
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 06 December 2023
Sec. Cancer Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1238086
This article is part of the Research TopicMetabolic and Senescence Characteristics Associated with the Immune Microenvironment in Tumor CellsView all 18 articles
 Suzheng Zheng1†
Suzheng Zheng1† Hai Yu1†
Hai Yu1† Xinkai Zheng1
Xinkai Zheng1 U Tim Wu2
U Tim Wu2 Wai-kit Ming3
Wai-kit Ming3 Hui Huang1
Hui Huang1 Jiaxin Song1
Jiaxin Song1 Xiaoxi Zhang1
Xiaoxi Zhang1 Jun Lyu4,5*
Jun Lyu4,5* Liehua Deng1,6*
Liehua Deng1,6*Background: The survival and prognosis of patients are significantly threatened by cutaneous melanoma (CM), which is a highly aggressive disease. It is therefore crucial to determine the most recent survival rate of CM. This study used population-based cancer registry data to examine the 5-year relative survival rate of CM in the US.
Methods: Period analysis was used to assess the relative survival rate and trends of patients with CM in the Surveillance, Epidemiology, and End Results (SEER) database during 2004–2018. And based on the data stratified by age, gender, race and subtype in the SEER database, a generalized linear model was 12established to predict the 5-year relative survival rate of CM patients from 2019 to 2023.
Results: The 5-year relative survival increased to various degrees for both total CM and CM subtypes during the observation period. The improvement was greatest for amelanotic melanoma, increasing from 69.0% to 81.5%. The 5-year overall relative survival rates of CM were 92.9%, 93.5%, and 95.6% for 2004–2008, 2009–2013, and 2014–2018, respectively. Females had a marginally higher survival rate than males for almost all subtypes, older people had lower survival rates than younger people, white patients had higher survival rates than nonwhite ones, and urban locations had higher rates of survival from CM than rural locations did. The survival rate of CM was significantly lower for distant metastasis.
Conclusion: The survival rate of patients with CM gradually improved overall during 2004–2018. With the predicted survival rate of 96.7% for 2019–2023, this trend will still be present. Assessing the changes experienced by patients with CM over the previous 15 years can help in predicting the future course of CM. It also provides a scientific foundation that associated departments can use to develop efficient tumor prevention and control strategies.
Cutaneous melanoma (CM) is a highly malignant tumor with early metastasis and invasion, which seriously threatens the survival and prognosis of patients (1, 2) and has become a common skin malignancy. Studies have highlighted that its incidence continues to increase worldwide, with about 232,100 new cases (1.7% of all cancer-related deaths) and about 55,500 deaths (0.7% of all cancer-related deaths) annually (3). There are many pathogenic factors of CM, among which ultraviolet rays from sunlight and genetic susceptibility are considered to be important factors in melanoma pathogenesis (3–5). There are four main clinical subtypes of CM: lentigo maligna melanoma (LMM), acral lentiginous melanoma (ALM), nodular melanoma (NM), and superficial spreading melanoma (SSM) (6). SSM is the most prevalent, accounting for >70% of all CMs (7). There are also some rare subtypes, such as desmoplastic melanoma (DM), amelanotic melanoma (AM), and spindle cell melanoma (SCM).
The main treatment methods for CM currently include surgery, chemotherapy, immunotherapy, and targeted therapy. With the recent continuous progress of CM treatment, immunotherapy and targeted therapy have become the current focuses of research, and researchers continue to explore treatment options for obtaining the best prognoses for patients (8–10). Schadendorf et al. found that the 5-year overall survival rate of metastatic melanoma has increased markedly, from<10% to its current rate of 40–50% (3). The overall survival and cure rates of patients with melanoma can be significantly increased with prevention, early detection, and efficient adjuvant therapies (3).
The 5-year survival rate is frequently used in clinical settings to assess the effectiveness of cancer treatment and track the prognosis of patients. The most recent estimates for the survival rate of patients with cancer are reliable indicators of the general situation and changing patterns of long-term survival in certain residence locations. It is advantageous for clinical prevention and treatment to comprehend the long-term survival-rate trend of CM and its prognostic variables. The ratio between the observed survival rate of the population with cancer and the anticipated survival rate of the general population is known as the relative survival rate, which is most often used in cancer statistics and is a crucial metric for assessing patient prognoses (11, 12). Analyzing relative survival rates using the period analysis can help reveal cyclical patterns in the data, improve prediction accuracy, and provide more insights for decision making (13). It can help medical professionals better understand and utilize time series data to improve medical decisions and treatment strategies. Existing data can be used to precisely estimate survival rates, examine trends, and predict future survival rates through model-based period analysis.
After stratifying data by age, sex, race, residence location, histology, and metastatic stage, we employed period analysis to evaluate survival trends in patients with CM enrolled in the Surveillance, Epidemiology, and End Results (SEER) database during 2004–2018. We also applied a model-based period analysis approach to predict the survival rate for 2019–2023 and explore the potential causes of the survival rate discrepancy during this period.
The information used in this study were obtained from the SEER database, which is a large population-based data set that now includes information on around 50% of patients with cancer in the US. The SEER project uses population-based cancer registries to compile and disseminate data on cancer incidence, prevalence, and survival (14). It is a trustworthy source of cancer surveillance data, and its long-term, comprehensive, and up-to-date data greatly facilitate the ability of researchers worldwide to gather, analyze, and disseminate trustworthy population-based cancer analysis results. In this study, data on patients with CM during 2004–2018 were retrieved using SEER*Stat software (version 8.4.0.1) (15). Patient follow-up data were obtained up to December 2019.
The inclusion criteria of this study were (1) older than 15 years, (2) data from 2004–2018 available, and (3) primary tumor of CM. The exclusion criteria were (1) only an autopsy or death certificate confirming the CM diagnosis, (2) alive or no way to determine survival time, and (3) incomplete data. There were finally 104,784 cases that met the above criteria.
We selected CM subtypes using a classification based on ICD-0-3 codes. The histology, residence location, and malignant behavior of tumors are all encoded by the ICD-O-3 system. Some rare subtypes were excluded due to small numbers of cases, and we finally included seven pathological subtypes of CM in this study. Other indicators were classified as follows: sex (female and male), race (black, white, American Indian/Alaska Native, and Asian or Pacific Islander), age (15–44, 45–54, 55–64, 65–74, and ≥75 years), residence location (rural and urban), and metastatic stage (distant, regional, and localized).
The sociodemographic and clinical characteristics of each observation period were compiled using descriptive statistics. Patient prognoses were evaluated using relative survival rates. The ratio between the actual and expected survival rates is known as the relative survival rate and is calculated as
where and represent the observed and expected survival rates, respectively. The variable i typically represents individual time intervals or observation periods. It is used to distinguish different segments of time, such as years or timeframes, over which relative survival rates are calculated. A k value of 5 corresponds to determining the 5-year relative survival rate. The expected survival rates were derived from the SES/geography/race Annual Life Tables generated from the US mortality data in SEER, and was calculated using the Ederer II method.
The period analysis approach was used in this study to assess 5-year relative survival rates during 2004–2018 of patients with CM. The Greenwood method was used to produce point estimates of relative survival rate and their standard errors. Cases diagnosed during 2004–2008, 2009–2013, and 2014–2018 were included in the study, estimated the slope and interception of linear models, and a generalized linear model based on the period analysis was developed to predict the 5-year relative survival rate of patients diagnosed with CM during 2019–2023. The linear model is represented in the form of Y=β1X+β0+ϵ. In this equation, X represents the year, Y represents the relative survival rate, ϵ denotes the error term. The above analysis process was performed using the SEER*Stat software (version 8.4.0.1) to calculate the relative survival rate and the Joinpoint Regression Program (version 4.9.1.0) to fit the linear model.
We identified 104,784 patients diagnosed with CM during 2004–2018 in the SEER database, comprising 46,010 females and 58,774 males. The CM cases detected and entered into the SEER database for each observation period are listed in Table 1. Most patients were male, white, aged 55–64 years, urban residents, and had localized metastasis and the SSM. The four most common CM subtypes (ALM, LMM, NM, and SSM) accounted for nearly 95% of all CM cases, while white patients accounted for 93.8%. The numbers of cases within each observation period were generally consistent among ages, sexes, races, residence locations, and stages.
The 5-year relative survival rates for the various CM subtypes by sex are listed in Table 2. Based on the outcomes of the period analysis, compared with 2004–2008, the survival rate of both males and females increased during 2014–2018, except for patients with SCM. The relative survival rate was significantly higher for females than for males across almost all subtypes. Moreover, there were significant subtype differences in changes in relative survival rate. LMM (100.0%), SSM (99.5%), and DM (94.4%) had the highest 5-year relative survival rates during 2014–2018. The 5-year relative survival rates for both total CM and the CM subtypes increased during the observation period. The survival rate improved the most for the AM subtype, increasing from 69.0% to 81.5% during 2004–2018. Generalized linear models predicted that the 5-year relative survival rate for CM and its subtypes will continue to increase during 2019–2023. The changing trends of relative survival rate between different pathological subtypes and different sexes are shown in Figures 1, 2.
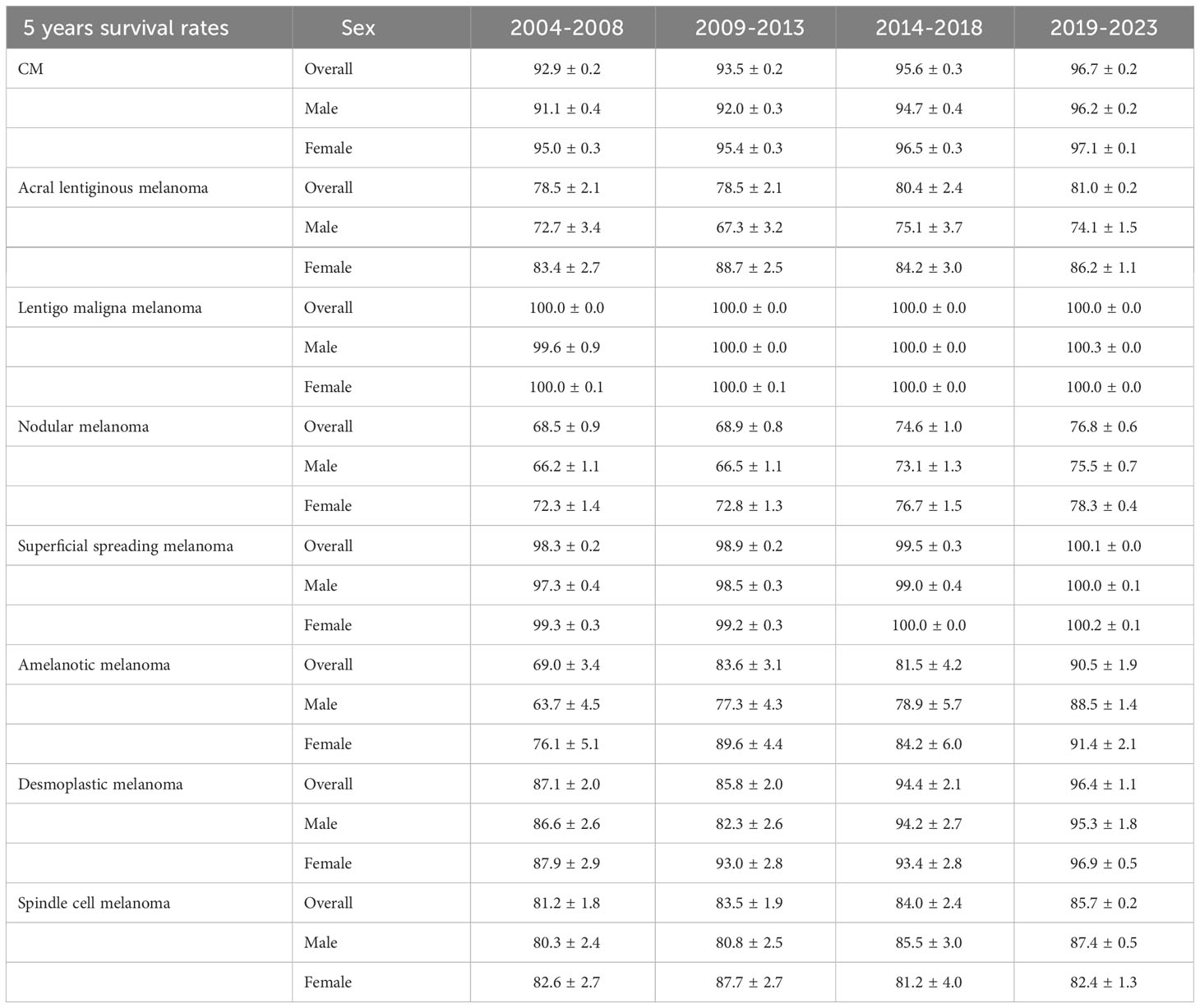
Table 2 5-year relative survival rates for patients with CM and its subtypes by sex from 2004 to 2018 and predicted relative survival rates for patients with CM and its subtypes from 2019 to 2023.
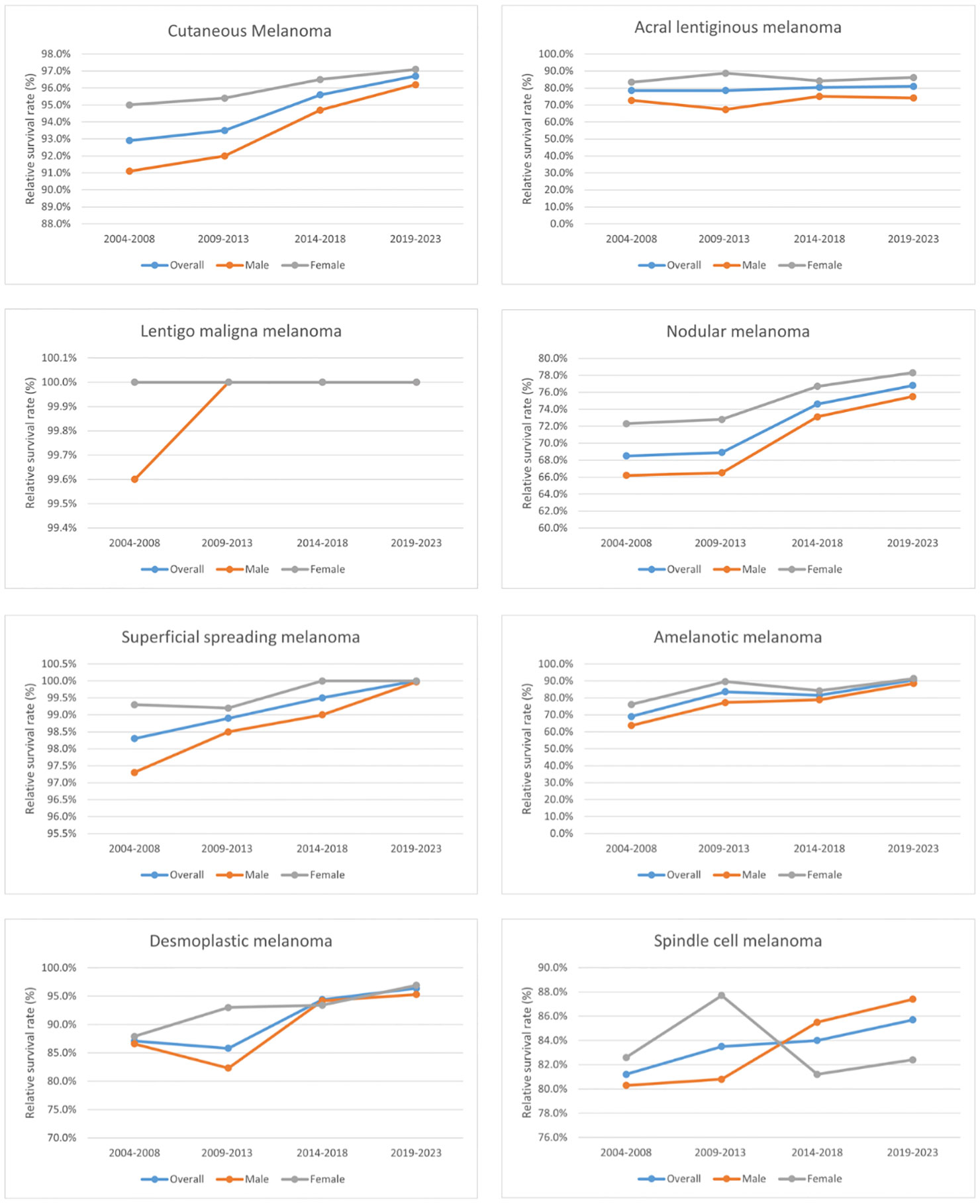
Figure 2 Trends in the 5-year relative survival rates of patients with each subtype of CM and between different sexes.
Table 3 lists the 5-year relative survival rate for CM by age. Survival rates improved across all age groups during 2004–2018. Younger age groups consistently had higher 5-year relative survival rates in CM than older age groups during the study period. The 5-year relative survival rates during 2004–2018 were 97.2%, 96.4%, 95.9%, 95.8%, and 92.1% for those aged 15–44, 45–54, 55–64, 65–74, and ≥75 years, respectively. The generalized linear model predicted that during the 2019–2023, patients with CM aged 15–44 years would have the highest 5-year relative survival rate of 97.6%, while those aged ≥75 years would have the lowest survival rate of 95.1%. Figure 3 displays the relative survival rate trend of patients with CM across various age groups.

Table 3 5-year relative survival rates of CM patients by age group from 2004 to 2018 and forecast of CM patients’ relative survival rates from 2019 to 2023.
The 5-year relative survival rates for patients with CM are listed in Table 4 according to race, residence location, and metastatic stage. White patients had the highest survival rate (95.1 ± 0.3%, mean ± SEM) during 2014–2018. The 5-year relative survival rates of white and American Indian/Alaska Native patients increased between 2004–2008 and 2014–2018; however, those of black and Asian or Pacific Islander patients decreased. The relative survival rates for white, black, American Indian/Alaska Native, and Asian or Pacific Islander patients during 2019–2023 were predicted using generalized linear models to be 96.0%, 68.1%, 88.9%, and 75.2%, respectively. Moreover, the CM survival rate was higher in urban locations than rural ones. The relative survival rate of CM has increased over time in both urban and rural locations, and generalized linear models indicated that this trend will persist in the future. Relative survival rates increased over time for all three metastatic stages, with generalized linear models predicting further increases during 2019–2023. However, the relative survival rate for regional and distant metastasis were still quite different from that for localized metastasis, with the relative survival rate of distant metastasis being<50%. Figures 4–6 depict the relative survival rate trends of patients with CM by race, residence location, and metastasis.
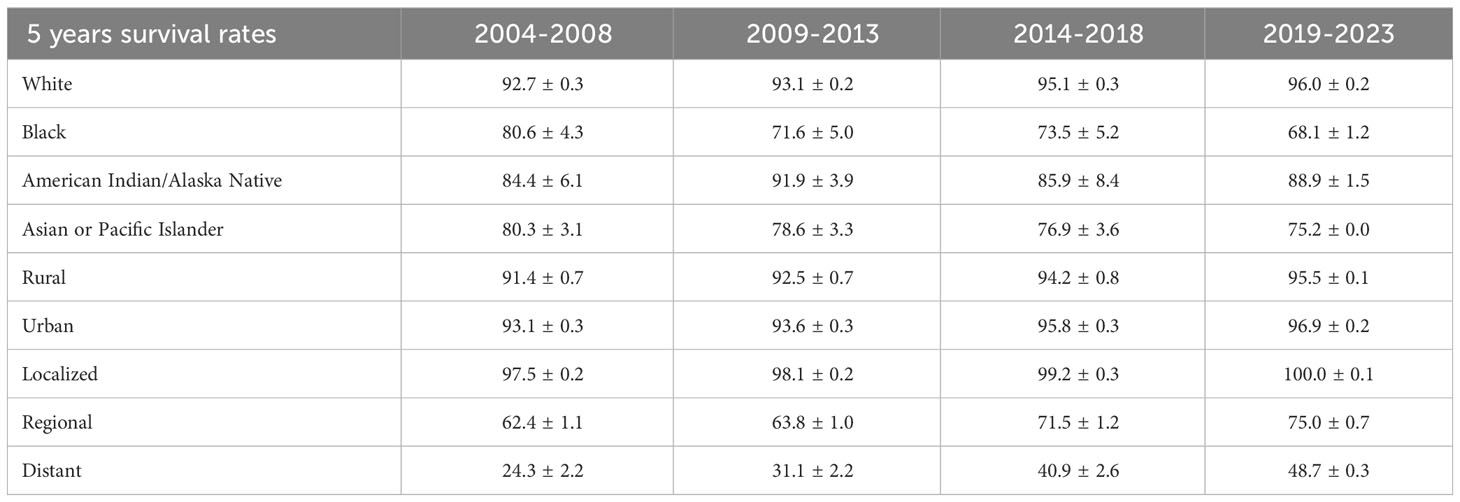
Table 4 5-year relative survival rates of CM patients by race, area and metastatic stage from 2004 to 2018 and forecast of CM patients’ relative survival rates from 2019 to 2023.
A period analysis was first applied in this study in order to assess the long-term survival trend and prognostic factors of patients with CM and to predict the survival rate during 2019–2023. The relative survival rates of CM and its distinct subtypes all increased to varying degrees during 2004–2018. The relative survival rate varied according to the classification of several criteria, including the CM subtype, sex, age, race, residence location, and metastatic stage.
The high rate of metastasis in CM has a significant impact on the probability of patient survival and poses a serious threat to human health. Among the various CM subtypes, factors such as high heterogeneity and wide variations in clinical and genetic characteristics lead to different degrees of malignancy and responses to treatment, resulting in the survival rates for different pathological subtypes also differing greatly (6, 16–18). NM had the highest degree of malignancy and the worst prognosis for patients in previous studies (19–21). We found variations in the survival rates of CM subtypes, but the relative survival rate of overall CM was high and had improved recently by varying degrees. This increasing trend in relative survival rate was observed for all CM subtypes during 2004–2018 in this study. Melanoma mortality has declined significantly since the US Food and Drug Administration approved the first immune-checkpoint inhibitor ipilimumab for improving late-term survival in 2011 (22). Developments in medical technologies, including immunotherapy and targeted therapy, have significantly increased the survival rate of patients due to improvements in the understanding of CM etiology (23–25). New immune-checkpoint inhibitors and targeted agents that improve immune-mediated antitumor modulation, particularly in advanced melanoma, are associated with improved overall survival rate. This may be a key factor that causes the relative survival rate of CM to continue its upward trend during 2019-2023. However, surgical treatment is still a key factor for the prognosis of patients with CM, with many previous studies finding that early surgical intervention can increase patient survival by reducing the risks of metastasis and infiltration in CM (2, 8, 26).
The sex of patients with CM has a significant impact on their prognosis. According to previous studies, female patients typically have a better prognosis than male patients, which was consistent with the results of the present study (18, 27). We found that, except for SCM that began to appear in 2014–2018, the relative survival rate was higher for male than female patients with CM, and the relative survival rates of CM and its subtypes were higher in females than in males. Although SCM is a variant of melanoma, it is easily misdiagnosed as other tumors due to the lack of traditional melanoma features and varying degrees of cytological atypia (28, 29). Because SCM is also aggressive, the prognosis of patients with SCM will be seriously affected when misdiagnosis and delayed diagnosis occur. Sex differences in the prognosis of CM patients. Existing studies have indicated that behavioral differences, hormone regulation, immune function, vitamin D metabolism, sex chromosome gene expression, and oxidative stress response are mechanisms underlying this difference in survival rates, but the exact mechanism remains unclear (30, 31). Future studies will help to explore these mechanisms in depth to more fully understand the role of gender in survival differences in CM patients and provide more scientific basis for the development of gender-specific treatment strategies.
Age was found to be an important factor affecting CM prognosis in this study. We found that most patients with CM were elderly, and that the survival rate decreased with age. The difference in relative survival rate between those aged 15–44 and ≥75 years was as high as 9.8%. This was consistent with the probability of cell senescence leading to mutations in its genetic material increasing with age, as does the risk of suffering from CM (32–34). At the same time, the response to treatment, tolerance, and recovery ability of the body are also reduced during the aging process, thereby affecting the survival of elderly patients (35, 36). The survival rates of all age groups increased by different degrees during the observation period, the most in patients aged ≥75 years, which may be related to recent developments in immunotherapy and targeted therapy and the application of new adjuvant therapies (37). The complex relationship between age and CM prognosis extends beyond biological factors to encompass the broader aspects of immune responses, health, and the outcomes of treatment. These findings underscore the importance of personalized treatment approaches that consider age as a critical determinant for improving therapeutic outcomes and enhancing survival rates.
We observed that white patients accounted for the vast majority of those with CM, and studies have indicated that this is related to various factors such as differences in gene expression, socioeconomic status, and living environment between races (38, 39). In our study, white patients not only had a higher incidence rate of CM than other races, but also had the highest survival rate. This was due to advances in treatment for and understanding of the disease in the US, a white-majority nation (37). It was also clear that the survival rate was lowest for black patients and trends downward over time. The lower socioeconomic level, education level, and participation in melanoma screening among black patients may explain some race-related differences in the outcomes of patients with cancer (39–42). The survival rate of Asian or Pacific Islander patients also exhibited a downward trend in our study, and we believe that the reasons are similar to those mentioned above. Sanchez et al. noted that melanoma education provided over the past 20 years has not significantly increased awareness of the disease, and that future education programs targeting early detection are more likely to benefit racial and ethnic minority individuals (43). However, due to the large proportion of white patients included in the SEER database and the small number of cases of other races, the data need to be interpreted with caution, and further large-sample research is necessary.
Advanced medical equipment, cutting-edge technology, and a pool of highly skilled healthcare professionals are more readily available in urban areas due to better economic conditions. This advantage not only facilitates early disease detection but also empowers healthcare professionals with advanced tools for precise diagnosis and effective treatment. Consequently, patients in urban areas often experience more favorable disease survival rates, which was consistent with our research findings (44–47). Our study also found that survival rates greatly differed between metastatic stages. The survival rate is lower when a cancer has metastasized farther. Patient survival rates will decrease significantly at each metastatic stage. Although the survival rates of regional and distant metastases increased significantly during the observation period, subsequent metastasis will still be fatal to patients. CM often metastasizes to important organs such as the liver, lung, and brain, which greatly complicates treatments and results in a poor prognosis (48, 49).
Our study highlights the significant advancements in CM survival rates and the impact of factors such as age, gender, race, and metastatic stage on prognosis. The rising trend in CM survival, driven by developments in immunotherapy and targeted therapy, provides hope for improved outcomes. As we move forward, it is crucial to delve deeper into understanding the underlying mechanisms behind these findings and to develop tailored treatments. Additionally, addressing disparities among different racial groups and focusing on early detection programs will be vital steps in enhancing the prognosis of CM patients in the future.
There were some limitations to this study. First, there were inherent drawbacks in its retrospective design. Second, the results of the study should be interpreted with caution because tumor stages change over time in the SEER database. Third, the proportion of nonwhite patients in the SEER database was relatively low, and caution is necessary when considering the analytical results for nonwhite patients given the smallness of the sample. Fourth, it was challenging to determine the influence on survival given the lack of potentially crucial elements in the SEER database, including treatment options, certain biological indicators, and behavioral habits. Fifth, the findings of this study were based on analyses of data from the US, so additional confirmation is required to ascertain whether they apply to other nations.
The relative survival rate of patients with CM in our study exhibited an overall upward trend with time, with only a few differences. This trend was predicted to persist during 2019–2023. These improvements may be connected to the efficacy of new therapeutic choices and improvements in risk factors due to elements such as setting and diagnostic techniques. Nonetheless, the prognosis of patients with CM was still impacted by race, with the survival rates being lower for nonwhite patients than for white patients, and lower for the elderly than for young patients, and for distant tumor metastases. It is necessary to improve tumor education, early diagnosis, treatment strategies, and other aspects to improve survival in the future. The study also found that females had a higher survival rate and that urban residents had a better prognosis than rural ones.
Understanding the survival rate of CM during the previous 15 years might be useful in predicting future trends as well as for designing better treatment programs and developing sensible health policies to improve the prognosis of CM.
Publicly available datasets were analyzed in this study. This data can be found at: https://seer.cancer.gov.
SZ and HY: Formal analysis, Visualization, Writing – original draft, Writing – review &editing. XKZ: Data curation, Writing – original draft, Writing – review & editing. UW and WM: Data curation, Formal analysis, Writing – review & editing. HH: Data curation, Formal analysis, Writing – review & editing. JS: Writing – original draft, Writing – review & editing. XZZ: Writing – original draft, Writing – review & editing. JL and LD: Visualization, Writing – original draft, Writing – review & editing. All authors contributed to the article and approved the submitted version.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by Key Scientific Problems and Medical Technical Problems Research Project of China Medical Education Association (2022KTZ009) and Guangdong Provincial Key Laboratory of Traditional Chinese Medicine Informatization (2021B1212040007).
We heartily thank all of the employees and scientists at each of the SEER registry locations.
Author UW was employed by Meng Yi Centre Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Négrier S, Fervers B, Bailly C, Beckendorf V, Cupissol D, Doré JF, et al. Cutaneous melanoma. Br J Cancer (2001) 84(Suppl 2):81–5. doi: 10.1054/bjoc.2001.1771
2. Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther (2019) 20(11):1366–79. doi: 10.1080/15384047.2019.1640032
3. SChadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet (2018) 392(10151):971–84. doi: 10.1016/S0140-6736(18)31559-9
4. Strashilov S, Yordanov A. Aetiology and pathogenesis of cutaneous melanoma: current concepts and advances. IJMS (2021) 22(12):6395. doi: 10.3390/ijms22126395
5. Lorenz A, Kozłowski M, Lenkiewicz S, Kwiatkowski S, Cymbaluk-Płoska A. Cutaneous melanoma versus vulvovaginal melanoma—Risk factors, pathogenesis and comparison of immunotherapy efficacy. Cancers (2022) 14(20):5123. doi: 10.3390/cancers14205123
6. Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. The 2018 world health organization classification of cutaneous, mucosal, and uveal melanoma: detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med (2020) 144(4):500–22. doi: 10.5858/arpa.2019-0561-RA
7. Forman SB, Ferringer TC, Peckham SJ, Dalton SR, Sasaki GT, Libow LF, et al. Is superficial spreading melanoma still the most common form of Malignant melanoma? J Am Acad Dermatol (2008) 58(6):1013–20. doi: 10.1016/j.jaad.2007.10.650
8. Leonardi G, Falzone L, Salemi R, Zanghì A, Spandidos DA, McCubrey JA, et al. Cutaneous melanoma: From pathogenesis to therapy (Review). Int J Oncol (2018) 52(4):1071–80. doi: 10.3892/ijo.2018.4287
9. Sun J, Carr MJ, Khushalani NI. Principles of targeted therapy for melanoma. Surg Clinics North America (2020) 100(1):175–88. doi: 10.1016/j.suc.2019.09.013
10. Comito F, Pagani R, Grilli G, Sperandi F, Ardizzoni A, Melotti B. Emerging novel therapeutic approaches for treatment of advanced cutaneous melanoma. Cancers (2022) 14(2):271. doi: 10.3390/cancers14020271
11. Henson DE, Ries LA. The relative survival rate. Cancer (1995) 76(10):1687–8. doi: 10.1002/1097-0142(19951115)76:10<1687::aid-cncr2820761002>3.0.co;2-i
12. Coleman MP, Quaresma M, Berrino F, Lutz J-M, De Angelis R, Capocaccia R, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol (2008) 9(8):730–56. doi: 10.1016/S1470-2045(08)70179-7
13. Lu Y, He M, Lian L, Lei H, Cheng Y, Wang L, et al. Use of period analysis to provide a timely assessment of 5-year relative survival for pancreatic cancer patients from Taizhou, eastern China. BMC Cancer (2023) 23(1):642. doi: 10.1186/s12885-023-11119-3
14. Yang J, Li Y, Liu Q, Li L, Feng A, Wang T, et al. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med (2020) 13(1):57–69. doi: 10.1111/jebm.12373
15. Wu WT, Li YJ, Feng AZ, Li L, Huang T, Xu AD, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Military Med Res (2021) 8(1):44. doi: 10.1186/s40779-021-00338-z
16. Lattanzi M, Lee Y, Simpson D, Moran U, Darvishian F, H.Kim R, et al. Primary melanoma histologic subtype: impact on survival and response to therapy. JNCI: J Natl Cancer Institute (2019) 111(2):180–8. doi: 10.1093/jnci/djy086
17. Howard MD, Xie C, Wee E, Wolfe R, McLean CA, Kelly JW, et al. Acral lentiginous melanoma: differences in survival compared with other subtypes. Br J Dermatol (2020) 182(4):1056–7. doi: 10.1111/bjd.18620
18. El Sharouni MA, van Diest PJ, Witkamp AJ, Sigurdsson V, van Gils CH. Subtyping cutaneous melanoma matters. JNCI Cancer Spectrum (2020) 4(6):pkaa097. doi: 10.1093/jncics/pkaa097
19. Lideikaitė A, Mozūraitienė J, Letautienė S. Analysis of prognostic factors for melanoma patients. AML (2017) 24(1):25–34. doi: 10.6001/actamedica.v24i1.3460
20. Dessinioti C, Geller AC, Whiteman DC, Garbe C, Grob JJ, Kelly JW, et al. Not all melanomas are created equal: a review and call for more research into nodular melanoma. Br J Dermatol (2021) 185(4):700–10. doi: 10.1111/bjd.20388
21. Mar V, Roberts H, Wolfe R, English DR, Kelly JW. Nodular melanoma: A distinct clinical entity and the largest contributor to melanoma deaths in Victoria, Australia. J Am Acad Dermatol (2013) 68(4):568–75. doi: 10.1016/j.jaad.2012.09.047
22. Teixido C, Castillo P, Martinez-Vila C, Arance A, Alos L. Molecular markers and targets in melanoma. Cells (2021) 10(9):2320. doi: 10.3390/cells10092320
23. Tímár J, Ladányi A. Molecular pathology of skin melanoma: epidemiology, differential diagnostics, prognosis and therapy prediction. IJMS (2022) 23(10):5384. doi: 10.3390/ijms23105384
24. Zhu Z, Liu W, Gotlieb V. The rapidly evolving therapies for advanced melanoma—Towards immunotherapy, molecular targeted therapy, and beyond. Crit Rev Oncology/Hematology (2016) 99:91–9. doi: 10.1016/j.critrevonc.2015.12.002
25. McDermott D, Srivastava N. Update on benefit of immunotherapy and targeted therapy in melanoma: the changing landscape. CMAR. Published Online June (2014) 6:279–89. doi: 10.2147/CMAR.S64979
26. Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet (2014) 383(9919):816–27. doi: 10.1016/S0140-6736(13)60802-8
27. Enninga EAL, Moser JC, Weaver AL, Markovic SN, Brewer JD, Leontovich AA, et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992-2011. Cancer Med (2017) 6(10):2203–12. doi: 10.1002/cam4.1152
28. Walia R, Jain D, Mathur SR, Iyer VK. Spindle cell melanoma: A comparison of the cytomorphological features with the epithelioid variant. Acta Cytologica (2013) 57(6):557–61. doi: 10.1159/000354405
29. Xu Z, Yibulayin F, Shi P, Feng L. Desmoplastic melanoma versus spindle cell melanoma. Med (Baltimore) (2018) 97(29):e11563. doi: 10.1097/MD.0000000000011563
30. Bellenghi M, Puglisi R, Pontecorvi G, De Feo A, Carè A, Mattia G. Sex and gender disparities in melanoma. Cancers (2020) 12(7):1819. doi: 10.3390/cancers12071819
31. Nosrati A, Wei ML. Sex disparities in melanoma outcomes: The role of biology. Arch Biochem Biophys (2014) 563:42–50. doi: 10.1016/j.abb.2014.06.018
32. Ribero S, Stucci L, Marra E, Marconcini R, Spagnolo F, Orgiano L, et al. Effect of age on melanoma risk, prognosis and treatment response. Acta Derm Venerol (2018) 98(7):624–9. doi: 10.2340/00015555-2944
33. Lasithiotakis KG, Petrakis IE, Garbe C. Cutaneous melanoma in the elderly: epidemiology, prognosis and treatment. Melanoma Res (2010) 20(3):163–70. doi: 10.1097/CMR.0b013e328335a8dd
34. Lasithiotakis K, Leiter U, Meier F, Eigentler T, Metzler G, Moehrle M, et al. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer (2008) 112(8):1795–804. doi: 10.1002/cncr.23359
35. Tsai S, Balch C, Lange J. Epidemiology and treatment of melanoma in elderly patients. Nat Rev Clin Oncol (2010) 7(3):148–52. doi: 10.1038/nrclinonc.2010.1
36. Pawelec G. Immunosenescence and cancer. Biogerontology (2017) 18(4):717–21. doi: 10.1007/s10522-017-9682-z
37. Ernst M, Giubellino A. The current state of treatment and future directions in cutaneous Malignant melanoma. Biomedicines (2022) 10(4):822. doi: 10.3390/biomedicines10040822
38. Dawes SM, Tsai S, Gittleman H, Barnholtz-Sloan JS, Bordeaux JS. Racial disparities in melanoma survival. J Am Acad Dermatol (2016) 75(5):983–91. doi: 10.1016/j.jaad.2016.06.006
39. Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer (2016) 16(1):691. doi: 10.1186/s12885-016-2747-6
40. Harvey VM, Patel H, Sandhu S, Wallington SF, Hinds G. Social determinants of racial and ethnic disparities in cutaneous melanoma outcomes. Cancer Control (2014) 21(4):343–9. doi: 10.1177/107327481402100411
41. Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo (2014) 28(6):1005–11.
42. Brady J, Kashlan R, Ruterbusch J, Farshchian M, Moossavi M. Racial disparities in patients with melanoma: A multivariate survival analysis. CCID (2021) Volume 14:547–50. doi: 10.2147/CCID.S311694
43. Sanchez DP, Maymone MBC, McLean EO, Kennedy KF, Sahni D, Secemsky EA, et al. Racial and ethnic disparities in melanoma awareness: A cross-sectional survey. J Am Acad Dermatol (2020) 83(4):1098–103. doi: 10.1016/j.jaad.2020.04.137
44. Coory M, Smithers M, Aitken J, Baade P, Ring I. Urban-rural differences in survival from cutaneous melanoma in Queensland. Aust New Z J Public Health (2006) 30(1):71–4. doi: 10.1111/j.1467-842X.2006.tb00089.x
45. Johnson-Obaseki SE, Labajian V, Corsten MJ, McDonald JT. Incidence of cutaneous Malignant melanoma by socioeconomic status in Canada: 1992–2006. J Otolaryngol - Head Neck Surg (2015) 44(1):53. doi: 10.1186/s40463-015-0107-1
46. Cortez JL, Vasquez J, Wei ML. The impact of demographics, socioeconomics, and health care access on melanoma outcomes. J Am Acad Dermatol (2021) 84(6):1677–83. doi: 10.1016/j.jaad.2020.07.125
47. Shah DR, Yang AD, Maverakis E, Martinez SR. Assessing rural–urban disparities in the use of sentinel lymph node biopsy for melanoma. J Surg Res (2013) 184(2):1157–60. doi: 10.1016/j.jss.2013.04.091
48. Kircher D, Silvis M, Cho J, Holmen S. Melanoma brain metastasis: mechanisms, models, and medicine. IJMS (2016) 17(9):1468. doi: 10.3390/ijms17091468
Keywords: cutaneous melanoma, period analysis method, relative survival rate, SEER, survival trend analyses
Citation: Zheng S, Yu H, Zheng X, Wu UT, Ming W-k, Huang H, Song J, Zhang X, Lyu J and Deng L (2023) Analysis and prediction of 5-year survival in patients with cutaneous melanoma: a model-based period analysis. Front. Endocrinol. 14:1238086. doi: 10.3389/fendo.2023.1238086
Received: 10 June 2023; Accepted: 30 October 2023;
Published: 06 December 2023.
Edited by:
Houjuan Zhu, Institute of Materials Research and Engineering (A*STAR), SingaporeReviewed by:
Ada Girnita, Karolinska Institutet (KI), SwedenCopyright © 2023 Zheng, Yu, Zheng, Wu, Ming, Huang, Song, Zhang, Lyu and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liehua Deng, bGllaHVhZGVuZ0AxMjYuY29t; Jun Lyu, bHl1anVuMjAyMEBqbnUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.