- 1Department of Gastroenterology, Shengjing Hospital of China Medical University, Shenyang, China
- 2Department of Endocrinology, Shengjing Hospital of China Medical University, Shenyang, China
Objectives: This study was performed to investigate the changes and influencing factors of liver controlled attenuation parameter (CAP) in obese patients with polycystic ovary syndrome (PCOS), and to determine the prevalence and risk factors of nonalcoholic fatty liver disease (NAFLD) in PCOS patients with obesity.
Methods: Forty-one PCOS patients with obesity and twenty age- and body mass index (BMI)-matched control women without PCOS were enrolled in this study. General data, body composition, biochemical parameters, sex hormones, and liver CAP in the two groups were collected and compared. Liver CAP was measured using transient elastography.
Results: NAFLD was more common in the Obese PCOS group than in the control group (75.61% vs. 45.00%, P=0.018). Compared to the control group, the obese PCOS group showed apparent increases in alanine transaminase (ALT), aspartate transaminase (AST), CAP, triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), totle testosterone (TT), free androgen index (FAI), fasting insulin (FIns), and homeostasis model assessment-insulin resistance (HOMA-IR), along with lower high-density lipoprotein cholesterol (HDL-C) and sex hormone binding globulin (SHBG) levels. In addition, as shown by Spearman analysis, liver CAP in PCOS patients with obesity had a positive correlation with ALT, AST, TG, TT, FAI, FIns, and HOMA-IR, and a negative correlation with SHBG. Logistic regression analysis showed that TG, TT, FIns, and HOMA-IR were risk factors for NAFLD, while TT was an independent risk factor for NAFLD in PCOS patients with obesity.
Conclusion: PCOS patients with obesity had a significantly higher prevalence of NAFLD. Furthermore, in PCOS patients with obesity, liver CAP was associated with disorders of lipid metabolism, insulin resistance, and hyperandrogenemia, with elevated testosterone levels being an independent risk factor for NAFLD in PCOS patients with obesity.
1 Introduction
Polycystic ovary syndrome (PCOS) is the most common reproductive, endocrine, and metabolic disorder among women of childbearing age. The prevalence of PCOS in adult Chinese women is 5.6% (1, 2). The most common clinical manifestations of PCOS include chronic anovulation, hirsutism or acne, menstrual cycle disorder, and infertility, which are closely related to obesity, insulin resistance (IR), and abnormal lipid metabolism (3). Up to 80% of PCOS patients are overweight or obese (4).
Recent studies have shown that the prevalence of non-alcoholic fatty liver disease (NAFLD) in PCOS patients is significantly higher than that in the normal population (5), and this phenomenon is more common in PCOS patients with obesity (6). NAFLD is defined as a liver fat accumulation ≥ 5% in patients in whom secondary causes, such as viral hepatitis, excessive drinking, drug-induced liver disease, autoimmune liver disease, and hereditary metabolic liver disease, can be excluded (7). Liver biopsy remains the gold standard for the diagnosis of NAFLD, but its invasive characteristics limit its clinical application (8). Ultrasonography, as a simple and convenient examination method, is the most commonly used screening method for NAFLD in the clinic; however, the screening results of ultrasonography are influenced by the subjective judgment of the examiners, and the sensitivity to the diagnosis of liver steatosis below 20% is obviously reduced (9). Therefore, a sensitive, non-subjective test is required to screen for NAFLD.
The Controlled Attenuation Parameter (CAP), which is based on the principle of instantaneous elastography, quantifies liver steatosis by measuring the attenuation of the ultrasound beam in direct correlation with liver fat content (10). High concordance between CAP and liver biopsy enables CAP to better reflect liver steatosis (11). FibroTouch, a third-generation liver transient elastography device (12), has been widely used in the diagnosis of NAFLD. Therefore, this study used FibroTouch to measure liver CAP in PCOS patients with obesity to explore the prevalence and risk factors of NAFLD in PCOS patients with obesity.
2 Materials and methods
2.1 Patients
Forty-one PCOS patients with obesity attending the endocrine clinic of the Shengjing Hospital of China Medical University from October 2020 to January 2022 were consecutively enrolled in the study. Twenty non-PCOS women matched for age and body mass index (BMI) were consecutively selected as control subjects during the same period. This study was audited by Medical Ethics Committee of Shengjing Hospital of China Medical University (Audite No. 2021PS647K), and informed consent was obtained from each patient.
2.2 Inclusion criteria and exclusion criteria
2.2.1 Inclusion criteria
(i) Premenopausal women aged over 18 who were diagnosed with obese PCOS for the first time. (ii) PCOS was diagnosed following the Rotterdam Standard (13), for which at least two of the following three conditions must be met: clinical manifestations of hyperandrogenemia (HA) and/or HA; Rare ovulation or anovulation; Ovarian polycystic changes (unilateral or bilateral ovaries 2–9 mm, follicles ≥12); or ovarian volume ≥10 ml (ovarian volume=0.5×length×width×thickness). (iii) Obesity was defined as a BMI ≥ 28 kg/m2, in accordance with the BMI classification criteria in China (14).
2.2.2 Exclusion criteria
(i) Women with other diseases that may cause HA or ovulation disorder, such as Cushing’s syndrome, thyroid diseases, congenital adrenal hyperplasia, hyperprolactinemia, androgen-secreting tumours of the adrenal gland or ovary, etc. (ii) Age under 18 years of age or postmenopausal. (iii) Previous clear diagnosis of PCOS. (iv) Chronic liver diseases, such as alcoholic liver disease (alcohol consumption ≥ 20 g per day), viral hepatitis, autoimmune liver disease, drug-induced liver disease, and hereditary metabolic liver disease, excluding patients with cardiopulmonary insufficiency, renal insufficiency, and malignant tumours. (v) A history of taking hepatoprotective drugs or drugs affecting glucose and lipid metabolism in the last 3 months.
2.3 CAP testing
FibroTouch (Haskell Medical Technology Co., Ltd., Wuxi, China), operated by two professionally trained and experienced operators, was blinded used to measure CAP of the PCOS patients and controls. First, the imaging site was selected with the machine’s B-ultrasound imaging probe, and the 7th, 8th, and 9th intercostal spaces between the right axillary front line and the axillary midline were selected as the detection area, while avoiding intrahepatic cysts, bile ducts, and great vessels, to confirm the clear structure of the imaged liver tissue. The probe coated with the coupling agent was close to the patient’s intercostal space at the positioning point and perpendicular to the skin surface. A small pressure was applied to ensure that the probe pressure was in a suitable area for measurement. At least 10 effective measurements were taken at the same measuring point, and the success rate was at least 75%. The relative deviation was less than 30%, the image required a straight A wave, uniform M wave layer, and normal elastogram waveform, and the CAP value (db/m) was obtained. NAFLD was defined as a CAP ≥ 244 db/m (15).
2.4 Measurement index
2.4.1 General data measurement
After fasting for 12 hours, patients and controls took off their normal shoes and put on light clothes, and their height and weight were measured during a quiet resting state in the morning. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared.
2.4.2 Body composition examination
Waist-to-hip ratio (WHR), body fat percentage, and visceral fat area (VFA) were measured using an InBody 770 scanner (Inbody Co., Seoul, Korea).
2.4.3 Measurement of biochemical indexes and sex hormone levels
Fasting blood samples were collected from the antecubital vein.
Biochemical parameters were measured using an automated biochemical analyzer (Abbott Architect ci 16200, Abbott, USA). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using NADH assays. Triglyceride (TG) levels were measured using deionization and enzymatic methods. Total cholesterol (TC) was measured using the cholesterol oxidase method. Low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) levels were measured using chemically modified enzymatic methods. Fasting blood glucose (FBG) levels were measured using the hexokinase/glucose-6-phosphate dehydrogenase method. Fasting insulin (FIns) levels were measured using the chemiluminescence (double antibody sandwich) method. Homeostasis model assessment-insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = FBG (mmol/L) ×FIns (mU/L)/22.5 (16). Fasting hyperinsulinemia and IR was defined by ≥95th percentile of our healthy reference population: that is, FIns > 11.8 mU/L, and HOMA-IR > 2.54 (17).
Sex hormone indicators were measured using an automated immunoassay (UnicelDxl800, Beckman Coulter, USA). The total testosterone (TT) level was assessed using an electrochemiluminescent immunoassay. The level of sex hormone-binding globulin (SHBG) was assessed using immunochemical luminescence assay. Free androgen index (FAI) was calculated using the following formula: FAI=TT (nmol/L) ×100/SHBG (nmol/L) (18).
2.5 Sample size estimation
For sample size calculation, a formula proposed by Charan et al. for cross-sectional studies was applied (19). The available data on CAP for PCOS and controls were used (287.3 ± 61.5db/m and 235 ± 39 db/m) (20), and with alpha set at 0.05, power at 90%, margin absolute error at 10%, and a sample size ratio of 2:1 for the PCOS group to the control group, we estimated that a minimum of 41 participants were needed (27 in the PCOS group and 14 in the control group).
2.6 Statistical methods
All statistical analyses were performed using the SPSS software (version 26.0; SPSS Inc., Chicago, IL, USA). Count data were analyzed using the chi-squared test. Kolmogorov-Smirnov statistics were used to test the normality of the measurement data. Variables with a normal distribution were expressed as mean ± standard deviation, and between-group comparisons were performed using an independent samples t-test. Non-normally distributed variables are presented as medians (25th and 75th percentiles), and between-group comparisons were performed using the Mann-Whitney U test. Spearman’s correlation analysis was used to verify correlations. Logistic regression was used to analyze risk factors. P-values<0.05 (P<0.05) were considered to indicate statistically significant differences.
3 Results
3.1 Patients screening process
A total of 126 PCOS patients based on the Rotterdam 2003 criteria were recruited from an outpatient endocrinology department. During the screening process, eighty-five patients were excluded for definite reasons: nine patients were under 18 years old; six patients were postmenopausal; seven patients had a BMI of less than 28 Kg/m2; five PCOS patients were comorbid with other chronic liver diseases; fifty-two patients with PCOS had not been diagnosed for the first time or had begun drug therapy; six patients declined to participate. Then, forty-one PCOS patients with obesity who met the inclusion criteria were enrolled in the study.
3.2 Comparison of the prevalence of NAFLD between the two groups
The prevalence of NAFLD in the obese PCOS and control groups was 75.61% (31/41) and 45.00% (9/20), respectively (χ2 = 5.579, P=0.018).
3.3 Comparison of general information, body composition, CAP, biochemical indexes, and sex hormone levels between the two groups
Compared to the control group, the obese PCOS group had higher levels of ALT, AST, CAP, TG, TC, LDL-C, TT, FAI, FIns, and HOMA-IR, and lower levels of HDL-C and SHBG (P <0.05). There were no statistically significant differences in age, BMI, waist circumference, WHR, body fat percentage, VFA, or FBG between the two groups (P > 0.05). These differences are shown in Table 1.
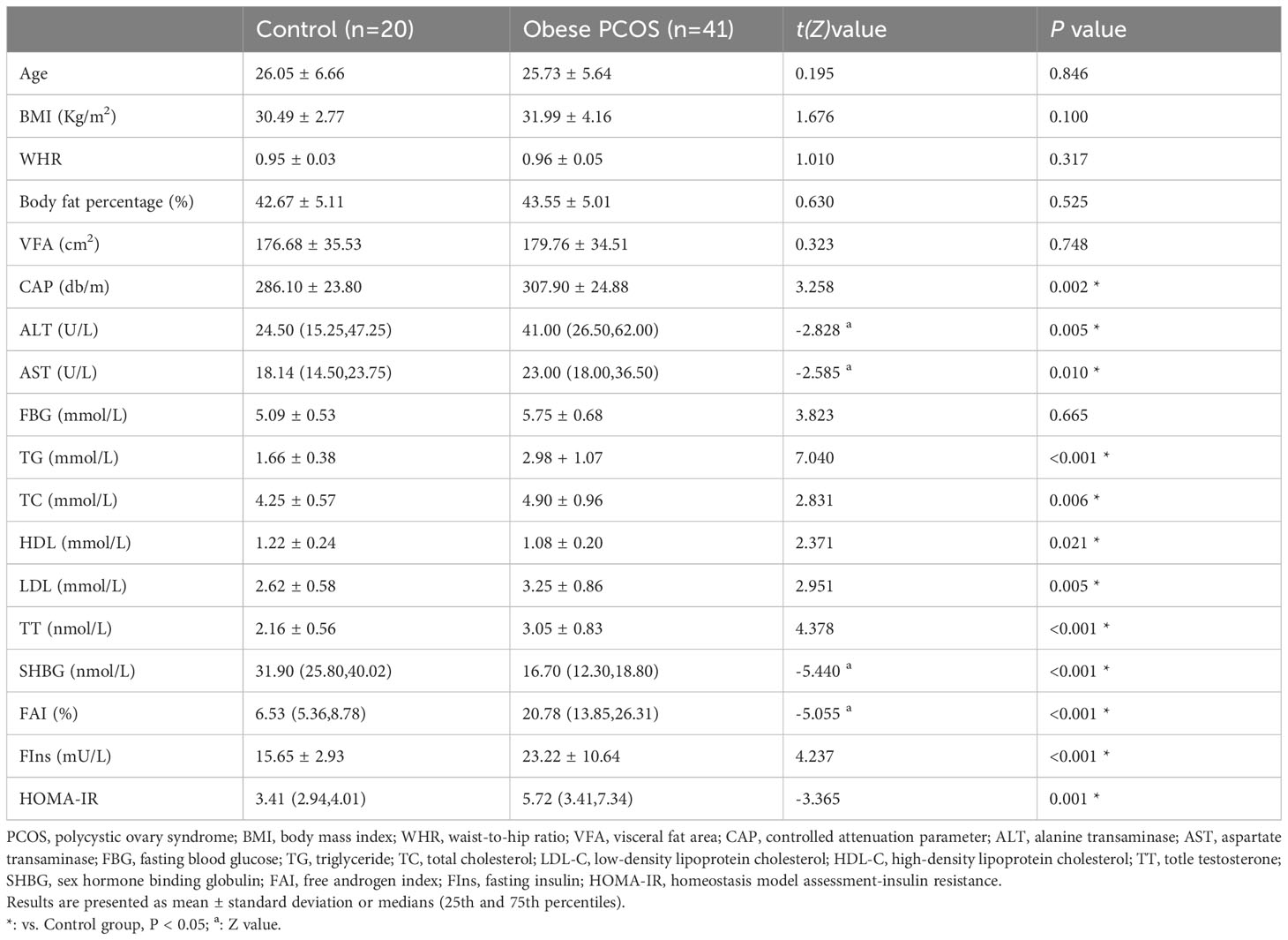
Table 1 Comparison of general information, body composition, CAP, biochemical indexes and sex hormone levels between control group and obese PCOS group.
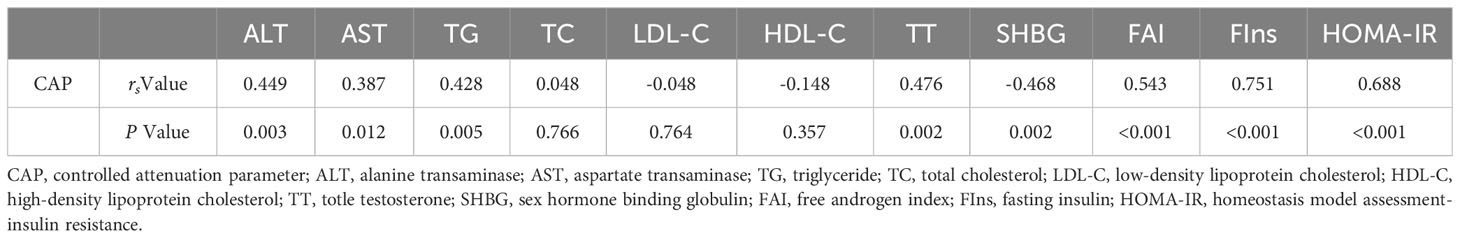
3.4 Correlation between liver CAP and differential indicators in PCOS patients with obesity
Liver CAP was positively correlated with ALT, AST, TG, TT, FAI, FIns, and HOMA-IR (P < 0.05), and negatively correlated with SHBG (P < 0.05). There were no significant correlations between liver CAP and TC, LDL-C, or HDL-C levels (P >0.05). The results of correlation analysis are shown in Table 2.
3.5 Analysis of risk factors for NAFLD in PCOS patients with obesity
The presence of NAFLD (defined as NAFLD with CAP ≥ 244 db/m) in PCOS patients with obesity was selected as the dependent variable. TG, TT, SHBG, FAI, FIns, and HOMA-IR, which were statistically significant in the correlation analysis, were selected as independent variables. The results of univariate logistic regression suggested that TG, TT, FIns, and HOMA-IR were risk factors for NAFLD in PCOS patients with obesity. Variables with statistical significance in the univariate analysis were further analyzed using multivariate logistic regression. The results revealed that TT was an independent risk factor for NAFLD in obese patients with PCOS. The results of risk factor analysis are shown in Table 3.
4 Discussion
This cross-sectional study aimed to investigate the prevalence and risk factors of NAFLD in PCOS patients with obesity by analyzing the changes in liver CAP in PCOS patients with obesity and the factors that influence it. To our knowledge, this is the first study to enroll obese Chinese women with PCOS as subjects for the FibroTouch test, and is further the first study with a weight- and age-matched control group included to observe and analyze changes in liver CAP. Our findings further showed that the prevalence of NAFLD was significantly higher in the obese PCOS group than that in the non-PCOS group (75.61% vs. 45.00%). Liver steatosis, transaminase levels, sex hormone disorders, and glucolipid metabolism were all found to be more severe in PCOS patients with obesity, as evidenced by the significant elevation in CAP, ALT, AST, TG, TC, LDL-C, TT, FAI, FIns, and HOMA-IR, and the significant decrease in HDL-C and SHBG levels in PCOS patients with obesity. Further analysis showed that hypertriglyceridaemia, HA, and IR were associated with elevated liver CAP in PCOS patients with obesity, while TT was an independent risk factor for the prevalence of NAFLD in PCOS patients with obesity.
PCOS can result in several metabolic disorders, and NAFLD is closely related to metabolic syndrome. Prior research has also indicated that NAFLD is the hepatic manifestation of metabolic syndrome; therefore, there is a close relationship between PCOS and NAFLD (21–23). In a previous study, the prevalence of NAFLD in PCOS, using the Rotterdam criteria as diagnostic criteria, was distributed between 32.9% and 77.0% (24). In the present study, the prevalence of NAFLD in PCOS patients with obesity was 75.61%, and the levels of liver CAP as well as ALT and AST activities in PCOS patients with obesity were significantly higher than those in controls, which further confirms that PCOS could exacerbate hepatic steatosis and liver function damage. These results suggest that liver CAP has important implications for investigating the factors affecting hepatic steatosis in obese patients with PCOS.
It is well known that obesity can lead to NAFLD (25). Therefore, in the present study, we enrolled an age- and BMI-matched control group with no statistical difference in BMI, WHR, body fat percentage, or VFA to the experimental group, thereby reducing the bias in the results potentially caused by obesity. The results showed that, compared to the control group, the obese PCOS group had more severe disorders of lipid metabolism (TG, TC, LDL-C, HDL-C), insulin resistance (FIns, HOMA-IR), and sex hormone disorders (TT, FAI, SHBG), further confirming that there is a correlation between PCOS and endocrine and metabolic disorders.
Similar to our results which showed higher TG, TC, and LDL-C levels and lower HDL-C levels in obese patients with PCOS, a previous study by Liu et al. noted the presence of an abnormal lipid pattern in patients with PCOS (26), including low levels of HDL-C and high levels of TG, TC, and LDL-C. Correlation analysis and univariate logistic regression showed that the TG level was a risk factor for NAFLD in PCOS patients with obesity. Hypertriglyceridaemia induces inflammatory responses and apoptosis in hepatocytes, thereby increasing both the susceptibility to and severity of NAFLD (27). Lipotoxic hepatocyte-derived extracellular vesicle-encapsulated miR-9-5p downregulated the expression of transglutaminase2 and promoted M1 polarisation in macrophages, thereby facilitating NAFLD progression (28). Hypertriglyceridemia also affected pancreatic α and β cells, leading to hyperinsulinaemia and IR (29), further aggravating metabolic disorders and promoting NAFLD.
IR occurs in approximately 75% of PCOS patients, with a higher proportion of IR in PCOS patients with obesity, and IR interacts with PCOS to worsen this situation (30, 31). Overall, our results showed that hyperinsulinaemia and IR were more pronounced and were risk factors for NAFLD in PCOS patients with obesity, consistent with the findings of Zhang et al. (32). Reduced insulin sensitivity of peripheral tissues and diminished inhibition of lipolysis by insulin in IR leads to ectopic deposition of excess free fatty acids in the liver (33). Furthermore, IR promotes de novo hepatic lipogenesis (34), increased lipotoxicity, oxidative stress, and inflammatory cascade activation, leading to liver damage (35). IR also activates hepatic stellate cells and contributes to hepatic fibrogenesis (36). Gangale et al. showed that patients with PCOS treated with the insulin sensitizer metformin had improved IR and reduced ALT levels (37). Riemann et al. demonstrated that metformin reduced the hepatic steatosis index in PCOS patients (38). This suggests that insulin sensitizers may provide an important future direction for the treatment of NAFLD in PCOS patients with obesity.
HA is an important clinical feature in patients with PCOS. The pulse frequency of gonadotropin-releasing hormone at sustained high frequencies in PCOS patients causes an increase in luteinizing hormone (LH) pulse amplitude, an overproduction of LH, and a relative lack of follicle-stimulating hormone, resulting in HA (39). Increases in TT and FAI levels were observed in PCOS patients with obesity, with a simultaneous decline in SHBG levels in our study. SHBG binds androgens with high affinity (40), and a reduction in SHBG can further worsen HA. Previous studies have shown that HA is an independent risk factor and predictor of NAFLD in patients with PCOS (41, 42), while PCOS patients with HA have more pronounced hepatic steatosis than those without HA (43). Similar results were observed in the present study. HA promotes the development and progression of NAFLD by increasing hepatic fatty acid de novo synthesis (44), inducing apoptosis and autophagy imbalance (45), impairing hepatic branched-chain amino acid metabolism (46), inducing mitochondrial β-oxidation imbalance, and upregulating the expression of inflammatory factors in hepatocytes (47). Overall, our results suggest that liver conditions should be systematically assessed in PCOS patients with obesity with HA, as improvement of HA not only restores reproductive and endocrine disorders in patients with PCOS, but also contributes to improved liver function.
Nevertheless, this study has several limitations which should be noted. First, this was a single-center cross-sectional study with a small sample size and a relatively limited number of patients, which may have contributed to the difference in the results between univariate logistic regression and multivariate logistic regression. There might be some instability in this result. Second, this study did not capture information on the patients’ dietary structure, small intake of alcohol, exercise, and other lifestyle factors, which could potentially influence the patients’ liver steatosis and affect the accuracy of the results. Future studies should validate the effects of liver CAP and risk factors for NAFLD in PCOS patients with obesity by conducting multicenter, large-sample-size studies based on the inclusion of influencing factors, such as diet and lifestyle.
5 Conclusion
In summary, PCOS patients with obesity had a significantly higher prevalence of NAFLD and liver CAP and transaminase levels, suggesting a more pronounced hepatic steatosis and liver damage. Hypertriglyceridaemia, IR, and HA were associated with changes in liver CAP in PCOS patients with obesity, and TT was an independent risk factor for NAFLD in PCOS patients with obesity.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was audited by Medical Ethics Committee of Shengjing Hospital of China Medical University (Audite No. 2021PS647K). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DW and BH conceived, designed, and performed the research. NN and HB conducted the FibroTouch examination for the PCOS patients and control subjects. DW wrote the paper. BH reviewed and edited the fifinal manuscript. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (grant no. 82271667), and Outstanding Scientific Fund of Shengjing Hospital.
Acknowledgments
We thank Professor Lianjie Lin for providing us with the FibroTouch equipment to ensure the successful completion of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Deswal R, Narwal V, Dang A, Pundir CS. The prevalence of polycystic ovary syndrome: A brief systematic review. J Hum Reprod Sci (2020) 13(4):261–71. doi: 10.4103/jhrs.JHRS_95_18
2. Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod (2013) 28(9):2562–9. doi: 10.1093/humrep/det262
3. Paschou SA, Polyzos SA, Anagnostis P, Goulis DG, Kanaka-Gantenbein C, Lambrinoudaki I, et al. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Endocrine (2020) 67(1):1–8. doi: 10.1007/s12020-019-02085-7
4. Barber TM, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) (2021) 95(4):531–41. doi: 10.1111/cen.14421
5. Wu J, Yao XY, Shi RX, Liu SF, Wang XY. A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: an update meta-analysis. Reprod Health (2018) 15(1):77. doi: 10.1186/s12978-018-0519-2
6. Tantanavipas S, Vallibhakara O, Sobhonslidsuk A, Phongkitkarun S, Vallibhakara SA, Promson K, et al. Abdominal obesity as a predictive factor of nonalcoholic fatty liver disease assessed by ultrasonography and transient elastography in polycystic ovary syndrome and healthy women. BioMed Res Int (2019) 2019:9047324. doi: 10.1155/2019/9047324
7. Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol (2017) 23(47):8263–76. doi: 10.3748/wjg.v23.i47.8263
8. Francisco V, Sanz MJ, Real JT, Marques P, Capuozzo M, Ait Eldjoudi D, et al. Adipokines in non-alcoholic fatty liver disease: are we on the road toward new biomarkers and therapeutic targets? Biol (Basel) (2022) 11(8):1237. doi: 10.3390/biology11081237
9. Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol (2009) 51(6):1061–7. doi: 10.1016/j.jhep.2009.09.001
10. Ferraioli G. Quantitative assessment of liver steatosis using ultrasound controlled attenuation parameter (Echosens). J Med Ultrason (2001) (2021) 48(4):489–95. doi: 10.1007/s10396-021-01106-1
11. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of fibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology (2019) 156(6):1717–30. doi: 10.1053/j.gastro.2019.01.042
12. Wong GL. Prediction of fibrosis progression in chronic viral hepatitis. Clin Mol Hepatol (2014) 20(3):228–36. doi: 10.3350/cmh.2014.20.3.228
13. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril (2004) 81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004
14. Chinese Nutrition Society Obesity Prevention and Control Section, Chinese Nutrition Society Clinical Nutrition Section, Chinese Preventive Medicine Association Behavioral Health Section, Chinese Preventive Medicine Association Sports and Health Section. Expert consensus on obesity prevention and treatment in China. Chin J Epidemiol (2022) 43(5):609–26. doi: 10.3760/cma.j.cn112338-20220402-00253
15. Qu Y, Song YY, Chen CW, Fu QC, Shi JP, Xu Y, et al. Diagnostic performance of fibroTouch ultrasound attenuation parameter and liver stiffness measurement in assessing hepatic steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Clin Transl Gastroenterol (2021) 12(4):e00323. doi: 10.14309/ctg.0000000000000323
16. Lahelma M, Luukkonen PK, Qadri S, Ahlholm N, Lallukka-Brück S, Porthan K, et al. Assessment of lifestyle factors helps to identify liver fibrosis due to non-alcoholic fatty liver disease in obesity. Nutrients (2021) 13(1):169. doi: 10.3390/nu13010169
17. Li M, Feng D, Zhang K, Gao S, Lu J. Disproportionately elevated proinsulin levels as an early indicator of β-cell dysfunction in nondiabetic offspring of chinese diabetic patients. Int J Endocrinol (2016) 2016:4740678. doi: 10.1155/2016/4740678
18. Zhang J, Xing C, Cheng X, He B. Canagliflozin combined with metformin versus metformin monotherapy for endocrine and metabolic profiles in overweight and obese women with polycystic ovary syndrome: A single-center, open-labeled prospective randomized controlled trial. Front Endocrinol (Lausanne) (2022) 13:1003238. doi: 10.3389/fendo.2022.1003238
19. Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med (2013) 35(2):121–6. doi: 10.4103/0253-7176.116232
20. Salva-Pastor N, López-Sánchez GN, Chávez-Tapia NC, Audifred-Salomón JR, Niebla-Cárdenas D, Topete-Estrada R, et al. Polycystic ovary syndrome with feasible equivalence to overweight as a risk factor for non-alcoholic fatty liver disease development and severity in Mexican population. Ann Hepatol (2020) 19(3):251–7. doi: 10.1016/j.aohep.2020.01.004
21. Platko K, Lebeau PF, Nederveen JP, Byun JH, MacDonald ME, Bourgeois JM, et al. A metabolic enhancer protects against diet-induced obesity and liver steatosis and corrects a pro-atherogenic serum profile in mice. Nutrients (2023) 15(10):2410. doi: 10.3390/nu15102410
22. Gómez-Pérez AM, Ruiz-Limón P, Salas-Salvadó J, Vioque J, Corella D, Fitó M, et al. Gut microbiota in nonalcoholic fatty liver disease: a PREDIMED-Plus trial sub analysis. Gut Microbes (2023) 15(1):2223339. doi: 10.1080/19490976.2023.2223339
23. Genazzani AD, Genazzani AR. Polycystic ovary syndrome as metabolic disease: new insights on insulin resistance. touchREV Endocrinol (2023) 19(1):71–7. doi: 10.17925/EE.2023.19.1.71
24. Wang D, He B. Current perspectives on nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr Obes (2022) 15:1281–91. doi: 10.2147/DMSO.S362424
25. Kosmalski M, Frankowski R, Deska K, Różycka-Kosmalska M, Pietras T. Exploring the impact of nutrition on non-alcoholic fatty liver disease management: unveiling the roles of various foods, food components, and compounds. Nutrients (2023) 15(13):2838. doi: 10.3390/nu15132838
26. Liu Q, Xie YJ, Qu LH, Zhang MX, Mo ZC. Dyslipidemia involvement in the development of polycystic ovary syndrome. Taiwan J Obstet Gynecol (2019) 58(4):447–53. doi: 10.1016/j.tjog.2019.05.003
27. Paiva AA, Raposo HF, Wanschel AC, Nardelli TR, Oliveira HC. Apolipoprotein CIII overexpression-induced hypertriglyceridemia increases nonalcoholic fatty liver disease in association with inflammation and cell death. Oxid Med Cell Longev (2017) 2017:1838679. doi: 10.1155/2017/1838679
28. Liu H, Niu Q, Wang T, Dong H, Bian C. Lipotoxic hepatocytes promote nonalcoholic fatty liver disease progression by delivering microRNA-9-5p and activating macrophages. Int J Biol Sci (2021) 17(14):3745–59. doi: 10.7150/ijbs.57610
29. Guo H, Ma C, Wu X, Pan C. Functional status of pancreatic α and β Cells in type 2 diabetes mellitus patients with different plasma triglyceride levels: A retrospective analysis. Int J Endocrinol (2021) 2021:9976067. doi: 10.1155/2021/9976067
30. Moghetti P, Tosi F. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest (2021) 44(2):233–44. doi: 10.1007/s40618-020-01351-0
31. Tong C, Wu Y, Zhang L, Yu Y. Insulin resistance, autophagy and apoptosis in patients with polycystic ovary syndrome: Association with PI3K signaling pathway. Front Endocrinol (Lausanne) (2022) 13:1091147. doi: 10.3389/fendo.2022.1091147
32. Zhang J, Hu J, Zhang C, Jiao Y, Kong X, Wang W. Analyses of risk factors for polycystic ovary syndrome complicated with non-alcoholic fatty liver disease. Exp Ther Med (2018) 15(5):4259–64. doi: 10.3892/etm.2018.5932
33. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism (2016) 65(8):1038–48. doi: 10.1016/j.metabol.2015.12.012
34. Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol (2013) 48(4):434–41. doi: 10.1007/s00535-013-0758-5
35. Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci (2014) 15(5):8591–638. doi: 10.3390/ijms15058591
36. Fujii H, Kawada N, Japan Study Group Of Nafld Jsg-Nafld. The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int J Mol Sci (2020) 21(11):3863. doi: 10.3390/ijms21113863
37. Gangale MF, Miele L, Lanzone A, Sagnella F, Martinez D, Tropea A, et al. Long-term metformin treatment is able to reduce the prevalence of metabolic syndrome and its hepatic involvement in young hyperinsulinaemic overweight patients with polycystic ovarian syndrome. Clin Endocrinol (Oxf) (2011) 75(4):520–7. doi: 10.1111/j.1365-2265.2011.04093.x
38. Riemann A, Blaschke M, Jauho-Ghadimi A, Siggelkow H, Gollisch KSC. Metformin improves the hepatic steatosis index in non-obese patients with polycystic ovary syndrome. J Clin Med (2022) 11(15):4294. doi: 10.3390/jcm11154294
39. McCartney CR, Campbell RE. Abnormal gnRH pulsatility in polycystic ovary syndrome: recent insights. Curr Opin Endocr Metab Res (2020) 12:78–84. doi: 10.1016/j.coemr.2020.04.005
40. Laurent MR, Hammond GL, Blokland M, Jardí F, Antonio L, Dubois V, et al. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: validation of the free hormone hypothesis. Sci Rep (2016) 6:35539. doi: 10.1038/srep35539
41. Cai J, Wu CH, Zhang Y, Wang YY, Xu WD, Lin TC, et al. High-free androgen index is associated with increased risk of non-alcoholic fatty liver disease in women with polycystic ovary syndrome, independent of obesity and insulin resistance. Int J Obes (Lond) (2017) 41(9):1341–7. doi: 10.1038/ijo.2017.116
42. Harsha Varma S, Tirupati S, Pradeep TVS, Sarathi V, Kumar D. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr (2019) 13(2):1065–9. doi: 10.1016/j.dsx.2018.12.020
43. Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, Aziz N, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab (2012) 97(10):3709–16. doi: 10.1210/jc.2012-1382
44. Seidu T, McWhorter P, Myer J, Alamgir R, Eregha N, Bogle D, et al. DHT causes liver steatosis via transcriptional regulation of SCAP in normal weight female mice. J Endocrinol (2021) 250(2):49–65. doi: 10.1530/JOE-21-0040
45. Cui P, Hu W, Ma T, Hu M, Tong X, Zhang F, et al. Long-term androgen excess induces insulin resistance and non-alcoholic fatty liver disease in PCOS-like rats. J Steroid Biochem Mol Biol (2021) 208:105829. doi: 10.1016/j.jsbmb.2021.105829
46. Anzai Á, Marcondes RR, Gonçalves TH, Carvalho KC, Simões MJ, Garcia N, et al. Impaired branched-chain amino acid metabolism may underlie the nonalcoholic fatty liver disease-like pathology of neonatal testosterone-treated female rats. Sci Rep (2017) 7(1):13167. doi: 10.1038/s41598-017-13451-8
Keywords: polycystic ovary syndrome, non-alcoholic fatty liver disease, controlled attenuation parameter, hyperandrogenemia, testosterone
Citation: Wang D, Nan N, Bing H and He B (2023) Controlled attenuation parameters to assess liver steatosis in obese patients with polycystic ovary syndrome. Front. Endocrinol. 14:1241734. doi: 10.3389/fendo.2023.1241734
Received: 19 June 2023; Accepted: 14 August 2023;
Published: 31 August 2023.
Edited by:
Ozlem Celik, Acıbadem University, TürkiyeReviewed by:
Aleksandra Klisic, Primary Health Care Center Podgorica, MontenegroAlper Sonmez, Güven Hospital, Türkiye
Copyright © 2023 Wang, Nan, Bing and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing He, aGViaW5nNzU1N0AxNjMuY29t
 Dongxu Wang
Dongxu Wang Nan Nan1
Nan Nan1 Bing He
Bing He