- 1Department of Endocrinology, The Fifth Medical Center of PLA General Hospital, Beijing, China
- 2Department of Orthopedics, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Background: Hepatitis B virus (HBV) infection is a global health concern that can potentially affect bone health. However, the specific association between resolved HBV infection and bone mineral density (BMD) remains unclear. This cross-sectional study aimed to investigate the potential association between resolved HBV infection and femoral and spinal BMD in adults in the United States.
Methods: This cross-sectional study included participants aged 20-79 years with negative HBV surface antigen (HBsAg) from the 2005-2010, 2013-2014, and 2017-2018 cycles of the National Health and Nutrition Examination Survey. Resolved HBV infection was defined as negative HBsAg with positive HBV core antibody. BMD was measured using dual-energy X-ray absorptiometry. Propensity score matching (PSM) was performed to balance baseline characteristics.
Results: A total of 10,333 eligible participants were identified and matched, of whom 737 (7.1%) had resolved HBV infection. Men with resolved HBV infection had significantly lower femoral and spinal BMD compared to those with no HBV infection, both before and after PSM. In the matched population, resolved HBV infection in men was negatively associated with femoral BMD (β= -0.024, 95% CI: -0.047 to -0.002, p = 0.0332) and spinal BMD (β= -0.025, 95% CI: -0.048 to -0.002, p = 0.0339). Postmenopausal women exhibited similar trends to men, while premenopausal women showed a tendency towards higher BMD, although statistical significance was not consistently achieved. Subgroup and sensitivity analyses supported the robustness of the findings.
Conclusion: The study suggests a negative association between resolved HBV infection and femoral and spinal BMD in adult men in the United States. It highlights the importance of routine bone density assessments and the consideration of anti-osteoporotic therapy, if necessary, in individuals with resolved HBV infection.
1 Introduction
Hepatitis B virus (HBV) infection is a global health problem, affecting approximately 240 million people worldwide (1) and 2.2 million in the United States (2). In addition to its potential to cause severe liver disease and liver-related mortality, HBV infection can also lead to extrahepatic complications that significantly impact patients’ prognosis (3, 4). One such complication is osteoporosis, a systemic bone disease characterized by low bone mineral density (BMD). Osteoporosis is a prevalent health concern, with an estimated 12.3 million Americans currently affected by the condition (5). Individuals with chronic hepatitis B infection face a higher risk, with prevalence rates of osteoporosis and osteopenia at 11.5%-12.8% and 23%-24.3%, respectively, compared to lower rates of 4.1%-4.7% for osteoporosis and 9.5%-10.1% for osteopenia in healthy controls (6). Furthermore, individuals with chronic hepatitis B infection have 1.09 times higher odds of developing osteoporosis or experiencing fractures compared to matched controls (7). There is also a trend toward an increase prevalence of osteoporosis among HBV-infected individuals (8). Osteoporosis is associated with a heightened risk of fragility fractures and significantly affects patients’ quality of life. Specifically, femoral region osteoporosis is linked to an elevated risk of all-cause mortality (9).
Resolved HBV infection refers to a state in which an individual who was previously infected with HBV has cleared the virus from their bloodstream and achieved a non-infectious state. This state is typically characterized by the absence of detectable HBV surface antigen (HBsAg) and the presence of HBV core antigen antibody (HBcAb) (10, 11). It is not uncommon, and an estimated 9.87 million U.S. adults are affected (12). Resolved HBV infection is considered a functional cure of hepatitis B, leading to considerable neglect in health management. Nevertheless, individuals in this group do not appear to be fully treated as a normal condition due to the presence of covalently closed circular DNA (cccDNA) in the liver, which can cause ongoing liver damage (12) and potential viral reactivation, especially under conditions of immune suppression (10). Therefore, individuals with resolved HBV infection require special medical monitoring and regular checkups to detect any signs of liver damage and viral reactivation. They may also need antiviral therapy as a preventive measure during immune suppression to prevent viral reactivation. Consequently, there is a possibility that individuals with resolved HBV infection are at risk of developing osteoporosis, similar to those with active HBV infection. However, currently, there is a lack of research on the association between resolved HBV infection and osteoporosis.
In this cross-sectional study, we aimed to investigate the association between resolved HBV infection and osteoporosis in a large population-based study, utilizing data from the National Health and Nutrition Examination Survey (NHANES).
2 Methods
2.1 Data source and subjects
This cross-sectional study is based on data from NHANES, a significant program conducted by the National Center for Health Statistics. NHANES aims to evaluate the health and nutritional status of individuals, including both adults and children, across the United States. The survey collects a wide range of information, including demographic, dietary, examination, laboratory, and questionnaire data, from a nationally representative sample of approximately 5,000 people each year and publishes its findings biennially.
Starting from the 2005-2006 cycle, the NHANES survey began including measurements of femoral and spinal BMD, with the exception of the 2011-2012 and 2015-2016 cycles, during which femoral BMD was not assessed. As a result, this study incorporated data from five NHANES cycles: 2005-2006, 2007-2008, 2009-2010, 2013-2014, and 2017-2018.
In this cross-sectional study, we included individuals aged 20-79 years with negative HBsAg. To minimize potential age bias, we did not include individuals aged 80 years or older due to the limitation of categorizing them all as 80 years old. We excluded participants with incomplete or invalid data on femoral or spinal BMD, as well as those with missing information on covariates and menstrual history. Women were classified into two groups based on menopausal status, following previous literature: premenopausal, if they were still experiencing menstruation or had amenorrhea due to factors such as pregnancy, breastfeeding, or medical conditions/treatments during the previous 12 months; postmenopausal, if they had not had menstrual periods for at least 12 months due to natural menopause or hysterectomy (13, 14). No HBV infection was defined as testing negative for both HBsAg and HBcAg, while resolved HBV infection was defined as testing negative for HBsAg but positive for HBcAg.
The study protocol was approved by the National Center for Health Statistics Research Ethics Review Board. Written informed consent was obtained from all participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
2.2 BMD measurement
Femoral and spinal BMD measurements were conducted using dual-energy X-ray absorptiometry (DXA) in the NHANES. For femoral BMD, Hologic QDR-4500A fan-beam densitometers (Hologic, Inc., Bedford, Massachusetts) were used in all five cycles. As for spinal BMD, Hologic QDR-4500A fan-beam densitometers were used in the 2005-2010 cycles and Hologic Discovery model A densitometers (Hologic, Inc., Bedford, Massachusetts) were used in the 2013-2014 and 2017-2018 cycles. The initial analysis of femoral and spinal BMD scans was performed using Hologic Discovery v12.4 software and then reanalyzed using Hologic APEX v3.0 software in the 2005-2008 cycles. Subsequently, the scans were analyzed with Hologic APEX v3.0 software in the 2009-2010 cycle, and with Hologic APEX v4.0 software in the 2013-2014 and 2017-2018 cycles.
2.3 Covariates
Based on previous literature, clinical experience, and the availability of data within NHANES, the following covariates were included in the analysis: age, race, educational level, marital status, poverty income ratio, physical activity, smoking status, body mass index (BMI), hepatitis B surface antibody (HBsAb), platelets, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), total bilirubin, alkaline phosphatase, estimated glomerular filtration rate (eGFR), cholesterol, triglycerides, fasting glucose (FBG), glycosylated hemoglobin (HbA1c), uric acid, total calcium, phosphorus, history of hypertension, and history of diabetes (4, 15, 16).
According to NHANES, the ethnic groups included in the study are categorized as follows: Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, and other races. Educational level was divided into three categories: under high school, high school or equivalent, and above high school. Marital status was categorized into two groups based on whether individuals lived with a partner. The poverty income ratio was categorized into three levels: ≤1.3, 1.31 to 3.5, and >3.5 (17, 18). Physical activity was classified as either vigorous/moderate or sedentary based on responses to the questions “average level of physical activity each day” or “moderate work activity”. Participants who had smoked 100 cigarettes or more were categorized as smokers, while those who had never smoked or had smoked fewer than 100 cigarettes during their lifetime were classified as non-smokers (4, 19). BMI was calculated as body weight (kg) divided by body height squared (m2), and eGFR was calculated using the CKD-EPI formula. Subjects were considered to have hypertension if they answered “yes” to the questions “have been told you have high blood pressure” or “take a prescription for high blood pressure”. They were considered to have diabetes if they responded “yes” to the questions “doctor has told you have diabetes”, “take insulin now”, or “take diabetic pills to lower blood sugar”.
2.4 Statistical analysis
Descriptive analysis was performed on all participants, where categorical variables were presented as numbers with proportions (%), and continuous variables were reported as means with standard deviation (SD) or medians with interquartile range (IQR), depending on their distribution. To evaluate differences between groups, appropriate statistical tests were applied. The chi-squared test was used for categorical variables, one-way analysis of variance (ANOVA) was employed for normally distributed variables, and the Kruskal-Wallis test was utilized for variables with skewed distributions.
To minimize allocation bias and confounding, linear regression with propensity score matching (PSM) was employed. A 1:1 nearest neighbor matching algorithm with a caliper width of 0.3 was used. All covariates were included in creating the propensity score. The standardized mean difference (SMD) was used to assess the degree of propensity score matching, with a threshold of less than 0.1 considered acceptable. Using the estimated propensity scores as weights, the standardized mortality ratio weighting (SMRW), pairwise algorithmic (PA), and overlap weight (OW) models were used to generate a weighted cohort.
In the subgroup analysis, multivariate linear regression was utilized to evaluate the heterogeneity among subgroups. The regression models included the covariates used in the PSM algorithm for sample matching. Additionally, a likelihood ratio test was conducted to assess the interaction between subgroups and resolved HBV infection.
To assess the robustness of the findings to the matching method, additional analyses were performed using the full cohort. Multivariate linear regression analyses were conducted to examine the independent associations after adjusting for all covariates listed in Tables 1–3. The propensity score was also included as a covariate in the analysis. In addition, stricter exclusion criteria were applied. Participants with a history of chronic liver disease, chronic kidney disease, and cancer were excluded from the analysis, as well as those using steroids, anti-osteoporotic drugs, and hormone replacement therapy, and individuals who were classified as heavy drinkers. Medical history and use of osteoporosis-related medications were assessed through a questionnaire. Heavy drinking was defined as consuming more than 4 standard drinks for men or 3 drinks for women on any day, according to the National Institute on Alcohol Abuse and Alcoholism (20). Further study was conducted, excluding participants with elevated serum ALT or Fibrosis-4 (FIB-4) index > 1.45, to eliminate the potential confounding effects of active hepatitis or cirrhosis. Serum ALT can serve as an indicator of necroinflammatory activity and is a relevant marker available in the NHANES study. The FIB-4 index is a simple and widely used non-invasive tool for assessing liver fibrosis and cirrhosis. It is more precise than AST-to-platelet ratio index (APRI), another commonly used non-invasive tool for assessing liver fibrosis, in chronic hepatitis B (21). It is calculated using the formula: FIB-4 index = [Age (years) × AST (U/L)]/[Platelet count (109 cells/L) × √ALT (U/L)]. The cut-off value of FIB-4 index ≤1.45 was used to differentiate moderate fibrosis from severe fibrosis (21).
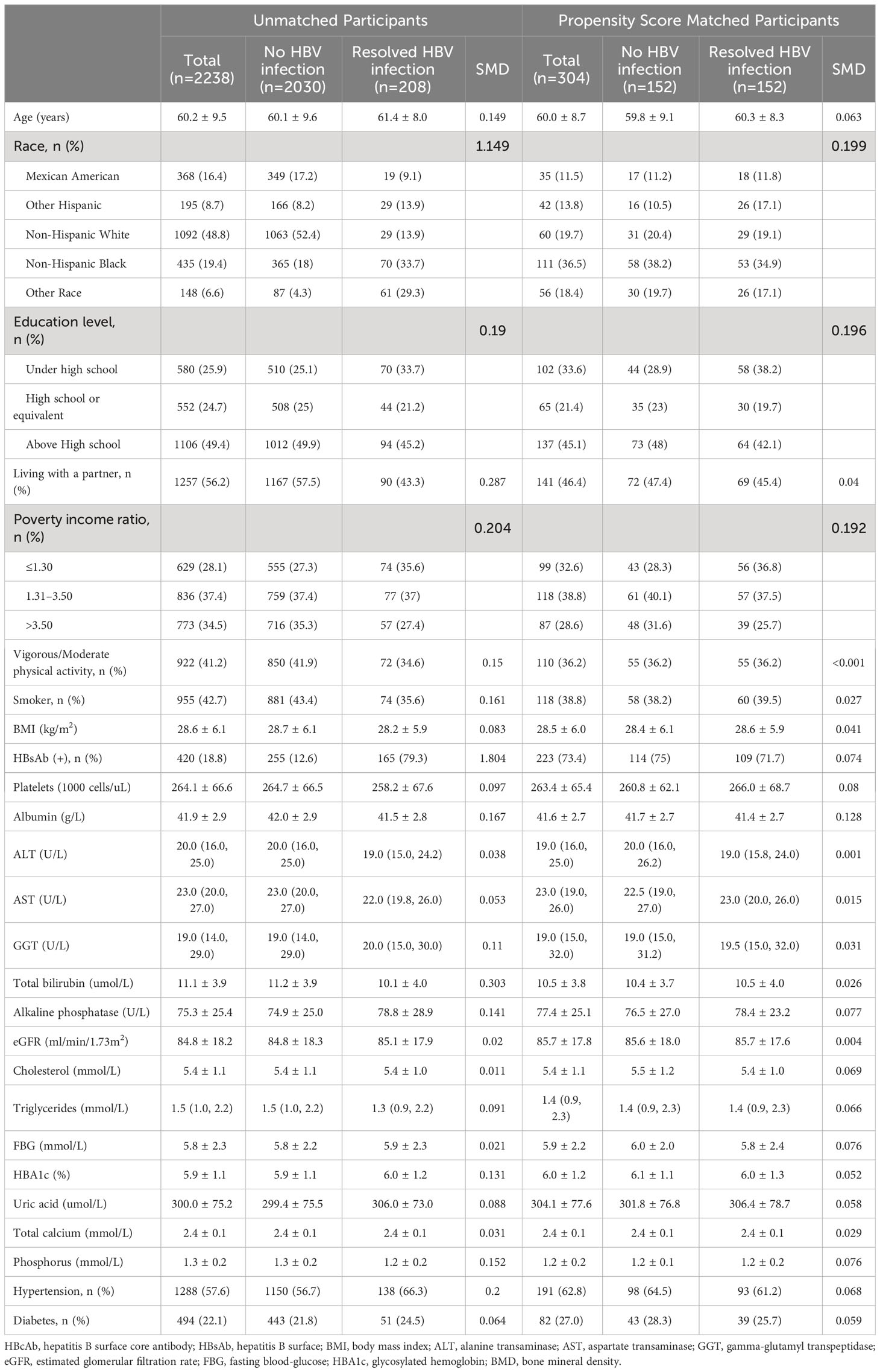
Table 2 Baseline characteristics of postmenopausal women participants before and after propensity score matching.
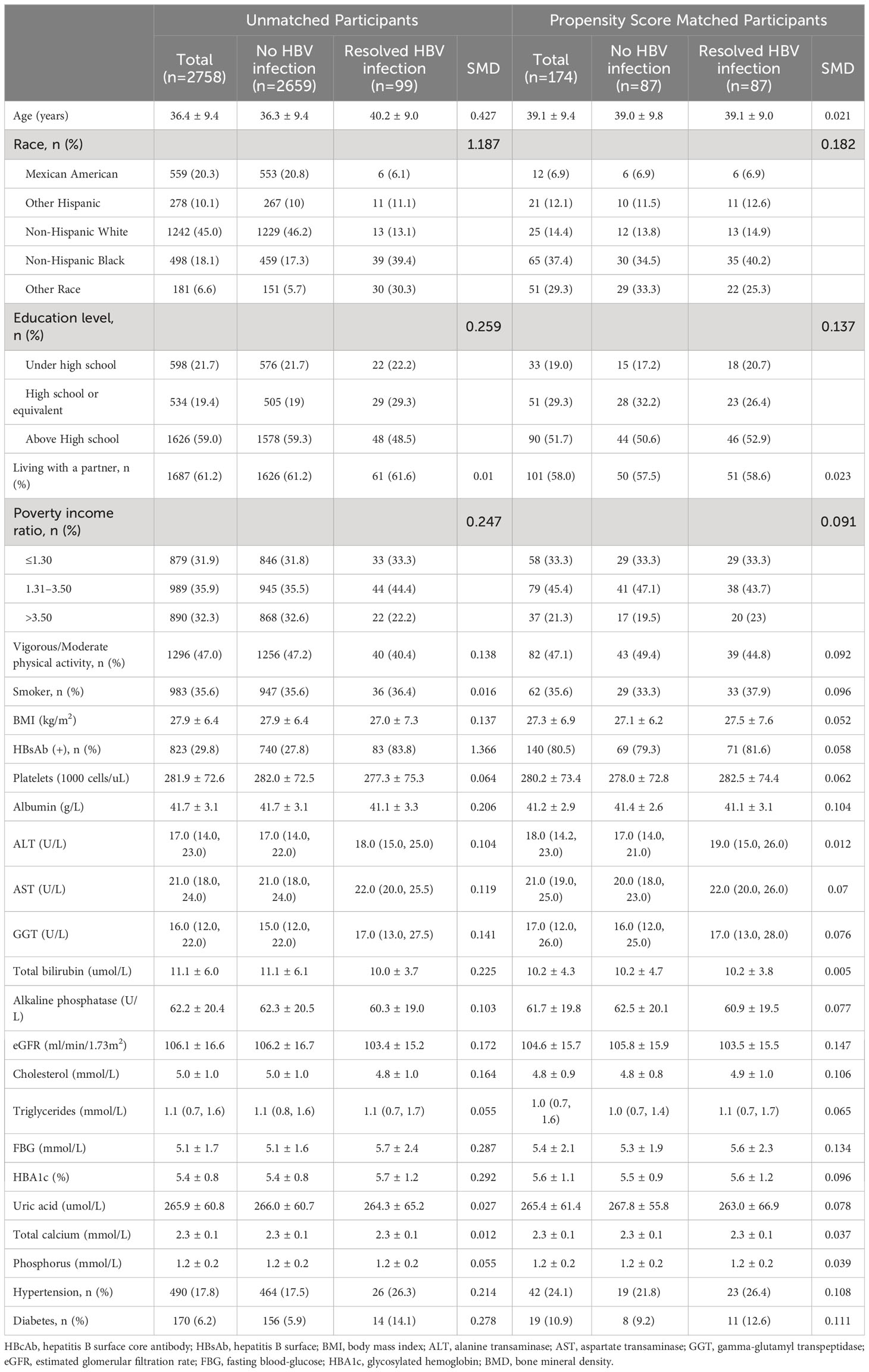
Table 3 Baseline characteristics of premenopausal women participants before and after propensity score matching.
All the analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and Free Statistics software version 1.7. By a two-tailed testing, a p-value of <0.05 was declared significant.
3 Results
3.1 Study population
A total of 19,791 individuals aged 20-79 years with negative HBsAg from the NHANES cycles of 2005-2010, 2013-2014, and 2017-2018 were initially included in this cross-sectional study. After excluding individuals with missing data on femoral BMD (n=4,894), spinal BMD (n=3,200), covariates (n=1,057), and menstrual history (n=307), a final sample of 10,333 participants were included in the analysis. This final sample consisted of 5,337 men, 2,238 postmenopausal women, and 2,758 premenopausal women. Following PSM, 344, 152, and 87 pairs were matched in men, postmenopausal women, and premenopausal women, respectively. The enrollment flowchart is presented in Figure 1.
3.2 Baseline characteristics
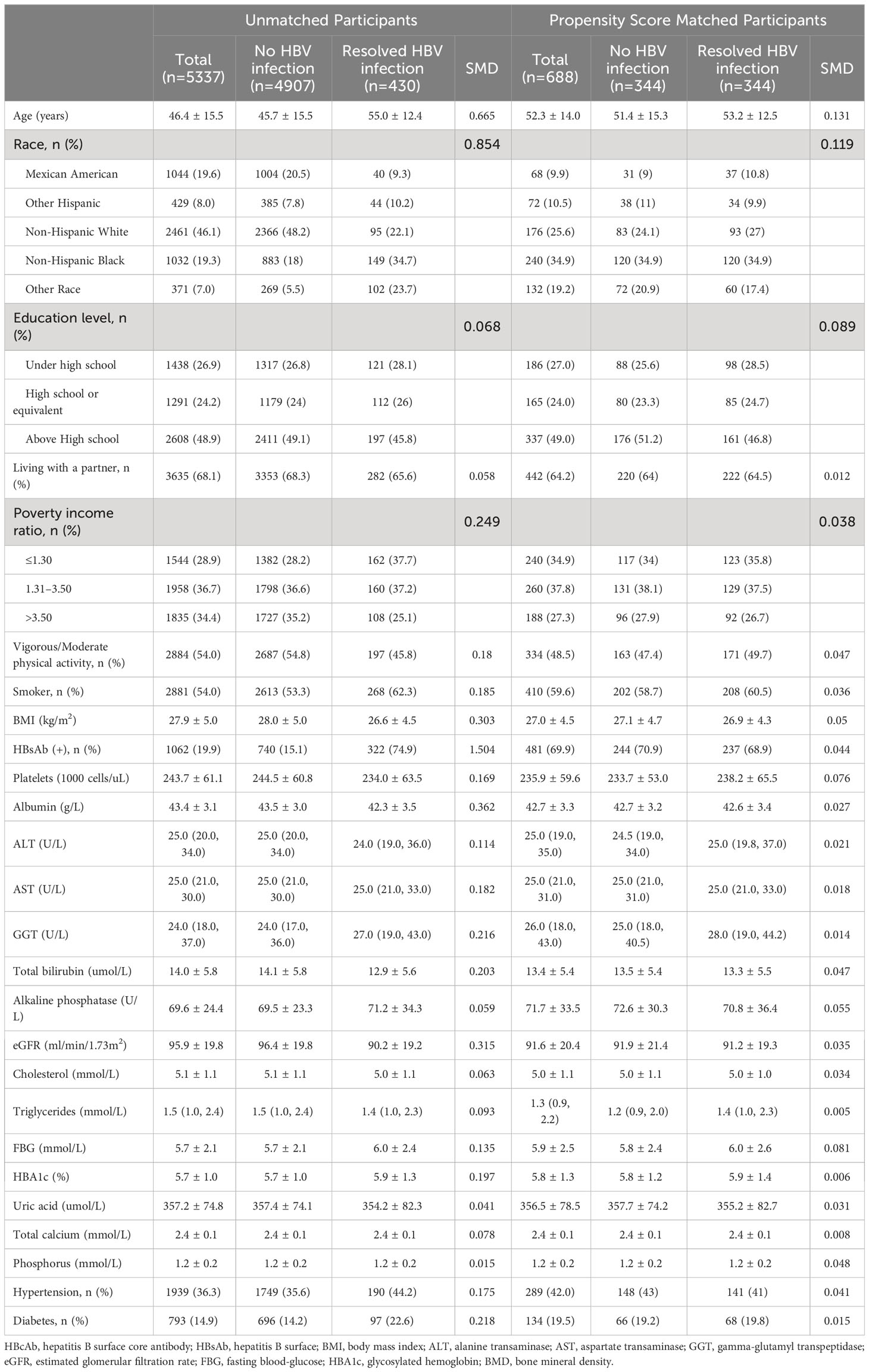
Out of the 10,333 participants, 737 (7.1%) were found to have resolved HBV infection. This included 430 (8.1%) men, 208 (9.3%) postmenopausal women, and 99 (3.6%) premenopausal women.
After PSM, participant characteristics were well-balanced between the no HBV infection and resolved HBV infection groups. In men, postmenopausal women, and premenopausal women, the mean age was 52.3 ± 14.0 years, 60.0 ± 8.7 years, and 39.1 ± 9.4 years, respectively; the mean BMI was 27.0 ± 4.5 kg/m2, 28.5 ± 6.0 kg/m2, and 27.3 ± 6.9 kg/m2, respectively; and the HBsAb positive rate was 69.9%, 73.4%, and 80.5%, respectively. Detailed demographic characteristics of all participants and the matched cohort are presented in Tables 1 to 3.
3.3 Association of resolved HBV infection with femoral and spinal BMD
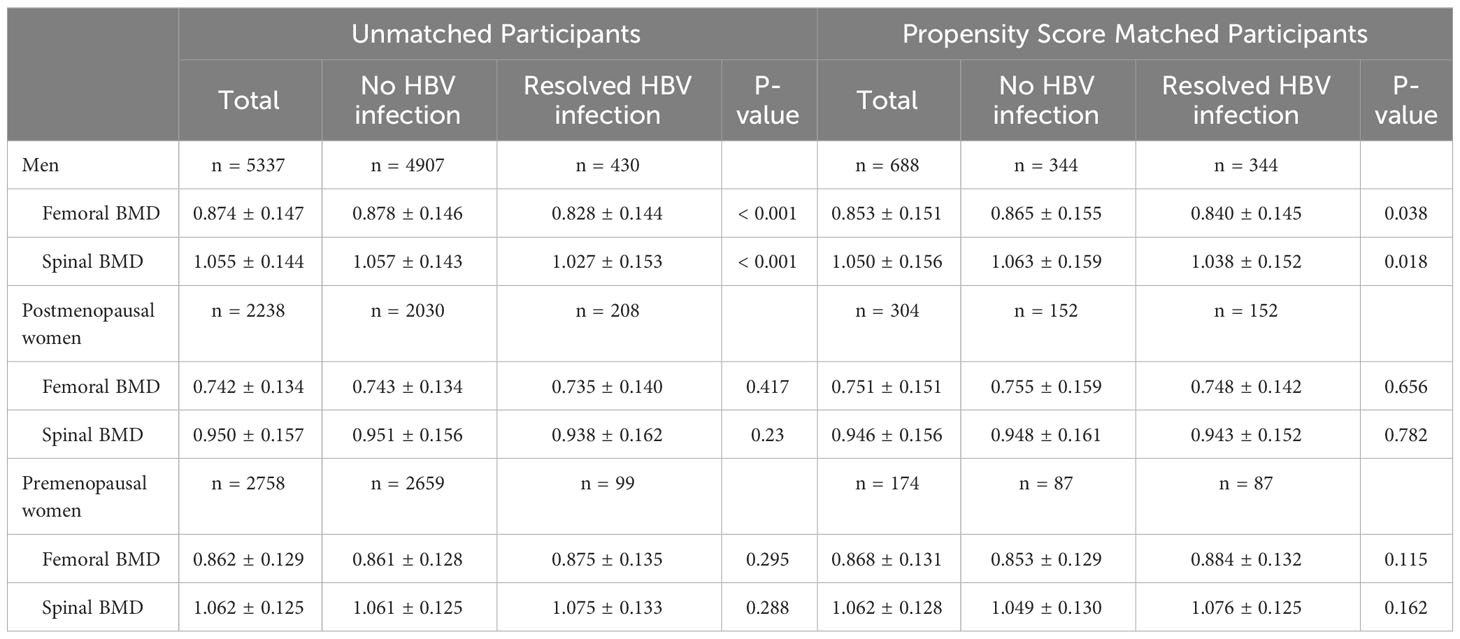
Before PSM, men with resolved HBV infection showed significantly lower femoral and spinal BMD compared to those with no HBV infection (0.828 ± 0.144 g/cm2 vs. 0.878 ± 0.146 g/cm2, p<0.001 and 1.027 ± 0.153 g/cm2 vs. 1.057 ± 0.143 g/cm2, p<0.001, respectively). After PSM, similar findings were still observed (0.840 ± 0.145 g/cm2 vs. 0.865 ± 0.155 g/cm2, p=0.038 and 1.038 ± 0.152 g/cm2 vs. 1.063 ± 0.159 g/cm2, p=0.018, respectively). In postmenopausal women, similar to men, the resolved HBV infection group exhibited lower femoral and spinal BMD both before and after PSM compared to the no HBV infection group. In premenopausal women, the trend was the opposite, with higher BMD observed in the resolved HBV infection group. However, these differences were not statistically significant (Table 4).
In men, after propensity score matching, a negative association was observed between resolved HBV infection and both femoral BMD (β=-0.024, 95%CI: -0.047 to -0.002, p=0.0332) and spinal BMD (β=-0.025 95%CI: -0.048 to -0.002, p=0.0339). Consistent results were obtained using weighting analysis with SMRW, PA, and OW. In postmenopausal women, there were trends suggesting a potential negative effect of resolved HBV infection on femoral and spinal BMD, although not all reached statistical significance. Conversely, in premenopausal women, a positive association between resolved HBV infection and femoral and spinal BMD was observed, reaching statistical significance in certain models (Figure 2).
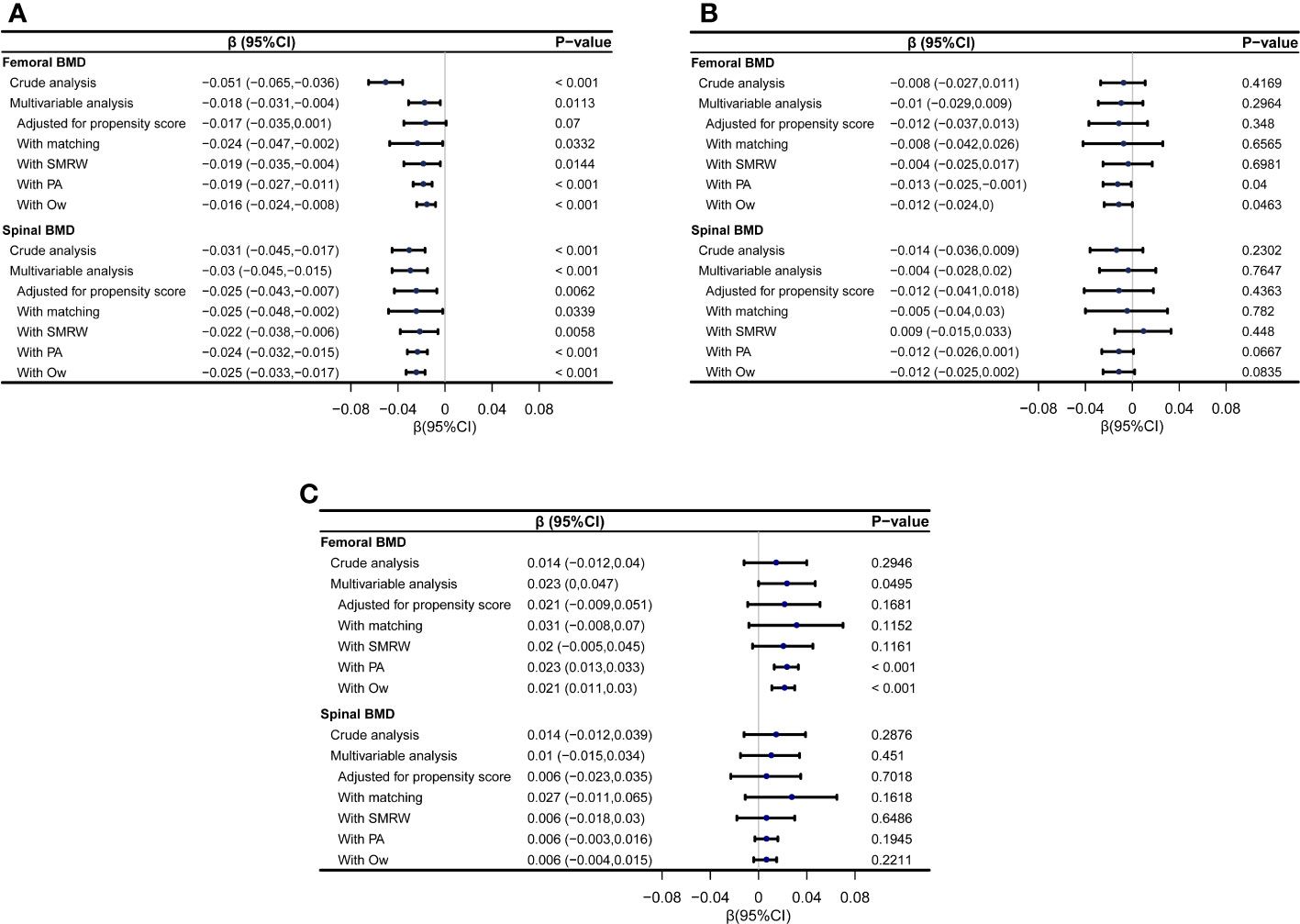
Figure 2 Forest plot showing the associations between resolved HBV infection and femoral and spinal BMD (g/cm2) using different statistical models. (A) the associations in men; (B) the associations in postmenopausal women; (C) the associations in premenopausal women. SMRW, the standardized mortality ratio weighting; PA, pairwise algorithmic; Ow, overlap weight.
3.4 Subgroup analyses
The subgroup analyses in men consistently demonstrated a negative association between resolved HBV infection and femoral and spinal BMD, irrespective of the subgroup being considered. This association was particularly notable in individuals with negative HBsAb and those with coexisting hypertension or diabetes (Figure 3).
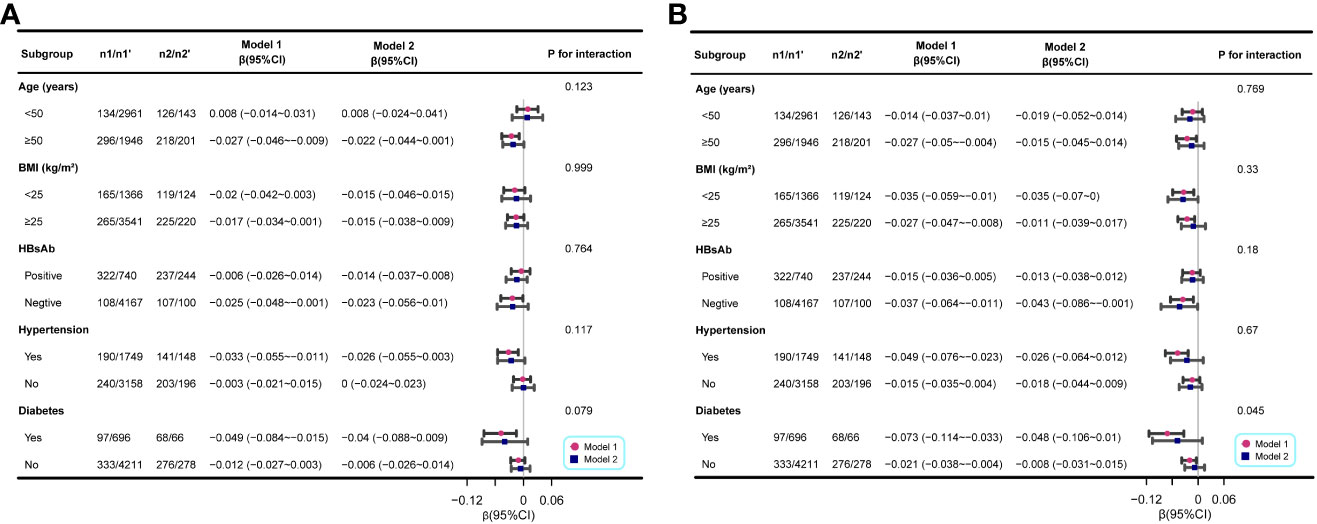
Figure 3 Associations between resolved HBV infection and femoral and spinal BMD (g/cm2) in different stratifications. (A) the associations between resolved HBV infection and femoral BMD; (B) the associations between resolved HBV infection and spinal BMD. The analysis was performed separately for the entire population and the matched population generated through PSM. n1, the number of participants with resolved HBV infection in the entire population; n1’, the number of participants with no HBV infection in the entire population; n2, the number of participants with resolved HBV infection in the matched population; n2’, the number of participants with no HBV infection in the matched population. Each stratification was adjusted for all other variables listed in Tables 1 to 3, except the specific stratification factor itself.
3.5 Sensitivity analyses
Upon adjusting for all covariates listed in Tables 1 to 3, multivariate linear regression analyses in the full-participant cohort yielded consistent findings. In men, resolved HBV infection demonstrated a negative association with femoral BMD (β=-0.018, 95% CI: -0.031 to -0.004, p=0.0113) and spinal BMD (β=-0.03, 95% CI: -0.045 to -0.015, p<0.001). This trend persisted in postmenopausal women, although statistical significance was not reached. In contrast, in premenopausal women, a positive association was observed between resolved HBV infection and femoral BMD (β=0.023, 95% CI: 0 to 0.047, p=0.0495), while no significant association was found with spinal BMD. After adjusting for the propensity score, the results remained stable, although statistical significance was not reached in some models (Figure 2).
The robustness of the results was maintained when applying stricter exclusion criteria. After propensity score matching, the β values for femoral BMD and spinal BMD were as follows: for men, -0.028 (95% CI: -0.055 to -0.002, p=0.0384) and -0.033 (95% CI: -0.061 to -0.006, p=0.0184), respectively; for postmenopausal women, -0.004 (95% CI: -0.059 to 0.05, p=0.8735) and -0.016 (95% CI: -0.072 to 0.041, p=0.5805), respectively; and for premenopausal women, 0.049 (95% CI: 0.003 to 0.096, p=0.0359) and 0.015 (95% CI: -0.03 to 0.059, p=0.5068), respectively. After excluding participants with elevated serum ALT or FIB-4 index > 1.45, the results remained robust, as demonstrated in Supplementary Table 1.
4 Discussion
In this cross-sectional study utilizing a propensity score-matched design involving US adults, the findings indicated a negative association between resolved HBV infection and femoral and spinal BMD in men. The impact of resolved HBV infection on BMD in women was influenced by menopausal status, with postmenopausal women demonstrating similar trends to men. However, premenopausal women showed a tendency towards higher BMD, although statistical significance was not consistently achieved. Subgroup analyses in men consistently supported these results and further revealed that the association was more pronounced in individuals with negative HBsAb and those with coexisting hypertension or diabetes. These results remained robust when the full cohort of participants was analyzed using different adjustment models or when different exclusion criteria were applied. To the best of our knowledge, this is the first study to specifically investigate the relationship between resolved HBV infection and BMD.
Despite the substantial population affected by HBV infection, only a limited number of studies have explored the relationship between HBV infection and BMD. Analyzing data from the NHANES database, which did not differentiate between patients with and without cirrhosis, serologic HBsAg was found to be positively associated with an increased risk of reduced bone mass. The β value for femoral BMD was -0.018 (95% CI: -0.026 to -0.009, P < 0.01), and for spinal BMD was -0.020 (95% CI: -0.030 to -0.010, p < 0.01) (22). Only a few studies have specifically focused on non-cirrhotic HBV infection, which can help elucidate the direct effects of hepatitis B infection itself on BMD, excluding the influence of cirrhosis, a known high-risk factor for osteoporosis (23, 24). One study found that non-cirrhotic chronic hepatitis B infection did not pose a risk for osteoporosis or low BMD. However, this study was limited by its measurement of BMD in the phalanges and the small number of patients (n=15) (25). Other studies have reported contradictory findings. An early German study with a small sample size of non-cirrhotic HBV patients reported lower femoral and spinal BMD (26). This conclusion was supported by two subsequent large-scale clinical studies: one conducted in Korea with 11,306 participants, demonstrating a significant association between HBsAg seropositivity and lower BMD (4), and the other conducted in Taiwan with 51,144 subjects, showing an inverse association between HBV infection and BMD after adjusting for confounding factors (15). In both studies, the negative association between HBV infection and BMD was more pronounced in men, while in women, it did not reach statistical significance and even showed a positive trend with BMD in premenopausal women (4, 15). In our study involving participants with resolved HBV infection, a milder form of hepatitis B infection, the results align with those of previous studies, suggesting that even the short-term presence of hepatitis B virus or persistent cccDNA in men may have an adverse effect on bone mineral density.
The exact mechanism underlying the association between hepatitis B infection and reduced BMD is not yet fully understood. One possible cause is the activation of the immune response and the release of cytokines associated with viral hepatitis, such as interleukin-6, interleukin-1β, and tumor necrosis factor-alpha. These cytokines can interfere with the nuclear factor kappa ligand-nuclear factor kappa-osteoprotegerin (RANKL-RANK-OPG) system, which plays a crucial role in regulating bone homeostasis (24). The medication tenofovir disoproxil fumarate, commonly used to treat hepatitis B, may also directly or indirectly affect BMD (24). In some individuals with advanced liver disease, impaired liver function can lead to decreased hydroxylation of vitamin D3 to its active form (D25), resulting in elevated levels of parathyroid hormone. This hormonal imbalance can increase bone turnover and contribute to bone loss (27). Additionally, impaired liver-produced insulin-like growth factor 1, which is important for bone health, may inhibit osteoblast differentiation and proliferation, leading to bone mass loss (28). Other potential mechanisms that have been suggested including alterations in the gut microbiota and the presence of sarcopenia, a condition characterized by loss of skeletal muscle mass and strength, which can indirectly affect bone health (24).
The inconsistent results across gender and menopausal status may be attributed to the important role of estrogen in osteoprotective effects (29). This is supported by similar trends observed in studies on TSH suppression therapy after surgery for differentiated thyroid cancer, highlighting the protective properties of estrogen on bone density (30). In addition, liver damage caused by HBV infection has the potential to interfere with estrogen inactivation, resulting in elevated estrogen levels, especially in premenopausal women who naturally have higher estrogen levels, and a consequent increase in the osteoprotective effects of estrogen. Moreover, younger patients, who receive advanced health education, tend to adopt healthier lifestyles, contributing to higher bone density. Furthermore, the shorter duration of current HBV infection and cccDNA in premenopausal women, due to advances in medical treatments and their younger age, has a relatively minor impact on bone metabolism. Further research is needed to confirm these hypotheses.
HBsAb seroconversion indicates stable viral control, and its positivity is associated with a higher likelihood of sustained HBsAg loss and a lower occurrence of occult HBV/HBV reactivation (12).In our study, we observed that participants who tested positive for HBsAb had less BMD loss compared to those who tested negative for HBsAb, suggesting a protective effect of HBsAb on BMD. Previous studies have reported an association between hypertension, diabetes, and increased bone loss (31–33). In our subgroup analyses, we observed a more significant decrease in BMD among participants with comorbidities of hypertension or diabetes. These findings emphasize the importance of paying special attention to these high-risk groups.
This study has several limitations. Firstly, due to the inherent limitations of cross-sectional studies, a causal relationship between resolved HBV infection and decreased BMD cannot be established. Future well-designed cohort studies are needed to confirm this relationship. Secondly, like all retrospective analyses, there is a potential for residual confounding. We made efforts to adjust for various potential confounders and achieved a good balance in the propensity score-matched cohorts. Additionally, we conducted stratified and sensitivity analyses to minimize this bias. Thirdly, the presence of occult hepatitis B infection (OBI) could not be definitively excluded. OBI is characterized by the presence of replication-competent HBV DNA in the liver, with or without detectable HBV DNA in the blood, in individuals who test negative for serum HBsAg using current assays (34). Unfortunately, assessing HBV DNA in the liver is challenging in large-scale epidemiological studies, and data on both liver and blood HBV DNA levels were not available in NHANES. However, the combination of negative HBsAg and positive HBcAb has been widely used as a reliable surrogate marker for resolved HBV infection (35), demonstrating a specificity of over 95% and an overall accuracy of 90% in previous studies (36). In addition, it is worth noting that the duration of active HBV infection before the loss of HBs antigen could potentially influence bone demineralization. However, due to the unavailability of relevant data in the NHANES database, we were unable to include this analysis in our study. Future research should focus on investigating this aspect.
5 Conclusions
The findings of the cross-sectional study reveal a significant negative association between resolved HBV infection and BMD in the femoral and spinal regions among adult men in the United States. This discovery is of particular importance due to the tendency to overlook resolved HBV infection. Consequently, it is crucial to underscore the significance of routine bone density assessments and, if necessary, consider implementing anti-osteoporotic therapy in individuals with resolved HBV infection.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by The National Center for Health Statistics Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YY, ZQ, and YF conceived and designed the study. XF, QW, and XW collected the data. YY, JZ, and TZ analysed the data and drafted the manuscript. JW assisted in data analysis and proofread the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This is a self-funded study.
Acknowledgments
We would like to express our gratitude to our colleagues in the Department of Endocrinology at the Fifth Medical Center of PLA General Hospital for their valuable assistance in the present study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1237618/full#supplementary-material
References
1. Liang TJ, Block TM, McMahon BJ, Ghany MG, Urban S, Guo JT, et al. Present and future therapies of hepatitis B: From discovery to cure. Hepatology (2015) 62:1893–908. doi: 10.1002/hep.28025
2. Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the advisory committee on immunization practices. MMWR Recomm Rep (2018) 67:1–31. doi: 10.15585/mmwr.rr6701a1
3. Liang TJ. Hepatitis B: the virus and disease. Hepatology (2009) 49:S13–21. doi: 10.1002/hep.22881
4. Baeg MK, Yoon SK, Ko SH, Han KD, Choi HJ, Bae SH, et al. Males seropositive for hepatitis B surface antigen are at risk of lower bone mineral density: the 2008-2010 Korea National Health and Nutrition Examination Surveys. Hepatol Int (2016) 10:470–7. doi: 10.1007/s12072-015-9672-7
5. LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int (2022) 33:2049–102. doi: 10.1007/s00198-021-05900-y
6. Huang Z, Wei H, Cheng C, Yang S, Wang J, Liu X. Low bone mineral density in chronic hepatitis B virus infection: A case-control study. Pak J Med Sci (2017) 33:457–61. doi: 10.12669/pjms.332.12099
7. Oh H, Jun DW, Lee IH, Ahn HJ, Kim BO, Jung S, et al. Increasing comorbidities in a South Korea insured population-based cohort of patients with chronic hepatitis B. Aliment Pharmacol Ther (2020) 52:371–81. doi: 10.1111/apt.15867
8. Liu A, Le A, Zhang J, Wong C, Wong C, Henry L, et al. Increasing co-morbidities in chronic hepatitis B patients: experience in primary care and referral practices during 2000-2015. Clin Transl Gastroenterol (2018) 9:141. doi: 10.1038/s41424-018-0007-6
9. Cai S, Fan J, Zhu L, Ye J, Rao X, Fan C, et al. Bone mineral density and osteoporosis in relation to all-cause and cause-specific mortality in NHANES: A population-based cohort study. Bone (2020) 141:115597. doi: 10.1016/j.bone.2020.115597
10. Mucke MM, Backus LI, Mucke VT, Coppola N, Preda CM, Yeh ML, et al. Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol (2018) 3:172–80. doi: 10.1016/S2468-1253(18)30002-5
11. Kusumoto S, Arcaini L, Hong X, Jin J, Kim WS, Kwong YL, et al. Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood (2019) 133:137–46. doi: 10.1182/blood-2018-04-848044
12. Huang SW, Chen C, Kong HY, Huang JQ. Prevalence of cirrhosis/advanced fibrosis among HBsAg-negative and HBcAb-positive US adults: A nationwide population-based study. Infect Dis Ther (2022) 11:1906–16. doi: 10.1007/s40121-022-00680-2
13. Huang Y, Guo J, Lv N, Li S, Wu Y, Bai R, et al. Associations of urinary polycyclic aromatic hydrocarbons with age at natural menopause in U.S. women aged 35-65, NHANES 2003-2012. Environ pollut (2018) 243:1878–86. doi: 10.1016/j.envpol.2018.09.109
14. Taylor KW, Hoffman K, Thayer KA, Daniels JL. Polyfluoroalkyl chemicals and menopause among women 20-65 years of age (NHANES). Environ Health Perspect (2014) 122:145–50. doi: 10.1289/ehp.1306707
15. Chen YY, Fang WH, Wang CC, Kao TW, Chang YW, Yang HF, et al. Crosssectional assessment of bone mass density in adults with hepatitis B virus and hepatitis C virus infection. Sci Rep (2019) 9:5069. doi: 10.1038/s41598-019-41674-4
16. Ciardullo S, Muraca E, Zerbini F, Manzoni G, Perseghin G. NAFLD and liver fibrosis are not associated with reduced femoral bone mineral density in the general US population. J Clin Endocrinol Metab (2021) 106:e2856–e65. doi: 10.1210/clinem/dgab262
17. Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013-2014. Environ Int (2019) 131:105057. doi: 10.1016/j.envint.2019.105057
18. Hu Z, Li Y, Yang Y, Yu W, Xie W, Song G, et al. Serum lipids mediate the relationship of multiple polyaromatic hydrocarbons on non-alcoholic fatty liver disease: A population-based study. Sci Total Environ (2021) 780:146563. doi: 10.1016/j.scitotenv.2021.146563
19. Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. Trends in osteoporosis and low bone mass in older US adults, 2005-2006 through 2013-2014. Osteoporos Int (2017) 28:1979–88. doi: 10.1007/s00198-017-3996-1
20. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology (2018) 67:328–57. doi: 10.1002/hep.29367
21. Mallet V, Dhalluin-Venier V, Roussin C, Bourliere M, Pettinelli ME, Giry C, et al. The accuracy of the FIB-4 index for the diagnosis of mild fibrosis in chronic hepatitis B. Aliment Pharmacol Ther (2009) 29:409–15. doi: 10.1111/j.1365-2036.2008.03895.x
22. Tao J, Yan Z, Huang W, Feng T. Seropositive for hepatitis B and C viruses is associated with the risk of decreased bone mineral density in adults: An analysis of studies from the NHANES database. Front Med (Lausanne) (2023) 10:1120083. doi: 10.3389/fmed.2023.1120083
23. Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut (2002) 50 Suppl 1:i1–9. doi: 10.1136/gut.50.suppl_1.i1
24. Jeong HM, Kim DJ. Bone diseases in patients with chronic liver disease. Int J Mol Sci (2019) 20:4270. doi: 10.3390/ijms20174270
25. Yenice N, Gumrah M, Mehtap O, Kozan A, Turkmen S. Assessment of bone metabolism and mineral density in chronic viral hepatitis. Turk J Gastroenterol (2006) 17:260–6.
26. Schiefke I, Fach A, Wiedmann M, Aretin AV, Schenker E, Borte G, et al. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J Gastroenterol (2005) 11:1843–7. doi: 10.3748/wjg.v11.i12.1843
27. Wintermeyer E, Ihle C, Ehnert S, Stockle U, Ochs G, de Zwart P, et al. Crucial role of vitamin D in the musculoskeletal system. Nutrients (2016) 8:319. doi: 10.3390/nu8060319
28. Kawai M, Rosen CJ. The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol Metab Clin North Am (2012) 41:323–33, vi. doi: 10.1016/j.ecl.2012.04.013
29. Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest (2016) 126:2049–63. doi: 10.1172/JCI86062
30. Ku EJ, Yoo WS, Lee EK, Ahn HY, Woo SH, Hong JH, et al. Effect of TSH suppression therapy on bone mineral density in differentiated thyroid cancer: A systematic review and meta-analysis. J Clin Endocrinol Metab (2021) 106:3655–67. doi: 10.1210/clinem/dgab539
31. Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Study Osteoporotic Fractures Res Group Lancet (1999) 354:971–5. doi: 10.1016/s0140-6736(99)01437-3
32. Cakmak HA, Cakmak BD, Yumru AE, Aslan S, Enhos A, Kalkan AK, et al. The relationships between blood pressure, blood glucose, and bone mineral density in postmenopausal Turkish women. Ther Clin Risk Manag (2015) 11:1641–8. doi: 10.2147/TCRM.S95017
33. Hofbauer LC, Busse B, Eastell R, Ferrari S, Frost M, Muller R, et al. Bone fragility in diabetes: novel concepts and clinical implications. Lancet Diabetes Endocrinol (2022) 10:207–20. doi: 10.1016/S2213-8587(21)00347-8
34. Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol (2019) 71:397–408. doi: 10.1016/j.jhep.2019.03.034
35. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol (2017) 67:370–98. doi: 10.1016/j.jhep.2017.03.021
Keywords: resolved hepatitis B virus infection, femoral bone mineral density, spinal bone mineral density, adults, NHANES
Citation: Yang Y, Zeng J, Zhang T, Wang J, Fan X, Wang Q, Wang X, Qi Z and Fang Y (2023) Association between resolved hepatitis B virus infection and femoral and spinal bone mineral density in American adults: a cross-sectional study. Front. Endocrinol. 14:1237618. doi: 10.3389/fendo.2023.1237618
Received: 09 June 2023; Accepted: 18 August 2023;
Published: 27 September 2023.
Edited by:
Antonino Catalano, University of Messina, ItalyReviewed by:
Luciana Diniz Silva, Federal University of Minas Gerais, BrazilAndre Lyra, Federal University of Bahia (UFBA), Brazil
Copyright © 2023 Yang, Zeng, Zhang, Wang, Fan, Wang, Wang, Qi and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengrong Qi, cWl6aGVuZ3JvbmdAY2NtdS5lZHUuY24=; Yi Fang, ZmFuZ3lpNXpob25neGluQDE2My5jb20=
†These authors have contributed equally to this work
 Yan Yang
Yan Yang Jing Zeng
Jing Zeng Tingting Zhang
Tingting Zhang Jinjing Wang
Jinjing Wang Xiaojing Fan
Xiaojing Fan Qiaomin Wang1
Qiaomin Wang1 Xuan Wang
Xuan Wang