are shown in Figures 2, 3. Regarding the risk of bias, in the 34 included studies, all studies described in detail random sequence generation, incomplete outcome data, selective reporting, and the fact that they were all considered low-risk studies. In the allocation concealment bias, 10 (29.4%) studies and 1 (2.9%) study were classified as unclear and high risk because they were not reported, and an open random allocation table was used. Regarding blinding of patients and personnel and blinding of outcome assessment, 11 (32.4%) were considered high risk because they were not blinded, and 2 (5.9%) were regarded as unclear risk because they were not reported in the study. In other biases, all studies were not reported and were considered of unclear risk.
3.3 Results of network meta-analysis
The network plot is shown in Figure 4. In the 34 included studies, 34 (100%) studies with 12993 (100%) participants reported a reduction in HbA1c, 32 (94%) studies with 12906 (99%) for weight loss, 27 (79%) studies with 11608 (89%) for the rate of adverse events, 29 (85%) studies with 12558 (96%) for the rate of serious adverse events, 30 (88%) studies with 12522 (96%) for the hypoglycemic events, 30 (88%) studies with 12638 (97%) for the rate of withdrawals due to adverse events. Each evidence network has direct and indirect comparisons, with 15 closed loops for all outcome indicators except hypoglycemia, which has 10 closed loops.
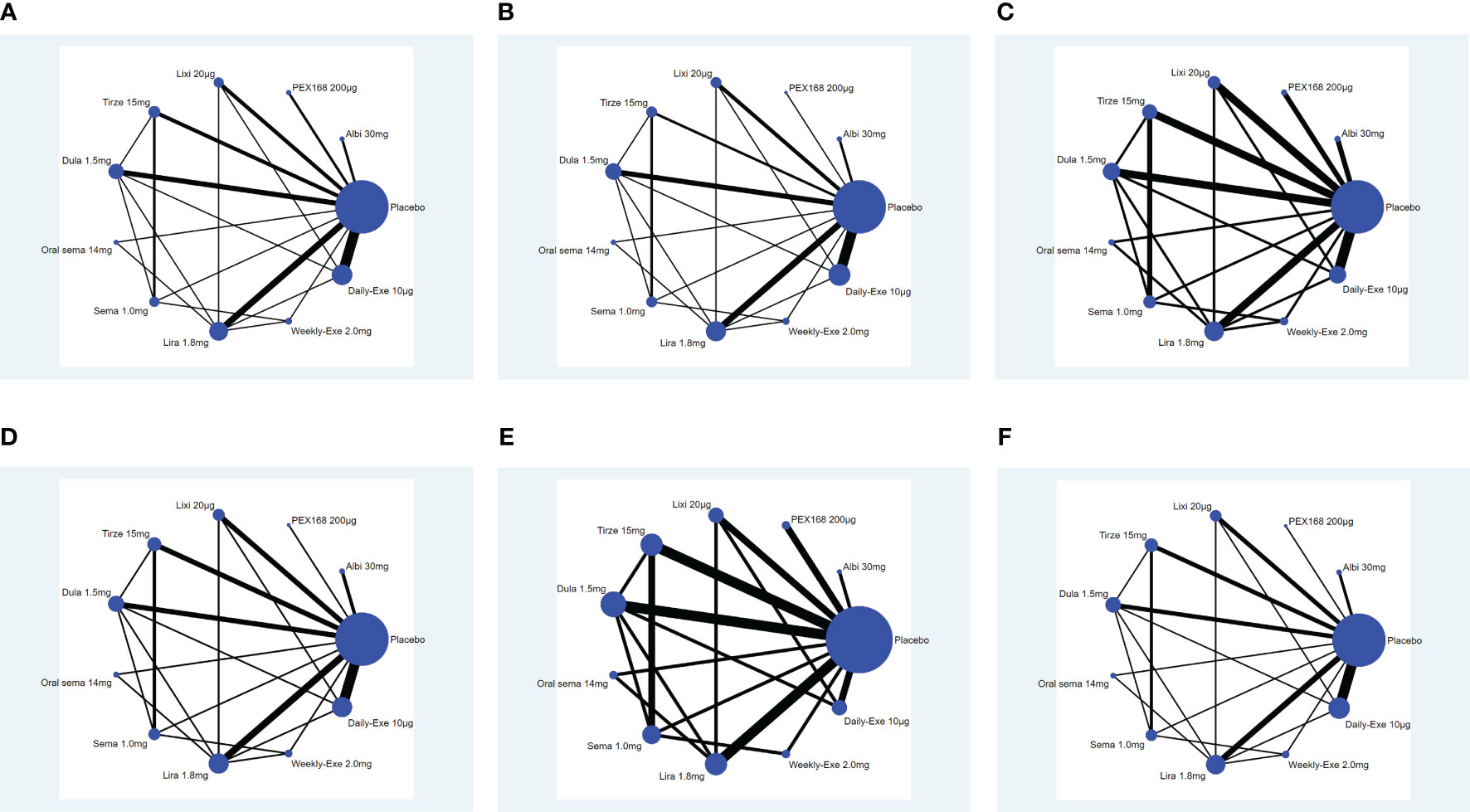
Figure 4 Network plot. (A) (Decreased HbA1c), (B) (Weight loss), (C) (The rate of adverse events), (D) (The rate of serious adverse events), (E) (Hypoglycemic events); (F) (AE withdrawal); (Note: Each node represents a specific intervention, the size of the nodes corresponds to the number of participants assigned to each treatment).
3.4 Result of the inconsistency analysis
The results of the global inconsistency test are shown in Supplementary File 3 (Table 1). The p-values of the global inconsistency test results for the six outcome indicators of HbA1c reduction, weight loss, total adverse events, serious adverse events, hypoglycemic events, and AE withdrawal were 0.99, 0.96, 0.52, 0.28, 0.48, and 0.80, respectively, indicating that there was no inconsistency. In the local inconsistency test (node splitting method), there was inconsistency between the semaglutide 1.0 mg group and the placebo group for weight loss and AE withdrawal, and there were no significant inconsistent differences between the other group comparisons. (p ≥ 0.05).
In the loop inconsistency analysis, there were four loops for decreased HbA1c and weight loss outcome indicators and one loop of inconsistency for serious adverse events; other closed loops are not significantly inconsistent (p ≥0.05 or CI_95 include 0). The heterogeneity of each outcome indicator was calculated using I-squared; the results of the heterogeneity and loop inconsistency tests are shown in Supplementary File 4.
3.5 Decreased HbA1c (%)
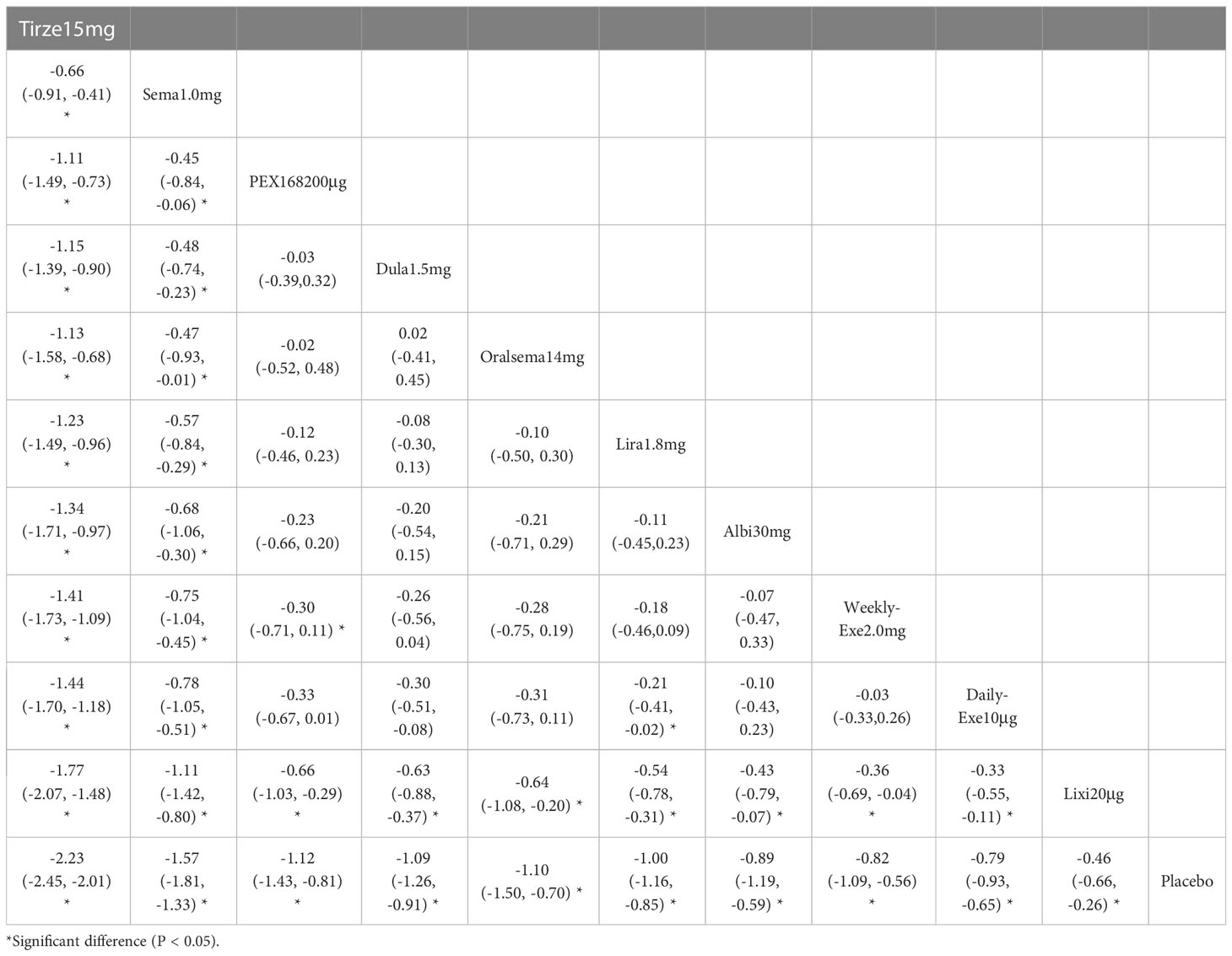
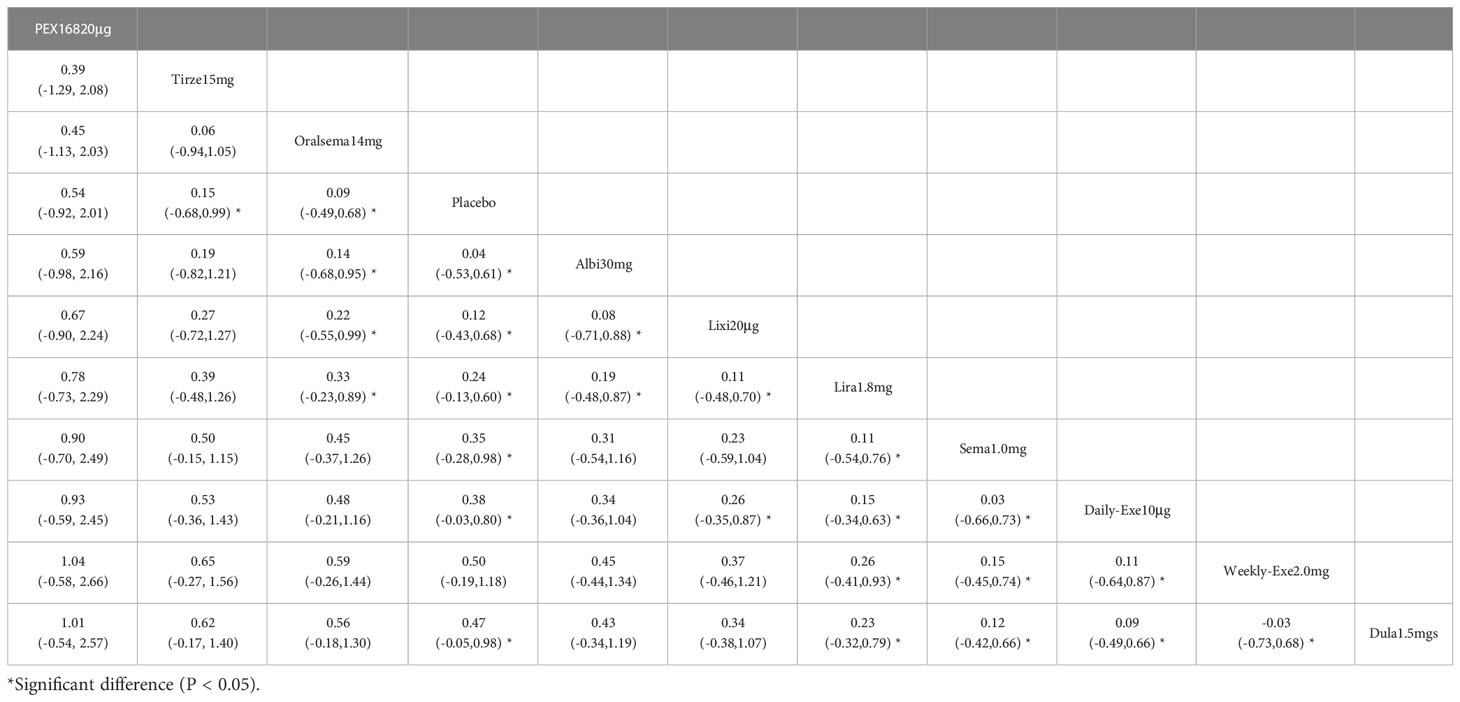
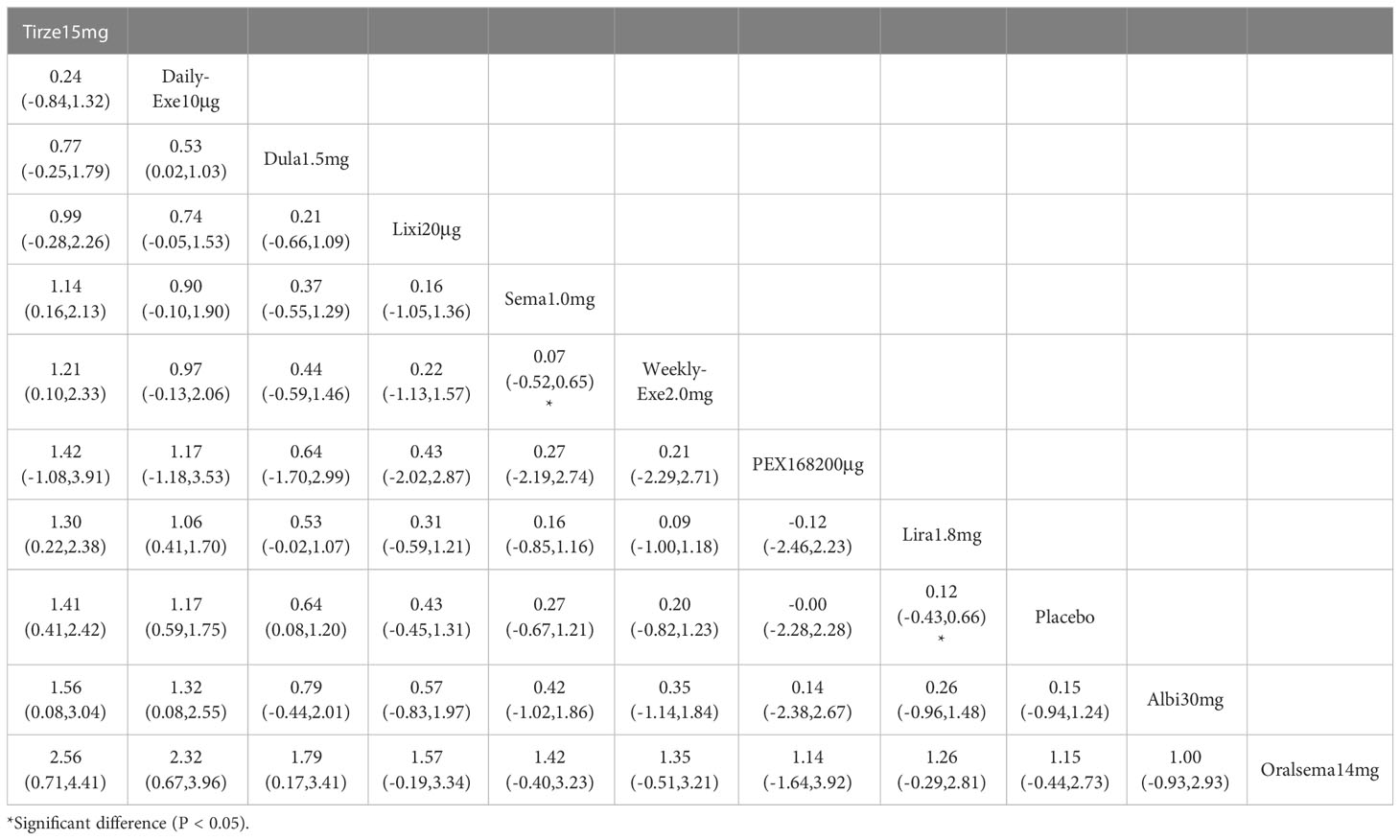
The results of HbA1c reduction for 10 interventions are shown in Table 2. Compared with placebo, all 10 interventions were effective in reducing HbA1c (e.g., tirzepatide 15mg (MD=-2.23%, 95%_CI [-2.45, -2.01])). All 10 GLP-1RAs were significantly more effective than placebo in reducing HbA1c (p≥0.05), and tirzepatide 15mg and semaglutide 1.0mg were significantly more effective than the other GLP-1RAs.
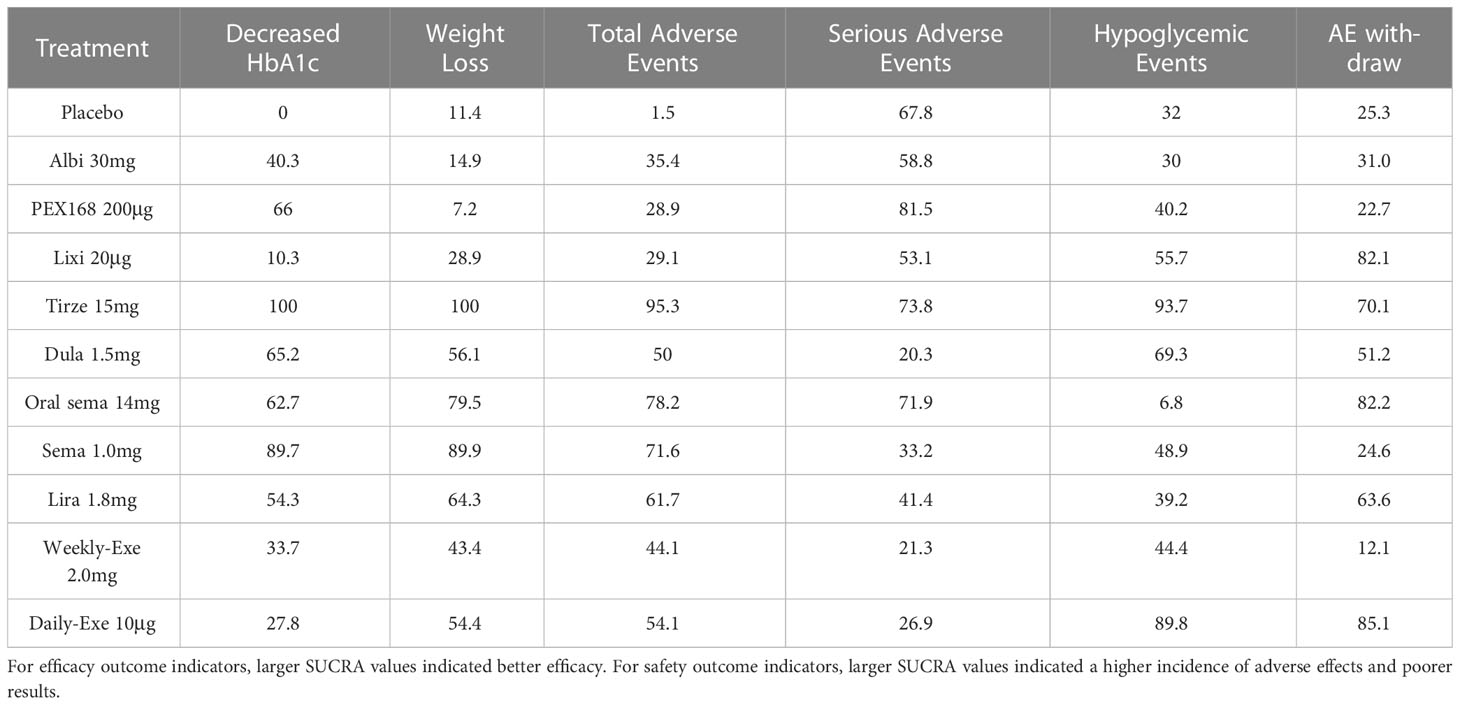
The results of SUCRA of 10 interventions and cumulative probability plots are shown in Table 3 and Figure 5. According to the SUCRA results, the ranking of HbA1c reduction from highest to lowest was as follows: tirzepatide15mg>semaglutide1.0mg>PEX168-200μg>dulaglutide1.5mg>oral semaglutide14mg>liraglutide1.8mg>albiglutide30mg>weekly-exenatide2.0mg>daily-exenatide10μg>lixisenatide20μg>placebo.
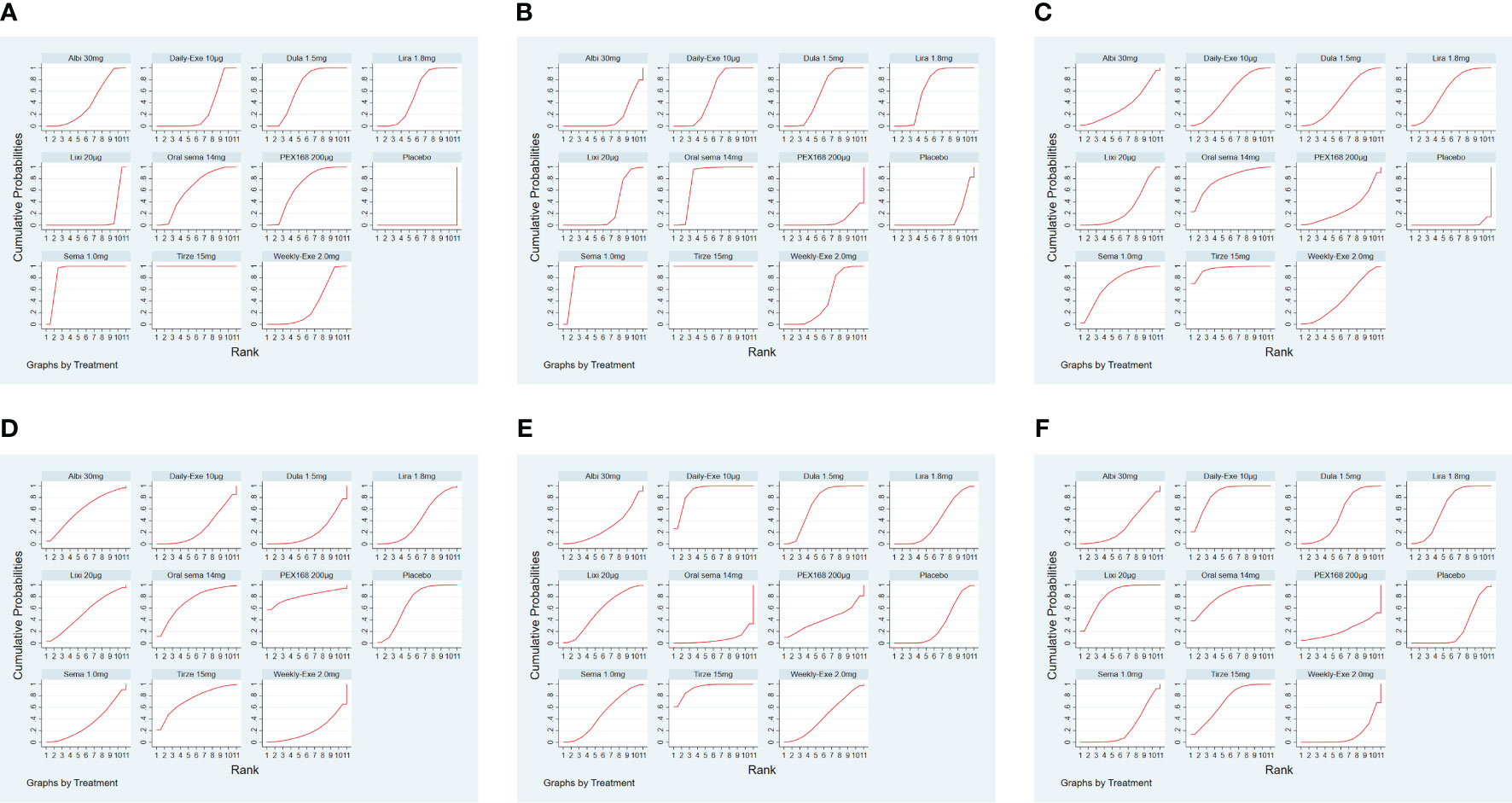
Figure 5 The ranking of GLP-1RA based on cumulative probability plots and SUCRA. (A) (Decreased HbA1c), (B) (Weight loss), (C) (The rate of adverse events), (D) (The rate of serious adverse events), (E) (Hypoglycemic events), (F) (AE withdrawal).
3.6 Weight loss (kg)
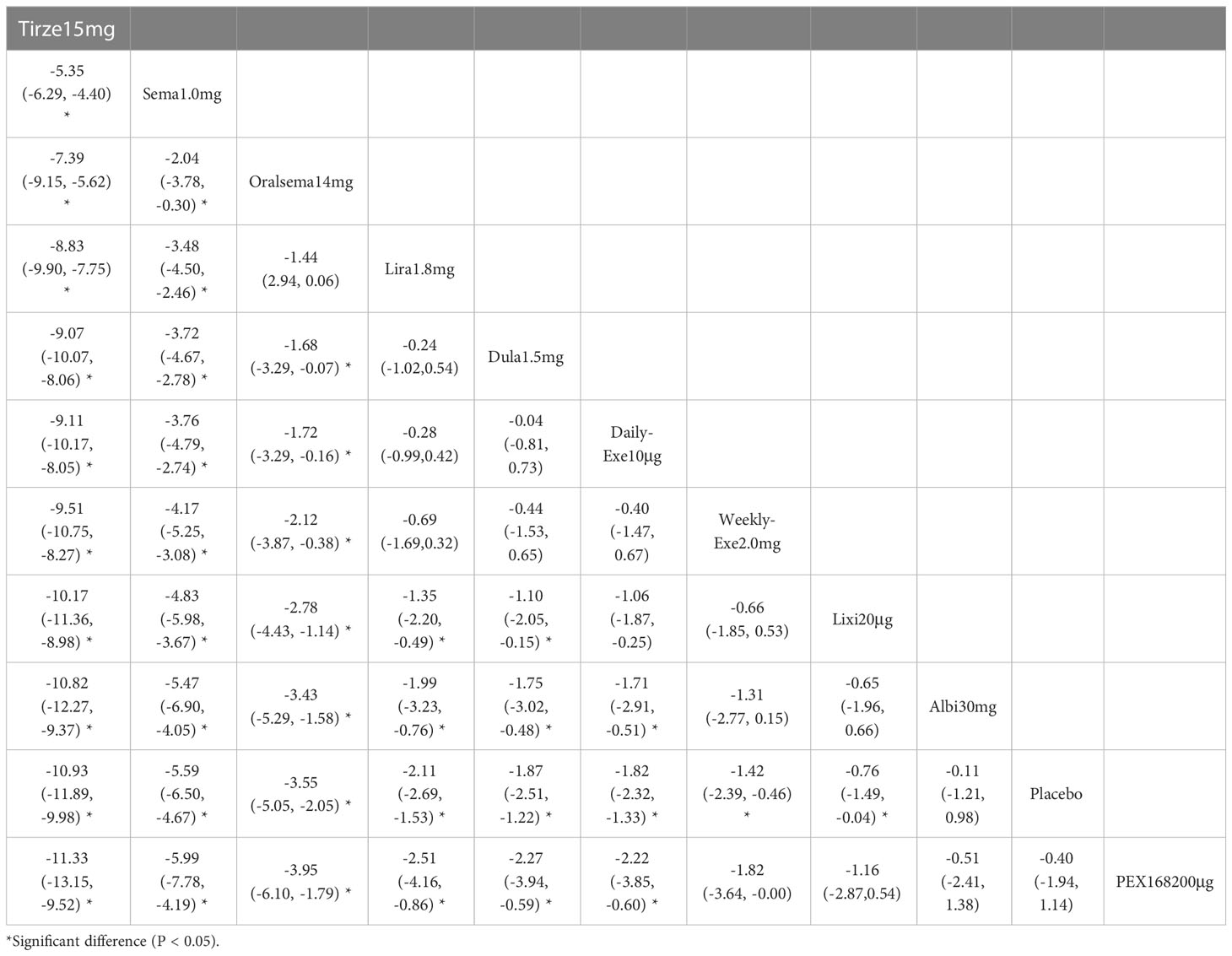
The weight loss results for 10 interventions are shown in Table 4. Compared with placebo, all 10 interventions were associated with weight loss (e.g., tirzepatide15mg (MD=-10.93kg, 95%_CI [-11.89, -9.98]). Compared with the placebo, the other 8 GLP-1RAs were more effective in reducing weight (kg), except for albiglutide 30 mg and PEX-200 μg, which did not show significant changes in weight reduction. In addition, tirzepatide 15 mg and semaglutide 1.0 mg were significantly more effective than the other interventions in reducing weight.
As shown in Table 3 and Figure 5, the SUCRA results show that the ranking of weight loss from highest to lowest was as follows: Tirzepatide15mg>Semaglutide1.0mg>Oral semaglutide14mg>Liraglutide1.8mg>Dulaglutide1.5mg>Daily exenatide10μg>Weekly exenatide2.0mg>Lixisenatide20μg>Albiglutide30mg>Placebo>PEX168-200μg.
3.7 The rate of adverse events
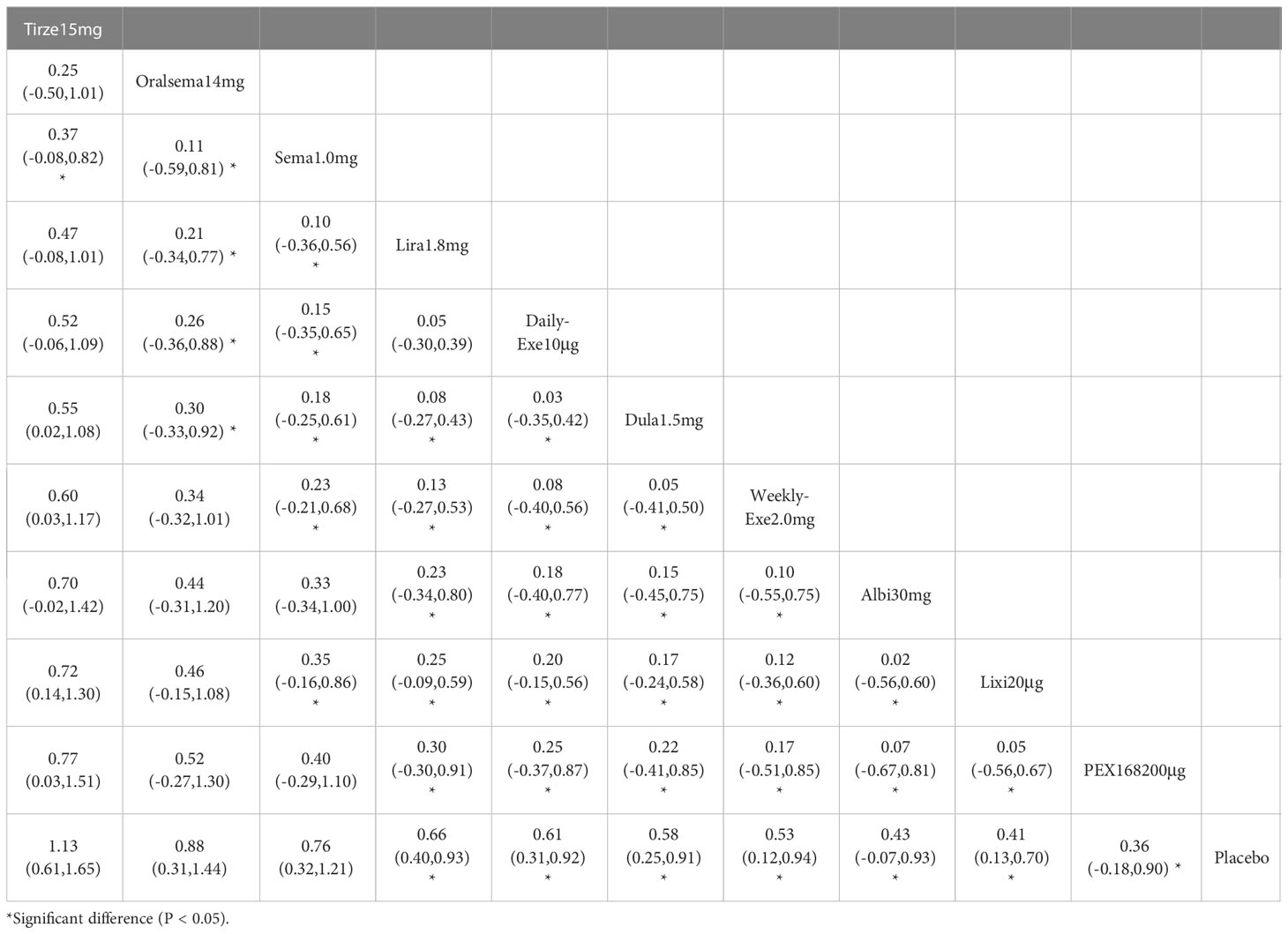
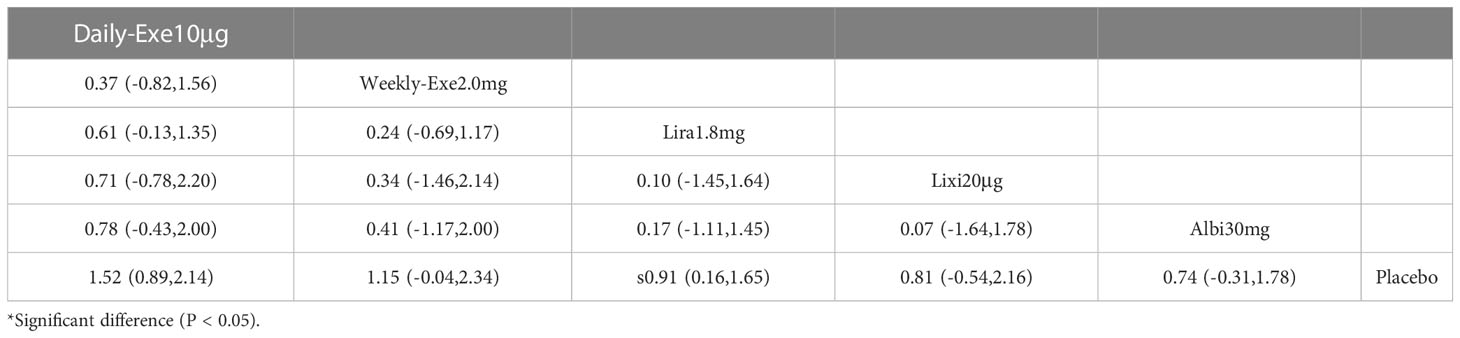
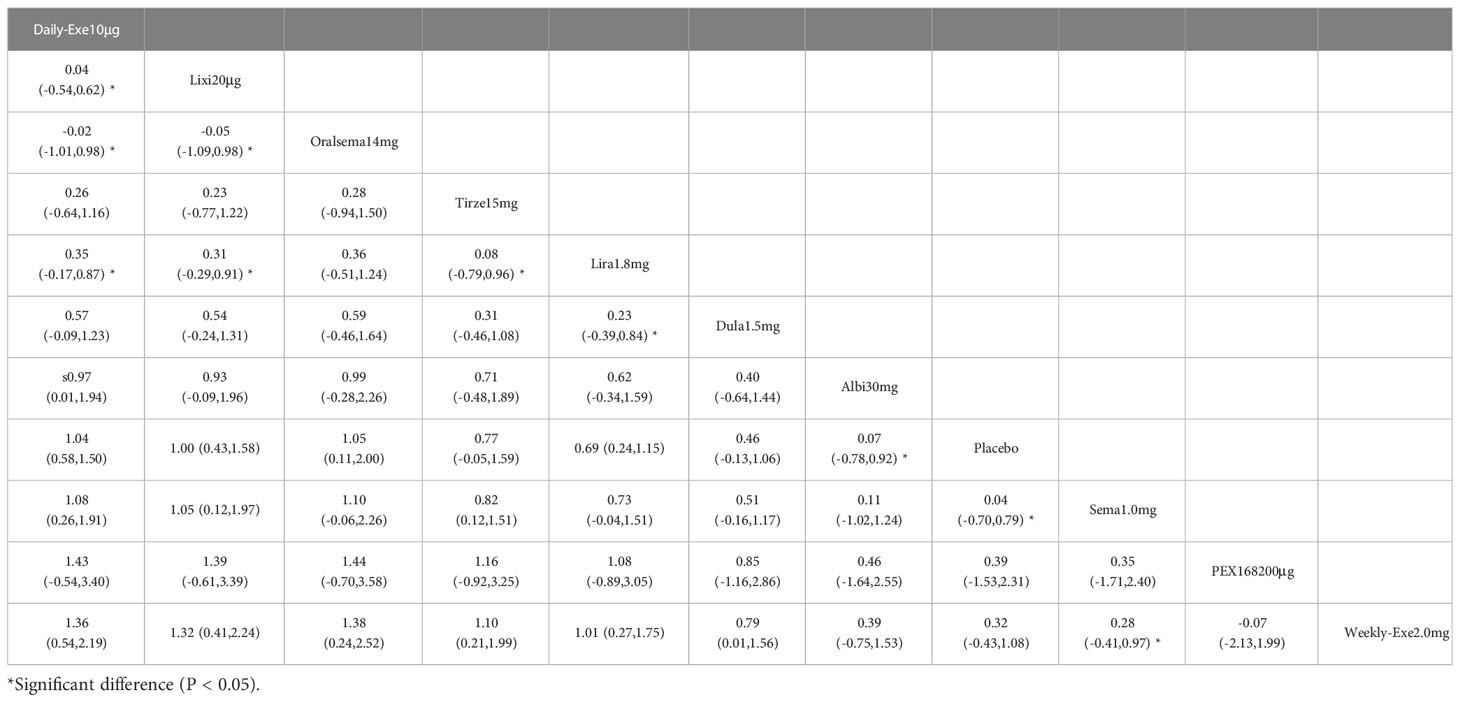
The results of the adverse events rate for 10 interventions are shown in Table 5. Tirzepatide 15 mg, oral semaglutide 14 mg, and semaglutide 1.0 mg did not differ significantly from placebo in the rate of adverse events, while the other seven GLP-1RAs did. Semaglutide1.0mg, tirzepatide15mg had a significantly higher rate of adverse events compared with the other GLP-1RAs, and oral semaglutide14mg had a significantly higher rate of adverse events compared with weekly exenatide2.0mg, albiglutide30mg, lixisenatide20μg, and PEX168-200μg. while all other head-to-head comparisons between GLP-1RAs were not significantly higher in terms of adverse event rates.
As shown in Table 3 and Figure 5, the SUCRA results show that the ranking of the rate of adverse events from highest to lowest was as follows: Tirzepatide15mg>Oral semaglutide14mg>Semaglutide1.0mg>Liraglutide1.8mg>Daily exenatide10μg>Dulaglutide1.5mg>Weekly exenatide2. 0mg>Albiglutide30mg>Lixisenatide20μg>PEX168-200μg>Placebo, this result indicates that tirzepatide15mg had the highest incidence of adverse events compared to all other interventions.
3.8 The rate of serious adverse events
The results of the serious adverse event rate for 10 interventions are shown in Table 6. Compared to the placebo, there was a significant difference in the incidence of serious adverse events between tirzepatide 15 mg and oral semaglutide 14 mg. In addition, the SUCRA values for PEX 168-200 μg, tirzepatide 15 mg, and oral semaglutide 14 mg were higher than placebo, meaning that PEX 168-200 μg, tirzepatide 15 mg and oral semaglutide 14 mg had the highest incidence of serious adverse events compared to placebo.
As shown in Table 3 and Figure 5, the SUCRA results suggest that the ranking of the rate of serious adverse events from high to low was as follows: PEX168-200μg>Tirzepatide15mg>Oralsemaglutide14mg>Placebo>Albiglutide30mg>Lixisenatide20μg>Liraglutide1.8mg>Semaglutide1.0mg>Daily-Exenatide10μg>Weekly-Exenatide2. 0mg>Dulaglutide1.5mg, it means that PEX168-200μg had the highest incidence of serious adverse events compared to all other interventions.
3.9 Hypoglycemic events
The results of hypoglycemic events for 5 interventions including sulfonylureas, and 10 interventions excluding sulfonylureas are shown in Tables 7, 8. There was no significant difference in hypoglycemic events when sulfonylureas were included in combination therapy with the 5 GLP-1RAs compared with placebo. There was no significant difference in hypoglycemic events in head-to-head comparisons. When sulfonylureas were not included among the drugs in combination therapy, there was a higher rate of hypoglycemic events with semaglutide 1.0 mg versus exenatide 2.0 mg and liraglutide 1.8 mg versus placebo, and no significant difference of hypoglycemic events in the other head-to-head comparisons. However, the hypoglycemic events (OR value) were significantly higher when the combination therapy included a sulfonylurea than when there was no sulfonylurea, suggesting that sulfonylureas can dramatically increase the incidence of hypoglycemia events. Meanwhile, there was no significant difference in the incidence of hypoglycemic events with or without sulfonylureas compared to placebo, suggesting that GLP-1RAs do not increase the incidence of hypoglycemic events.
As shown in Table 3 and Figure 5, the SUCRA results indicated that the ranking of the hypoglycemic events from high to low excluding sulfonylureas as follows: Tirzepatide15mg>Daily-Exenatide10μg>Dulaglutide1.5mg>Lixisenatide20μg>Semaglutide1.0mg>Weekly-Exenatide2.0mg>PEX168-200μg>Liraglutide1. 8mg>Placebo>Albiglutide30mg>Oral semaglutide14mg, and it suggested that tirzepatide15mg had the highest incidence of hypoglycemic events compared with all other GLP-1RAs.
3.10 AE withdrawal
The results of the AE discontinuation rate for 10 interventions are shown in Table 9. Compared to the placebo, the other eight GLP-1RAs were similar regarding adverse event withdrawal rates, except for a significant difference between albiglutide 30 mg and semaglutide 1.0 mg. Semaglutide 1.0 mg versus exenatide 2.0 mg, oral semaglutide 14 mg versus exenatide 10 μg, and lixisenatide 20 μg showed significant differences in adverse event withdrawal rates. Lixisenatide 20 μg significantly differed from dulaglutide 1.5 mg, tirzepatide 15 mg, lixisenatide 20 μg, and exenatide 10 μg. The other head-to-head comparisons between GLP-1RAs did not show a significantly higher rate of adverse event withdrawal.
As shown in Table 3 and Figure 5, the SUCRA results indicate that the ranking of AE withdrawals from highest to lowest was as follows: Daily exenatide10μg>Lixisenatide20μg>Oral semaglutide14mg>Tirzepatide15mg>Liraglutide1.8mg>Dulaglutide1.5mg>Albiglutide30mg>Placebo>Semaglutide1. 0mg>PEX168-200μg>weekly-exenatide2.0mg, it suggested that daily-exenatide10μg had the highest incidence of AE withdrawal compared to all other interventions.
3.11 Cluster analysis
Cluster analysis was performed based on the SUCRA values for clinical efficacy (reduction in HbA1c) and safety (the rate of adverse events), and the results of the cluster analysis are shown in Supplementary File 3 (Figure 1). Compared with other interventions, 15 mg of tirzeptide had a significant advantage in efficacy, but it had a higher rate of adverse events than other GLP-1RAs. Semaglutide1.0mg, oral semaglutide14mg, liraglutide1.8mg, dulaglutide1.5mg, PEX168-200μg, albiglutide30mg, daily exenatide10μg, weekly exenatide2.0mg have a similar advantage in terms of efficacy and safety. Lixisenatide 20 µg has an efficacy advantage but not in security (the rate of adverse events).
3.12 Sensitivity analysis
Supplementary File 3 (Table 2) shows the sensitivity analysis results. Stata software was used to perform sensitivity analyses for the primary outcome indicator (HbA1c reduction) after excluding 10 high-risk studies (5, 8, 10, 12, 16, 19, 20, 25, 32, 33) according to results 3.2 Quality assessment of included studies. Supplementary File 3 (Table 2) shows that the network meta-analysis did not change significantly without inversion, indicating that the results were reliable.
3.13 Publication bias analysis
Stata software generated funnel plots for six outcome measures: HbA1c reduction, weight loss, total adverse events, serious adverse events, hypoglycemic events, and AE discontinuation. The funnel plots for efficacy and safety are shown in Figure 6. Figure 6 shows that the distribution of studies in the funnel plot is approximately symmetric, suggesting no publication or other bias between studies. However, in Figures 6A, B, there is heterogeneity in some studies with scatter plots outside the funnel plot, possibly due to minor sample effects.
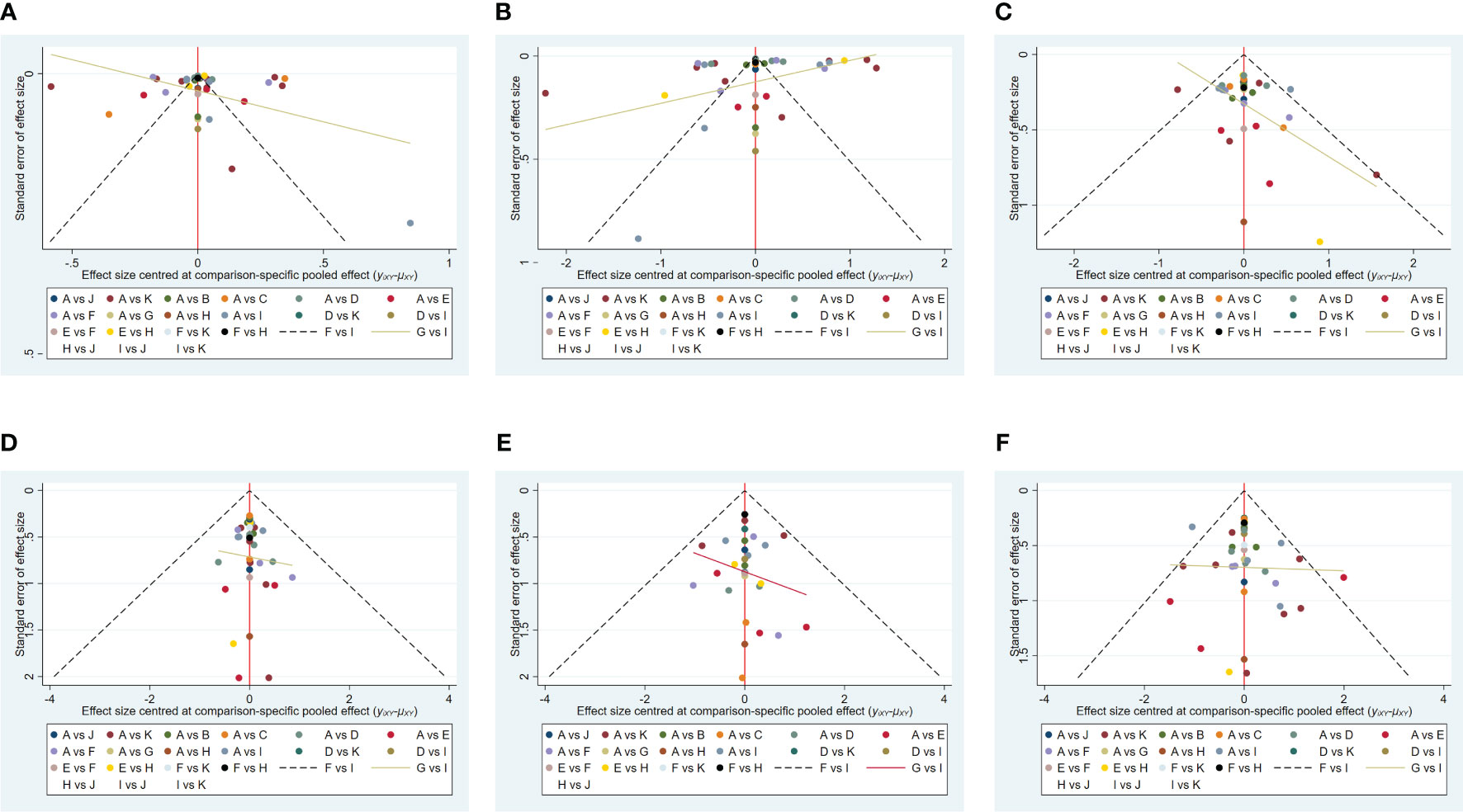
Figure 6 The funnel plots for efficacy and safety. (A) (Decreased HbA1c), (B) (Weight loss), (C) (The rate of adverse events), (D) (The rate of serious adverse events), (E) (Hypoglycemic events), (F) (AE withdrawal), (Note: A: Placebo; B: Albi30mg; C: PEX168-200μg; D: Lixisenatide20μg; E: Tirze15mg; F: Dulaglutide1.5mg; G: Oralsema14mg; H: Sema1.0mg; I: Lira1.8mg; J: Weekly-Exenatide2.0mg; K: Daily-Exenatide10μg).
4 Discussion
This review was based on 34 RCTs involving 12,993 patients with type 2 diabetes who had poor glycemic control on metformin and were randomized to 10 GLP-1RAs or placebo. In the 34 included studies, 18 used metformin + GLP-1RA as the therapeutic agent, and 16 used metformin + GLP-1RA ± OAD (e.g., sulfonylurea, thiazolidinedione, and sodium-glucose cotransporter-2 inhibitors). According to the NMA results, after treatment (82.4%≥ 24 weeks), all GLP-1RAs were superior to placebo in efficacy. In terms of HbA1c reduction, the most effective drug was tirzepatide 15 mg (-2.23%), followed by semaglutide 1.0 mg (-1.57%) and PEX 168200 (-1.12%). In terms of weight loss, tirzepatide 15 mg was the best (-11.3 kg), followed by semaglutide 1.0 mg (-5.99 kg) and oral semaglutide 14 mg (-3.95 kg). This result suggests that tirzepatide 15mg and semaglutide 1.0mg have a clear advantage in reducing blood glucose and weight in patients with T2D.
In terms of safety, tirzepatide 15mg (OR=1.13), oral semaglutide 14mg (OR=0.88), and semaglutide 1.0mg (OR=0.76) were more frequent than the other GLP-1RAs in the rate of adverse events (e.g., nausea, vomiting, and diarrhea, etc.). The OR values for tirzepatide 15mg were more significant than 1, indicating a higher risk of adverse events. In addition, tirzepatide 15mg, oral semaglutide 14mg, and semaglutide 1.0mg did not show a significantly higher incidence of adverse events. The incidence of serious adverse events (e.g., cardiovascular events, severe gastrointestinal reactions, infections, etc.) was higher for PEX168 (OR=0.54), tirzepatide 15mg (OR=0.15), and oral semaglutide 14mg (OR=0.09) than for other GLP-1RAs, and tirzepatide 15mg and oral semaglutide 14mg will have serious adverse events but at a lower risk.
GLP-1RAs generally do not cause hypoglycemia in patients with type 2 diabetes who are not taking insulin (54). However, it is noteworthy that when the combination included sulfonylureas, hypoglycemia (OR) incidence was significantly higher with GLP-1RAs, suggesting that sulfonylureas are an essential contributor to hypoglycemia (55). The 10 GLP-1RAs did not exhibit a significantly higher incidence of AE withdrawal.
Tirzepatide is a novel GIP and GLP-1 dual receptor agonist that the U.S. FDA approved for treating T2D in 2022. it has demonstrated potent HbA1c and body weight reduction. In a recently completed Phase 3, double-blind, randomized, controlled trial of once-weekly tirzepatide in the treatment of obesity, tirzepatide 15 mg achieved a mean percentage change in body weight of -20.9% (-21.9 kg) over 72 weeks of treatment, suggesting that tirzepatide 15 mg is also a potential weight loss agent (56). The result is a significant breakthrough in dual-targeted GLP-1 and GIP drugs that reduce HbA1c and body weight more effectively than GLP-1 RA. In addition, a phase 2 RCT demonstrated that retatrutide, a GIP, GLP-1, and glucagon receptor agonist achieved clinically meaningful improvements in glycemic control (2.02% reduction in HbA1c with 24 weeks of treatment) and significant weight reduction (17.18kg reduction in weight with 36 weeks of treatment), and its safety profile was consistent with that of GLP-1 receptor agonists as well as GIP and GLP-1 receptor agonists (57). In the future, GIP, GLP-1, and glucagon tri-agonists are promising for patients with T2D and/or obesity.
Semaglutide1.0mg and oral semaglutide 14mg was inferior to tirzepatide in reducing HbA1c and body weight but had significant advantages over other GLP-1RAs. In addition, oral semaglutide offers excellent convenience to patients and improves compliance. The results were generally consistent with Xia L et al. 's (58) efficacy in lowering HbA1c and body weight. However, oral semaglutide treatment increases the incidence of nausea, vomiting, and diarrhea (Comparison with placebo or other OAD (e.g., Sitagliptin, empagliflozin)), and the oral route of administration is strongly associated with an increase in gastrointestinal adverse events (59). The higher AE withdrawal rate for oral semaglutide than for subcutaneous semaglutide and tirzepatide may be related to gastrointestinal adverse events.
The global and local inconsistency test results showed no significant inconsistency for each outcome indicator and two comparison groups. In the closed loop inconsistency test, the same four closed loops showed significant inconsistency in the two outcome indicators of HbA1c reduction and weight loss. The other closed loops were those for which there was no evidence of inconsistency (p >0.05 or CI_95 includes 0). In conclusion, the results for the inconsistency of the whole network are reliable.
In sensitivity analyses, the NMA results did not change significantly and were reliable. Overall, the risk of the included studies was low, the quality was good, and the NMA results were reliable. The publication bias results suggest that there may be some bias in the effectiveness due to differences in the drugs used and the effect of a small sample size. The heterogeneity results indicate a significant difference in efficacy, and the main reason for this was the difference in the use of background medication (metformin +/-OAD). In conclusion, heterogeneity does not significantly impact the NMA results.
T2D places a heavy burden on the body, causing various complications (cardiovascular and cerebrovascular complications, microvascular and neurological lesions, diabetic foot, etc.) that can reduce the quality of life and life expectancy (60). The anti-inflammatory effects of metformin and GLP-1RAs contribute to their beneficial effects, as T2DM is characterized by chronic low-grade inflammation. In addition to glycemic control, aggressive cardiac risk reduction is a priority for all patients with T2D. Evidence suggests (61, 62) that aggressive reduction of multiple risk factors (weight loss or maintenance, smoking cessation, blood pressure control, lipid-lowering, dietary modification, and exercise) reduces the risk of microvascular and macrovascular complications in patients with diabetes.
GLP-1RAs are safe and effective and can reduce weight and the risk of major cardiovascular disease (63). Semaglutide 2.4mg and liraglutide 3.0mg have been approved by the U.S. Food and Drug Administration (FDA) for weight management in people who are overweight or obese. A related weight loss meta-analysis (15) showed that treatment with semaglutide 2.4 mg and liraglutide 3.0 mg for more than 20 weeks in combination with daily diet and exercise resulted in weight loss of 12.47 kg and 5.24 kg, respectively, in people with overweight and obesity. Tirzepatide 15 mg is expected to be approved shortly for weight control in adults with obesity or overweight (with >= 1+ obesity-related comorbidity). Liraglutide, dulaglutide, and semaglutide have been shown to have cardiovascular benefits, and the only other glucose-lowering agents for T2D that have been shown to have cardiovascular benefits are the SGLT-2 inhibitors empagliflozin, canagliflozin and dapagliflozin (64, 65). In clinical practice, selecting a GLP-1RA with cardiovascular efficacy, good glucose-lowering, and weight-loss (kg) effects, and a high safety profile based on the patient’s medical condition and needs is critical. Based on efficacy and safety results, tirzepatide 15mg, semaglutide 1.0mg, and oral semaglutide 14mg are good options for patients with T2DM.
The main objective of this study is to, directly and indirectly compare the safety and efficacy of 10 GLP-1RAs in combination with metformin, to address the wide variety of medications encountered in clinical practice, and to provide evidence-based support and reference for the use of GLP-1RAs in clinical practice. Meanwhile, being overweight and obese are risk factors for diabetes, and this study evaluated the effect of weight loss with different GLP-1RAs and glucose lowering. However, there are limitations to this study: First, the range of drug treatment cycles is vast, mostly between 12 and 48 weeks, which affects the safety assessment results. Second, in this review, there were some studies where the background drug was metformin and others where it was metformin +/- OAD, which is the source of the heterogeneity in the efficacy assessment. Third, the populations included in this study include Caucasian, African and Asian populations. However, there are some differences in the physical condition of patients with type 2 diabetes from different regions or races, and their actual clinical efficacy is also biased. Finally, there were small samples of studies and high-risk studies in the included studies, which may make our results insubstantial and incomplete. Even though there are some limitations in this study, more and more large-sample, high-quality studies will gradually emerge over time so that our evaluation results will improve and become more convincing overall.
5 Conclusion
This study results showed that GLP-1RAs were effective in lowering HbA1c and reducing body weight without leading to increased incidence of hypoglycemic reactions. In addition, this study may provide reference and evidence-based medical evidence for clinicians to select GLP-1RAs in patients with T2DM and high BMI. Tirzepatide 15mg, semaglutide 1.0 mg, and oral semaglutide 14 mg may be preferred for treating patients with T2DM. Finally, there is an urgent need for more high-quality, large-sample randomized controlled trials to make our study results more comprehensive and reliable.
Author contributions
All co-authors made substantial contributions to this article and this version is published as the final version, agreeing to submit this study to the journal and taking responsibility for all aspects of the article. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key Specialty Construction Project (Clinical Pharmacy) and the High-level Clinical Key Specialty of Guangdong Province, and funded by the Central Finance Subsidy Fund for the Improvement of Medical Services and Guarantee Capacity, code Z155080000004; Guangzhou Minsheng Science and Technology Research Program Project, code 201803010096.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1244432/full#supplementary-material
References
1. American Diabetes Association. Economic costs of diabetes in the U.S. @ in 2017. Diabetes Care (2018) 41:917–28. doi: 10.2337/dci18-0007
2. Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross-sectional study. BMJ (2020) 369:m997. doi: 10.1136/bmj.m997
3. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA (2013) 310(9):948 959. doi: 10.1001/jama.2013.168118
4. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA (2017) 317(24):2515 2523. doi: 10.1001/jama.2017.7596
5. Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988-2014. Lancet Diabetes Endocrinol (2018) 6:392–403. doi: 10.1016/S2213-8587(18)30027-5
6. Pan Y, Chen W, Wang Y. Prediabetes and outcome of ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis (2019) 28:683–92. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.008
7. Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all-cause mortality: systematic review and meta-analysis. BMJ (2016) 355:i5953. doi: 10.1136/bmj.i5953
8. American Diabetes Association. Introduction: standards of medical care in diabetes—2022. Diabetes Care (2022) 45(Supplement_1):S1–2. doi: 10.2337/dc22-Sint
9. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med (2016) 375(4):311–22. doi: 10.1056/NEJMoa1603827
10. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet (2019) 394(10193):121–30. doi: 10.1016/S0140-6736(19)31149-3
11. Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol (2019) 7(10):776–85. doi: 10.1016/250S2213-8587(19)30249-9
12. Sattar N, Rawshani A, Franzen S, Rawshani A, Svensson AM, Rosengren A, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation (2019) 139:2228–37. doi: 10.1161/CIRCULATIONAHA.118.037885
13. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by 255 representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J (2016) 37:2315–81. doi: 10.1093/eurheartj/ehw106
14. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, EXSCEL Study Group, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med (2017) 377:1228–39. doi: 10.1056/NEJMoa1612917
15. Xie Z, Yang S, Deng W, Li J, Chen J. Efficacy and safety of liraglutide and semaglutide on weight loss in people with obesity or overweight: A systematic review. Clin Epidemiol (2022) 14:1463–76. doi: 10.2147/CLEP.S391819
16. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9
17. International Diabetes Federation. IDF Diabetes Atlas. 10th edn. Brussels, Belgium: International Diabetes Federation (2021).
18. Mosenzon O, Alguwaihes A, Leon JLA, Bayram F, Darmon P, Davis TME, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol (2021) 20(1):154. doi: 10.1186/s12933-021-01344-0
19. Chinese Medical Association, Diabetes Branch. Chinese guidelines for the prevention and treatment of type 2 diabetes mellitus (2020 edition). Int J Endocrinol Metab (2021) 41(05):482–548. doi: 10.19538/j.nk2021080106
20. Ahrén B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care (2014) 37(8):2141–8. doi: 10.2337/dc14-0024
21. Home PD, Shamanna P, Stewart M, Yang F, Miller M, Perry C, et al. Efficacy and tolerability of albiglutide versus placebo or pioglitazone over 1 year in people with type 2 diabetes currently taking metformin and glimepiride: HARMONY 5. Diabetes Obes Metab (2015) 17(2):179–87. doi: 10.1111/dom.12414
22. Gao F, Lv X, Mo Z, Ma J, Zhang Q, Yang G, et al. Efficacy and safety of polyethylene glycol loxenatide as add-on to metformin in patients with type 2 diabetes: A multicentre, randomized, double-blind, placebo-controlled, phase 3b trial. Diabetes Obes Metab (2020) 22(12):2375–83. doi: 10.1111/dom.14163
23. Chen X, Lv X, Yang G, Lu D, Piao C, Zhang X, et al. Polyethylene glycol loxenatide injections added to metformin effectively improve glycemic control and exhibit favorable safety in type 2 diabetic patients. J Diabetes (2017) 9(2):158–67. doi: 10.1111/1753-0407.12397
24. Nauck M, Rizzo M, Johnson A, Bosch-Traberg H, Madsen J, Cariou B, et al. Once-daily liraglutide versus lixisenatide as add-on to metformin in type 2 diabetes: A 26-week randomized controlled clinical trial. Diabetes Care (2016) 39(9):1501–9. doi: 10.2337/dc15-2479
25. Yu Pan C, Han P, Liu X, Yan S, Feng P, Zhou Z, et al. Lixisenatide treatment improves glycaemic control in Asian patients with type 2 diabetes mellitus inadequately controlled on metformin with or without sulfonylurea: a randomized, double-blind, placebo-controlled, 24-week trial (GetGoal-M-Asia). Diabetes Metab Res Rev (2014) 30(8):726–35. doi: 10.1002/dmrr.2541
26. Bolli GB, Munteanu M, Dotsenko S, Niemoeller E, Boka G, Wu Y, et al. Efficacy and safety of lixisenatide once daily vs. placebo in people with Type 2 diabetes insufficiently controlled on metformin (GetGoal-F1). Diabetes Med (2014) 31(2):176–84. doi: 10.1111/dme.12328
27. Rosenstock J, Raccah D, Korányi L, Maffei L, Boka G, Miossec P, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care (2013) 36(10):2945–51. doi: 10.2337/dc12-2709
28. Ahrén B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care (2013) 36(9):2543–50. doi: 10.2337/dc12-2006
29. Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med (2021) 385(6):503–15. doi: 10.1056/NEJMoa2107519
30. Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet (2018) 392(10160):2180–93. doi: 10.1016/S0140-6736(18)32260-8
31. Heise T, Mari A, DeVries JH, Urva S, Li J, Pratt EJ, et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol (2022) 10(6):418–29. doi: 10.1016/S2213-8587(22)00085-7
32. Frias JP, Nauck MA, Van J, Benson C, Bray R, Cui X, et al. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes Metab (2020) 22(6):938–46. doi: 10.1111/dom.13979
33. Frias JP, Wynne AG, Matyjaszek-Matuszek B, Bartaskova D, Cox DA, Woodward B, et al. Efficacy and safety of an expanded dulaglutide dose range: A phase 2, placebo-controlled trial in patients with type 2 diabetes using metformin. Diabetes Obes Metab (2019) 21(9):2048–57. doi: 10.1111/dom.13764
34. Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab (2015) 17(9):849–58. doi: 10.1111/dom.12479
35. Dungan KM, Povedano ST, Forst T, González JG, Atisso C, Sealls W, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet (2014) 384(9951):1349–57. doi: 10.1016/S0140-6736(14)60976-4
36. Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care (2014) 37(8):2159–67. doi: 10.2337/dc13-2760
37. Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet (2019) 394(10192):39–50. doi: 10.1016/S0140-6736(19)31271-1
38. Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol (2018) 6(4):275–86. doi: 10.1016/S2213-8587(18)30024-X
39. Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): A 56-week, open-label, randomized clinical trial. Diabetes Care (2018) 41(2):258–66. doi: 10.2337/dc17-0417
40. van Eyk HJ, Paiman EHM, Bizino MB, de Heer P, Geelhoed-Duijvestijn PH, Kharagjitsingh AV, et al. A double-blind, placebo-controlled, randomised trial to assess the effect of liraglutide on ectopic fat accumulation in South Asian type 2 diabetes patients. Cardiovasc Diabetol (2019) 18(1):87. doi: 10.1186/s12933-019-0890-5
41. Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care (2009) 32(1):84–90. doi: 10.2337/dc08-1355
42. Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care (2009) 32(7):1224–30. doi: 10.2337/dc08-2124
43. Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia (2009) 52(10):2046–55. doi: 10.1007/s00125-009-1472-y
44. Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet (2009) 374(9683):39–47. doi: 10.1016/S0140-6736(09)60659-0
45. Lu CH, Wu TJ, Shih KC, Ni E, Reed V, Yu M, et al. Safety and efficacy of twice-daily exenatide in Taiwanese patients with inadequately controlled type 2 diabetes mellitus. J Formos Med Assoc (2013) 112(3):144–50. doi: 10.1016/j.jfma.2012.02.027
46. Derosa G, Franzetti IG, Querci F, Carbone A, Ciccarelli L, Piccinni MN, et al. Exenatide plus metformin compared with metformin alone on β-cell function in patients with Type 2 diabetes. Diabetes Med (2012) 29(12):1515–23. doi: 10.1111/j.1464-5491.2012.03699
47. Apovian CM, Bergenstal RM, Cuddihy RM, Qu Y, Lenox S, Lewis MS, et al. Effects of exenatide combined with lifestyle modification in patients with type 2 diabetes. Am J Med (2010) 123(5):468.e9–17. doi: 10.1016/j.amjmed.2009.11.019
48. Gao Y, Yoon KH, Chuang LM, Mohan V, Ning G, Shah S, et al. Efficacy and safety of exenatide in patients of Asian descent with type 2 diabetes inadequately controlled with metformin or metformin and a sulphonylurea. Diabetes Res Clin Pract (2009) 83(1):69–76. doi: 10.1016/j.diabres.2008.09.037
49. Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care (2005) 28(5):1083–91. doi: 10.2337/diacare.28.5.1083
50. DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care (2005) 28(5):1092–100. doi: 10.2337/diacare.28.5.1092
51. Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet (2013) 381(9861):117–24. doi: 10.1016/S0140-6736(12)61267-7
52. Gadde KM, Vetter ML, Iqbal N, Hardy E, Öhman P. DURATION-NEO-2 study investigators. Efficacy and safety of autoinjected exenatide once-weekly suspension versus sitagliptin or placebo with metformin in patients with type 2 diabetes: The DURATION-NEO-2 randomized clinical study. Diabetes Obes Metab (2017) 19(7):979–88. doi: 10.1111/dom.12908
53. Kadowaki T, Namba M, Yamamura A, Sowa H, Wolka AM, Brodows RG. Exenatide exhibits dose-dependent effects on glycemic control over 12 weeks in Japanese patients with suboptimally controlled type 2 diabetes. Endocr J (2009) 56(3):415–24. doi: 10.1507/endocrj.k08e-296
54. Østoft SH, Bagger JI, Hansen T, Pedersen O, Faber J, Holst JJ, et al. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care (2014) 37(7):1797–805. doi: 10.2337/dc13-3007
55. Matsuoka A, Hirota Y, Takeda A, Kishi M, Hashimoto N, Ohara T, et al. Relationship between glycated hemoglobin level and duration of hypoglycemia in type 2 diabetes patients treated with sulfonylureas: A multicenter cross-sectional study. J Diabetes Investig (2020) 11(2):417–25. doi: 10.1111/jdi.13132
56. Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med (2022) 387(3):205–16. doi: 10.1056/NEJMoa2206038
57. Rosenstock J, Frias J, Jastreboff AM, Du Y, Lou J, Gurbuz S, et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet (2023) 402(10401):529–44. doi: 10.1016/S0140-6736(23)01053-X
58. Xia L, Shen T, Dong W, Su F, Wang J, Wang Q, et al. Comparative efficacy and safety of 8 GLP-1RAs in patients with type 2 diabetes: a network meta-analysis. Diabetes Res Clin Pract (2021) 177:108904. doi: 10.1016/j.diabres.2021.108904
59. Avgerinos I, Michailidis T, Liakos A, Karagiannis T, Matthews DR, Tsapas A, et al. Oral semaglutide for type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab (2020) 22(3):335–45. doi: 10.1111/dom.13899
60. Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society; Geriatric Professional Committee of Beijing Medical Award Foundation; National Clinical Medical Research Center for Geriatric Diseases (PLA General Hospital). Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition). Zhonghua Nei Ke Za Zhi (2022) 61(1):12–50. Chinese. doi: 10.3760/cma.j.cn112138-20211027-00751
61. Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med (2018) 379(7):633–44. doi: 10.1056/NEJMoa1800256
62. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med (2003) 348(5):383–93. doi: 10.1056/NEJMoa021778
63. Jing G. Research progress of glucagon-like peptide-1 receptor agonists in cardiovascular complications of type 2 diabetes mellitus. Modern Diagnosis Ther (2020) 31(19):3041–3042+3190.
64. Yanlan L, Aiwen H, Guanxu C, Chen T, Zhao L, Liao X, et al. Cardiovascular benefit of SGLT-2 inhibitors and GLP-1 receptor agonists in the treatment of type 2 diabetes: a systematic review and reticulated Meta-analysis. J Pharm Pract (2022) 40(04):354–8.
Keywords: glucagon-like peptide-1 receptor agonists, safety, efficacy, systematic review, randomized controlled trials, type 2 diabetes
Citation: Xie Z, Hu J, Gu H, Li M and Chen J (2023) Comparison of the efficacy and safety of 10 glucagon-like peptide-1 receptor agonists as add-on to metformin in patients with type 2 diabetes: a systematic review. Front. Endocrinol. 14:1244432. doi: 10.3389/fendo.2023.1244432
Received: 22 June 2023; Accepted: 07 August 2023;
Published: 28 August 2023.
Edited by:
Isabela Lovizutto Iessi, Indiana Biosciences Research Institute, United StatesReviewed by:
Eric Morris Bomberg, Medical School, University of Minnesota, United StatesFranciane Gallego, Sao Paulo State University, Brazil
Copyright © 2023 Xie, Hu, Gu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jisheng Chen, Y2pzbHltQDE2My5jb20=
†ORCID: Zeyu Xie, orcid.org/0000-0002-1609-4377
 Zeyu Xie
Zeyu Xie Jia Hu
Jia Hu Hangye Gu
Hangye Gu